Abstract
We previously reported that a vaccine composed of liposome-mannan complexes of Candida albicans (L-mann) stimulates mice to produce protective antibodies against disseminated candidiasis. An immunoglobulin M (IgM) monoclonal antibody (MAb), B6.1, specific for a β-1,2-mannotriose in the complexes protects against the disease, whereas MAb B6 does not. In the present study, the vaccine and MAbs B6.1 and B6 were tested for the ability to protect against Candida vaginal infection, established by intravaginal (i.vg.) inoculation of yeast cells in mice maintained in pseudoestrus. Fungal CFU in each vagina was determined to assess the severity of infection. Mice vaccinated before infection developed about 62% fewer vaginal CFU than nonimmunized controls. Naive mice that received polyclonal antiserum (from vaccinated mice) i.vg. before infection had 60% fewer CFU than controls. The serum protective factor was stable at 56°C, but C. albicans cells absorbed this factor. Mice given MAb B6.1 i.vg. after infection was established had fewer Candida CFU in vaginal tissue than control mice given buffer instead of antibody. MAbs B6.1 and B6 given intraperitoneally before infection protected mice, but MAbs preabsorbed with yeast cells did not. MAb B6.1 also protected against C. tropicalis vaginal infection, but MAb B6 did not. The protective activities of MAbs B6.1 and B6 appeared to be specific because an irrelevant IgM carbohydrate-specific MAb and an irrelevant IgG protein-specific MAb were not protective; also, MAb B6.1 did not affect development of vaginal chlamydial infection. These studies show that an appropriate antibody response, or administration of protective antibodies, can help the host to resist Candida vaginal infection.
Vaginal candidiasis, a mucosal infection caused by Candida species (39), is one of the most common infections in women (41). An estimated 75% of all females experience at least one episode of the disease during their lifetime (40). In the United States, there are approximately 13 million cases of vaginal candidiasis annually (34). C. albicans is the most common etiologic agent (14, 22), but other Candida species such as C. tropicalis, C. glabrata, and C. parapsilosis also cause the disease (22, 30).
Topical and/or oral administration of antifungal drugs is used for the prevention and treatment of vaginal candidiasis (2, 5, 41). In otherwise healthy individuals, however, antifungal drugs are used after the onset of disease; thus, these patients must suffer symptoms before seeking therapy, and in some the disease will recur after discontinuation of the drug (22, 30). Newly developed triazoles have been beneficial in prevention and treatment of candidiasis; however, azole-resistant strains of C. albicans are emerging (9, 36, 42), and prolonged preventive use of antifungal drugs in healthy individuals is unwarranted. These problems led us to consider alternative preventive and therapeutic approaches.
Host immunological defenses that protect against Candida vaginal infection are not well defined and may involve both cell- and antibody-mediated mechanisms. Vaginal immunization with C. albicans protected pseudoestrous mice against experimental vaginal infection (11), and local cell-mediated immunity may have a role in host defense against this condition (16). The role of a specific antibody in host defense against Candida vaginitis has been questioned because patients with this condition are likely to have antibodies of various isotypes in vaginal secretions (15, 31, 37). Cassone et al. (8) showed, however, that antibodies, apparently against mannan and secretory aspartyl proteinases of C. albicans, mediated protection against infection in ovariectomized and estrogen-treated rats.
In previous work we focused on the role of antibodies to Candida in host defense against disseminated candidiasis. Vaccination with liposome-encapsulated C. albicans surface mannan (L-mann) provoked a protective antibody response against disseminated disease due to either C. albicans or C. tropicalis (16). A monoclonal antibody (MAb), B6.1, specific for the Candida mannan, enhanced resistance of normal and SCID mice against disseminated candidiasis (16) and had a protective effect in neutropenic mice (17). A second MAb, B6, did not show protective activity (16, 17). Both MAbs are immunoglobulin M (IgM), and both agglutinated yeast cells (16). MAb B6.1 is specific for a β-1,2-mannotriose (18), which is an acid-labile component in the phosphomannoprotein complex of the cell wall (38). MAb B6 is specific for a mannan epitope in the acid-stable part of the complex (unpublished data).
In this study, we tested the ability of the L-mann vaccine and the MAbs to enhance resistance of mice to Candida vaginal infection. All of these reagents showed protective effects.
MATERIALS AND METHODS
Organism and culture conditions.
C. albicans CA-1, previously characterized as a serotype A strain by use of rabbit anti-C. albicans serum developed by Hasenclever et al. (19, 20), is a serotype B strain according to the Candida-Check system (Iatron Laboratories Inc., Tokyo, Japan). C. tropicalis CT-4 is from our stock culture collection. Species classification was confirmed by API 20C yeast identification strips (BioMerieux Vitek, Inc., Hazelwood, Mo.), and this strain was used in a previous study (16). Stock cultures were stored at −20°C. New yeast suspensions were started each week from the stock cultures and grown as hydrophilic stationary-phase yeast cells in glucose-yeast extract-peptone broth at 37°C as previously described (21). Yeast cells were harvested from the broth cultures by centrifugation, washed in cold (0 to 4°C) sterile deionized water, suspended to the desired yeast cell concentration in cold sterile Dulbecco’s phosphate-buffered saline (DPBS; Sigma Chemical Co., St. Louis, Mo.), and used immediately to infect the test mice.
Mice.
BALB/c female mice obtained from either The Jackson Laboratory (Bar Harbor, Maine) or Charles River Laboratories (Kingston, N.Y.) were used at 6 to 9 weeks of age. Three days before infection with C. albicans, each mouse received a subcutaneous (s.c.) injection of estradiol valerate (0.5 mg per mouse; Sigma) suspended in sterile sesame oil (Sigma) to induce pseudoestrus (12, 13). Chlamydia infection experiments are described below. In all experiments, mice were maintained in accordance with institutional regulations in an Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facility at Montana State University (MSU).
MAbs.
MAbs B6.1 and B6 were isolated and characterized as previously described (3, 16, 18); both were produced in serum-free medium by LigoCyte Pharmaceuticals, Inc., Bozeman, Mont. Starting antibody concentrations and purity of the MAbs were estimated as before (18). The concentrations of MAbs B6.1 and B6 in the final preparations were estimated at 3.5 and 3.4 mg/ml, respectively. As a control antibody, an irrelevant IgM MAb, S9, which is specific for type III group B streptococcal polysaccharide (32), was used. The S9 hybridoma clone was a generous gift from Seth Pincus, MSU, and MAb S9 was produced in the same way at LigoCyte Pharmaceuticals. The starting concentration of MAb S9 was estimated at 4.6 mg/ml. An irrelevant IgG MAb, termed 54.1 and specific for human neutrophil cytochrome b (4) (a generous gift from Jim Burritt and Al Jesaitis, MSU), was used as an additional negative control at a starting concentration of 5.0 mg/ml, as estimated by the above methods. MAbs S9 and 54.1 were used in experiments at the same concentrations as MAbs B6.1 and B6.
All antibody preparations were tested for the presence of endotoxin contamination by the Limulus amebocyte lysate test (E-Toxate kit; Sigma). This test was performed according to the manufacturer’s guidelines.
L-mann vaccine.
The L-mann vaccine was prepared as previously described (16). In brief, a C. albicans cell surface mannan-enriched fraction was isolated by a β-mercaptoethanol treatment of yeast cells and encapsulated into multilamellar liposomes. Control liposomes (L-DPBS) were prepared by adding diluent buffer (DPBS) instead of mannan during the preparation as previously described (16). Control and test liposomes were identical with respect to microscopic appearance.
Preventive effect of active immunization.
Before vaginal infection with C. albicans, mice were immunized with the L-mann vaccine by intravenous (i.v.) injection once a week for a total of 5 weeks as previously described (16). Each injection consisted of 0.2 ml of L-mann, which contained 178 μg of the enriched mannan fraction. Control mice received L-DPBS or buffer (DPBS) alone. Four days after the fifth immunization, all mice were given estradiol s.c. Three days later, they were infected with C. albicans (5 × 105 yeast cells) intravaginally (i.vg.) by gentle insertion of a thin micropipette tip into the vaginal tract and delivery of 10 μl containing the desired number of the yeast cells. Two days after the infection, the animals were sacrificed and CFU of vaginal tissue was determined as indicated below.
Passive transfer of PAbs.
To determine if antibodies in the sera from vaccinated mice were responsible for the protection induced by active immunization, polyclonal antisera (PAbs) were obtained from L-mann-vaccinated mice and pooled. The pooled PAb had an agglutinin titer of 40 to 80 (16) and was either immediately stored at −20°C, heated at 56°C for 30 min, and stored at −20°C or absorbed with C. albicans yeast cells and stored as previously described (16). As an additional control, one mouse group received normal mouse serum (NMS) obtained from mice that were given only DPBS. Three days after being given estradiol s.c., naive mice were divided into groups of four; each group received one of the various test sera (30 μl per mouse) i.vg. by gentle insertion of a thin micropipette tip into the vaginal tract. Four hours later, all mice were challenged i.vg. with C. albicans (5 × 105 yeast cells). All animals received a second dose (10 μl) of serum or buffer i.vg. 24 h after the first dose. Forty-four hours after the first dose of serum, vaginal CFU was determined and evaluated as indicated below.
Passive transfer of MAbs by the i.vg. or intraperitoneal (i.p.) route.
The preventive effects of MAbs B6.1 and B6 were examined by the same injection schedules as above for experiments on PAbs. Intravaginal doses of either MAb for the first (i.e., at 4 h before infection) and second (i.e., at 20 h after infection) treatments were 35 and 10 μg per mouse in volumes of 30 and 10 μl, respectively. Control mice received equivalent volumes of the DPBS diluent.
The therapeutic effect of MAb B6.1 was also tested by the i.vg. route. Mice were given estradiol s.c., and 3 days later these pseudoestrous mice were infected with C. albicans (5 × 105 yeast cells/mouse) i.vg. At 4 and 24 h after infection, the animals were given 35 and 10 μg, respectively, of MAb B6.1. Forty-eight hours after the infection, vaginal CFU was measured and evaluated as indicated below.
In some experiments, groups of mice received the antibody or diluent i.p. instead of i.vg. In this test, the antibodies were given again in two doses at 4 h before and 20 h after infection, as described above and previously for experimental disseminated candidiasis (16). The doses of antibodies for the first and second treatments were approximately 106 and 42.5 μg per mouse in volumes of 0.5 and 0.2 ml, respectively. In addition to the various MAb preparations, the hybridoma serum-free medium (HB101) that was concentrated and prepared as for antibody production was tested for its effect on vaginal infection.
The preventive effect of the i.p. administration of MAbs B6.1 and B6 against vaginal infection due to C. tropicalis was also determined. Dosages of antibodies and injection schedules prior to infection were identical to those for the above experiments except that each animal was infected i.vg. with 106 C. tropicalis yeast cells in a 10-μl volume.
Assessment of resistance and susceptibility to Candida vaginal infection.
To determine relative susceptibility or resistance to vaginal infection, the number of Candida CFU per gram of vaginal tissue was determined as previously described for kidney CFU measurements (16, 17, 33). In brief, the entire vagina was removed from each infected mouse, weighed, and homogenized in 1 ml of DPBS with a hand-held glass tissue homogenizer. Appropriate dilutions of the vaginal tissue homogenates were plated onto Mycobiotic agar (Difco Laboratories, Detroit, Mich.) and incubated at 37°C; 48 h later, Candida colonies were counted. A minimum of four mice per group was tested, and each experiment was repeated at least three times.
C. trachomatis genital infections.
As an additional test for specificity, MAbs B6.1 and B6 were tested for protective effects against C. trachomatis infection in mice. Vaginal infection with the mouse pneumonitis C. trachomatis strain was performed as described previously (27), with the following modifications. Three groups of five mice received 2.5 mg of Dep-provera (medroxy-progesterone acetate) subcutaneously at 10 and 3 days prior to vaginal infection. Four hours before and 20 h after infection, mice were injected i.p. as described above with either MAb B6.1, MAb B6, or DPBS. The course of infection was monitored by swabbing the vaginal tract at various times after infection and enumerating inclusion-forming units by isolation onto HeLa cell monolayers. Inclusions were visualized by indirect immunofluorescent staining (44).
Detection of antibody in vaginal fluid and tissue.
Mice received MAb B6.1 or B6 by the i.p. route and were infected i.vg. as described above. Control mice received DPBS instead of antibody. For immunofluorescence detection of antibody, vaginal fluid containing Candida cells was collected by vaginal lavage with cold sterile DPBS (400 μl per mouse) 5 or 24 h after the first dose and 24 h after the second dose of antibody. The C. albicans cells present in the vaginal fluid were washed twice with 400 μl of cold DPBS. The washed yeast cell pellet from the second wash was suspended in 50 μl of goat anti-mouse IgM (μ-chain specific; 1.0 mg/ml; diluted 1:30 in DPBS; Sigma) in a microcentrifuge tube. The mixture in the tube was plunged into ice and incubated for 30 min. The fungal cells were washed three times in 1 ml of cold DPBS, suspended in 500 μl of fluorescein isothiocyanate-conjugated rabbit anti-goat IgG (anti-whole molecule; 3.0 mg/ml; diluted 1:200 in DPBS; Sigma), incubated in an ice bath for 30 min, and washed three times as described above. Evidence of antibody on the fungal cell surface was determined by examination of the cells by confocal fluorescence microscopy (Nikon DVC-250 scanning confocal argon-krypton laser [488-nm excitation wavelength] microscope, equipped with a 60× oil immersion lens, numerical aperture 1.4, as obtained from Bio-Rad, Hercules, Calif.).
In other experiments, vaginal fluid was collected from mice that received MAb B6.1 or B6 by the i.p. route as described above, but these animals were not infected with C. albicans. The amount of antibody given to mice was usually the same as indicated above, but some groups of mice received a 16-fold-higher dose of MAb B6.1 before vaginal lavage. The vaginal lavage fluid from each animal was collected by washing the vagina with sterile DPBS (400 μl in total) 4 h after the first dose or immediately after the second dose of antibody, and the material was clarified by centrifugation at 300 × g. In some cases, the 400-μl vaginal wash was concentrated to approximately 50 μl by use of a C30 concentrator (Pierce, Rockford, Ill.) and centrifugation at 5,000 × g for 20 min. Forty microliters of each preparation was mixed with 40 μl from a 106/ml yeast cell suspension in DPBS and then incubated in an ice bath for 30 min; the yeast cells in the suspension were washed three times in DPBS. The cells were suspended in 200 μl of the goat anti-mouse IgM preparation and incubated in an ice bath for 30 min. After washings, the cells were suspended in fluorescein isothiocyanate-conjugated rabbit anti-goat IgG (diluted 1:200 in DPBS) and examined by confocal fluorescence microscopy as described above. As a positive control in both fluorescence tests, C. albicans yeast cells were reacted with stock MAb B6.1 or B6, and the yeast cells were processed as described above.
In addition to the immunofluorescence method, an enzyme-linked immunosorbent assay (ELISA) was used as a further means of detecting antibody in vaginal lavage fluids. Mice in pseudoestrus were given approximately equal amounts of MAbs B6.1, B6, and S9 i.p. as described above. At various times after antibody administration, the animals were sacrificed and the vaginal lumen was washed as described above. An ELISA, as described previously for the isolation of MAb B6.1 (16), was used to test vaginal washes for the presence of antibody. Briefly, one half of the number of wells in an ELISA 96-microtiter plate (Corning Glass, Corning, N.Y.) received 50 μl of the enriched mannan fraction, which was used for preparation of the L-mann vaccine. The mannan fraction was prepared at 1 μg/ml in 0.06 M carbonated buffer, pH 9.6. For negative controls, each of the remaining wells received 50 μl of buffer solution. The plate was incubated at 37°C for 3 h followed by 5 to 8°C for 16 h. The wells were washed with deionized water three times, blocked with 3% bovine serum albumin (BSA) in Tris (Sigma)-buffered saline solution (TBS; 0.01 M Tris-HCl, 0.15 M NaCl [pH 7.4]) (TBS-BSA) at room temperature (RT; 22 to 24°C) for 1 h, washed with TBS plus 0.05% Tween 20 (TBS-Tween) three times, and washed with TBS three times. Fifty microliters of vaginal lavage obtained as indicated above was added to the appropriate wells, which were then incubated at 37°C for 1 h and washed with TBS-Tween and TBS as described above. Peroxidase-conjugated anti-mouse polyvalent immunoglobulins (1.6 mg/ml; diluted 1:1,000 in TBS-BSA; Sigma) were added to the wells, which were then incubated at RT for 1 h and washed as described above. The substrate was prepared by dissolving 25 mg of o-phenylenediamine (Sigma) in 25 ml of 0.1 M citric acid solution (pH 5.0) and then adding 10 μl of 30% hydrogen peroxide. One hundred microliters of substrate was added to each well, the plate was incubated at RT for color development, the reaction was stopped by addition of 100 μl of 10% H2SO4 per well, and the results were read at 490 nm in a microtiter plate reader (model 450; Bio-Rad). As a positive control, MAb B6.1 was used under conditions identical to those described above. A known amount of MAb B6.1 (23.5 μg) was serially diluted in TBS-BSA to determine the sensitivity of the ELISA method for antibody detection.
Statistical analyses.
In all cases, the number of animals per group was 5, and averages and standard errors were computed from independent observations run at least two times. Statistical significance of differences between test and control groups was determined by Student’s t test.
RESULTS
Depending on the route of treatment, i.vg. or i.p., the final vaginal Candida CFU count varied. For each experimental protocol, however, the numbers of CFU were highly reproducible within each group. Furthermore, the vaccine and antibodies had a similar magnitudes of effect on CFU values.
Prophylaxis potential of the L-mann vaccine against Candida vaginal infection.
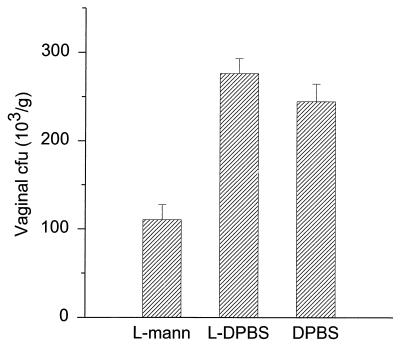
L-mann-vaccinated mice showed greater resistance to vaginal infection than DPBS control mice (Fig. 1). Vaccinated animals challenged with the yeast cells had (110 ± 38) × 103 CFU/g of vaginal tissue, while mice that received DPBS instead of the vaccine had (240 ± 44) × 103 CFU/g (mean ± standard error). The difference between vaccinated and unvaccinated mice was significant (P < 0.01). The vaginal count from L-DPBS control mice was (280 ± 38) × 103 CFU/g, which was slightly higher than that from DPBS controls, but the differences were not significant (P > 0.3).
FIG. 1.
Vaccination with L-mann enhances protection of mice against vaginal infection. Pseudoestrus mice were vaccinated with L-mann i.v. and challenged with C. albicans (5 × 105 yeast cells) i.vg. Control mice were given L-DPBS or DPBS (diluent) only. Vaginal CFU per gram of tissue were determined by plating on Mycobiotic agar. The L-mann-vaccinated mice developed approximately 62% less CFU than mice that received DPBS (P < 0.01). CFU from L-DPBS control-vaccinated mice were slightly higher than CFU from DPBS controls, but the difference was not statistically significant (P > 0.3). Bars denote standard errors.
Polyclonal antiserum given i.vg. transfers enhanced resistance.
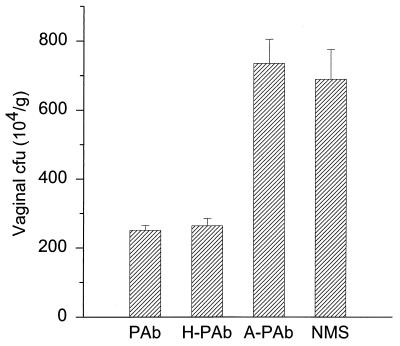
Pooled PAbs from L-mann-vaccinated mice enhanced resistance of mice against vaginal infection (Fig. 2). Treatment of mice with unheated PAb, heated PAb (H-PAb), C. albicans-absorbed PAb (A-PAb), or NMS (negative control) i.vg. and challenge with C. albicans i.vg. resulted in CFU per gram of vaginal tissue of (250 ± 24) × 104 for PAb, (260 ± 37) × 104 for H-PAb, (740 ± 120) × 104 for A-PAb, and (690 ± 150) × 104 for NMS (mean ± standard error). When the CFU values for both PAb- and H-PAb-treated mice were compared to values for NMS-treated control mice, the differences were significant (P < 0.01). Antiserum preabsorbed with C. albicans yeast cells did not confer resistance (Fig. 2).
FIG. 2.
PAb protects mice against Candida vaginal infection. PAb from L-mann-vaccinated mice was administered to pseudoestrus mice i.vg. before and after an i.vg. challenge with C. albicans. The resulting vaginal CFU were compared with CFU from animals H-PAb, A-PAb, or NMS. Mice given the unheated or heated PAb had 60% fewer CFU than animals that received NMS (P < 0.01). Mice that received the absorbed serum developed almost the same number of CFU as the NMS groups. Bars denote standard errors.
MAb B6.1 given i.vg. has both preventive and therapeutic effects.
MAb B6.1 or B6 was given i.vg. as described above (i.e., before and after infection with yeast cells) to test for a preventive effect (Fig. 3A) or only after an i.vg. infection to test for a therapeutic effect (Fig. 3B). Mice given MAb B6.1 or B6 before infection had, respectively, about 90 and 65% fewer vaginal CFU than DPBS control mice. The differences in CFU between MAb B6.1-treated or B6-treated mice and the control animals were statistically significant (P < 0.001 and P < 0.01, respectively) (Fig. 3A). Mice given MAb B6.1 therapeutically at 4 and 24 h after infection developed 48% fewer CFU than DPBS control animals (P < 0.02). MAb B6 did not have a therapeutic effect at a dose either comparable to that of MAb B6.1 (Fig. 3B) or 16-fold higher (data not shown). In fact, CFU values obtained after therapeutic application of MAb B6 were higher than CFU from DPBS control mice (P < 0.05) (Fig. 3B).
FIG. 3.
MAb B6.1 given i.vg. has preventive and therapeutic effects. Pseudoestrous mice were given MAb B6.1 i.vg. either before and after an i.vg. challenge with yeast cells (A) or only after infection (B). Mice given MAb B6.1 and those given MAb B6 before and after infection developed about 90 and 65%, respectively, fewer CFU than DPBS control mice (P < 0.001 and (P < 0.01, respectively) (A). MAb B6.1 also reduced by 48% the CFU in animals in which vaginal infection was established before MAb treatment (P < 0.02), whereas MAb B6 did not show this therapeutic effect (B), even at a 16-fold-higher dose (data not shown). Bars denote standard errors.
MAb B6.1 given i.p. enhances resistance against vaginal infection due to C. albicans and C. tropicalis.
Mice that received MAb B6.1 i.p. before and after infection with C. albicans developed 91% fewer vaginal CFU than DPBS control mice (P < 0.001) (Fig. 4A). In addition, enhanced resistance against vaginal infection was noted in mice that received either PAb from vaccinated mice or MAb B6. These animals developed 43 and 55%, respectively, fewer CFU compared to DPBS controls. Neither of the two irrelevant MAbs, S9 and 54.1, nor the hybridoma culture medium, HB101, had an effect (Fig. 4A). All preparations had an endotoxin content of less than 0.15 ng/ml (Escherichia coli O55:B5 lipopolysaccharide was the positive control). In addition, the protective ability of MAbs B6.1 and B6 was eliminated by absorption with C. albicans yeast cells (data not shown).
FIG. 4.
MAb B6.1 given i.p. protects mice against vaginal infection due to C. albicans (A) or C. tropicalis (B). Pseudoestrus mice were given MAb B6.1 or B6 i.p. before an i.vg. challenge with yeast. Negative controls were treated with DPBS alone or with hybridoma culture medium (HB101) that was concentrated and prepared as for antibody production. In panel A, additional controls received either MAb S9, which is specific for type III group B streptococcus polysaccharide, or MAb 54.1, which is specific for human neutrophil cytochrome b. MAb B6.1-treated and MAb B6-treated mice developed approximately 91 and 51% fewer CFU, respectively, than the DPBS negative controls (P < 0.001 and P < 0.01, respectively). The medium alone and the irrelevant MAbs were not protective (A). A similar result was observed with vaginal infection due to C. tropicalis (B). The differences between MAb B6.1-treated mice and control mice were statistically significant (P < 0.01), but the differences between MAb B6-treated and control mice were not statistically significant (P > 0.2), in vaginal infection due to C. tropicalis. Bars denote standard errors.
MAb B6.1 i.p. also reduced vaginal infection due to C. tropicalis, but MAb B6 did not show a significant protective effect (Fig. 4B). That is, CFU determinations for pseudoestrous mice given either MAb B6.1, MAb B6, or buffer (DPBS) and challenged with C. tropicalis resulted in (110 ± 24) × 103, (200 ± 19) × 103, or (230 ± 33) × 103 CFU/g of vaginal tissue (mean ± standard error), respectively. The differences between values for MAb B6.1-treated and buffer control mice were significant (P < 0.01), but the differences between values for MAb B6-treated and buffer (DPBS)-treated control mice were not significant (P > 0.2).
MAbs B6.1 and B6 do not affect C. trachomatis vaginal infection.
The experiment was done as an additional control for specificity of the protective effects due to the two MAbs. Neither MAb B6.1 nor MAb B6 had any effect on the course of a chlamydial vaginal infection. The numbers of infectious chlamydiae shed throughout the course of infection and the duration of infection were not different among antibody-treated and control groups (data not shown).
Detection of antibody in vaginal fluid and tissue.
To determine if MAb B6.1 given to mice by the i.p. route can directly interact with C. albicans yeast cells on vaginal surfaces, we attempted to detect the antibody in vaginal lavage fluid. Although the ELISA was capable of detecting as little as 1.8 pg of MAb B6.1, as determined by in vitro titrations of the antibody, MAb B6.1 was not detected in tissues either by ELISA or by immunofluorescence tests. The same negative results were obtained for mice given i.p. a dose of MAb B6.1 16-fold higher than that used in the protection experiments (data not shown).
DISCUSSION
The L-mann vaccine, which was previously shown to induce protective responses in mice against disseminated candidiasis (16, 17), caused enhanced resistance against experimental vaginal infection in mice. The prevailing belief is that Th1-type responses are important in the host defense against vaginal candidiasis (35). Cassone and workers have observed that antimannan antibodies given i.vg. to rats enhance their resistance to vaginal infection (8). We confirmed this observation in the mouse model of Candida vaginal infection. Our finding that serum from immunized animals conferred protection when given i.p. was, however, surprising because the predominant protective antibody response in vaccinated mice is IgM (reference 16 and our unpublished observations). This observation was strengthened by the finding that the protective factor in the polyclonal immune serum is removed by preabsorption with C. albicans yeast cells. Furthermore, MAbs specific for Candida mannan epitopes are also effective in enhancing resistance against vaginal infection when given either i.vg. or i.p.
As in previous studies on disseminated candidiasis (16, 17), MAb B6.1 was effective in protecting naive animals against vaginal infection. This antibody had protective activity when given before infection and therapeutic activity when given after infection. MAb B6.1 is specific to a β-1,2-linked mannotriose, which is an acid-labile component of the phosphomannan complex of C. albicans (18). This epitope appears to be a major surface marker, as indicated by confluent distribution patterns revealed by immunofluorescence (17), electron microscopy, and relative abundance data obtained by gel electrophoresis (our unpublished findings). MAb B6.1 protected mice against vaginal infection due to C. tropicalis (Fig. 4), which was expected because this species produces a β-1,2-mannotriose as part of its phosphomannan complex (25) and the antibody also protected animals against disseminated disease (16, 17).
Although MAb B6 was not effective in prevention of disseminated candidiasis (16), it could enhance resistance against vaginal infection, albeit not to the same extent as MAb B6.1. MAb B6 did not, however, protect mice against vaginal infection due to C. tropicalis even though the antibody reacts with this species. MAb B6 is specific for an acid-stable component of the phosphomannan complex (reference 17 and our unpublished data), but epitope distribution is patchy on the cell surface and appears less concentrated than the B6.1 epitope (18). A possible explanation for the protective activity of MAb B6 against vaginal infection is that the B6 epitope is more exposed due to lowered expression of the acid-labile β-1,2-oligomannosyl residues. In agreement with this hypothesis is the finding that expression of these acid-labile components is reduced when fungal cells are grown in an acidic environment (24, 26). Removing Candida cells from the vaginal tract of infected animals and assessing expression of the B6 and B6.1 epitopes will test this hypothesis.
Since MAb B6 enhanced resistance to vaginal infection, it did not serve as a negative control for these studies. Additional controls were thus added to ensure that protection due to MAbs B6.1 and B6 was specific. First, none of the preparations had detectable endotoxin contamination by the Limulus amebocyte lysate test (<0.15 ng/ml). Second, uninoculated hybridoma growth medium, which was concentrated in the same way as the MAb preparations, did not have protective activity. Third, the protective activity of both antibodies was removed by preabsorption with Candida yeast cells. Fourth, an irrelevant IgM MAb, specific for a group B streptococcal polysaccharide and prepared exactly like MAbs B6.1 and B6, was not protective. Fifth, neither MAb B6.1 nor MAb B6 protected mice against vaginal infection due to C. trachomatis. For this latter experiment, susceptibility to disease was heightened by pretreatment of the mice with progesterone, rather than estradiol as used in the Candida infection model, but the antibodies were ineffective nonetheless.
The mechanism(s) by which the vaccine and MAbs B6.1 and B6 protect against vaginal infection is unknown. Active immunization of mice may well lead to general stimulation of the immune system, but the protective value of immune sera indicated that protective antibodies were produced. In our studies on disseminated candidiasis, MAb B6.1 promoted in vitro neutrophil candidacidal activity (6). Extrapolation of these observations to the in vivo situation may make sense for host defense against disseminated candidiasis but not for resistance against vaginal infection. Hematoxylin-and-eosin-stained tissue sections of infected vaginal tissue did not reveal the presence of many neutrophils (our unpublished findings), and others have provided evidence that these phagocytes may not alter the outcome of experimental vaginal candidiasis (1). In our studies, the vaccine was given i.v. and the MAbs were given either i.vg. or i.p. As alluded to above, protection by the i.vg. route is not surprising, as others have shown that this route is an effective way to protect rats against Candida vaginal infection (8). An explanation of protection induced by vaccination is complex, as plasma and secretory antibodies and cell-mediated immune responses may participate in the host defense. We have chosen, instead, to focus on mechanisms by which protective antibodies may be administered i.p. yet exert their effect on the vaginal epithelium.
Protection of mice against vaginal infection by i.p. administration of antibody was an unexpected result. The implication is that MAbs B6.1 and B6 are somehow transported from the peritoneal cavity to the vaginal epithelial surface, but we have thus far failed to find the antibodies in vaginal lavage fluids. We are currently considering the possibility that the antibodies are found in subsurface locations within the vaginal epithelium.
The role of antibodies in host defense against fungal diseases is worth further investigation (7). Strong evidence for protective antibodies against cryptococcosis has been demonstrated (29), and a role for antibodies against blastomycosis has been suggested (23). The important point related to our work is that the presence of Candida-specific antibodies in the sera or on the vaginal epithelium of patients with candidiasis does not imply that antibodies are not protective. Indeed, as others have found with experimental cryptococcosis (10, 28, 43), the most important considerations may be the titer of the appropriate specific antibody and the antibody isotype.
ACKNOWLEDGMENTS
Special thanks go to Sandra Morrison for performing the C. trachomatis studies. We deeply appreciate the gifts of control MAbs from Seth Pincus (MAb S9) and from Jim Burritt and Al Jesaitis (MAb 54.1).
This work was supported by individual (RO1) and program project (PO1) grants from the National Institute of Allergy and Infectious Diseases (to M.P.M., RO1 AI 38991; to J.E.C., RO1 AI24912 and PO1 AI37194).
REFERENCES
- 1.Black C A, Eyers F M, Russell A, Dunkley M, Clancy R L, Beagley K W. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66:1273–1275. doi: 10.1128/iai.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brammer K W, Feczko J M. Single-dose oral fluconazole in the treatment of vaginal candidosis. Ann N Y Acad Sci. 1988;544:561–563. doi: 10.1111/j.1749-6632.1988.tb40452.x. [DOI] [PubMed] [Google Scholar]
- 3.Brawner D L, Cutler J E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986;51:337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burritt J B, Quinn M T, Jutila M A, Bond C W, Jesaitis A J. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 5.Bushell T E C, Evans E G V, Llewellyn P A, Meaden J D, Milne J D, Warnock D W. Prophylactic use of clotrimazole in recurrent vaginal candidosis. Ann N Y Acad Sci. 1988;544:558–560. doi: 10.1111/j.1749-6632.1988.tb40451.x. [DOI] [PubMed] [Google Scholar]
- 6.Caesar-TonThat T C, Cutler J E. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun. 1997;65:5354–5357. doi: 10.1128/iai.65.12.5354-5357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–2624. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning D W. Can we prevent azole resistance in fungi. Lancet. 1995;346:454–455. doi: 10.1016/s0140-6736(95)91314-9. [DOI] [PubMed] [Google Scholar]
- 10.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 11.Fidel P L, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel P L, Lynch M E, Sobel J D. Candida-specific Th-1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993a;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidel P L, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993b;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleury F J. Adult vaginitis. Clin Microbiol Rev. 1981;9:335–348. [Google Scholar]
- 15.Gough P M, Warnock D W, Richardson M D, Mansell N J, King J M. IgA and IgG antibodies to Candida albicans in the genital tract secretions of women with or without vaginal candidosis. Sabouraudia J Med Vet Mycol. 1984;22:265–271. [PubMed] [Google Scholar]
- 16.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Y, Cutler J E. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–1175. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenclever H F, Mitchell W O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasenclever H F, Mitchell W O, Loewe J. Antigenic studies of Candida. II. Antigenic relation of Candida albicans group A and group B to Candida stellatoidea and Candida tropicalis. J Bacteriol. 1961;82:574–577. doi: 10.1128/jb.82.4.574-577.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazen K C, Brawner D L, Riesselman M H, Jutila M A, Cutler J E. Differential adherence between hydrophobic and hydrophilic yeast cells of Candida albicans. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horowitz B J. Mycotic vulvovaginitis: a broad overview. Am J Obstet Gynecol. 1991;165:1188–1192. doi: 10.1016/s0002-9378(12)90725-5. [DOI] [PubMed] [Google Scholar]
- 23.Klein B S. Role of cell surface molecules of Blastomyces dermatitidis in the pathogenesis and immunobiology of blastomycosis. Semin Respir Med Infect. 1997;12:198–205. [PubMed] [Google Scholar]
- 24.Kobayashi H, Giummelly P, Takahashi S, Ishida M, Sato J, Takaku M, Nishidate Y, Shibata N, Okawa Y, Suzuki S. Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without beta-1,2 linkage and phosphate group. Biochem Biophys Res Commun. 1991;175:1003–1009. doi: 10.1016/0006-291x(91)91664-x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Matsuda K, Ikeda T, Suzuki M, Takahashi S, Suzuki A, Shibata N, Suzuki S. Structures of cell wall mannans of pathogenic Candida tropicalis IFO 0199 and IFO 1647 yeast strains. Infect Immun. 1994;62:615–622. doi: 10.1128/iai.62.2.615-622.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi H, Takahashi S-I, Shibata N, Miyauchi M, Ishida M, Sato J, Maeda K, Suzuki S. Structural modification of cell wall mannans of Candida albicans serotype A strains grown in yeast extract-Sabouraud liquid medium under acidic conditions. Infect Immun. 1994;62:968–973. doi: 10.1128/iai.62.3.968-973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee J, Nussbaum G, Scharff M D, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odds F C. Candida and candidosis. London, England: Bailliere Tindall; 1988. [Google Scholar]
- 31.Ott A K, Franklyn K, Warmington J R, Ashman R B. A Candida-specific antibody in patients with vaginitis. Med J Aust. 1990;152:390–391. doi: 10.5694/j.1326-5377.1990.tb125254.x. [DOI] [PubMed] [Google Scholar]
- 32.Pincus S H, Cole R L, Kamanga-Sollo E, Fischer S H. Interaction of group B streptococcal opacity variants with the host defense system. Infect Immun. 1993;61:3761–3768. doi: 10.1128/iai.61.9.3761-3768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Q, Jutila M A, van Rooijen N, Cutler J E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–5008. [PubMed] [Google Scholar]
- 34.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romani L. The T cell response against fungal infections. Curr Opin Immunol. 1997;9:484–490. doi: 10.1016/s0952-7915(97)80099-4. [DOI] [PubMed] [Google Scholar]
- 36.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal E, Vardinon N, Foldes J, Schwartz J, Eylan E. Serum anti-candida albicans antibodies in candidal and non-candidal vaginitis. Zentralbl Bakteriol Hyg. 1977;239:548–553. [PubMed] [Google Scholar]
- 38.Shibata N, Ikuta K, Imai T, Satoh Y, Satoh R, Suzuki A, Kojima C, Kobayashi H, Hisamichi K, Suzuki S. Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. J Biol Chem. 1995;270:1113–1122. doi: 10.1074/jbc.270.3.1113. [DOI] [PubMed] [Google Scholar]
- 39.Sobel J D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152:924–935. doi: 10.1016/s0002-9378(85)80003-x. [DOI] [PubMed] [Google Scholar]
- 40.Sobel J D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 41.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14(Suppl. 1):S148–S153. [DOI] [PubMed]
- 42.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 44.Yuan Y, Lyng K, Zhang Y-X, Rockey D D, Morrison R P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]