Abstract
Chemotherapy-induced thrombocytopenia (CIT) is a common complication of antineoplastic therapy, resulting in antineoplastic therapy dose reductions, treatment delays, treatment discontinuation, and morbid bleeding events. Despite several decades of research into thrombopoietic growth factors in CIT, there are presently no available U.S. FDA- or EMA-approved agents to treat CIT. However, a respectable body of evidence has been published evaluating the thrombopoietin receptor agonists (TPO-RAs) for the management and prevention of CIT in patients with solid tumors, and critical studies are ongoing with the TPO-RAs romiplostim and avatrombopag. When employed in the appropriate patient population and used properly, TPO-RAs can successfully and safely manage CIT for extended periods of time with minimal apparent risks. This comprehensive review discusses the evidence for TPO-RAs in CIT in patients with solid tumors, provides detailed guidance for their use in the clinic, and discusses ongoing essential clinical trials in management of CIT.
Keywords: Chemotherapy, chemotherapy-induced thrombocytopenia, persistent CIT, nadir CIT, thrombocytopenia, supportive care, romiplostim, avatrombopag, thrombopoietin, thrombopoietin receptor agonist, bleeding
INTRODUCTION
Thrombocytopenia is a common occurrence in patients with cancer and most commonly occurs following administration of antineoplastic therapy, a clinical entity known as chemotherapy-induced thrombocytopenia (CIT) [1]. While historically CIT occurred exclusively in the setting of cytotoxic and myelosuppressive chemotherapeutics, antineoplastic therapy additionally now includes countless targeted therapies, which can also result in CIT due to alternative mechanisms [2, 3]. While hypoproliferative thrombocytopenia is not generally associated with spontaneous bleeding complications in patients with aplastic anemia or acute leukemia until the platelet count is profoundly low (<10–20 × 109/L) [4], patients with solid tumors invading organs and tissues and disrupting mucosal surfaces (such as gastrointestinal and genitourinary tracts) may experience significant spontaneous bleeding at higher platelet count thresholds (<30–40 × 109/L) [5]. Because thromboembolic complications, and therefore a need for anticoagulation, is very common in these patients, the platelet counts at which risk for major bleeding increases is higher still in patients requiring anticoagulation and/or antiplatelet therapies [6, 7]. Significant bleeding events in patients with CIT may result in hospitalizations, irreversible declines in performance status, and halting or discontinuation of antineoplastic therapy, and therefore are associated with rather dramatic reductions in overall survival [5].
In an effort to avoid these morbid complications, cancer care providers commonly resort to chemotherapy dose reductions and treatment delays when patients develop CIT.[8] This compromises the relative dose intensity (RDI) of chemotherapy. As would be expected, reduced RDI has been clearly linked to inferior oncologic outcomes across numerous studies [5, 9–13]. As one example, in a pooled analysis of patients from multiple clinical trials of FOLFIRI [folinic acid, irinotecan, and fluorouracil], a common fluorouracil-based regimen for gastrointestinal cancer, modest reductions in irinotecan RDI of only 20% were associated with significant reductions in both progression-free survival (HR for disease progression, 3.18, 95% CI 1.47–6.88, P<0.01) and overall survival (HR for death, 2.72, 95% CI 11.22–6.04, P<0.01) [13]. Every dose reduction and treatment delay imposed upon a patient reduces the overall RDI of that patient’s antineoplastic regimen, which compromises oncologic outcomes. Cancer therapy continues to evolve, but hematologic adverse events, particularly cytopenias, remain among the most common complications of treatment [1, 14]. Red cell transfusions, intravenous iron infusions, and/or erythropoiesis-stimulating agents adequately address chemotherapy-induced anemia and neutrophil growth factors such as granulocyte colony stimulating factors very effectively treat and prevent chemotherapy-induced neutropenia [14]. Conversely, outside of China, there are no available regulatory agency-approved thrombopoietic growth factors to address CIT in the majority of the world.
The development of thrombopoietin receptor agonists (TPO-RAs) for immune thrombocytopenia (ITP) and other etiologies of thrombocytopenia has resulted in substantial interest in the use of these agents in CIT [15]. At present, none of the five commercially available TPO-RAs are approved for CIT, but an extensive published off-label body of evidence as well as completed clinical trials offer insight into the clinical situations in which TPO-RA treatment of CIT is likely and unlikely to be beneficial, as well as important lessons on how these agents are used effectively [16–19]. In addition, important clinical trials are presently ongoing evaluating romiplostim and avatrombopag for the treatment of CIT [15]. This review will discuss the evidence for TPO-RA use in CIT, optimal off-label use of these agents to treat CIT, and highlight the ongoing clinical trials (NCT03362177, NCT03937154, and NCT05772546) evaluating TPO-RAs in CIT. The use of thrombopoietic agents to manage myeloid malignancies (such as myelodysplastic syndrome or acute myeloid leukemia), hasten hematologic recovery following stem cell transplant, or treat other etiologies of thrombocytopenia is beyond the scope of this review and will not be discussed.
A BRIEF HISTORICAL PERSPECTIVE
Given the limitations of platelet transfusions and their relative scarcity as a resource, thrombopoietic growth factor support of cancer patients receiving chemotherapy is not a new concept. As with granulocyte colony-stimulating factors and erythropoiesis-stimulating agents, investigation into the use of thrombopoietic growth factors to support cancer therapy goes back several decades. Recombinant human thrombopoietin (rhTPO) and pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) were first-generation thrombopoietic agents that began development in the 1990s shortly after the first purification of human thrombopoietin [20, 21]. rhTPO and PEG-rHuMGDF, administered as daily subcutaneous injections, demonstrated promising activity in treatment and prevention of CIT in phase I and II clinical trials. However, the development of antibodies to PEG-rHuMGDF capable of cross-reacting with and neutralizing endogenous TPO, thereby causing severe chronic thrombocytopenia necessitating immunosuppressive therapy in a small minority of patients, halted development of both drugs in most of the world (despite the fact that rhTPO had not led to this serious immunologic complication in any patients) [22, 23]. Ultimately, rhTPO completed development in China, where it is approved and used routinely in the management of CIT and where its use is recommended by Chinese oncological practice guidelines [24]. Because of the very limited global availability of this agent, it will not be discussed further in this review.
Oprelvekin (recombinant human interleukin-11, Neumega, Pfizer), a recombinant human thrombopoietic cytokine that increased platelet counts without direct agonism of the thrombopoietin receptor, did achieve regulatory approval in the United States (U.S.) in the 1990s for CIT management [25, 26]. However, the adverse event burden of this agent, which includes constitutional symptoms, atrial arrhythmias, and fluid retention, as well as its considerable monetary cost [27], limited its use and it was subsequently withdrawn from the market by the manufacturer in the U.S.
Therefore, the accepted standard of care in most of the world to manage CIT is RDI reduction (or more drastic antineoplastic treatment compromise): dose reductions, treatment delays, drug discontinuations, switching to an therapeutically inferior but less myelosuppressive regimen, or discontinuation of systemic antineoplastic therapy altogether. Recurrent platelet transfusion is rarely practical, results in eventual alloimmunization, exposes the patient to the risk of transfusion reactions and infection, and is a scare resource typically reserved for actively bleeding patients or those with profound thrombocytopenia, so is not generally considered a viable option for routine CIT management. The published data on TPO-RAs in CIT, however, provide another treatment option, on which the rest of this review article will focus.
DIAGNOSIS AND MODERN CLASSIFICATION OF CHEMOTHERAPY-INDUCED THROMBOCYTOPENIA
While diagnosis of CIT is usually straightforward—the occurrence of new thrombocytopenia in association with cytotoxic or myelosuppressive antineoplastic therapy, it is critical to consider alternative causes of thrombocytopenia in the cancer patient prior to a CIT diagnosis [2]. These largely include infection, non-chemotherapy drug effect (including heparin-induced thrombocytopenia [HIT]), development of clinically significant liver disease (through malignant involvement, toxicity of cancer therapy, or both), myelophthisis, thrombotic microangiopathy (TMA, due to malignancy or antineoplastic therapy) and malignancy-associated disseminated intravascular coagulation (DIC). Most of these diagnoses are readily ruled out with a brief patient history and physical examination as well as a review of pre-existing liver imaging (previously obtained for oncologic purposes) and basic laboratory studies, including complete blood count with differential, peripheral blood film, liver function testing, blood chemistries, and basic coagulation studies. Most of these studies are readily routinely obtained in cancer patients receiving systemic therapy and are therefore readily available in the medical record at the time of CIT presentation. ITP can complicate cancer or its therapy (particularly immunotherapies such as immune checkpoint inhibitors), but usually presents as rapidly fluctuating, often profound thrombocytopenia with increased mean platelet volume and no temporal relationship to chemotherapy administration [28]. Occasionally specific chemotherapy agents may provoke toxicities that result in thrombocytopenia in association with their administration, but via a different mechanism (e.g. oxaliplatin-induced splenomegaly [29], temozolomide-induced aplastic anemia [30], gemcitabine or mitomycin C-induced TMA) and these rare occurrences should also be considered. Confidence that the patient does not have active infection, HIT, DIC, or TMA as a contributor to thrombocytopenia is required prior to consideration of thrombopoietic support.
Modern studies recognize two distinct presentations of CIT [1, 8, 17, 19], which have substantial relevance in the potential use of TPO-RAs to treat it.
Nadir CIT.
Nadir CIT occurs when a patient with a normal or near-normal platelet count on day 1 of a chemotherapy cycle has an excessive drop in the platelet count during the mid-cycle count nadir. The platelet count threshold for concern is often patient-dependent: for example, in a patient receiving therapeutic anticoagulation for prior cancer-associated venous thromboembolism and/or antiplatelet therapy for pre-existing heart disease, a drop to <40–50 × 109/L may be considered unacceptable, whereas in a patient not on antithrombotic therapy at a lower bleeding risk this threshold may be closer to <20–30 × 109/L. Patients with nadir CIT recover to a normal or near-normal platelet count by day 1 of the following chemotherapy cycle. Because routine laboratory monitoring mid-cycle is not standard for many chemotherapy regimens (beyond a customary toxicity check during the first cycle of therapy), most cases of nadir CIT not resulting in bleeding likely go undetected clinically. As has been observed in a randomized clinical trial [17], nadir CIT may not recur reliably with subsequent cycles of the same regimen, even when the chemotherapy dose is kept stable.
Persistent CIT.
Persistent CIT occurs when a patient presents with moderate to severe thrombocytopenia on day 1 of a chemotherapy cycle, such that administration of full dose treatment on schedule is deemed unsafe due to the risk of profound and/or prolonged severe thrombocytopenia during the cycle. The threshold for clinically relevant persistent CIT also varies depending on characteristics of the patient as well as the chemotherapy regimen in question, but is generally <70–100 × 109/L. Moderate-to-severe persistent CIT tends to provoke a treatment delay of at least 1 week, with the hope that the patient’s platelet count will recover adequately after the delay so treatment can be administered safely. Mild persistent CIT often results in dose reduction of one or more agents in the regimen to avoid a treatment delay. Persistent CIT usually recurs in later cycles despite these dose reductions and treatment delays and often worsens over time with further cytotoxic treatment. Absent thrombopoietic support, adequate management of persistent CIT usually requires a drastic regimen alteration (such as discontinuation of one drug altogether from a multidrug regimen or transition to another regimen entirely) to allow for consistent ongoing treatment.
While both nadir CIT and persistent CIT may occur in relatively chemotherapy-naïve patients as well as those that are more chemotherapy-experienced, persistent CIT is much more common in patients who have already received numerous cycles of multiagent chemotherapy, often after patients have received 2 or more prior multiagent regimens [16, 19]. Patients with thrombocytopenia at baseline prior to initiation of chemotherapy, such as those with thrombocytopenia of chronic liver disease, chronic mild immune thrombocytopenia, clonal hematopoiesis, idiopathic cytopenia of undetermined significance or persistent isolated mild thrombocytopenia of unknown etiology [31] are at particular risk of worsened thrombocytopenia and bleeding complications upon initiation of chemotherapy and may additionally be considered for thrombopoietic support of their cancer treatment.
THE THROMBOPOIETIN RECEPTOR AGONISTS
The TPO-RAs are the second-generation thrombopoietic agents. Five TPO-RAs have been approved by regulatory authorities in various countries worldwide: the subcutaneously administered “peptibody” agent, romiplostim, and the orally administered small molecules eltrombopag, avatrombopag, lusutrombopag, and hetrombopag [32, 33]. Table 1 highlights and contrasts the features, indications, and potential advantages and disadvantages of these agents. TPO-RAs are presently approved for various thrombocytopenic conditions, including ITP [34–36], aplastic anemia [37, 38] and periprocedural thrombocytopenia [39–42], among others.
Table 1.
Comparison of the thrombopoietin receptor agonists.
| Hetrombopag | |||||
|---|---|---|---|---|---|
| Molecular structure | Peptide | Small molecule | Small molecule | Small molecule | Small molecule |
| TPO receptor site of action | Extracellular domain | Transmembrane domain | Transmembrane domain | Transmembrane domain | Transmembrane domain |
| Route of administration | Subcutaneous | Oral | Oral | Oral | Oral |
| Dosing frequency a | Weekly | Daily | Daily or less frequently than daily | Daily | Daily |
| Relevant food interactions | N/A | Yes | No | No | Yes |
| Known hepatotoxicity | No | Yes | No | No | Yes |
| Possible plasma, skin, and sclerae discoloration | No | Yes | No | No | No |
| Iron chelator | No | Yes | No | No | Yes |
| May require lower starting dose in persons of East Asian ethnicity | No | Yes | No | No | Yes |
| Requires dose-reduction in hepatic dysfunction | No | Yes | No | No | Yes |
| Demonstrated increased thrombosis risk in chronic liver disease | No | Yes | No | No | No |
| Data exists for use in pregnancy b | Yes | Yes | No | No | No |
| Safety data available for extended durationc use | Yes | Yes | No | No | No |
| Current FDA-approved indications | Immune thrombocytopenia (adults and children) Acute radiation syndrome | Immune thrombocytopenia (adults and children) Hepatitis C-associated thrombocytopenia Severe aplastic anemia | Periprocedural thrombocytopenia in CLD patients Immune thrombocytopenia (adults) | Periprocedural thrombocytopenia in CLD patients | None (approved in China for management of immune thrombocytopenia and severe aplastic anemia) |
Per drug label. Like avatrombopag, eltrombopag can be dosed less frequently than once daily [73] though this is not in the drug label.
No TPO-RAs are approved for use in pregnancy for any indication. Available data in pregnancy is in patients with ITP.
Defined here as 5 or more years of continuous use.
TPO, thrombopoietin. CLD, chronic liver disease. N/A, not applicable.
Two approaches to CIT management have been studied with thrombopoietic agents: management of existing CIT in patients who have the diagnosis and prevention of CIT in a broad population of cancer patients who have not yet developed CIT. All CIT studies of eltrombopag have been performed evaluating the latter (Table 2), an approach that has been largely abandoned in the research space and one that is not endorsed by any clinical practice guidelines. Therefore, the potential use of TPO-RAs in broad non-CIT cancer patient populations for CIT prevention is not appropriate outside of the setting of a clinical trial and will not be further discussed in this review (notably, there are no such ongoing trials at present). No studies, clinical trials or otherwise, evaluating hetrombopag (which is currently only approved in China) or lusutrombopag for CIT have been published to date. So, while five TPO-RAs are available, just two have been formally evaluated for the treatment of CIT: romiplostim and avatrombopag [16–19]. Outside of China, these are also the only two TPO-RAs with clinical trials ongoing evaluating safety and efficacy for the treatment of CIT. The data published for the use of these agents in CIT, as well as the ongoing clinical trials evaluating their use, are described in detail in Table 2 and discussed further in later sections of this review.
Table 2.
Findings of representative studies (including 20 or more patients) of thrombopoietin receptor agonists for the treatment of CIT in adults. Adapted with permission from Al-Samkari 2022 [8].
| Study | Patient Population | Chemotherapy Regimen | Principal Findings |
|---|---|---|---|
| Romiplostim | |||
| Parameswaran 2014[52] CIT Treatment |
20 patients with various solid tumors who developed CIT (Plt <100×109/L for ≥6 weeks) Observational cohort |
Various regimens | 95% of patients achieved Plt >100×109/L 75% resumed cytotoxic chemotherapy and all but one of these patients tolerated at least 2 additional chemotherapy cycles on romiplostim support without recurrence of dose-limiting CIT 3 patients developed VTE; bleeding not reported |
| Al-Samkari 2018[18] CIT Treatment |
22 patients with various solid tumors who developed CIT (as defined by treating physician) Observational cohort |
Various regimens | 95% of patients achieved Plt >100×109/L 100% resumed cytotoxic chemotherapy, receiving 2 or more cycles (range, 2–18) on romiplostim Significant reduction in dose reductions and treatment delays on romiplostim, with some patients able to dose-escalate No patients developed VTE; 3 developed bleeding |
| Soff 2019[19] CIT Treatment |
60 patients with various solid tumors who developed persistent CIT (Plt <100×109/L for ≥4 weeks without chemotherapy treatment) randomized to romiplostim or untreated observation; ultimately 52 received romiplostim Randomized phase 2 trial |
Various regimens | 85% of patients treated with romiplostim achieved Plt >100×109/L compared to 12.5% untreated observation Only 7% of patients of patients who achieved Plt >100×109/L experienced recurrent chemotherapy dose-reduction or treatment delay 10.2% of patients had VTE over 12 months; bleeding not reported Subsequent publication [53] confirmed similar safety and efficacy with extended duration use in the study population |
| Al-Samkari 2021[16] CIT Treatment |
173 patients with various malignancies (153 solid tumor, 20 lymphoma/myeloma) who developed persistent CIT (Plt <100×109/L for ≥3 weeks or chemotherapy delay of ≥1 week due to thrombocytopenia) Observational cohort |
Various regimens | 85% achieved Plt >100×109/L (95% without predictors of non-response) 71% achieved Plt >75×109/L and ≥30×109/L higher than pretreatment baseline (82% without predictors of non-response) 79% avoided chemotherapy dose-reduction or treatment delay; 89% avoided platelet transfusion Bone marrow tumor invasion, prior pelvic irradiation, prior temozolomide predicted romiplostim non-response VTE rate 14 per 100 patient-years; bleeding rate 23 per 100 patient-years (1% of 1063 cycles supported with romiplostim) |
| Eltrombopag | |||
| Kellum 2010[74] CIT Prevention |
183 chemotherapy-naïve patients with various advanced solid tumors randomized to placebo or eltrombopag 50, 75, or 100 mg; 134 patients completed at least 2 cycles and could be evaluated Randomized phase 2 trial |
Carboplatin and paclitaxel | Primary endpoint (significant difference in the change in platelet count from day 1 in cycle 2 to the platelet nadir in cycle 2 between eltrombopag and placebo-treated patients) not met Eltrombopag-treated patients had higher platelet counts at start of subsequent treatment cycles (higher counts with higher eltrombopag dose) |
| Winer 2015[75] CIT Prevention |
26 patients with pancreatic cancer randomized to receive eltrombopag 100 mg or placebo Randomized phase 1 trial |
Gemcitabine or gemcitabine plus cisplatin or carboplatin | Mean platelet nadirs significantly higher in eltrombopag-treated patients Chemotherapy dose reduction or treatment delay occurred in 50% of placebo-treated patients vs. 14% of eltrombopag-treated patients |
| Winer 2017[76] CIT Prevention |
75 patients with various solid tumors randomized to receive eltrombopag 100 mg or placebo; only 26 of the enrolled patients completed the planned number of cycles Randomized phase 2 trial |
Gemcitabine or gemcitabine plus cisplatin or carboplatin | Eltrombopag-treated patients had higher platelet counts, lower frequencies of grade 3 or 4 CIT, more rapid platelet count recovery, and fewer dose reductions/treatment delays or missed doses due to thrombocytopenia Rates of grade 3 or 4 CIT remained high overall in both arms |
| Avatrombopag | |||
| Al-Samkari 2022[16] CIT Treatment |
122 patients with lung, ovarian, or bladder cancer with primarily nadir CIT randomized to receive avatrombopag 40 mg daily or placebo for 1 chemotherapy cycle Randomized phase 3 trial |
Various regimens | Similar proportions of patients achieved the primary endpoint (of avoidance of chemotherapy treatment delay, dose reduction, bleeding, or platelet transfusion) in avatrombopag (70%) and placebo (73%) groups, due to high rates of unexpected higher platelet count nadirs in placebo arm during the interventional chemotherapy cycle Avatrombopag-treated patients had higher platelet counts Avatrombopag overall safe and well-tolerated in cancer patients with a safety profile similar to placebo Arterial thromboembolism rate 2.4% in avatrombopag arm versus 2.5% in placebo arm No venous thromboembolic events in either arm |
Most clinical studies of TPO-RAs have been performed in patients with ITP. Early concerns for potential TPO-RA-induced myelofibrosis or leukemogenicity have been laid to rest in long-term studies of patients with ITP and myelodysplastic syndrome receiving these agents for extended periods (many years in patients with ITP) and therefore should not be a source of concern in the management of CIT in patients with solid tumor malignancies or lymphoid malignancies receiving these agents over a comparably short period of time (generally a few months to a year).
The theoretical risk of thromboembolic complications with TPO-RA use remains in all populations receiving these drugs, and is of particular concern in patients with cancer, who have a substantially elevated risk of thromboembolism at baseline. Prior studies have not shown platelet hyperreactivity, increased platelet activation, or spontaneous platelet aggregation in patients with ITP receiving romiplostim, eltrombopag, or avatrombopag [43–45]. Reassuringly, the largest clinical studies evaluating TPO-RAs in this population, including patients receiving them for extended durations (>1 year), did not find an apparent increase in risk for venous or arterial thromboembolic events. A large observational study of 173 patients with solid tumors or lymphoid malignancies receiving romiplostim for persistent CIT and a phase 2 clinical trial of 60 patients with solid tumors receiving romiplostim for persistent CIT both reported thromboembolism incidences and/or rates consistent with historical similar cancer patient populations [16, 19]. Similarly, a phase 3 randomized, controlled trial of 122 patients with lung, ovarian, or bladder cancers receiving avatrombopag for CIT (no specific restriction on CIT type, but the eligibility criteria strongly favored enrollment of patients with nadir CIT and the vast majority of enrolled patients indeed had nadir CIT) reported a 2.4% thromboembolic rate in the avatrombopag arm and a 2.5% thromboembolic rate in the placebo arm [17]. However, it is worth noting that multiple randomized controlled trials of eltrombopag in patients with chronic liver disease did demonstrate higher thromboembolic rates in the eltrombopag arm [39, 46]. Therefore, while thromboembolic complications remain a theoretical risk with TPO-RAs in any population, the data to date does not support an increased thromboembolism risk with TPO-RA support of patients with CIT.
Beyond the potential risks discussed above, clinically relevant adverse events with the use of TPO-RAs are rare in clinical practice [47]. With the exception of thrombocytosis, which may increase thromboembolic risk (though it has only been demonstrated to do so in primate studies of thrombopoietic agents at extreme platelet counts well over 1,000 × 109/L [48, 49]), randomized trials of TPO-RAs have consistently demonstrated similar adverse event profiles between TPO-RA and placebo arms [17, 36, 50, 51]. Headache or arthralgia, which is usually mild and readily managed with acetaminophen, may occur in a small minority of patients, but other routine adverse events are rare.
PATIENT SELECTION FOR TPO-RA TREATMENT OF CIT
TPO-RAs are expensive, may require weekly clinic visits (in the case of romiplostim), and may have side-effects, so it is crucial that only patients likely to benefit from TPO-RA support for CIT are considered for it. Two primary dimensions must be considered in selecting proper patients for TPO-RA treatment of CIT: the presentation of the CIT (nadir versus persistent) and the characteristics of the patient.
First, one must consider the presentation of the CIT. Modest nadir CIT (nadir platelet count >30 × 109/L) does not require treatment in most cases [8, 17], at least in patients not receiving antithrombotic therapy or experiencing bleeding symptoms. High-quality evidence to support this recommendation comes from a phase 3 global randomized, controlled clinical trial of avatrombopag for the treatment of nadir CIT (platelet count <50 × 109/L) [17]. Patients in this study qualified with a platelet count measured <50 × 109/L over the qualifying chemotherapy cycle and could recover their platelet count to up to 175 × 109/L on day 1 of the on-treatment chemotherapy cycle (cycle being supported by study drug) and remain eligible. Platelet counts were measured several times over the course of the on-treatment cycle to detect recurrent nadir CIT. In this study, which randomized 82 patients with CIT to receive avatrombopag and 40 patients with CIT to receive matched placebo, patients in the avatrombopag had higher platelet counts during the on-treatment cycle than those receiving placebo (Figure 1). However, similar numbers of patients in each arm achieved the primary endpoint of avoidance of platelet transfusion, chemotherapy dose reduction of 15% or more, or chemotherapy treatment delay of 4 or more days because of strikingly high rates of spontaneous “recovery” (lack of nadir CIT recurrence in the on-treatment cycle once cycle after it had occurred in the qualifying cycle) in the placebo arm (>75%), Figure 1. Notably, this study restricted enrollment to patients with no more than 1 prior chemotherapy treatment regimen and no prior history of CIT before the episode in the qualifying cycle that enabled entrance into the trial, so it is possible that more chemotherapy-experienced patients may have higher rates of nadir CIT recurrence. Ultimately, the author recommends consideration of TPO-RA therapy to treat or prevent recurrence of nadir CIT only in the setting of known bleeding occurring with count nadirs or in the setting of recurrent profound nadir thrombocytopenia (platelet count <20 × 109/L in patients not receiving antithrombotic therapy and <30–40 × 109/L in patients receiving antithrombotic therapy), Figure 2.
Figure 1. Mean platelet counts over time in a randomized, controlled phase 3 clinical trial evaluating avatrombopag versus placebo for the treatment of nadir CIT.
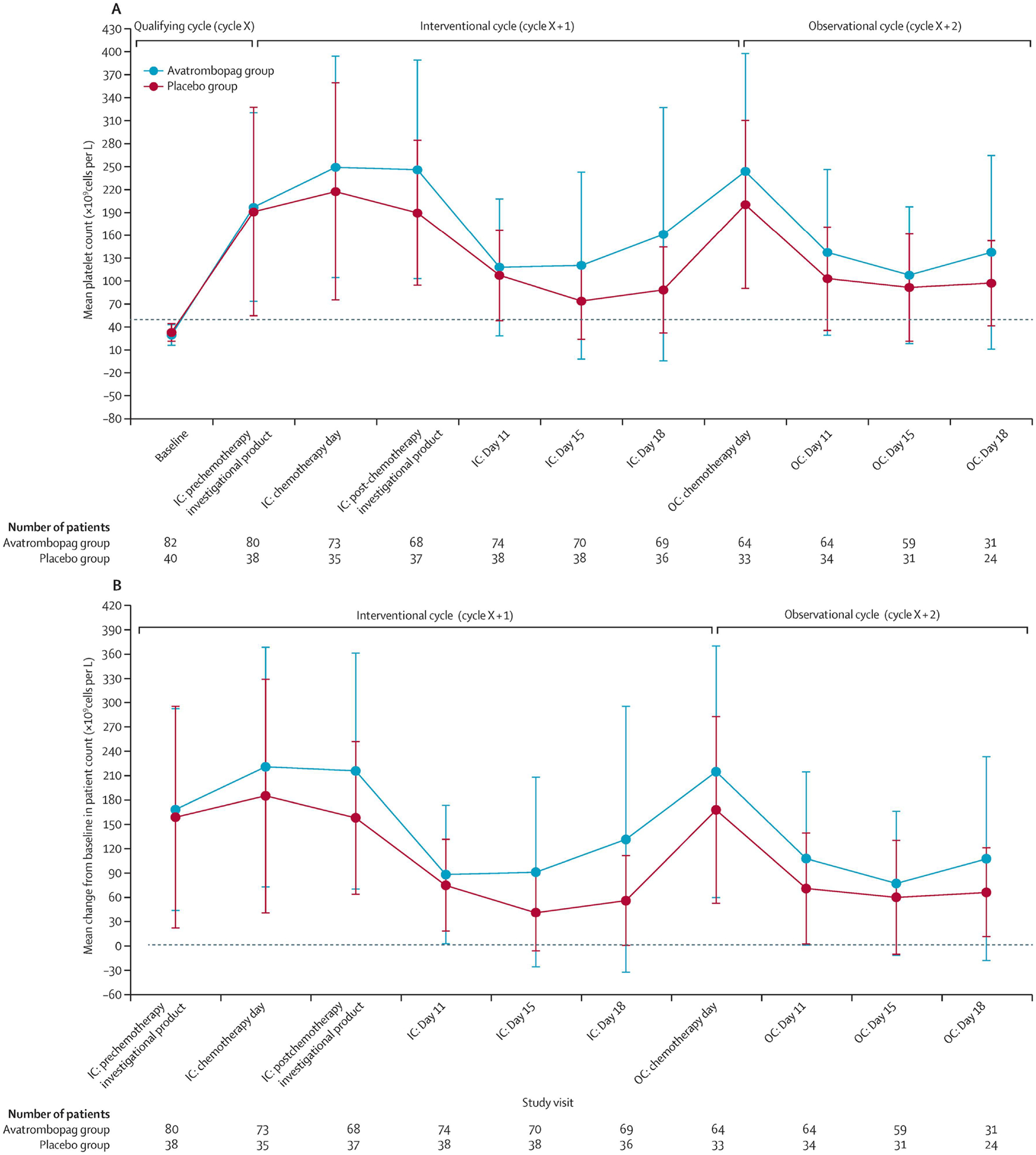
(A) Mean platelet count over time. Note that nadir during qualifying cycle (baseline) is much lower than nadir for either avatrombopag arm (as expected) or placebo arm (unexpected, signifying “spontaneous recovery”, or lack of nadir CIT reoccurrence, in a majority of patients). (B) Mean change from baseline in platelet count over time. Note the numerical outperformance of the avatrombopag arm over the placebo arm at all time points. Error bars show SDs. Cycle X is the qualifying cycle. IC=interventional cycle (cycle X + 1). OC=observational cycle (cycle X + 2). Reproduced with permission from Al-Samkari et al., 2022 [17].
Figure 2. Graphical summary of nadir CIT and persistent CIT along with the author’s recommended treatment algorithm when TPO-RA therapy is considered.
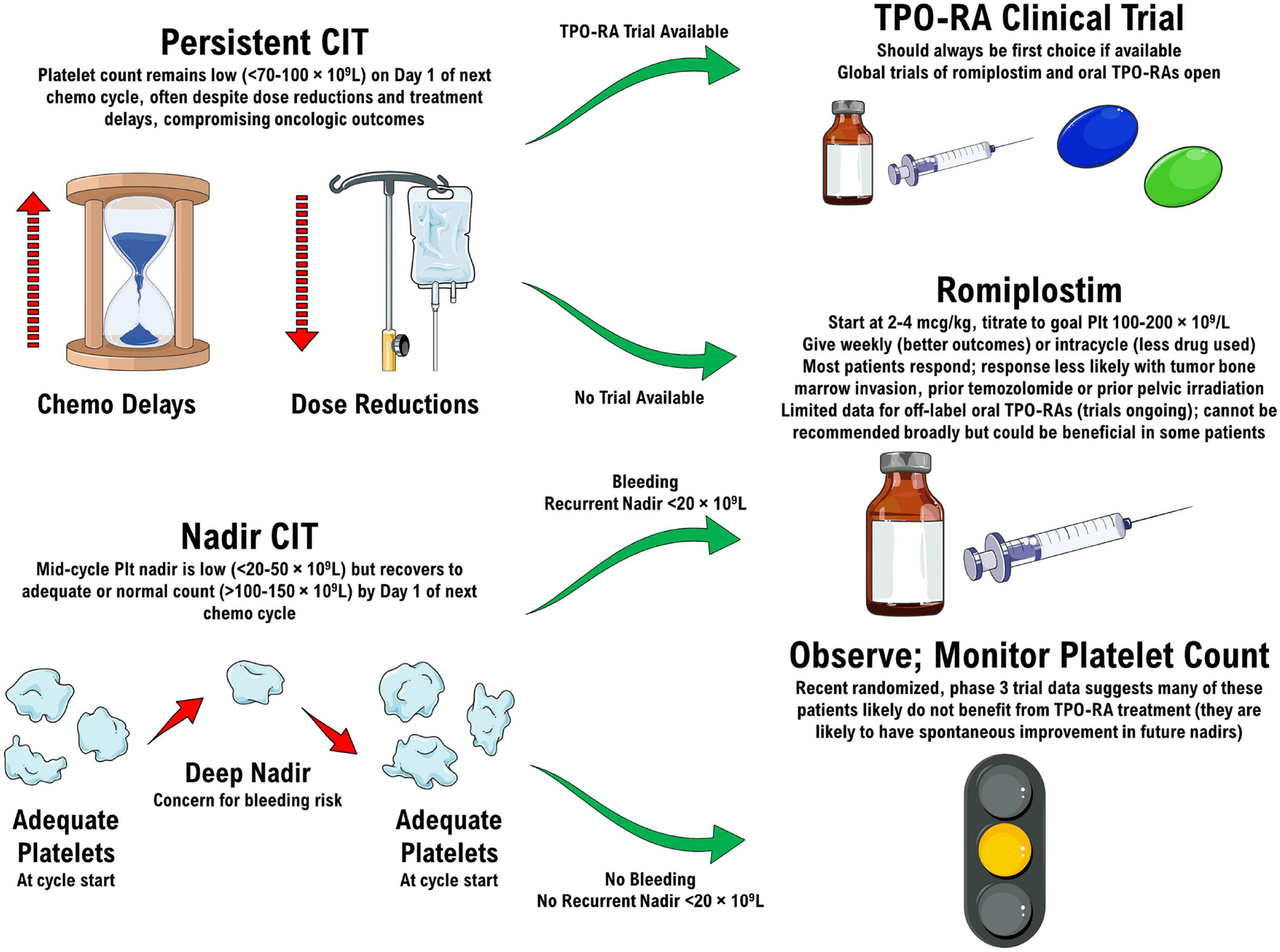
Adapted with permission from Al-Samkari 2022 [8].
Multiple studies evaluating romiplostim for persistent CIT, including a positive phase 2 clinical trial, support TPO-RA treatment of persistent CIT (Table 2) [16, 18, 19, 52, 53]. When the patient presents on day 1 of a chemotherapy cycle with a platelet count inadequate for safe administration of chemotherapy at full dose and on schedule (typically <70–100 × 109/L, depending on the regimen and the underlying bleeding risk of the patient), TPO-RA support of patients with persistent CIT should be considered. This approach is additionally recommended by the National Comprehensive Cancer Network (NCCN) guidelines [14]. Patient characteristics then dictate whether the patient is a good or poor candidate for TPO-RA support.
Table 3 highlights patient characteristics that support the use of TPO-RA support and those that do not. Patients with prior pelvic irradiation, prior temozolomide exposure, and bone marrow infiltration by tumor (solid tumor or lymphoid malignancy) have been observed to have substantially lower rates of response to romiplostim support (Figure 3) [16]. Temozolomide is exceptionally myelotoxic in certain individuals (in whom it may cause temozolomide-induced aplastic anemia [30]) and generally more myelotoxic than typical alkylating agents in the broader population [54], likely depleting the marrow progenitor pool more severely than other treatments. Known bony metastatic involvement by tumor or evidence of myelophthisis on peripheral blood film (leukoerythroblastosis, abundant dacrocytes/teardrop cells) can serve as a reasonable surrogate for a bone marrow biopsy in patients suspected of having bone marrow involvement of their cancer (most common in breast, prostate, lung, gastric, thyroid, and renal cancers).
Table 3.
Factors influencing the likelihood of romiplostim response in CIT.
| Response Less Likely | Response More Likely |
|---|---|
| Bone marrow infiltration by tumor | Solid tumor with no bone metastasis |
| Dramatic elevations of endogenous thrombopoietin (>10–20 times upper limit of normal) | Normal or modest elevation (<5 times upper limit of normal) of endogenous thrombopoietin |
| Prior temozolomide treatment | |
| Prior pelvic irradiation | |
| Diffuse bone metastases | |
| Lymphoid malignancy involving bone marrow |
Figure 3. Median weekly platelet counts for various patient populations treated for CIT with romiplostim from a study of 173 patients with CIT.
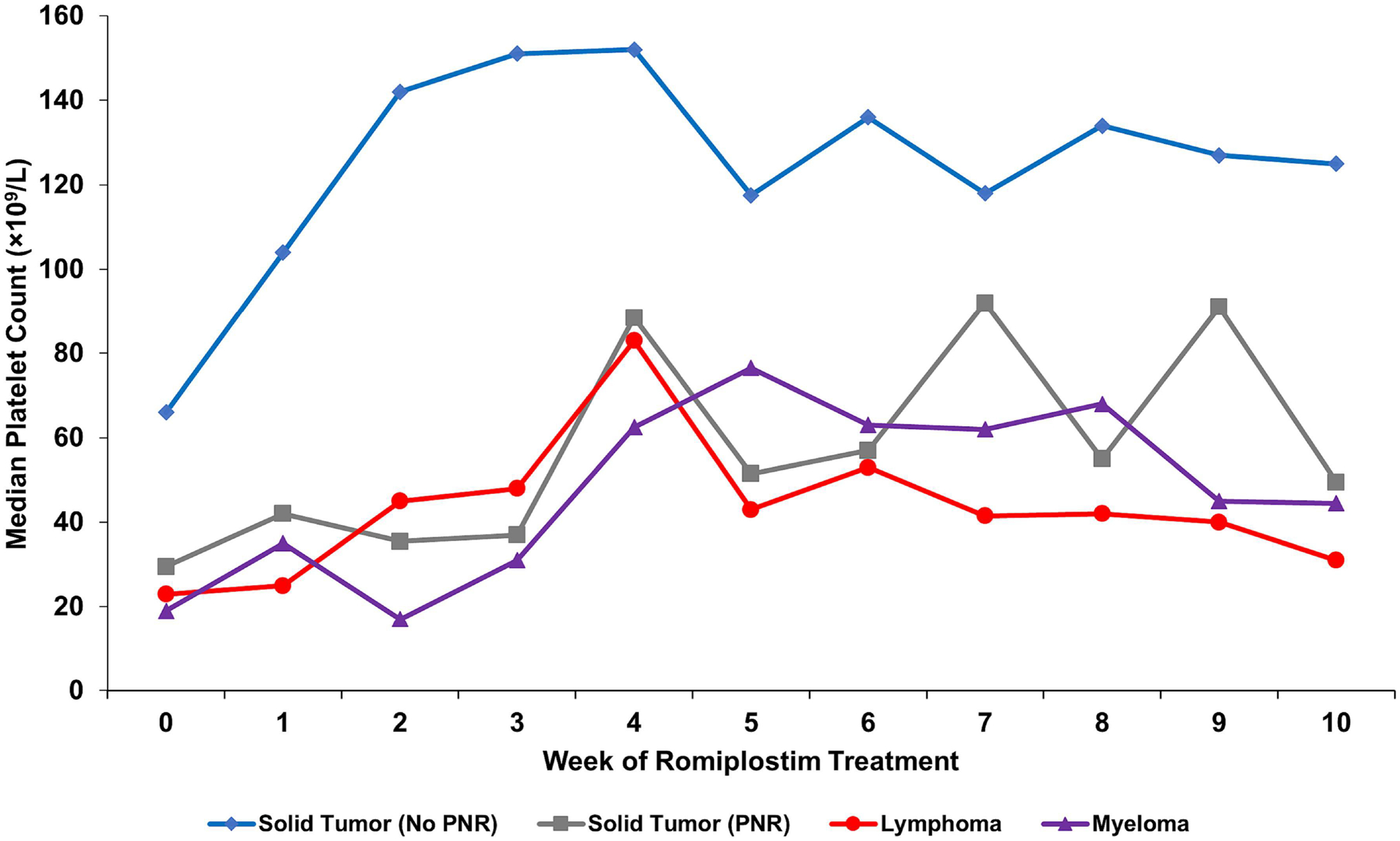
Solid tumor patients with no predictors of romiplostim non-response (N=122, blue); solid tumor patients with predictors of romiplostim non-response (N=31, gray) including bone marrow invasion by tumor, prior pelvic irradiation, or prior temozolomide treatment; aggressive lymphoma patients (N=13, red); and myeloma patients (N=7, purple). All lymphoma and myeloma patients had known marrow involvement by tumor. PNR, predictors of non-response. Reproduced with permission from Al-Samkari et al., 2021 [16].
The endogenous thrombopoietin level has been observed to predict response to TPO-RAs across numerous conditions, including ITP, CIT, and thrombocytopenia of chronic liver disease [55–58]. Therefore, in those with access to endogenous thrombopoietin testing, dramatic elevations of the endogenous serum thrombopoietin level to greater than 10–20 times the upper limit of the normal range (>1000–2000 pg/mL in one study using a widely-available well-validated assay with a normal range of 7–99 pg/mL) strongly predicts nonresponse to romiplostim: 25% likelihood of response was observed in patients with TPO levels >1040 pg/mL declining to 7% in patients with TPO levels >1978 pg/mL [55]. Conversely, patients with normal or mild elevations in endogenous thrombopoietin (<5 times the upper limit of normal) were more likely to respond to romiplostim: 91% likelihood of response was observed in patients with TPO levels <101 pg/mL declining to 61% in patients with TPO levels <457 pg/mL. Patients with striking elevations in endogenous thrombopoietin are very likely to have either bone marrow failure or myelophthisis [58].
There are few absolute contraindications to TPO-RA support beyond known hypersensitivity or prior serious adverse event attributable to TPO-RA use. A prior history of remote venous or arterial thromboembolism is not a contraindication to TPO-RA use for CIT. A recent history of such an event is not either, as long as the patient is on appropriate antithrombotic therapy. However, the author avoids TPO-RA use in cancer patients with a very strong thrombophilia or major history of thromboembolism, such as patients with triple-positive antiphospholipid antibody syndrome, multiply recurrent venous thromboembolism (unless the patient is on therapeutic anticoagulation) or multiply recurrent arterial thromboembolism (unless the patient is on adequate antiplatelet therapy).
MANAGEMENT OF PERSISTENT CIT WITH THROMBOPOIETIN RECEPTOR AGONISTS
All patients with persistent CIT being considered for TPO-RA support should first be offered a clinical trial if available (Figure 2). Ongoing clinical trials of romiplostim and avatrombopag treatment of persistent CIT are described later in this review. Where a clinical trial is not available or a patient is not a clinical trial candidate, off-label use of romiplostim may be considered. Because there are no significant data published describing use of any of the oral TPO-RAs to treat persistent CIT, these agents are not presently recommended for this use outside the setting of a clinical trial. Therefore, the remainder of this section will focus on off-label use of romiplostim in persistent CIT in adults. While the recommendations below are likely also applicable in children, the data for off-label use of romiplostim for CIT in children is limited to small case series [59–61] and therefore their applicability in children is uncertain.
Initial Dose.
Studies to date have used a starting dose of romiplostim of 2–4 mcg/kg weekly to treat persistent CIT [16, 18, 19, 52]. This includes a 173 patient cohort study as well as a 60 patient phase II clinical trial, among others. The ongoing phase 3 clinical trials utilize a 2 mcg/kg weekly initial dose. With this dosing, adequate response is achieved frequently without further titration and risk of thrombocytosis is very low. The author obtains baseline thrombopoietin levels as a part of initial evaluation [55] and therefore has the results of this test available at the time of initial dosing; patients with normal TPO levels are initiated at 2 mcg/kg, those with mild elevations (up to 5 times the ULN) are initiated at 3 mcg/kg, and those with more significant elevations (over 5 times the ULN) are initiated at 4 mcg/kg. Those with over 10 times the ULN may be initiated at higher doses, such as 5 mcg/kg, with more aggressive titration (see below) given their high likelihood of nonresponse. Where baseline TPO levels are not available or not used, the author recommends initiation at 2 mcg/kg in patients with baseline platelet counts above 50 × 109/L on day 1 of a chemotherapy cycle and 3–4 mcg/kg in patients with baseline platelet counts below this threshold. Patients with profound thrombocytopenia (<20–30 × 109/L) on day 1 of a chemotherapy cycle are likely to have complicating additional etiologies of thrombocytopenia, such as myelophthisis, particularly if these patients have a history of many weeks of a platelet count <75–100 × 109/L. When this is suspected, even higher initial doses of romiplostim can be considered to minimize delays to further chemotherapy administration, accounting for the potential risk of thrombocytosis.
It is worth noting that the FDA prescribing information for romiplostim in ITP advises a starting dose of 1 mcg/kg/week even though some randomized trials of romiplostim for ITP used starting doses of 3 mcg/kg/week without issue (which is the author’s preferred starting dose of romiplostim in ITP) [36, 62]. Starting CIT patients at 1 mcg/kg/week likely only delays achievement of platelet count response and restoration of RDI with little to no meaningful reduction in thrombocytosis or thromboembolism risk.
Dose Titration.
The aforementioned studies of romiplostim for CIT employed titration of the dose up or down by 1–2 mcg/kg/week to achieve a goal platelet count of 100–200 × 109/L, and this approach is recommended by the author, Table 4. Thrombocytosis is quite rare when following the dosing recommendations for CIT described herein, and when encountered it is even more rare for it to be severe enough to merit holding a dose of romiplostim; most of the time, reduction of the dose by 2 mcg/kg is adequate to resolve the thrombocytosis by the following week.
Table 4.
The author’s recommended weekly romiplostim dose titration procedure for CIT.
| Platelet Count | Recommended Weekly Romiplostim Dose Titration |
|---|---|
| <50×109/L | Increase dose by 2 mcg/kg |
| 50–99×109/L | Increase dose by 1 mcg/kg |
| 100–200×109/L | Maintain current dose |
| 201–400×109/L | Decrease dose by 1 mcg/kg |
| >400×109/L | Decrease dose by 1–2 mcg/kg or consider holding romiplostim. If holding, resume romiplostim at 1–2 mcg/kg lower than prior dose once platelet count drops to <200–300×109/L |
Frequency of Administration.
Romiplostim is approved for weekly dosing in ITP, and this has been the sole approach taken in the majority of published CIT clinical studies, including the prospective phase 2 clinical trial [19]. In the published 173 patient cohort [16], approximately half of the solid tumor patients (N=73) were treated with an alternative dosing regimen, dubbed ‘intracycle dosing’, and the remaining solid tumor patients (N=80) were treated with standard weekly dosing. Intracycle dosing is characterized by romiplostim administration primarily on chemotherapy off-weeks, on average twice per month. In patients receiving weekly chemotherapy or daily oral targeted therapy, patients managed with intracycle dosing received romiplostim every other week. Ultimately, patients receiving standard weekly dosing had higher platelet counts, higher rates of response to romiplostim, lower rates of CIT recurrence, chemotherapy RDI reduction, or bleeding; rates of thromboembolism and platelet transfusion were similar between the two groups. Therefore, with the exception of resource-limited settings, in which intracycle dosing may be preferred, the author advises standard weekly dosing.
Declaring Futility.
Patients not adequately responsive to high (8–10 mcg/kg) doses of romiplostim should be considered for bone marrow biopsy to evaluate for additional complicating etiologies or alternative etiologies of thrombocytopenia, such as myelophthisis or a bone marrow failure syndrome such as amegakaryocytic thrombocytopenia, myelodysplastic syndrome or aplastic anemia. Endogenous thrombopoietin levels are also likely to be strikingly elevated to 10–20 times the ULN in these patients as well. Inadequate response to 10 mcg/kg doses for 2–4 weeks should prompt discontinuation and alternative management. While combination thrombopoietic therapy [63] or switching from one TPO-RA to another [64–66] has been employed with success in other thrombocytopenic conditions, the rationale for treatment failure in CIT is different and no data exists to support these approaches in this scenario.
Addressing Potential Toxicity.
Patients developing cancer-associated thromboembolism while receiving romiplostim should be managed with appropriate antithrombotic therapy [6, 67] and given the likelihood of thrombocytopenia recurrence with discontinuation of romiplostim, continued romiplostim treatment concurrent with antithrombotic therapy and ongoing cancer therapy is usually prudent. Venous thromboembolism in particular is common in patients with cancer, especially those with metastatic disease who are also the most likely to develop CIT given the extended duration of chemotherapy treatment they require [68]. Risk of VTE is further increased by cytotoxic chemotherapy itself, indwelling lines and ports, and other patient characteristics very common in patients with cancer [6]. Therefore, VTE will occur relatively commonly in patients receiving romiplostim irrespective of whether the romiplostim is meaningfully contributing to the VTE risk or not. Once a patient is therapeutically anticoagulated, any potential contribution of romiplostim therapy to VTE risk should be negligible. If the thromboembolism occurred in the setting of thrombocytosis, the romiplostim dose should be carefully adjusted to prevent subsequent platelet counts above 200 × 109/L.
Low-grade adverse events attributable to romiplostim, such as headache or bone pain, can usually be managed with standard symptomatic treatments such as acetaminophen. If a patient develops a persistent adverse event that is not responsive to these approaches, the a trial of romiplostim discontinuation to assess if the event resolves is reasonable, followed by re-challenge to see if it subsequently returns. If so, shared decision-making between patient and provider should determine if the adverse effect is tolerable or not and the benefits of ongoing therapy outweigh the risks.
Discontinuation for Therapy Completion.
In patients completing chemotherapy, the final dose of romiplostim may be administered one week prior to the final day of the last chemotherapy cycle. Rebound thrombocytopenia, sometimes observed in patients with ITP following romiplostim discontinuation, has not been reported in the CIT literature to date and therefore post-chemotherapy romiplostim tapers are not necessary.
Management of Patients with Solid Tumors and Predictors of Nonresponse or Lymphoid Malignancies.
Use of romiplostim in patients with known bone marrow solid tumor infiltration, prior pelvic irradiation, prior temozolomide exposure, or lymphoid malignancies involving the bone marrow is not always futile, and a trial of romiplostim treatment can be considered though responses are often attenuated (Figure 3). This is particularly true in patients with tumor marrow involvement that may regress with effective therapy, and for whom even a modest response to romiplostim may be enough to facilitate safer administration of chemotherapy. In such patients, the author usually employs a starting dose of 5 mcg/kg and does not hesitate to rapidly increase the dose if this initial dose does not result in an adequate platelet response (i.e. to 8–10 mcg/kg for the second dose).
ONGOING CLINICAL TRIALS OF THROMBOPOIETIN RECEPTOR AGONISTS TO TREAT PERSISTENT CIT
Three major clinical trials evaluating TPO-RA management of CIT are currently ongoing and enrolling patients.
RECITE.
RECITE (Study of Romiplostim for Chemotherapy-induced Thrombocytopenia in Adult Subjects with Gastrointestinal, Pancreatic or Colorectal Cancer, NCT03362177) is a pivotal global multicenter industry-sponsored phase III randomized, controlled trial enrolling 162 patients with persistent CIT receiving oxaliplatin-based chemotherapy regimens for esophageal, gastric, colorectal, or pancreatic cancers, randomizing subjects 2:1 to receive romiplostim or placebo once weekly for 3 chemotherapy cycles. Patients must have persistent CIT with a platelet count ≤85 × 109/L, must be receiving an oxaliplatin-based multiagent cytotoxic chemotherapy regimen containing either fluorouracil or capecitabine (or receiving an alternative regimen at the time of developing CIT with plan to transition to an oxaliplatin + fluorouracil/capecitabine regimen upon trial enrollment), and have at least 3 remaining planned cycles of chemotherapy at study enrollment to be eligible. The study’s primary endpoint is RDI reduction (dose reduction, delay, or omission) or treatment discontinuation due to thrombocytopenia in the second and third cycles of chemotherapy on trial. Secondary endpoints include overall survival, the depth of the observed platelet nadir, the time to platelet response, the duration-adjusted rate of bleeding events of grade 2 or higher, incidence of platelet transfusions, the proportion of patients achieving a platelet response, and safety. As of writing, the study’s current estimated primary completion date is April of 2025.
PROCLAIM.
PROCLAIM (Study of Romiplostim for Chemotherapy-induced Thrombocytopenia in Adult Subjects with Non-small Cell Lung Cancer, Ovarian Cancer, or Breast Cancer, NCT03937154) is a pivotal global multicenter industry-sponsored phase III randomized, controlled trial enrolling 162 patients with persistent CIT receiving cytotoxic chemotherapy for non-small cell lung cancer, breast cancer, or ovarian cancer (including fallopian tube epithelial carcinomas and peritoneal epithelial carcinoma of unknown primary), randomizing subjects 2:1 to receive romiplostim or placebo once weekly for 3 chemotherapy cycles. Patients must have persistent CIT with a platelet count ≤85 × 109/L, must be receiving a single agent chemotherapy regimen including carboplatin, gemcitabine, pemetrexed, liposomal doxorubicin, paclitaxel, nab-paclitaxel, and/or docetaxel, a multi-agent carboplatin doublet (carboplatin/gemcitabine, carboplatin/pemetrexed, carboplatin/liposomal doxorubicin, or carboplatin/taxane), or receiving an alternative regimen at the time of developing CIT with plan to transition to one of the aforementioned regimens, and have at least 3 remaining planned cycles of chemotherapy at study enrollment to be eligible. The study’s primary endpoint is RDI reduction (dose reduction, delay, or omission) or treatment discontinuation due to thrombocytopenia in the second and third cycles of chemotherapy on trial. Secondary endpoints include overall survival, the depth of the observed platelet nadir, the time to platelet response, the duration-adjusted rate of bleeding events of grade 2 or higher, incidence of platelet transfusions, the proportion of patients achieving a platelet response, and safety. As of writing, the study’s current estimated completion date is February of 2027.
ACT-GI.
ACT-GI (Avatrombopag for Chemotherapy-induced Thrombocytopenia in Gastrointestinal cancers, NCT05772546) is a national U.S. multicenter investigator-initiated randomized, controlled phase II clinical trial evaluating the safety and efficacy of the oral TPO-RA avatrombopag in patients with gastrointestinal cancers receiving cytotoxic chemotherapy who develop persistent CIT. Avatrombopag is a newer oral thrombopoietin receptor agonist with several advantages over eltrombopag, including a lack of dietary restrictions (ideal in patients with cancer), no signal for hepatotoxicity, possible higher potency, and no effect on the color of patient plasma, skin, or sclerae (Table 1) [69–72]. ACT-GI will enroll and randomize in a 1:1 ratio up to 60 patients with gastrointestinal cancers to avatrombopag or placebo. Patients with esophageal, gastric, small bowel, hepatobiliary (cholangiocarcinoma, gallbladder carcinoma, hepatocellular carcinoma), pancreatic, or colorectal cancer receiving treatment with a 14, 21, or 28-day cycle length chemotherapy regimen including at least one of the eligible chemotherapeutic agents (fluorouracil, capecitabine, trifluridine/tipiracil, gemcitabine, cisplatin, carboplatin, oxaliplatin, irinotecan, liposomal irinotecan, paclitaxel, nanoalbumin-bound paclitaxel, docetaxel, epirubicin, or doxorubicin) who present with persistent CIT defined as a platelet count ≤80 × 109/L and no platelet count ≥100 × 109/L in the preceding four weeks are eligible. Enrolled patients will enter a 2-week lead-in period during which time they will receive study drug and their platelet count will be monitored; patients will be eligible to resume chemotherapy after the platelet count recovers to ≥100 × 109/L, at which time they will receive one cycle of chemotherapy on study drug support. Patients who fail to achieve platelet count recovery in the lead-in period will be deemed treatment failures and complete study participation without completing one cycle of chemotherapy on study drug support. All patients who complete study participation per protocol have access to avatrombopag following completion. The study’s primary endpoint is achievement and maintenance of a response, defined as achieving a platelet count ≥100 × 109/L within the 2 week lead-in period and then finishing at least 1 cycle of chemotherapy without CIT recurrence (no on-cycle dose-reduction or treatment delay due to thrombocytopenia and ability to receive another cycle of chemotherapy without dose-reduction or treatment delay, defined as platelet count of ≥100 × 109/L at the start of the following cycle whether or not an additional cycle is planned). Secondary endpoints include proportion of patients achieving platelet count recovery, incidence of platelet transfusions, rate of bleeding events of grade 2 or higher, rate of thromboembolic events, and safety. As of writing, the study’s current estimated completion date is July of 2025.
FUTURE CONSIDERATIONS AND CONCLUSIONS
CIT remains a common clinical problem with no available regulatory agency-approved medications in the majority of the world. CIT may result in major bleeding events or reduction of chemotherapy RDI, both of which have been demonstrated to reduce overall survival of patients with cancer and compromise oncologic therapy and outcomes. Patients with nadir CIT, who by definition recover to a normal or near normal platelet count by the start of their next cycle, usually do not require treatment for CIT unless they have developed bleeding in association with the CIT or they have profound nadir thrombocytopenia (typically a platelet count <20–30 × 109/L). Patients with persistent CIT, who present with a platelet count <70–100 × 109/L on day 1 of a chemotherapy cycle and cannot be safely treated at full dose and on schedule without intervention may benefit from TPO-RA support. At present, this is best accomplished in the setting of a clinical trial where available, but if a clinical trial is not available it can be accomplished through the proper and judicious use of off label romiplostim, given the published data supporting the use of this agent for this indication. Treatment of persistent CIT with TPO-RAs clearly allows resumption of delayed or discontinued chemotherapy and restoration of RDI, which may therefore improve oncologic and patient outcomes such as progression-free survival, overall survival, and health-related quality of life. Ongoing major clinical trials may ultimately facilitate regulatory agency approval of TPO-RA therapy to manage CIT. If these trials are successful, future studies—high-quality large observational studies, as the requisite size randomized trials are not feasible—will be necessary to definitively quantify the impact of TPO-RA on overall survival in patients with CIT.
PRACTICE POINTS.
CIT is categorized clinically into two main presentations/phenotypes: nadir CIT, in which a patient has an excessive mid-chemotherapy cycle platelet nadir and recovers to normal or near normal by day 1 of the next cycle, and persistent CIT, in which a patient presents with an inadequate platelet count on day 1 of a chemotherapy cycle for treatment at full dose on schedule.
Persistent CIT usually recurs over and over again without drastic modification to the chemotherapy regimen (with accordant compromise of oncologic therapeutic outcomes) or initiation of TPO-RA support. This should be done in the setting of a clinical trial where available, and with off-label romiplostim where not available.
Patients with solid tumors and no marrow infiltration by tumor or prior pelvic irradiation treated with off-label romiplostim usually respond well to treatment, which is initiated at 2–4 mcg/kg and dosed weekly, titrated based on the patient’s platelet count.
Evidence to date over multiple prospective clinical trials, including a randomized clinical trial, and a large body of observational evidence suggests that TPO-RA use does not appear to increase venous or arterial thromboembolic risk in patients with cancer, but additional study is needed.
Nadir CIT is usually clinically silent, does not reliably recur in the same patient even with the same chemotherapy regimen, and generally does not require treatment with TPO-RAs to prevent recurrence in subsequent cycles except in specific circumstances: deep nadirs resulting in bleeding, recurrent profoundly deep nadirs, or deep nadirs in patients on antithrombotic therapy.
RESEARCH AGENDA.
Three major clinical trials of TPO-RA management in persistent CIT are ongoing: RECITE (romiplostim for persistent CIT in gastrointestinal cancers), PROCLAIM (romiplostim for persistent CIT in lung, breast, and ovarian cancers), and ACT-GI (avatrombopag for persistent CIT in gastrointestinal cancers).
The results of these trials will be pivotal in establishing the long-term treatment guidelines for TPO-RAs in CIT and possibly earning formal regulatory approval for these agents in CIT.
Additional well-powered observational studies will be necessary to confirm that treatment of CIT and enabling administration of ongoing chemotherapy at full dose and on schedule ultimately improves overall survival. Current practice relies on extrapolation from the fact that RDI reduction generally worsens outcomes and that restoration of RDI through the use of TPO-RAs should then improve outcomes.
ACKNOWLEDGEMENTS
H.A. is funded by the National Heart, Lung, and Blood Institute (1K23HL159313). Artwork in the Figure 2 was reproduced and modified from Servier Medical Art (https://smart.servier.com/) in accordance with the Creative Commons license CC BY 3.0 (permission given for use and adaptation for any purpose, medium, or format).
FUNDING INFORMATION
H.A. is funded by the National Heart, Lung, and Blood Institute (1K23HL159313).
UNIVERSAL DISCLOSURES:
H. Al-Samkari (universal disclosures): Research funding to institution (Agios, Sobi, Novartis, Vaderis, Amgen) and consultancy (Agios, Sobi, Moderna, Novartis, Rigel, argenx, Forma, Pharmacosmos).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Off-label use: Use of romiplostim, eltrombopag, and avatrombopag for chemotherapy-induced thrombocytopenia
REFERENCES
- [1].Al-Samkari H, Soff GA. Clinical challenges and promising therapies for chemotherapy-induced thrombocytopenia. Expert Rev Hematol. 2021;14:437–48. [DOI] [PubMed] [Google Scholar]
- [2].Kuter DJ. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica. 2022;107:1243–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheloff AZ, Al-Samkari H. Romiplostim for PARP inhibitor-induced thrombocytopenia in solid tumor malignancies. Platelets. 2022;33:1312–3. [DOI] [PubMed] [Google Scholar]
- [4].Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771–80. [DOI] [PubMed] [Google Scholar]
- [5].Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–46. [DOI] [PubMed] [Google Scholar]
- [6].Al-Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood advances. 2019;3:3770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leiva O, Newcomb R, Connors JM, Al-Samkari H. Cancer and thrombosis: new insights to an old problem. J Med Vasc. 2020;45:6S8–6S16. [DOI] [PubMed] [Google Scholar]
- [8].Al-Samkari H Thrombopoietin receptor agonists for chemotherapy-induced thrombocytopenia: a new solution for an old problem. Hematology Am Soc Hematol Educ Program. 2022;2022:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Denduluri N, Patt DA, Wang Y, Bhor M, Li X, Favret AM, et al. Dose Delays, Dose Reductions, and Relative Dose Intensity in Patients With Cancer Who Received Adjuvant or Neoadjuvant Chemotherapy in Community Oncology Practices. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13:1383–93. [DOI] [PubMed] [Google Scholar]
- [10].Aspinall SL, Good CB, Zhao X, Cunningham FE, Heron BB, Geraci M, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203–10. [DOI] [PubMed] [Google Scholar]
- [12].Hanna RK, Poniewierski MS, Laskey RA, Lopez MA, Shafer A, Van Le L, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129:74–80. [DOI] [PubMed] [Google Scholar]
- [13].Nakayama G, Tanaka C, Uehara K, Mashita N, Hayashi N, Kobayashi D, et al. The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2014;73:847–55. [DOI] [PubMed] [Google Scholar]
- [14].Network NCC. NCCN Guidelines Version 1.2022: Hematopoietic Growth Factors. 2022.
- [15].Song AB, Al-Samkari H. Emerging data on thrombopoietin receptor agonists for management of chemotherapy-induced thrombocytopenia. Expert Rev Hematol. 2023;16:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Al-Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021;106:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Samkari H, Kolb-Sielecki J, Safina SZ, Xue X, Jamieson BD. Avatrombopag for chemotherapy-induced thrombocytopenia in patients with non-haematological malignancies: an international, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Haematology. 2022;9:e179–e89. [DOI] [PubMed] [Google Scholar]
- [18].Al-Samkari H, Marshall AL, Goodarzi K, Kuter DJ. The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103:e169–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soff GA, Miao Y, Bendheim G, Batista J, Mones JV, Parameswaran R, et al. Romiplostim Treatment of Chemotherapy-Induced Thrombocytopenia. J Clin Oncol. 2019;37:2892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vadhan-Raj S. Clinical experience with recombinant human thrombopoietin in chemotherapy-induced thrombocytopenia. Seminars in hematology. 2000;37:28–34. [DOI] [PubMed] [Google Scholar]
- [21].Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109:4607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–8. [DOI] [PubMed] [Google Scholar]
- [23].Basser RL, O’Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599–602. [DOI] [PubMed] [Google Scholar]
- [24].Consensus Committee of Chemotherapy Induced Thrombocytopenia CSoCO. [Consensus on clinical diagnosis, treatment and prevention management of chemotherapy induced thrombocytopenia in China(2018)]. Zhonghua Zhong Liu Za Zhi. 2018;40:714–20. [DOI] [PubMed] [Google Scholar]
- [25].Wilde MI, Faulds D. Oprelvekin: a review of its pharmacology and therapeutic potential in chemotherapy-induced thrombocytopenia. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 1998;10:159–71. [DOI] [PubMed] [Google Scholar]
- [26].Tepler I, Elias L, Smith JW 2nd, Hussein M, Rosen G, Chang AY, et al. A randomized placebo-controlled trial of recombinant human interleukin-11 in cancer patients with severe thrombocytopenia due to chemotherapy. Blood. 1996;87:3607–14. [PubMed] [Google Scholar]
- [27].Cantor SB, Elting LS, Hudson DV Jr., Rubenstein EB. Pharmacoeconomic analysis of oprelvekin (recombinant human interleukin-11) for secondary prophylaxis of thrombocytopenia in solid tumor patients receiving chemotherapy. Cancer. 2003;97:3099–106. [DOI] [PubMed] [Google Scholar]
- [28].Song F, Al-Samkari H. Management of Adult Patients with Immune Thrombocytopenia (ITP): A Review on Current Guidance and Experience from Clinical Practice. J Blood Med. 2021;12:653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ji R, Huang G, Xu J, Yu X, Zhou A, Du C. Splenomegaly during oxaliplatin-based chemotherapy: impact on blood parameters and anti-neoplastic treatment. Transl Cancer Res. 2022;11:1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park AK, Waheed A, Forst DA, Al-Samkari H. Characterization and prognosis of temozolomide-induced aplastic anemia in patients with central nervous system malignancies. Neuro Oncol. 2022;24:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ayad N, Grace RF, Kuter DJ, Al-Samkari H. Long-term risk of developing immune thrombocytopenia and hematologic neoplasia in adults with mild thrombocytopenia. Blood. 2022;140:2849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Therapeutic advances in hematology. 2019;10:2040620719841735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Virk ZM, Kuter DJ, Al-Samkari H. An evaluation of avatrombopag for the treatment of thrombocytopenia. Expert Opin Pharmacother. 2021;22:273–80. [DOI] [PubMed] [Google Scholar]
- [34].Al-Samkari H, Grace RF, Kuter DJ. The role of romiplostim for pediatric patients with immune thrombocytopenia. Therapeutic advances in hematology. 2020;11:2040620720912992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ayad N, Grace RF, Al-Samkari H. Thrombopoietin receptor agonists and rituximab for treatment of pediatric immune thrombocytopenia: A systematic review and meta-analysis of prospective clinical trials. Pediatr Blood Cancer. 2022;69:e29447. [DOI] [PubMed] [Google Scholar]
- [36].Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–99. [DOI] [PubMed] [Google Scholar]
- [37].Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peffault de Latour R, Kulasekararaj A, Iacobelli S, Terwel SR, Cook R, Griffin M, et al. Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. N Engl J Med. 2022;386:11–23. [DOI] [PubMed] [Google Scholar]
- [39].Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442–52 e1. [DOI] [PubMed] [Google Scholar]
- [40].Al-Samkari H, Marshall AL, Goodarzi K, Kuter DJ. Romiplostim for the management of perioperative thrombocytopenia. Br J Haematol. 2018;182:106–13. [DOI] [PubMed] [Google Scholar]
- [41].Nagrebetsky A, Al-Samkari H, Davis NM, Kuter DJ, Wiener-Kronish JP. Perioperative thrombocytopenia: evidence, evaluation, and emerging therapies. British journal of anaesthesia. 2019;122:19–31. [DOI] [PubMed] [Google Scholar]
- [42].Lindquist I, Olson SR, Li A, Al-Samkari H, Jou JH, McCarty OJT, et al. The efficacy and safety of thrombopoietin receptor agonists in patients with chronic liver disease undergoing elective procedures: a systematic review and meta-analysis. Platelets. 2021:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Michelson AD, Smolensky Koganov E, Forde EE, Carmichael SL, Frelinger AL 3rd. Avatrombopag increases platelet count but not platelet activation in patients with thrombocytopenia resulting from liver disease. J Thromb Haemost. 2018;16:2515–9. [DOI] [PubMed] [Google Scholar]
- [44].Psaila B, Bussel JB, Linden MD, Babula B, Li Y, Barnard MR, et al. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: no evidence of platelet activation. Blood. 2012;119:4066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Al-Samkari H, Van Cott EM, Kuter DJ. Platelet aggregation response in immune thrombocytopenia patients treated with romiplostim. Ann Hematol. 2019;98:581–8. [DOI] [PubMed] [Google Scholar]
- [46].Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716–24. [DOI] [PubMed] [Google Scholar]
- [47].Al-Samkari H, Kuter DJ. Immune Thrombocytopenia in Adults: Modern Approaches to Diagnosis and Treatment. Semin Thromb Hemost. 2020;46:275–88. [DOI] [PubMed] [Google Scholar]
- [48].Harker LA, Marzec UM, Hunt P, Kelly AB, Tomer A, Cheung E, et al. Dose-response effects of pegylated human megakaryocyte growth and development factor on platelet production and function in nonhuman primates. Blood. 1996;88:511–21. [PubMed] [Google Scholar]
- [49].Harker LA, Hunt P, Marzec UM, Kelly AB, Tomer A, Hanson SR, et al. Regulation of platelet production and function by megakaryocyte growth and development factor in nonhuman primates. Blood. 1996;87:1833–44. [PubMed] [Google Scholar]
- [50].Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–8. [DOI] [PubMed] [Google Scholar]
- [51].Jurczak W, Chojnowski K, Mayer J, Krawczyk K, Jamieson BD, Tian W, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Parameswaran R, Lunning M, Mantha S, Devlin S, Hamilton A, Schwartz G, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22:1217–22. [DOI] [PubMed] [Google Scholar]
- [53].Wilkins CR, Ortiz J, Gilbert LJ, Yin S, Mones JV, Parameswaran R, et al. Romiplostim for chemotherapy-induced thrombocytopenia: Efficacy and safety of extended use. Res Pract Thromb Haemost. 2022;6:e12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Villano JL, Letarte N, Yu JM, Abdur S, Bressler LR. Hematologic adverse events associated with temozolomide. Cancer Chemother Pharmacol. 2012;69:107–13. [DOI] [PubMed] [Google Scholar]
- [55].Song AB, Goodarzi K, Karp Leaf R, Kuter DJ, Al-Samkari H. Thrombopoietin level predicts response to treatment with romiplostim in chemotherapy-induced thrombocytopenia. Am J Hematol. 2021;96:1563–8. [DOI] [PubMed] [Google Scholar]
- [56].Al-Samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol. 2018;93:1501–8. [DOI] [PubMed] [Google Scholar]
- [57].Al-Samkari H Avatrombopag maleate for the treatment of periprocedural thrombocytopenia in patients with chronic liver disease. Drugs of today. 2018;54:647–55. [DOI] [PubMed] [Google Scholar]
- [58].Makar RS, Zhukov OS, Sahud MA, Kuter DJ. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol. 2013;88:1041–4. [DOI] [PubMed] [Google Scholar]
- [59].Jacobson AE, Shah N, Setty BA. Romiplostim for therapy-related thrombocytopenia in pediatric malignancies. Pediatr Blood Cancer. 2017;64. [DOI] [PubMed] [Google Scholar]
- [60].Fassel H, Bussel JB, Roberts SS, Modak S. Romiplostim for Immune Thrombocytopenia in Neuroblastoma Patients Receiving Chemotherapy. J Pediatr Hematol Oncol. 2019;41:e257–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Merjaneh N, Young J, Mangoli A, Olsen M, Setty B, Lane A, et al. Chemotherapy-induced thrombocytopenia in Ewing sarcoma: Implications and potential for romiplostim supportive care. Pediatr Blood Cancer. 2022;69:e29548. [DOI] [PubMed] [Google Scholar]
- [62].Amgen I. Nplate (romiplostim) [prescribing information]. Thousand Oaks, CA: Amgen; October 2017. [Google Scholar]
- [63].Park AK, Park JC, Al-Samkari H. Pembrolizumab-Induced Acquired Amegakaryocytic Thrombocytopenia and Successful Combination Treatment With Eltrombopag, Romiplostim and Cyclosporine: A Brief Communication. J Immunother. 2022;45:321–3. [DOI] [PubMed] [Google Scholar]
- [64].Gonzalez-Porras JR, Godeau B, Carpenedo M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Therapeutic advances in hematology. 2019;10:2040620719837906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Al-Samkari H, Jiang D, Gernsheimer T, Liebman H, Lee S, Wojdyla M, et al. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study. Br J Haematol. 2022;197:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Al-Samkari H, Jiang D, Gernsheimer T, Liebman H, Lee S, Bernheisel C, et al. Durability of platelet response after switching to avatrombopag from eltrombopag or romiplostim in immune thrombocytopenia. Res Pract Thromb Haemost. 2023;7:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Song AB, Rosovsky RP, Connors JM, Al-Samkari H. Direct oral anticoagulants for treatment and prevention of venous thromboembolism in cancer patients. Vascular health and risk management. 2019;15:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Al-Samkari H, Connors JM. The Role of Direct Oral Anticoagulants in Treatment of Cancer-Associated Thrombosis. Cancers (Basel). 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Al-Samkari H, Kuter DJ. Relative potency of the thrombopoietin receptor agonists eltrombopag, avatrombopag and romiplostim in a patient with chronic immune thrombocytopenia. Br J Haematol. 2018;183:168. [DOI] [PubMed] [Google Scholar]
- [70].Song AB, Al-Samkari H. An updated evaluation of avatrombopag for the treatment of chronic immune thrombocytopenia. Expert Rev Clin Immunol. 2022;18:783–91. [DOI] [PubMed] [Google Scholar]
- [71].Al-Samkari H, Nagalla S. Efficacy and safety evaluation of avatrombopag in immune thrombocytopenia: analyses of a phase III study and long-term extension. Platelets. 2022;33:257–64. [DOI] [PubMed] [Google Scholar]
- [72].Cheloff AZ, Al-Samkari H. Avatrombopag for the treatment of immune thrombocytopenia and thrombocytopenia of chronic liver disease. J Blood Med. 2019;10:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Al-Samkari H, Kuter DJ. An alternative intermittent eltrombopag dosing protocol for the treatment of chronic immune thrombocytopenia. Br J Clin Pharmacol. 2018;84:2673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kellum A, Jagiello-Gruszfeld A, Bondarenko IN, Patwardhan R, Messam C, Mostafa Kamel Y. A randomized, double-blind, placebo-controlled, dose ranging study to assess the efficacy and safety of eltrombopag in patients receiving carboplatin/paclitaxel for advanced solid tumors. Curr Med Res Opin. 2010;26:2339–46. [DOI] [PubMed] [Google Scholar]
- [75].Winer ES, Safran H, Karaszewska B, Richards DA, Hartner L, Forget F, et al. Eltrombopag with gemcitabine-based chemotherapy in patients with advanced solid tumors: a randomized phase I study. Cancer medicine. 2015;4:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Winer ES, Safran H, Karaszewska B, Bauer S, Khan D, Doerfel S, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine-based chemotherapy: a randomized, placebo-controlled phase 2 study. Int J Hematol. 2017;106:765–76. [DOI] [PubMed] [Google Scholar]


