Abstract
Introduction:
Lysine-specific histone demethylase 1A (KDM1A/LSD1) has emerged as an important therapeutic target in various cancer types. LSD1 regulates a wide range of biological processes that influence cancer development, progression, metastasis, and therapy resistance. However, recent studies have revealed novel aspects of LSD1 biology, shedding light on its involvement in immunogenicity, antitumor immunity, and DNA damage response. These emerging findings have the potential to be leveraged in the design of effective LSD1-targeted therapies.
Areas covered:
This paper discusses the latest developments in the field of LSD1 biology, focusing on its role in regulating immunogenicity, antitumor immunity, and DNA damage response mechanisms. The newfound understanding of these mechanisms has opened possibilities for the development of novel LSD1-targeted therapies for cancer treatment. Additionally, the paper provides an overview of LSD1 inhibitor-based combination therapies for the treatment of cancer.
Expert opinion:
Exploiting LSD1 role in antitumor immunity and DNA damage response provides cues to not only understand the LSD1 resistant mechanisms but also rationally design new combination therapies that are more efficient and less toxic than monotherapy. The exploration of LSD1 biology and the development of LSD1-targeted therapies hold great promise for the future of cancer treatment.
Keywords: LSD1/KDM1A, antitumor immunity, DNA damage response, DNA repair, immune therapies, epigenetics, combination therapies
1. Introduction
Histone methylation is a dynamic process regulated by histone methylases and demethylases, and alterations in histone methylation play a vital role in oncogenic processes[1]. Histone demethylases are classified into one of two distinct groups: the flavin adenine dinucleotide (FAD)-dependent family and the Jumonji-C (JmjC) family. Lysine-specific demethylase 1 (LSD1, also known as KDM1A) is one of the members of the FAD-dependent demethylase family. LSD1 was the first histone demethylase discovered in 2004 by Shi’s group[2]. Specifically, LSD1 demethylates both mono- and di-methylated lysine residues 4 and 9 on histone H3 (H3K4me1/2 and H3K9me1/2) promoting a myriad of global gene expression changes[3,4]. LSD1 demethylates its histone substrates in a context-dependent manner, and its specificity towards its target histone marks is dictated by its interacting protein complexes [2–4]. Studies have shown that H3K4 methylation near transcriptional start sites is associated with actively transcribed genes and LSD1 in association with corepressor of REST (CoREST) and nucleosome remodeling and deacetylase (NuRD) complex demethylates H3K4 residues and represses the gene transcription[3]. Likewise, H3K9 methylation is a repressive epigenetic mark and LSD1 in association with androgen receptor (AR) or estrogen receptor (ER) demethylates H3K9 residues and activates the gene transcription[4,5]. The role of LSD1, either as a transcriptional co-repressor or co-activator, is of significant importance in promoting the progression of cancer[6]. Recent studies suggest that in addition to its demethylase activity at gene promoters, LSD1 contributes to aberrant enhancer silencing/activation and transcriptional regulation [7,8]. LSD1 also demethylates non-histone substrates such as DNA-methyltransferase 1 (DNMT1), tumor suppressor protein p53, transcription factor E2F1, signal transducer and activator of transcription 3 (STAT3), retinoblastoma protein (RB1), myocyte enhancer factor 2 D (MEF2D), metastasis-associated 1 (MTA1), estrogen receptor alpha (ERα), heat shock protein 90 (HSP90), hypoxia-inducible factor 1 (HIF1), and argonaute RISC catalytic component 2 (AGO2)[9]. Increased expression of LSD1 has been found in several cancers and is associated with poor prognosis [10–12]. Outside of LSD1’s role in cancer, LSD1 plays an essential role in various cellular processes such as embryonic development, stem cell maintenance, tissue-specific differentiation, inflammation, neuronal plasticity, thermogenesis, adipogenesis, and metabolism[13].
1.1. LSD1 Structure
LSD1 is a 110 kDa protein encoded by the KDM1A gene located on chromosome 1 at 1p36.12. LSD1 is an 852 amino acids long protein consisting of three main structural motifs: a Swi3p, Rsc8p, and Moira (SWIRM) domain, a bisected amine oxidase domain, and a tower domain. The SWIRM domain is formed by six-helical bundles and is a common motif found in other chromatin remodeling enzymes (Fig. 1). The amine oxidase domain is very similar to that of other MAOs but varies in that it possesses an additional binding site that makes it more specific for the Histone H3 tail [14]. Additionally, the amine oxidase domain has two primary subdomains: a FAD-binding domain and a substrate-binding domain. In the center of this amine oxidase pocket is the LSD1 catalytic site. The tower domain is an extension of the amine oxidase domain, though technically non-essential for in vitro demethylase activity [15], most importantly serves as a binding area for known LSD1 binding partners such as CoREST, CtBP1, HDAC1/2, and AR [16].
Figure 1.
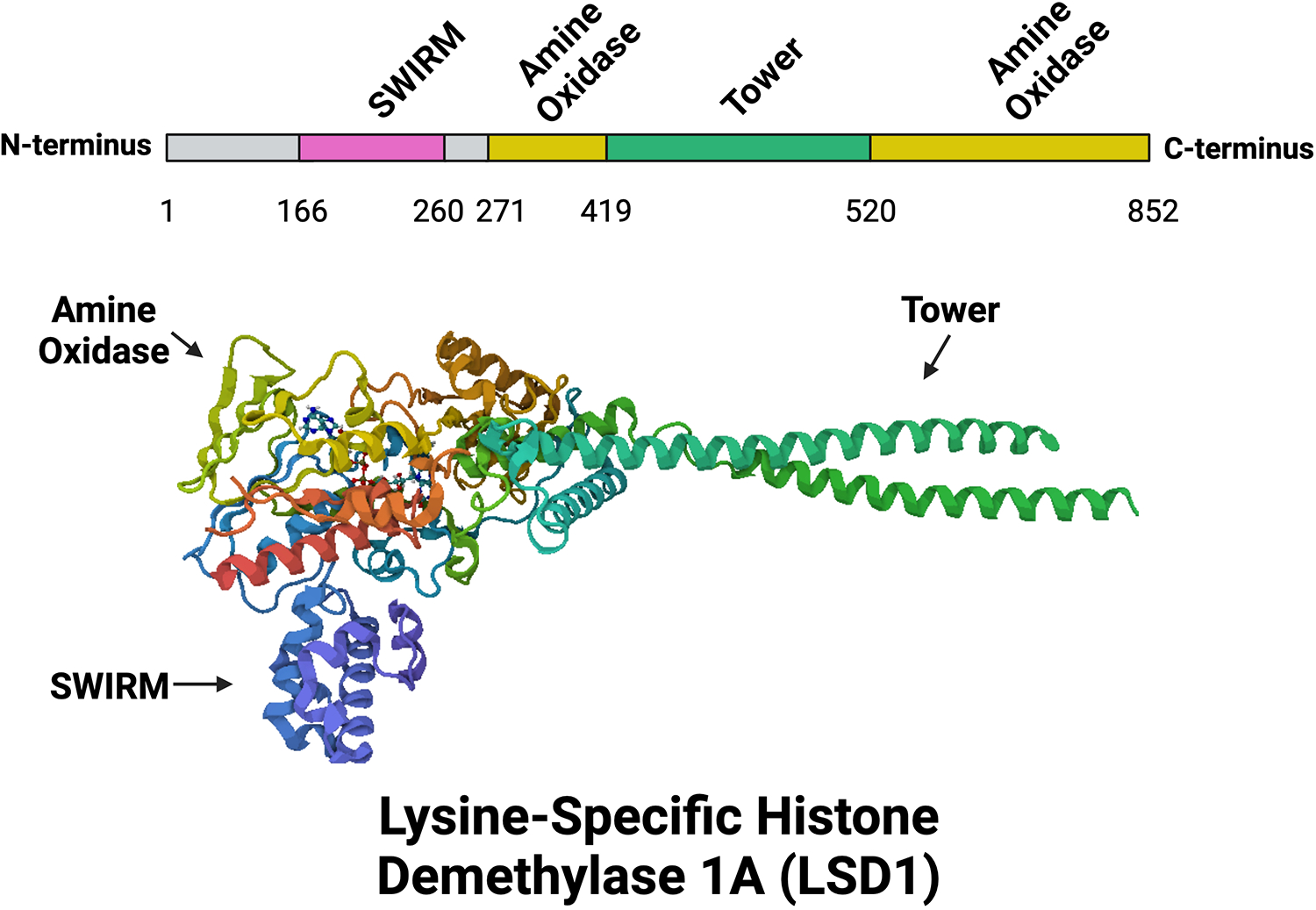
Motifs and Structure of LSD1. A linear representation of LSD1 motifs (above) and ribbon-style cartoon 3D structure of LSD1 (below). LSD1 consists of three major domains: a DNA-binding SWIRM domain (purple), a protein-protein interacting Tower domain (green), and a catalytically active enzymatic demethylase amine oxidase domain (gold). Structure was obtained from Protein Data Bank (PDB) Entry ID 6NQM as generated by PISA software under Creative Commons license. Created with BioRender.com.
Given the extensive literature on LSD1’s involvement in various oncogenic processes, this review specifically concentrates on the latest advancements in the field of LSD1 biology, focusing on its role in regulating immunogenicity, antitumor immunity, DNA damage response mechanisms, and LSD1-based combination therapies.
2. Role of LSD1 in tumor immune evasion and antitumor immunity
Although several cancer-associated mechanisms are regulated by LSD1, recent studies suggest that LSD1 also plays a crucial role in the regulation of immune evasion and antitumor immunity. Loss of LSD1 or its pharmacological inhibition enhances antitumor immunity through various mechanisms. These mechanisms include modifying the expression of immune checkpoint molecules like programmed cell death protein 1 (PD-1), programmed-death ligand 1 (PD-L1), chemokines, endogenous retroviruses (ERVs), major histocompatibility complexes (MHCs), and activating interferon (IFN) and transforming growth factor beta (TGFβ) signaling.
2.1. LSD1 regulates PD-1/PD-L1 immune checkpoint blockade therapy
PD-L1 is commonly present on tumor cell surfaces, hindering CD8+ T cell cytotoxicity and causing CD8+ T cell exhaustion by binding to PD-1 on T cells, resulting in immune evasion by tumor cells[17]. Blockade of PD-1/PD-L1 pathway promotes anti-tumor immunity and tumor cell elimination. However, some patients develop drug resistance and tumor progression over time despite an initial positive response to anti-PD-(L)1 therapy. Epigenetic modulators have been shown to be potential partners with PD-1/PD-L1 blockade, leading to improved antitumor efficacy.
For instance, in head and neck squamous carcinoma cells (HNSCC), LSD1 deletion leads to increased PD-L1 levels, compromising antitumor immunity. Combining LSD1 inhibitor SP2509 with anti-PD-1 monoclonal antibody significantly inhibits tumor growth[18]. In oral squamous cell carcinoma, LSD1 inhibition upregulates PD-L1 expression and enhances the efficacy of PD-1 and PD-L1 antibody immunotherapy[19]. In GDF1-positive hepatocellular carcinoma (HCC) tumors, LSD1 inhibitor GSK-LSD1 boosts the expression of cancer-testis antigens (CTAs) and further enhances the therapeutic effects of PD-1 immune-checkpoint inhibitors[20]. Additionally, combining ORY-1001 with anti-PD-L1 antibody augments the antitumor immune response in refractory small cell lung cancer (SCLC) models[21]. In a syngeneic model of SCLC, IMG-7289 (Bomedemstat) was found to enhance responses to PD-1 inhibition [22]. LSD1 depletion in cancer cells elicits significant responses in checkpoint blockade-refractory mouse melanoma to anti-PD-1 therapy[23]. In HCC cells, LSD1 promotes immunosuppression by regulating PD-L1 expression via demethylation of MEF2D, which activates PD-L1 expression[24]. Further simultaneous inhibition of LSD1 and TGFβ increases CD8+ T-cell infiltration and cytotoxicity, leading to eradication of poorly immunogenic tumors upon anti-PD-1 treatment[25]. SP-2577, an LSD1 inhibitor, promotes PD-L1 expression in small cell carcinoma of the ovary hypercalcemic type (SCCOHT) and ovarian clear cell carcinomas [26]. Interestingly, in immunotherapy-resistant metastatic melanoma patients and immunotherapy-resistant mice, nuclear-phosphorylated LSD1 (nLSD1p) is enriched in PD-1+CD8+ T cells. Targeting the LSD1p nuclear axis induces IFN-γ/TNF-α-expressing CD8+ T cell infiltration into tumors which is further augmented by combined immunotherapy [27]. LSD1, in association with BLIMP-1, epigenetically represses PDCD1 (PD-1) expression in CD8+ T cells during acute lymphocytic choriomeningitis virus (LCMV) infection[28] and in a murine melanoma model, the proportion of PD-1-positive cells is increased in tumors from mice with LSD1-deficient CD8+ T cells [28]. All these studies support that LSD1 depletion or inhibition enhances PD-(L)1 expression.
Contrary to these findings, in some cancers, inhibiting LSD1 has been shown to decrease PD-1 and/or PD-L1 expression. Interestingly, in gastric cancer, LSD1 inhibits T-cell response by inducing the accumulation of PD-L1 in exosomes, while membrane PD-L1 remains constant in gastric cancer cells. LSD1 deletion decreases exosomal PD-L1 and restores T cell response in gastric cancer [29]. In cervical cancer, LSD1 knockdown directly downregulates the expression of CD47 and PD-L1. Combining ORY-1001 with anti-CD47/PD-L1 antibodies effectively inhibits tumor growth in a xenograft model[30]. A Phenothiazine-Based LSD1 inhibitor downregulates PD-L1 expression in gastric cancer cell lines, enhancing T-cell killing response [31]. Interestingly, He et al., demonstrated the role of LSD1 in PD-L1 non-immune regulatory functions in lung adenocarcinoma cells. In these models, LSD1 inhibition reduces tumor growth and metastasis via downregulation of PD-L1 through the JAK pathway[32]. Altogether, these studies demonstrate PD-(L)1 regulation by LSD1 and that combining PD-(L)1 antibodies alongside LSD1 inhibitors could enhance anti-tumor immune responses.
2.2. LSD1 regulates T-cell trafficking
Lymphocytes that infiltrate tumors are called tumor-infiltrating lymphocytes (TILs). Tumors can be classified as “cold tumors” or “hot tumors” based on the abundance of TILs. “Cold tumors” have a lack of T lymphocyte infiltration, leading to poor clinical response. In contrast, “hot tumors” are characterized by the infiltration of CD8+ cytotoxic T cells, which is associated with a favorable prognosis[33]. Therefore, it is crucial to find ways to activate the infiltration of CD8+ T cells into the tumor microenvironment (TME).
Emerging studies have shown that LSD1 blockade increases CD8+ T cell infiltration in tumor tissue and promotes antitumor immunity. TCGA data analysis indicates an inverse correlation between LSD1 expression and CD8+ T cell infiltration in various human cancers[23]. LSD1 ablation has been demonstrated to promote the infiltration of CD4+ and CD8+ T cells in poorly immunogenic tumors and elicit significant responses to anti-PD-1 therapy in checkpoint blockade-refractory mouse melanoma[23]. Furthermore, a hydrogel formulation of GSK-LSD1 has been found to increase CD8+ T cell infiltration as well as the ratio of CD8+ T cells to Treg cells within the TME of triple-negative breast cancer (TNBC)[34]. In the context of HNSCC, the combination of SP2509 with anti-PD-1 monoclonal antibody has been shown to augment CD8+ T cell infiltration and greatly inhibit tumor growth in immunocompetent tumor-bearing mouse models[18]. Sheng et al., showed that simultaneous inhibition of LSD1 and TGFβ has been found to increase both CD8+ T cell infiltration and cytotoxicity, resulting in the eradication of poorly immunogenic tumors upon anti-PD-1 treatment [25]. Treatment with SP-2577 has led to the infiltration of CD8+, CD4+, CD4+CD8+ double-positive T cells, and CD56+ NKT cells in SCCOHT [26]. Moreover, LSD1 has been shown to counteract TCF1-mediated progenitor maintenance and promote the terminal differentiation of progenitor-exhausted CD8+ T cells. Genetic ablation of LSD1 or pharmacological inhibition with GSK2879552 has been found to enhance the persistence of progenitor-exhausted CD8+ T cells, providing a sustained source for exhausted T cells with tumor-killing cytotoxicity, leading to effective and durable responses to anti-PD1 therapy[35].
Furthermore, circulating tumor cells isolated from immunotherapy-resistant metastatic melanoma patients express higher levels of nuclear LSD1 (nLSD1). Additionally, nLSD1p is enriched in PD-1+CD8+ T cells from both resistant melanoma patients and 4T1 immunotherapy-resistant mice. Targeting the LSD1p nuclear axis has been shown to induce infiltration of IFN-γ/TNF-α-expressing CD8+ T cells into the tumors of 4T1 immunotherapy-resistant mice, and this effect is further enhanced when combined with immunotherapy [27]. In HNSCC, the combination of SP2509 and anti-PD-1 monoclonal antibody augmented CD8+ T cell infiltration and greatly inhibited tumor growth in immunocompetent tumor-bearing mouse models [18]. Further, in a syngeneic model of SCLC, IMG-7289 (Bomedemstat) was found to enhance increased infiltration of CD8+ T-cells and significant inhibition of tumor growth [22].
Although LSD1 inhibition shows promising potential in increasing CD8+ T cell infiltration and antitumor immune responses, it is essential to acknowledge that it may also induce TGFβ expression, which could have an inhibitory effect on T-cell immunity and limit the antitumor effects of LSD1 inhibition-induced T-cell infiltration in some scenarios[25]. Ablation of the LSD1 in multiple tumor cells induces TGF-β expression, which exerts an inhibitory effect on T-cell immunity by suppressing the cytotoxicity of intratumoral CD8+ T cells[25]. Qin et al. demonstrated that LSD1 expression is inversely associated with cytotoxic T cell-attracting chemokines (CCL5, CXCL9, CXCL10) and PD-L1 in clinical TNBC TCGA datasets. LSD1 inhibition was found to reactivate chemokines via increased H3K4me2 levels at their proximal promoter regions and enhance CD8+ T cell trafficking to the TME. Additionally, combining HCI-2509 (SP2509) with PD-1 antibody significantly suppressed tumor growth and pulmonary metastasis in TNBC xenograft models, associated with augmented CD8+ T cell infiltration in xenograft tumors[36].
Furthermore, the combination of the LSD1 inhibitor phenelzine with nab-paclitaxel has been found to promote an innate, M1 macrophage-like tumoricidal immune response in breast cancer [37]. Tan et al. demonstrated that treatment with phenelzine increased the expression of genes associated with macrophage polarization toward an M1 phenotype and checkpoint molecules in vitro and in a murine TNBC model[38]. Additionally, inhibition of LSD1 using catalytic inhibitors has been shown to promote an immune gene signature that augmented natural killer (NK) cell infusion leading to reduced tumor burden in pediatric high-grade glioma models[39].
Overall, LSD1 inhibition has the potential to increase CD8+ T cell infiltration by inducing tumor cells to secrete CD8+ T cell-attracting chemokines, potentially transforming “cold tumors” into “hot tumors” and rendering them more responsive to immunotherapy. However, further research is needed to fully understand the complex effects of LSD1 inhibition on the TME and immune response.
2.3. LSD1 ablation/inhibition enhances tumor immunogenicity
Tumor immunogenicity is influenced by the expression of tumor-associated antigens (TAA) and tumor-specific antigens (TSA), as well as the efficiency of tumor antigen presentation[40]. However, low or non-immunogenic tumor cells evade recognition and destruction by immune cells due to their weaker antigen expression and presentation capabilities, often resulting in a poor prognosis. Therefore, a potential immunotherapy strategy involves enhancing the immunogenicity of tumors. Emerging studies demonstrate that loss/inhibition of LSD1 improves tumor immunogenicity in low or non-immunogenic tumors.
Inhibition of LSD1 in SCLC has been shown to restore MHC-I cell surface expression, activate interferon signaling, induce tumor-intrinsic immunogenicity, and sensitize SCLC cells to MHC-I-restricted T cell cytolysis[21]. Further, LSD1 inhibitor bomedemstat was observed to elevate MHC class I expression in mouse SCLC tumor cells and promote MHC-I induction by IFNɣ[22]. Sheng et al. demonstrated that LSD1 depletion in cancer cells increases ERVs, and reduces expression of RNA-induced silencing complex (RISC) components, leading to double-stranded RNA (dsRNA) stress and activation of type 1 interferon. This ultimately stimulates anti-tumor T cell immunity and restrains tumor growth[23]. Moreover, GSK-LSD1-loaded hydrogel activates antitumor T cell immunity by eliciting MDA5-dependent innate sensing of ERV expression in tumor cells[34]. Similarly, Zhou et al. found that ectopic expression of LSD1 attenuates the induction of IFNs and IFN-related genes. Inhibition of LSD1 by p53 leads to ERV derepression and dsRNA stress, which activates a viral mimicry response and the antigen processing and presentation pathway in cancer cells [41]. Additionally, SP-2577 promotes ERV expression and activates the IFNβ pathway in SCCOHT cell lines[26]. These studies suggest that LSD1 inhibition could be a potential strategy to reactivate the TAA and TSA on tumor cells and this could be exploited by T cell-mediated killing.
3. Role of LSD1 in DNA damage response
The DNA damage response (DDR) is a cellular process that detects and repairs DNA damage to maintain genome integrity. Chemo and radiation therapies induce cell death in cancer cells by inducing DNA damage. Several lines of evidence suggest that efficient DNA repair mechanisms in cancer cells often contribute to therapy resistance[40].
Emerging studies implicate LSD1 in the regulation of DDR in a cell/tissue-specific manner. Much of the studies supporting the role of LSD1 in homologous recombination (HR) come from the U2OS osteosarcoma cells. It has been shown that LSD1 is potentially recruited to sites of DNA damage in an RNF168-dependent manner and that LSD1 knockdown resulted in a modest increase in HR in U2OS cells[42]. Interestingly, LSD1 depletion sensitizes tumor cells to γ-irradiation [42]. LSD1 demethylates UHRF1, an essential protein in DDR pathway including HR, and knockdown of LSD1 increases UHRF1 methylation in U2OS cells which increases HR efficiency whereas reconstitution of LSD1 diminishes HR efficiency[43]. Interestingly, LSD1 is recruited to double-strand breaks and generates reactive oxygen species (ROS) in response to DNA damage in U2OS cells and the localized production of H202 affects the recruitment of DNA repair proteins, specifically, the recruitment of non-homologous end joining (NHEJ) protein Ku80 to DNA damage sites is reduced, while HR end-binding protein NBS1 is increased, indicating that the redox state of the cell can influence the choice of DNA repair pathway [44]. However, a recent study showed that LSD1 is recruited to the promoters of DNA-DSB repair genes in glioma stem cells (GSCs), and knockdown or knockout or inhibition of LSD1 reduces the expression of DNA DSB repair genes, attenuated both HR and NHEJ repair activity of GSCs, enhanced the alkylating agent temozolomide (TMZ) mediated DNA damage and sensitizes GSCs to TMZ mediated killing [45].
LSD1 has also been demonstrated to play a vital role in NHEJ by facilitating the recruitment of 53BP1 to the sites of DNA damage, enhancing cell proliferation and survival in response to DNA damage [46]. Moreover, LSD1 destabilizes the tumor suppressor E3 ubiquitin ligase FBXW7, thereby abrogating its functions in growth suppression, NHEJ repair, and radioprotection in lung cancer cells [47]. In chronic myeloid leukemia (CML) cells, LSD1 binds to NHEJ protein Ku70, and knockdown or inhibitor (2-PCPA) treatment enhanced NHEJ repair efficiency whereas overexpression of WT LSD1 but not demethylase-defective K661A reduced NHEJ repair efficiency[48]. Further, silencing of LSD1 markedly induced H2AX phosphorylation and reduced 53BP1 DNA repair foci after oxaliplatin treatment in colorectal cancer (CRC). Besides, several genes that participated in DNA repair such as PNKP, UBA2, and KHDRBS1/Sam68 were upregulated in LSD1-silenced cells, whereas the TARDBP, a member of the hnRNP family involved in NHEJ and DNA repair, was downregulated[49].
LSD1 has been shown to regulate HP1-positive chromatin in endothelial cells, thereby stimulating DNA repair and regulation of proliferation machinery [50]. LSD1 has also been linked to the regulation of mismatch repair in leukemia and hypoxic stress-mediated silencing of DNA Mismatch Repair Gene MLH1 is dependent on LSD1 [51]. LSD1 is a regulator of cellular UV response and LSD1 knockdown or inhibition by phenelzine sensitizes skin cancer cells to UVA radiation-induced cell death and enhances photodynamic therapy efficacy[52].
Although LSD1 has been shown to regulate several oncogenic signaling pathways such as MYC, ATF4, HIF, STAT3, β-catenin, EGFR, TLX, NOTCH, AKT/mTOR in cancer cells[53–56], DNA damage/repair is the target mechanism for several first-line therapeutic regimens of multiple cancers such as platinum drugs, anthracyclines, alkylating agents, radiation, and PARP inhibitors, and exploiting this phenomenon in cancer cells provides a distinct advantage over other targets and is readily translatable for cancer therapy. Such combinations could potentially enhance treatment efficacy by simultaneously disrupting critical oncogenic pathways while also inducing DNA damage in cancer cells, leading to a synergistic effect.
4. LSD1 inhibitors: Overview
LSD1 is overexpressed in several cancer types and associated with poor prognosis[57]. LSD1 plays a vital role in a wide variety of oncogenic mechanisms including, tumor initiation, tumor progression, metastases, therapy resistance, autophagy, senescence, apoptosis, cell cycle, epithelial-mesenchymal transition (EMT), immunogenicity, DNA repair, metabolism, and cancer stemness[6,57]. Since the establishment of LSD1 as a valid target in multiple cancer types, there has been significant progress in the development of potent and selective LSD1 inhibitors for cancer treatment. To date, several LSD1 inhibitors that act through either covalent or non-covalent mechanisms, as well as dual inhibitors, have entered clinical trials for the treatment of hematological and/or solid cancers, as well as neurological disorders. These include the irreversible inhibitor of LSD1 such as tranylcypromine (TCP, PCPA, 2-PCPA), ORY-1001 (iadademstat), ORY-2001 (vafidemstat), IMG-7289 (bomedemstat), GSK2879552, INCB059872, TAK-418; reversible LSD1 inhibitors such as CC-90011 (pulrodemstat), and SP-2577 (seclidemstat); dual LSD1/HDAC6/8 inhibitor(JBI-802), dual LSD1/HDAC6 inhibitor (JBI-097); ZY0511 and MOA inhibitor phenelzine are being evaluated in clinical trials as either monotherapy or in combination therapy for various diseases[58–60]. In addition, several groups developed LSD1 inhibitors that have still not yet reached the clinical stage and showed promising activity in preclinical models. The development of LSD1 inhibitors is extensively reviewed in a recent update on LSD1 inhibitors[60]. These findings highlight the diversity of approaches taken to develop potent LSD1 inhibitors and demonstrate the potential of these compounds as promising candidates for cancer treatment. Clinical and preclinical status of LSD1 inhibitors in different types of cancers are shown in Table 1.
Table 1.
LSD1 inhibitors in clinical/preclinical trials.
| S. No | LSD1 Inhibitor | Mode of Action | Single/Dual Inhibitor | Indications | Phase | Trial Number/Ref | Status |
|---|---|---|---|---|---|---|---|
| 1 | Bomedemstat (IMG-7289) |
Irreversible | Single Inhibitor | AML SCLC AML |
Phase I Phase I Phase I |
NCT05597306
NCT05191797 NCT02842827 |
Recruiting Recruiting Completed |
| 2. | SP2509/SP2577 | Reversible | Single Inhibitor | ES Advanced Solid Tumors Gynecologic Cancers ES, CCS, LGFMS, SEF, EMC, AFH |
Phase I Phase I Phase I Phase I/II |
NCT03600649
NCT03895684 NCT04611139 NCT05266196 |
Recruiting Completed Withdrawn Enrolling |
| 3. | GSK2879552 | Irreversible | Single Inhibitor | SCLC MDS Leukemia |
Phase I Phase I Phase I |
NCT02034123
NCT02929498 NCT02177812 |
Terminated Terminated Terminated |
| 4. | Tranylcypromine | Irreversible | Single inhibitor | AML AML AML |
Phase I Phase I Phase I |
NCT02273102
NCT02261779 NCT02717884 |
Completed Unknown Unknown |
| 5. | ORY-1001 | Irreversible | Single Inhibitor | AML AML SCLC AML |
Phase I Phase II Phase II Phase I |
NCT05546580 2018–000482-36 2018–000469-35 2013–002447-29 |
Recruiting Completed Completed Completed |
| 6 | Corin | Irreversible | Dual Inhibitor LSD1/HDACs |
NSCLC | Phase II | NCT02264210 | Recruiting |
| 7 | INCB059872 | Irreversible | Single Inhibitor | Solid Tumors Solid Tumors, Hematologic Malignancy Relapsed ES |
Phase II Phase I Phase I |
NCT02959437
NCT02712905 NCT03514407 |
Terminated Terminated Terminated |
| 8 | GSK-LSD1 | Irreversible | Single Inhibitor | BC HCC |
Pre-Clinical Pre-Clinical |
86 20 |
- |
| 9 | JBI-097 | Irreversible | Dual Inhibitor LSD1/HDAC6 |
MM, Leukaemia | Pre-Clinical | 62 | - |
| 10 | ZY0511 | Reversible | Single Inhibitor | HCC, CC | Pre-Clinical | 94, 99 | - |
| 11 | GSK690 | Reversible | Single Inhibitor | NA | NA | 69 | - |
| 12 | NCD38 | Irreversible | Single Inhibitor | GBM, EC | Pre-Clinical | 45,88 | - |
| 13 | Compound 5 | Reversible | NA | NA | NA | 64 | - |
| 14 | Pargyline | Irreversible | Single inhibitor |
KC | Pre-Clinical | 97 | - |
| 15 | MC_2580 DDP_38003 | Irreversible | Single inhibitor | AML | Pre-Clinical | 76 | - |
| 16 | Clorgyline | Irreversible | Single inhibitor |
PC | Pre-Clinical | 98 | - |
5. LSD1 inhibitor-based combination therapies for cancer treatment
Combination therapy regimes have consistently demonstrated advantages in cancer treatment. LSD1 inhibitors have been investigated in clinical trials as monotherapy or combination therapies for several cancers. Emerging studies have highlighted the importance of combining LSD1 inhibitors with other epigenetic drugs, targeted therapies, as well as chemotherapy agents.
5.1. Combination with HDAC inhibitors
LSD1 and histone deacetylases (HDACs) are promising drug targets for cancers. Recent studies reveal an important functional interplay between LSD1 and HDACs, suggesting a synergistic effect of combined LSD1 and HDAC inhibitors on cancers. Therefore, developing combination therapies of LSD1 and HDAC inhibitors, or dual inhibitors targeting both LSD1 and HDACs, could be a promising strategy for epigenetic cancer therapy. Dual et al. synthesized a series of tranylcypromine derivatives as LSD1/HDAC dual inhibitors, and their compound (compound 7) displayed the most potent inhibitory activity against HDAC1 and HDAC2, as well as against LSD1. It demonstrated stronger anti-proliferative activities against MGC-803, MCF-7, SW-620, PC-3, and A-549 human cancer cell lines [61]. JBI-097, a dual LSD1/HDAC6 inhibitor, showed strong potency in inhibiting LSD1 and HDAC6 enzymatic activities. It exhibited a superior anti-proliferative profile against hematological and solid tumor cell lines in vitro and had a stronger effect in erythroleukemia, multiple myeloma xenograft models, and the CT-26 syngeneic model in vivo. Moreover, it showed efficacy as monotherapy and additive or synergistic efficacy in combination with standard care or immune checkpoint inhibitors[62]. The LSD1 and HDAC6 selective dual inhibitor (compound 1) showed superior potency in multiple myeloma cell lines and xenograft models compared to single-agent LSD1 and HDAC6 inhibitors [63]. In another study, the novel dual LSD1 and HDAC inhibitor (compound 5) showed promising anticancer effects in gastric cancer via LSD1 and HDAC dual inhibition [64]. In diffuse intrinsic pontine glioma (DIPG) cells, a CRISPR screen revealed that the knockout of LSD1 sensitizes DIPG cells to HDAC inhibitors and bifunctional inhibitor of HDACs and LSD1, Corin, potently inhibits DIPG growth by increasing HDAC-targeted H3K27ac and LSD1-targeted H3K4me1 at differentiation-associated genes[65]. These studies highlight the development and utility of dual LSD1 and HDAC inhibitors for cancer therapy.
Several studies have also demonstrated that the combination of LSD1 and HDAC inhibitors has therapeutic efficacy. In Ewing sarcoma cells, a synergistic effect of SP2509 was observed with the combination of HDAC inhibitor romidepsin[66]. Further, a combination of pan-HDAC inhibitor panobinostat and SP2509 was synergistically lethal against cultured and primary acute myeloid leukemia (AML) blasts and significantly improved the survival of mice engrafted with human AML cells[67]. Inhibition of the HDAC5-LSD1 axis with the combination of the natural bioactive HDAC inhibitor sulforaphane and the LSD1 inhibitor HCI-2509 resulted in synergistic growth inhibition in breast cancer cells and exhibited a superior inhibitory effect on MDA-MB-231 xenograft tumor growth [68]. Moreover, a synergistic interaction of LSD1 inhibitors (GSK690, Ex917) and HDAC inhibitors (JNJ-26481585, Suberoylanilide hydroxamic acid (SAHA)) was shown to induce cell death in rhabdomyosarcoma (RMS) cells[69]. Combined treatment with the LSD1 inhibitor pargyline and the HDAC inhibitor SAHA (Vorinostat) led to superior growth inhibition and apoptotic death in TNBC cells [70]. Similarly, in glioblastoma (GBM) models, combined inhibition of HDAC and LSD1 using vorinostat and tranylcypromine, respectively, led to synergistic apoptotic cell death in GBM cells, reduced GSC viability, and GBM growth in xenograft models [71,72]. LSD1 binds to the 3’ domain of lncRNA HOTAIR, specifically removes mono- and di-methyl marks from H3K4, and the combination of the HOTAIR-EZH2 disrupting agent AC1Q3QWB and an LSD1 inhibitor GSK-LSD1 inhibits cell cycle processes, promoting apoptosis in vitro, and shows enhanced anti-tumor efficacy in GBM models [73]. Altogether, these studies suggest that a combination of these two classes of epigenetic inhibitors could potentially enhance the efficacy of cancer treatment by targeting multiple signaling pathways and gene expression patterns simultaneously.
5.2. LSD1-Based Combination Therapies in Leukemia Models
Since LSD1 is an attractive therapeutic target in hematological malignancies, several studies investigated the potential of combining LSD1 inhibitors with other agents. All-trans retinoic acid (ATRA) is currently approved for use in acute promyelocytic leukemia (APL), where it promotes the differentiation of abnormal blast cells into normal white blood cells. Combined treatment with ATRA and GSK2879552 results in synergistic effects on cell proliferation, markers of differentiation, and, most importantly, cytotoxicity [74]. Notably, treatment with ATRA plus tranylcypromine (TCP) markedly diminished the engraftment of primary human AML cells in vivo, suggesting that ATRA in combination with TCP may target leukemia-initiating cells [75]. Other studies showed that APL cells are resistant to LSD1 inhibition or knockout, but targeting LSD1 sensitizes them to retinoic acid (RA) without altering PML-RAR levels, and extends survival of leukemic mice upon RA treatment. The combination of RA with LSD1 inhibition (or knockout) is also effective in other non-APL and AML cells[76]. Co-treatment with LSD1 inhibitor and the JAK inhibitor ruxolitinib was also synergistically lethal against post-MPN secondary AML cells [77]. Furthermore, a CRISPR screen in LSD1 inhibitor INCB059872-treated AML cells identified BRD4, MOZ, HDAC3, and DOT1L among the codependencies. Co-treatment with INCB059872 and BET inhibitor (OTX-015) or ruxolitinib exerted superior in vivo efficacy against post-MPN secondary AML cells[77]. The combination of the ruxolitinib and LSD1 inhibitors demonstrated synergy in vitro and improved mice survival in CSF3R/CEBPA mutant AML [78].
Moreover, a genome-wide CRISPR-Cas9 dropout screen identified multiple components of the mTORC1 signaling, such as RRAGA, MLST8, WDR24, and LAMTOR2, as cellular sensitizers to LSD1 inhibition, and combined pharmacologic inhibition of LSD1 using OG-86 and mTORC1 using RAD001 impairs primary AML cell clonogenic activity in vivo[79]. N-alkylated TCP derivatives S2116 and S2157 significantly retarded the growth of T-cell acute lymphoblastic leukemia cells in xenotransplanted mice, and S2157 in combination with the conventional anti-leukemic agent dexamethasone prolonged the survival of recipient mice [80]. Although EZH2 and LSD1 have opposite histone methylation functions, these enzymes were paradoxically up-regulated in AML cells. Importantly, the combination of LSD1 inhibitor SP2509 and EZH2 inhibitor EPZ-6438 resulted in a synergistic activity against AML in vitro and in vivo[81]. It has also been shown that drug resistance could be overcome in hematopoietic stem cell-derived leukemias by combining LSD1 inhibitor IMG-7289 with the BCL-2 inhibitor venetoclax[82]. Altogether, these studies suggest that LSD1 inhibitor-based combination therapies could be a promising therapeutic strategy for hematological malignancies.
5.3. LSD1-based combination therapies in hormonal cancers
In castration-resistant prostate cancer (CRPC) models, LSD1 drives prostate cancer progression by activating super-enhancer-mediated oncogenic programs, which can be targeted with the combination of LSD1 and BRD4 inhibitors to suppress the growth of CRPC[83]. The LSD1 antagonist HCI-2509 exhibited a synergistic effect when combined with docetaxel in PC3 cells [84]. Further, the combination of SP-2509 and JQ1 treatments synergistically inhibits the growth of CRPC cells and inhibits tumor invasion in prostate cancer (PCa)[85]. It has been shown that LSD1 plays an important role in the chemoresistance of breast cancer cells, and LSD1 inhibitors, 2-PCPA and GSK-LSD1, in combination with doxorubicin and paclitaxel, are more efficacious than monotherapy alone in eliminating tumor cells in 3D tumorsphere models of breast cancer [86]. He et al, reported that dual-acting agent 11g, which targets estrogen receptor α (ERα) and LSD1, showed higher antiproliferative efficacy and induced apoptosis in the MCF-7 breast cancer cell line [87]. In endometrial cancer (EC), LSD1 knockdown or inhibition downregulated the expression of genes involved in rapamycin-induced activation of Akt, including the mTORC2 complex. The combination therapy of the LSD1 inhibitor NCD38 and rapamycin reduced tumor growth in EC xenograft and patient-derived xenograft models in vivo, as well as in patient-derived tumor explants ex vivo[88].
Interestingly, a few other studies indicated that the presence of LSD1 is essential for chemotherapy response. Yang et al, reported that depletion of LSD1 in MCF-7 cells resulted in the loss of sensitivity to camptothecin, doxorubicin, and paclitaxel, suggesting it may play an essential role in maintaining sensitivity to chemotherapeutic drugs for breast cancer [89]. Overexpression of LSD1 reduces BRCA1 expression in triple-negative or basal-like breast cancer and increases sensitivity to PARP inhibitors in basal-like breast cancer[90].
5.4. LSD1-based combination therapies in other solid tumors
LSD1 inhibitors SP2509 and tranylcypromine enhance the antitumor effect of regorafenib in HCC cells [91]. LSD1 inhibitors, pargyline, and GSK2879552, by derepressing the expression of multiple upstream negative regulators of the Wnt signaling pathway to downregulate the β-catenin pathway, resensitizes sorafenib-resistant HCC cells to sorafenib [92]. LSD1 upregulates LncRNA LINC01134 in HCC cells and confers Oxaliplatin resistance through SP1-induced p62 transcription in HCC [93]. Further, ZY0511, an LSD1 inhibitor, combined with DTP3, a GADD45β/MKK7 inhibitor, effectively inhibits HCC cell proliferation in vitro and in vivo[94]. In non-small cell lung cancer (NSCLC) models, LSD1 knockdown or its inhibition with SP2509 suppressed tumor growth and enhanced gefitinib response in in vivo xenograft models[95]. LSD1 inhibition with bizine increased ROS generation and enhanced PDT efficacy in HaCat and FaDu cell lines[52]. Further, LSD1 activation by vitamin B2 attenuates the efficacy of apatinib for proliferation and migration of gastric cancer cells [96]. In kidney cancer cells, LSD1 inhibitor pargyline delayed growth and repressed EMT markers, and these effects were additively increased by co-treatment with the AR inhibitor enzalutamide[97]. Combining DNMT inhibitor 5-Aza-CdR with LSD1 inhibitor clorgyline has synergistic effects on reactivating aberrantly silenced genes by enriching H3K4me2 and H3K4me1 and improving the efficacy of epigenetic therapy in bladder cancer, leukemia, and colon cancer cell lines [98]. Further, LSD1 inhibitor ZY0511 in combination with 5-FU has shown promise as a potential therapy for CRC as the combination significantly reduced CRC xenograft tumor growth as well as lung and liver metastases in vivo[99]. The detailed summary of combination drugs is depicted in Table 2.
Table 2.
List of LSD1 inhibitors in combination with other anticancer drugs.
| S.No | LSD1 KD/ Inhibitor | Combination Drug | Class of drug combination | Indication | Ref |
|---|---|---|---|---|---|
|
| |||||
| 1 | GSK-LSD1 | Doxorubicin | Alkylant agent | Breast Cancer | 86 |
| anti-PD-1 | Immunotherapy | HCC | 20 | ||
| AC1Q3QWB | HOTAIR-EZH2 Inhibitor | Glioblastoma | 73 | ||
|
| |||||
| 2. | Bomedemstat (IMG-7289) | anti-PD-1 | Immunotherapy | Small Cell Lung Cancer | 22 |
| Venetoclax | BCL-2 Inhibitor | HSC-derived leukemias | 82 | ||
|
| |||||
| 3. | SP2509 | Panobinostat | HDAC inhibitor | Acute myeloid leukemia | 67 |
| Tazemetostat | EZH2 Inhibitor | Acute myeloid leukemia | 81 | ||
| Romidepsin | HDAC inhibitor | Ewing Sarcoma | 66 | ||
| Etoposide | Topoisomerase II inhibitor | Ewing Sarcoma | 66 | ||
| Verteporfin | YAP inhibitor | OSCC | 19 | ||
| anti-PD-1 | Immunotherapy | HNSCC, OSCC, TNBC | 18, 19, 36 | ||
| JQ1 | BET bromodomain inhibitor | CRPC | 83 | ||
| Sulforaphane | HDAC inhibitor | Breast Cancer | 68 | ||
| EPZ6438 | EZH2 Inhibitor | Acute myeloid leukemia | 81 | ||
| Docetaxel | Taxans | Prostate cancer | 84 | ||
| Regorafenib | Multi-kinase inhibitor | HCC | 91 | ||
|
| |||||
| 4. | JBI-097 | Dual inhibitor LSD1 Inhibitor | HDAC6, and Leukemia SCLC Melanoma HCC | Multiple Myeloma | 62 |
|
| |||||
| 5. | GSK2879552 | anti-PD-1 | Immunotherapy | Colorectal cancer Melanoma | 35 |
| Sorafenib | Multikinase Inhibitor | HCC | 92 | ||
| ATRA | Retinoids | Acute myeloid leukemia | 74 | ||
|
| |||||
| 6 | ZY0511 | DTP3 | Vaccine | HCC | 94 |
| 5-fluorouracil | Anti-metabolites | Colorectal cancer | 99 | ||
|
| |||||
| 7 | GSK690 | JNJ-26481585 | HDAC inhibitor | Rhabdomyosarcoma | 69 |
|
| |||||
| 8 | Ex917 | Vorinostat | HDAC inhibitor | Rhabdomyosarcoma | 69 |
|
| |||||
| 9 | LSD1 KD | Vorinostat | HDAC inhibitor | Glioblastoma | 71 |
| anti-PD-1 | Immunotherapy | Melanoma, | 23 | ||
| UVA radiation | Radiation | Skin Cancer | 52 | ||
|
| |||||
| 10 | Tranylcypromine | Vorinostat | HDAC inhibitor | Glioblastoma | 71,72 |
| ATRA | Retinoids | Acute myeloid leukemia | 75 | ||
| Tranylcypromine (Compound 7) | Dual inhibitor | HDAC1/2, and LSD1 Inhibitor | Gastric cancer, Breast cancer, Colorectal cancer, Lung cancer, Prostate cancer | 61 | |
| OG-86 (Tranylcypromin e -derivatives) | RAD001 | mTOR Inhibitor | Acute myeloid leukemia | 79 | |
|
| |||||
| 11 | ORY-1001 | anti-PD-1 | Immunotherapy | SCLC | 21 |
| anti-CD47/PD-L1 | Immunotherapy | Cervical Cancer | 30 | ||
| Ruxolitinib | JAK inhibitor | Acute myeloid leukemia | 78 | ||
| I-BET-762 | BET inhibitor | CRPC | 83 | ||
|
| |||||
| 12 | NCD38 | Temozolomide | Alkylant agent | Glioblastoma | 45 |
| Rapamycin | mTOR Inhibitor | Endometrial cancer | 88 | ||
|
| |||||
| 13 | Compound 5 | Dual inhibitor | HDAC/LSD1 Inhibitor | Gastric cancer | 64 |
|
| |||||
| 14 | Corin | Dual inhibitor | HDAC/LSD1 Inhibitor | DIPG | 65 |
|
| |||||
| 15 | Pargyline | Vorinostat | HDAC inhibitor | TNBC | 70 |
| Enzalutamide | Androgen Receptor Inhibitor | Kidney Cancer | 97 | ||
|
| |||||
| 16 | MC_2580 DDP_38003 | ATRA | Retinoids | Acute myeloid leukemia | 76 |
|
| |||||
| 17 | INCB059872 | OTX-015 | BET inhibitor | Acute myeloid leukemia | 77 |
|
| |||||
| 18 | Clorgyline | 5-Aza-CdR | DNMT inhibitor | Bladder Cancer | 98 |
Collectively, these studies suggest combination of LSD1 inhibitors with other therapeutic molecules simultaneously or synergistically regulates multiple pathways leading to enhanced tumor cell killing which offers effective cancer treatment.
6. Conclusions:
In summary, the increasing understanding of LSD1 biology has identified its potential as a promising target for cancer treatment. Emerging studies unveiled LSD1 functions in the regulation of immune evasion, antitumor immunity, and DNA damage response, opening opportunities for therapeutic applications. Since new LSD1 inhibitors are continuously being developed and evaluated in clinical trials, there is potential for combining them with other targeted agents like immune checkpoint blockers, DNA damage response modulators, chemotherapeutic drugs, and epigenetic therapies for improved treatment outcomes. Further, tailoring LSD1-based combination therapies to specific cancer types holds significant promise in enhancing effectiveness while reducing toxicity.
7. Expert Opinion:
LSD1 is overexpressed in several cancer types and associated with poor prognosis. It plays a vital role in various oncogenic mechanisms, including tumor initiation, progression, metastases, therapy resistance, autophagy, senescence, apoptosis, cell cycle, EMT, immunogenicity, DNA repair, metabolism, and cancer stemness. As a result, LSD1 has been established as a valid target in multiple cancer types, and there has been significant progress in developing potent and selective LSD1 inhibitors for cancer treatment. Several LSD1 inhibitors have entered clinical trials for hematological and solid cancers, as well as neurological disorders. In addition to its role in oncogenic mechanisms, recent studies suggest that LSD1 also plays a crucial role in the regulation of immune evasion and antitumor immunity. LSD1 inhibition enhances antitumor immunity by altering the expression of immune checkpoint molecules like PD-(L)1, chemokines, ERVs, and MHCs, and activating IFN and TGFβ signaling. LSD1 is also involved in DDR, affecting DNA repair processes. Inhibition of LSD1 has been shown to sensitize cancer cells to DNA-damaging agents, making it a potential target for combination therapies. Given that LSD1 is a key regulator of stem cell programs and is often overexpressed in cancer stem cells compared to normal/tumor cells, which are associated with a resistance phenotype, it’s not surprising that blocking LSD1 functions could enhance chemo/radiation therapy and prevent therapy resistance. Additionally, LSD1 regulates several other processes such as senescence, EMT program, and hypoxia signaling, all of which are known to play a role in therapy resistance. We firmly believe that instead of using LSD1 inhibitors alone, combining them with other therapeutic molecules, such as DNA-damaging agents, targeted therapies, or immunotherapies, could serve as a multi-pronged approach, thereby increasing treatment efficacy and decreasing treatment resistance. The following are important avenues where LSD1 inhibitors could be combined with other agents for cancer therapy. Antitumor Immunity: As LSD1 regulates immune pathways, combining LSD1 inhibitors with immune checkpoint inhibitors could potentially improve treatment outcomes. Targeting Multiple Pathways: As LSD1 regulates several pathways involved in cancer progression, combining LSD1 inhibitors with other targeted therapies can potentially improve treatment outcomes. Overcoming Resistance: Cancer cells can develop resistance to single-agent therapies over time and LSD1 is known to play a vital role in cancer stemness, an important contributor to therapy resistance. Combining LSD1 inhibitors with other drugs that target different pathways or mechanisms of resistance may help overcome this problem and prevent tumor recurrence. Synergistic Effects: Some combinations of drugs can have synergistic effects, particularly in combination with other epigenetic drugs and DNA damaging agents and such combinations could lead to enhanced tumor cell killing and improved therapeutic response.
Overall, the findings highlight the potential of LSD1 inhibitors as promising candidates for cancer treatment, either as monotherapy or in combination with other agents to target multiple pathways and enhance antitumor immunity and DNA damage response. However, further research and more data from ongoing clinical trials are needed to fully explore the therapeutic potential of LSD1 inhibitors in cancer treatment.
Article highlights.
LSD1 is a recognized and established target for cancer therapy, and numerous LSD1 inhibitors are presently undergoing evaluation in clinical trials.
LSD1 plays a critical role in governing a diverse array of biological processes. Recent research has illuminated its involvement in both antitumor immunity and the DNA damage response.
Combining LSD1 inhibition with immune checkpoint inhibitors and agents that induce DNA damage holds significant promise as a strategy for cancer treatment.
The potential therapeutic benefits of combining LSD1 inhibitors with other therapies warrant thorough investigation and further study.
Funding
This manuscript was funded by National Institutes of Health (NIH) grants 1R01NS106173-01A1 (GRS), T32CA148724 (JDJ), and American Cancer Society Research Scholar Grant 132931-RSG-18-187-01-TBG (GRS). Department of Defense (DoD) Breast Cancer Research Program (BCRP) grant W81XWH-22-1-0013 (GRS), and METAvivor Translational Research Award (GRS).
Footnotes
Declaration of Interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- [1].Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nature Reviews Genetics. 2012. 2012/05/01;13(5):343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004. Dec 29;119(7):941–53. [DOI] [PubMed] [Google Scholar]
- [3].Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol. 2008. Jun;20(3):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Metzger E, Wissmann M, Yin N, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005. Sep 15;437(7057):436–9. [DOI] [PubMed] [Google Scholar]
- [5].Perillo B, Ombra MN, Bertoni A, et al. DNA Oxidation as Triggered by H3K9me2 Demethylation Drives Estrogen-Induced Gene Expression. Science. 2008;319(5860):202–206. [DOI] [PubMed] [Google Scholar]
- [6].Perillo B, Tramontano A, Pezone A, et al. LSD1: more than demethylation of histone lysine residues. Experimental & Molecular Medicine. 2020. 2020/12/01;52(12):1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maiques-Diaz A, Spencer GJ, Lynch JT, et al. Enhancer Activation by Pharmacologic Displacement of LSD1 from GFI1 Induces Differentiation in Acute Myeloid Leukemia. Cell Rep. 2018. Mar 27;22(13):3641–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vinckier NK, Patel NA, Geusz RJ, et al. LSD1-mediated enhancer silencing attenuates retinoic acid signalling during pancreatic endocrine cell development. Nat Commun. 2020. Apr 29;11(1):2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang X, Wang X, Wu T, et al. Therapeutic potential of targeting LSD1/ KDM1A in cancers. Pharmacol Res. 2022. Jan;175:105958. [DOI] [PubMed] [Google Scholar]
- [10].Hosseini A, Minucci S. A comprehensive review of lysine-specific demethylase 1 and its roles in cancer. Epigenomics. 2017. Aug;9(8):1123–1142. [DOI] [PubMed] [Google Scholar]
- [11].Majello B, Gorini F, Saccà CD, et al. Expanding the Role of the Histone Lysine-Specific Demethylase LSD1 in Cancer. Cancers (Basel). 2019. Mar 7;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sareddy GR, Nair BC, Krishnan SK, et al. KDM1 is a novel therapeutic target for the treatment of gliomas. Oncotarget. 2013. Jan;4(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim D, Kim KI, Baek SH. Roles of lysine-specific demethylase 1 (LSD1) in homeostasis and diseases. Journal of Biomedical Science. 2021. 2021/06/04;28(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Yang Y, Wang F, et al. Crystal structure of human histone lysine-specific demethylase 1 (LSD1). Proc Natl Acad Sci USA. 2006;103(38):13956–13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burg JM, Makhoul AT, Pemble CW, et al. A rationally-designed chimeric KDM1A/KDM1B histone demethylase tower domain deletion mutant retaining enzymatic activity. FEBS Letters. 2015;589(18):2340–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hino S, Kohrogi K, Nakao M. Histone demethylase LSD1 controls the phenotypic plasticity of cancer cells. Cancer Sci. 2016;107(9):1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nature Reviews Clinical Oncology. 2021. 2021/06/01;18(6):345–362. [DOI] [PubMed] [Google Scholar]
- [18].Han Y, Xu S, Ye W, et al. Targeting LSD1 suppresses stem cell-like properties and sensitizes head and neck squamous cell carcinoma to PD-1 blockade. Cell Death Dis. 2021. Oct 23;12(11):993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alhousami T, Diny M, Ali F, et al. Inhibition of LSD1 Attenuates Oral Cancer Development and Promotes Therapeutic Efficacy of Immune Checkpoint Blockade and YAP/TAZ Inhibition. Mol Cancer Res. 2022. May 4;20(5):712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng W, Li HL, Xi SY, et al. Growth differentiation factor 1-induced tumour plasticity provides a therapeutic window for immunotherapy in hepatocellular carcinoma. Nat Commun. 2021. Dec 8;12(1):7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nguyen EM, Taniguchi H, Chan JM, et al. Targeting Lysine-Specific Demethylase 1 Rescues Major Histocompatibility Complex Class I Antigen Presentation and Overcomes Programmed Death-Ligand 1 Blockade Resistance in SCLC. J Thorac Oncol. 2022. Aug;17(8):1014–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hiatt JB, Sandborg H, Garrison SM, et al. Inhibition of LSD1 with Bomedemstat Sensitizes Small Cell Lung Cancer to Immune Checkpoint Blockade and T-Cell Killing. Clin Cancer Res. 2022. Oct 14;28(20):4551–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sheng W, LaFleur MW, Nguyen TH, et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell. 2018. Jul 26;174(3):549–563.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Cao K. KDM1A Promotes Immunosuppression in Hepatocellular Carcinoma by Regulating PD-L1 through Demethylating MEF2D. J Immunol Res. 2021;2021:9965099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sheng W, Liu Y, Chakraborty D, et al. Simultaneous Inhibition of LSD1 and TGFβ Enables Eradication of Poorly Immunogenic Tumors with Anti-PD-1 Treatment. Cancer Discov. 2021. Aug;11(8):1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Soldi R, Ghosh Halder T, Weston A, et al. The novel reversible LSD1 inhibitor SP-2577 promotes anti-tumor immunity in SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian cancer. PLoS One. 2020;15(7):e0235705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tu WJ, McCuaig RD, Tan AHY, et al. Targeting Nuclear LSD1 to Reprogram Cancer Cells and Reinvigorate Exhausted T Cells via a Novel LSD1-EOMES Switch. Front Immunol. 2020;11:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bally APR, Neeld DK, Lu P, et al. PD-1 Expression during Acute Infection Is Repressed through an LSD1-Blimp-1 Axis. J Immunol. 2020. Jan 15;204(2):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen DD, Pang JR, Bi YP, et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol Cancer. 2022. Mar 16;21(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu S, Wang X, Yang Y, et al. LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer. Cell Death Dis. 2021. Mar 17;12(4):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dai XJ, Zhao LJ, Yang LH, et al. Phenothiazine-Based LSD1 Inhibitor Promotes T-Cell Killing Response of Gastric Cancer Cells. J Med Chem. 2023. Mar 23;66(6):3896–3916. [DOI] [PubMed] [Google Scholar]
- [32].He P, Du L, Hao P, et al. Inhibition of lysine-specific demethylase 1 (LSD1) prevented tumor growth and metastasis by downregulating PD-L1 expression in lung adenocarcinoma. Genes Dis. 2023. Sep;10(5):1779–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang J, Huang D, Saw PE, et al. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends in Immunology. 2022;43(7):523–545. [DOI] [PubMed] [Google Scholar]
- [34].Ji X, Guo D, Ma J, et al. Epigenetic Remodeling Hydrogel Patches for Multidrug-Resistant Triple-Negative Breast Cancer. Advanced Materials. 2021;33(18):2100949. [DOI] [PubMed] [Google Scholar]
- [35].Liu Y, Debo B, Li M, et al. LSD1 inhibition sustains T cell invigoration with a durable response to PD-1 blockade. Nat Commun. 2021. Nov 24;12(1):6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Qin Y, Vasilatos SN, Chen L, et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019. Jan;38(3):390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boulding T, McCuaig RD, Tan A, et al. LSD1 activation promotes inducible EMT programs and modulates the tumour microenvironment in breast cancer. Sci Rep. 2018. Jan 8;8(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tan AHY, Tu W, McCuaig R, et al. Lysine-Specific Histone Demethylase 1A Regulates Macrophage Polarization and Checkpoint Molecules in the Tumor Microenvironment of Triple-Negative Breast Cancer. Front Immunol. 2019;10:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bailey CP, Figueroa M, Gangadharan A, et al. Pharmacologic inhibition of lysine-specific demethylase 1 as a therapeutic and immune-sensitization strategy in pediatric high-grade glioma. Neuro Oncol. 2020. Sep 29;22(9):1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haen SP, Löffler MW, Rammensee H-G, et al. Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire. Nature Reviews Clinical Oncology. 2020. 2020/10/01;17(10):595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou X, Singh M, Sanz Santos G, et al. Pharmacologic Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Antitumor Immunity. Cancer Discovery. 2021;11(12):3090–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mosammaparast N, Kim H, Laurent B, et al. The histone demethylase LSD1/KDM1A promotes the DNA damage response. J Cell Biol. 2013. Nov 11;203(3):457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hahm JY, Kim JY, Park JW, et al. Methylation of UHRF1 by SET7 is essential for DNA double-strand break repair. Nucleic Acids Res. 2019. Jan 10;47(1):184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Duquette ML, Kim J, Shi LZ, et al. LSD1 mediated changes in the local redox environment during the DNA damage response. PLOS ONE. 2018;13(8):e0201907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Alejo S, Palacios BE, Venkata PP, et al. Lysine-specific histone demethylase 1A (KDM1A/LSD1) inhibition attenuates DNA double-strand break repair and augments the efficacy of temozolomide in glioblastoma. Neuro Oncol. 2023. Jul 6;25(7):1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Peng B, Wang J, Hu Y, et al. Modulation of LSD1 phosphorylation by CK2/WIP1 regulates RNF168-dependent 53BP1 recruitment in response to DNA damage. Nucleic Acids Res. 2015. Jul 13;43(12):5936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lan H, Tan M, Zhang Q, et al. LSD1 destabilizes FBXW7 and abrogates FBXW7 functions independent of its demethylase activity. Proc Natl Acad Sci U S A. 2019. Jun 18;116(25):12311–12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Roth M, Wang Z, Chen WY. SIRT1 and LSD1 competitively regulate KU70 functions in DNA repair and mutation acquisition in cancer cells. Oncotarget. 2016. Aug 2;7(31):50195–50214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Antona A, Leo G, Favero F, et al. Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation. Cell Death Discovery. 2023. 2023/06/29;9(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wojtala M, Dąbek A, Rybaczek D, et al. Silencing Lysine-Specific Histone Demethylase 1 (LSD1) Causes Increased HP1-Positive Chromatin, Stimulation of DNA Repair Processes, and Dysregulation of Proliferation by Chk1 Phosphorylation in Human Endothelial Cells. Cells. 2019. Oct 7;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lu Y, Wajapeyee N, Turker Mitchell S, et al. Silencing of the DNA Mismatch Repair Gene MLH1 Induced by Hypoxic Stress in a Pathway Dependent on the Histone Demethylase LSD1. Cell Reports. 2014;8(2):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mudambi S, Fitzgerald M, Pera P, et al. KDM1A inhibition increases UVA toxicity and enhances photodynamic therapy efficacy. Photodermatol Photoimmunol Photomed. 2023. May;39(3):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sareddy GR, Viswanadhapalli S, Surapaneni P, et al. Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene. 2017. Apr 27;36(17):2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Faletti S, Osti D, Ceccacci E, et al. LSD1-directed therapy affects glioblastoma tumorigenicity by deregulating the protective ATF4-dependent integrated stress response. Sci Transl Med. 2021. Dec 8;13(623):eabf7036. [DOI] [PubMed] [Google Scholar]
- [55].Saccà CD, Gorini F, Ambrosio S, et al. Inhibition of lysine-specific demethylase LSD1 induces senescence in Glioblastoma cells through a HIF-1α-dependent pathway. Biochim Biophys Acta Gene Regul Mech. 2019. May;1862(5):535–546. [DOI] [PubMed] [Google Scholar]
- [56].Kozono D, Li J, Nitta M, et al. Dynamic epigenetic regulation of glioblastoma tumorigenicity through LSD1 modulation of MYC expression. Proc Natl Acad Sci U S A. 2015. Jul 28;112(30):E4055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hosseini A, Minucci S. A comprehensive review of lysine-specific demethylase 1 and its roles in cancer. Epigenomics. 2017;9 8:1123–1142. [DOI] [PubMed] [Google Scholar]
- [58].Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. Journal of Hematology & Oncology. 2019. 2019/12/04/;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Song Y, Zhang H, Yang X, et al. Annual review of lysine-specific demethylase 1 (LSD1/KDM1A) inhibitors in 2021. Eur J Med Chem. 2022. Jan 15;228:114042. [DOI] [PubMed] [Google Scholar]
- [60].Zhang C, Wang Z, Shi Y, et al. Recent advances of LSD1/KDM1A inhibitors for disease therapy. Bioorg Chem. 2023. May;134:106443. [DOI] [PubMed] [Google Scholar]
- [61].Duan YC, Ma YC, Qin WP, et al. Design and synthesis of tranylcypromine derivatives as novel LSD1/HDACs dual inhibitors for cancer treatment. Eur J Med Chem. 2017. Nov 10;140:392–402. [DOI] [PubMed] [Google Scholar]
- [62].Gajendran C, Tantry SJ, M NS, et al. Novel dual LSD1/HDAC6 inhibitor for the treatment of cancer. PLoS One. 2023;18(1):e0279063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Naveen Sadhu M, Sivanandhan D, Gajendran C, et al. Novel dual LSD1/HDAC6 inhibitors for the treatment of multiple myeloma. Bioorg Med Chem Lett. 2021. Feb 15;34:127763. [DOI] [PubMed] [Google Scholar]
- [64].Duan YC, Jin LF, Ren HM, et al. Design, synthesis, and biological evaluation of novel dual inhibitors targeting lysine specific demethylase 1 (LSD1) and histone deacetylases (HDAC) for treatment of gastric cancer. Eur J Med Chem. 2021. Aug 5;220:113453. [DOI] [PubMed] [Google Scholar]
- [65].Anastas JN, Zee BM, Kalin JH, et al. Re-programing Chromatin with a Bifunctional LSD1/HDAC Inhibitor Induces Therapeutic Differentiation in DIPG. Cancer Cell. 2019. Nov 11;36(5):528–544.e10. [DOI] [PubMed] [Google Scholar]
- [66].Welch D, Kahen E, Fridley B, et al. Small molecule inhibition of lysine-specific demethylase 1 (LSD1) and histone deacetylase (HDAC) alone and in combination in Ewing sarcoma cell lines. PLoS One. 2019;14(9):e0222228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fiskus W, Sharma S, Shah B, et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014. Nov;28(11):2155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cao C, Wu H, Vasilatos SN, et al. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int J Cancer. 2018. Sep 15;143(6):1388–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Haydn T, Metzger E, Schuele R, et al. Concomitant epigenetic targeting of LSD1 and HDAC synergistically induces mitochondrial apoptosis in rhabdomyosarcoma cells. Cell Death Dis. 2017. Jun 15;8(6):e2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vasilatos SN, Katz TA, Oesterreich S, et al. Crosstalk between lysine-specific demethylase 1 (LSD1) and histone deacetylases mediates antineoplastic efficacy of HDAC inhibitors in human breast cancer cells. Carcinogenesis. 2013. Jun;34(6):1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Singh MM, Manton CA, Bhat KP, et al. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro-Oncology. 2011;13(8):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Singh MM, Johnson B, Venkatarayan A, et al. Preclinical activity of combined HDAC and KDM1A inhibition in glioblastoma. Neuro Oncol. 2015. Nov;17(11):1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhao J, Jin W, Yi K, et al. Combination LSD1 and HOTAIR-EZH2 inhibition disrupts cell cycle processes and induces apoptosis in glioblastoma cells. Pharmacol Res. 2021. Sep;171:105764. [DOI] [PubMed] [Google Scholar]
- [74].Smitheman KN, Severson TM, Rajapurkar SR, et al. Lysine specific demethylase 1 inactivation enhances differentiation and promotes cytotoxic response when combined with all-trans retinoic acid in acute myeloid leukemia across subtypes. Haematologica. 2019. Jun;104(6):1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schenk T, Chen WC, Göllner S, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012. Mar 11;18(4):605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ravasio R, Ceccacci E, Nicosia L, et al. Targeting the scaffolding role of LSD1 (KDM1A) poises acute myeloid leukemia cells for retinoic acid-induced differentiation. Sci Adv. 2020. Apr;6(15):eaax2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fiskus W, Mill CP, Nabet B, et al. Superior efficacy of co-targeting GFI1/KDM1A and BRD4 against AML and post-MPN secondary AML cells. Blood Cancer J. 2021. May 20;11(5):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Braun TP, Coblentz C, Curtiss BM, et al. Combined inhibition of JAK/STAT pathway and lysine-specific demethylase 1 as a therapeutic strategy in CSF3R/CEBPA mutant acute myeloid leukemia. Proc Natl Acad Sci U S A. 2020. Jun 16;117(24):13670–13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Deb G, Wingelhofer B, Amaral FMR, et al. Pre-clinical activity of combined LSD1 and mTORC1 inhibition in MLL-translocated acute myeloid leukaemia. Leukemia. 2020. May;34(5):1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Saito S, Kikuchi J, Koyama D, et al. Eradication of Central Nervous System Leukemia of T-Cell Origin with a Brain-Permeable LSD1 Inhibitor. Clin Cancer Res. 2019. Mar 1;25(5):1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wen S, Wang J, Liu P, et al. Novel combination of histone methylation modulators with therapeutic synergy against acute myeloid leukemia in vitro and in vivo. Cancer Lett. 2018. Jan 28;413:35–45. [DOI] [PubMed] [Google Scholar]
- [82].Cai SF, Chu SH, Goldberg AD, et al. Leukemia Cell of Origin Influences Apoptotic Priming and Sensitivity to LSD1 Inhibition. Cancer Discov. 2020. Oct;10(10):1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li M, Liu M, Han W, et al. LSD1 Inhibition Disrupts Super-Enhancer-Driven Oncogenic Transcriptional Programs in Castration-Resistant Prostate Cancer. Cancer Res. 2023. May 15;83(10):1684–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gupta S, Weston A, Bearrs J, et al. Reversible lysine-specific demethylase 1 antagonist HCI-2509 inhibits growth and decreases c-MYC in castration- and docetaxel-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2016. Dec;19(4):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wang J, Yu Q, Qiu Z, et al. The combined effect of epigenetic inhibitors for LSD1 and BRD4 alters prostate cancer growth and invasion. Aging (Albany NY). 2020. Jan 5;12(1):397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Verigos J, Karakaidos P, Kordias D, et al. The Histone Demethylase LSD1/ΚDM1A Mediates Chemoresistance in Breast Cancer via Regulation of a Stem Cell Program. Cancers (Basel). 2019. Oct 17;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].He M, Ning W, Hu Z, et al. Design, synthesis and biological evaluation of novel dual-acting modulators targeting both estrogen receptor α (ERα) and lysine-specific demethylase 1 (LSD1) for treatment of breast cancer. Eur J Med Chem. 2020. Jun 1;195:112281. [DOI] [PubMed] [Google Scholar]
- [88].Venkata PP, Chen Y, Alejo S, et al. KDM1A inhibition augments the efficacy of rapamycin for the treatment of endometrial cancer. Cancer Lett. 2022. Jan 1;524:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yang Y, Huang W, Qiu R, et al. LSD1 coordinates with the SIN3A/HDAC complex and maintains sensitivity to chemotherapy in breast cancer. J Mol Cell Biol. 2018. Aug 1;10(4):285–301. [DOI] [PubMed] [Google Scholar]
- [90].Nagasawa S, Sedukhina AS, Nakagawa Y, et al. LSD1 overexpression is associated with poor prognosis in basal-like breast cancer, and sensitivity to PARP inhibition. PLoS One. 2015;10(2):e0118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu LW, Zhou DM, Zhang ZY, et al. Suppression of LSD1 enhances the cytotoxic and apoptotic effects of regorafenib in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2019. May 14;512(4):852–858. [DOI] [PubMed] [Google Scholar]
- [92].Huang M, Chen C, Geng J, et al. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett. 2017. Jul 10;398:12–21. [DOI] [PubMed] [Google Scholar]
- [93].Ma L, Xu A, Kang L, et al. LSD1-Demethylated LINC01134 Confers Oxaliplatin Resistance Through SP1-Induced p62 Transcription in HCC. Hepatology. 2021. Dec;74(6):3213–3234. [DOI] [PubMed] [Google Scholar]
- [94].Sang N, Zhong X, Gou K, et al. Pharmacological inhibition of LSD1 suppresses growth of hepatocellular carcinoma by inducing GADD45B. MedComm (2020). 2023. Jun;4(3):e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lu Y, Liu Y, Oeck S, et al. Hypoxia Promotes Resistance to EGFR Inhibition in NSCLC Cells via the Histone Demethylases, LSD1 and PLU-1. Mol Cancer Res. 2018. Oct;16(10):1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ma JL, Zhang T, Suo FZ, et al. Lysine-specific demethylase 1 activation by vitamin B2 attenuates efficacy of apatinib for proliferation and migration of gastric cancer cell MGC-803. J Cell Biochem. 2018. Jun;119(6):4957–4966. [DOI] [PubMed] [Google Scholar]
- [97].Lee KH, Kim BC, Jeong SH, et al. Histone Demethylase LSD1 Regulates Kidney Cancer Progression by Modulating Androgen Receptor Activity. Int J Mol Sci. 2020. Aug 24;21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Han H, Yang X, Pandiyan K, et al. Synergistic re-activation of epigenetically silenced genes by combinatorial inhibition of DNMTs and LSD1 in cancer cells. PLoS One. 2013;8(9):e75136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Peng W, Zhang H, Tan S, et al. Synergistic antitumor effect of 5-fluorouracil with the novel LSD1 inhibitor ZY0511 in colorectal cancer. Ther Adv Med Oncol. 2020;12:1758835920937428. [DOI] [PMC free article] [PubMed] [Google Scholar]


