Abstract
Introduction:
Asthma is a chronic lung disease influenced by environmental and inflammatory triggers and involving complex signaling pathways across resident airway cells such as epithelium, airway smooth muscle, fibroblasts, and immune cells. While our understanding of asthma pathophysiology is continually progressing, there is a growing realization that cellular microdomains play critical roles in mediating signaling relevant to asthma in the context of contractility and remodeling. Mechanosensitive pathways are increasingly recognized as important to microdomain signaling, with Piezo and transient receptor protein (TRP) channels at the plasma membrane considered important for converting mechanical stimuli into cellular behavior. Given their ion channel properties, particularly Ca2+ conduction, a question becomes whether and how mechanosensitive channels contribute to Ca2+ microdomains in airway cells relevant to asthma.
Areas covered:
Mechanosensitive TRP and Piezo channels regulate key Ca2+ regulatory proteins such as store operated calcium entry (SOCE) involving STIM and Orai channels, and sarcoendoplasmic (SR) mechanisms such as IP3 receptor channels (IP3Rs), and SR Ca2+ ATPase (SERCA) that are important in asthma pathophysiology including airway hyperreactivity and remodeling.
Expert opinion:
Physical and/or functional interactions between Ca2+ regulatory proteins and mechanosensitive channels such as TRP and Piezo can towards understanding asthma pathophysiology and identifying novel therapeutic approaches.
Keywords: STIM, Orai, Piezo channels, TRPV receptor, Airway smooth muscle, Asthma
1. Introduction
Asthma is one of the most common chronic and non-communicable respiratory disorders affecting as many as 339 million people globally (1). Furthermore, asthma is correlated with a higher risk of other diseases like hypertension, diabetes, obesity, and cancer (2–8). Research has highlighted that a number of environmental and inflammatory factors are involved in asthma pathophysiology, as are different resident cells of the airway along with immune cells, making it difficult to identify single or limited sets of mechanisms that could be targeted towards asthma therapy (9, 10). Regardless, at least one common factor relevant to different airway cells is intracellular Ca2+ concentration ([Ca2+]i). Ca2+ is an essential second messenger and plays an important role not only normal cellular function but specifically in asthma. For example, increased Ca2+ can lead to exaggerated contraction of airway smooth muscle (ASM) cells (11, 12), release of inflammatory mediators (13) and mucus hypersecretion from airway epithelial cells (14) along with increased vascular permeability of endothelial cells (15) overall contributing to airway hyperreactivity, remodeling and inflammation (16). Thus, understanding the mechanisms of Ca2+ regulation and dysregulation in airway cells in the context of asthma pathophysiology becomes critical towards development of novel therapies (17–22).
An evolving concept of increasing interest in lung disease pathogenesis is that of mechanobiology, involving the impact of mechanical forces on cellular structure and behavior, and the interaction between intracellular components or that between intracellular and extracellular components in the context of mechanical stimuli. Tschumperlin and Drazen were the first to hypothesize that mechanical stimulation plays a role in airway remodeling (23). In this regard, ASM cells experience substantial mechanical forces in the context of normal breathing and ongoing airway contractions and relaxation. Mechanical stretch can influence the secretory properties of ASM (24, 25). For patients with asthma, who undergo repeated episodes of bronchoconstriction, show pathological airway remodelling such as goblet cell hyperplasia and increased collagen deposition (25), changes in airway structure and airway reactivity alter the mechanical relationships between cells, and the responsiveness of airway cells to mechanical forces. However, the mechanisms by which mechanical forces lead to structural or functional changes, or those that respond to mechanical forces are only recently being understood.
There is increasing recognition that mechanosensitive Piezo and transient receptor potential (TRP) ion channels, which influence entry of cations, particularly Ca2+, in response to mechanical stimuli, are potentially important in airways (26, 27) and might play a role in airway remodeling and airway hyperreactivity in asthma (28, 29). What is less known is how these plasma membrane channels interact with intracellular pathways to induce structural or functional changes in the airway and whether and how they are detrimental vs. alleviating towards airway physiology (28, 29). We review the current state of knowledge regarding interactions between mechanosensitive ion channels and key Ca2+ regulatory proteins that influence both Ca2+ homeostasis and airway hyperreactivity and remodeling in the airways, particularly in the context of asthma. Information gleaned from studies to date have the potential to help understand the relevance of mechanosensitive pathways in other lung diseases such as COPD and pulmonary fibrosis that involve altered structure and function of bronchial and parenchymal airways.
2. Ca2+ regulatory proteins in asthma
A balance between [Ca2+]i and extracellular Ca2+ is critical to cellular homeostasis and cell survival which involves regulation of Ca2+ fluxes across the plasma membrane vs. intracellular regulation of Ca2+ availability via several proteins (17, 18). The mechanisms of [Ca2+]i regulation in airway cells has been recently reviewed (30–40). The sarcoendoplasmic reticulum (SR) is the main intracellular Ca2+ store (19, 41, 42). The sarcoendoplasmic reticulum calcium ATPase (SERCA) pumps Ca2+ from the cytosol into the SR lumen in an ATP-dependent manner (20) and thus helps to maintain low baseline Ca2+ levels. Upon G protein-coupled receptor (GPCR) stimulation by agonists (e.g. acetylcholine and muscarinic receptors; histamine and histaminergic receptors), second messenger cascades involving inositol 1,4,5-trisphosphate (IP3) or cyclic ADP ribose trigger Ca2+ from the SR through IP3 receptors (IP3Rs) or ryanodine receptors (RyRs), respectively. Depletion of SR stores under these conditions activates store-operated Ca2+ entry (SOCE) (21). SOCE is mediated by stromal interaction molecule (STIM, particularly STIM1) situated on the SR membrane. STIM functions as a luminal Ca2+ sensor which is activated by decreased SR Ca2+ and translocated into junctions formed between SR and the plasma membrane. Upon binding to the plasma membrane channel Orai1, there is an increase in Ca2+ influx. Supporting Ca2+ regulatory mechanisms include plasma membrane Ca2+ ATPase (PMCA), voltage gated Ca2+ channel (VGCC), Na+/Ca2+ exchanger (NCX, 3Na+:1Ca2+) and non-specific cation channels (22). Here, we explore some of the major Ca2+ regulatory pathways in the context of asthma and their potential to interact with mechanosensitive pathways.
2.1. STIM
STIM is one of the major intracellular components and the initiator of SOCE (43). There are two homologous proteins, STIM1 and STIM2, which are multi-domain, single-pass transmembrane proteins residing on the SR membrane and sensing changes in SR luminal Ca2+ levels (44, 45), communicating such changes to Orai1 channel proteins in the plasma membrane (46). In the case of STIM1, a 90-kDa protein, located in the SR membrane and plasma membrane with an NH2-terminal luminal low-affinity EF-hand acts as an SR sensor for Ca2+ and communication with Orai1 occurs through interaction between the cytosolic COOH-terminal STIM-Orai1 activating region (SOAR) of STIM and the COOH-terminal coiled-coil domain of Orail when Ca2+ depletion occurs in the SR (47). STIM proteins sense the Ca2+ depletion in the SR, oligomerize, and redistribute into discrete puncta located in junctional SR sites near the plasma membrane and directly interact with Orai1 resulting in sustained Ca2+ entry that allows for refilling of the SR Ca2+ stores. Despite the overall high sequence similarity between STIM1 and STIM2 luminal N-terminal domains, STIM2 is only expressed in the SR. This is due to the EF-hand of STIM2 having a lower affinity for Ca2+, which makes STIM2 a weaker activator of Orai1 and results in lower distinct stability and Ca2+-sensitivity (48, 49). As a result, while STIM1 acts as a sensor for SR Ca2+ depletion, STIM2 can be activated during sub-maximal reductions in SR Ca2+.
STIM1 has been implicated in the pathogenesis and development of asthma (16, 21). Enhanced expression of STIM1 protein is associated with airway remodeling in asthma (16). One study reported that exposure of mice to the allergen, ovalbumin (OVA) results in increased expression of STIM and Orai1 (50). Hyperplasia of ASM cells is a feature in asthma, and proliferation and migration of ASM cells are promoted by STIM1 and may thus contribute to airway remodeling (51). Knockdown of STIM1 inhibits platelet derived growth factor (PDGF)-induced activation of SOCE and attenuates ASM cell proliferation and migration in OVA challenged mice (50). Interestingly, in a rat model of asthma, aerobic exercise can improve ASM contractile function by downregulating the expression of STIM1, Orai1, and Orai2, blunting excessive SOCE (52). ASM-mediated airway hyperresponsiveness (AHR) and airway remodeling are also significantly blunted in STIM1 knockout mice (21).
We and others have previously shown the importance of ASM Ca2+ oscillations in airway contractility and AHR including in human airways (53, 54), where the frequency of Ca2+ oscillations determines the extent of ASM contraction (55). Higher airway responsiveness in BALB/c than C57BL/6 mice has been attributed to increased expression of ASM STIM1, which is associated with faster Ca2+ oscillations (56). STIM1 is also necessary for house dust mite (HDM)-induced Ca2+ oscillations, where STIM1 knockout significantly decreases ASM Ca2+ oscillations (21).
Previous studies from our laboratory have also shown that inflammatory triggers on STIM1 aggregates contributes to AHR. The asthma-relevant pro-inflammatory cytokines interleukin (IL)-13 and tumor necrosis factor alpha (TNF-α) increase Ca2+ release and SOCE in ASM by upregulating STIM1 and Orai1 (57). Johnson and colleagues found that increased STIM1 expression in ASM can also trigger AHR and remodeling by activation of nuclear factor of activated T cells (NFAT) and secretion of IL-6 (16). BTP2, an efficient inhibitor of SOCE, has been shown to attenuate allergic inflammation induced allergic asthma (52). Beyond ASM, STIM1 protein has been implicated in the activation of mast cells, which are involved in the early phase of asthma pathogenesis (58). Overall, these data underline the role of STIM1 in the pathogenesis of asthma and suggest that inhibition of STIM1 represents a novel therapeutic target.
While STIM2 has similar functional effects to STIM1 in some respects, there is not much known regarding the contributions of STIM2 to airway contractility, AHR, or asthma. Interestingly, one study reported that the expression and function of STIM2 was significantly exaggerated in asthmatic patients (59), but the implications relative to changes in STIM1, or impact on remodeling are unknown. Given the high likelihood that maximal SR depletion does not occur consistently in ASM cells even during substantial contraction, and thus a sub-maximal reduction of Ca2+ is important, the role of STIM2 is likely more important, which can be activated even by mild depletion of SR Ca2+ stores and can drive activation of constitutive Ca2+ influx (60). Expression of STIM2 has been found to be higher in non-allergic asthmatic children compared to healthy controls following anti-CD3/28 stimulation (61). STIM2 is also expressed in WI-38 fibroblasts and can enhance the percentage of cells showing Ca2+ oscillations upon stimulation with agonists that activate Gq (62, 63).
2.2. ORAI
Orai proteins are highly Ca2+ selective plasma membrane channels that are regulated by STIM (64). The Orai family consists of three homologs, Orai1, Orai2 and Orai3, which function as thermo-molecular components of the SOCE pathway. Orai expression is associated with airway pathology in asthma, including airway remodeling, inflammation, and bronchoconstriction (65). Orai1 expression is upregulated in ASM from asthmatic mice and in PDGF-mediated ASM cell proliferation, while knockdown of Orai1 attenuates ASM proliferation and SOCE (50, 51). Long-term administration of an Orai1 antagonist (3-fluoropyridine-4-carboxylic acid, FPCA) resulted in bronchodilation in a pig asthma model (66, 67). Orai1 knockdown also significantly inhibited ASM proliferation and chemotactic migration in response to PDGF (50). Beyond ASM, in human bronchial epithelial cells, the allergen house dust mite (HDM) activates STIM1-Orai1-dependent SOCE and drives expression of genes involved in remodeling (50, 68).
Increases in [Ca2+]i resulting from the interaction of STIM and Orai are also involved in the pathogenesis of inflammation in asthma (69, 70). IL-4, a type 2 cytokine, is reduced in T cells of patients with loss-of-function mutations in Orai1 (71). Pretreatment of ASM with IL-4 upregulates the expression of Orai1 and Orai2, promoting ASM contraction, which can be inhibited by aerobic exercise (52). T cell-specific deletion of Orai1 protects mice from HDM-induced allergic airway inflammation, while Orai1 controls the expression of cell cycle regulators and T cell proliferation during allergic airway inflammation (72). Short palate lung and nasal epithelial clone 1 (SPLUNC1), an asthma gene modifier that inhibits Orai1 via its C-terminal α6 region, decreases eosinophilic inflammation in OVA-induced asthma mice and a murine allergic asthma model (73–75). Lung mast cells also express Orai1, wherein shRNA knockdown of Orai1 reduces Ca2+ influx (76). Inhibition of SOCE by GSK-7975A and synta-66 suppresses mast cell secretion of IL-5, IL-3 and TNF-α (77). IL-13 regulates airway remodeling in asthma mouse model by regulating Orai1 expression (78). Orai1 inhibition can thus exert broad effects on inflammatory activity and may have therapeutic potential in asthma. Other Orai family members (Orai2 and 3) are also involved in Ca2+ regulation in ASM but to a lesser extent than Orai1. Arachidonic acid induced Ca2+ oscillations in cultured ASMCs, while unaffected by SOCE inhibitors, are inhibited by knockdown of Orai3 (13, 40). In comparison, knockdown of Orai2 results in only marginal reductions of Ca2+ influx (76). Overall, these disparate data underline the importance of different Orai isoforms in airway structure and function in the context of inflammation and asthma.
2.3. IP3R
IP3Rs are located on SR and mitochondrial-associated-membranes (79). It is a tetrameric Ca2+ release channel consisting of four subunits that binds to IP3 triggering Ca2+ release into the cytosol. The increase in cytosolic Ca2+ may regulate cytosolic effectors or uptake by other organelles, while the associated decrease in SR Ca2+, depending on the extent of depletion, activates STIM2 and/or STIM1 and SOCE (80). Cytosolic Ca2+ in turn can also regulate the level of IP3Rs, where higher concentrations of cytosolic Ca2+ inhibits IP3Rs (81).
IP3R is strongly associated with asthma (52, 82, 83). Both mRNA and protein levels of IP3R are increased in interleukin-stimulated ASM cells and in HDM-induced asthma models (52). IP3R levels was also found to be reduced in lipopolysaccharide (LPS) stimulated human bronchial epithelial cells from asthma patients. This reduction was correlated with increased SR Ca2+ release (84). In ASM, IP3R inhibition with 2-APB or Xestospongin C reduces Ca2+ oscillation frequency, leading to relaxation (85, 86) while, conversely, IP3R-dependent Ca2+ oscillations and resultant contraction are increased in rat model of asthma (87). Separately, suppressed IP3R-mediated Ca2+ signaling by traniterol, a relaxant of ASM also blunts ASM proliferation (88).
IP3R has also been linked to airway inflammation in asthma. Activation of IP3R can trigger release of pro-inflammatory cytokines and chemokines, which attract immune cells to the airways and contribute to development of asthma symptoms (84). Inhibition of IP3R activity reduces production of pro-inflammatory cytokines in airway epithelial cells (89). Interactions with IP3R with B-cell lymphoma 2 (Bcl-2), a mitochondrial apoptosis protein, modulates apoptotic pathways in asthma (90, 91). In contrast, B-cell lymphoma-extra-large (Bcl-xL) promotes Ca2+ oscillations by increasing sensitization to IP3R (92, 93).
Studies have also shown that mitochondrial dysfunction is associated with the development of asthma (94, 95). Mitochondria plays a critical role in energy metabolism and are also involved in regulating Ca2+ signaling (96). Dysfunctional mitochondria can lead to increased oxidative stress, inflammation, and impaired Ca2+ handling, all of which have been implicated in the pathogenesis of asthma (97, 98). IP3R -mediated Ca2+ release from the SR can activate mitochondrial Ca2+ uptake, which can further modulate mitochondrial function (99, 100). IP3R regulates mitochondrial Ca2+ concentration, resulting in mitochondrial reactive oxygen species generation and inflammation (101). Here, mitochondria can relay feedback to regulate nearby SR Ca2+ channels, thereby maintaining Ca2+ homeostasis (102). Carbachol, via IP3R aggravates mitochondrial disfunction and NLRP3 inflammasome activation (89). IP3R can also communicate with RyR Ca2+ channels and mitochondria to regulate SR Ca2+ release, mitochondrial dysfunction, and reactive oxygen species generation (83).
2.4. SERCA
SERCA works to transport cytosolic Ca2+ back into the SR (103), and thus contributes to muscle relaxation (104). There are 3 tissue-specific members: SERCA1, SERCA2 and SERCA3 (105). SERCA2 is most highly expressed in smooth muscle (106, 107). In both asthmatic and healthy ASM cells, inhibition of SERCA2 leads to an increase in [Ca2+]i levels, which results in enhanced ASM contraction, proliferation, and secretory function (108). Diminished expression of SERCA2 is also correlated with severity of asthma and airway inflammation (104). The ability of SERCA2 to replete SR Ca2+ stores is also decreased in asthmatic ASM cells (108). Expression of SERCA2b shows an inverse association with airway tone (104), which is an underlying contributor to airway contractile capacity (109). In addition, AHR is also related to downregulated expression and function of SERCA2b (108).
We have previously shown that inflammatory cytokines such as TNF-α and IL-13 decrease expression of SERCA2 in ASM (110). Brain-derived neurotrophic factor (BDNF), a growth factor associated with asthma that enhances ASM proliferation and contractility (111, 112), also promotes the expression of SERCA, while inhibition of SERCA prevents BDNF enhancement of Ca2+ responses to histamine (113).
SERCA expression in immune cells is also relevant to asthma. For example, expression of SERCA shows an inverse association between the ability of human basophils to respond to IgE-dependent stimulation, confirmed by pharmacological inhibitor (thapsigargin) vs. activator (disulfiram) (114). Interestingly bodily symptoms such as muscle weakness and atrophy in asthma is also in part driven by SERCA dysfunction, including decreased expression of SERCA2 (115).
2.5. Caveolin
The relevance of caveolins and caveolar proteins lies in their ability to integrate plasma membrane signals and modulate the interactions of plasma membrane proteins with intracellular components. There are three caveolin family members: Caveolin-1 (Cav-1), Cav-2, and Cav-3 (116). Cav-2 is co-expressed with Cav-1 in multiple cell types including epithelial and ASM cells, while Cav-3 is expressed mainly in cardiac and skeletal muscle cells. Cav-1 is most related to asthma (117). Cav-1 is a 178-amino acid hairpin structural protein with a hydrophobic transmembrane domain (118). Caveolae harbor Ca2+ regulatory proteins including receptors for bronchoconstrictor agonists and SOCE proteins such as Orai1. Thus Cav-1 is required for the association of STIM1 with Orai1, and suppression of Cav-1 reduces the expression of Orail1 but not STIM1 (119). Functionally, Cav-1 has been shown to regulate ASM [Ca2+]i by modulating SOCE, thereby regulating cell contraction (119). By virtue of harboring agonist receptors, disruption of caveolae using siRNA attenuates [Ca2+]i responses to agonists such as histamine, acetylcholine, and bradykinin (120). Thus Cav-1 plays a critical role in ASM cell function, and thus the assumption would be that increased Cav-1 is associated with increased ASM contractility. However, this is not consistently the case. For example, Cav-1 plays an important role in airway inflammation by mediated the effect of TNF-α and IL-13 on enhanced [Ca2+]i in ASM (121). Cav-1 expression in ASM induced by OVA is also correlated to the degree of airway obstruction and hyperresponsiveness (122, 123). However, patients with asthma have lower Cav-1 expression, and this decrease is associated with enhanced expression of extracellular matrix (ECM) proteins (collagen, tenascin and periostin deposition) (123, 124). A drug caveolin scaffolding domain (CSD) that restores Cav-1 function in Cav-1-deficient cells is protective in ASM (125).
The role of Cav-1 in epithelium is also protective for the most part. IL-4 is required for allergen induced mucus production and airway inflammation (126, 127). Cav-1 plays an important role in mucus in asthmatics (128). Blocking Cav-1 prevents Ca2+ influx and MUC5AC synthesis induced by IL-4 in bronchial epithelial cells (129). However, Cav-1 deficient mice show increased thickness of subepithelial collagen layer and develop asthma-like responses to OVA (31, 130). In addition, silencing GATA6 increases Cav-1 and reduces inflammation and mucus production in asthmatic mouse model (128). Cav-1 expression acts to stabilize E-cadherin and β-catenin at adherens junctions to maintain epithelial barrier function (131). Suppression of Cav-1 results in delocalization of E-cadherin and barrier dysfunction in 16HBE epithelial cells (131). Cav-1 knockout mice exhibit activation of TGF-β, mediating subepithelial airway fibrosis (132). Overall, the preponderance of data suggest Cav-1 plays a protective role in asthma.
2.6. NCX
NCX is a bidirectional transporter using the electrochemical gradient driven by Na+ to respond to elevated [Ca2+]i and contribute to Ca2+ extrusion (133). NCX is encoded by three genes: NCX1, NCX2, and NCX3 (134). The expression, function, and regulation of NCX differ across tissues and species. Only NCX1 has been detected in ASM so far. While largely known for Ca2+ extrusion, under extreme conditions, NCX works in reverse mode (NCXREV), and promotes Ca2+ influx with Na+ efflux, resulting in increased Ca2+ and enhancing contraction. NCX operating in reverse mode is involved in ASM contraction (135) and is thought to contribute to asthma and AHR (136, 137), via mechanisms involving neurokinin receptors (138). Inflammatory cytokines TNF-α and IL-13 increase the expression of NCX protein further enhancing Ca2+ fluxes (139). NCX also contributes to ASM cell proliferation and migration, by preventing excessive mitochondrial Ca2+ overload and supporting the entry of Ca2+ through SOCE pathways. NCX also activates Ca2+/calmodulin-dependent kinase II, leading to transcriptional and reprogramming. Furthermore, a model of asthma involving NCX knockout in smooth muscle exhibits reduced airway remodeling, AHR and airway fibrosis (140). Overall, these limited studies suggest a detrimental role of NCX in asthma.
3. Mechanosensitive Ion Channels
3.1. Piezo channels
Mechanosensitive pathways transmit mechanical signals into electrochemical signals essential for cellular function (141). Relevance of mechanosensitive pathways lie in their potential ability to influence both airway contractility and remodeling (142). In this regard, there is substantial interest in the role of the more recently identified Piezo channels, Piezo 1 (Fam38a) and Piezo 2 (Fam38b), that are sensitive to various forms of mechanical stimuli including stretch, compression, and shear (143–146). Recent studies have shown that Piezo channels play critical roles in regulating physiological and pathological functions across different cell types and organs (147). Piezo channels are expressed in lung, bladder, skin, and neurons and are involved in a range of functions such as regulating RBC volume, organ development, cell proliferation, and migration (148–150). Their interaction with the plasma membrane, and/or ECM components as well intracellular signaling proteins make Piezo channels appealing in terms of understanding their potential importance in airway function and asthma. Mechanical forces exerted on the cytoskeleton and ECM causes the opening of the Piezo1 channel, leading to the influx of extracellular Ca2+ and transducing mechanical signals into electrical and chemical signals in the cell (Figure 1). Piezo channel mediation of cellular functions through plasma membrane and/or ECM interactions is evident in multiple cell types. For example, dendritic cell activation via changes in stiffness is mediated through Piezo (149). Piezo channels are involved in macrophage polarization (150). Piezo1 modulates matrix degradation in vascular smooth muscle cells (97). In the absence of ECM proteins, Piezo1 is not sensitive to mechanical forces (151).
Figure 1.
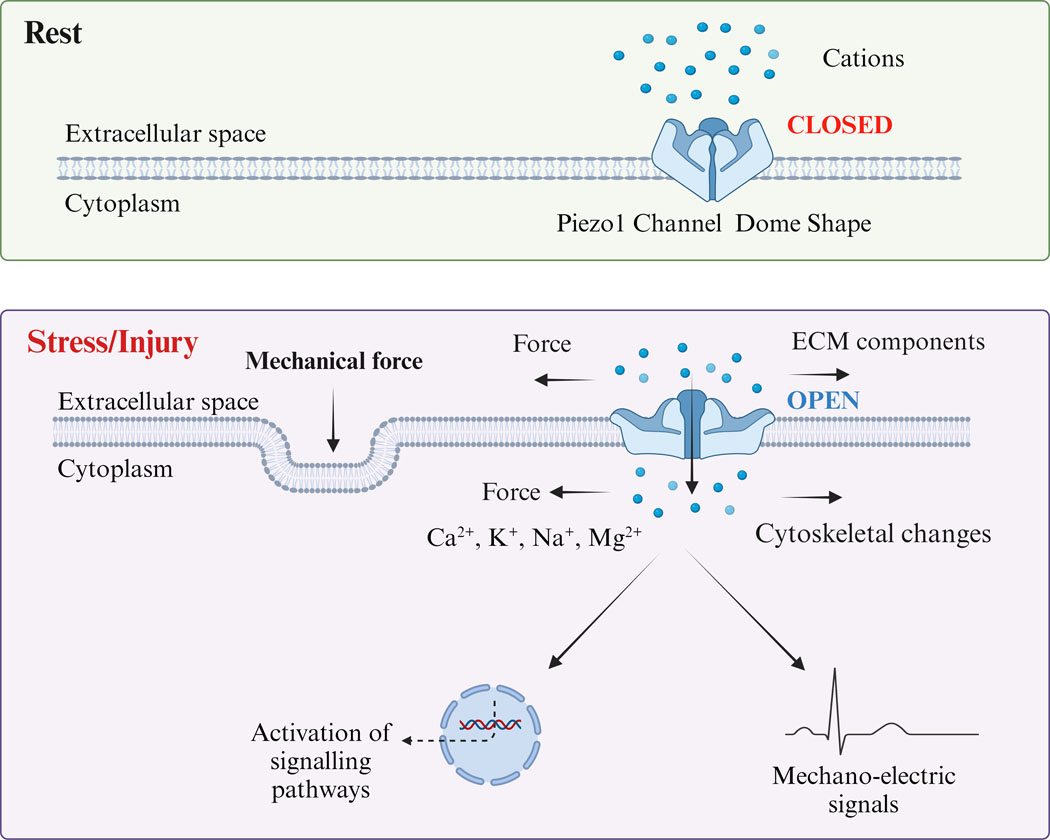
Schematic diagram of Piezo1 Channel activation by mechanical force. The Piezo1 channel is a trimeric structure located in the plasma membrane. The mechanical force exerted on the cytoskeleton and extracellular matrix (ECM) causes the opening of the Piezo1 channel, leading to the influx of extracellular Ca2+ and transducing mechanical signals into electrical and chemical signals in the cell.
In the lung, Piezo1 is expressed in pulmonary endothelial cells (152), ASM (153) and alveolar epithelial cells (154), although the data are limited often to gene expression or protein without localization. In rat ASM, Piezo1 activation by the specific agonist Yoda1 decreases cell stiffness and traction force, disrupting stress fibers and cell migration (153). Our previous study found that Piezo1 and Piezo2 are expressed in human fetal ASM cells, where activation of Piezo1 by agonist Yoda or stretch causes significant [Ca2+]i responses and increased ECM production (155, 156). Piezo2 has been found in neurons that are responsive to mechanical forces including those in the lung (157–159). In a preliminary study, Piezo2 staining was localized in bronchial epithelial cells, macrophages, and smooth muscle cells (160). How Piezo1 and Piezo2 in lungs interact in the context of airway structure and function or in asthma has not been extensively investigated. Piezo1 in bronchial epithelial cells is higher in asthmatic mice (26). Impairment of airway epithelial function and disordered tight junction expression relevant to asthma (161) may be tied to Piezo1 (26). Piezo1 is activated by auto-positive end-expiratory alveolar pressure, resulting in an increase in [Ca2+]i and aggravates the degradation of tight junction in a Piezo1 dependent manner (26).
3.2. TRP channels
TRP proteins are a group of relatively non-specific cationic channels located mainly on the plasma membrane (162). TRP proteins contains an intracellular-N and C-termini, 6 membrane-spanning helices, and a presumed pore-forming loop (163). These channels respond to various heterogeneous stimuli, including physical stimuli (mechanical force and temperature), endogenous and exogenous chemical mediators, depletion of Ca2+ stores in SR and free cytosolic Ca2+. Seven subfamilies have been identified: TRPA, TRPC, TRPV, TRPM, TRPP, TRPML and TRPN. The expression and function of all these families have not been uniformly explored in the airway or lung disease. Nonetheless, TRPC and TRPV channels appear to be most relevant, including for mechanotransduction.
TRPC channels have been more highly explored in the airway in the context of Ca2+ regulation but are also relevant to mechanosensation as discussed further below. TRPC channels can be activated by IP3 and inactivated by calmodulin under conditions of high intracellular Ca2+. TRPC can also serve in a store operated activation role like STIM1-Orai1. TRPC channels can also be activated downstream of mechanosensitive GPCRs signaling through phospholipase C. Deformation of the plasma membrane can also activate TRPCs. Here, TRPC1, TRPC5, and TRPC6 are thought to be mechanosensitive. TRPC1 mechanosensitivity has been shown in a variety of cell types including ganglionic neurons (164), cardiac cells (165), and bronchial epithelial cells (29, 166). Furthermore, TRPC1 and TRPC5 can heterodimerize (167) and remain mechanosensitive, while such properties may change when complexing with TRPC4 which is not mechanosensitive (168, 169). TRPC5 can be activated by membrane stretching or osmotic pressure changes (170, 171). TRPC5 is localized to the apical membrane of airway epithelial cells is important for mechanotransduction changes with osmotic pressure (172). TRPC6 is mechanosensitive in arterial smooth muscle cells in response to intravascular pressure (173), but is also expressed in other tissues where its role is less known. TRPC3 is a key molecular component of nonselective cation channels found in ASM cells, which is important in regulating resting membrane potential and [Ca2+]i in ASM cells, as well as membrane depolarization and hyperresponsive in OVA-sensitized/-challenged cells (174). In guinea pig ASM cells, TRPC3 is important for maintaining [Ca2+]i basal levels and preserving smooth muscle basal tone. Blocking TRPC3 with 2-aminoethoxydipheny1(2-APB) and Pyr3 significantly decreases baseline [Ca2+]i (175). Expression of TRPC3 is also enhanced in ASM cells of sensitized mice, resulting in elevation of baseline [Ca2+]i, potentially contributing to the development of AHR relevant to asthma (174). TRPC3 can been activated by IP3 resulting in Ca2+ influx, in addition to conversely opening IP3Rs to induce Ca2+ release from intracellular Ca2+ stores (176). While there is evidence for TRPC3 in regulation of [Ca2+]i in the airways (177), it is important to emphasize that links to mechanosensitivity as not yet known.
The TRPV family (TRPV1-TRPV6) is a group of polymodal channels that sense thermal, acidic, mechanical stress, and osmotic stimuli and can be activated by endogenous ligands (178). TRPV1 to TRPV4 are non-selective cation channels that exhibit a preference for Ca2+ influx over Na+, TRPV5 and TRPV6 are selective channels that specifically permit Ca2+ (28). Under the TRPV family, TRPV1, TRPV2 and TRPV4 are considered relevant to development and exacerbation of asthma (179).
TRPV1 increases airway inflammation (180–182), AHR (183), mucus production (184), ASM contraction, cough, and airway remodeling (185, 186), all potentially relevant to asthma. Capsazepine, a TRPV1 antagonist inhibits AHR, airway remodeling and airway inflammation in mice (186) and prevents bronchoconstriction in guinea pig ASM (187), similar to SB-705498 and PF-04065463 (188). Both endogenous and exogenous TRPV1 agonists (ROS and bradykinin) can induced cough in response to tissue inflammation in asthma (189).The antagonist HC-030031 reduces cough response (146), while GDC-0334a, an orally bioavailable TRPV1 antagonist, inhibits allergic airway inflammation (190). Airway infiltration of leukocytes, IL-13, IL-5, IL-4, IL-13 and TNF-α are reduced in TRPV1 deleted mice (186, 191, 192). In addition, TRPV1 gene mutation was closely related to bronchial asthma in children and provide a new treatment and prognosis of children with bronchial asthma.
TRPV2 has been found to be a potential new biomarker for diagnosis of childhood asthma with typical IgE levels (28, 180, 193) using peripheral lymphocytes which show upregulation of TRPV2 (180). Blockade of TRPV2 with SKF-96365 decreases secretion of inflammatory cytokines (TNF-α, IL-13 and IL-17A) (194). A traditional Chinese prescription, San-ao decoction used to treat asthma, reduced the expression of TRPV2 in the lungs of OVA-induced asthmatic mice, and diminished the levels of IL-4 and IL-10 in BALF (195). TRPV4 is expressed in airway epithelial cells and ASM cells. Activation of TRPV4 by warm temperatures, osmotic, and mechanical stimuli induces proliferation of ASM (196, 197), allergic inflammation (198), and airway remodeling (199), TRPV4 has been implicated in non-atopic asthma, where stimulation of TRPV4 increases [Ca2+]i and releases ATP, which activates P2X4 receptors on mast cells, and further evokes the release of leukotrienes thus promoting ASM contraction (179). TRPV4 can also function as an osmolarity sensor in airways when stimulated by hypotonic solutions (196). GSK222069 and GSK2337429A, antagonists of TRPV4, attenuate lung inflammation by reducing neutrophils, macrophages and associated cytokines (200). TRPV4 contributes to Ca2+ regulation by forming a complex with NCX and with IP3R with downstream effects on airway tone as shown in mice (201). TRPV4 can also modulate ASM contraction in exercise and with inspiration of humid air in the context of hypoosmotic stimulus (202). In a murine model of HDM-induced asthma, activation of TRPV4/Rho/MRTF-A signal pathway results in increased remodeling and ECM deposition (203).
Ambient temperature is another factor that may trigger asthma. TRPs are sensitive to temperature, where TRPV1 is heat-sensitive while TRPM8 channels are cold-sensitive. Cold air stimulus induced airway inflammatory and remodeling by increasing TRPM8 expression while knockdown TRPM8 attenuates this response (204). TRPV1 responds to thermal stimuli exceeding 42˚C (205). TRPM8 and TRPA1 are decreased in ASM of the rat asthma model, while activation of TRPM8 and TRPA1 inhibits ASM proliferation (206).
4. Crosstalk between mechanosensitive channels and Ca2+ regulatory proteins
Given that a number of Ca2+ regulatory pathways exist within the plasma membrane or either physically or functionally interact with it, it would be reasonable to assume that mechanosensitive channels also within the plasma membrane could potentially modulate Ca2+ in a number of ways. Such crosstalk between mechanosensitive channels and Ca2+ regulatory proteins was recently summarized in the context of cardiovascular health and disease (207) demonstrating the importance of many of the plasma membrane intracellular pathways that are also relevant to the airways. However, there are also clear differences in the expression and functionality of these pathways in the cardiovascular vs. pulmonary systems. Yet there is only scattered and newly emerging information relevant to the role of mechanosensitivity in the airways. Understanding these interactions in the context of airway structure and function and airway diseases holds potential for identification of novel disease mechanisms as well as potential targets for intervention.
4.1. Piezo and Ca2+ regulatory proteins
Given the relative novelty of Piezo channels, there is obviously little known regarding their interactions with intracellular Ca2+ proteins. Recently, SERCA2 has been found to interact with Piezo1 channels via a 14-residue intracellular linker region at PM-ER junction to regulate cellular mechanotransduction processes. Mutating this linker reduces this interaction between Piezo1 and SERCA2 and abolishes SERCA2-mediated inhibition of mechanosensitive currents (208). Piezo1 interactions with SERCA2 are thought to modulate Piezo1-induced Ca2+ entry in the context of stretch (208). Whether such effects are relevant for example to ASM cells is unknown. Eisenhoffer et al. found that Piezo 1 dependent Ca2+ influx appears to activate two opposing processes in epithelial cells dependent on where and how Piezo1 is activated. Piezo1 accumulates in the plasma membrane to activate epithelial cell division in regions with sparse epithelial cells, while Piezo1 localizes in cytoplasm in cell dense regions allowing cell extrusion to maintain cell number at a stable homeostatic level (209).
Piezo1 also appears to modulate SR Ca2+ release dynamics via IP3R2 at least in the cardiovascular system (210). The release dynamics downstream of Piezo1 are independent of the initial increase in SR lumen Ca2+ under sheer stress, but deletion of IP3R2 by siRNA reduces the rate constant of SR Ca2+ decay without affecting rate constant of SR Ca2+ increase (210). Thus, IP3R2 appears important for release of SR Ca2+ by activation of Piezo1 (210). The Piezo1 activator Yoda1 increases generation of cAMP, prevented by inhibition of soluble adenylate cyclase (210) and thus Piezo1 induced rapid mobilization of intracellular Ca2+ into the SR followed by SR Ca2+ release is thought to involve both sAC-cAMP and IP3R2 (210). However, it is important to note that while IP3R2 is expressed in the CV system, its expression and function in airways seems less clear. In fact, ASM is more likely to express IP3R1 and IP3R3 (176), but there is currently no information on the interactions between Piezo1 and these isoforms.
4.2. TRPs and Ca2+ regulatory proteins
In differentiated, normal bronchial epithelial cells which express TRPM4 and all isoforms of NCX, suppression of these proteins blunts MUC5AC and mucus secretion (211). In goblet cells, TRPM5 links to NCXRev, such that the NCX inhibitor KB-R9743 significantly reduces mucus secretion (212).
TRPC channels can also function in STIM1-dependent and STIM1-independent modes (213). Cav-1 helps to retain TRPC1 within STIM1 punctiform domains after storage depletion. This enables the interaction of TRPC1 with STIM1, facilitating TRPC1-mediated SOCE. At baseline, Cav-1 binding to the N-terminal region of TRPC1 keeps the channel in an inactive state (214). STIM1 replaces Cav-1 for binding to TRPC1 to activate this channel. Cav-1 re-binds to TRPC1 following refilling of SR Ca2+ stores (214, 215). Knockdown of Cav-1 results in dislocation of TRPC1, preventing STIM1 from gating the channel (214). Cav-1−/− mice exhibit disruption of TRPC1 localization in endothelial cells (216). Thus, Cav-1 acts as a scaffold for inactive TRPC1 and facilitates activation of TRPC1 by STIM1.
TRPC1 colocalizes and interacts with STIM1 after storage depletion, in contrast, refilling of SR-Ca2+ stores results in dissociation of STIM1 from TRPC1 and functional inactivation of TRPC1 (217, 218). These interactions involve aspartate residues in TRPC1 with polybasic domain of STIM1 (219). The ERM (ezrin/radixin/moesin) domain of STIM1 mediates the selective binding of STIM1 to TRPC1, 2 and 4, helping with the gating of TRPC1 (220). In human ASM, TRPC channels form complexes with STIM1 and Orai1, and this complex regulates Ca2+ influx (221). Orai1 also plays a key role in TRPC1 activation by store depletion. Orai1-mediated Ca2+ entry triggers recruitment of TRPC1 into the plasma membrane where it is activated by STIM1 (222, 223).
IP3R is not only a link between the plasma membrane and Ca2+ but also a sensor of the degree of filling the store. The Ca2+-binding site for IP3R is located in lumen (224). When Ca2+ dissociates from it, triggering exposure of a cytosolic signal-transfer domain, TRP-based Ca2+ entry channels are activated by IP3R (225, 226). 2-APB which inhibits SOCE also inhibits IP3Rs, SERCA, and TRP channels. TRPC1 links to the IP3 receptor in the context of regulating Ca2+ filling status of the SR (219). TRPP2 can strongly interact with IP3R by binding to positively charged amino acids in the N-terminal ligand-binding domain of IP3R, and increase local cytosolic Ca2+, enhancing smooth muscle contraction (227). In addition, TRPP2 would inhibit the binding of IP3 to IP3R due to a conformational change of N-terminal ligand-binding domain of IP3R. However, at higher dose of IP3, this inhibition will be overcome (228, 229). These data indicate that TRP proteins are integral parts of agonist and store depletion activated Ca2+ entry channels and that these channels are regulated directly by IP3Rs.
The interaction between TRP channels and SERCA in ASM is essential for regulating Ca2+ homeostasis and preventing excessive smooth muscle contraction (230). Sustained activation of TRP channels with excessive Ca2+ influx and smooth muscle hypercontractility contributes to airway hyperresponsiveness (231). Activation of TRPC7 and TRPC3 channels is blocked by the SERCA pump inhibitor thapsigargin (232) and thus prevents sustained TRP activation. Activation of TRPV4 channels in ASM can lead to an increase in Ca2+ uptake by SERCA, suggesting that SERCA plays a role in regulating the activation of TRP channels in this tissue (231, 233).
5. Expert opinion
Ca2+ signaling plays an important role in airway structure and function and in the airway hyperreactivity and remodeling characteristic of asthma. Here, beyond the many pathways that regulate Ca2+ in cells such as epithelium and ASM, there is increasing recognition that interactions with mechanosensitive pathways can modulate Ca2+ regulation and thus increase the complexity of how Ca2+ contributes to airway physiology. Thus, in the context of ultimately treating asthma, there remains much to understand regarding the interplay between mechanical forces and Ca2+ regulation. Here, an important aspect is to determine whether such interplay leads to enhancement of features of asthma such as AHR (in particular, given the importance of Ca2+ in contractility) or even remodeling in the long term, vs. any alleviating effects when Piezo or TRP channels are activated. Certainly Piezo and TRPs interact with a number of mechanisms that increase [Ca2+]i such as STIM1, STIM2, Orai1 and IP3Rs and in that sense there are multiple pathways via which mechanical stimulation can lead to increased [Ca2+]i and contractility of smooth muscle, and even have Ca2+ mediated stimulatory effects in other cell types such as epithelium (towards mucus production) and immune cells (towards inflammation). On the other hand, mechanosensitive channels can also interact with NCX and SERCA that could reduce [Ca2+]i under normal circumstances, although it is difficult to predict whether the channels would blunt these regulatory mechanisms towards increasing [Ca2+]i or whether they would enhance their function and thus reduce [Ca2+]i. Beyond Ca2+ and contractility, it is also increasingly apparent that mechanical forces can modulate epithelial barrier function, mucus production, ECM production, and airway remodeling, many of which may also be Ca2+ dependent. Responses to mechanical forces could also include production of inflammatory and growth factors, themselves Ca2+ dependent. Thus, understanding the role of mechanosensitive pathways such as Piezo and TRP channels becomes important, especially given emerging data in the cardiovascular system for crosstalk between these mechanisms and the Ca2+ regulatory pathways. Even here, there remain significant gaps in our knowledge of the interactive mechanisms at play, particularly for Piezo channels. This review summarized current understanding, albeit limited, of crosstalk between mechanosensitive channels and Ca2+ regulatory pathways in asthma, given the more direct links between this airway disease and Ca2+ in the context of AHR and even remodeling. However, it is likely that Piezo and TRP channels also play a role in other lung diseases such as COPD and pulmonary fibrosis (155, 156). Even here, the data are only emerging in that such mechanosensitive channels are expressed in other lung areas, and show altered responses to mechanical forces, and contribute in particular to fibrosis. However, what is not known is whether there are any interactions with Ca2+ regulatory pathways as we summarize here. Future studies will need to consider further investigation of mechanical forces in the airway and other parts of the lung, to better understanding how these pathways interface with Ca2+, contractility, and remodeling towards creating novel interventions for asthma and perhaps beyond (Figure 2).
Figure 2.
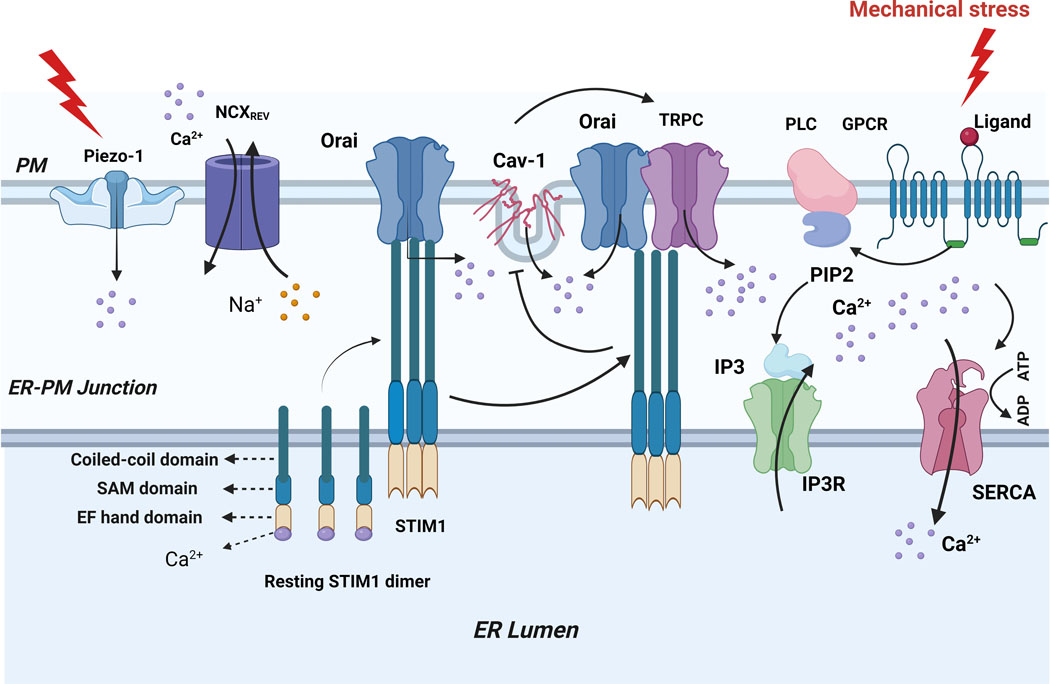
Crosstalk between mechanical and calcium regulatory channels in the airway. Mechanical overstretch induces Piezo1 channels to open, which causes endoplasmic reticulum (ER) pressure to facilitate Ca2+ release and ER store depletion, As stores deplete, Ca2+ dissociates from the luminal EF-hand domain of STIM1 proteins, which in turn causes STIM1 oligomerize and translocate to ER-plasma membrane junction and activate Ca2+ release- Ca2+ (CRAC) channels formed by Orai protein causing Ca2+ influx, this is also trigger the recruitment of TRP and activated by STIM1, TRP, STIM1 and Orail form complex to regulates Ca2+ influx, this complex suppress the bind of Cav-1 to TRP, but loss of effect after refill of ER-Ca2+ stores. In addition, mechanical overstretch stimulate G-protein coupled receptors (GPCR) associated with phospholipase-C(PLC) produces IP3. IP3 binds to IP3R to facilitate Ca2+ release and ER store depletion.
Article highlights.
In the context of diseases such as asthma, regulation of intracellular Ca2+ has several downstream effects towards airway contractility and even remodeling (proliferation and fibrosis).
There is increasing recognition for the role of mechanosensitive pathways in airway cell structure and function, with particular interest in Piezo and transient receptor protein (TRP) channels that are permeant to Ca2+.
A number of pathways regulate Ca2+ in airway cells, including Ca2+ release via IP3 receptors and reuptake via Ca2+ ATPase in the sarcoendoplasmic reticulum, and Ca2+ influx pathways that respond to depletion of Ca2+ stores, involving STIM proteins and Orai channels. Additional pathways such as sodium-calcium exchange and caveolins provide further modulation.
Piezo and TRP channels physically or functionally interact with Ca2+ regulatory pathways, typically enhancing their function.
Crosstalk between mechanosensitive Piezo or TRP channels and Ca2+ regulatory pathways in the lung in the context of asthma is only now being recognized and provides an opportunity to identify novel targets to address airway hyperreactivity and remodeling.
Funding
This paper was supported by NIH grants R01-HL142061 (CMP and YSP), R01-HL088029 and R01-HL056470 (YSP) and Foundation of Anesthesia Education and Research Mentored Research Training Grant (FAER MRTG; ERV), Science and Technology Program of Shaanxi Province (NO.2020SF-062; YY), 2023 Shaanxi University Youth Innovation Team (Prevention and Treatment of Acute Lung Injury) (79; Shengyu Wang), Science and Technology Department of Guizhou Province (ZK [2021]351; MZ).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter of materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.El-Husseini ZW, Vonk JM, van den Berge M, Gosens R, Koppelman GH. Association of asthma genetic variants with asthma-associated traits reveals molecular pathways of eosinophilic asthma. Clin Transl Allergy. 2023;13(4):e12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergantin LB. The Interplay Between Asthma and Other Diseases: Role of Ca2+/cAMP Signalling. Endocr Metab Immune Disord Drug Targets. 2020;20(3):321–7. [DOI] [PubMed] [Google Scholar]
- 3.Zeng R, Wang Z, Zhang J, Liang Z, Xu C, Wang J, et al. Type 1 diabetes and asthma: a systematic review and meta-analysis of observational studies. Endocrine. 2022;75(3):709–17. [DOI] [PubMed] [Google Scholar]
- 4.Cazzola M, Rogliani P, Ora J, Calzetta L, Matera MG. Asthma and comorbidities: recent advances. Pol Arch Intern Med. 2022;132(4). [DOI] [PubMed] [Google Scholar]
- 5.Gergen PJ. Adult-onset asthma and cancer: Causal or coincidental? J Allergy Clin Immunol. 2021;147(1):52–3. [DOI] [PubMed] [Google Scholar]
- 6.Woodrow JS, Sheats MK, Cooper B, Bayless R. Asthma: The Use of Animal Models and Their Translational Utility. Cells. 2023;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkar NA, Roos B, Prakash YS, Sathish V, Pabelick CM. Nicotinic alpha7 acetylcholine receptor (alpha7nAChR) in human airway smooth muscle. Arch Biochem Biophys. 2021;706:108897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalfaoui L, Mukhtasimova N, Kelley B, Wells N, Teske JJ, Roos BB, et al. Functional alpha7 nicotinic receptors in human airway smooth muscle increase intracellular calcium concentration and contractility in asthmatics. Am J Physiol Lung Cell Mol Physiol. 2023;325(1):L17–L29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borkar NA, Combs CK, Sathish V. Sex Steroids Effects on Asthma: A Network Perspective of Immune and Airway Cells. Cells. 2022;11(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkar NA, Sathish V. Sex Steroids and Their Influence in Lung Diseases Across the Lifespan. Silveyra P, Tigno XT, editor: Springer; 2021. [Google Scholar]
- 11.Perusquia M, Flores-Soto E, Sommer B, Campuzano-Gonzalez E, Martinez-Villa I, Martinez-Banderas AI, et al. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE 2 in guinea pig airway smooth muscle. Pflugers Arch. 2015;467(4):767–77. [DOI] [PubMed] [Google Scholar]
- 12.Bazan-Perkins B, Sanchez-Guerrero E, Carbajal V, Barajas-Lopez C, Montano LM. Sarcoplasmic reticulum Ca2+ depletion by caffeine and changes of [Ca2+](i) during refilling in bovine airway smooth muscle cells. Arch Med Res. 2000;31(6):558–63. [DOI] [PubMed] [Google Scholar]
- 13.Jairaman A, Maguire CH, Schleimer RP, Prakriya M. Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci Rep. 2016;6:32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genovese M, Borrelli A, Venturini A, Guidone D, Caci E, Viscido G, et al. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. J Physiol. 2019;597(24):5859–78. [DOI] [PubMed] [Google Scholar]
- 15.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39(4–5):173–85. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MT, Xin P, Benson JC, Pathak T, Walter V, Emrich SM, et al. STIM1 is a core trigger of airway smooth muscle remodeling and hyperresponsiveness in asthma. Proc Natl Acad Sci U S A. 2022;119(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicencio JM, Lavandero S, Szabadkai G. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 2010;47(2):112–21. [DOI] [PubMed] [Google Scholar]
- 18.Eisner DA, Caldwell JL, Kistamas K, Trafford AW. Calcium and Excitation-Contraction Coupling in the Heart. Circ Res. 2017;121(2):181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23(1):10–4. [DOI] [PubMed] [Google Scholar]
- 20.Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267(20):14483–9. [PubMed] [Google Scholar]
- 21.Thakore P, Earley S. STIM1 is the key that unlocks airway smooth muscle remodeling and hyperresponsiveness during asthma. Cell Calcium. 2022;104:102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Jin Z, Yang X, Lou J, Shan W, Hu Y, et al. Plasma membrane Ca(2+)-permeable channels and sodium/calcium exchangers in tumorigenesis and tumor development of the upper gastrointestinal tract. Cancer Lett. 2020;475:14–21. [DOI] [PubMed] [Google Scholar]
- 23.Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S90–4. [DOI] [PubMed] [Google Scholar]
- 24.Asano S, Ito S, Morosawa M, Furuya K, Naruse K, Sokabe M, et al. Cyclic stretch enhances reorientation and differentiation of 3-D culture model of human airway smooth muscle. Biochem Biophys Rep. 2018;16:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(6 Suppl):320S–6S. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Zhou XD, Xu R, Du XZ, Li Q, Li B, et al. The Degradation of Airway Epithelial Tight Junctions in Asthma Under High Airway Pressure Is Probably Mediated by Piezo-1. Front Physiol. 2021;12:637790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao A, Gao W, Sawada T, Yoshimoto RU, Aijima R, Ohsaki Y, et al. Transient Receptor Potential Channel Vanilloid 1 Contributes to Facial Mechanical Hypersensitivity in a Mouse Model of Atopic Asthma. Lab Invest. 2023;103(6):100149. [DOI] [PubMed] [Google Scholar]
- 28.Reyes-Garcia J, Carbajal-Garcia A, Montano LM. Transient receptor potential cation channel subfamily V (TRPV) and its importance in asthma. Eur J Pharmacol. 2022;915:174692. [DOI] [PubMed] [Google Scholar]
- 29.Li N, He Y, Yang G, Yu Q, Li M. Role of TRPC1 channels in pressure-mediated activation of airway remodeling. Respir Res. 2019;20(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mitochondria in lung diseases. Expert Rev Respir Med. 2013;7(6):631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aravamudan B, VanOosten SK, Meuchel LW, Vohra P, Thompson M, Sieck GC, et al. Caveolin-1 knockout mice exhibit airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiarella SE, Cardet JC, Prakash YS. Sex, Cells, and Asthma. Mayo Clin Proc. 2021;96(7):1955–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kistemaker LEM, Prakash YS. Airway Innervation and Plasticity in Asthma. Physiology (Bethesda). 2019;34(4):283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer CA, Roos B, Teske J, Wells N, Martin RJ, Chang W, et al. Calcium-sensing receptor and CPAP-induced neonatal airway hyperreactivity in mice. Pediatr Res. 2022;91(6):1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol (1985). 2001;91(1):488–96. [DOI] [PubMed] [Google Scholar]
- 36.Prakash YS. Asthma without borders. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L1001–L3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305(12):L912–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prakash YS. Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol. 2016;311(6):L1113–L40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesler AM, Wicher SA, Ravix J, Britt RD Jr., Manlove L, Teske JJ, et al. Calcium sensing receptor in developing human airway smooth muscle. J Cell Physiol. 2019;234(8):14187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson MA, Prakash YS, Pabelick CM. The role of caveolae in the pathophysiology of lung diseases. Expert Rev Respir Med. 2014;8(1):111–22. [DOI] [PubMed] [Google Scholar]
- 41.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev. 2010;90(1):113–78. [DOI] [PubMed] [Google Scholar]
- 42.Rossi D, Barone V, Giacomello E, Cusimano V, Sorrentino V. The sarcoplasmic reticulum: an organized patchwork of specialized domains. Traffic. 2008;9(7):1044–9. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Wang Z, Liu Y, Zhao L, Fu W. Stromal Interaction Molecule 1 (STIM1) is a Potential Prognostic Biomarker and Correlates with Immune Infiltrates in Solid Tumors. J Environ Pathol Toxicol Oncol. 2023;42(2):11–30. [DOI] [PubMed] [Google Scholar]
- 44.Horvath F, Berlansky S, Maltan L, Grabmayr H, Fahrner M, Derler I, et al. Swing-out opening of stromal interaction molecule 1. Protein Sci. 2023;32(3):e4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacheco J, Sampieri A, Vaca L. STIM1: The lord of the rings? Cell Calcium. 2023;112:102742. [DOI] [PubMed] [Google Scholar]
- 46.Novello MJ, Zhu J, Feng Q, Ikura M, Stathopulos PB. Structural elements of stromal interaction molecule function. Cell Calcium. 2018;73:88–94. [DOI] [PubMed] [Google Scholar]
- 47.Gudlur A, Zeraik AE, Hirve N, Rajanikanth V, Bobkov AA, Ma G, et al. Calcium sensing by the STIM1 ER-luminal domain. Nat Commun. 2018;9(1):4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng L, Stathopulos PB, Schindl R, Li GY, Romanin C, Ikura M. Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci U S A. 2011;108(4):1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins SR, Meyer T. Evolutionary origins of STIM1 and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol. 2011;21(4):202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spinelli AM, Gonzalez-Cobos JC, Zhang X, Motiani RK, Rowan S, Zhang W, et al. Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration. Pflugers Arch. 2012;464(5):481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca(2)(+) entry in airway smooth muscle cell proliferation. J Appl Physiol (1985). 2011;110(5):1256–63. [DOI] [PubMed] [Google Scholar]
- 52.Huang JH, Gao HW, Gao DD, Yang WY, Zhao MK, Shen B, et al. Exercise Reduces Airway Smooth Muscle Contraction in Asthmatic Rats via Inhibition of IL-4 Secretion and Store-Operated Ca(2+) Entry Pathway. Allergy Asthma Immunol Res. 2023;15(3):361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125(6):535–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc. 2008;5(1):23–31. [DOI] [PubMed] [Google Scholar]
- 55.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274(6):C1653–60. [DOI] [PubMed] [Google Scholar]
- 56.Zeng Z, Cheng M, Li M, Wang T, Wen F, Sanderson MJ, et al. Inherent differences of small airway contraction and Ca(2+) oscillations in airway smooth muscle cells between BALB/c and C57BL/6 mouse strains. Front Cell Dev Biol. 2023;11:1202573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalidhindi RSR, Katragadda R, Beauchamp KL, Pabelick CM, Prakash YS, Sathish V. Androgen Receptor-Mediated Regulation of Intracellular Calcium in Human Airway Smooth Muscle Cells. Cell Physiol Biochem. 2019;53(1):215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231(1):210–24. [DOI] [PubMed] [Google Scholar]
- 59.Deng F, Yu C, Zhong S, Liang Z, Lin C, Zou F, et al. Store-operated calcium entry enhances the polarization and chemotaxis of neutrophils in the peripheral venous blood of patients with bronchial asthma by upregulating ERM protein. J Thorac Dis. 2023;15(4):2051–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131(7):1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boeck A, Landgraf-Rauf K, Vogelsang V, Siemens D, Prazeres da Costa O, Klucker E, et al. Ca(2+) and innate immune pathways are activated and differentially expressed in childhood asthma phenotypes. Pediatr Allergy Immunol. 2018;29(8):823–33. [DOI] [PubMed] [Google Scholar]
- 62.Berra-Romani R, Vargaz-Guadarrama A, Sanchez-Gomez J, Coyotl-Santiago N, Hernandez-Arambide E, Avelino-Cruz JE, et al. Histamine activates an intracellular Ca(2+) signal in normal human lung fibroblast WI-38 cells. Front Cell Dev Biol. 2022;10:991659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoast RE, Emrich SM, Zhang X, Xin P, Johnson MT, Fike AJ, et al. The native ORAI channel trio underlies the diversity of Ca(2+) signaling events. Nat Commun. 2020;11(1):2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spinelli AM, Trebak M. Orai channel-mediated Ca2+ signals in vascular and airway smooth muscle. Am J Physiol Cell Physiol. 2016;310(6):C402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dwivedi R, Drumm BT, Griffin CS, Dudem S, Bradley E, Alkawadri T, et al. Excitatory cholinergic responses in mouse primary bronchial smooth muscle require both Ca(2+) entry via l-type Ca(2+) channels and store operated Ca(2+) entry via Orai channels. Cell Calcium. 2023;112:102721. [DOI] [PubMed] [Google Scholar]
- 66.Sutovska M, Kocmalova M, Franova S, Vakkalanka S, Viswanadha S. Pharmacodynamic evaluation of RP3128, a novel and potent CRAC channel inhibitor in guinea pig models of allergic asthma. Eur J Pharmacol. 2016;772:62–70. [DOI] [PubMed] [Google Scholar]
- 67.Sutovska M, Kocmalova M, Joskova M, Adamkov M, Franova S. The effect of long-term administered CRAC channels blocker on the functions of respiratory epithelium in guinea pig allergic asthma model. Gen Physiol Biophys. 2015;34(2):167–76. [DOI] [PubMed] [Google Scholar]
- 68.Samanta K, Bakowski D, Parekh AB. Key role for store-operated Ca2+ channels in activating gene expression in human airway bronchial epithelial cells. PLoS One. 2014;9(8):e105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esteve C, Gonzalez J, Gual S, Vidal L, Alzina S, Sentellas S, et al. Discovery of 7-azaindole derivatives as potent Orai inhibitors showing efficacy in a preclinical model of asthma. Bioorg Med Chem Lett. 2015;25(6):1217–22. [DOI] [PubMed] [Google Scholar]
- 70.Komlosi ZI, van de Veen W, Kovacs N, Szucs G, Sokolowska M, O’Mahony L, et al. Cellular and molecular mechanisms of allergic asthma. Mol Aspects Med. 2022;85:100995. [DOI] [PubMed] [Google Scholar]
- 71.Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165(1):297–305. [DOI] [PubMed] [Google Scholar]
- 72.Wang YH, Noyer L, Kahlfuss S, Raphael D, Tao AY, Kaufmann U, et al. Distinct roles of ORAI1 in T cell-mediated allergic airway inflammation and immunity to influenza A virus infection. Sci Adv. 2022;8(40):eabn6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wrennall JA, Ahmad S, Worthington EN, Wu T, Goriounova AS, Voeller AS, et al. A SPLUNC1 Peptidomimetic Inhibits Orai1 and Reduces Inflammation in a Murine Allergic Asthma Model. Am J Respir Cell Mol Biol. 2022;66(3):271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thaikoottathil JV, Martin RJ, Di PY, Minor M, Case S, Zhang B, et al. SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice. Am J Respir Cell Mol Biol. 2012;47(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T, Huang J, Moore PJ, Little MS, Walton WG, Fellner RC, et al. Identification of BPIFA1/SPLUNC1 as an epithelium-derived smooth muscle relaxing factor. Nat Commun. 2017;8:14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashmole I, Duffy SM, Leyland ML, Bradding P. The contribution of Orai(CRACM)1 and Orai(CRACM)2 channels in store-operated Ca2+ entry and mediator release in human lung mast cells. PLoS One. 2013;8(9):e74895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashmole I, Duffy SM, Leyland ML, Morrison VS, Begg M, Bradding P. CRACM/Orai ion channel expression and function in human lung mast cells. J Allergy Clin Immunol. 2012;129(6):1628–35 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang LL, Wan QQ, Wang YM, He SJ, Xu WJ, Ding M, et al. IL-13 Regulates Orai1 Expression in Human Bronchial Smooth Muscle Cells and Airway Remodeling in Asthma Mice Model via LncRNA H19. J Asthma Allergy. 2022;15:1245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demydenko K, Ekhteraei-Tousi S, Roderick HL. Inositol 1,4,5-trisphosphate receptors in cardiomyocyte physiology and disease. Philos Trans R Soc Lond B Biol Sci. 2022;377(1864):20210319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW. IP(3) receptors and Ca(2+) entry. Biochim Biophys Acta Mol Cell Res. 2019;1866(7):1092–100. [DOI] [PubMed] [Google Scholar]
- 81.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351(6329):751–4. [DOI] [PubMed] [Google Scholar]
- 82.Huang AS, Tong BC, Hung HC, Wu AJ, Ho OK, Kong AH, et al. Targeting calcium signaling by inositol trisphosphate receptors: A novel mechanism for the anti-asthmatic effects of Houttuynia cordata. Biomed Pharmacother. 2023;164:114935. [DOI] [PubMed] [Google Scholar]
- 83.Zhao C, Wu AY, Yu X, Gu Y, Lu Y, Song X, et al. Microdomain elements of airway smooth muscle in calcium regulation and cell proliferation. J Physiol Pharmacol. 2018;69(2). [DOI] [PubMed] [Google Scholar]
- 84.An TJ, Kim JH, Hur J, Park CK, Lim JU, Kim S, et al. Tiotropium Bromide Improves Neutrophilic Asthma by Recovering Histone Deacetylase 2 Activity. J Korean Med Sci. 2023;38(12):e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007(74):9–22. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto H, Hirata Y, Otsuka K, Iwata T, Inazumi A, Niimi A, et al. Interleukin-13 enhanced Ca2+ oscillations in airway smooth muscle cells. Cytokine. 2012;57(1):19–24. [DOI] [PubMed] [Google Scholar]
- 87.Tao FC, Tolloczko B, Mitchell CA, Powell WS, Martin JG. Inositol (1,4,5)trisphosphate metabolism and enhanced calcium mobilization in airway smooth muscle of hyperresponsive rats. Am J Respir Cell Mol Biol. 2000;23(4):514–20. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Zhang Y, Li Q, Zhuang Q, Zhu X, Pan L, et al. An improved method for guinea pig airway smooth muscle cell culture and the effect of SPFF on intracellular calcium. Mol Med Rep. 2014;10(3):1309–14. [DOI] [PubMed] [Google Scholar]
- 89.Ye L, Zeng Q, Ling M, Ma R, Chen H, Lin F, et al. Inhibition of IP3R/Ca2+ Dysregulation Protects Mice From Ventilator-Induced Lung Injury via Endoplasmic Reticulum and Mitochondrial Pathways. Front Immunol. 2021;12:729094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosa N, Shabardina V, Ivanova H, Sebe-Pedros A, Yule DI, Bultynck G. Tracing the evolutionary history of Ca(2+)-signaling modulation by human Bcl-2: Insights from the Capsaspora owczarzaki IP(3) receptor ortholog. Biochim Biophys Acta Mol Cell Res. 2021;1868(12):119121. [DOI] [PubMed] [Google Scholar]
- 91.Ivanova H, Vervliet T, Monaco G, Terry LE, Rosa N, Baker MR, et al. Bcl-2-Protein Family as Modulators of IP(3) Receptors and Other Organellar Ca(2+) Channels. Cold Spring Harb Perspect Biol. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7(10):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosa N, Ivanova H, Wagner LE 2nd, Kale J, La Rovere R, Welkenhuyzen K, et al. Bcl-xL acts as an inhibitor of IP(3)R channels, thereby antagonizing Ca(2+)-driven apoptosis. Cell Death Differ. 2022;29(4):788–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang T, Wang M, Xiao H, Wei X. Mitochondrial dysfunction and chronic lung disease. Cell Biol Toxicol. 2019;35(6):493–502. [DOI] [PubMed] [Google Scholar]
- 95.Sachdeva K, Do DC, Zhang Y, Hu X, Chen J, Gao P. Environmental Exposures and Asthma Development: Autophagy, Mitophagy, and Cellular Senescence. Front Immunol. 2019;10:2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boyman L, Karbowski M, Lederer WJ. Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends Mol Med. 2020;26(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qian L, Mehrabi Nasab E, Athari SM, Athari SS. Mitochondria signaling pathways in allergic asthma. J Investig Med. 2022;70(4):863–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chellappan DK, Paudel KR, Tan NW, Cheong KS, Khoo SSQ, Seow SM, et al. Targeting the mitochondria in chronic respiratory diseases. Mitochondrion. 2022;67:15–37. [DOI] [PubMed] [Google Scholar]
- 99.Tagashira H, Bhuiyan MS, Shioda N, Fukunaga K. Fluvoxamine rescues mitochondrial Ca2+ transport and ATP production through sigma(1)-receptor in hypertrophic cardiomyocytes. Life Sci. 2014;95(2):89–100. [DOI] [PubMed] [Google Scholar]
- 100.Diaz-Vegas AR, Cordova A, Valladares D, Llanos P, Hidalgo C, Gherardi G, et al. Mitochondrial Calcium Increase Induced by RyR1 and IP3R Channel Activation After Membrane Depolarization Regulates Skeletal Muscle Metabolism. Front Physiol. 2018;9:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Decuypere JP, Monaco G, Missiaen L, De Smedt H, Parys JB, Bultynck G. IP(3) Receptors, Mitochondria, and Ca Signaling: Implications for Aging. J Aging Res. 2011;2011:920178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.NavaneethaKrishnan S, Law V, Lee J, Rosales JL, Lee KY. Cdk5 regulates IP3R1-mediated Ca(2+) dynamics and Ca(2+)-mediated cell proliferation. Cell Mol Life Sci. 2022;79(9):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–78. [DOI] [PubMed] [Google Scholar]
- 104.Alvarez-Santos MD, Alvarez-Gonzalez M, Eslava-De-Jesus E, Gonzalez-Lopez A, Pacheco-Alba I, Perez-Del-Valle Y, et al. Role of airway smooth muscle cell phenotypes in airway tone and obstruction in guinea pig asthma model. Allergy Asthma Clin Immunol. 2022;18(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax. 2010;65(6):547–52. [DOI] [PubMed] [Google Scholar]
- 106.Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004;322(4):1192–203. [DOI] [PubMed] [Google Scholar]
- 107.Hovnanian A. SERCA pumps and human diseases. Subcell Biochem. 2007;45:337–63. [DOI] [PubMed] [Google Scholar]
- 108.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci U S A. 2009;106(26):10775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rieg AD, Suleiman S, Anker C, Bunting NA, Verjans E, Spillner J, et al. Platelet-derived growth factor (PDGF)-BB regulates the airway tone via activation of MAP2K, thromboxane, actin polymerisation and Ca(2+)-sensitisation. Respir Res. 2022;23(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012;16(4):812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009;41(5):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abcejo AJ, Sathish V, Smelter DF, Aravamudan B, Thompson MA, Hartman WR, et al. Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One. 2012;7(8):e44343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Selno ATH, Sumbayev VV, Gibbs BF. IgE-dependent human basophil responses are inversely associated with the sarcoplasmic reticulum Ca(2+)-ATPase (SERCA). Front Immunol. 2022;13:1052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qaisar R, Qayum M, Muhammad T. Reduced sarcoplasmic reticulum Ca(2+) ATPase activity underlies skeletal muscle wasting in asthma. Life Sci. 2021;273:119296. [DOI] [PubMed] [Google Scholar]
- 116.Kruglikov IL, Scherer PE. Caveolin as a Universal Target in Dermatology. Int J Mol Sci. 2019;21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wicher SA, Prakash YS, Pabelick CM. Caveolae, caveolin-1 and lung diseases of aging. Expert Rev Respir Med. 2019;13(3):291–300. [DOI] [PubMed] [Google Scholar]
- 118.Llano M, Kelly T, Vanegas M, Peretz M, Peterson TE, Simari RD, et al. Blockade of human immunodeficiency virus type 1 expression by caveolin-1. J Virol. 2002;76(18):9152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca(2+) influx in human airway smooth muscle. Eur Respir J. 2012;40(2):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gosens R, Stelmack GL, Dueck G, Mutawe MM, Hinton M, McNeill KD, et al. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1406–18. [DOI] [PubMed] [Google Scholar]
- 121.Sathish V, Abcejo AJ, VanOosten SK, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alvarez-Santos M, Ramos-Ramirez P, Gutierrez-Aguilar F, Sanchez-Hernandez S, Lascurain R, Olmos-Zuniga R, et al. Antigen-induced airway hyperresponsiveness and obstruction is related to caveolin-1 expression in airway smooth muscle in a guinea pig asthma model. Clin Transl Allergy. 2015;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen CM, Wu MY, Chou HC, Lang YD, Wang LF. Downregulation of caveolin-1 in a murine model of acute allergic airway disease. Pediatr Neonatol. 2011;52(1):5–10. [DOI] [PubMed] [Google Scholar]
- 124.Bains SN, Tourkina E, Atkinson C, Joseph K, Tholanikunnel B, Chu HW, et al. Loss of caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67(12):1601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vogel ER, Britt RD Jr., Faksh A, Kuipers I, Pandya H, Prakash YS, et al. Moderate hyperoxia induces extracellular matrix remodeling by human fetal airway smooth muscle cells. Pediatr Res. 2017;81(2):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maspero J, Adir Y, Al-Ahmad M, Celis-Preciado CA, Colodenco FD, Giavina-Bianchi P, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scott G, Asrat S, Allinne J, Keat Lim W, Nagashima K, Birchard D, et al. IL-4 and IL-13, not eosinophils, drive type 2 airway inflammation, remodeling and lung function decline. Cytokine. 2023;162:156091. [DOI] [PubMed] [Google Scholar]
- 128.Fang P, Shi HY, Wu XM, Zhang YH, Zhong YJ, Deng WJ, et al. Targeted inhibition of GATA-6 attenuates airway inflammation and remodeling by regulating caveolin-1 through TLR2/MyD88/NF-kappaB in murine model of asthma. Mol Immunol. 2016;75:144–50. [DOI] [PubMed] [Google Scholar]
- 129.Xia Y, Cai PC, Yu F, Xiong L, He XL, Rao SS, et al. IL-4-induced caveolin-1-containing lipid rafts aggregation contributes to MUC5AC synthesis in bronchial epithelial cells. Respir Res. 2017;18(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gabehart KE, Royce SG, Maselli DJ, Miyasato SK, Davis EC, Tang ML, et al. Airway hyperresponsiveness is associated with airway remodeling but not inflammation in aging Cav1−/− mice. Respir Res. 2013;14(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hackett TL, de Bruin HG, Shaheen F, van den Berge M, van Oosterhout AJ, Postma DS, et al. Caveolin-1 controls airway epithelial barrier function. Implications for asthma. Am J Respir Cell Mol Biol. 2013;49(4):662–71. [DOI] [PubMed] [Google Scholar]
- 132.Le Saux CJ, Teeters K, Miyasato SK, Hoffmann PR, Bollt O, Douet V, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283(9):5760–8. [DOI] [PubMed] [Google Scholar]
- 133.Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium. 2007;42(4–5):467–76. [DOI] [PubMed] [Google Scholar]
- 134.DiPolo R, Beauge L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Rev. 2006;86(1):155–203. [DOI] [PubMed] [Google Scholar]
- 135.Algara-Suarez P, Romero-Mendez C, Chrones T, Sanchez-Armass S, Meza U, Sims SM, et al. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L191–8. [DOI] [PubMed] [Google Scholar]
- 136.Li M, Shang YX. Inhaled corticosteroids inhibit substance P receptor expression in asthmatic rat airway smooth muscle cells. BMC Pulm Med. 2012;12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rahman M, Inman M, Kiss L, Janssen LJ. Reverse-mode NCX current in mouse airway smooth muscle: Na(+) and voltage dependence, contributions to Ca(2+) influx and contraction, and altered expression in a model of allergen-induced hyperresponsiveness. Acta Physiol (Oxf). 2012;205(2):279–91. [DOI] [PubMed] [Google Scholar]
- 138.Li M, Shang YX. Neurokinin-1 receptor antagonist decreases [Ca(2+)]i in airway smooth muscle cells by reducing the reverse-mode Na(+)/Ca(2+) exchanger current. Peptides. 2019;115:69–74. [DOI] [PubMed] [Google Scholar]
- 139.Sathish V, Delmotte PF, Thompson MA, Pabelick CM, Sieck GC, Prakash YS. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS One. 2011;6(8):e23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johnson MT, Benson JC, Pathak T, Xin P, McKernan AS, Emrich SM, et al. The airway smooth muscle sodium/calcium exchanger NCLX is critical for airway remodeling and hyperresponsiveness in asthma. J Biol Chem. 2022;298(8):102259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S. Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol. 2021;22(1):22–38. [DOI] [PubMed] [Google Scholar]
- 142. Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. (**-of considerable interest-demonstrated mechanosensitivity of Piezo1 via force)
- 143.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taberner FJ, Prato V, Schaefer I, Schrenk-Siemens K, Heppenstall PA, Lechner SG. Structure-guided examination of the mechanogating mechanism of PIEZO2. Proc Natl Acad Sci U S A. 2019;116(28):14260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu J, Lewis AH, Grandl J. Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels. Trends Biochem Sci. 2017;42(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fang Z, Yi F, Peng Y, Zhang JJ, Zhang L, Deng Z, et al. Inhibition of TRPA1 reduces airway inflammation and hyperresponsiveness in mice with allergic rhinitis. FASEB J. 2021;35(5):e21428. [DOI] [PubMed] [Google Scholar]
- 147.Zhang EY, Bartman CM, Prakash YS, Pabelick CM, Vogel ER. Oxygen and mechanical stretch in the developing lung: risk factors for neonatal and pediatric lung disease. Front Med (Lausanne). 2023;10:1214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song S, Zhang H, Wang X, Chen W, Cao W, Zhang Z, et al. The role of mechanosensitive Piezo1 channel in diseases. Prog Biophys Mol Biol. 2022;172:39–49. [DOI] [PubMed] [Google Scholar]
- 149.Chakraborty M, Chu K, Shrestha A, Revelo XS, Zhang X, Gold MJ, et al. Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell Rep. 2021;34(2):108609. [DOI] [PubMed] [Google Scholar]
- 150.Atcha H, Jairaman A, Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat Commun. 2021;12(1):3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gaub BM, Muller DJ. Mechanical Stimulation of Piezo1 Receptors Depends on Extracellular Matrix Proteins and Directionality of Force. Nano Lett. 2017;17(3):2064–72. [DOI] [PubMed] [Google Scholar]
- 152.Friedrich EE, Hong Z, Xiong S, Zhong M, Di A, Rehman J, et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc Natl Acad Sci U S A. 2019;116(26):12980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo M, Ni K, Gu R, Qin Y, Guo J, Che B, et al. Chemical Activation of Piezo1 Alters Biomechanical Behaviors toward Relaxation of Cultured Airway Smooth Muscle Cells. Biol Pharm Bull. 2023;46(1):1–11. [DOI] [PubMed] [Google Scholar]
- 154.Diem K, Fauler M, Fois G, Hellmann A, Winokurow N, Schumacher S, et al. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020;34(9):12785–804. [DOI] [PubMed] [Google Scholar]
- 155.Kelley B, Zhang EY, Khalfaoui L, Schiliro M, Wells N, Pabelick CM, et al. Piezo channels in stretch effects on developing human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Migulina N, Kelley B, Zhang EY, Pabelick CM, Prakash YS, Vogel ER. Mechanosensitive Channels in Lung Health and Disease. Compr Physiol. 2023;13(4):5157–78. [DOI] [PubMed] [Google Scholar]
- 157.Chesler AT, Szczot M. Portraits of a pressure sensor. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nickolls AR, Lee MM, Espinoza DF, Szczot M, Lam RM, Wang Q, et al. Transcriptional Programming of Human Mechanosensory Neuron Subtypes from Pluripotent Stem Cells. Cell Rep. 2020;30(3):932–46 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romero LO, Caires R, Nickolls AR, Chesler AT, Cordero-Morales JF, Vasquez V. A dietary fatty acid counteracts neuronal mechanical sensitization. Nat Commun. 2020;11(1):2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hambright P EK, Rau K, Chappell J, Binks AP, LeClair R. Determining the Presence and Expression of Piezo2 in Human Lung Tissue Across Various Pathologies. American Thoracic Society 2020. p. A5538. [Google Scholar]
- 161.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120(6):1233–44; quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 162.Zhong T, Zhang W, Guo H, Pan X, Chen X, He Q, et al. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin B. 2022;12(4):1761–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66(3):676–814. [DOI] [PubMed] [Google Scholar]
- 164.Staaf S, Franck MC, Marmigere F, Mattsson JP, Ernfors P. Dynamic expression of the TRPM subgroup of ion channels in developing mouse sensory neurons. Gene Expr Patterns. 2010;10(1):65–74. [DOI] [PubMed] [Google Scholar]
- 165.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105(10):1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang H, Cheng X, Tian J, Xiao Y, Tian T, Xu F, et al. TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharmacol Ther. 2020;209:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29(3):645–55. [DOI] [PubMed] [Google Scholar]
- 168.Chen J, Barritt GJ. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca(2+)-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem J. 2003;373(Pt 2):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–85. [DOI] [PubMed] [Google Scholar]
- 170.Shen B, Wong CO, Lau OC, Woo T, Bai S, Huang Y, et al. Plasma membrane mechanical stress activates TRPC5 channels. PLoS One. 2015;10(4):e0122227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol. 2008;586(23):5633–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Timmermans JP, Adriaensen D. Neuroepithelial bodies as mechanotransducers in the intrapulmonary airway epithelium: involvement of TRPC5. Am J Respir Cell Mol Biol. 2012;47(3):315–23. [DOI] [PubMed] [Google Scholar]
- 173.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90(3):248–50. [DOI] [PubMed] [Google Scholar]
- 174.Xiao JH, Zheng YM, Liao B, Wang YX. Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;43(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Flores-Soto E, Reyes-Garcia J, Carbajal-Garcia A, Campuzano-Gonzalez E, Perusquia M, Sommer B, et al. Sex steroids effects on guinea pig airway smooth muscle tone and intracellular Ca(2+) basal levels. Mol Cell Endocrinol. 2017;439:444–56. [DOI] [PubMed] [Google Scholar]
- 176.Song T, Hao Q, Zheng YM, Liu QH, Wang YX. Inositol 1,4,5-trisphosphate activates TRPC3 channels to cause extracellular Ca2+ influx in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2015;309(12):L1455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reyes-Garcia J, Flores-Soto E, Carbajal-Garcia A, Sommer B, Montano LM. Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review). Int J Mol Med. 2018;42(6):2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Himmel NJ, Cox DN. Transient receptor potential channels: current perspectives on evolution, structure, function and nomenclature. Proc Biol Sci. 2020;287(1933):20201309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bonvini SJ, Birrell MA, Dubuis E, Adcock JJ, Wortley MA, Flajolet P, et al. Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur Respir J. 2020;56(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cai X, Yang YC, Wang JF, Wang Q, Gao J, Fu WL, et al. Transient receptor potential vanilloid 2 (TRPV2), a potential novel biomarker in childhood asthma. J Asthma. 2013;50(2):209–14. [DOI] [PubMed] [Google Scholar]
- 181.Li J, Chen Y, Chen QY, Liu D, Xu L, Cheng G, et al. Role of transient receptor potential cation channel subfamily V member 1 (TRPV1) on ozone-exacerbated allergic asthma in mice. Environ Pollut. 2019;247:586–94. [DOI] [PubMed] [Google Scholar]
- 182.Samivel R, Kim DW, Son HR, Rhee YH, Kim EH, Kim JH, et al. The role of TRPV1 in the CD4+ T cell-mediated inflammatory response of allergic rhinitis. Oncotarget. 2016;7(1):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li C, Zhang H, Wei L, Liu Q, Xie M, Weng J, et al. Role of TRPA1/TRPV1 in acute ozone exposure induced murine model of airway inflammation and bronchial hyperresponsiveness. J Thorac Dis. 2022;14(7):2698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang J, Yu HM, Zhou XD, Kolosov VP, Perelman JM. Study on TRPV1-mediated mechanism for the hypersecretion of mucus in respiratory inflammation. Mol Immunol. 2013;53(1–2):161–71. [DOI] [PubMed] [Google Scholar]
- 185.Millqvist E. TRPV1 and TRPM8 in Treatment of Chronic Cough. Pharmaceuticals (Basel). 2016;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Choi JY, Lee HY, Hur J, Kim KH, Kang JY, Rhee CK, et al. TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model. Allergy Asthma Immunol Res. 2018;10(3):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rousseau E, Cloutier M, Morin C, Proteau S. Capsazepine, a vanilloid antagonist, abolishes tonic responses induced by 20-HETE on guinea pig airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L460–70. [DOI] [PubMed] [Google Scholar]
- 188.Delescluse I, Mace H, Adcock JJ. Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanaesthetized, ovalbumin-sensitized guinea pig. Br J Pharmacol. 2012;166(6):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772(8):915–27. [DOI] [PubMed] [Google Scholar]
- 190.Balestrini A, Joseph V, Dourado M, Reese RM, Shields SD, Rouge L, et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J Exp Med. 2021;218(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song J, Kang J, Lin B, Li J, Zhu Y, Du J, et al. Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci Rep. 2017;7(1):11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen CL, Li H, Xing XH, Guan HS, Zhang JH, Zhao JW. Effect of TRPV1 gene mutation on bronchial asthma in children before and after treatment. Allergy Asthma Proc. 2015;36(2):e29–36. [DOI] [PubMed] [Google Scholar]
- 194.Xu J, Yang Y, Hou Z, Jia H, Wang Y. TRPV2-spike protein interaction mediates the entry of SARS-CoV-2 into macrophages in febrile conditions. Theranostics. 2021;11(15):7379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang JJ, Li MW, Fan XS, Zhu Y. [Effect of San’ao Decoction on ovalbum induced asthmatic mice and expression of TRPV2 in lung]. Zhongguo Zhong Yao Za Zhi. 2020;45(11):2619–25. [DOI] [PubMed] [Google Scholar]
- 196.Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, et al. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L272–8. [DOI] [PubMed] [Google Scholar]
- 197.Zhao L, Sullivan MN, Chase M, Gonzales AL, Earley S. Calcineurin/nuclear factor of activated T cells-coupled vanilliod transient receptor potential channel 4 ca2+ sparklets stimulate airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2014;50(6):1064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yao L, Chen S, Tang H, Huang P, Wei S, Liang Z, et al. Transient Receptor Potential Ion Channels Mediate Adherens Junctions Dysfunction in a Toluene Diisocyanate-Induced Murine Asthma Model. Toxicol Sci. 2019;168(1):160–70. [DOI] [PubMed] [Google Scholar]
- 199.Gombedza F, Kondeti V, Al-Azzam N, Koppes S, Duah E, Patil P, et al. Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J. 2017;31(4):1556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang J, Wei Y, Bai S, Ding S, Gao H, Yin S, et al. TRPV4 Complexes With the Na(+)/Ca(2+) Exchanger and IP(3) Receptor 1 to Regulate Local Intracellular Calcium and Tracheal Tension in Mice. Front Physiol. 2019;10:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aggarwal B, Mulgirigama A, Berend N . Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med. 2018;28(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Al-Azzam N, Teegala LR, Pokhrel S, Ghebreigziabher S, Chachkovskyy T, Thodeti S, et al. Transient Receptor Potential Vanilloid channel regulates fibroblast differentiation and airway remodeling by modulating redox signals through NADPH Oxidase 4. Sci Rep. 2020;10(1):9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu H, Liu Q, Hua L, Pan J. Inhibition of transient receptor potential melastatin 8 alleviates airway inflammation and remodeling in a murine model of asthma with cold air stimulus. Acta Biochim Biophys Sin (Shanghai). 2018;50(5):499–506. [DOI] [PubMed] [Google Scholar]
- 205.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. [DOI] [PubMed] [Google Scholar]
- 206.Zhang L, An X, Wang Q, He M. Activation of Cold-Sensitive Channels TRPM8 and TRPA1 Inhibits the Proliferative Airway Smooth Muscle Cell Phenotype. Lung. 2016;194(4):595–603. [DOI] [PubMed] [Google Scholar]
- 207. Wang Y, Shi J, Tong X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int J Mol Sci. 2021;22(16). (*-of interest-comprehensive review article on crosstalk between mechanosensitive ion channels and calcium regulatory proteins in cardiovascular diseases)
- 208. Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun. 2017;8(1):1797. (*-of interest, showed calcium regulatory mediated Piezo interactions)
- 209.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Santana Nunez D, Malik AB, Lee Q, Ahn SJ, Coctecon-Murillo A, Lazarko D, et al. Piezo1 induces endothelial responses to shear stress via soluble adenylyl Cyclase-IP(3)R2 circuit. iScience. 2023;26(5):106661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cantero-Recasens G, Butnaru CM, Brouwers N, Mitrovic S, Valverde MA, Malhotra V. Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca(2+)-induced mucin secretion from goblet cells. J Biol Chem. 2019;294(3):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mitrovic S, Nogueira C, Cantero-Recasens G, Kiefer K, Fernandez-Fernandez JM, Popoff JF, et al. TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. Elife. 2013;2:e00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bodnar D, Chung WY, Yang D, Hong JH, Jha A, Muallem S. STIM-TRP Pathways and Microdomain Organization: Ca(2+) Influx Channels: The Orai-STIM1-TRPC Complexes. Adv Exp Med Biol. 2017;993:139–57. (*-of interest, demonstrated STIM1-dependent and independent Ca2+ dependent functions)
- 214.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, et al. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106(47):20087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330(6000):101–5. [DOI] [PubMed] [Google Scholar]
- 216.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282(22):16631–43. [DOI] [PubMed] [Google Scholar]
- 217.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE). J Biol Chem. 2008;283(25):17333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32(3):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114(6):777–89. [DOI] [PubMed] [Google Scholar]
- 220.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8(9):1003–10. [DOI] [PubMed] [Google Scholar]
- 221.Ong HL, Ambudkar IS. STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca(2+) Entry: Impact on Ca(2+) Signaling and Cell Function. Adv Exp Med Biol. 2017;993:159–88. [DOI] [PubMed] [Google Scholar]
- 222.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283(19):12935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1. J Biol Chem. 2009;284(15):9733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Taylor CW. Inositol trisphosphate receptors: Ca2+-modulated intracellular Ca2+ channels. Biochim Biophys Acta. 1998;1436(1–2):19–33. [DOI] [PubMed] [Google Scholar]
- 225.Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, et al. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271(43):27005–12. [DOI] [PubMed] [Google Scholar]
- 226.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, et al. Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc Natl Acad Sci U S A. 1999;96(26):14955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, et al. Unraveling the role of polycystin-2/inositol 1,4,5-trisphosphate receptor interaction in Ca signaling. Commun Integr Biol. 2010;3(6):530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tovey SC, de Smet P, Lipp P, Thomas D, Young KW, Missiaen L, et al. Calcium puffs are generic InsP(3)-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J Cell Sci. 2001;114(Pt 22):3979–89. [DOI] [PubMed] [Google Scholar]
- 229.Kasri NN, Bultynck G, Smyth J, Szlufcik K, Parys JB, Callewaert G, et al. The N-terminal Ca2+-independent calmodulin-binding site on the inositol 1,4,5-trisphosphate receptor is responsible for calmodulin inhibition, even though this inhibition requires Ca2+. Mol Pharmacol. 2004;66(2):276–84. [DOI] [PubMed] [Google Scholar]
- 230.Ong HL, Barritt GJ. Transient receptor potential and other ion channels as pharmaceutical targets in airway smooth muscle cells. Respirology. 2004;9(4):448–57. [DOI] [PubMed] [Google Scholar]
- 231.Jha A, Sharma P, Anaparti V, Ryu MH, Halayko AJ. A role for transient receptor potential ankyrin 1 cation channel (TRPA1) in airway hyper-responsiveness? Can J Physiol Pharmacol. 2015;93(3):171–6. [DOI] [PubMed] [Google Scholar]
- 232.Lemonnier L, Trebak M, Lievremont JP, Bird GS, Putney JW, Jr. Protection of TRPC7 cation channels from calcium inhibition by closely associated SERCA pumps. FASEB J. 2006;20(3):503–5. [DOI] [PubMed] [Google Scholar]
- 233.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006;112(3):744–60. [DOI] [PubMed] [Google Scholar]


