Abstract
Our previous studies have shown that miR-511-3p treatment has a beneficial effect in alleviating allergic airway inflammation. Here, we sought to explore its therapeutic potential in animal models and gain a deeper understanding of its therapeutic value for asthma. miR-511-3p knockout mice (miR-511-3p−/−) were generated by CRISPR/Cas and showed exacerbated airway hyper-responsiveness and Th2-associated allergic airway inflammation compared with wild-type (WT) mice after exposed to cockroach allergen. RNA nanoparticles with mannose decorated EV-miR-511-3p were also created by loading miR-511-3p mimics into the mannose decorated EVs with engineered RNA nanoparticle PRNA-3WJ (Man-EV-miR-511-3p). Intra-tracheal inhalation of Man-EV-miR-511-3p, which could effectively penetrate the airway mucus barrier and deliver functional miR-511-3p to lung macrophages, successfully reversed the increased airway inflammation observed in miR-511-3p−/− mice. Through microarray analysis, complement C3 (C3) was identified as one of the major targets of miR-511-3p. C3 was increased in LPS-treated macrophages but decreased after miR-511-3p treatment. Consistent with these findings, C3 expression was elevated in the lung macrophages of an asthma mouse model but decreased in mice treated with miR-511-3p. Further experiments, including miRNA-mRNA pulldown and luciferase reporter assays, confirmed that miR-511-3p directly binds to C3 and activates the C3 gene. Thus, miR-511-3p represents a promising therapeutic target for asthma, and RNA nanotechnology reprogrammed EVs are efficient carriers for miRNA delivery for disease treatment.
Keywords: Asthma, Inflammation, macrophage, miRNA, complement C3
Graphic summary.
A lack of miR-511-3p can evoke allergen-induced airway inflammation using our newly generated miR-511-3p−/− mice. In contrast, our newly developed RNA nanoparticles with mannose decorated EV as a miR-511-3p carrier significantly inhibit allergen-induced airway inflammation. Mechanistically, miR-511-3p protects against allergic airway inflammation through regulating macrophage polarization in interaction with its target genes ( e.g., C3, PTGDS, ROCK2, LTBP1).
Graphical abstract

INTRODUCTION
Cockroaches are a potent source of allergens that are known to induce sensitization and drive allergic respiratory symptoms 1–8. Cockroach allergen is present in 85% of inner-city American homes 4, and 60–80% of inner-city children with asthma are sensitized to cockroach allergen 9. Recent advances have demonstrated that a single intervention by controlling cockroach exposure significantly reduced asthma morbidity in children 10, and cockroach immunotherapy for cockroach allergy holds promise as a treatment strategy 11. However, the fundamental mechanisms underlying cockroach allergen-induced allergic inflammation and asthma still remain largely elusive, and no specific treatments have been developed to target cockroach allergen-induced allergic sensitization and asthma.
We have made several major findings in unraveling an important link between cockroach allergen and the development of allergic asthma 6,12–15. We found that MRC1 plays a role in allergen clearance as a natural defense mechanism and in limiting the progression and severity of cockroach allergen-induced airway inflammation 12. MRC1 is one of the key C-type lectin receptors (CLRs) expressed by macrophages16,17 that recognizes complex glycan structures on various pathogens and facilitates pathogen endocytosis and presentation18–20. Most importantly, we identified miR-511-3p, which is encoded by MRC1 and plays an important role in macrophage polarization and allergic airway inflammation 12,13. It has been reported that miR-511-3p is transcriptionally co-regulated with the MRC1 gene in macrophages, regulates the activation of tumor-associated macrophages21, and attenuates intestinal inflammation by targeting Toll-like receptor 4 (TLR4) 22. Further studies suggest that miR-511-3p may shape the balance of M1 and M2 macrophage polarization and modulate downstream immune responses 12,13. A recent study suggested that miR-511-3p may represent a key miRNA in mesenchymal stem cells (MSCs)-derived extracellular vesicles (EVs) that participates in apoptosis and inflammatory processes during spinal cord injury23. We found that patients with asthma have reduced levels of plasma miR-511-3p compared with those without asthma, suggesting that miR-511-3p may be a therapeutic target for asthma. Indeed, mice treated with adeno-associated virus (AAV)-miR-511-3p showed a significant reduction in cockroach allergen-induced airway inflammation12,13. However, it is unknown whether a lack of miR-511-3p could lead to increased allergic airway inflammation. It also remains a big challenge for the efficient delivery of miR-511-3p specifically to the target cells for airway inflammation suppression and asthma treatment.
EVs have been shown to be critical in intercellular communication and inter-organ crosstalk, especially through miRNAs 24,25. EVs are more stable in biological fluids, which allows them to travel long distances within the body and release their cargo into the cytosol to regulate target genes directly 26–29. Thus, miR-511-3p in EVs may represent a functional miR-511-3p that can be used for the treatment of diseases. Furthermore, recent advances have suggested that decorating EVs with RNA nanoparticles that harbor ligands against specific cell surface markers can reprogram natural EVs for specific delivery of miRNAs to target recipient cells and mouse models 25,29,30.
In the present study, we generated a new miR-511-3p knockout mice (miR-511-3p−/−), which enables us to test the essential role of miR-511-3p in protecting against allergic airway inflammation. More importantly, we developed RNA nanoparticles with mannose decorated EV-miR-511-3p (Man-EV-miR-511-3p). We demonstrated that Man-EV-miR-511-3p could penetrate the airway mucus barrier and target lung macrophages, polarize macrophages into M2, and suppress allergic airway inflammation. Mechanistically, we identified C3, a well-recognized target for asthma treatment, as one of the major targets of miR-511-3p that plays a role in mediating allergic airway inflammation.
METHODS
Mice
Wild type (C57BL/6J) mice were purchased from the Jackson Laboratory, while the miR-511-3p knockout mice in the C57BL/6J background were generated in-house. All mice were maintained under specific pathogen-free conditions at the animal facility of the Johns Hopkins University School of Medicine. The animal care and experiments were performed in compliance with the institutional and US National Institutes of Health (NIH) guidelines and were reviewed and approved by the Institutional Animal Care and Use Committee (ACUC) of the Johns Hopkins University. All mice were used at 6–8 weeks of age with age and sex-matched controls for all experiments.
Generation of miR-511-3p knockout mice
We have generated miR-511-3p−/− mice that remain Mrc1 gene expression normally by using Dharmacon Edit-R CRISPR/Cas-mediated genome engineering 31 at the Johns Hopkins Transgenic Core Facility. Briefly, synthetic guide RNA (gRNA) sequences flanking the murine miR-511-3p (MIMAT0004940) were generated using the sgDNA Designer CRISPRko portal (Broad Institute) and synthesized by Dharmacon (GE). When gRNA(s) designed to target specific site(s) and Cas9 were co-injected into fertilized mouse eggs, cleavage at the target site(s) followed by imperfect repair resulted in the targeted deletion of miR-511-3p. These fertilized eggs were transferred into recipient mothers. Mouse genomic DNA was extracted from tails of offspring and genotyped using the Mouse Direct PCR kit (Bimake, Houston TX). Identified mutants were confirmed by Sanger Sequencing and then backcrossed with C57BL/6J mice for at least 6 generations to generate the desired homozygous deleted mutant mice.
Cockroach allergen-induced asthma mouse model with miR-511-3p−/− mice
The cockroach allergen-induced asthma mouse model was established with the protocol as described previously13 and also illustrated in Fig 1, A. Briefly, mice were sensitized by intra-tracheal (i.t.) inhalation of 20 μg cockroach extract (CRE, B46, Stallergenes Greer, NC) in 50 μL of PBS once on day 1, 3, and 5 and challenged with the same dosage of CRE for 3 successive days (day 11–13). Control mice received the same volume of PBS. Mice were sacrificed and bronchoalveolar fluids (BALF), serum, and lung tissues were collected on the next day (Day 14) after the last allergen challenge 32. Particularly, BALF was harvested by two consecutive flushes of the lung with 1.0 ml ice-cold PBS. In some experiments, mice were pre-treated with 50 μL of a 1.0 μM solution containing either miR-511-3p loaded mannose-modified nanoparticles or empty controls 1 h before the allergen challenge. The analysis of lung inflammation was established as previously described13,32.
Fig. 1. Generation of miR-511-3p knockout mice using the CRISPR-Cas9 system.

(A) Schematic illustration of the process for the generation of miR-511-3p−/− mice by using CRISPR/Cas9 system with guide RNA (gRNA) sequences flanking the murine miR-511-3p. (B-C) Confirmation of the newly generated miR-511-3p−/− mice by Sanger Sequencing (B) and genotyping (C). (D) RT-PCR confirmation of miR-511-3p deletion in the lung tissues of miR-511-3p−/− mice (n=10/group). (E-F) Expression of Mrc1 in lung tissue macrophages of wild-type (WT) and miR-511-3p−/− mice analyzed by flow cytometry analysis (E) and co-immunostaining with Mrc1 and F4/80 (F). (G) Representative of FITC-CRE (green) in macrophages (red) of lung tissues from WT and miR-511-3p−/− mice (n = 3 per group). All data were expressed as the means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. ***P < 0.001.
Airway hyperresponsiveness
Mice were anesthetized with a mixture of 90 mg/kg ketamine and 18 mg/kg xylazine, and then inserted with a tracheotomy tube. Ventilation was initiated with a volume-cycled ventilator (Flexivent, SCIREQ Scientific) with a positive-end expiratory pressure of 2 cm H2O. Airway hyperresponsiveness (AHR) was measured by challenging mice with increasing concentration of aerosolized methacholine (0–30 mg/mL). The airway resistance was detected with the Flexivent software and exported to Pulmodyn data-acquisition system (Hugo Sachs Electronic) for data analysis 13.
Histological analysis
Mouse lungs were perfused with 10 mL of ice-cold PBS injected into the right ventricle followed by excision, fixed with 4% formalin, and embedding in paraffin. Sections (4 μm) were then prepared from these paraffin-embedded lungs and subjected to hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining to evaluate general morphology and mucus production as previously described 32.
Immunofluorescence staining
Immunofluorescence staining was performed as previously reported 13. Briefly, after de-paraffin and dehydration, sectioned lung tissues were first blocked using 5% w/v BSA at room temperature for 1 h, followed by incubation with the primary antibodies as listed in the online repository (Table S1) overnight at 4°C. The sample sections were then incubated with secondary antibodies conjugated with fluorescence at room temperature for 1 h. Isotype-matched negative control antibodies were used under the same conditions. The sections were mounted with the Fluoromount Mounting medium (Sigma) with DAPI (ThermoFisher) and then observed by a NIKON ECLIPSE Ti-U microscope equipped with DS-Fi2 camera (NIKON, USA).
Flow cytometry analysis
Single-cell suspensions were prepared from harvested BALFs and total cells in BALFs were counted by Countless II (ThermoFisher). Cellular differential percentages in BALFs were measured by flow cytometry as previously described 33. Single-cell suspensions from whole lung tissues were prepared from minced lung tissues. Classically (M1) or alternatively (M2) activated macrophages were analyzed by staining the suspended cells with antibodies against F4/80 (BM8, BioLegend), CD11c (HL3, BD), Arg-1 (D4E3M, Cell Signaling Technology), or iNOS (NB300–605, Novus). These stained samples were then analyzed on an Accuri C6 Plus Flow Cytometer (BD Biosystems). All data from flow cytometer were analyzed with FlowJo software (Tree Star Inc.) as previously described 33.
Enzyme-linked immunosorbent assay
IL-4, IL-5, IL-12, IL-13, and IFN-γ in cell-free BAL fluid or supernatant were quantified by using the Ready-Set-Go! ELISA sets (ThermoFisher) according to the manufacturer’s instructions. Serum levels of cockroach allergen-specific IgE and IgG1 were analyzed by ELISA as previously described 34. In brief, 96-well plate were coated with diluted capture antibodies or cockroach allergen overnight at 4°C. Then samples were incubated at room temperature for 4 hours, followed by detection antibody incubation at room temperature for 1 h. After reaction with avidin-HRP, the plate was added stop solution and absorbance value were measured with micro-plate reader (BioRad).
Bone marrow-derived macrophage culture
Murine bone marrow (BM) cells were obtained from the femurs and tibias of 6–8-week-old mice. The BM cells were cultured at a starting density of 5×106 cells/mL at 37°C and differentiated into bone marrow-derived macrophages (BMDMs) in DMEM containing 10% FBS, 1% penicillin /streptomycin, and 20 ng/mL of recombinant murine M-CSF (BioLegend) for 7 days.
Macrophage polarization
BMDMs were seeded onto a 6-well tissue culture plate (CellTreat, Pepperell MA) at a density of 1×105 cells/well 24 h prior to treatment at 37°C, 5% CO2 incubator. To induce M1/M2 polarization, BMDMs were treated with fresh medium containing either LPS (100 ng/mL, ThermoFisher) or murine recombinant IL-4 (20 ng/mL, ThermoFisher) for 6 or 24 hours, respectively. Macrophage polarization was determined by analyzing M1 and M2 macrophage markers with quantitative real-time PCR (qPCR) as previously described 12,13.
RNA isolation and quantitative real-time PCR analysis
Total RNA was isolated using the Monarch Total RNA Miniprep Kit (New England BioLabs) and cDNAs were synthesized with the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher). The qPCR analysis was performed using Power SYBR Green PCR Master Mix (ThermoFisher) on an ABI Prism 7300 detection system. mRNA levels were normalized to the internal control gene (β-actin) and analyzed using the 2−ΔΔCT method relative to the housekeeping gene Actin 35. Primer sequences are provided in the Online Repository (See Table S2 and S3).
Generation of mannose receptor-targeted nanoparticles for the delivery of miR-511-3p
We constructed Mannose-EV-miR-511-3p (Man-EV-miR-511-3p) by loading miR-511-3p mimics with alexa647 fluorescent labeling into the exosomes (EV) purified from HEK-293T cells, and then decorated the surface with arrow-shaped PRNA-3WJ nanoparticles with mannose modification as described previously 25,29,36. In brief, HEK293T supernatant cultured for 48 h in EV-depleted FBS was spun at 300×g for 10 min to remove dead cells, followed by centrifugation at 10,000×g for 30 min at 4 °C to remove cell debris and/or microvesicles. EVs were concentrated from the culture medium by using the OptiPrep cushion procedure 37 and suspended in 1 mL of sterile PBS. EVs and synthetic miR-511-3p, conjugated with either Alexa647 or Alexa488, were mixed in 100 μL of PBS with 10 μL of ExoFect Exosome transfection (System Biosciences) followed by a heat-shock protocol. Three-way junction (3WJ) DNA molecules were modified with a mannose glycan at the arrowhead to bind target cells and a single cholesterol-triethylene glycol molecule attached at the arrowtail to assist anchorage of the 3WJ onto the EV membrane. The resulting miR-511-3p loaded mannose-decorated EVs (Man-EV-miR-511-3p) were analyzed using a Malvern NanoSight NS300 system (Malvern Panalytical, Westborough MA). miR-511-3p loading efficiency into Man-EVs was determined by calculating the fluorescent intensity between the input RNA minus the free RNA and dividing by the input RNA. transmission electron micrograph (TEM) imaging was acquired using negative staining methods as reported 36. In brief, Copper grids (400 mesh, TED PELLA) were immersed into exosomes for 5 min and then stain with 1% uranyl acetate for 30s. Image was taken by FEI Tecnai G2 Spirit TEM at the Microscopy & Imaging Facility (CMIF) in the Ohio State University. The successful delivery of miR-511-3p into macrophages was confirmed by confocal microscopy. Furthermore, we constructed a psi-check2-anti-miR-511-3p vector to detect the intracellular miR-511-3p level38. The miR-511-3p reverse complementary sequence is cloned into the 3’UTR of renilla luciferase gene to test intracellular activity of miR-511-3p levels (Fig. S3).
Microarray analysis
mRNA expression in BMDMs untreated (M0), treated with IL-4(M2) or IL-4 and miR-511-3p mimic (M2+miR-511-3p) was performed by using MouseRef-8 vs Expression ReadChip arrays (Illumina). Gene expression array data have been deposited to the NCBI’s Gene Expression Omnibus (GEO GSE137120). Total RNA extraction and RAW data generation were previously described12. The Raw signal was read, filtered, and normalized by employing embedded approaches for Illumina microarrays in limma R package 39(version 3.52.4). Differential expression analyses were performed with multiple linear regression models and empirical Bayes moderation39. The differential expressed genes (DEGs) were defined as any genes with absolute log 2-fold change [abs(log2FC)] > 1 and adjusted p value < 0.05. All microarray related plots were visualized by ComplexHeatmap40 (version 2.12.1) and ggplot241 (version 3.3.6) in R 4.2.1 statistical language.
miR-511-3p and C3 interaction
The miR-511-3p and C3 interaction was analyzed as previously described13. Briefly, a synthetic miR-511-3p containing biotin at the 3′end was annealed with a complementary strand (Integrated DNA Technologies). Twenty nanograms of the duplex was incubated with cell lysates from IL-4-treated BMDM cells. The miR-511-3p complexes were isolated using streptavidin beads and then reverse-transcribed using the High-Capacity Reverse Transcription Kit (Thermo Fisher Scientific). The bounded C3 mRNA was determined by RT-PCR with C3 primers. The enrichment was analyzed by the fold-change in miR-511-3p pulldown versus that of the negative control miR (cel-miR-39 biotinylated miR).
Luciferase activity
The WT and mutant C3 fragment containing miR-511-3p binding site were fused into psicheck2 vector (Promega, USA) using Xho I and Not I restriction enzyme (NEB, USA). The WT and mutant C3 fragment were synthesized by Tsingke Biotechnology (Beijing, China). The fused recombinant vectors were verified by sanger sequencing. The miR-511-3p mimics and NC mimics were purchased from GenePharma (Shanghai, China). HEK-293T cells were seeded into a 96-well plate at a density of 10,000 cells/well. The recombinant vector or blank vector and miR-511-3p mimics or NC mimics were co-transfected simultaneously into HEK-293T cells using Lipofectamine 2000 (Invitrogen, USA). Luciferase activity was determined 24h after transfection using a dual luciferase reporter assay kit (Promega, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8.2.1 (GraphPad Software, La Jolla, CA). All data were expressed as the means ± SEM for each group. Comparison of two groups was performed by student’s two-tailed unpaired t-test. Comparison of more than two groups was carried out by ordinary one-way ANOVA followed by Tukey’s post-hoc test. False discovery rate (FDR) in microarray analysis was controlled by Benjamini-Hochberg procedure42. Two-way ANOVA was applied for the analysis of AHR. The statistical significance was set as *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Generation of miR-511-3p knockout mice using the CRISPR-Cas9 system
Mice treated with adeno-associated virus (AAV)-miR-511-3p showed a significant reduction in cockroach allergen-induced airway hyper-responsiveness and lung inflammation 13. To further determine the role of miR-511-3p in allergic airway inflammation, we have generated miR-511-3p−/− mice by using the CRISPR/Cas9 system with guide RNA (gRNA) sequences flanking murine miR-511-3p (Fig. 1, A). Direct sequencing (Fig. 1, B) and genotyping (Fig. 1, C) were performed to confirm the deletion of miR-511-3p. Consistently, RT-PCR analyses demonstrated that miR-511-3p was nearly undetectable in the lung tissues of miR-511-3p−/− mice (Fig. 1, D). Lack of miR-511-3p expression was also confirmed in other tissues, including liver, kidney, and heart (Fig. S1). Next, we examined whether miR-511-3p deletion affects the expression of Mrc1 in macrophages. Mrc1 expression in lung macrophages from WT and miR-511-3p−/− mice was examined by flow cytometry analysis. As expected, Mrc1 is highly expressed in lung macrophages (F4/80+CD11c+) and at similar levels in WT and miR-511-3p−/− mice (Fig. 1, E). Same observation was obtained by co-immunostaining of lung tissue sections with Mrc1 and F4/80 antibodies (Fig. 1, F), indicating that miR-511-3p deletion has no effect on the expression of Mrc1 in macrophages. Furthermore, we have previously demonstrated that Mrc1 plays a role in antigen uptake by macrophages12. By using the same protocol, we found no difference in CRE uptake in miR-511-3p−/− compared with WT mice (Fig. 1, G). Taken together, these newly generated miR-511-3p−/− mice represent an ideal mouse model to examine the functional role of miR-511-3p in allergic airway inflammation.
Lack of miR-511-3p exacerbates cockroach allergen-induced airway hyper-responsiveness and lung inflammation
We then examined whether miR-511-3p deficiency affects allergen-induced airway and lung responses using the newly generated miR-511-3p−/− mice. A well-established cockroach allergen (CRE)-induced asthma mouse model was used as illustrated in Fig. 2, A. Consistent with our previous studies 33,34, CRE-treated mice showed a significant increase in airway resistance (Fig. 2, B). Intriguingly, the increased airway resistance was further potentiated in CRE-treated miR-511-3p−/− mice when compared with WT mice. The same pattern was observed for airway inflammation as assessed by histological analysis and inflammatory cytokine measurements. Particularly, histological analysis demonstrated that miR-511-3p−/− mice showed a significantly increased recruitment of inflammatory cells to the lung with dense peri-bronchial infiltrates (Fig. 2, C, upper panel, H&E) and goblet cell hyperplasia (Fig. 2, C, lower panel, PAS). Compared to WT mice, miR-511-3p−/− mice had increased total inflammatory cells and eosinophils in the bronchoalveolar lavage fluids (BALFs) (Fig. 2, D), No statistically differences were noted in BALFs for macrophages, lymphocytes, and neutrophils (Fig. S2). Higher levels of cockroach allergen-specific IgE and IgG1 were also detected in serum of CRE-treated miR-511-3p−/− mice (Fig. 2, E). Furthermore, miR-511-3p−/− mice had higher levels of IL-4, IL-5, IL-13 but lower levels of IFN-γ in BALFs (Fig. 2, F). In contrast, no clear change was observed for IL-12. Collectively, these findings indicate that lack of miR-511-3p exacerbates cockroach allergen-induced airway hyper-responsiveness and Th2-associated allergic airway inflammation.
Fig. 2. miR-511-3p−/− mice show exacerbation of cockroach allergen-induced airway hyper-responsiveness and lung inflammation.

(A) Experimental protocol for the generation of cockroach allergen-induced mouse model of asthma with WT and miR-511-3p−/− mice. (B) Lung resistance in response to increasing concentrations of methacholine using the forced oscillation technique (FlexiVent, SCIREQ) (n=5/group). (C) Histological examination of mouse paraffin lung sections stained with hematoxylin and eosin (H&E, upper panel) and Periodic acid–Schiff (PAS, lower panel) staining. (D) Bronchoalveolar lavage (BAL) fluid total and eosinophil cell counts as assessed by flow cytometry. (E) Serum levels of cockroach allergen-specific IgE and IgG1. (F) Levels of cytokines in BAL fluids. D-F, n=6–16/group. All data were expressed as the means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001. CRE: cockroach extract. WT: wild-type.
Lack of miR-511-3p leads to macrophage polarization to M1 phenotypes
We previously demonstrated that miR-511-3p can polarize macrophages toward the M2 phenotypes by using AAV-miR-511-3p (In vivo) and lentivirus (LV)-miR-511-3p (In vitro), respectively 12,13 We then tested whether deletion of miR-511-3p may affect the macrophage polarization using miR-511-3p−/− mice following the procedure illustrated in Fig. 3, A. Lung tissue M1 and M2 macrophages were initially assessed by flow cytometry analyses as previously described13 (Fig. 3, B). miR-511-3p−/− mice showed more M1 (F4/80+CD11c+iNOS+, Fig. 3, C) but fewer M2 macrophages (F4/80+CD11c+Arg-1+, Fig. 3, D) in the lung tissues. This was further supported by co-immunostainings of lung tissue sections using antibodies against F4/80 and iNOS (M1) (Fig. 3, E) or F4/80 and Arg-1 (M2) (Fig. 3, F). We found increased M1 macrophages (iNOS+ cells, Fig. 3, E) but decreased M2 macrophages (Arg-1+ cells, Fig. 3, F) in CRE-treated miR-511-3p−/− mice relative to WT mice. To further support the in vivo findings, murine bone marrow-derived macrophages (BMDMs) from WT and miR-511-3p−/− mice were cultured and then treated under either M1 (LPS stimulation) or M2 (IL-4 stimulation) condition. BMDMs from miR-511-3p−/− mice cultured under the M1 condition had an increased expression of M1-associated genes (IL-1β, TNF-α, and iNOS) compared to those from WT mice (Fig. 3, G). In contrast, BMDMs from miR-511-3p−/− mice cultured under the M2 condition had a decreased expression of M2-associated genes (Arg-1, Chi3l3, and Ym1/2) compared to those from WT mice (Fig. 3, H). Taken together, these results suggest that lack of miR-511-3p leads to the polarization of macrophages toward the M1 phenotypes.
Fig. 3. Lack of miR-511-3p leads macrophage polarization to M1 phenotypes.

(A) Schematic of preparation of single cells from lung tissues for flow cytometry analysis. (B) Gating strategies for the flow cytometry analysis of M1/M2 macrophages in the lung tissues of wild-type (WT) and miR-511-3p−/− mice. (C-D) Quantitation of lung tissue M1 and M2 macrophages as indicated by the percentage of F4/80+CD11c+iNOS+ cells (%M1, C) and F4/80+CD11c+Arg-1+ cells (%M2, D) among total lung single cells. n=4/group. (E) Representative image of immune fluorescence staining with the primary antibodies against iNOS. Nuclei were counterstained with 4’-6-diamidino-2-phenylindole dihydrochloride (DAPI). Right panel: quantification of immunostainings for F4/80+iNOS+ in total F4/80+ cells. n=5/group. (F) Representative image of immune fluorescence staining with the primary antibodies against Arg-1. Right panel: quantification of immunostainings for F4/80+Arg-1+ in total F4/80+ cells. n=5. (G-H) Quantitative RT-PCR analyses for M1 (G) and M2 (H)-associated genes in bone marrow-derived macrophages (BMDMs) from WT and miR-511-3p−/− mice cultured under M1 or M2 condition. n=4. Data represent means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Generation of RNA nanoparticles with mannose decorated extracellular vesicle-miR-511-3p for targeted delivery
EV miRNAs are biologically active and can directly engage in regulating the protein levels of targeted genes27,28,43. Thus, we used RNA nanotechnology to generate RNA nanoparticles with mannose decorated extracellular vesicle-miR-511-3p for specific delivery of miR-511-3p to the target recipient cells and mouse models. As illustrated in Fig. 4, A, we constructed Man-EV-miR-511-3p by loading miR-511-3p mimics with AF-647 fluorescent labeling into the EVs from HEK293T cell culture supernatants 44. These EVs were then decorated with engineered RNA nanoparticle PRNA-3WJ (Three-way junction of the bacteriophage phi29 motor packaging RNA) with mannose modification on the surface. The mannose decorated EVs were designed to target macrophages through the mannose receptor Mrc1 for specific delivery of EV miR-511-3p (Fig. 4, B). Man-EV-miR-511-3p was displayed in a 3D structure (Fig. 4, C), and the size and concentration of Man-EV-miR-511-3p were detected by NTA (50–150 nm, Fig. 4, D) and actual morphology was observed with transmission electron microscope (Fig. 4, E). To test whether the Man-EV-miR-511-3p was efficiently delivered into macrophages, RAW 264.7 cells were incubated with Man-EV-miR-511-3p containing Alexa647 labelled miR-511-3p mimics at 200nM for 2h, and then stained with DAPI (nucleus) and AF488-phalloidin (cytoplasm). RAW 264.7 cells incubated with Man-EVs serves as controls. Alexa647 labelled miR-511-3p mimics were detected in Man-EV-miR-511-3p loaded macrophages by Olympus FV1000 confocal microscopy (Fig. 4, F). To further confirm whether the delivered mannose-EV-miR-511-3p in macrophages has functional activity, we used a luciferase-based miR-511-3p reporter system in which a binding sequence of miR-511-3p was cloned into the 3’-UTR of Renilla luciferase gene (psi-Check2-anti-miR-511-3p) (Fig. 4, G). Successfully delivered miR-511-3p can bind to the anti-miR-511-3p in the 3’-UTR of Renilla luciferase gene and block its translation, thus resulting in a decreased expression of Renilla luciferase38. RAW 264.7 were transfected with the newly generated plasmid and a co-expressed Firefly luciferase gene as the internal control. These cells were then incubated with miR-511-3p mimics, EV-miR-511-3p, and Man-EV-miR-511-3p, respectively, for 24 h. As expected, Man-EV-miR-511-3p showed better inhibition of Renilla luciferase expression when compared with EV-miR-511-3p or miR-511-3p mimic as assessed by Renilla/firefly luciferase ratio (Fig. 4, H). Therefore, mannose decorated EV-511-3p can successfully deliver miR-511-3p into macrophages with functional activity.
Fig. 4. Generation of RNA nanoparticles with mannose decorated extracellular vesicle-miR-511-3p for targeted delivery.
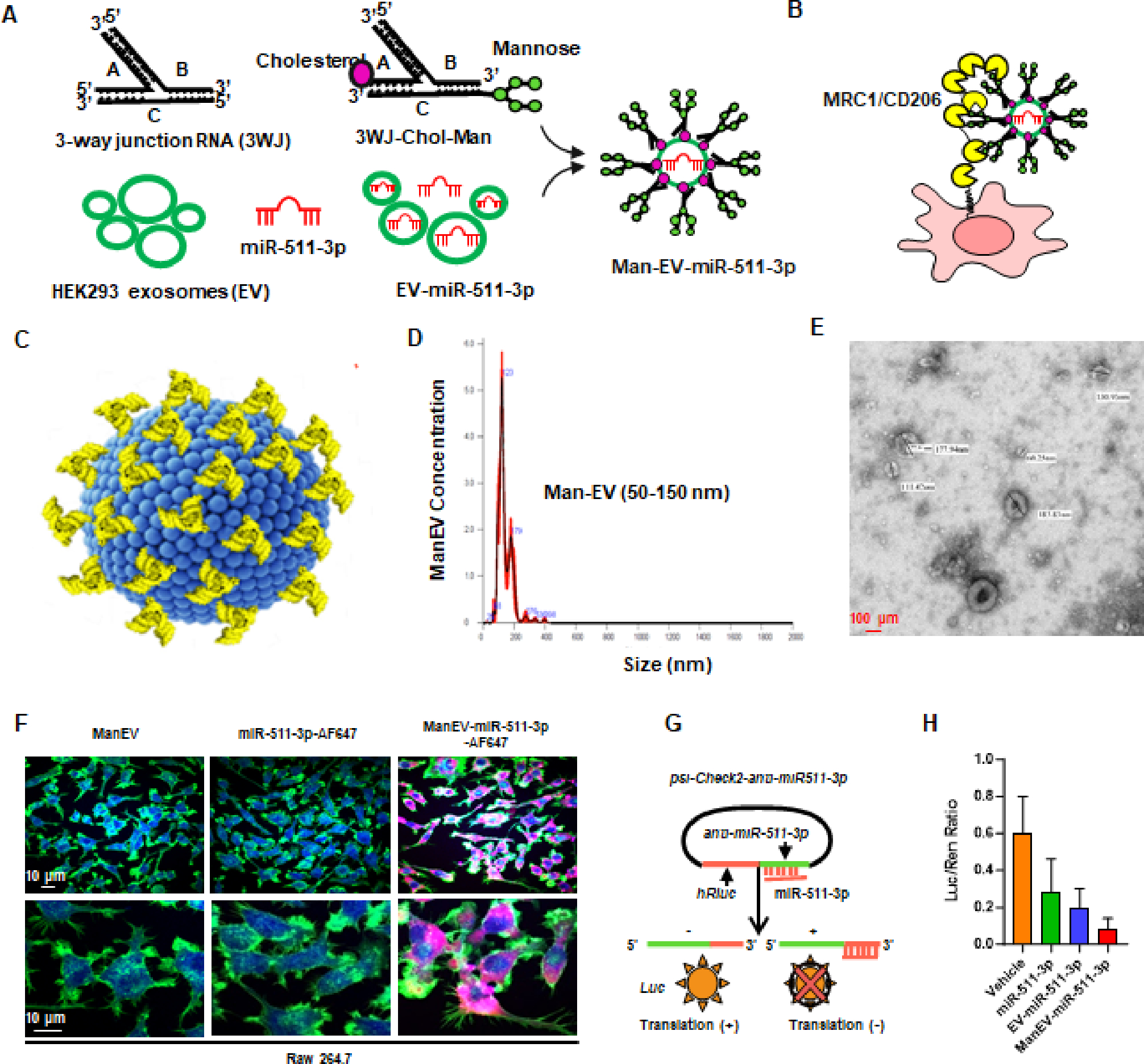
(A) Schematic of generation of RNA nanoparticles with mannose decorated extracellular vesicle-miR-511-3p for specific delivery of miR-511-3p. (B) Schematic of the mannose decorated EVs with miR-511-3p targeting macrophages through mannose receptor Mrc1. (C) 3D structure of Man-EV-miR-511-3p with EVs (blue) and RNA nanoparticles harboring mannose (yellow). (D) Size and concentration of Man-EV-miR-511-3p detected by Nanoparticle tracking analysis (NTA). (E) Actual morphology was observed with transmission electron microscope. (F)Representative confocal images show miR-511-3p uptake by RAW 264.7 cells. Nucleus (blue), cytoplasm (green), and miR-511-3p (red). (G) Schematic of a luciferase-based miR-511-3p reporter system in which a binding sequence of miR-511-3p was cloned into the 3’-UTR of Renila luciferase gene (psi-Check2-anti-miR-511-3p). (H) Dual luciferase assay to test the delivery of miR-511-3p into RAW 264.7 cells and shows a better inhibition as assessed by renilla/firefly luciferase ratio.
Successful delivery of Man-EV-miR-511-3p into lung macrophages of asthma mouse model
Next, we examined whether ManEV-miR-511-3p can be delivered into lung macrophages of the asthma mouse model via intratracheal installation. Mice were injected with Man-EV-miR-511-3p (1.0 μM/50 μL, i.t.), and the Man-EV-miR-511-3p in the lung tissues was analyzed 24 h after administration by detecting AF-488 (Fig. S3, A). Flow cytometry analysis showed that Man-EV-miR-511-3p was not only detected in lung macrophages (CD45+CD11b+, 59.1%) but also found in DCs (CD45+CD11c+, 34.8%) (Fig. S3, B). The expression of Man-EV-miR-511-3p in lung macrophages was further confirmed by co-immunostaining with F4/80 (Fig. S3, C). Thus, Man-EV-miR-511-3p can penetrate the airway mucus barrier, and enabling effective delivery of miR-511-3p to lung macrophages in mice.
Man-EV-miR-511-3p recovers the increased allergic airway inflammation caused by miR-511-3p deficiency
Given that miR-511-3p−/− mice showed an exacerbation of cockroach allergen-induced airway inflammation compared to WT mice, we asked whether delivery of Man-EV-miR-511-3p into the lung can reverse the increased allergic airway inflammation observed in miR-511-3p−/− mice. Man-EV-miR-511-3p was administered via intratracheal installation 1 h prior to every single challenge of the asthma mouse model by using the protocol as illustrated in Fig. 5, A. Consistent with our previous findings, miR-511-3p−/− mice showed increased airway inflammation as compared to WT mice. When the miR-511-3p−/− mice were pre-treated with Man-EV-miR-511-3p, the CRE-induced airway inflammation was significantly attenuated. In particular, Man-EV-miR-511-3p pre-treatment inhibited CRE-induced peribronchial inflammation (Fig. 5, B, upper panel) and goblet cell hyperplasia (Fig. 5, B, lower panel) as determined by histological analyses. These Man-EV-miR-511-3p pre-treated mice also demonstrated reduced numbers of total inflammatory cells and eosinophils in BALFs (Fig. 5, C). No significant changes were observed for macrophage, neutrophils, and lymphocytes. Moreover, reduced levels of cockroach allergen-specific IgE and IgG1 were observed in serum of those pre-treated miR-511-3p−/− mice (Fig. 5, D). A similar pattern was noted for cytokine levels in BALFs. Man-EV-miR-511-3p pre-treatment inhibited the secretion of IL-4, IL-5, and IL-13, but no significant change was noted for IL-12 and IFN-γ in BALFs (Fig. 5, E). Taken together, the results suggest that Man-EV-miR-511-3p can suppress allergic airway inflammation, representing the clinical potential of RNA nanotechnology-based delivery of miR-511-3p for the treatment of asthma.
Fig. 5. Man-EV-miR-511-3p recovers the increased airway inflammation caused by miR-511-3p deficiency.

(A) Experimental protocol for the use of Man-EV-miR-511-3p in asthma mouse model. (B) (C) Histological examination of mouse paraffin lung sections stained with hematoxylin and eosin (H&E, upper panel) and Periodic acid-Schiff (PAS, lower panel) staining. (C) Flow cytometry analyses of inflammatory cells in bronchoalveolar lavage (BAL) fluids. (D) ELISA analyses of cockroach allergen-specific IgE and IgG1 levels in serum. (E) ELISA analyses of cytokine levels in BAL fluids. C-E, n=6–11/group. All data were expressed as the means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001. CRE: cockroach extract. WT: wild-type. ManEV: mannose decorated extracellular vesicle.
Man-EV-miR-511-3p polarizes macrophages into M2 phenotypes
We also examined whether treatment with Man-EV-miR-511-3p can modulate lung macrophage polarization using the approach illustrated in Fig. 6, A. Flow cytometry analyses were performed to identify M1 and M2 macrophages in lung tissue cells from CRE-challenged mice with or without Man-EV-miR-511-3p treatment (Fig. 6, B) as previously described13. Compared to those untreated mice, Man-EV-miR-511-3p-treated mice showed reduced M1 (F4/80+CD11c+iNOS+, Fig. 6, C) but increased M2 macrophages (F4/80+CD11c+Arg-1+, Fig. 6, D). This was further supported by co-immunostainings of lung tissue sections with antibodies against F4/80 and M1 (iNOS, Fig. 6, E) or M2 (Arg-1, Fig. 6, F) markers. To further support the in vivo findings, BMDMs were pre-treated with Man-EV-miR-511-3p for 24 h and then cultured under either M1 (LPS) or M2 (IL-4). Man-EV-miR-511-3p-treated BMDMs showed reduced expression of M1-associated genes (IL-1β, TNF-α, and iNOS, Fig. 6, G) but increased expression of M2-associated genes (Arg-1, Chi3l3, and Yim1/2, Fig. 6, H). Collectively, our data from both in vivo and in vitro analyses suggest that Man-EV-miR-511-3p can deliver miR-511-3p to target cells and modulate macrophage polarization.
Fig. 6. Man-EV-miR-511-3p polarizes macrophages into M2 phenotypes.

(A) Schematic of delivered Man-EV-miR-511-3p targeting lung macrophages via Mrc1 to modulating macrophage polarization. (B) Gating strategies for the flow cytometry analysis of M1/M2 macrophages in the lung tissues. (C-D) Quantitation of lung tissue M1 and M2 macrophages as indicated by the percentage of F4/80+CD11c+iNOS+ cells (%M1, C) and F4/80+CD11c+Arg-1+ cells (%M2, D) among total lung single cells. n=5/group. (E) Representative image of immune fluorescence staining with the primary antibodies against iNOS. Nuclei were counterstained with 4’-6-diamidino-2-phenylindole dihydrochloride (DAPI). Right panel: uantification of immunostainings for F4/80+iNOS+ in total F4/80+ cells. n=3–4/group (F) Representative image of immune fluorescence staining with the primary antibodies against Arg-1. Right panel: uantification of immunostainings for F4/80+Arg-1+ in total F4/80+ cells. n=3–4/group (G-H) Quantitative RT-PCR analyses for M1 (G) and M2 (H)-associated genes in Man-EV-miR-511-3p pre-treated bone marrow-derived macrophages (BMDMs) cultured under M1 or M2 condition. n=3/group. Data represent means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001. ManEV: mannose decorated extracellular vesicle.
Microarray analysis identifies C3 as a major miR-511-3p target gene
To better understand the mechanisms underlying miR-511-3p-modulated macrophage polarization, we performed microarray analysis of the macrophages with miR-511-3p pre-treatment followed by further culture under M1 culture conditions as illustrated in Fig. 7, A. The gene array was performed by using the MouseRef-8 v2.0 BeadChip and reported previously12. Volcano plots showed the pattern of the up- and down-regulated genes in the macrophages treated versus un-treated with M1 culture conditions (Fig. 7, B) and in the macrophages in M1 culture conditions with or without miR-511-3p pre-treatment (Fig. 7, C). Results revealed approximately 2476 mRNAs that were differentially expressed in macrophages treated with or without LPS stimulation (|Log2FC ≥1|, FDR-corrected P < 0.05), including 1268 downregulated genes and 1208 upregulated genes. A total of 110 mRNAs were differentiated in LPS-treated macrophages with or without miR-511-3p pre-treatment, including 51 downregulated and 60 upregulated genes. Of these, complement component 3 (C3), a potential therapeutic target for asthma45,46, was up-regulated under the M1 condition but significantly inhibited when these M1 macrophages were pretreated with miR-511-3p. The pattern of C3 up- and downregulation in macrophages is shown by heatmap (Fig. 7, D) and its relative expression in 3 groups was shown in Fig 7, E. The changes of C3 expression were further validated in AAV-miR-511-3p treated BMDCs (Fig 7, F) and mouse model (Fig 7, G) by qPCR. Particularly, C3 expression was increased in the lung tissues of the CRE-induced mouse model of asthma but significantly decreased after treatment with AAV-miR-511-3p. To determine the expression pattern of C3 in lung tissue in situ, C3 was co-immunostained with macrophage marker F4/80 in lung tissues. As expected, C3 expression was increased in the lung macrophages of CRE-induced mice as compared to PBS-treated mice (Fig 7, H). The expression of C3 was significantly inhibited when mice were pre-treated with Man-EV-miR-511-3p (Fig 7, I). The inhibition of C3 expression was further confirmed by RT-PCR (Fig. S4). Similar inhibitions by Man-EV-miR-511-3p were also observed for several identified target genes Ptgds, Rock2, and Ltbp1. No change was found for CCL2. Additionally, miR-511-3p−/− mice showed increased expression of C3 after CRE treatment as compared to WT mice, (Fig. S5). These results provide evidence that C3 is one of the major target genes of miR-511-3p.
Fig. 7. Microarray analysis identifies C3 as one of the major miR-511-3p target genes.

(A) Schematic of microarray analysis for miR-511-3p pre-treated macrophages under M1 culture condition using the mouseRef-8 v2.0 BeadChip. (B-C) Volcano plot of differentially expressed genes in bone marrow-derived macrophages (BMDMs) cultured with (M1) or without (M0) LPS (B) or LPS-treated BMDMs with or without miR-511-3p pretreatment (C). The logarithms of the fold changes of individual genes (x axis) are plotted against the negative logarithm of false discover rate (FDR) to base 10 (y axis). (D-E) Heatmap (D) and gene expression (E) of complement C3 gene in undifferentiated BMDMs and M1 macrophages with or without miR-511-3p pretreatment. n=3/group. (F-G) qPCR analysis of C3 expression in BMDMs (F) and lung tissues of asthma mouse model (G) with or without AAV-miR-511-3p treatment. n=6/group. (H) Expression of C3 in macrophages defined by the representative image of C3 (red) co-immune fluorescence staining with F4/80 (green). Nuclei were counterstained with 4’-6-diamidino-2-phenylindole dihydrochloride (DAPI). Right panel: quantification of double immunostainings for C3 and F4/80. n=9–12/group. (I) Expression of C3 in macrophages defined by the representative image of C3 (red) co-immune fluorescence staining with F4/80 (green) in miR-511-3p−/− mice treated with/without ManEV-miR-511-3p. Nuclei were counterstained with 4’-6-diamidino-2-phenylindole dihydrochloride (DAPI). Right panel: quantification of double immunostainings for C3 and F4/80. n=4–6/group. All data were expressed as the means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, **P < 0.01, and ***P < 0.001. CRE: cockroach extract. ManEV: mannose decorated extracellular vesicle.
miR-511-3p is directly bounded to C3 and regulates its activity
To determine whether the inhibitory effect miR-511-3p on C3 expression is through direct binding, we performed the miRNA-mRNA pulldown assay as illustrated in Fig. 8, A. Compared to miR control, a significant amount of C3 mRNA was detected in miR-511-3p–biotin-mRNA complexes from IL-4–treated BMDMs (Fig. 8, B). The miR-511-3p–C3 binding was further supported by in silico analysis using BiBiServ RNAhybrid program47. A binding sequence in C3 mRNA was predicted for miR-511-3p as shown in Fig. 8, C. The predicted miR-511-3p–C3 binding was confirmed by luciferase reported assays in HEK293T cells as previously described48. Overexpression of miR-511-3p markedly suppressed C3–3’UTR-driven luciferase activity (Fig. 8, D). However, no significant changes in luciferase activity were noted when the miR-511-3p binding sequence in the C3 fragment was mutated (Fig. 8, E). As a negative control, no luciferase activity was observed in the empty vector psicheck2 overexpressed cells (Fig. 8, F). Collectively, the results suggest that miR-511-3p is directly bound to and activates C3 gene.
Fig. 8. miR-511-3p illustrates a direct binding to C3 and regulates C3 activity.

(A) Schematic representation of miR-511-3p-C3 mRNA biotin pulldown assay. (B) qPCR analysis of C3 expression in miR-511-3p-C3 mRNA complexes. n=4. (C) In silico prediction of miR-511-3p binding site in C3 mRNA by BiBiServ RNAhybrid. (D-F) Dual-luciferase assay in HEK293T cells transfected with C3-psicheck2 plasmid containing the binding site (D), mutant binding site (E), and control (F) and then treated with either NC-mimics or miR-511-3p mimics. n=3. All data were expressed as the means ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s post-hoc test. *P < 0.05, ***P < 0.001.
DISCUSSION
Our previous studies have well established that mannose receptor CD206 on macrophages is critical in allergen clearance as a natural defense mechanism and in limiting the progression and severity of cockroach allergen-induced allergic inflammation12. miR-511-3p, which is encoded by MRC1, is transcriptionally co-regulated with the MRC1 gene in macrophages12,13,49. Of interest, we found that miR-511-3p was significantly lower in the blood of patients with asthma compared with those in non-asthmatics12. Further studies suggest that miR-511-3p has the ability to control the balance of M1 and M2 macrophage polarization and skew immune response12,13. Importantly, we have demonstrated that targeted miR-511-3p delivery by using adeno-associated virus inhibited the increased allergic airway inflammation observed in Mrc1 deficient mice13.
In this study, we generated miR-511-3p knockout mice, in which Mrc1 gene expression remains, by CRISPR/Cas-mediated genome engineering to define the critical role of miR-511-3p in allergen-induced airway and lung responses. In miR-511-3p−/− mice, the whole primary miR-511, including both miR-511-3p and miR-511–5p, is removed. However, the miR-511-3p sequence, but not miR-511–5p, is the active strand of miR-511 in mouse and human precursor21. Because miR-511-3p is an intronic miRNA located in the fifth intron of mouse Mrc1 genes, deletion of miR-511-3p doesn’t affect Mrc1 gene expression. Indeed, these newly generated miR-511-3p−/− mice had the same expression of Mrc1 as WT mice with the same capability for allergen uptake as WT mice, and miR-511-3p deficiency is the only cause of the phenotypic changes observed. As expected, miR-511-3p−/− mice after allergen treatment showed a significant increase in airway resistance and airway inflammation as indicated by increased recruitment of inflammatory cells to the lung, goblet cell hyperplasia, and higher levels of IL-4, IL-5, and IL-13 in serum. These results suggest that miR-511-3p confers protection against allergen-induced airway hyper-responsiveness and Th2-associated allergic airway inflammation. These findings, however, were based on miR-511-3p global knockout mice, and deletion of miR-511-3p specifically in macrophages might be essential to understand the significance of miR-511-3p specifically in macrophages in contributing the increased airway resistance and inflammation. Furthermore, while our studies are mainly focusing on macrophages, miR-511-3p in DCs may also be equally important since miR-511-3p is also expressed by DCs50. The expression of miR-511– 3p in human DCs has been shown to play a key role in regulating DC phenotype and function partly by regulating MRC1 expression50. These findings warrant further investigation into determining whether mice with miR-511-3p knockout specifically in macrophages or DCs show exacerbation of allergen-induced allergic asthma.
With exciting development of novel therapeutic strategies and drug delivery51, it holds promise for improving the treatment of various lung diseases, including lung injury and fibrosis52, cellular senescence53,54, and asthma55, with improved patient compliance, reduced the required dosage of the medication, and enhanced the therapeutic effectiveness of treatments. Of these, recent studies have suggested that EV miRNAs are biologically active and can directly regulate the protein expression of targeted genes27,28,43. These findings raise the possibility that EV miRNAs may serve as novel regulators that play a role in immune cell activation and function. However, EVs may not target specific recipient cells due to a lack of their specificity. Thus, we used RNA nanotechnology to reprogram natural EVs for miR-511-3p delivery only to lung macrophages of the mouse model25. To do this, we constructed Man-EV-miR-511-3p by loading miR-511-3p mimics into the EVs from HEK293T cell culture supernatants44. The surface of EVs was decorated with RNA nanoparticle pRNA-3WJ constructed from a three-way junction motif of packaging RNA (pRNA) molecules, which have been shown to serve as a platform for building larger, multifunctional nanoparticles for the treatment of different diseases25,56. The RNA nanoparticle was also modified with mannose with the purpose of targeting macrophages through its receptor Mrc1 that is dominantly expressed by macrophages. The newly constructed Man-EV-miR-511-3p was characterized by its size and concentration as assessed by Nanoparticle Tracking Analysis. As expected, Man-EV-miR-511-3p can penetrate the airway mucus barrier and effectively deliver miR-511-3p into lung macrophages.
However, reducing the adhesion of big molecule drugs to the airway mucosa after inhalation through the airways with minimized dosage and maximized therapeutic benefit is a significant challenge in the development of inhaled biologics 51. Even in vitro mucosal penetration experiments may not always accurately reflect drug penetration observed in mouse models or in vivo systems. Our approach involves a combination of in vitro and in vivo experiments to assess the delivery efficiency and therapeutic effects of Man-EV-miR-511-3p. First of all, we conducted in vitro experiments using Alexa647 labeled miR-511-3p and Man-EV-miR-511-3p to assess macrophage uptake and functional activity. The results showed that Man-EV-miR-511-3p could successfully deliver miR-511-3p into macrophages and exhibited functional activity. Second, we used intratracheal installation to deliver Man-EV-miR-511-3p into the airways and lung macrophages of an asthma mouse model. Both flow cytometry analysis and immunostaining confirmed the effective delivery of miR-511-3p via intratracheal installation to lung macrophages. Lastly, we provided evidence that the delivery of Man-EV-miR-511-3p into lung macrophages could reverse the increased allergic airway inflammation observed in miR-511-3p-deficient mice. We also noticed that Man-EV-miR-511-3p was also detected in DCs. Thus, the potential role of Man-EV-miR-511-3p in DCs or possibly other cells needs to be considered for the inhibition of allergic airway inflammation in those treated mice. Due to the limited availability of Man-EV-miR-511-3p, we mainly focused on the delivery of miR-511-3p to lung macrophages and its potential impact on airway inflammation in asthma. Future studies will investigate whether the administration of Man-EV-miR-511-3p can rescue the CRE-induced airway hyperresponsiveness observed in the miR-511-3p−/− mice. Additionally, further studies are warranted to characteriseMan-EV-miR-511-3p, explore different delivery approaches for better penetration of airway mucosa, consider different experimental settings for asthma prevention or treatment, and the retention rate and half-life of Man-EV-miR-511-3p in the lungs after inhalation. We also investigated the molecular mechanisms by which miR-511-3p confers protection against allergic inflammation. While there are many possible mechanisms, our pervious study has suggested that miR-511-3p prevents allergen-induced inflammatory responses through polarizing macrophages into M2 phenotypes that have been associated with the resolution of inflammation and the clearance of dead cells57,58. Several genes have been reported to be either direct (e.g., ROCK2, LTBP121, PTGDS 12, and CCL213) or indirect targets (e.g., TLR4, an asthma-associated gene59) of miR-511-3p that are linked with macrophage polarization or the pathogenesis of asthma. Of these, PTGDS, hematopoietic prostaglandin D synthase, is encoding gene that catalyzes the conversion of PGH2 to PGD212. Ccl2 and its receptor Ccr2 has been shown to regulate macrophage polarization through RhoA signaling13. With the microarray dataset that we have previously generated12, we identified the genes that showed up-regulation under M1 conditions but down-regulation in M1 macrophages pretreated with miR-511-3p. Among these genes, complement C3, a central player in the complement activation pathway, was significantly regulated by miR-511-3p. Thus, C3 is a potentially novel target gene for miR-511-3p that may participate in miR-511-3p-regulated downstream immune responses in allergic asthma.
Interestingly, enormous studies have suggested that C3 is a potential therapeutic target for asthma60. For example, C3 activation at the airway surface in asthma has been considered to serve as a common pathway for the induction of Th2-associated immune responses to environmental triggers such as allergens, pollutants, viral infections, and cigarette smoke61. Mice with C3 deficiency showed significant attenuation of airway hyperresponsiveness and Th2-associated inflammation as compared to WT mice62. Furthermore, high levels of plasma C3 have been associated with increased risk of asthma hospitalizations in the general population and with high risk of asthma exacerbations in individuals with allergic asthma46. Our present study provided evidence that C3 as a miR-511-3p downstream target is highly expressed in lung macrophages of asthma mouse model. Collectively, these evidences from both human and mouse studies strongly support that activation of C3 may play an essential role in Th2-associated inflammation and asthma. Additionally, our mechanistic study demonstrated that miR-511-3p confers protection against airway inflammation through inhibiting C3 expression and activation. Indeed, our miRNA-mRNA pulldown assay demonstrated a direct interaction between miR-511-3p and C3 fragment as evident by the significant amount of C3 mRNA detected in miR-511-3p-biotin-mRNA complexes from macrophages. Additionally, we found that miR-511-3p can suppress C3–3’UTR-driven luciferase activity. The findings further support that C3 is a novel target gene for miR-511-3p and mediates miR-511-3p–regulated allergic airway inflammation and development of asthma. However, the mechanisms by which C3 and its pathway contribute to macrophage polarization and the increased allergic airway inflammation remain to be fully elucidated.
In summary, our research provides evidence regarding the role of miR-511-3p in allergic airway inflammation and its therapeutic potential for asthma. By utilizing miR-511-3p knockout mice, we demonstrated that the absence of miR-511-3p exacerbated allergen-induced airway inflammation. In contrast, the use of RNA nanoparticles with mannose-decorated EVs as carriers effectively delivered miR-511-3p to lung macrophages, resulting in a significant reduction of allergen-induced airway inflammation and modulation of macrophage polarization. Furthermore, we identified complement C3 as a major target of miR-511-3p, suggesting its involvement in miR-511-3p-mediated allergic airway inflammation. This finding is significant as C3 is a well-recognized target for asthma treatment (See graphic summary). The use of RNA nanotechnology reprogrammed EVs as efficient carriers for miRNA delivery into the lungs highlights the potential of this approach for treating diseases like asthma. The therapeutic implications of our work extend beyond mechanistic understanding, offering promising targets for the development of novel treatments for allergies and asthma. Overall, our findings contribute valuable insights into the role of miR-511-3p in asthma pathogenesis and highlights its potential as a therapeutic target. The use of advanced delivery systems such as RNA nanoparticles and EVs opens up new avenues for miRNA-based treatments in various respiratory diseases.
Supplementary Material
Highlight.
CRISPR/Cas gene editing created miR-511-3p knockout mice, which serve as a valuable model for investigating the role of miR-511-3p in asthma.
RNA nanoparticles loaded with miR-511-3p and decorated with mannose demonstrates an innovative approach to miRNA delivery, facilitating effective penetration of the airway mucus barrier.
Intra-tracheal inhalation of Man-EV-miR-511-3p successfully reverses the increased airway inflammation in miR-511-3p−/− mice.
Complement C3 as one of the major targets of miR-511-3p, and miR-511-3p binds to C3 and activates the C3 gene.
miR-511-3p as a Therapeutic Target for asthma.
ACKNOWLEDGEMENTS
We thank Professor Peixuan Guo at the Center for RNA Nanobiotechnology and Nanomedicine, the Ohio State University, for his guidance for the generation of mannose receptor-targeted nanoparticles for the delivery of miR-511-3p.
Funding:
The research leading to these results has received funding from the US National Institutes of Health (NIH) (R01AI153331, R01AI141642).
ABBREVIATIONS
- CRE
Cockroach extract
- Mrc1
Mannose receptor/CD206
- miR-511-3p
microRNA-511-3p
- Man
Mannose
- BALF
Bronchoalveolar lavage fluid
- BMDM
Bone marrow-derived macrophage
- M1
Classically activated macrophage
- M2
Alternatively activated macrophage
- LPS
Lipopolysaccharide
- WT
Wild-type
- EV
Extracellular vesicle
- CLR
C-type lectin receptor
- gRNA
Guide RNA
- 3WJ
Three-way junction
- pRNA
Packaging RNA
- RT-PCR
real-time PCR
- FDR
False Discovery Rate
- DEG
differential expressed gene
- AAV
Adeno-associated virus
- H&E
hematoxylin and eosin
- PAS
periodic acid-Schiff
- DAPI
4’-6-diamidino-2-phenylindole dihydrochloride
Footnotes
Disclosure of potential conflict of interest:
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. The American review of respiratory disease 1993; 147(3): 573–8. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. The New England journal of medicine 1997; 336(19): 1356–63. [DOI] [PubMed] [Google Scholar]
- 3.Chew GL, Perzanowski MS, Canfield SM, et al. Cockroach allergen levels and associations with cockroach-specific IgE. The Journal of allergy and clinical immunology 2008; 121(1): 240–5. [DOI] [PubMed] [Google Scholar]
- 4.Olmedo O, Goldstein IF, Acosta L, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. The Journal of allergy and clinical immunology 2011; 128(2): 284–92 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang B, Vellody D, Homburger H, Yunginger JW. Cockroach cause of allergic asthma. Its specificity and immunologic profile. The Journal of allergy and clinical immunology 1979; 63(2): 80–6. [DOI] [PubMed] [Google Scholar]
- 6.Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy 2016; 71(4): 463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glesner J, Filep S, Vailes LD, et al. Allergen content in German cockroach extracts and sensitization profiles to a new expanded set of cockroach allergens determine in vitro extract potency for IgE reactivity. The Journal of allergy and clinical immunology 2019; 143(4): 1474–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomes A, Glesner J, Calatroni A, et al. Cockroach allergen component analysis of children with or without asthma and rhinitis in an inner-city birth cohort. The Journal of allergy and clinical immunology 2019; 144(4): 935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. The Journal of allergy and clinical immunology 2005; 115(3): 478–85. [DOI] [PubMed] [Google Scholar]
- 10.Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. The Journal of allergy and clinical immunology 2017; 140(2): 565–70. [DOI] [PubMed] [Google Scholar]
- 11.Wood RA, Togias A, Wildfire J, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. The Journal of allergy and clinical immunology 2014; 133(3): 846–52 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Do DC, Ishmael FT, et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. The Journal of allergy and clinical immunology 2018; 141(1): 350–64 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do DC, Mu J, Ke X, et al. miR-511-3p protects against cockroach allergen-induced lung inflammation by antagonizing CCL2. JCI Insight 2019; 4(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do DC, Yang S, Yao X, Hamilton RG, Schroeder JT, Gao P. N-glycan in cockroach allergen regulates human basophil function. Immun Inflamm Dis 2017; 5(4): 386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdeva K, Do DC, Zhang Y, Hu X, Chen J, Gao P. Environmental Exposures and Asthma Development: Autophagy, Mitophagy, and Cellular Senescence. Frontiers in immunology 2019; 10: 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32(5): 593–604. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Pomares L The mannose receptor. Journal of leukocyte biology 2012; 92(6): 1177–86. [DOI] [PubMed] [Google Scholar]
- 18.Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 2011; 34(5): 651–64. [DOI] [PubMed] [Google Scholar]
- 19.Emara M, Royer PJ, Abbas Z, et al. Recognition of the major cat allergen Fel d 1 through the cysteine-rich domain of the mannose receptor determines its allergenicity. The Journal of biological chemistry 2011; 286(15): 13033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royer PJ, Emara M, Yang C, et al. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol 2010; 185(3): 1522–31. [DOI] [PubMed] [Google Scholar]
- 21.Squadrito ML, Pucci F, Magri L, et al. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell reports 2012; 1(2): 141–54. [DOI] [PubMed] [Google Scholar]
- 22.Heinsbroek SE, Squadrito ML, Schilderink R, et al. miR-511-3p, embedded in the macrophage mannose receptor gene, contributes to intestinal inflammation. Mucosal immunology 2016; 9(4): 960–73. [DOI] [PubMed] [Google Scholar]
- 23.Huang T, Jia Z, Fang L, et al. Extracellular vesicle-derived miR-511-3p from hypoxia preconditioned adipose mesenchymal stem cells ameliorates spinal cord injury through the TRAF6/S1P axis. Brain research bulletin 2022; 180: 73–85. [DOI] [PubMed] [Google Scholar]
- 24.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21(4): 575–81. [DOI] [PubMed] [Google Scholar]
- 25.Pi F, Binzel DW, Lee TJ, et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol 2018; 13(1): 82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014; 19(2): 1568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracia T, Wang X, Su Y, et al. Urinary Exosomes Contain MicroRNAs Capable of Paracrine Modulation of Tubular Transporters in Kidney. Scientific reports 2017; 7: 40601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reza AMM, Choi Y-JJ, Yasuda H, Kim J-HH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Scientific reports 2016; 6: 38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z, Li Z, Xu C, Guo B, Guo P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J Control Release 2019; 311-312: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo S, Vieweger M, Zhang K, et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nature communications 2020; 11(1): 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura H, Quadros RM, Gurumurthy CB, Ohtsuka M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat Protoc 2018; 13(1): 195–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Shen Y, Zhao Y, et al. Epithelial Aryl Hydrocarbon Receptor Protects From Mucus Production by Inhibiting ROS-Triggered NLRP3 Inflammasome in Asthma. Frontiers in immunology 2021; 12(4810): 767508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do DC, Zhang Y, Tu W, et al. Type II alveolar epithelial cell-specific loss of RhoA exacerbates allergic airway inflammation through SLC26A4. JCI Insight 2021; 6(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Do DC, Hu X, et al. CaMKII oxidation regulates cockroach allergen-induced mitophagy in asthma. The Journal of allergy and clinical immunology 2021; 147(4): 1464–77 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4): 402–8. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Scientific reports 2018; 8(1): 14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasinski DL, Schwartz CT, Haque F, Guo P. Large scale purification of RNA nanoparticles by preparative ultracentrifugation. Methods Mol Biol 2015; 1297: 67–82. [DOI] [PubMed] [Google Scholar]
- 38.Shu D, Li H, Shu Y, et al. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015; 9(10): 9731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research 2015; 43(7): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016; 32(18): 2847–9. [DOI] [PubMed] [Google Scholar]
- 41.Wickham H ggplot2: Elegant Graphics for Data Analysis.: Springer-Verlag; New York; 2016. [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 1995; 57(1): 289–300. [Google Scholar]
- 43.Kuipers ME, Nolte-’t Hoen ENM, van der Ham AJ, et al. DC-SIGN mediated internalisation of glycosylated extracellular vesicles from Schistosoma mansoni increases activation of monocyte-derived dendritic cells. J Extracell Vesicles 2020; 9(1): 1753420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol Pharm 2015; 12(10): 3650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizutani N, Nabe T, Yoshino S. Complement C3a regulates late asthmatic response and airway hyperresponsiveness in mice. Journal of immunology (Baltimore, Md : 1950) 2009; 183(6): 4039–46. [DOI] [PubMed] [Google Scholar]
- 46.Vedel-Krogh S, Rasmussen KL, Nordestgaard BG, Nielsen SF. Complement C3 and allergic asthma: a cohort study of the general population. The European respiratory journal 2021; 57(2). [DOI] [PubMed] [Google Scholar]
- 47.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA 2004; 10(10): 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue P, Huang S, Han X, et al. Exosomal miR-101–3p and miR-423–5p inhibit medulloblastoma tumorigenesis through targeting FOXP4 and EZH2. Cell death and differentiation 2022; 29(1): 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Squadrito ML, Pucci F, Magri L, et al. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell reports 2012; 1(2): 141–54. [DOI] [PubMed] [Google Scholar]
- 50.Awuah D, Alobaid M, Latif A, Salazar F, Emes RD, Ghaemmaghami AM. The Cross-Talk between miR-511-3p and C-Type Lectin Receptors on Dendritic Cells Affects Dendritic Cell Function. J Immunol 2019; 203(1): 148–57. [DOI] [PubMed] [Google Scholar]
- 51.Banat H, Ambrus R, Csoka I. Drug combinations for inhalation: Current products and future development addressing disease control and patient compliance. Int J Pharm 2023; 643: 123070. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang B, Deng L, Yang F, et al. Albumin-based formononetin nanomedicines for lung injury and fibrosis therapy via blocking macrophage pyroptosis. Mater Today Bio 2023; 20: 100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie C, Ya Likun MM, Luo QL, Dong JC. Role of cellular senescence in inflammatory lung diseases. Cytokine Growth Factor Rev 2023; 70: 26–40. [DOI] [PubMed] [Google Scholar]
- 54.Wan R, Srikaram P, Guntupalli V, Hu C, Chen Q, Gao P. Cellular senescence in asthma: from pathogenesis to therapeutic challenges. EBioMedicine 2023; 94: 104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaqoubi S, Adibkia K, Nokhodchi A, et al. Co-electrospraying technology as a novel approach for dry powder inhalation formulation of montelukast and budesonide for pulmonary co-delivery. Int J Pharm 2020; 591: 119970. [DOI] [PubMed] [Google Scholar]
- 56.Khisamutdinov EF, Bui MN, Jasinski D, Zhao Z, Cui Z, Guo P. Simple Method for Constructing RNA Triangle, Square, Pentagon by Tuning Interior RNA 3WJ Angle from 60 degrees to 90 degrees or 108 degrees. Methods Mol Biol 2015; 1316: 181–93. [DOI] [PubMed] [Google Scholar]
- 57.Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res 2018; 191: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ross EA, Devitt A, Johnson JR. Macrophages: The Good, the Bad, and the Gluttony. Frontiers in immunology 2021; 12: 708186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shalaby KH, Al Heialy S, Tsuchiya K, et al. The TLR4-TRIF pathway can protect against the development of experimental allergic asthma. Immunology 2017; 152(1): 138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan MA, Nicolls MR, Surguladze B, Saadoun I. Complement components as potential therapeutic targets for asthma treatment. Respiratory medicine 2014; 108(4): 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wills-Karp M Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc 2007; 4(3): 247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol 2001; 167(8): 4141–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


