SUMMARY
Resolution of cohesion between sister telomeres in human cells depends on TRF1-mediated recruitment of the polyADP-ribosyltransferase tankyrase to telomeres. In human aged cells, due to insufficient recruitment of TRF1/tankyrase to shortened telomeres, sisters remain cohered in mitosis. This persistent cohesion plays a protective role, but the mechanism by which sisters remain cohered is not well understood. Here we show that telomere repeat-containing RNA (TERRA) holds sister telomeres together through RNA-DNA hybrid (R-loop) structures. We show that a tankyrase-interacting partner, the RNA-binding protein C19orf43, is required for repression of TERRA R-loops. Persistent telomere cohesion in C19orf43-depleted cells is counteracted by RNaseH1, confirming that RNA-DNA hybrids hold sisters together. Consistent with a protective role for persistent telomere cohesion, depletion of C19orf43 in aged cells reduces DNA damage and delays replicative senescence. We propose that the inherent inability of shortened telomeres to recruit R-loop-repressing machinery permits a controlled onset of senescence.
Graphical Abstract

In brief
In aged human cells, sister telomeres remain cohered into mitosis. Sze et al. show that sister telomeres are held together by RNA-DNA hybrid (R-loop) structures. Depletion of the tankyrase-interacting, RNA-binding protein C19orf43 increases R-loops at telomeres and delays senescence onset, indicating a protective role for telomeric R-loops in aged cells.
INTRODUCTION
Human telomeres consist of tandem arrays of double-stranded TTAGGG repeats, telomere repeat-containing RNA (TERRA), and the shelterin complex. Shelterin comprises six subunits, two of which, TRF1 and TRF2, bind directly and specifically to the double-stranded TTAGGG repeats.1 Shelterin and associated factors collaborate to protect chromosome ends from being viewed as double-strand breaks, regulate telomere length and replication, and control maintenance and resolution of telomere cohesion.2–4
Resolution of cohesion between sister telomeres prior to mitosis requires the polyADP-ribosyltransferases, tankyrase 1 and tankyrase 2. A distinguishing feature of tankyrases is their ankyrin repeat domains, which can serve as platforms for multiple binding proteins through a consensus tankyrase-binding motif (TBM), Rxx(G/P/A)xGxx.2,5–8 Tankyrases localize to telomeres by binding TRF1 in late S/G2 to resolve cohesion.9,10 In cells depleted of tankyrase 1 and/or 2 or where the TBM in endogenous TRF1 is mutated, sister telomeres remain cohered in mitosis despite resolution of arms and centromeres.11–14 Cells with persistent telomere cohesion undergo a prolonged anaphase, but, ultimately, telomeres resolve and cells exit mitosis.15
Despite the positive actions of shelterin and associated factors, telomere function becomes compromised in normal human cells. Due to the end-replication problem and nucleolytic processing, telomeres shorten following each round of DNA replication.16,17 This shortening can be counteracted by telomerase, which adds telomere repeats de novo to chromosomes ends.18,19 However, telomerase is repressed in the human soma.20 Thus, as cells age, the resulting shortened telomeres are unable to recruit sufficient shelterin to protect the ends, leading to a persistent DNA damage response that signals replicative senescence.21 Cancer cells must acquire a telomere maintenance mechanism to override senescence; most upregulate telomerase, but 10%–15% activate a recombination-based, alternative lengthening of telomeres (ALT) mechanism.22,23 As a result of the recombination, ALT cells have very long heterogeneous telomeres, but a fraction of their telomeres are critically short, like aged cells.24
Interestingly, ALT cancer cells and normal aged cells have another feature in common: persistent telomere cohesion. Thus, similar to tankyrase-depleted or TRF1-mutated cells, telomeres of aged and ALT cells are persistently cohered into mitosis.15,25–27 Persistent cohesion is a direct consequence of telomere shortening; it can be counteracted by introduction of telomerase into aged or ALT cells11,27 and, conversely, be induced by long-term inhibition of telomerase in telomerase-positive cancer cells.11 Short telomeres in aged and ALT cells do not recruit sufficient TRF1/tankyrase to resolve cohesion. Forced resolution of cohesion by overexpression of TRF1 results in subtelomere recombination with non-sisters, DNA damage, and a senescent-like growth arrest.11,26 Thus, persistent cohesion is a naturally occurring protective state at short telomeres.
In addition to shelterin and associated factors, telomeres are bound by the telomere repeat-containing RNA (TERRA).28–30 TERRA is transcribed by RNA polymerase II from subtelomeric promoters into the telomere tract toward the ends of chromosomes using the telomeric C-rich strand as the template.28,31–34 TERRA is retained at telomeres through association with telomeric proteins or by base-pairing with telomeric DNA to form R-loop structures consisting of an RNA-DNA hybrid and a displaced DNA strand.35,36 R-loops are found throughout the genome and impact genome integrity in multiple ways.37,38 TERRA R-loops can form during transcription in cis or post transcriptionally in trans.39 TERRA is implicated in multiple functions, including telomere length regulation by telomerase, telomere replication, and the telomeric DNA damage response.40,41 TERRA R-loops also appear to play important roles in telomerase-negative cells where they are regulated by RNaseH, a family of endonucleases that cleave the RNA in an RNA-DNA hybrid.42 In ALT cells, TERRA levels are high and TERRA R-loops are regulated by the RNA endonuclease RNaseH1 to control recombination.43,44 In telomerase-negative budding yeast, TERRA R-loops are regulated by RNaseH1 and RNaseH2. Here, R-loops accumulate at short telomeres and slow the rate of replicative senescence.45,46
Compelled by the similarities between the role of TERRA RNA-DNA hybrids (in ALT cells and in telomerase-negative budding yeast) and in persistent telomere cohesion (in ALT and aged human cells), we investigated a role for TERRA R-loops in persistent telomere cohesion. We show that overexpression of RNaseH1 in cells or incubation of purified RNaseH1 in situ can resolve sister telomere cohesion. We further identify the RNA-binding protein C19orf43 as a tankyrase binding partner whose depletion results in persistent telomere cohesion, increased TERRA R-loops, and delayed replicative senescence, indicating a beneficial role for R-loops at aged human telomeres.
RESULTS
C19orf43 is a tankyrase-binding protein that is required for resolution of telomere cohesion
Tankyrase is recruited to telomeres by TRF1 to resolve cohesion. Its ankyrin repeat domain allows binding to multiple TBM-containing partners at once, imparting a scaffolding capacity that we reasoned could play a role in the resolution of cohesion. Two proteomic screens identified the uncharacterized protein C19orf43 as a potential tankyrase-binding protein.12,47 C19orf43 was also identified as a component of a partially purified nuclear extract and shown to have exoribonuclease activity in vitro.48 It was named human telomerase RNA interacting RNase (hTRIR), despite no presented evidence for a specific association with hTR. It was shown to have 5′ and 3′ exoribonuclease activity in vitro across a wide range of substrates, including hTR. A subsequent study identified C19orf43 as a protein phosphatase 4 (PP4) interacting protein (IP) and renamed it PP4IP.49 In our study, motivated by its potential interaction with tankyrase and RNA, we queried the role of C19orf43 in telomere cohesion.
C19orf43 has two potential TBMs, but no other obvious motifs (Figure 1A). To validate the interaction between C19orf43 and tankyrase, HEK293T cells extracts were immunoprecipitated with anti-TNKS or control IgG. As shown in Figure 1B, endogenous C19orf43 specifically co-immunoprecipitated with endogenous TNKS. We generated stable cell lines depleted for C19orf43 using two different C19orf43 shRNA-expressing lentiviruses (#1 and #4). Immunoblot analysis showed efficient depletion with both shRNAs (Figure 1C). We then asked if the potential TBMs in C19orf43 were required for interaction with tankyrase. We generated a triple FLAG epitope-tagged allele of C19orf43 and mutated the essential R in each of the binding motifs to generate mutants R7G and R106G. Vector control, C19orf43.wild-type (C19orf43.WT), and the C19orf43 mutant plasmids R7G and R106G were each transfected into the HEK293T C19orf43 shRNA#1 cell line. The shRNA#1 targets the 3′ untranslated region of C19orf43 and therefore does not affect the transfected constructs as they contain only C19orf43 coding sequence. Transfected cell extracts were immunoprecipitated with anti-FLAG antibody. As shown in Figure 1D, FLAGC19orf43.WT and R106G, but not R7G, co-immunoprecipitated endogenous tankyrase, indicating that the amino terminal (but not the internal) TBM is required for C19orf43 interaction with tankyrase. Treatment of the immunoprecipitates with nucleases RNaseA, DNase1, or RNaseH1 did not affect the binding, confirming a protein-protein interaction between C19orf43 and tankyrase (Figure S1A).
Figure 1. C19orf43 is a tankyrase-binding protein that is required for resolution of telomere cohesion.
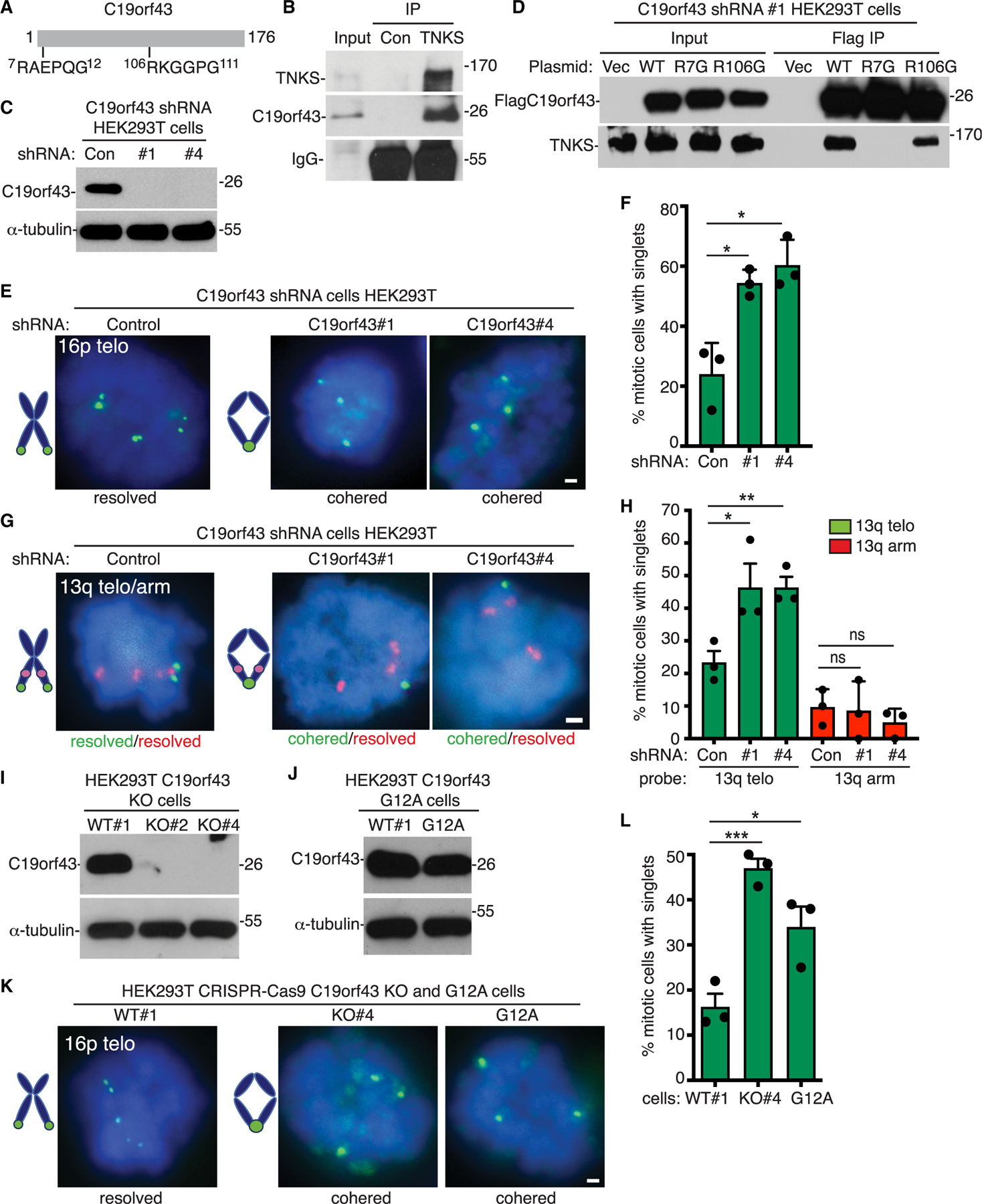
(A) Schematic of C19orf43 with tankyrase-binding motifs (TBMs) indicated.
(B) Immunoblot analysis of HEK293T cell extracts immunoprecipitated (IP) with control or anti-TNKS antibody.
(C) Immunoblot analysis of cell extracts from HEK293T cells stably expressing control or C19orf43 shRNAs #1 or #4.
(D) Immunoblot analysis of cell extracts from C19orf43 shRNA #1 HEK293T cells transfected with vector or C19orf43 WT, R7G, or R106G and immunoprecipitated with anti-FLAG antibody (FLAG IP).
(E) FISH analysis of HEK293T control or C19orf43 shRNA (#1 or #4) mitotic cells using a 16p subtelo probe (green).
(F) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 37–69 cells each) ± SEM. *p ≤ 0.05, Student’s unpaired t test.
(G) FISH analysis of HEK293T control or C19orf43 shRNA (#1 or #4) mitotic cells with a dual 13q subtelo (green)/arm (red) probe.
(H) Quantification of the frequency of mitotic cells with cohered telomeres and arms. Three independent experiments (n = 26–29 cells each) ± SEM. *p ≤ 0.05, **p≤ 0.01, Student’s unpaired t test; ns, not significant.
(I) Immunoblot analysis of cell extracts from CRISPR-Cas9-generated HEK293T knockout (KO) cell lines #2 and #4.
(J) Immunoblot analysis of cell extracts from CRISPR-Cas9-generated HEK293T mutant G12A cell line.
(K) FISH analysis of WT, KO#4, or G12A HEK293T mitotic cells using a 16p subtelo probe (green).
(L) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 44–56 cells each) ± SEM. *p ≤ 0.05, ***p ≤ 0.001, Student’s unpaired t test. (E, G, K) DNA was stained with DAPI (blue). Scale bars represent 2 μm.
To determine if C19orf43 is required for resolution of cohesion, we analyzed the shRNA cell lines using fluorescent in situ hybridization (FISH). Mitotic cells were isolated from asynchronous cultures by shake-off, fixed, collected in a cytospin onto cover slips, and probed with the subtelomere-specific chromosome probe 16p. As shown in Figures 1E and 1F, in control mitotic cells, telomeres appeared as doublets (resolved). However, in C19orf43-depleted cell lines #1 and #4, telomeres appeared as singlets (cohered) in mitosis, indicating persistent telomere cohesion. To determine if the persistent cohesion was specific to telomeres, we performed FISH using a dual telomere/arm 13q probe. Note, HEK293T cells have a triple locus for the 13q arm, but due to a subtelomere deletion, only a single locus for the 13q-telomere. FISH analysis showed that the telomere, but not the arms, exhibited persistent telomere cohesion in C19orf43-depleted cells (Figures 1G and 1H). Thus, C19orf43 is required specifically for telomere cohesion.
We further validated the role of C19orf43 in persistent telomere cohesion using CRISPR-Cas9 to generate C19orf43 HEK293T knockout cell lines: KO#2 and KO#4 (Figure 1I) and knockin cell lines where the essential G in the amino terminal TBM of the endogenous C19orf43 gene was mutated to A to generate a C19orf43 mutant cell line G12A (Figure 1J). FISH analysis showed that both the C19orf43 KO#4 and the C19orf43.G12A cell lines exhibited persistent telomere cohesion (Figures 1K and 1L). Depletion of tankyrase using siRNA in C19orf43 KO#4 cells did not have an additive effect on persistent cohesion (Figures S1B–S1D).
C19orf43 counteracts persistent telomere cohesion in C19orf43-depleted and aged cells
To confirm that C19orf43 itself can counteract persistent telomere cohesion and that its N-terminal TBM is required, we transfected vector or C19orf43 (WT, R7G, or R106G) into the C19orf43-depleted (shRNA#1) cell line, performed immunoblot analysis (Figure 2A), and measured telomere cohesion by FISH analysis. As shown in Figures 2B and 2C, FLAGC19orf43.WT and R106G (but not R7G) counteracted persistent telomere cohesion, indicating that C19orf43 (and its amino terminal TBM) is required for resolution of cohesion. We further demonstrated that in cells deleted of C19orf43 (KO#4 cells), reintroduction of C19orf43.WT counteracted the persistent cohesion (Figures 2D–2F).
Figure 2. C19orf43 (wild type, but not mutant) counteracts persistent telomere cohesion in C19orf43-depleted and aged cells.
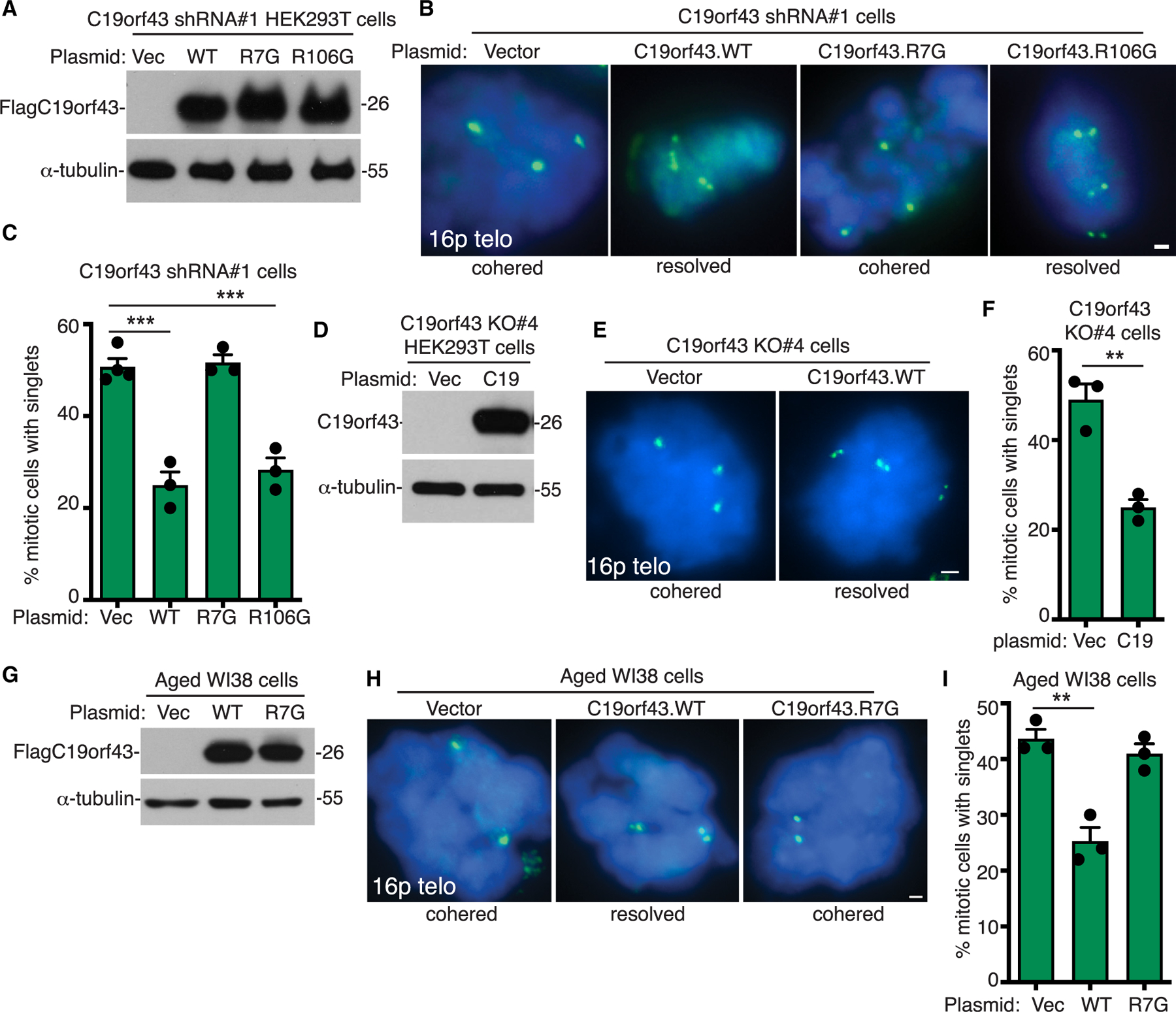
(A) Immunoblot analysis of cell extracts from C19orf43 shRNA#1 HEK293T cells transfected with vector or C19orf43 (WT, R7G, or R106G).
(B) FISH analysis of vector or C19orf43 (WT, R7G, or R106G) transfected C19orf43 shRNA#1 HEK293T mitotic cells with a 16p subtelo probe (green).
(C) Quantification of the frequency of mitotic cells with cohered telomeres. Three to four independent experiments (n = 27–68 cells each) ± SEM. ***p ≤ 0.001, Student’s unpaired t test.
(D) Immunoblot analysis of cell extracts from C19orf43 KO#4 cells transfected with a vector control or C19orf43 WT.
(E) FISH analysis of vector or C19orf43 WT transfected C19orf43 KO#4 cells using a 16p subtelo probe (green).
(F) Quantification of FISH analysis showing the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50–56 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test.
(G) Immunoblot analysis of cell extracts from vector or C19orf43 (WT or R7G) transfected aged WI38 cells.
(H) FISH analysis of vector or C19orf43 (WT or R7G) transfected aged WI38 mitotic cells using a 16p subtelo probe (green).
(I) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 36–50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test. (B, E, H) DNA was stained with DAPI (blue). Scale bars represent 2 μm.
Next, we asked if C19orf43 plays a role in the persistent telomere cohesion that is a naturally occurring feature of aged cells. We transfected pre-senescent WI38 cells with a vector control or C19orf43 (WT or R7G) and performed immunoblot (Figure 2G) and FISH analysis. As shown in Figures 2H and 2I, C19orf43.WT (but not R7G) counteracted persistent telomere cohesion. Similar results were obtained with transfection into ALT GM847 cells. C19orf43.WT (but not R7G) counteracted persistent telomere cohesion (Figures S2A–S2C). Thus, C19orf43 is required for resolution of telomere cohesion, and its overexpression in aged or ALT cells can counteract the persistent telomere cohesion phenotype, dependent on its ability to interact with tankyrase. Finally, we asked if C19orf43-mediated resolution of telomere cohesion was fully dependent on tankyrase for its resolving activity. For this, we used HEK293T TNKS1/2 DKO (double knockout) cells generated previously by CRISPR-Cas9 deletion of TNKS1 and TNKS2.12 TNKS1/2 DKO cells were transfected with vector or C19orf43 (WT or R7G) and analyzed by immunoblot and FISH. As shown in Figures S2D–S2F, C19orf43 counteracted the persistent cohesion, but here (unlike in the cell types above that express tankyrase), it was not dependent on its TBM. This validates that the TBM requirement is due to tankyrase and further shows that C19orf43 (at least under conditions of overexpression in the absence of tankyrase) can counteract persistent telomere cohesion, independent of its TBM. Altogether, these data indicate that C19orf43 is an essential component of the persistent telomere cohesion phenotype.
C19orf43 binds TERRA, protects TERRA levels, and counteracts TERRA R-loops
We next asked if depleting C19orf43 would impact TERRA levels. Purified C19orf43 was shown to have exoribonuclease activity in vitro on a broad range of substrates.48 One prediction is that loss of a nuclease activity might lead to increased TERRA levels. We evaluated TERRA levels in C19orf43 KO cell lines #2 and #4 by RNA dot blot analysis. Blots were probed with 32P-labeled (CCCTAA)4 Telo C probe that hybridizes to TERRA and normalized to an 18S rRNA probe. As shown in Figures 3A and 3B, we observed a significant reduction in TERRA levels. Additionally, we measured TERRA levels by northern blot analysis of C19orf43 shRNA lines #1 and #4. Blots were probed using the Telo C probe and normalized to 18S rRNA. As shown in Figures 3C and 3D, we observed a reduction in TERRA. Finally, we measured TERRA using qRT-PCR in C19orf43 shRNA lines #1 and #4 cell lines using a set of subtelomere-specific (2p, 9p, 16p, and 18p) and telomere-specific (Telo TTAGGG) primers, normalizing to the reference gene GAPDH. As shown in Figure 3E, we observed a reduction in TERRA levels. Together, these data indicate that TERRA levels are reduced in C19orf43-depleted cells.
Figure 3. C19orf43 depletion leads to a decrease in TERRA and an increase in TERRA R-loops.
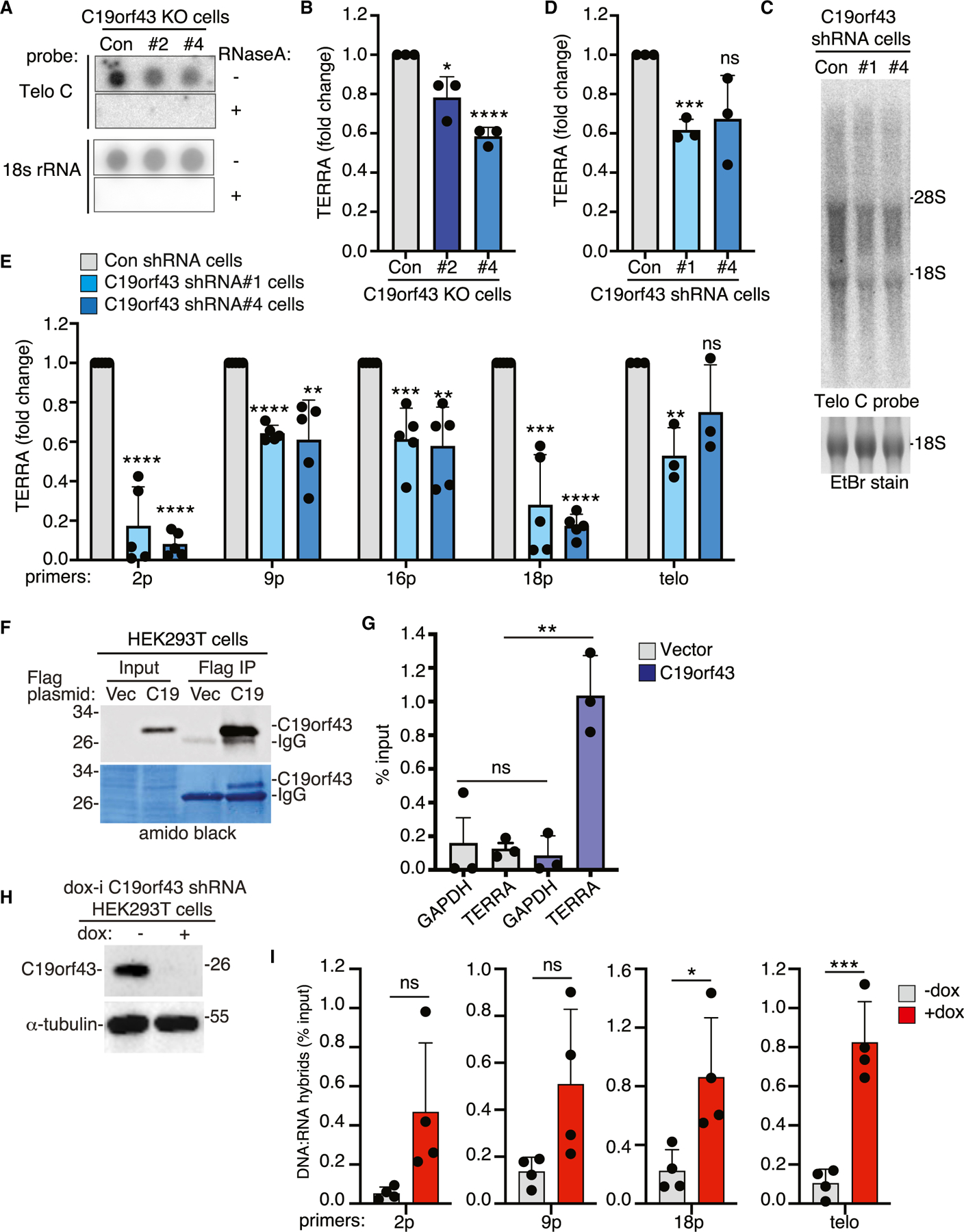
(A) RNA dot blot analysis of TERRA or 18S rRNA levels in control or C19orf43 KO#2 and #4 HEK293T cell lines. Samples were treated (−) or (+) RNaseA as a control. Blots were probed with a Telo C TERRA or 18S rRNA probe.
(B) Quantification of TERRA levels normalized to 18S rRNA levels plotted as fold change over control cells. Three independent experiments ± SEM. *p ≤ 0.05, ****p ≤ 0.0001, Student’s unpaired t test.
(C) Northern blot analysis of TERRA levels in control or C19orf43 shRNA #1 and #4 HEK293T cell lines. Blots were probed with a Telo C TERRA probe.
(D) Quantification of TERRA levels normalized to 18S rRNA levels plotted as fold change over control cells. Three independent experiments ± SEM. ***p ≤ 0.001, Student’s unpaired t test. ns, not significant.
(E) qRT-PCR analysis of RNA samples from control or C19orf43 shRNA #1 and #4 HEK293T cell lines. TERRA levels from individual sub-telomeres (2p, 9p, 16p, 18p) and all telomeres (Telo) were measured from total RNA, normalized to GAPDH, and plotted as fold change over control cells. The bars represent the average value from three to five independent experiments ± SEM. **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, Student’s unpaired t test. ns, not significant.
(F) Immunoblot analysis of cell extracts from HEK293T cells transfected with vector or C19orf43 and immunoprecipitated with anti-FLAG antibody (FLAG IP).
(G) RNA immunoprecipitation (RIP) analysis using anti-FLAG antibody in extracts from vector or C19orf43 transfected HEK293T cells. Input and immunoprecipitated samples were analyzed by qRT-PCR using GAPDH and Telo primers and plotted as percent input. Three independent experiments ± SEM. **p ≤ 0.01, Student’s unpaired t test. ns, not significant.
(H) Immunoblot analysis of dox-inducible (dox-i) C19orf43 shRNA HEK293T cells following 48 h of treatment (−) or (+) dox.
(I) DNA:RNA immunoprecipitation (DRIP) analysis using S9.6 antibody in extracts from −dox and +dox dox-i C19orf43 HEK293T shRNA cells. In vitro digestion with RNaseH1 prior to immunoprecipitation served as a negative control for the specificity of S9.6 antibody. Immunoprecipitates and input samples were analyzed by qPCR with primer sets amplifying 2p, 9p, 18p subtelomeric DNA or telomeric DNA and plotted as percent input. Four independent experiments ± SEM. *p ≤ 0.05, ***p ≤ 0.001, Student’s unpaired t test. ns, not significant. See also Figure S3 and Table S1.
The reduction in TERRA levels observed upon C19orf43 depletion raised the possibility that C19orf43 might bind and protect TERRA. To determine if C19orf43 is bound to TERRA in cells, we performed RNA immunoprecipitation (RIP) on FLAGC19orf43 versus FLAGvector transfected HEK293T cells using anti-FLAG antibody (Figure 3F). Immunoprecipitated RNA was analyzed by qRT-PCR using GAPDH or telomere TTAGGG-specific primers and plotted as percent input. As shown in Figure 3G, C19orf43 associated specifically with TERRA.
TERRA is also found as R-loops, triple-stranded structures that comprise an RNA-DNA hybrid and a DNA single strand. We thus asked if loss of C19orf43 would impact TERRA R-loop levels. For this, we created a doxycycline-inducible lentiviral shRNA plasmid (dox-i C19orf43 shRNA) using the C19orf43 shRNA #1 sequence described above and generated a stable dox-i cell line in HEK293T cells. Cells were induced with doxycycline (dox) for 48 h and analyzed by immunoblot. As shown in Figure 3H, C19orf43 levels were significantly reduced. RNA-DNA hybrid levels were assayed using DNA:RNA immunoprecipitation (DRIP) with the S9.6 antibody, which detects R-loops.50 As a control, prior to immunoprecipitation, the nucleic acids were treated in situ with or without ribonuclease RNaseH1, which hydrolyzes the RNA of RNA-DNA hybrids.42 The DNA component of the immunoprecipitated hybrid and input samples was then quantified by qPCR with primer sets amplifying 2p, 9p, 18p subtelomeric DNA or telomeric DNA and plotted as percent input. As shown in Figure 3I, we observed an increase in TERRA R-loops in the +dox (C19orf43-depleted) compared to the −dox (control) cells. DRIP using the Telo and 18p primers reached statistical significance; while 2p and 9p did not (p values of 0.057 and 0.061, respectively), there was a clear trend toward an increase in R-loops. Analysis using previously characterized R-loop-positive (RPL13A) and -negative (SNRPN) control loci51 showed a slight (not statistically significant) trend toward an increase in R-loops at the RPL13A (but not the SNRPN) locus in +dox (C19orf43-depleted) cells compared to the −dox (control) cells, suggesting that C19orf43 might influence other loci (Figure S3A).
RNaseH1 counteracts persistent telomere cohesion
Our data above, indicating that depletion of C19orf43 leads to persistent telomere cohesion and to an increase in TERRA R-loops, raised the possibility that increased TERRA R-loop levels account for the persistent telomere cohesion phenotype. We thus asked if overexpression of RNaseH1 impacts persistent telomere cohesion. C19orf43 shRNA #1 cells were transfected with a vector control, RNaseH1.WT, or RNaseH1.CD (a catalytically dead mutant with a D145 mutation),43 analyzed by immunoblot (Figure 4A), and subjected to FISH analysis. As shown in Figures 4B and 4C, RNaseH1 WT, but not CD, counteracted persistent telomere cohesion. We next asked if the persistent cohesion observed in TNKS1/2 DKO cells was due to R-loops. TNKS1/2 DKO cells were transfected with a vector control, RNaseH1.WT, or RNaseH1.CD, analyzed by immunoblot (Figure 4D), and subjected to FISH analysis. As shown in Figures 4E and 4F, RNaseH1 WT, but not CD, counteracted the persistent telomere cohesion.
Figure 4. RNaseH1 counteracts persistent telomere cohesion.
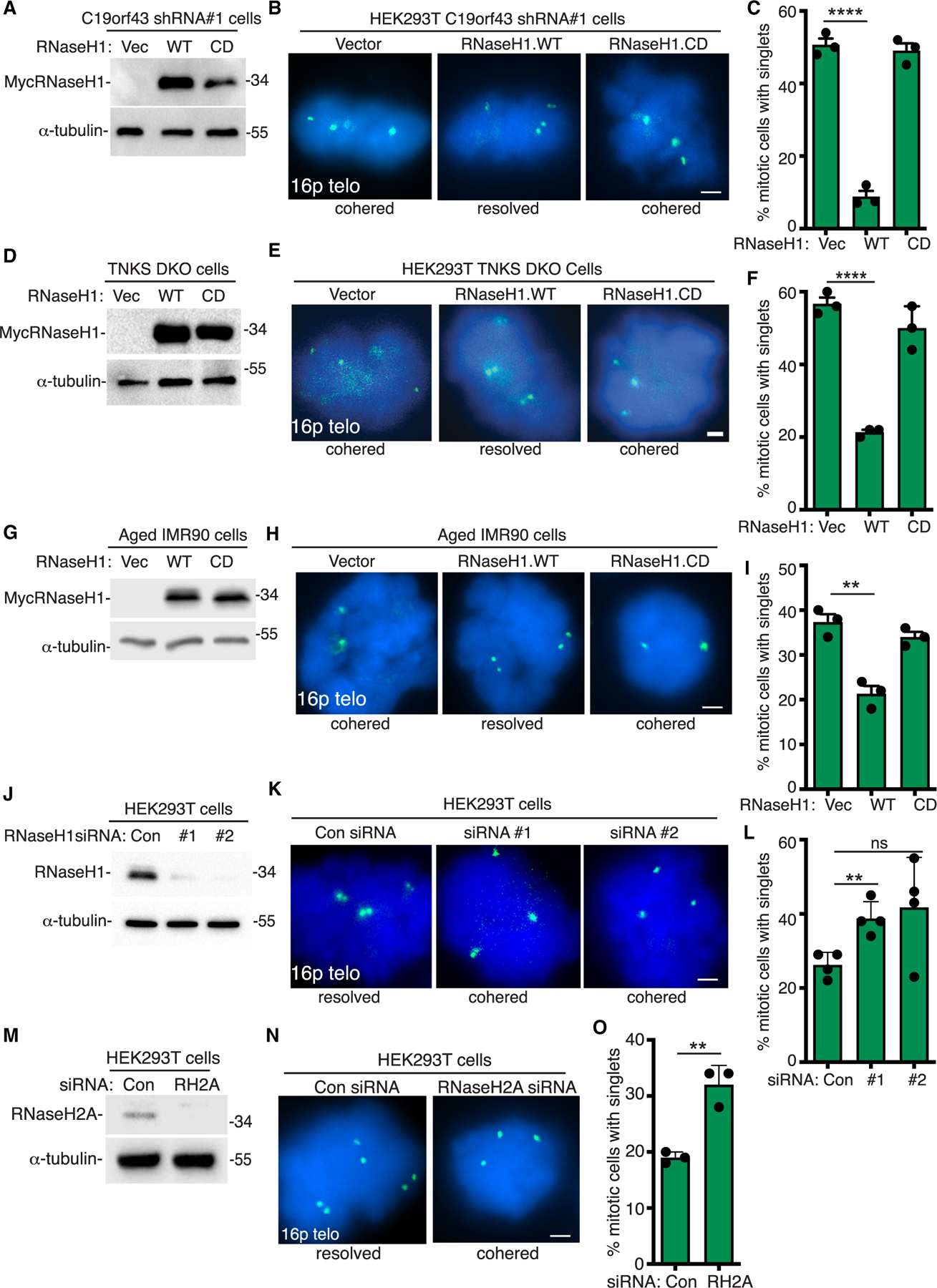
(A) Immunoblot analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected C19orf43 shRNA#1 HEK293T cell extracts.
(B) FISH analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected C19orf43 shRNA#1 HEK293T mitotic cells using a 16p subtelo probe (green).
(C) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. ****p ≤ 0.0001, Student’s unpaired t test.
(D) Immunoblot analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected HEK293T TNKS1/2 DKO cell extracts.
(E) FISH analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected HEK293T TNKS1/2 DKO mitotic cells using a 16p subtelo probe (green).
(F) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. ****p ≤ 0.0001, Student’s unpaired t test.
(G) Immunoblot analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected aged IMR90 cell extracts.
(H) FISH analysis of vector, RNaseH1.WT, or RNaseH1.CD transfected aged IMR90 mitotic cells using a 16p subtelo probe (green).
(I) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test.
(J) Immunoblot analysis of control, RNaseH1#1, or RNaseH1#2 siRNA transfected HEK293T cell extracts.
(K) FISH analysis of control, RNaseH1#1, or RNaseH1#2 siRNA transfected HEK293T mitotic cells using a 16p subtelo probe (green).
(L) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 49–87 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test. ns, not significant.
(M) Immunoblot analysis of control or RNaseH2A siRNA transfected HEK293T cell extracts.
(N) FISH analysis of control or RNaseH2A siRNA transfected HEK293T mitotic cells using a 16p subtelo probe (green).
(O) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 41–50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test. (B, E, H, K, N) DNA was stained with DAPI (blue). Scale bars represent 2 μm.
We next assessed the impact of RNaseH1 in cells with naturally occurring persistent cohesion. We transfected pre-senescent IMR90 cells with a vector control, RNaseH1.WT, or RNaseH1.CD, performed immunoblot analysis (Figure 4G), and subjected cells to FISH analysis. As shown in Figures 4H and 4I, persistent telomere cohesion in aged IMR90 cells was counteracted by overexpression of RNaseH1.WT but not RNaseH1.CD. Similar results were obtained with transfection into ALT GM847 cells; RNaseH1.WT (but not CD) counteracted persistent telomere cohesion (Figures S4A–S4C).
These results showed that overexpression of RNaseH1 in C19orf43-depleted, TNKS1/2 DKO, aged, or ALT cells counteracted persistent telomere cohesion. To determine if RNaseH1 activity is important to resolve cohesion in WT cells, we measured the impact of RNaseH1 depletion in HEK293T cells. We introduced two distinct RNaseH1 siRNAs (#1 and #2). Immunoblot analysis showed efficient depletion of RNaseH1 (Figure 4J), and FISH analysis showed induction of persistent telomere cohesion in the RNaseH1-depleted cells (Figures 4K and 4L). We also tested the impact of RNaseH2A depletion using siRNA in HEK293T cells. Immunoblot analysis showed efficient depletion of RNaseH2A (Figure 4M), and FISH analysis showed induction of persistent telomere cohesion in the RNaseH2A-depleted cells (Figures 4N and 4O). Altogether, these data, showing that RNaseH1 overexpression counteracts persistent telomere cohesion and RNaseH1 or RNaseH2A depletion induces persistent cohesion, are consistent with a role for RNA-DNA hybrids in telomere cohesion.
Telomeres are cohered in mitotic cells through RNA-DNA hybrids and single-stranded DNA
As described above, overexpression of RNaseH1 counteracted persistent telomere cohesion in cells. In these experiments, RNaseH1 was transfected into C19orf43-depleted cells for 24 h prior to harvesting mitotic cells and subjecting them to FISH analysis. We next queried whether RNaseH1 could resolve telomere cohesion if the purified enzyme was added directly to the cohered telomeres in situ. For this approach, untransfected C19orf43-depleted mitotic cells were harvested, fixed, and collected in a cytospin onto cover slips. The cover slips were then incubated with or without RNaseH1 enzyme and analyzed by FISH (see schematized process in Figure 5A). As shown in Figures 5B and 5C, treatment with RNaseH1 in situ resolved persistent telomere cohesion, indicating that the cohered telomeres (singlets) were held together by RNA-DNA hybrids. We sought to further query the nature of the cohered telomeres by incubating the cover slips with other enzymes in situ. We tested RNaseA, an endoribonuclease that specifically degrades single-stranded RNA and not the RNA-DNA hybrid.52,53 As shown in Figures 5B and 5C, incubation with RNaseA in situ did not resolve cohered telomeres.
Figure 5. RNaseH1 or nuclease S1 resolves cohered sister telomeres in situ, and RAD51 is required for induction of persistent cohesion in cells.
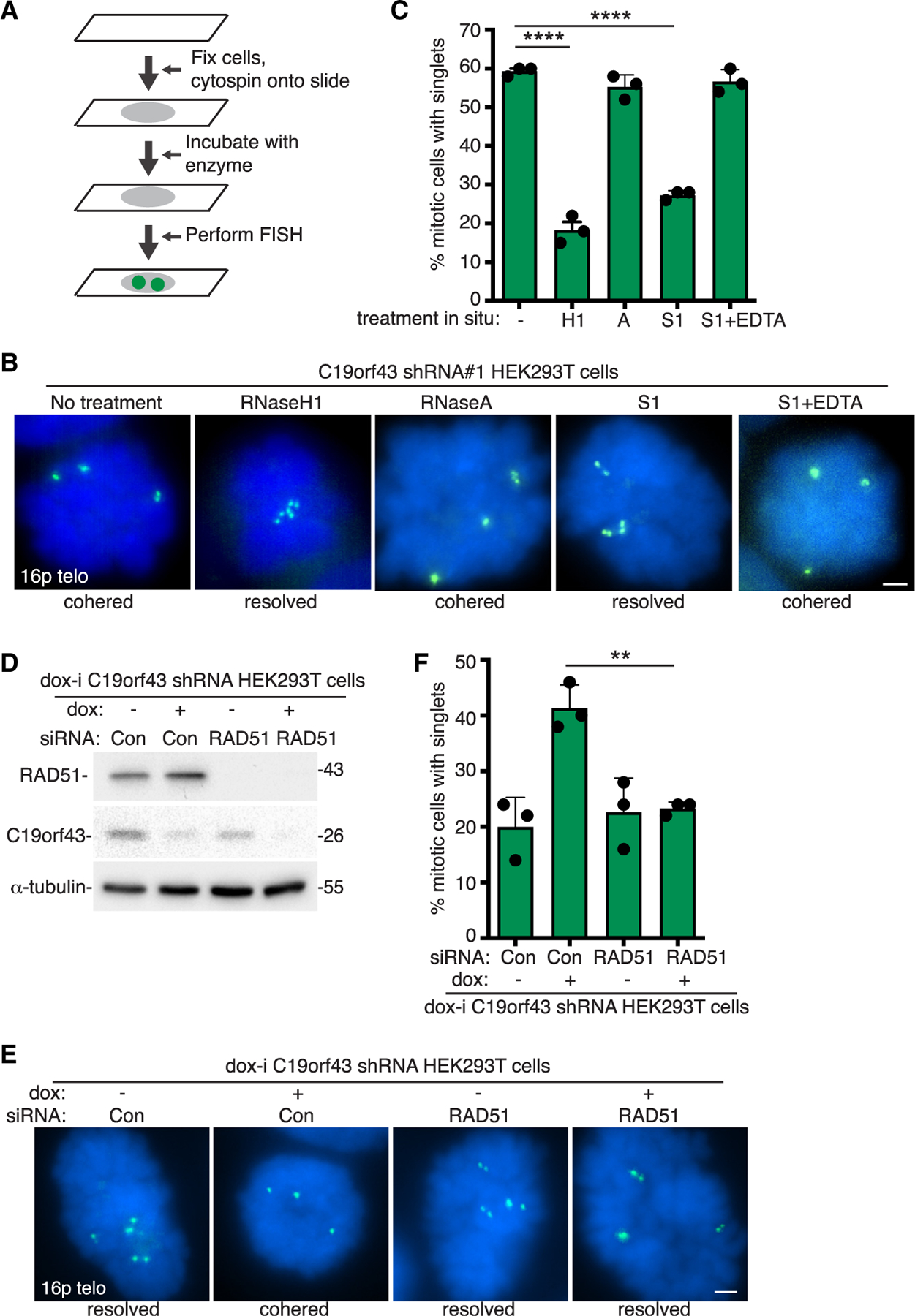
(A) Schematic of experimental approach for enzyme incubations in situ prior to FISH analysis.
(B) FISH analysis of C19orf43 shRNA#1 HEK293T mitotic cells following incubation in situ with no treatment, RNaseH1, RNaseA, nuclease S1, or S1 plus EDTA inhibitor, using a 16p subtelo probe (green).
(C) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. ****p ≤ 0.0001, Student’s unpaired t test.
(D) Immunoblot analysis of dox-i C19orf43 shRNA HEK293T cells following 64 h of treatment with control or RAD51 siRNA. Cells were treated with dox for 48 h prior to harvest.
(E) FISH analysis of mitotic dox-i C19orf43 shRNA HEK293T cells following treatment with control or RAD51 siRNA and − or + dox using a 16p subtelo probe (green).
(F) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test. (B, E) DNA was stained with DAPI (blue). Scale bars represent 2 μm.
We performed additional in situ analysis on cohered telomeres from TNKS1/2 DKO cells and aged WI38 cells. RNaseH1 resolved persistent telomere cohesion on fixed cells from TNKS1/2 DKO (Figures S5A and S5B) and aged WI38 (Figures S5C and S5D) cells. Resolution was blocked by inclusion of the chelator EDTA, which inhibits RNaseH1. Incubation with RNaseA in situ did not resolve cohered telomeres. Together, our data indicate that persistent telomere cohesion in C19orf43-depleted, TNKS1/2 DKO, and aged WI38 cells is mediated by RNA-DNA hybrids.
We additionally incubated fixed mitotic C19orf43-depleted cells with purified recombinant C19orf43 protein and/or recombinant tankyrase in situ. However, as shown in Figures S5E and S5F, unlike RNaseH1, neither C19orf43 nor tankyrase resolved persistent telomere cohesion in situ. Thus, while C19orf43 and tankyrase (like RNaseH1) counteract persistent telomere cohesion when overexpressed in cells, they (unlike RNaseH1) cannot promote resolution of the cohered sisters when incubated with fixed mitotic cells in situ.
Finally, we tested S1, an endonuclease that cleaves single-stranded DNA, on cohered telomeres in situ. As shown in Figures 5B and 5C, sister telomere cohesion was resolved by S1. Resolution was blocked by inclusion of the chelator EDTA, which inhibits S1. Altogether, the in situ analysis indicates that persistent telomere cohesion is mediated by RNA-DNA hybrids and single-stranded DNA but not single-stranded RNA.
Formation of R-loops leads to a displaced single strand. The observation that single-stranded DNA connects sister telomeres suggested the possibility that persistent cohesion could result from a RAD51-mediated invasion between sister telomeres. We thus asked if persistent telomere cohesion was dependent on RAD51. Dox-i C19orf43 shRNA HEK293T cells were treated with control or RAD51 siRNA, followed by dox induction to deplete C19orf43. When C19orf43 was depleted in control siRNA-treated cells, we observed persistent telomere cohesion as expected. By contrast, when C19orf43 was depleted in RAD51 siRNA-treated cells, persistent telomere cohesion was not observed (Figures 5D–5F). Thus, persistent telomere cohesion induced by C19orf43 depletion depends upon RAD51.
C19orf43 depletion extends replicative lifespan of aged IMR90 cells
Persistent telomere cohesion occurs naturally in normal human cells as they approach senescence. We showed previously that this has a protective role: forced resolution of cohesion in aged cells by overexpression of TRF1 led to DNA damage and a senescent-like growth arrest.11 We wondered if inducing persistent cohesion in young cells at an early population doubling (PD) might confer benefits as the cells aged. For this, we generated a stable dox-i C19orf43 shRNA IMR90 cell line at an early PD (PD25). Cells were induced with dox for 48 h and analyzed by immunoblot. As shown in Figure 6A, C19orf43 levels were significantly reduced in the +dox (C19orf43-depleted) cells. FISH analysis showed induction of persistent telomere cohesion in the +dox cells (Figures 6B and 6C). The cells were then passaged in culture −dox or +dox for 120 days. Initially (for the first 40 days), the cells grew at the same rate with or without dox (Figure 6D). However, after 40 days (PD37), the growth rate of the +dox (C19orf43-depleted) cells increased compared to the −dox (control) cells. The −dox (control) cells’ growth rate began to slow and ultimately ceased at 65 days (PD48). The +dox (C19orf43-depleted) cells continued to divide for an additional 55 days (PD62) (Figure 6D). Thus, depletion of C19orf43 imparted a growth advantage to the aged cells. Similar results (demonstrating a growth advantage in C19orf43-depleted cells) were obtained in two additional independent analyses (Figures S6A and S6B).
Figure 6. C19orf43 depletion delays replicative senescence.
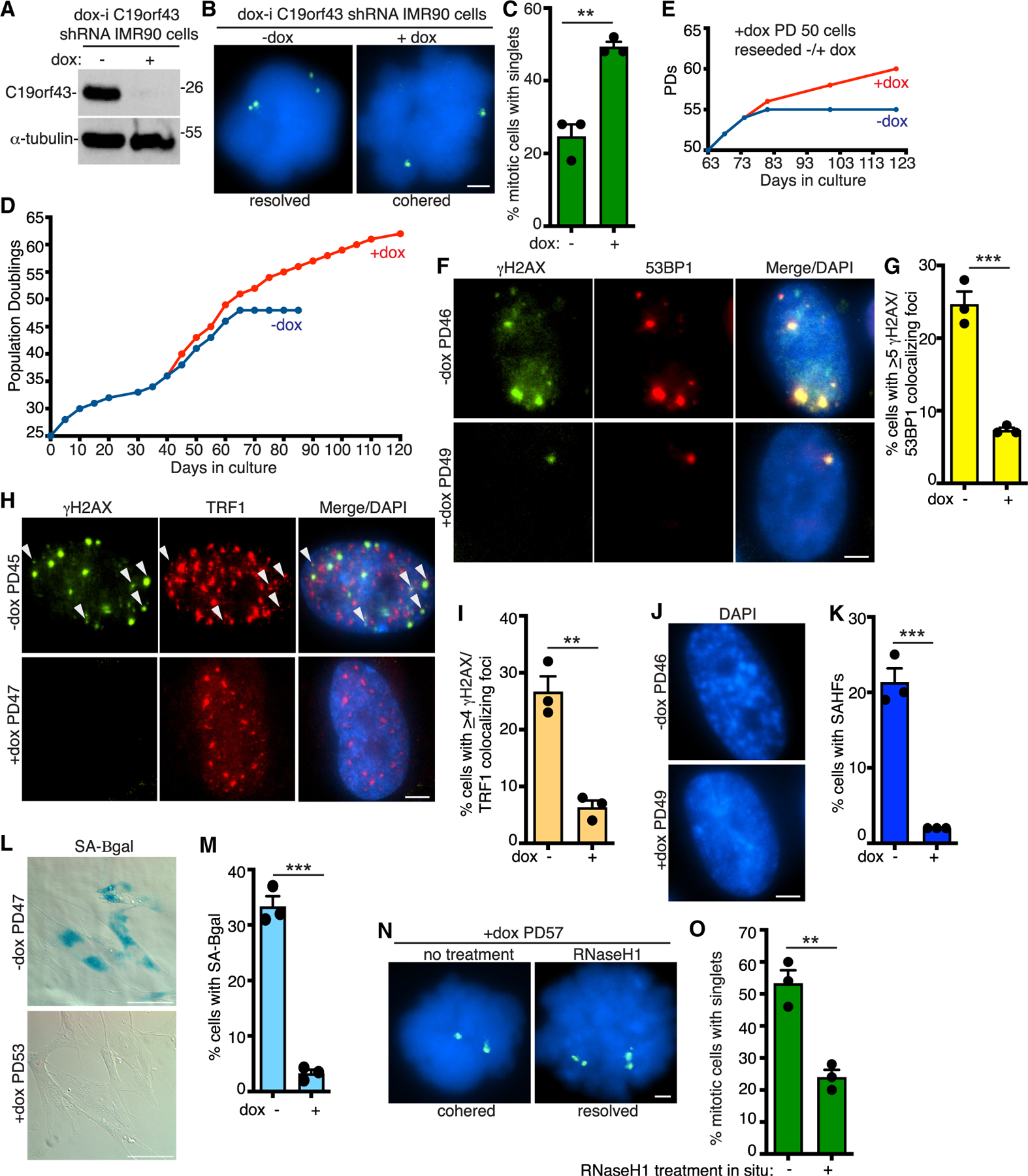
(A) Immunoblot analysis of dox-i C19orf43 shRNA IMR90 cells (−dox PD43) following 48 h (−) or (+) dox.
(B) FISH analysis of mitotic dox-i C19orf43 shRNA IMR90 cells (−dox PD39) following 48 h (−) or (+) dox using a 16p subtelo probe (green).
(C) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test.
(D) Growth curve analysis of dox-i C19orf43 shRNA IMR90 (PD25) cells generated by lentiviral infection and passaged (−) or (+) dox for 120 days.
(E) Growth curve analysis of dox-i C19orf43 shRNA IMR90 (+dox PD50) cells reseeded (−) or (+) dox and passaged for an additional 60 days.
(F) Immunofluorescence analysis of dox-i C19orf43 shRNA IMR90 −dox PD46 or +dox PD49 cells stained with γH2AX (green) and 53BP1 (red) antibodies.
(G) Quantification of the frequency of cells displaying ≥5 γH2AX/53BP1 colocalizing foci from −dox PD43/45/46 or +dox PD45/47/49 cells. Three independent experiments (n = 105–124 cells each) ± SEM. ***p ≤ 0.001, Student’s unpaired t test.
(H) Immunofluorescence analysis of dox-i C19orf43 shRNA IMR90 −dox PD45 or +dox PD47 cells stained with γH2AX (green) and TRF1 (red) antibodies. Arrowheads indicate colocalizing foci.
(I) Quantification of the frequency of cells displaying ≥4 γH2AX/TRF1 colocalizing foci from −dox PD45 or +dox PD47 cells. Three independent experiments (n = 94–134 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test.
(J) Detection of senescence-associated heterochromatin foci (SAHF) in DAPI-stained dox-i C19orf43 shRNA IMR90 −dox PD46 or +dox PD49 cells.
(K) Quantification of SAHF-positive DAPI-stained dox-i C19orf43 shRNA IMR90 −dox PD43/46 or +dox PD45/49 cells. Three independent experiments (n = 104–215 cells each) ± SEM. ***p ≤ 0.001, Student’s unpaired t test.
(L) SA-β-gal analysis of dox-i C19orf43 shRNA IMR90 −dox PD47 or +dox PD53 cells. Scale bars represent 100 μm.
(M) Quantification of SA-β-gal-positive cells. Three independent experiments (n = 173–207 cells each) ± SEM. ***p ≤ 0.001, Student’s unpaired t test.
(N) FISH analysis using a 16p subtelo probe (green) of mitotic dox-i C19orf43 shRNA IMR90 +dox (PD57) cells following no treatment or RNaseH1 treatment in situ.
(O) Quantification of the frequency of mitotic cells with cohered telomeres. Three independent experiments (n = 50 cells each) ± SEM. **p ≤ 0.01, Student’s unpaired t test. (B, F, H, J, N) DNA was stained with DAPI (blue). Scale bars represent 2 μm. See also Figure S6.
To determine if the growth advantage conferred by C19orf43 depletion was reversible, PD50 +dox (C19orf43-depleted) cells were reseeded with or without dox (Figure 6E). Initially (for the first 10 days), the cells grew at the same rate with or without dox. However, after 10 days, the growth rate of the +dox (C19orf43-depleted) cells increased compared to that of the −dox (control) cells. The −dox (control) cells began to slow down and ultimately ceased growth at 81 days (PD55), while the +dox (C19orf43-depleted) cells continued to divide. Similar results were obtained in two additional independent analyses (Figure S6C).
Aged human cells accumulate DNA damage that signals senescence.21 We thus asked if the DNA damage signal was attenuated in the +dox (C19orf43-depleted) versus the −dox (control) cells by measuring the frequency of DNA damage foci at late PDs (~day 60). As shown in Figures 6F and 6G, − dox (control) (PD46) cells displayed a high level of DNA damage foci that was dramatically reduced in the +dox (C19orf43-depleted) (PD49) cells. We also observed a reduction in DNA damage foci associated with telomeres in the +dox (C19orf43-depleted) cells (Figures 6H and 6I). However, we did not observe a dramatic difference in telomere length between +dox (C19orf43-depleted) (PD45, 47, 53) and −dox (control) (PD43, 45, and 51) cells, based on telomere restriction fragment analysis (Figure S6D).
To determine if senescence was delayed in the +dox (C19orf43-depleted) versus the −dox (control) cells, we measured senescence-associated heterochromatin foci (SAHF)54 in late PD cells and observed a reduction in SAHFs in +dox (C19orf43-depleted) versus −dox (control) cells (Figures 6J and 6K). Measurement of the senescence-associated marker β-galactosidase (SA-β-gal)55 revealed a reduction in SA-β-gal-positive cells in +dox (C19orf43-depleted) (PD53) versus −dox (control) (PD47) cells, indicating delayed senescence (Figures 6L and 6M).
Finally, to determine the status and nature of the cohesion in cells with extended lifespan, we performed FISH analysis on the +dox (C19orf43-depleted) cells at PD57 (day 90). Prior to FISH, we incubated the cells in situ without or with RNaseH1. As shown in Figures 6N and 6O, the +dox (C19orf43-depleted) cells (without treatment) exhibited a high level of persistent telomere cohesion. Incubation of the cover slips with RNaseH1 in situ led to resolution of cohesion. Together these data indicate that aged C19orf43-depleted cells exhibit cohered telomeres in mitosis that are held together by RNA-DNA hybrids and are protected from DNA damage and senescence.
DISCUSSION
Telomere shortening robs normal human cells of their potential for immortality and thus serves as a roadblock to tumorigenesis. Chromosome replication in the absence of telomerase erodes the reservoirs of TTAGGG repeats, as well as the proteins that bind them. Ultimately, this loss culminates in cessation of growth. This does not occur suddenly, but rather through the gradual process known as replicative senescence. We describe a mechanism inherent to the loss of telomere repeats (and associated proteins) that shepherds chromosome ends through this process. The integrity of the genome relies upon mechanisms that prevent accumulation of potentially toxic structures such as R-loops. Shortened telomeres lose their ability to prevent R-loop buildup. Surprisingly, these otherwise toxic structures afford protection to critically short telomeres, promoting a gradual replicative senescence.
Persistent telomere cohesion in mitosis is a state that occurs naturally in cells that lack telomerase: normal aged cells and ALT cancer cells. This state can also be created artificially in telomerase-positive cells by depletion of tankyrase, mutation of the TBM in TRF1, and now in this report, by depletion of C19orf43 or mutation of its TBM. We suggest the following model. When telomeres are sufficiently long, C19orf43 is recruited and binds TERRA to limit R-loop formation. Upon telomere shortening, the TRF1/tankyrase/C19orf43 axis is diminished, leading to R-loop accumulation and persistent telomere cohesion.
Unexpectedly, the increase in TERRA R-loops was accompanied by a decrease in TERRA levels. This was surprising because in other examples, increased R-loops were accompanied/induced by increased TERRA levels.43,46,56 There are likely multiple ways to regulate R-loops at telomeres. A recent study showed that the THOC complex binds TERRA and negatively regulates TERRA R-loops without significantly changing TERRA levels.57 C19orf43 could work in a similar fashion, binding TERRA and preventing R-loop formation. In the case of C19orf43 depletion, the increased R-loops could block TERRA transcription, leading to a reduction in TERRA levels. Interestingly, a recent study used a proximity labeling-based approach to identify the RNase H proximal proteome and found that C19orf43 (among a number of other proteins) was significantly enriched.58 The study suggested that there were multiple classes of R-loop regulators that use distinct mechanisms. C19orf43 could be part of an R-loop repressing cellular machinery that through its association with TRF1/tankyrase limits R-loops at long, but not critically short, telomeres.
What actually holds persistently cohered sister telomeres together? We showed that overexpression of RHaseH1 in living cells counteracts persistent telomere cohesion, indicating a role for RNA-DNA hybrids. Conversely and in support, depletion of RNaseH1 led to increased persistent telomere cohesion. Remarkably, the action of RNaseH1 could be recapitulated in situ; incubation of fixed cohered telomeres with purified RNaseH1 in situ resolved telomere cohesion. Thus, RNA-DNA hybrids hold sisters together. R-loops are composed of RNA-DNA hybrids and a displaced single strand. The displaced DNA single strand could invade the sister telomere and connect them. In support of this, treatment of cohered telomeres in situ with the S1 endonuclease, which cleaves single-stranded DNA, resolved telomere cohesion. We suggest that persistent cohesion reflects TERRA R-loop-mediated associations between sister telomeres. These associations could protect critically short telomeres from being viewed as DNA breaks in G2/M. The question remains how these associations would ultimately get resolved in mitotic cells. We showed previously by live cell imaging that persistent telomere cohesion (in tankyrase-depleted or aged IMR90 cells) led to anaphase delay.15 Cells struggled briefly to segregate their chromosomes but ultimately resolved them and proceeded through the cell cycle without damaged telomeres. The R-loop-mediated interactions described here could (with the aid of spindle forces in live cells) enable resolution in anaphase without significant damage.
As telomerase-negative yeast cells approach senescence, TERRA R-loop abundance increases, and this correlates with a delayed onset of replicative senescence.45,46 The increase in R-loops is accompanied by increased homology directed repair, and it has been proposed that recombination between sister chromatids at critically short telomeres can partially compensate for the loss of telomeric repeats and thereby buffer against early onset senescence.45,46,59,60 The persistent telomere cohesion observed in human pre-senescent cells could reflect formation of R-loop-promoted recombination intermediates between short sister telomeres. Future studies will determine whether these structures, in addition to serving a protective role, also facilitate homology-directed repair. Interestingly, we found that persistent telomere cohesion in C19orf43-depleted cells was RAD51 dependent. RAD51 could act in multiple ways. It could associate with TERRA and stimulate formation of R-loops and/or associate with the displaced DNA strand of the R-loop and stimulate strand invasion and recombination.35,39
When young IMR90 cells were depleted of C19orf43 (+dox) and passaged in culture, they grew for 55 days (14 PDs) more than their uninduced −dox counterpart. When +dox (C19orf43-depleted) cells at PD50 were reseeded with or without dox, the −dox (control) cells rapidly ceased growth. Thus, the persistent cohesion (R-loop connection) affords only a transient protection. Ultimately, even the +dox (C19orf43-depleted) cells succumb to senescence. We showed previously that at the senescence point (the final PD), sister telomere cohesion is lost, likely due to the inability of short telomeres to establish cohesion.11 Thus, persistent telomere cohesion offers a transient protective state, but it cannot ultimately override telomere loss. Nonetheless, delaying or prolonging senescence could impact organismal fitness. While replicative senescence is an essential barrier to tumorigenesis, accumulation of senescent cells can impact aging and age-related diseases, including cancer.61,62 Thus, the ability to modulate/lengthen the senescence window could offer therapeutic opportunities.
Limitations of the study
We used DRIP coupled with qPCR to show that C19orf43 counteracts R-loops at telomeres. It is possible that C19orf43 may act at other genomic regions. This could be addressed using DRIP-seq.
We used telomere restriction fragment analysis to show that telomere length was not increased in C19orf43-depleted aged cells. It is possible that minor lengthening could occur in a subset of specific (critically short) telomeres. This could be addressed with single-telomere length analysis.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Susan Smith (susan.smith@med.nyu.edu)
Materials availability
Plasmids and cell lines generated in this study will be shared upon request.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Cell lines
All cells were grown in standard conditions. WI38 (ATCC) and IMR90 (ATCC) fibroblast cell lines were supplemented with 20% FBS, HEK293T (ATCC) and TNKS1/2 DKO12 cell lines were supplemented with 10% DBS, and 293FT cells (Invitrogen) were supplemented with 10% FBS. For dox induction, cells were treated with 0.1 μg/ml for 48 h.
Generation of C19orf43 tankyrase binding motif (TBM) mutant and knockout (KO) cell lines using CRISPR/Cas9
C19orf43 TBM mutant and KO cell lines were generated using RNA-guided CRISPR associated nuclease Cas9. A 20 bp target sequence directed against the first exon of the human C19orf43 gene (C19 guide DNA 5′–GACGGGCGGAGCCTCAGGGC–3′) was inserted into the guide sequence insertion site using Bbs1 site of the CRISPR plasmid pX330 comprised of Cas9 and a chimeric guide RNA.68 To generate C19orf43 TBM clones, a single-stranded DNA homology template was designed to change Gly12 to Ala (C19orf43.G12A) and also to introduce an XhoI restriction site to screen the potential clones. To generate the C19orf43 KO clones, a single-stranded DNA homology template was designed to change Arg6 to a stop codon (TAA), and also to introduce an XhoI restriction site to screen the potential clones. HEK293T cells were transfected with the pX330 plasmid and homology template ssDNA as described previously.69 Following transfection, cells were re-plated for single cell cloning, propagated and screened by a PCR strategy designed to screen for gain of an XhoI site in the target site, using the forward primer Fwd 5’ – CTTCCCGGCATGCATTGTTC −3′ or Fwd1 5′-AACCCGCGAGACGGGGGCT-3′ and the reverse primer Rev 5′-CACGGCTCCTTACGAAGCTA-3′. Three independent homozygous C19orf.G12A clones (#3, #4, and #6) were isolated and confirmed by DNA sequencing of the PCR products; C19orf.G12A #3 is shown in Figure 1. Two independent homozygous KO clones (#2 and #4) were isolated and confirmed by DNA sequencing of the PCR products.
Plasmids
The shRNA plasmids against C19orf43 were generated by cloning hairpins targeting the following sequences into the pLKO.1 puro Vector: C19orf43#1 against the 3′ UTR 5′- GCCTCGTGAGACTTCATAGAA-3′ and C19orf43#4 against the coding sequence 5′-AGACGGAGGATGAGGTATTAA-3’. For dox-i C19orf43 the same hairpin as for #1, the 3′ UTR 5′- GCCTCGTGAGACTTCATAGAA-3′, was cloned into pLKO-Tet-On (Addgene #21915).
RNaseH1 plasmids containing C-terminally myc-tagged human full length RNaseH1, wild type (WT) or catalytically dead (CD) (D145A), cloned into the retroviral Vector pLHCX (Clontech), were kindly provided by Claus Azzalin.43 TNKS1 plasmid was described previously.65 The C19orf43.WT plasmid is comprised of an N-terminal 3x FLAG tag followed by human full length C19orf43 cloned into the p3XFlag-CMV-10 Vector (Sigma). C19orf43.R7G was obtained by mutating the arginine at position 7 to glycine and C19orf43.R106G was obtained by mutating the arginine at position 106 to glycine. Mutagenesis was performed using Q5 Site-Directed Mutagenesis Kit (NEB) according to the manufacturer’s instructions. For expression in E. coli, C19orf43 was cloned into the pET-22b Vector (Novagen) with a C-terminal His tag and expressed without the pelB leader sequence.
METHODS DETAILS
siRNA and plasmid transfection
For plasmids, cells were transfected with Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol for 20 h.
For siRNAs, cells were transfected with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol for 48–72 h. The final concentration of siRNA was 20 nM. The following target sequences were used for siRNAs: RNaseH1#1 (5′- UCCUUUAAAUGUAGGCAUUAGACUU-3′) described previously as RNaseH1c43; RNaseH1#2 (5′-GGGAAAGAGGUGAUCAACA-3′) described previously as RNAseH1–2 siRNA66; RNaseH2A (5′-AUAAUCAGUAUCCAAGUCC-3′) described previously67; TNKS1 siRNA (5-CAAUUCACCGUCGUCCUCU-3) described previously14; and RAD51 siRNA (5′-CCAGAUCUGUCAUACGCUA-3) described previously as RAD51#270 (Dharmacon). The control siRNA is the GFP duplex I (Dharmacon).
Lentiviral infection
shRNA cell lines were generated by introducing lentiviruses expressing GFP (Control), C19orf43#1, C19orf43#4 or dox-i C19orf43 into HEK293T or IMR90 cells. For lentivirus generation, 293FT cells (Invitrogen) were transfected using Lipofectamine 3000 (Invitrogen) with 1 μg each lentiviral Vector and pCMVΔR8.9 packaging plasmid, and 100 ng pMD.G envelope plasmid. Forty-eight hours after transfection, supernatants were collected, filtered with a 0.45-μm filter (Millipore), supplemented with 8 μg/mL polybrene (Sigma-Aldrich), and used to infect target cells. Following 48–72 h infection, cells were sub-cultured 1:2 into medium containing 2 μg/mL puromycin.
Preparation of cell extracts
Cells were resuspended in four volumes of TNE buffer [10 mM Tris (pH 7.8), 1% Nonidet P-40, 0.15 M NaCl, 1 mM EDTA, and 2.5% protease inhibitor cocktail (PIC) (Sigma)] and incubated for 1 h on ice. Suspensions were pelleted at 10,000 × g for 10 min at 4°C. Equal amounts of supernatant proteins (determined by Bio-Rad protein assay) were fractionated by SDS- PAGE and analyzed by immunoblotting.
Immunoblot analysis
Immunoblots were incubated separately with the following primary antibodies: mouse anti-Myc05-724 (0.1 μg/mL; Millipore), mouse anti-Flag F3165 (3.8 μg/mL, Sigma), rabbit anti- C19orf43 PA5–63805 (0.4 μg/mL, Invitrogen), rabbit anti C19orf43 19420-1-AP (1.1 μg/mL; Proteintech), rabbit anti-TNKS1 762 (1 μg/mL),63 rabbit anti-RNaseH1 15606-AP (0.5 μg/mL, Proteintech), rabbit anti RNaseH2A PA5–78330 (0.82 μg/mL, Invitrogen), rabbit anti-RAD51 (H-92) sc-8349 (0.4 μg/mL, Santa Cruz), or mouse anti-α-tubulin ascites (1:20,000, Sigma), followed by horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG (1:3,000, Amersham). Bound antibody was detected with Super Signal West Pico (Thermo Scientific).
Immunoprecipitation
Cells were lysed as above and supernatants precleared with Protein G-Sepharose rotating at 4°C for 30 min. Nonspecific protein aggregates were removed by centrifugation and the supernatant was used for immunoprecipitation analysis or fractionated directly on SDS-PAGE (indicated as input, ~5% of the amount used in the immunoprecipitation). For immunoprecipitation of Flag epitope-tagged proteins, supernatants were incubated with 20 μL of Flag M2 agarose (Sigma, A2220) for 3 h. For TNKS immunoprecipitations supernatants were incubated for 3 h with 1 μg rabbit anti-tankyrase 46565 or IgG, followed by Protein G Sepharose for 1 h. For all immunoprecipitations, beads were washed three times with 1mL of TNE buffer, fractionated by SDS-PAGE, and processed for immunoblotting as described above.
For treatment with nucleases prior to fractionation on SDS-PAGE, immunoprecipitates were incubated with RNaseA and RNaseH1 as described below for in situ FISH. For DNase treatment, we used 2 units DNaseI (NEB) in DNase Buffer (10 mM Tris-HCl pH 7.6, 0.5 mM CaCl2, 2.5 mM MgCl2). All samples were incubated for 15 min at 37°C.
Chromosome-specific FISH
Cells were fixed and processed as described previously.14 Briefly, cells were isolated mechanically by mitotic shake-off, fixed twice in methanol:acetic acid (3:1) for 15 min, in a cytospin (Shandon Cytospin) at 2,000 rpm for 2 min onto slides, rehydrated in 2X SSC at 37°C for 2 min, and dehydrated in an ethanol series of 70%, 85%, and 100% for 2 min each. Cells were denatured at 75°C for 2 min and hybridized overnight at 37°C with FITC-conjugated 16ptelo subtelomere probe or FITC-conjugated subtelomere and TRITC-conjugated arm 13q14.3 deletion probe (13qtelo/13qarm) from Cytocell. Cells were washed in 0.4X SSC at 72°C for 2 min, and in 2X SSC with 0.05% Tween 20 at RT for 30 s. DNA was stained with 0.2 μg/mL DAPI. For FISH analysis of WI38 and IMR90 fibroblasts, cells were treated with 50 ng/mL nocodazole (Sigma) for 16 h prior to shake-off. Mitotic cells were scored as having telomeres cohered (singlets) if 50% or more of their loci appeared as singlets, i.e., one out of two or two out of three.
For in situ treatment with purified enzymes, following cytospin samples were rehydrated in 50 μL and subjected to the following treatments. Coverslips were incubated with: 5 units RNaseH1 (NEB) in RNaseH1 Buffer (50 mM Tris-HCl pH 8.3, 75 mM KCl, 3 mM MgCl2,10 mM DTT) and incubated for 30 to 60 min without or with 6 mM EDTA at 37°C; RNaseA (0.2 mg/ml final concentration) (Invitrogen) in H2O for 30 to 60 min without or with 6 mM EDTA at 37°C; 10 units S1 Nuclease (Invitrogen) in S1 Buffer (30 mM sodium acetate pH 4.6, 50 mM NaCl, 1 mM zinc acetate, and 5% glycerol) without or with 30 mM EDTA (pretreated for 10 min at 70°C) for 30 min at 37°C; 6 μg C19orf43 (purified from E. coli BL21 through Qiagen Ni-TA agarose using standard procedures) in RNaseH1 buffer for 30 min at RT; with 2 μg tankyrase (purified from baculovirus as described previously)65 with 100 mM NAD+ in 50 mM Tris pH 8.0, 4mM MgCl2, 0.2 mM DTT for 30 min at RT, or with 6 μg C19orf43 and 2 μg tankyrase following a 15 min incubation at RT. Following treatment cells were washed 2X with 2X SSC and processed as described above beginning with the 2X SSC treatment at 37°C for 2 min.
Indirect immunofluorescence
Cells were fixed in 2% paraformaldehyde in PBS for 10 min at RT, permeabilized in 0.5% NP-40/PBS for 10 min at RT, blocked in 1% BSA/PBS, and incubated with mouse anti-γH2AX #05 636 (20 μg/mL; Millipore), rabbit anti-53BP1 NB 100–304 (1 μg/mL; Novus Biologicals) or rabbit anti-TRF1 415 (0.2 μg/mL).64 Primary antibodies were incubated at RT for 2 h, followed by detection with FITC-conjugated or TRITC-conjugated donkey anti-rabbit or anti-mouse antibodies (1:100; Jackson Laboratories). DNA was stained with 0.2 μg/mL DAPI. Detection of Senescence-associated heterochromatin foci (SAHF) SAHF was analyzed on coverslips processed for γH2AX and 53BP1 immunofluorescence described above. A cell was scored as SAHF-positive if its DAPI counterstain had a characteristic punctate pattern.54
Senescence associated β-galactosidase assay
For the SA- β-galactosidase assay,55 cells were fixed in 2% formaldehyde and 0.2% glutaraldehyde in PBS for 5 min, washed three times in PBS, and stained for 5 h at 37°C in staining solution (1 mg/mL X-gal, 150 mmol/L NaCl, 2 mmol/L MgCl2, 5 mmol/L K3Fe[CN] 6, 5 mmol/L K4Fe[CN]6, and 40 mmol/L NaPi, pH 6.0).
RNA isolation and dot blot
RNA was isolated from cells using RNeasy Mini Kit (Qiagen). Two on-column (Qiagen) and one in-solution (NEB) DNase digestions were performed. Purified RNA was digested with RNase (DNase-free) (Invitrogen), as a control. All samples were denatured at 65°C for 5 min, cooled on ice 5 min, and blotted (10 μg per sample) onto a Hybond-XL membrane (Amersham) using a dot-blot apparatus (Bio-Rad). RNA was UV-crosslinked to the membrane. The membrane was then blocked in Church buffer (0.5 M NaHPO4, 1 mM EDTA, pH 8.0, 1% (w/v) BSA, 7% SDS) for at least 2 h at 55°C, and then hybridized to a 32P end-labeled (CCCTAA)4 oligonucleotide Telo C probe in Church Buffer o/n 55°C. The membrane was washed 3–4 times, 15 min each with 4X SSC at RT and exposed to a phosphorimager screen. Radioactive signal was detected with a Typhoon Biomolecular Imager (GE). After signal detection, the membrane was stripped by incubation with boiling 0.1% SDS, 2mM EDTA three times. The membrane was then blocked in Church buffer for 2 h at 55°C, and then hybridized to a 32P end-labeled (CCATCCAATCGGTAGTAGCG) 18s rRNA probe at 55°C overnight and processed as described for the Telo C probe.
Northern blot analysis
RNA was isolated as described above. 15 μg total RNA per sample was subjected to electrophoresis on a 1.3% agarose formaldehyde gel. The gel was stained with ethidium bromide, the RNA visualized by UV, and (capillary) transferred to Hybond-XL membrane (Amersham). The RNA was UV-crosslinked and processed as described above for the dot blot with Telo C probe.
Telomere restriction fragment analysis
Genomic DNA was isolated from IMR90 cells using a Blood and Cell Culture DNA mini kit (Qiagen 13323) and digested with HhaI, HinfI, Alu1, MspI, and RsaI. Approximately 2.5 μg of the digested DNA was fractionated on a 0.7% agarose gel. The gel was denatured, dried, neutralized, and prehybridized in Church buffer (0.5 M NaHPO4, 1 mM EDTA, pH 8.0, 1% (w/v) BSA, 7% SDS) for 30 min at 42°C, and then hybridized to a 32P end-labeled (CCCTAA)4 oligonucleotide Telo C probe in Church Buffer o/n at 42°C. The gel was washed once in 2X SSC, 0.1% SDS at 42°C for 15 min and 3–4 times, 15 min each with 0.5X SSC, 0.1% SDS at 42°C and exposed to a phosphorimager screen. Radioactive signal was detected with a Typhoon Biomolecular Imager (GE).
RT-qPCR
TERRA RT-qPCR was performed as previously described with some modifications.71 Cells were treated with 25 ng/mL nocodazole for 16 h prior to harvest. RNA was isolated from cells using RNeasy Mini Kit (Qiagen). Two on-column (Qiagen) and one in-solution DNase (NEB) digestions were performed. Five micrograms of RNA was reverse-transcribed using 200 U SuperScript III Reverse transcriptase (Thermo Fisher Scientific), and GAPDH (5′-GCCCAATACGACCAAATCC-3′) and TERRA (5′- CCCTAACCCTAACCCTAACCCTAACCCTAA-3′) reverse primers in a 20 μL reaction. Reverse transcription was performed at 55°C for 1 h, followed by heat inactivation at 70°C for 15 min. Sample was diluted to 80 μL with H2O. For each qPCR, 3 μL was mixed with Power SYBR Green PCR Master mix (Applied Biosystems) and 0.5 μM forward and reverse qPCR primers. qPCR consisted of 10 min at 95°C followed by 40 cycles at 95°C for 15 s and 60°C for 1 min in an Applied Biosystems StepOne Real-Time System. The following primers were used: subtelomeric primers33: 2p (forward primer: 5′- GTAAAGGCGAAGCAGCATTCTCC-3′, reverse primer: 5′- TAAGCCGAAGCCTAACTCGTGTC −3′); 9p (forward primer: 5′- GAGATTCTCCCAAGGCAAG −3′, reverse primer: 5′- ACATGAGGAATGTGGGTGTTAT −3′); 16p (forward primer: 5′- TGCAACCGGGAAAGATTTTATT-3′, reverse primer: 5′- GCCTGGCTTTGGGACAACT −3′); 18p (forward primer: 5′- TACCTCGCTTTGGGACAAC–3′, reverse primer: 5′- CCTAACCCTCACCCTTCTAAC-3′); telomeric primers72: (forward primer: 5′- GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′, reverse primer: 5′-TCC CGACTATCCCTATCCCTATCCCTATCCCTATCCCTA −3′); and DRIP control primers51: RPL13A positive control (forward primer: 5′–AGGTGCCTTGCTCACAGAG’-3′, reverse primer: 5′- GGTTGCATTGCCCTCATTAC-3′) and SNRPN negative control (forward primer: 5′- GCCAAATGAGTGAGGATGGT-3′, reverse primer: 5′- TCCTCTCTGCCTGACTCCAT-3′).
DNA–RNA immunoprecipitation (DRIP)
DRIP was performed as previously described with some modifications.39,73 Cells were treated with 25 ng/mL nocodazole for 16 h prior to harvest. Cells were harvested, counted, and washed with PBS. Up to ten million cells were dissolved in 175 μL of ice-cold RLN buffer (50 mM Tris-HCl pH 8.0, 140 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40, 1 mM dithiothreitol DTT, and 100 U ml SuperaseIn (Ambion), incubated on ice for 5 min, and pelleted 300 × g for 5 min 4°C. The nuclear pellet was warmed to RT and lysed in 500 μL RA1 buffer (Machery Nagel) containing 5 μL of β-mercaptoethanol, and homogenized by passing through a 20 G x 1–1/2 inch hypodermic needle (BD). The nucleic-acid-containing extracts (500 μL) were mixed with 250 μL H2O and 750 μL phenol:chloroform:isoamylalcohol (25:24:1) in a Phase Lock Gel heavy (5PRIME) 2 mL tube and pelleted (13,000 × g, 5 min, RT). The upper aqueous phase was mixed with 1500 μL of ice-cold isopropanol and 30 μl 5M NaCl (to 50 mM), then incubated on ice for 30 min. Precipitated nucleic acids were pelleted at 10,000 × g for 30 min at 4°C. The pellets were washed twice with 70% ice-cold ethanol, air-dried, dissolved in 140 μL of H2O, and sonicated with Covaris R230 Focused-Ultrasonicator with R230_500750 PSU Rack 96 microTube Plate +0.5 offset (25% duty factor, 450 peak incident power, 600 cycles per burst, 70 s, 130 μL) to obtain fragments of 350 bp. Nucleic acid concentration was determined by nanodrop. Five micrograms of sonicated nucleic acids in 40 μL of 1X RNaseH1 buffer (20 mM HEPES-KOH pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT) were treated with 3 μL (15 units) RNaseH1 (NEB) or H2O as a control and incubated at 37°C for 2 h with slight agitation (300 rpm). Samples were diluted to 1400 μL with IP buffer (16.6 mM Tris pH 8.0, 166 mM NaCl, 1.2 mM EDTA pH 8.0, 1.1% Triton X-100, 0.01% SDS) and pre-cleared with 40 μL of Sepharose protein G beads (50% slurry) for 1 h, on a rotating wheel, at 4°C. 650 μL was used for each IP and 1% (6.5 μL) of the nucleic acids were kept as input. 650 μL of sample (roughly 2.5 μg of nucleic acids) was incubated with 1 μg of S9.6 antibody (Kerafest) or mouse IgG and 30 μL of Sepharose protein G beads on a rotating wheel at 4°C overnight. The next day the samples were washed for 5 min on a rotating wheel at 4°C with 1 mL each of the following buffers: A (20 mM Tris pH 8.0, 165 mM NaCl, 2 mM EDTA pH 8.0, 1% Triton X-100, 0.1% SDS), B (50 mM 20 mM Tris pH 8.0, 500 mM NaCl, 2 mM EDTA pH 8.0, 1% Triton X-100, 0.1% SDS), C (10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0, 250 mM LiCl, 1% NP-40, 1% Na-deoxycholate), and D (10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0). Beads were incubated with 50 μL IP elution buffer (100 mM NaHCO3, 1% SDS), twice 60 min each at 65°C shaking (1050 rpm). Inputs were adjusted to 100 μL with IP elution buffer. The 100 μL samples were digested with RNaseA DNase-free (Thermofisher) 10 μg each at 37°C for 1 h and then with 4 μg Proteinase K each at 45°C for 1 h, and purified using GFX PCR DNA purification kit (Cytiva). DNA was eluted twice with 30 μL H2O and 1 μL was used for qPCR as described above.
RNA immunoprecipitation (RIP)
Cells were harvested by trypsinization 20 h post-plasmid DNA transfection, counted, and washed with 1X PBS. Cells were resuspended in 2 mL of freshly prepared nuclear isolation buffer (1.28 M Sucrose, 40 mM Tris-HCl pH 7.5, 20 mM MgCl2, 4% Triton X-100) and 6 mL of cold DEPC-treated water, incubated on ice with frequent mixing for 20 min, and pelleted by centrifugation at 2500 × g for 15 min at 4°C. Nuclei were lysed with Nuclear Lysis Buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl pH 8.0), supplemented with 20 U/ml of SUPERase•In RNase Inhibitor and a protease inhibitor cocktail (Sigma) (6 × 106 cells/ml of Nuclear Lysis Buffer). Lysates were sonicated with Covaris R230 Focused-Ultrasonicator with R230_500750 PSU Rack 96 microTube Plate +0.5 offset (25% duty factor, 450 peak incident power, 600 cycles per burst, 70 s, 130 μL), and centrifuged at high speed for 10 min at 4°C. The supernatant was collected, diluted with 1 mL of RIP buffer (150 mM KCl, 25 mM Tris pH 7.4, 10 mM EDTA, 0.5 Mm DTT, 0.5% NP40), supplemented with 20 U/ml of SUPERase•In RNase Inhibitor and a protease inhibitor cocktail (Sigma), and pre-cleared with 40 μL of Sepharose protein G beads (50% slurry) on a rotating wheel for 1 h at 4°C. Pre-cleared extracts equivalent to 2 × 106 cells were used per immunoprecipitation. 40 μL of Flag M2 agarose (Sigma, A2220) were added to each immunoprecipitation sample and incubated on a rotating wheel overnight at 4°C. Input samples equivalent to 5% of each immunoprecipitation were also collected. Beads were then washed three times at 4°C with RIP buffer and one time with PBS, 15 min/wash. 1 mL of TRIzol Reagent and 400 μL of chloroform were added to immunoprecipitation samples and inputs, vortexed, and centrifuged at max speed for 15 min at 4°C. The aqueous phase was then transferred into a new tube. 510 μL isopropanol, 50 μL of 2M NaCl, and 5 μl of glycogen were added to the aqueous phase, mixed thoroughly, and incubated at −80°C for 30 min. Samples were centrifuged at max speed at 4°C to precipitate RNA, followed by two washes of 70% cold ethanol. After air drying, pellets were resuspended in 20 μL of water and subjected to RT-qPCR as described above.
Image acquisition
FISH images were acquired with a Zeiss Axioplan 2 microscope with a Plan Apochrome 63X NA 1.4 oil immersion lens and a digital camera (C4742–95; Hamamatsu Photonics) using Openlab software (PerkinElmer) or a Nikon Eclipse Ti microscope with a Nikon Plan APo TIRF 60X 1.45 oil immersion lensand a digital camera (Andor Neo) using NIS-Elements AR software. For chromosome specific FISH, if foci fell in more than one optical plane of focus, multiple planes were merged using Openlab software for the Zeiss images or the Fiji application for the Nikon images. Immunofluorescence images were acquired using a Nikon Eclipse Ti microscope. SA-β-galstained images were imaged with simple brightfield at 203 magnification using a Zeiss AxioObserver.Z1 microscope and a Axiocam 503 camera.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis was performed using Prism 9 software. Data are shown as mean ± SEM. Student unpaired t test was applied. p < 0.05 values were considered significant: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, not significant.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Anti-Myc Tag Antibody, clone 4A6 | Millipore Sigma | Cat#05-724; RRID:AB_309938 |
| Monoclonal ANTI-FLAG® M2 antibody produced in mouse | Millipore Sigma | Cat#F3165; RRID:AB_259529 |
| C19orf43 Polyclonal Antibody | Invitrogen | Cat#PA5-63805; RRID:AB_2638871 |
| C19orf43 Polyclonal antibody | Proteintech | Cat#19420-1-AP; RRID:AB_10640586 |
| rabbit anti-TNKS1 | Scherthan et al.63 | 762 |
| RNaseH1 Polyclonal Antibody | Proteintech | Cat#15606-AP; RRID:AB_2238624 |
| RNaseH2A Polyclonal Antibody | Invitrogen | Cat#PA5-78330; RRID:AB_2736528 |
| Rad51 Antibody (H-92) | Santa Cruz | Cat#sc-8349; RRID:AB_2253533 |
| Monoclonal Anti-α-Tubulin antibody produced in mouse | Sigma | Cat#T5168; RRID:AB_477579 |
| Amersham ECL Mouse IgG, HRP-linked whole Ab (from sheep) | Cytvia | Cat#NA931; RRID:AB_772210 |
| Amersham ECL Rabbit IgG, HRP-linked whole Ab (from donkey) | Cytvia | Cat#NA934; RRID:AB_772206 |
| Anti-phospho-Histone H2A.X (Ser139) Antibody, clone JBW301 | Millipore | Cat#05636; RRID:AB_309864 |
| 53BP1 Antibody - BSA Free | Novus Biologicals | Cat#NB100-304; RRID:AB_10003037 |
| rabbit anti-TRF1 | Cook et al.64 | 415 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| C19orf43 | This paper | N/A |
| Tankyrase | Smith et al.65 | N/A |
|
| ||
| Critical commercial assays | ||
|
| ||
| 16pter Subtelomere Specific Probe (15μL) | Oxford Gene Technology | Cat#LPT16PG-A |
| 13q14.3 Deletion Probe | Oxford Gene Technology | Cat#LPH006-A |
| Blood & Cell Culture DNA Mini Kit (25) | Qiagen | Cat#13323 |
| RNeasy Mini Kit (50) | Qiagen | Cat#74104 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| WI-38 | ATCC | Cat#CCL-75; RRID:CVCL_0579 |
| IMR-90 | ATCC | Cat#CCL-186; RRID:CVCL_0347 |
| 293 [HEK-293] | ATCC | Cat#CRL-1573; RRID:CVCL_0045 |
| TNKS1/2 DKO | Bhardwaj et al.12 | N/A |
| 293FT Cell Line | Invitrogen | Cat#R70007; RRID:CVCL_6911 |
|
| ||
| Oligonucleotides | ||
|
| ||
| siRNAs: RNaseH1#1: UCCUUUAAAUGUAGGCAUUAGACUU | Arora et al.43 | N/A |
| siRNAs: RNaseH1#2: GGGAAAGAGGUGAUCAACA | Yadav et al.66 | N/A |
| siRNAs: RNaseH2A: AUAAUCAGUAUCCAAGUCC | Daley et al.67 | N/A |
| siRNAs: TNKS1: CAAUUCACCGUCGUCCUCU | Dynek et al.14 | N/A |
| siRNAs: RAD51: CCAGAUCUGUCAUACGCUA | Dharmacon | N/A |
| GFP duplex I | Dharmacon | N/A |
| CRISPR guides: C19 guide: GACGGGCGGAGCCTCAGGGC | This paper | N/A |
| PCR primer screen XhoI site fwd: CTTCCCGGCATGCATTGTTC | This paper | N/A |
| PCR primer screen XhoI site fwd: AACCCGCGAGACGGGGGCT | This paper | N/A |
| PCR primer screen XhoI site rev: CACGGCTCCTTACGAAGCTA | This paper | N/A |
| Telo C Probe: (CCCTAA)4 | This paper | N/A |
| 18s rRNA probe: CCATCCAATCGGTAGTAGCG | This paper | N/A |
| For primers for qPCR | This paper | see Table S1 |
|
| ||
| Recombinant DNA | ||
|
| ||
| pLKO.1 puro Vector | Broad Institute | N/A |
| pLKO.1.shC19orf43#1_5′- GCCTCGTGAGACTTCATAGAA-3′ | This paper | N/A |
| pLKO.1.shC19orf43#4_5′-AGACGGAGGATGAGGTATTAA-3′ | This paper | N/A |
| pLKO-tet-on vector | Addgene | #21915 |
| pLKO-Tet-On_shC19orf43#1 | This paper | N/A |
| pLHCX vector | Clontech | N/A |
| pLHCX_RNaseH1_WT | Arora et al.43 | N/A |
| pLHCX_RNaseH1_CD_D145A | Arora et al.43 | N/A |
| p3XFlag-CMV-10 Vector | Sigma | N/A |
| p3XFlag-CMV-10_C19orf43.WT | This paper | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| ImageJ- Fiji | NIH | https://fiji.sc |
| GraphPad Prism v9.0 | GraphPad Software Inc. | https://www.graphpad.com |
Highlights.
RNA-binding protein C19orf43 represses formation of TERRA R-loops at telomeres
TERRA R-loop accumulation prevents resolution of sister telomeres in mitosis
RNaseH1 overexpression counteracts persistent cohesion in C19orf43-depleted cells
C19orf43 depletion delays senescence and boosts replicative lifespan in aged cells
ACKNOWLEDGMENTS
We thank Jerome Perrard for helpful discussions and critical reading of the manuscript. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01GM129780 and R01GM141292.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113235.
DECLARATION OF INTERESTS
There are no competing interests.
REFERENCES
- 1.de Lange T (2005). Shelterin: the protein complex that shapes and sfeguards human telomeres. Genes Dev 19, 2100–2110. [DOI] [PubMed] [Google Scholar]
- 2.Azarm K, and Smith S (2020). Nuclear PARPs and genome integrity. Genes Dev 34, 285–301. 10.1101/gad.334730.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lange T (2018). Shelterin-Mediated Telomere Protection. Annu. Rev. Genet 52, 223–247. 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 4.Lim CJ, and Cech TR (2021). Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization. Nat. Rev. Mol. Cell Biol 22, 283–298. 10.1038/s41580-021-00328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisemann T, McCauley M, Langelier MF, Gupta K, Roy S, Van Duyne GD, and Pascal JM (2016). Tankyrase-1 Ankyrin Repeats Form an Adaptable Binding Platform for Targets of ADP-Ribose Modification. Structure 24, 1679–1692. 10.1016/j.str.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Guettler S, LaRose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, and Sicheri F (2011). Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell 147, 1340–1354. 10.1016/j.cell.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Sbodio JI, and Chi NW (2002). Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1. NuMA contains this RXXPDG motif and is a novel tankyrase partner. J. Biol. Chem 277, 31887–31892. [DOI] [PubMed] [Google Scholar]
- 8.Seimiya H, and Smith S (2002). The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182). J. Biol. Chem 277, 14116–14126. 10.1074/jbc.M112266200. [DOI] [PubMed] [Google Scholar]
- 9.Bisht KK, Daniloski Z, and Smith S (2013). SA1 binds directly to DNA through its unique AT-hook to promote sister chromatid cohesion at telomeres. J. Cell Sci 126, 3493–3503. 10.1242/jcs.130872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisht KK, Dudognon C, Chang WG, Sokol ES, Ramirez A, and Smith S (2012). GDP-mannose-4,6-dehydratase is a cytosolic partner of tankyrase 1 that inhibits its poly(ADP-ribose) polymerase activity. Mol. Cell Biol 32, 3044–3053. 10.1128/MCB.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azarm K, Bhardwaj A, Kim E, and Smith S (2020). Persistent telomere cohesion protects aging cells from premature senescence. Nat. Commun 11, 3321. 10.1038/s41467-020-17133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj A, Yang Y, Ueberheide B, and Smith S (2017). Whole proteome analysis of human tankyrase knockout cells reveals targets of tankyrase-mediated degradation. Nat. Commun 8, 2214. 10.1038/s41467-017-02363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canudas S, Houghtaling BR, Kim JY, Dynek JN, Chang WG, and Smith S (2007). Protein requirements for sister telomere association in human cells. Embo J 26, 4867–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dynek JN, and Smith S (2004). Resolution of sister telomere association is required for progression through mitosis. Science 304, 97–100. [DOI] [PubMed] [Google Scholar]
- 15.Kim MK, and Smith S (2014). Persistent telomere cohesion triggers a prolonged anaphase. Mol. Biol. Cell 25, 30–40. 10.1091/mbc.E13-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffman KE, Levene SD, Tesmer VM, Shay JW, and Wright WE (2000). Telomere shortening is proportional to the size of the G-rich telomeric 3’-overhang. J. Biol. Chem 275, 19719–19722. 10.1074/jbc.M002843200. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Takai H, and de Lange T (2012). Telomeric 3’ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell 150, 39–52. 10.1016/j.cell.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greider CW, and Blackburn EH (1985). Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413. [DOI] [PubMed] [Google Scholar]
- 19.Greider CW, and Blackburn EH (1987). The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898. [DOI] [PubMed] [Google Scholar]
- 20.Wright WE, Piatyszek MA, Rainey WE, Byrd W, and Shay JW (1996). Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet 18, 173–179. [DOI] [PubMed] [Google Scholar]
- 21.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, and Jackson SP (2003). A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198. [DOI] [PubMed] [Google Scholar]
- 22.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, and Reddel RR (1997). Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines [see comments]. Nat. Med 3, 1271–1274. [DOI] [PubMed] [Google Scholar]
- 23.Bryan TM, Englezou A, Gupta J, Bacchetti S, and Reddel RR (1995). Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J 14, 4240–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henson JD, Neumann AA, Yeager TR, and Reddel RR (2002). Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610. [DOI] [PubMed] [Google Scholar]
- 25.Ofir R, Yalon-Hacohen M, Segev Y, Schultz A, Skorecki KL, and Selig S (2002). Replication and/or separation of some human telomeres is delayed beyond S-phase in pre-senescent cells. Chromosoma 111, 147–155. [DOI] [PubMed] [Google Scholar]
- 26.Ramamoorthy M, and Smith S (2015). Loss of ATRX Suppresses Resolution of Telomere Cohesion to Control Recombination in ALT Cancer Cells. Cancer Cell 28, 357–369. 10.1016/j.ccell.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yalon M, Gal S, Segev Y, Selig S, and Skorecki KL (2004). Sister chromatid separation at human telomeric regions. J. Cell Sci 117, 1961–1970. [DOI] [PubMed] [Google Scholar]
- 28.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, and Lingner J (2007). Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318, 798–801. 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 29.Luke B, Panza A, Redon S, Iglesias N, Li Z, and Lingner J (2008). The Rat1p 5’ to 3’ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 32, 465–477. 10.1016/j.molcel.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Schoeftner S, and Blasco MA (2009). A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J 28, 2323–2336. 10.1038/emboj.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azzalin CM, and Lingner J (2015). Telomere functions grounding on TERRA firma. Trends Cell Biol 25, 29–36. 10.1016/j.tcb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Nergadze SG, Farnung BO, Wischnewski H, Khoriauli L, Vitelli V, Chawla R, Giulotto E, and Azzalin CM (2009). CpG-island promoters drive transcription of human telomeres. RNA 15, 2186–2194. 10.1261/rna.1748309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feretzaki M, Renck Nunes P, and Lingner J (2019). Expression and differential regulation of human TERRA at several chromosome ends. RNA 25, 1470–1480. 10.1261/rna.072322.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porro A, Feuerhahn S, Delafontaine J, Riethman H, Rougemont J, and Lingner J (2014). Functional characterization of the TERRA transcriptome at damaged telomeres. Nat. Commun 5, 5379. 10.1038/ncomms6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes RV, Feretzaki M, and Lingner J (2021). The makings of TERRA R-loops at chromosome ends. Cell Cycle 20, 1745–1759. 10.1080/15384101.2021.1962638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toubiana S, and Selig S (2018). DNA:RNA hybrids at telomeres - when it is better to be out of the (R) loop. FEBS J 285, 2552–2566. 10.1111/febs.14464. [DOI] [PubMed] [Google Scholar]
- 37.Brickner JR, Garzon JL, and Cimprich KA (2022). Walking a tightrope: The complex balancing act of R-loops in genome stability. Mol. Cell 82, 2267–2297. 10.1016/j.molcel.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Muse T, and Aguilera A (2019). R Loops: From Physiological to Pathological Roles. Cell 179, 604–618. 10.1016/j.cell.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 39.Feretzaki M, Pospisilova M, Valador Fernandes R, Lunardi T, Krejci L, and Lingner J (2020). RAD51-dependent recruitment of TERRA lncRNA to telomeres through R-loops. Nature 587, 303–308. 10.1038/s41586-020-2815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettin N, Oss Pegorar C, and Cusanelli E (2019). The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells 8. 10.3390/cells8030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalonde M, and Chartrand P (2020). TERRA, a Multifaceted Regulator of Telomerase Activity at Telomeres. J. Mol. Biol 432, 4232–4243. 10.1016/j.jmb.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Cerritelli SM, and Crouch RJ (2009). Ribonuclease H: the enzymes in eukaryotes. FEBS J 276, 1494–1505. 10.1111/j.1742-4658.2009.06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora R, Lee Y, Wischnewski H, Brun CM, Schwarz T, and Azzalin CM (2014). RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat. Commun 5, 5220. 10.1038/ncomms6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva B, Arora R, Bione S, and Azzalin CM (2021). TERRA transcription destabilizes telomere integrity to initiate break-induced replication in human ALT cells. Nat. Commun 12, 3760. 10.1038/s41467-021-24097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balk B, Maicher A, Dees M, Klermund J, Luke-Glaser S, Bender K, and Luke B (2013). Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat. Struct. Mol. Biol 20, 1199–1205. 10.1038/nsmb.2662. [DOI] [PubMed] [Google Scholar]
- 46.Graf M, Bonetti D, Lockhart A, Serhal K, Kellner V, Maicher A, Jolivet P, Teixeira MT, and Luke B (2017). Telomere Length Determines TERRA and R-Loop Regulation through the Cell Cycle. Cell 170, 72–85.e14. 10.1016/j.cell.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Han H, Zhou MT, Yang B, Ta AP, Li N, Chen J, and Wang W (2017). Proteomic Analysis of the Human Tankyrase Protein Interaction Network Reveals Its Role in Pexophagy. Cell Rep 20, 737–749. 10.1016/j.celrep.2017.06.077. [DOI] [PubMed] [Google Scholar]
- 48.Xie J, Chen Z, Zhang X, Chen H, and Guan W (2017). Identification of an RNase that preferentially cleaves A/G nucleotides. Sci. Rep 7, 45207. 10.1038/srep45207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J, Lee J, and Lee DH (2019). Identification of Protein Phosphatase 4 Inhibitory Protein That Plays an Indispensable Role in DNA Damage Response. Mol. Cells 42, 546–556. 10.14348/molcells.2019.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boguslawski SJ, Smith DE, Michalak MA, Mickelson KE, Yehle CO, Patterson WL, and Carrico RJ (1986). Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J. Immunol. Methods 89, 123–130. 10.1016/0022-1759(86)90040-2. [DOI] [PubMed] [Google Scholar]
- 51.Sanz LA, and Chédin F (2019). High-resolution, strand-specific R-loop mapping via S9.6-based DNA-RNA immunoprecipitation and highthroughput sequencing. Nat. Protoc 14, 1734–1755. 10.1038/s41596-019-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laspata N, Kaur P, Mersaoui SY, Muoio D, Liu ZS, Bannister MH, Nguyen HD, Curry C, Pascal JM, Poirier GG, et al. (2023). PARP1 associates with R-loops to promote their resolution and genome stability. Nucleic Acids Res 51, 2215–2237. 10.1093/nar/gkad066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang ZZ, Pannunzio NR, Hsieh CL, Yu K, and Lieber MR (2015). Complexities due to single-stranded RNA during antibody detection of genomic rna:dna hybrids. BMC Res Notes 8, 127. 10.1186/s13104-015-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, and Lowe SW (2003). Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716. [DOI] [PubMed] [Google Scholar]
- 55.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367. 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sagie S, Toubiana S, Hartono SR, Katzir H, Tzur-Gilat A, Havazelet S, Francastel C, Velasco G, Chédin F, and Selig S (2017). Telomeres in ICF syndrome cells are vulnerable to DNA damage due to elevated DNA:RNA hybrids. Nat. Commun 8, 14015. 10.1038/ncomms14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandes RV, and Lingner J (2023). The THO complex counteracts TERRA R-loop-mediated telomere fragility in telomerase+ cells and telomeric recombination in ALT+ cells. Nucleic Acids Res 51, 6702–6722. 10.1093/nar/gkad448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan Q, Wulfridge P, Doherty J, Fernandez-Luna JL, Real PJ, Tang HY, and Sarma K (2022). Proximity labeling identifies a repertoire of site-specific R-loop modulators. Nat. Commun 13, 53. 10.1038/s41467-021-27722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguilera P, Whalen J, Minguet C, Churikov D, Freudenreich C, Simon MN, and Géli V (2020). The nuclear pore complex prevents sister chromatid recombination during replicative senescence. Nat. Commun 11, 160. 10.1038/s41467-019-13979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fallet E, Jolivet P, Soudet J, Lisby M, Gilson E, and Teixeira MT (2014). Length-dependent processing of telomeres in the absence of telomerase. Nucleic Acids Res 42, 3648–3665. 10.1093/nar/gkt1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campisi J (2013). Aging, cellular senescence, and cancer. Annu. Rev. Physiol 75, 685–705. 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossiello F, Jurk D, Passos JF, and d’Adda di Fagagna F (2022). Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol 24, 135–147. 10.1038/s41556-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scherthan H, Jerratsch M, Li B, Smith S, Hultén M, Lock T, and de Lange T (2000). Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol. Biol. Cell 11, 4189–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook BD, Dynek JN, Chang W, Shostak G, and Smith S (2002). Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell Biol 22, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith S, Giriat I, Schmitt A, and de Lange T (1998). Tankyrase, a poly(ADP-ribose) polymerase at human telomeres [see comments]. Science 282, 1484–1487. [DOI] [PubMed] [Google Scholar]
- 66.Yadav T, Zhang JM, Ouyang J, Leung W, Simoneau A, and Zou L (2022). TERRA and RAD51AP1 promote alternative lengthening of telomeres through an R- to D-loop switch. Mol. Cell 82, 3985–4000.e4. 10.1016/j.molcel.2022.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daley JM, Tomimatsu N, Hooks G, Wang W, Miller AS, Xue X, Nguyen KA, Kaur H, Williamson E, Mukherjee B, et al. (2020). Specificity of end resection pathways for double-strand break regions containing ribonucleotides and base lesions. Nat. Commun 11, 3088. 10.1038/s41467-020-16903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, and Zhang F (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, and Zhang F (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389. 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho NW, Dilley RL, Lampson MA, and Greenberg RA (2014). Interchromosomal homology searches drive directional ALT telomere movement and synapsis. Cell 159, 108–121. 10.1016/j.cell.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feretzaki M, and Lingner J (2017). A practical qPCR approach to detect TERRA, the elusive telomeric repeat-containing RNA. Methods 114, 39–45. 10.1016/j.ymeth.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Res 30, e47. 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatchi E, Skourti-Stathaki K, Ventz S, Pinello L, Yen A, Kamieniarz-Gdula K, Dimitrov S, Pathania S, McKinney KM, Eaton ML, et al. (2015). BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair. Mol. Cell 57, 636–647. 10.1016/j.molcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


