Abstract
Alcohol Use Disorder (AUD) is a significant public health issue in the United States. It affects millions of individuals and their families and contributes to substantial societal and economic burdens. Despite the availability of some pharmacological treatments, there is still a pressing need to develop more effective therapeutic strategies to address the diverse range of symptoms and challenges associated with AUD. Catechol-O-methyltransferase (COMT) inhibition recently emerged as a promising new approach to treating AUD due to its potential to improve cognitive effects commonly associated with AUD. Tolcapone, an FDA-approved COMT inhibitor, has shown some promise for treating AUD; however, its ability to decrease drinking in ethanol-dependent rats has not been well-established. In this study, we evaluated the effects of tolcapone on operant, oral ethanol self-administration in non-dependent and dependent rats, and in rats that self-administered oral saccharin. To induce dependence, rats underwent the chronic intermittent exposure to vapor model, and their drinking levels were assessed during acute withdrawal from ethanol. Our results demonstrated that tolcapone attenuated responding for ethanol in dependent rats only, without affecting self-administration in nondependent rats or rats self-administering saccharin. Moreover, we found that tolcapone was differentially effective in different estrous phases in female rats. These findings suggest that COMT inhibition, specifically using tolcapone, may be a valuable pharmacotherapy for treating AUD, particularly in individuals who are physically dependent on alcohol. Further research is needed to elucidate the precise mechanisms underlying the observed effects and to assess the potential of COMT inhibitors in a broader population of individuals with AUD.
Keywords: tolcapone, COMT, ethanol, alcohol use disorder, rat, estrous cycle
1. Introduction
Alcohol Use Disorder (AUD) is a significant public health problem, affecting millions of individuals worldwide and contributing to substantial societal and economic burdens. In the United States alone, it is estimated that approximately 15 million people suffer from AUD, with the disorder being responsible for nearly 100,000 deaths annually (Grant et al., 2015; Sacks et al., 2015). Despite the significant impact of AUD on individuals and society, there are currently only three FDA-approved treatments available, which are only modestly effective in a subset of individuals (Berrettini, 2013; Goldman et al., 2005; Mason and Lehert, 2012; Mason et al., 2013). Furthermore, only 10% of patients with AUD are prescribed any of the available pharmacotherapies (Jonas et al., 2014). These limitations highlight the urgent need for research into novel approaches for treating AUD.
Over the last few decades, little progress has been made in developing novel medications with previously identified targets (Litten et al., 2012), leading to a renewed interest in identifying new therapeutic targets with innovative mechanisms of action for the treatment of AUD. One such target is the regulation of cognitive control, as AUD is characterized by impaired cognitive control, including impulsivity and poor decision-making (Bechara, 2005; Mitchell et al., 2005; Phung et al., 2019). Medications that directly address these cognitive impairments associated with AUD could represent a groundbreaking treatment strategy.
Dopamine signaling in the prefrontal cortex plays a crucial role in cognitive function (Nieoullon, 2002), and catechol-O-methyltransferase (COMT) inhibitors have been investigated as cognitive enhancers due to their ability to modulate dopaminergic function in the prefrontal cortex (Apud et al., 2007; Bhakta et al., 2017; Gasparini et al., 1997). Recent studies have demonstrated that tolcapone, a clinically approved COMT inhibitor, can attenuate alcohol consumption and significantly reduce impulsive choices in individuals with AUD (Coker et al., 2020; Schacht et al., 2022). In preclinical research, tolcapone has been shown to decrease ethanol drinking in a cued access protocol paradigm in P rats (McCane et al., 2014; McCane et al., 2018); however, these studies only evaluated drinking in a non-dependent state. To date, the effects of COMT inhibitors like tolcapone have not been investigated in ethanol-dependent rats.
To address this knowledge gap, the present study utilized the chronic intermittent access to ethanol vapor (CIE) model, which has face, predictive, and construct validity to induce ethanol dependence in rats (Kononoff et al., 2018; Vendruscolo and Roberts, 2014). Although animal models cannot fully replicate the human experience of AUD, the CIE model allows for the investigation of specific elements of the addiction process, such as escalation of ethanol drinking, compulsive-like responding, vulnerability to relapse, and withdrawal symptoms (Rodd et al., 2004). The use of the CIE model enables the assessment of the effects of ethanol drinking during acute withdrawal (6-8 hours), a critical timepoint for withdrawal-induced escalation of ethanol drinking and the development of somatic and emotional signs of withdrawal.
In light of these considerations, the present study aimed to evaluate whether the administration of the COMT inhibitor tolcapone could decrease ethanol drinking in dependent and non-dependent rats using an operant self-administration procedure. As an additional control, the effects of tolcapone on responding maintained by saccharin were also evaluated. Finally, given that COMT levels fluctuate with the estrous cycle, the phase was assessed in dependent, female rats to determine whether tolcapone would be more effective during different phases of the cycle.
2. Methods
2.1. Subjects
Adult Wistar rats (n=20 males; n=24 females) were obtained from Charles River. Experiments began when rats were 10-12 weeks old. Rats had access to water and standard laboratory chow (PJ Noyes Company, Lancaster, NH, USA) ad libitum in their home cage. Rats were housed in a temperature- (20–22 °C) and humidity-controlled (45–55%) environment on a 12h/12h light/dark cycle, with lights on at 9 p.m. All the procedures were performed in accordance with the ARRIVE guidelines (Kilkenny et al., 2010), adhered National Research Council's Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
2.2. Operant ethanol self-administration
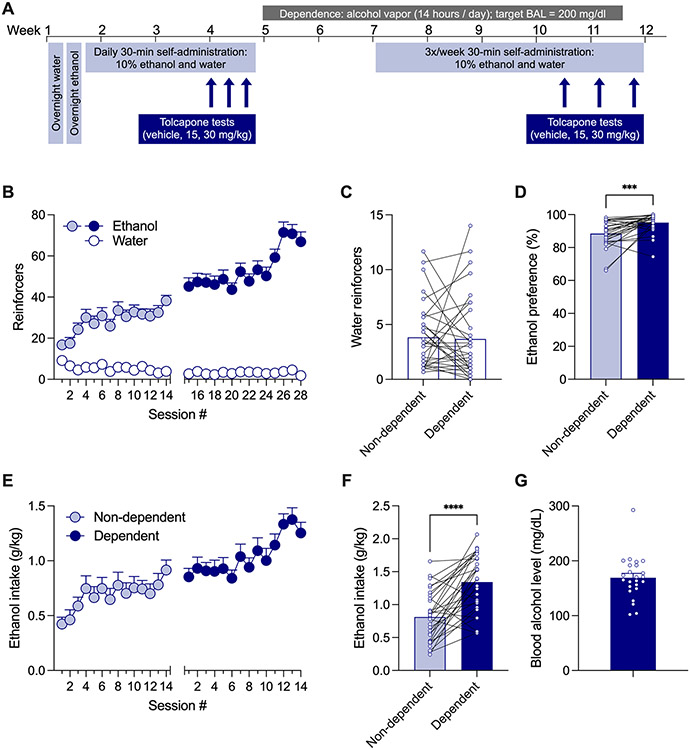
Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT, USA). For the ethanol self-administration studies, the rats (n=16 males; n=17 females) first self-administered water (0.1 ml/reinforcer) in a single overnight, 16-hour session on a (fixed-ratio 1 [FR1]) schedule of reinforcement. Then, rats self-administered an ethanol (10% v/v) solution (0.1 ml/reinforcer) on FR1 in a single overnight, 16-hour session. Food was available ad libitum in the operant chamber during both 16-hour sessions. Rats then self-administered ethanol only during three 30-min FR1 sessions. Finally, rats were allowed to self-administer ethanol (10% v/v) (right lever) or water (left lever) on FR1 during daily 30-min sessions, where each response resulted in delivery of 0.1 ml of solution. The non-dependent self-administration phase lasted at least 14 daily sessions (Monday-Friday) before testing of tolcapone began (Figure 1A).
Figure 1. Ethanol self-administration in non-dependent and dependent rats.
Wistar rats self-administered oral ethanol (10% v/v, 0.1 ml/reinforcer) and water (0.1 ml/reinforcer) on a FR1 schedule of reinforcement in 30-min sessions for at least 14 days when non-dependent and ethanol dependent. A. Schematic of the experimental timeline. B. Number of ethanol (colored circles) and water reinforcers (white circles) earned by rats in a nondependent state (light blue circles) and dependent rats self-administering ethanol during acute withdrawal (dark blue circles). C. Mean number of water reinforcers earned in the last three sessions. D. Mean ethanol preference (ethanol reinforcers / (ethanol + water reinforcers) *100) in the last three sessions. E. Ethanol intake as a function of bodyweight (g/kg) across sessions in rats a nondependent state (light blue circles) and dependent rats self-administering ethanol during acute withdrawal (dark blue circles). F. Mean ethanol intake during the last 3 self-administration sessions prior to testing with tolcapone in nondependent and dependent states. G. Mean blood alcohol level (BAL) at the end of the vapor exposure. Each data point or bar represents group mean and error bars represent SEM. Individual points on bars represent a mean of three days for individual subjects. Symbols indicate statistical significance in a paired t-test where *** is p < 0.001 and **** is p < 0.0001.
2.3. Ethanol dependence
After >3 weeks of self-administration in a non-dependent state (and after testing of tolcapone in a subset of non-dependent rats), self-administration sessions were temporarily stopped, and rats were made physically dependent using the chronic intermittent ethanol vapor exposure (CIE) model (Kononoff et al., 2018; Vendruscolo and Roberts, 2014). Rats were housed in ethanol vapor chambers, and ethanol vapor was on for 16 hours/day. Ethanol vapor levels were titrated to generate blood alcohol levels (BALs) of around 150-225 mg/dl. BALs were measured weekly using tail vein bleeds and quantified using gas chromatography, as previously described (de Guglielmo et al., 2023). Once target BALs were achieved, self-administration sessions were restarted on Mondays, Wednesdays, and Fridays during acute withdrawal (6-8 hours after the vapor was turned off) (Figure 1A). After at least 14 self-administration sessions, rats were pretreated with tolcapone and vehicle before sessions (see below for more details).
2.4. Tolcapone treatment
After initial self-administration of ethanol was stable, a subset of rats were tested in a non-dependent state (n=11 males; n=12 females), although a subset (n=3 males, n=2 females) were excluded from testing due to low ethanol intake (mean intake of <0.2 g/kg). All ethanol rats (n=16 males; n=17 females) were tested during dependence, although a subset of rats (n=2 males; n=1 female) were excluded from testing intake due to low ethanol intake (mean intake of <0.2 g/kg). All rats tested received injections of vehicle, 15 mg/kg and 30 mg/kg 60-min before the session, and the order of pretreatments was assigned using a Latin square design. One control session was conducted in between each test. The doses and pretreatment time were selected based on other studies (Lapish et al., 2009; McCane et al., 2014; McCane et al., 2018; Swerdlow et al., 2013) and the inhibition potency of tolcapone (Borges et al., 1997).
2.5. Saccharin self-administration
Another group of rats (n=7 females n=4 males) self-administered saccharin (0.04% w/v in tap water; 0.1 ml/reinforcer) on FR1 on the right lever and water (0.1 ml) on FR1 on the left lever. After stable responding, they received pretreatment injections of vehicle, 15 mg/kg tolcapone, and 30 mg/kg tolcapone 60-min before the session. Dose order was assigned using a Latin square design and one control (no injection) session was conducted between each test.
2.6. Estrous cycle
Dependent, female rats were vaginally swabbed immediately after self-administration sessions on test days. The samples air-dried and stained with Hema 3 stat pack staining kit (30-s/solution; Fisher Scientific, Pittsburgh, PA). Images of the smears were captured on a BZX700 microscope (Keyence, Itasca, IL), and phase determination was made by a blinded observer.
2.7. Drugs
Tolcapone ((3,4-dihydroxy-5-nitrophenyl)(p-tolyl)methanone)) was purchased from Combi-Blocks (San Diego, CA, USA). It was dissolved in 10% DMSO, 10% Tween-20, and 80% 0.9% sterile saline and generally administered intraperitoneally (i.p.) at a volume of 0.1 ml/kg. The vehicle was also 10% DMSO, 10% Tween-20 and 85% sterile saline and administered at a volume of 0.1 ml/kg. All pretreatments were administered i.p. 60-min before self-administration sessions began. The 10% v/v ethanol solution was 95% ethanol mixed with tap water and delivered at a volume of 0.1 ml/reinforcer. Saccharin was purchased from Sigma-Aldrich (Burlington, MA), dissolved in tap water to make a 0.04% w/v solution, and delivered at a volume of 0.1 ml/reinforcer.
2.8. Statistical analysis
A paired t-test was used to analyze differences in baseline ethanol intake, water reinforcers earned, and ethanol preference between the last three sessions of responding prior to tolcapone pretreatment in non-dependent or dependent rats (or last three sessions prior to the start of ethanol vapor exposure, in rats not tested in a non-dependent state). Ethanol preference was calculated by dividing number of ethanol reinforcers by number of total reinforcers (ethanol + water) and multiplying by 100. A three-way, repeated measures ANOVA was used to analyze the number of ethanol and water reinforcers earned in rats before and during dependence across the 14 sessions. A two-way, repeated measures ANOVA was used to analyze ethanol intake in male and female rats across the 28 sessions. A one-way, repeated measures ANOVA with Dunnett’s post-hoc was used to evaluate the effects of tolcapone in each dependent rats self-administering ethanol, non-dependent rats self-administering ethanol, and rats self-administering saccharin. A two-way, repeated measures ANOVA, with Dunnett’s post-hoc, was used to evaluate the effects of tolcapone in male and female rats. Tolcapone was considered “effective” for the estrous cycle data if it reduced ethanol intake by ≥30% of baseline levels of responding at either dose. Since both doses of tolcapone were significantly effective and due to low sample number, data for the estrous cycle were collapsed across both doses tested.
3. Results
3.1. Acquisition of Ethanol Self-Administration and the Development of Dependence
Rats self-administered oral ethanol (0.1 ml of 10% ethanol) and water (0.1 ml of tap water) on a FR1 schedule of reinforcement in 30-min sessions (Figure 1B). A three-way repeated measures ANOVA indicated there were main effects of reinforcer (F (1, 33) = 475.3; p < 0.0001), dependence state (F (1, 33) = 43.98; p < 0.0001), and session (F (13, 429) = 7.182; p < 0.0001), as well as interactions for reinforcer x dependence state (F (1, 33) = 66.14; p < 0.0001), reinforcer x session F (13, 429) = 10.37; p < 0.0001), and dependence x session (F (13, 429) = 4.445; p < 0.0001), suggesting rats earned more reinforcers of ethanol than water and earned more ethanol infusions during when dependent compared to before dependence. In the last three sessions after dependence compared to before dependence, there was no significant difference in number of water reinforcers earned (t=0.2135, df=31; p = 0.8323) (Figure 1C), but rats had higher preference for ethanol when in a dependent state (t=4.139, df=27; p = 0.0003) (Figure 1D). Rats generally escalated their ethanol intake (g/kg) throughout the experiment (Figure 1E) and had higher intake in the last 3 sessions when dependent compared to non-dependent (t=5.978, df=28; p < 0.0001) (Figure 1F). The average BAL induced by the ethanol vapor chambers was around 175 mg/dL, with some individual variability (Figure 1G).
3.2. Effects of Tolcapone on Ethanol and Saccharin Self-Administration in Nondependent and Dependent Rats
The effects of tolcapone pretreatment were evaluated in rats responding for ethanol in a non-dependent state as well as in dependent rats self-administering ethanol during acute withdrawal (Figure 2). There was a significant effect of dose in dependent rats when measuring both ethanol intake in g/kg (F (1.738, 48.66) = 6.080; p = 0.0062) and as a percentage of baseline level of responding (F (1.890, 52.92) = 7.192; p = 0.0021) (Figure 2A, D). In both cases, rats responded significantly less after receiving a pretreatment of 30 mg/kg tolcapone compared to vehicle (g/kg: p = 0.0016; % of baseline: p = 0.0016). In contrast, there was no effect of tolcapone dose in non-dependent rats, regardless of whether data were analyzed as ethanol intake (F (1.784, 30.34) = 0.3876; p = 0.6585) or percentage of baseline (F (1.881, 31.98) = 0.4844; p = 0.6091) (Figure 2B, E). Tolcapone also did not affect responding maintained by saccharin (reinforcers: F (1.600, 14.40) = 0.9745; p = 0.3824; % of baseline: F (1.663, 14.97) = 0.9452; p = 0.3945) (Figure 2C, F). There was no change in responding for water in any of the groups (dependent: F (1.706, 47.76) = 0.3680; p = 0.6605; non-dependent: F (1.590, 27.04) = 0.4195; p = 0.6157; saccharin: F (1.842, 18.42) = 0.2256; p = 0.7826) (Figure 2G-I).
Figure 2. Effects of tolcapone in dependent rats, non-dependent rats, and rats responding for saccharin.
Effects of tolcapone pretreatment (10 or 30 mg/kg, i.p.) on ethanol self-administration in dependent (dark blue bars) and non-dependent rats (light blue bars) and dependent rats and on saccharin self-administration (orange bars). Abscissae: vehicle or dose of tolcapone administered 60-min before the session. A, B. Ethanol intake (in g/kg) in dependent (A) and non-dependent (B) rats. C. Number of reinforcers earned of 0.04% saccharin solution. D, E. Ethanol reinforcers earned by dependent (D) and non-dependent rats (E) as a percentage of average responding in the three baseline sessions prior to pretreatments. F. Saccharin reinforcers earned as a percentage of baseline responding. G-I. Number of water reinforcers earned by dependent (G) and non-dependent rats (H) and rats responding for saccharin (I). Bars represent mean and error bars represent SEM. Data points are individual subjects. Symbols indicate statistical significance in a Dunnett’s post-hoc comparison where ** is p < 0.01 compared to vehicle
3.3. Sex Differences in Ethanol Intake and Response to Tolcapone Treatment
Ethanol intake and effects of tolcapone were also analyzed in male and female subjects (Figure 3). There was no main effect of sex in the number of reinforcers earned across the 28 self-administration sessions (F (1, 17) = 0.2306; p = 0.6372) (Figure 3A). However, there was a sex x session interaction (F (27, 459) = 1.562; p = 0.0374). There was a main effect of sex on ethanol intake (g/kg) (F (1, 17) = 19.45; p = 0.0004) where females had higher ethanol intake than males, but no sex x session interaction (F (27, 459) = 1.399; p = 0.0898). When the last three self-administration sessions before and after dependence were analyzed, there was a main effect of sex (F (1, 27) = 30.20; p < 0.0001) and dependence state (F (1, 27) = 33.80; p < 0.0001) where females had higher intake than males and dependent rats had higher intake than non-dependent rats (Figure 3C).
Figure 3. Ethanol self-administration and effects of tolcapone in female and male rats.
Ethanol self-administration and effects of tolcapone pretreatment (10 or 30 mg/kg, i.p.) in female and male rats on ethanol self-administration in dependent and non-dependent rats and on saccharin self-administration. A. Reinforcers of ethanol and water earned across 14 non-dependent (left) and 14 dependent (right) sessions. B. Ethanol intake in g/kg corresponding to number of reinforcers earned. C. Baseline ethanol intake (in g/kg) in nondependent and dependent rats prior to tolcapone pretreatments. D, E. Ethanol intake (in g/kg) in dependent (D) and non-dependent rats (E) during sessions in which tolcapone was administered. F. Number of reinforcers earned of 0.04% saccharin solution during sessions in which tolcapone was administered. G-I. Ethanol or saccharin intake as a percentage of average responding in the three baseline sessions prior to pretreatments. Bars represent mean and error bars represent SEM. Data points are individual subjects. Symbols in panel C represent significant main effect, where **** is p < 0.0001. Symbols in other panels indicate statistical significance in a Dunnett’s post-hoc comparison where * is p < 0.05
With regard to tolcapone pretreatments in dependent rats, there was a main effect of dose (F (2, 54) = 5.761; p = 0.0054) and a main effect of sex (F (1, 27) = 21.63; p < 0.001) in dependent rats (Figure 3D). Post-hoc analyses indicated there was a significant decrease in ethanol intake in female rats at both doses (15 mg/kg: p = 0.0339; 30 mg/kg: p = 0.0173), but only a trend in male rats (30 mg/kg: p = 0.0801). However, when data were analyzed as a percentage of baseline responding, there was a significant main effect of dose (F (2, 54) = 7.145; p = 0.0018) where tolcapone was effective at reducing responding in both female (15 mg/kg: p = 0.0319; 30 mg/kg: p = 0.0207) and male rats (30 mg/kg: p = 0.0179) (Figure 3G). In contrast to dependent rats, tolcapone was not effective in nondependent rats when analyzed in terms of ethanol intake (F (2, 32) = 0.4216; p = 0.6596) (Figure 3E) or percent of baseline responding (F (2, 32) = 0.7792; p = 0.4673) (Figure 3H); however, there was still a main effect of sex for ethanol intake (F (1, 16) = 20.76; p = 0.003) where females had greater ethanol intake than males (Figure 3E). Finally, there was no effect of tolcapone (ethanol intake: F (2, 16) = 1.015; p = 0.3844; % of baseline: F (2, 18) = 1.704; p = 0.2101) or sex (F (1, 8) = 0.2452; p = 0.6338), regardless of whether the number of reinforcers (Figure 3F) or percent of baseline was evaluated (Figure 3I).
3.4. Tolcapone Effectiveness as a Function of Estrous Phase
The effectiveness of tolcapone as a function of estrous phase was also evaluated in dependent female rats, collapsed across tolcapone dose (Figure 4). Representative images for each quantified phase are shown in Figure 4. Tolcapone was significantly more effective at reducing ethanol intake (as a percentage of baseline responding) in rats in estrus and metestrus (when estradiol is the lowest; (Goldman et al., 2005), compared to rats in proestrus and diestrus (when estradiol is the highest; t (20) = 2.441; p = 0.0241) (Figure 4).
Figure 4. Tolcapone effects during phases of the estrous cycle.
Representative images of stained estrous swab images and the proportion of rats in each phase when treated with tolcapone. Abscissae: estrous phase on day of tolcapone pretreatment. Ordinate: percentage decrease in ethanol intake after administration of tolcapone for individual rats in estrus and metestrus (left bar, black and dark grey circles) or in diestrus and proestrus (right bar, light grey and white circles). Bar represents mean and error represents S.E.M. * is p < 0.05 for a t-test.
4. Discussion
COMT inhibitors have been implicated as novel treatments for AUD due to their ability to improve cognitive function. Although tolcapone, an FDA-approved COMT inhibitor, has been used in randomized, double-blind, placebo-controlled studies in people (Coker et al., 2020; Schacht et al., 2022) and in rodent models of ethanol drinking (McCane et al., 2014; McCane et al., 2018), its ability to decrease operant ethanol self-administration specifically in dependent subjects had not been evaluated. In this study, we found that tolcapone can decrease ethanol drinking in rats made dependent using the CIE model, but not in nondependent rats or rats drinking saccharin. These findings suggest tolcapone, and perhaps other COMT inhibitors, may be effective treatments for people with AUD.
Tolcapone decreased ethanol self-administration in rats made dependent using the CIE model but did not affect ethanol intake in non-dependent rats or responding for saccharin in a different group of rats. There was also a nonsignificant trend towards tolcapone reducing alcohol drinking in male, nondependent rats. These findings are consistent with prior reports that, in a cued access protocol, tolcapone was ineffective at reducing ethanol intake in non-dependent Wistar rats, but was effective at decreasing ethanol intake in selectively bred, alcohol-preferring P rats (McCane et al., 2014; McCane et al., 2018). Tolcapone can also decrease cue seeking in the absence of an ethanol reinforcer delivery (McCane et al., 2018). It is notable that the same dose of tolcapone can decrease ethanol drinking in two, distinct models that capture different aspects of AUD (Kononoff et al., 2018; McBride et al., 2014; Murphy et al., 2002; Vendruscolo and Roberts, 2014) in different laboratories, especially since there have been pushes to evaluate potentially therapeutic compounds under conditions that model different of the human condition (Becker and Lopez, 2016; Lynch, 2018; Vendruscolo and Roberts, 2014). Using models of alcohol dependence, such as the CIE model, is a key factor in evaluating whether pharmacotherapies may be effective in the human population.
The mechanism through which tolcapone can decrease drinking in dependent rats is still unclear. Others have reported that tolcapone may be effective at suppressing excessive reward-seeking due to altered dopaminergic transmission in the prefrontal cortex (McCane et al., 2014). Given that CIE alters dopaminergic activity in the prefrontal cortex (Trantham-Davidson et al., 2014) and COMT inhibitors are used as cognitive enhancers (Apud et al., 2007; Bhakta et al., 2017; Gasparini et al., 1997), it is possible that regulating dopaminergic activity in the prefrontal cortex is responsible for the decrease in alcohol drinking.
Another potential mechanism could involve the interaction between tolcapone, and stress-related neurotransmitter systems implicated in alcohol dependence and withdrawal symptoms, such as the corticotropin-releasing factor (CRF) (Koob, 2008). Recent evidence suggests an interaction between the COMT and CRF systems in the context of withdrawal from other substances of abuse, such as methamphetamine (Garcia-Carmona et al., 2018). In recent work, García-Carmona, and colleagues (2018) demonstrated that methamphetamine withdrawal induces activation of CRF neurons in the brain stress system, which occurs in parallel with increased activity of cardiac sympathetic pathways, including COMT. Given the role of CRF in alcohol withdrawal and dependence (de Guglielmo et al., 2019; Funk and Koob, 2007; Funk et al., 2006; Gilpin et al., 2008; Koob, 2010; Roberto et al., 2010), a similar interaction between COMT and CRF systems could be an explanation for the selective effects of tolcapone observed in dependent rats. However, in the present study, we did not directly assess withdrawal symptoms such as anxiety or handling-induced convulsions. Future studies should investigate the effects of tolcapone on these withdrawal measures and explore the potential interactions between tolcapone, dopamine signaling, and stress-related neurotransmitter systems, particularly CRF, to provide further insight into the mechanisms underlying its selective action in dependent animals.
In this study, tolcapone was effective in both male and female subjects at similar doses. This is somewhat surprising given prior work with tolcapone in rats (McCane et al., 2018) as well as in a mouse model of COMT gene disruption (Tammimaki et al., 2008). In those studies, decreasing COMT activity pharmacologically or increasing COMT activity genetically altered drinking in male subjects without affecting drinking in female subjects. It has been long known that COMT activity changes as a function of estrous cycle (Parvez et al., 1978), so differences in these studies could be related to estrous cycle. In fact, COMT activity in the rat whole brain and hypothalamus specifically is highest during estrus, compared to the other phases (Parvez et al., 1978), when estrogen levels are lowest (Smith et al., 1975). Additionally, estrogens decrease COMT activity (Cohn and Axelrod, 1971). This is consistent with our findings that tolcapone administered during estrus and metestrus, when COMT activity is highest and estrogens are lowest, could increase the likelihood of a beneficial response to tolcapone compared to proestrus and estrus, when estrogen is increasing. It is important to note that tolcapone was also generally effective in the non-estrus phases (Goldman et al., 2005), and these studies have a low number of subjects, particularly in metestrus and diestrus. However, given these findings and that there are estrogen response elements in the promotor region of the COMT gene through which estrogen can inhibit the formation of COMT (Jiang et al., 2003; Xie et al., 1999), the use of COMT inhibitors as a treatment for AUD in women/female subjects and during different phases of the menstrual/estrous cycle needs to be further explored to understand the interaction between circulating estrogens and COMT inhibitors.
There is a well-characterized polymorphism in the COMT gene that substantially increases COMT activity in humans, which suggests COMT inhibitors may be more effective in a subset of individuals that are homozygous for the val allele at the rs4680 single nucleotide polymorphism (Bhakta et al., 2017; Boettiger et al., 2007; Coker et al., 2020; Comasco et al., 2015; Schacht et al., 2022). These individuals tend to be more impulsive (Boettiger et al., 2007; Coker et al., 2020) and drink more (Schacht et al., 2022), which is consistent with the genetic mouse model that increases COMT levels (Tammimaki et al., 2008). Importantly, these individuals are also more responsive to tolcapone (Schacht et al., 2022), suggesting a personalized medicine approach may be particularly useful when considering COMT inhibitors as a treatment for AUD or other psychiatric disorders.
In our study, we did not observe any notable side effects of tolcapone in our acute treatment, which is an encouraging finding. Moreover, tolcapone did not affect water or saccharin consumption, nor did it affect ethanol consumption in non-dependent rats. The fact that tolcapone did not affect water or saccharin consumption indicates that the drug is not causing a general suppression of consummatory behavior or locomotor activity, which are common concerns with pharmacological treatments for substance use disorders. Moreover, it was previously shown that tolcapone can increase locomotor activity at the doses tested (Mihaylova et al., 2019). Instead, the selective reduction of ethanol consumption in dependent rats suggests that tolcapone may be specifically targeting the neurobiological mechanisms underlying AUD.
An additional consideration in evaluating tolcapone as a treatment for AUD is the possibility that it might alter alcohol metabolism, which could influence its ability to reduce alcohol self-administration. Although we did not measure alcohol metabolism in our study, and there is no direct evidence in the literature suggesting that tolcapone could affect alcohol metabolism, it seems unlikely based on the available data. Behaviorally, tolcapone did not alter alcohol drinking in nondependent rats, which would be expected if it had a significant impact on ethanol metabolism. Furthermore, a comparison of the metabolism pathways for both tolcapone and alcohol reveals that they are both partially metabolized by UDP-glucuronosyltransferases. However, only ~15% of tolcapone is metabolized via this pathway (Jorga et al., 1999), and less than 0.1% of ethanol metabolism occurs through this pathway (Wurst et al., 2015), suggesting that tolcapone is unlikely to directly alter ethanol metabolism. No other overlapping metabolism pathways were identified that might interact.
It is important to note that the lack of side effects observed in our study may be due to the acute treatment paradigm. In clinical settings, tolcapone is often administered chronically, and it is during this long-term administration that concerns about liver dysfunction have arisen (Olanow and Panel, 2000). Moving forward, novel COMT inhibitors with better side effect profiles would be better candidates for a novel pharmacotherapy for AUD. Although tolcapone is FDA-approved to treat Parkinson’s disorder, there are concerns about liver dysfunction that limit its use (Olanow and Panel, 2000). Entacapone and opicapone are two other FDA-approved COMT inhibitors that are also suboptimal due to their poor bioavailability and low efficacy, or undesirable side effects such as dyskinesia and difficulty sleeping, respectively (Fabbri et al., 2018; Keränen et al., 1994). Future studies must evaluate novel COMT inhibitors with better side effect profiles to determine whether COMT inhibition is effective at treating AUD without problematic side effects. These next-generation COMT inhibitors should also have reduced toxicity and metabolic liability, while being blood-brain-barrier penetrant with improved efficacy and safety profiles. The present study combined with prior work in humans (Coker et al., 2020; Schacht et al., 2022) and rodents (McCane et al., 2014; McCane et al., 2018) suggests COMT inhibitors show promise for AUD treatment, but future studies should evaluate novel COMT inhibitors, particularly as a part of personalized medicine.
In conclusion, our findings contribute to the growing body of evidence supporting the use of COMT inhibitors, such as tolcapone, as a potential treatment for AUD. The selective reduction of ethanol consumption in dependent rats, coupled with the lack of side effects observed in our acute treatment paradigm, suggests that tolcapone may be a viable option for individuals with AUD, particularly those who are dependent. Further research is necessary to develop novel COMT inhibitors with better side effect profiles and to evaluate their efficacy in personalized medicine approaches for the treatment of AUD and other psychiatric disorders. This research should also explore the potential benefits of acute tolcapone treatment in comparison to chronic treatment, as our study indicates a promising direction for the use of tolcapone in AUD management.
Acknowledgements
The authors would like to thank Yicen Zheng and Xiuhan Li for their technical assistance in completing these studies.
Funding and Disclosures
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R21 AA030630 to SMC and GdG, R01 AA030048 to GdG, and T32 AA007456 to MRD], the National Institute on Drug Abuse [K00 DA057923 to EAS], and the Preclinical Addiction Research Consortium (PARC) at the University of California San Diego.
Abbreviations:
- AUD
Alcohol Use Disorder
- BALs
blood alcohol level
- COMT
catechol-O-methyltransferase
- CIE
chronic intermittent access to ethanol vapor
- FR
fixed ratio
Footnotes
The authors declare the following competing financial interest(s): SMC is a co-founder, has an equity interest, and receives income as member of the Scientific Advisory Board for Forge Therapeutics; is a co-founder, has an equity interest, and is a member of the Scientific Advisory Board for Blacksmith Medicines; and is a co-founder and has an equity interest Cleave Therapeutics (formerly Cleave Biosciences). These companies may potentially benefit from the research results of certain projects in the laboratory of SMC. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR, 2007. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Bechara A., 2005. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience 8, 1458–1463. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, 2016. An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. Int Rev Neurobiol 126, 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W., 2013. Opioid pharmacogenetics of alcohol addiction. Cold Spring Harb Perspect Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta SG, Light GA, Talledo JA, Balvaneda B, Hughes E, Alvarez A, Rana BK, Young JW, Swerdlow NR, 2017. Tolcapone-Enhanced Neurocognition in Healthy Adults: Neural Basis and Predictors. Int J Neuropsychopharmacol 20, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL, 2007. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci 27, 14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges N, Vieira-Coelho MA, Parada A, Soares-da-Silva P, 1997. Studies on the tight-binding nature of tolcapone inhibition of soluble and membrane-bound rat brain catechol-O-methyltransferase. J Pharmacol Exp Ther 282, 812–817. [PubMed] [Google Scholar]
- Cohn CK, Axelrod J, 1971. The effect of estradiol on catechol-O-methyltransferase activity in rat liver. Life Sci I 10, 1351–1354. [DOI] [PubMed] [Google Scholar]
- Coker AR, Weinstein DN, Vega TA, Miller CS, Kayser AS, Mitchell JM, 2020. The catechol-O-methyltransferase inhibitor tolcapone modulates alcohol consumption and impulsive choice in alcohol use disorder. Psychopharmacology 237, 3139–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Hellgren C, Olivier J, Skalkidou A, Sundström Poromaa I, 2015. Supraphysiological hormonal status, anxiety disorders, and COMT Val/Val genotype are associated with reduced sensorimotor gating in women. Psychoneuroendocrinology 60, 217–223. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, Koob GF, Messing RO, George O, 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun 10, 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Simpson S, Kimbrough A, Conlisk D, Baker R, Cantor M, Kallupi M, George O, 2023. Voluntary and forced exposure to ethanol vapor produces similar escalation of alcohol drinking but differential recruitment of brain regions related to stress, habit, and reward in male rats. Neuropharmacology 222, 109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Ferreira JJ, Lees A, Stocchi F, Poewe W, Tolosa E, Rascol O, 2018. Opicapone for the treatment of Parkinson's disease: A review of a new licensed medicine. Mov Disord 33, 1528–1539. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF, 2007. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res 1155, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF, 2006. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26, 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carmona JA, Georgiou P, Zanos P, Bailey A, Laorden ML, 2018. Methamphetamine withdrawal induces activation of CRF neurons in the brain stress system in parallel with an increased activity of cardiac sympathetic pathways. Naunyn Schmiedebergs Arch Pharmacol 391, 423–434. [DOI] [PubMed] [Google Scholar]
- Gasparini M, Fabrizio E, Bonifati V, Meco G, 1997. Cognitive improvement during Tolcapone treatment in Parkinson's disease. J Neural Transm (Vienna) 104, 887–894. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF, 2008. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res 32, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, O'Malley S, Anton R, 2005. COMBINE genetics study: the pharmacogenetics of alcoholism treatment response: genes and mechanisms. J Stud Alcohol Suppl, 56–64; discussion 33. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL, 2003. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology 45, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC, 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311, 1889–1900. [DOI] [PubMed] [Google Scholar]
- Jorga K, Fotteler B, Heizmann P, Gasser R, 1999. Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyltransferase. Br J Clin Pharmacol 48, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen T, Gordin A, Karlsson M, Korpela K, Pentikäinen PJ, Rita H, Schultz E, Seppälä L, Wikberg T, 1994. Inhibition of soluble catechol-O-methyltransferase and single-dose pharmacokinetics after oral and intravenous administration of entacapone. Eur J Clin Pharmacol 46, 151–157. [DOI] [PubMed] [Google Scholar]
- Kononoff J, Kallupi M, Kimbrough A, Conlisk D, de Guglielmo G, George O, 2018. Systemic and Intra-Habenular Activation of the Orphan G Protein-Coupled Receptor GPR139 Decreases Compulsive-Like Alcohol Drinking and Hyperalgesia in Alcohol-Dependent Rats. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2010. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG, 2009. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology (Berl) 202, 521–530. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A, 2012. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17, 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, 2018. Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Lehert P, 2012. Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res 36, 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A, 2013. Gabapentin Treatment for Alcohol Dependence: A Randomized Clinical Trial. JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK, 2014. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol 48, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCane AM, Czachowski CL, Lapish CC, 2014. Tolcapone suppresses ethanol intake in alcohol-preferring rats performing a novel cued access protocol. Alcohol Clin Exp Res 38, 2468–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCane AM, DeLory MJ, Timm MM, Janetsian-Fritz SS, Lapish CC, Czachowski CL, 2018. Differential COMT expression and behavioral effects of COMT inhibition in male and female Wistar and alcohol preferring rats. Alcohol 67, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova A, Zlatanova H, Doncheva N, Delev D, Kostadinov I, 2019. Catechol-o-methyltransferase inhibitor tolcapone improves learning and memory in naive but not in haloperidol challenged rats. Iran J Basic Med Sci 22, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA, 2005. Impulsive responding in alcoholics. Alcohol Clin Exp Res 29, 2158–2169. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK, 2002. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet 32, 363–388. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., 2002. Dopamine and the regulation of cognition and attention. Prog Neurobiol 67, 53–83. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Panel, a. t. T. A., 2000. Tolcapone and Hepatotoxic Effects. Archives of Neurology 57, 263–267. [DOI] [PubMed] [Google Scholar]
- Parvez S, Ismahan G, Raza-Bukhari A, Youdim MB, 1978. Activity of catechol-o-methyltransferase in brain regions and adrenal gland during the oestrus cycle. J Neural Transm 42, 305–312. [DOI] [PubMed] [Google Scholar]
- Phung QH, Snider SE, Tegge AN, Bickel WK, 2019. Willing to Work But Not to Wait: Individuals with Greater Alcohol Use Disorder Show Increased Delay Discounting Across Commodities and Less Effort Discounting for Alcohol. Alcoholism: Clinical and Experimental Research 43, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH, 2010. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 67, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ, 2004. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79, 439–450. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Yeongbin Im, Hoffman M, Voronin KE, Book SW, Anton RF, 2022. Effects of pharmacological and genetic regulation of COMT activity in alcohol use disorder: a randomized, placebo-controlled trial of tolcapone. Neuropsychopharmacology 47, 1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD, 1975. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96, 219–226. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hines SR, Herrera SD, Weber M, Breier MR, 2013. Opposite effects of tolcapone on amphetamine-disrupted startle gating in low vs. high COMT-expressing rat strains. Pharmacol Biochem Behav 106, 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammimaki A, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT, 2008. Increase in free choice oral ethanol self-administration in catechol-o-methyltransferase gene-disrupted male mice. Basic Clin Pharmacol Toxicol 103, 297–304. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ, 2014. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci 34, 3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ, 2014. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W, 2015. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res 39, 2060–2072. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D, 1999. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol 56, 31–38. [DOI] [PubMed] [Google Scholar]