Abstract
Introduction:
The Health and Retirement Study (HRS) has collected biomarker data over multiple waves. Such data can help improve our understanding of health changes in individuals and the causal pathways related to health. There are, however, technical challenges to using the HRS Dried Blood Spots (DBS) biomarker data due to changes over time in assay protocols, platforms, and laboratories. We provide technical and summary information on biological indicators collected as part of the HRS from 2006–2016 that should be useful to users of the data.
Methods:
We describe the opportunities and challenges provided by the HRS DBS data as well as insights provided by the data. The HRS collected dried blood spots from its nationally representative sample of respondents which is 51 years of age or older from 2006 to 2016. DBS-based biomarkers were collected from half the sample in 2006, 2010, and 2014, and from the other half of the sample in 2008, 2012, and 2016. These DBS specimens were used to assay total and HDL cholesterol, glycosylated hemoglobin, C-reactive protein, and cystatin C from 2006 to 2016, and Interleukin 6 was added in 2014/2016. Samples included approximately 6,000 individuals at each wave, and completion rates ranged from 81% to 90%. HRS transformed DBS values into venous blood equivalents to make them more comparable to those of the whole blood-based assays collected in most other studies and to facilitate longitudinal analysis.
Results:
Distribution of changes over time by age shows that total cholesterol levels decreased over time for each age, while HbA1c levels increased over time. Cystatin C shows a clear age gradient, but a number of other markers do not. Non-Hispanic Black persons and Hispanic respondents have higher risk of incident high biological risk except for CRP among non-Hispanic Black older persons.
Conclusion:
This public-use DBS data provides analysis opportunities that can be used to improve our understanding of health change with age in both populations and among individuals.
Keywords: DBS, HRS, longitudinal biomarker
INTRODUCTION
Collection of biomarker data has become nearly routine in social science surveys of aging populations; such data have contributed significantly to improving our understanding of health and contributors to it (Börsch-Supan et al., 2013, 2021; Chen et al., 2019; Christensen, 2004; Crimmins et al., 2020; Goldman et al., 2010; Harris & Schorpp, 2018; Mayeux, 2004; Rosero-Bixby & Dow, 2012). However, collection of biomarkers in community dwelling populations offers a number of challenges and only recently have national surveys been addressing some of these challenges with new technology. Dried blood spots (DBS) were heralded as a way to collect blood samples and preserve samples collected under a variety of field conditions while shipping to central laboratories (McDade, 2014). DBS had been used for specific medical uses for a long time, e.g., neonatal screening, but they had not been used for population assessment of biomarkers connected to aging routinely. With continuing development of assays for appropriate analytes, a number of national studies adopted the use of dried blood spots as a method of determining objective measures of health and disease over the last two decades. The Health and Retirement Study (HRS) began DBS collection in 2006 and is one such study that began DBS collection in 2006 and ended it in 2016. The HRS is one of a family of national studies and DBS collection has also been used in Europe for the Survey of Health, Ageing and Retirement in Europe (SHARE) (Borsch-Supan et al., forthcoming), India for the Longitudinal Ageing Study in India (LASI) (Bloom et al., 2014), Indonesia for the Indonesian Family Life Survey (IFLS) (Herningtyas et al., 2017), and China for the pilot for the China Health and Retirement Longitudinal Study (CHARLS) (Crimmins et al., 2011). A number of studies are continuing to adopt this approach even now. This paper provides a summary description of the HRS DBS data along with insights into their development and suggestions for use.
The HRS initiated data collection in 1992 and has continued collecting data every two years with the aim of creating a data resource from a nationally representative sample of Americans 51 years of age and older that captures the evolving health and economic circumstances linked to aging at both the individual and population levels (Sonnega et al., 2014). The HRS includes information on a comprehensive range of health measures pertinent to aging and well-being in middle-aged and older population including survey reported functioning difficulty and presence of chronic diseases. It also includes performance-based physical tests including lung function, grip strength, walking speed, balance as well as measured height, weight, waist circumference, blood pressure and heart rate. The HRS introduced the collection of blood based biomarker data in 2006 in order to supplement the survey data with more objective measures of health.
In health research, understanding the dynamics of health change within individuals is crucial to uncovering insights about links to risk factors and subsequent outcomes as well as identifying effects of and opportunities for intervention. While many large national studies provide valuable cross-sectional data, they often fall short in capturing the longitudinal aspect of health. The HRS longitudinal DBS collection allows researchers to examine change over time in health and causal pathways affecting it (Kumari & Benzeval, 2021; Weir, 2007). It also allows the examination of disease development and progression and the interplay of health with social and environmental changes (Tabák et al., 2010; Wu et al., 2021), suggesting opportunities for intervention.
The use of blood-based biomarkers to study changes in health over time, however, can be complicated. Measurement protocols, equipment, lab practices, and laboratories themselves can change over time, raising issues for longitudinal data analysis (Brown et al., 2019; Kumari & Benzeval, 2021). While survey researchers make considerable effort to minimize variation and maximize consistency across laboratories, (Plebani, 2013), assays performed by different laboratories may not be comparable or interchangeable (McLawhon, 2011). Even laboratories that produce good, valid assay results may see high variability in assays, especially those that are not used for diagnosis or drug prescription (Stepman et al., 2014; Warnick et al., 2008). Changes in reagents and analyzers can also affect longitudinal data (Kumari & Benzeval, 2021). Such changes can make it difficult to maintain the consistency of biomarker measurements over time; consequently, researchers who use longitudinal biomarker data face challenges in using and interpreting the data.
This paper provides technical and summary information on multiple waves of biological indicators collected in HRS DBS assays from 2006 to 2016 along with an indication of the value of these data for researchers. It indicates changes over time and across laboratories used to process these specimens that result in issues that researchers need to consider when using the data. In doing so, it emphasizes both the longitudinal opportunities and challenges provided by these data, which are available for public use (Crimmins et al., 2013; Crimmins et al., 2015, 2017, 2020).
MATERIALS & METHODS
Data collected
As indicated above, the HRS began collecting blood-based biomarkers using DBS in 2006 and ended this collection in 2016, when both DBS and venous blood samples (VBS) were collected. In 2006, the HRS asked a random half sample to provide a blood sample and repeated collection for this sample in 2010 and 2014. It asked the other half to provide a sample in 2008, 2012, and 2016.
The HRS sought blood samples from respondents who provided a face-to-face interview, excluding those who were interviewed by proxy or who were in a nursing home (Crimmins et al., 2013). The process included acquiring additional informed consent for blood acquisition. As Supplementary Table 1 shows, the percent consenting to and completing DBS collection varied by wave. While consent rates were above 80 percent in all waves, and nearly all those consenting to DBS collection completed it, the consent rate at the beginning of DBS collection in 2006 for the first half of the sample started at lower and increased over time while the second half sample begun in 2008 maintained a stable consent rate over the three waves. The increase in consent rates was due to increasing experience of the interviewers in collecting DBS as well as targeted efforts to increase participation rates. The experience gained in the initial years resulted in some changes in methods of collection, ways of requesting participation, and more focus on clarifying the value of participation for research. Across all waves, the average consent rate was lower for non-Hispanic Blacks (81%) and for those with a post-graduate degree (84%), which in turn led to lower average completion rates for non-Hispanic Blacks (80%) and those with a post-graduate degree (84%). The HRS provides an analysis weight for biomarkers in each wave that accounts for differences in consent and completion.
Biomarker measures based on DBS
For all 6 waves, DBS samples were assayed for 5 biomarkers: Total cholesterol (mg/dL), HDL cholesterol (mg/dL), glycosylated hemoglobin (HbA1c %), C-reactive protein (CRP) (ug/mL), and cystatin C (mg/L). These indicators reflect the functioning of multiple physiological and organ systems. Total and HDL cholesterol are indicators of lipid levels; glycosylated hemoglobin is an indicator of glycemic control over the past 2–3 months; C-reactive protein is a general marker of systemic inflammation; and cystatin C is an indicator of kidney functioning. For one cycle, 2014–2016, Interleukin 6 (IL-6) (pg/mL), a proinflammatory cytokine, was assayed.
Processing
Laboratories and assay protocols varied by wave. Altogether, five laboratories have assayed the HRS DBS. Supplementary Table 2 lists the names of these laboratories and the assays they performed. Initially, HRS staff sought to use one set of laboratories and assays; however, this was not possible for a variety of reasons that many studies will confront. In 2006, interviewers sent the DBS samples to Biosafe labs in Chicago for assays of total and HDL cholesterol and HbA1c, and to the University of Vermont for CRP and cystatin C assays. Biosafe declared bankruptcy while processing the 2008 samples and was replaced by FlexSite for HbA1c and by the University of Washington for the lipid assays. The University of Vermont continued to assay CRP and cystatin C. In 2010 and 2012, interviewers mailed the DBS cards directly to HRS staff at the University of Michigan, where they were sorted, frozen, and shipped (in batches) to the appropriate labs for processing (Heritage Laboratory, now owned by Clinical Reference Laboratory, Inc., and the University of Washington). In 2014 and 2016, the interviewers mailed the DBS cards directly to the Department of Laboratory Medicine at the University of Washington in Seattle for assay. In HRS, changes in laboratory procedures including the choice of assay and the analyzer used, both of which can vary across time and by lab, can potentially affect assay values (Börsch-Supan et al., 2021; Crimmins et al.,2020; Groh et al., 2022), thus we provide these details to illustrate the continuity issues that researchers may need to address.
Assay values from dried blood spots vs. whole blood
While dried blood spots (DBS) samples offer a valuable tool for data collection, DBS-based biomarker values may differ significantly from conventional venous blood-based assays. HRS have made efforts to ensure the validity and reliability of the assay results by comparing dried blood spot (DBS) samples to VBS samples through multiple comparisons, demonstrating strong agreement with high correlation coefficients and reproducibility, as detailed in the HRS documentation (Crimmins et al., 2013; Crimmins et al., 2015, 2017, 2020). However, the biomarker values based on DBS are generally quite different in value from the more conventional whole (venous) blood based assays such as those used in the NHANES (Börsch-Supan et al., 2021; Crimmins et al., 2014, 2020; McDade, 2014; McDade et al., 2007; Weir, 2007; Weiss et al., 2019). The results from the two methods can differ because of a number of factors including cell dilution, inconsistent blood application, differences in drying and reconstitution, as well as metabolic and circulatory dynamics, all of which contribute to the observed disparities in measured values (McDade, 2014). Because DBS values are collected in the field rather than a clinic, they are also affected by procedures of collection, shipping, and handling (Crimmins et all, 2020; Borsch-Supan et al., forthcoming). As a result, DBS assay values cannot be compared directly with VBS assays.
HRS has transformed DBS values into venous blood equivalents to make them more comparable to those of venous blood-based assays. NHANES was used as a standard to convert HRS DBS values to VBS equivalent values for each analyte assuming the values in this nationally representative sample of the same age provide appropriate benchmarks. In NHANES, HbA1c was measured in whole blood collected in vacuum collection tubes containing EDTA anticoagulant; the other analytes were assessed using serum or plasma EDTA or heparin anticoagulants. The conversion makes HbA1c equivalent to whole blood values and total cholesterol, HDL cholesterol, CRP, and cystatin C equivalent to serum values. The HRS has constructed and released NHANES equivalent values for total and HDL cholesterol, HbA1c, C-reactive protein, and cystatin C. HRS documentation compares HRS DBS values and the NHANES equivalent values for the blood assays from 2006 through 2016 (Crimmins et al., 2013; Crimmins et al., 2015, 2017, 2020). Because HRS collected venous blood-based as well as DBS samples in 2016, we can compare the distributions of 2016 HRS DBS and VBS with NHANES VBS samples and the HRS NHANES equivalent samples (supplementary Figure 1). Our conversion of DBS values to NHANES equivalent values produces comparable distributions for total and HDL cholesterol, and CRP, but cystatin C in NHANES and HRS is different resulting from the use of earlier NHANES data to make the equivalences and changes in assay over time. For IL-6, there is no standard available for a national population so the University of Washington has provided a plasma equivalent which is what is provided by HRS.
Constructing VBS equivalent values
The NHANES equivalent values for the HRS DBS assays reflect the levels and variance of conventional VBS assays in NHANES while preserving the individual variability within the HRS sample. To estimate these, we applied a version of linear equation based on the percentile distributions of values in each study. Specifically, we first calculated the values of the assays corresponding to (weighted) percentiles in HRS and in NHANES. To facilitate construction of percentiles when values were discrete and multiple individuals had the same value, we added a very small random number to each observed value, created the (weighted) percentiles based on the altered values, and then took the mean of the actual assay values at each percentile. We then had 100 percentiles for HRS and 100 percentiles for NHANES (for CRP, which had a highly skewed distribution, we worked with logged values). We then regressed the HRS value on the NHANES value to create an equation that can be used to convert HRS values into NHANES equivalent values. Supplementary Table 3 shows these equations. We followed this procedure for total and HDL cholesterol, HbA1c, CRP and cystatin C.
To make appropriate NHANES equivalent values that allow comparisons over time, we used pooled samples from NHANES surveys. The specific survey years used for each wave of HRS are presented in Supplementary Table 3. Average differences in the 2006/2010/2014 and 2008/2012/2016 HRS samples will reflect NHANES changes over time. The two exceptions are for cystatin C and CRP. Because cystatin C has not been regularly assayed in NHANES, the 1999–2002 NHANES data are used for computing the NHANES equivalent values for HRS from 2006 to 2016. This means the HRS values do not capture changes over time in cystatin C. For CRP, NHANES 2005–2006 and 2007–2008 data were used for the released HRS 2006 and 2008 values; NHANES 2009–2010 data for the HRS 2010, 2012, and 2014 assays (there is no NHANES CRP data for 2011–2012 and 2013–2014); and NHANES 2015–2016 data for the HRS 2016 assay. Subsequently, we noted a large drop in the NHANES CRP mean for older adults from 4.59 in 2005–2006 to 4.44 in 2007–2008 to 3.94 in 2009–2010. A drop of this magnitude likely reflects assay change at NHANES rather than actual changes; we have therefore readjusted CRP values and are releasing updated data on the HRS website, with NHANES equivalents based on NHANES 2005–2008 for all HRS waves. This means there is no time change in the average HRS CRP measures in the longitudinal file, but individual change can still be tracked.
The University of Washington supplied formulas to convert IL-6 DBS values to plasma values based on comparisons of DBS and plasma made in the laboratory. Because the original 2014 and 2016 IL-6 values derived from DBS were not comparable due to the use of different reagents for the assays, the University of Washington assayed the frozen 2014 samples again using the 2016 assay and produced 2014 equivalent values for the 2016 assays. The laboratory provided the 2014 plasma equivalent IL-6 values and the formula to convert DBS IL-6 values to plasma equivalent IL-6 values of 2016 (Crimmins et al., forthcoming).
RESULTS
Distributions of the biomarkers
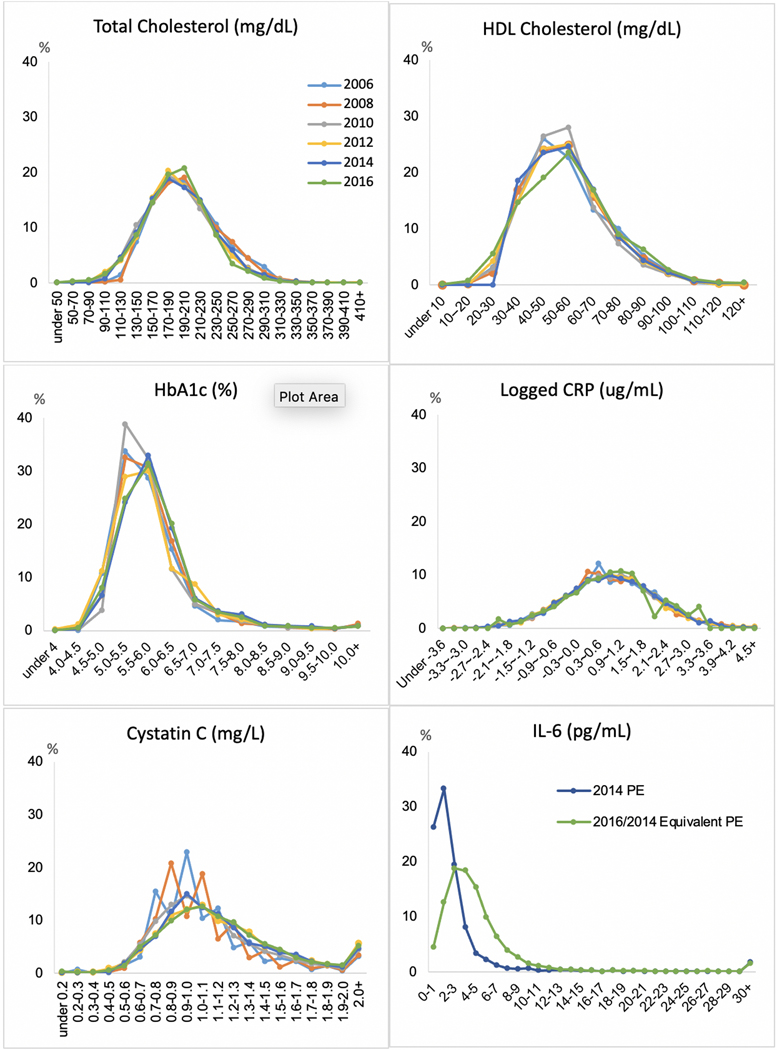
Figure 1 shows distributions of the NHANES equivalent values for five biomarkers at 6 waves and IL-6 plasma equivalent values in 2014 and 2016. The distributions of total and HDL cholesterol are quite similar across waves, with a normal distribution. For HbA1c, the distribution moved to the right somewhat, indicating more respondents with higher levels of glycosylated hemoglobin in 2014 and 2016. This is consistent with the increase of high-risk HbA1c observed in a NHANES-based study in this time period (Kim et al., 2019). The log of CRP has a normal distribution that was similar across waves as is the distribution for Cystatin C. Our attempts to harmonize values for IL-6 at two dates appear to be relatively unsuccessful as the figure indicates quite different patterns at the low end resulting from the changed assay.
Figure 1.
Distribution of NHANIS Equivalent Biomarker Values in Six Waves
Change in high-risk biomarkers over time
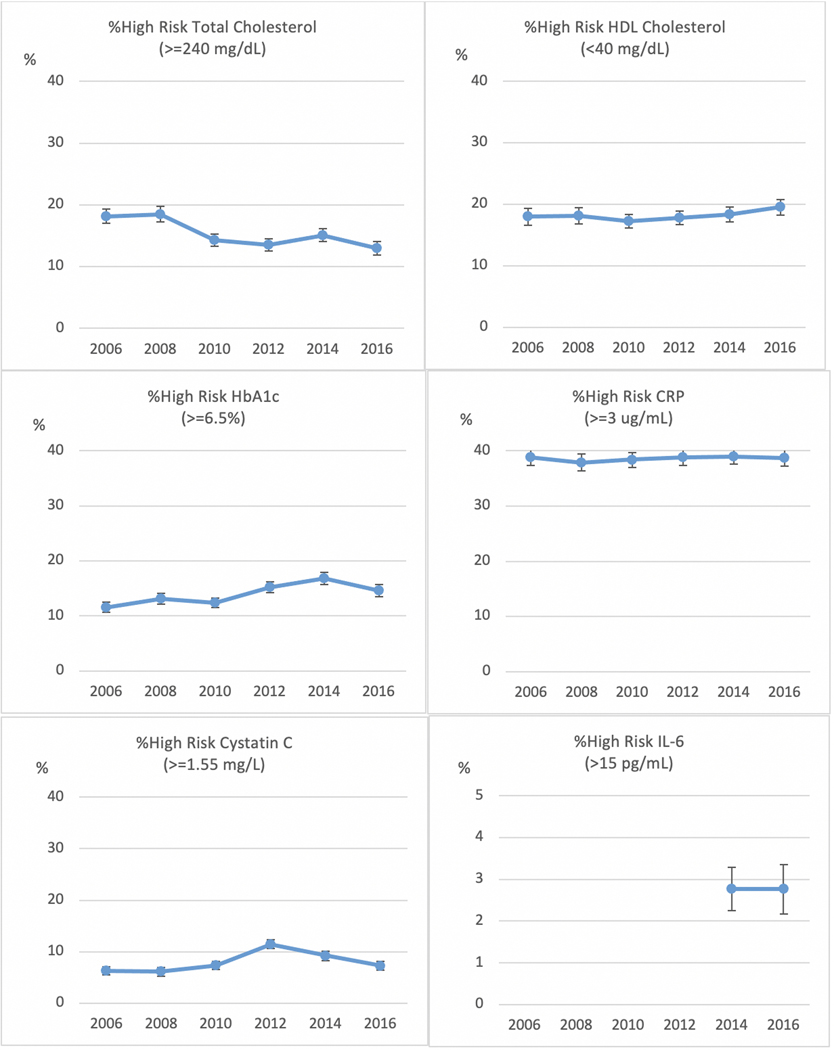
One way many users want to employ the biomarker data is to use cutoffs for high-risk levels. In Figure 2, we show the percent with high-risk levels, as determined by standard cutoff levels, of each of the biomarkers over 6 waves with controls for age and sex. Change over time is reflected in decreasing prevalence of high total cholesterol (>=240 mg/dL) and increasing percent with high HbA1c (>=6.5%), peaking at 16.8% prevalence in 2014. There is no change in HDL cholesterol. We have estimated the other three assays so that there should not be change and this is what is observed, although there is some variability in cystatin C.
Figure 2.
Age- and Sex- Adjusted Percent With High Biomarkers in Sex Waves
Difference in the level of biomarkers by age and over time
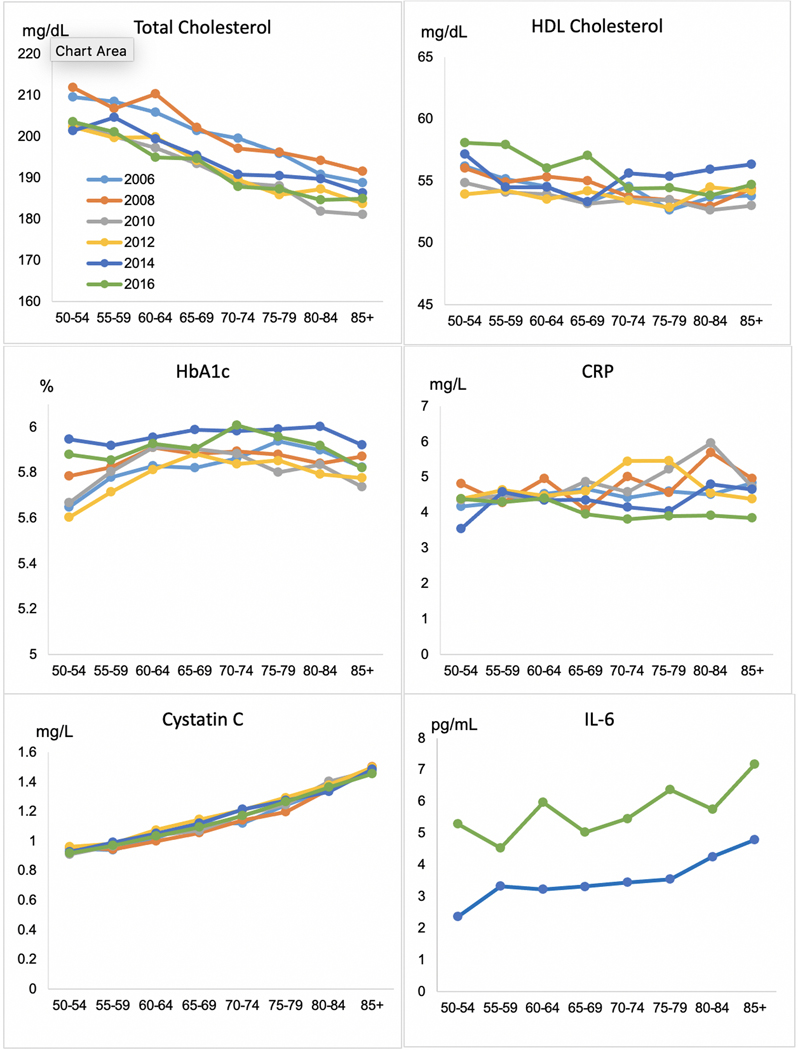
Because the HRS is focused on aging, it collects biomarkers that are expected to differ by age and to change over time with age. Figure 3 shows the levels of biomarkers by age for each measurement year. As age increases, the level of total cholesterol goes down. This age pattern is not what might be expected but it is very consistent throughout the measurement period, and points to new associations by age with risk factors. There are consistent reductions in total cholesterol across age over time, so that respondents of all ages in more recent waves tend to have lower levels of total cholesterol. The two other markers where we can observe differences by age and change over time – HDL cholesterol and HbA1c – do not show a clear pattern of age differences. HbA1c tends to increase with age but then levels off and decreases among older age groups, perhaps due to mortality among those with high HbA1c. The highest values of HbA1c for all ages occur in 2014 and 2016. Cystatin C clearly increases with age, reflecting declining kidney function associated with aging (Shlipak et al., 2009). IL-6 also clearly increases with age but with obvious differences in value in the two years of data collection. There is no clear age pattern to CRP.
Figure 3.
Mean Biomarkers by Age in Six Waves
Individual change in biomarkers over time.
The HRS biomarker variables offer the unique opportunity to examine change at a relative individual level in these biomarkers and how that varies for important subgroups of the population. As an example, we examine the effect of race/ethnicity on the onset of high risk levels of individual biomarkers over eight years for those who had normal levels at baseline using cox proportional hazard models (Table 1). This allows us to examine differences in health change in a given time period rather than differences that might have had onset at any point in the lifecycle. Our hypothesis is that persons who are non-Hispanic Black and those who are Hispanic will have higher levels of health deterioration. We find this to be true; compared to non-Hispanic White, non-Hispanic Black and Hispanic respondents have 1–6% (p<0.001) higher risk of onset of high biological risk in all biomarkers except CRP for non-Hispanic Blacks, who actually have lower risk (HR=0.96, p<.001). The value of this type of approach is in allowing researchers to link time appropriate events, behaviors, onset of other health conditions and use of health services to changing health.
Table 1.
Hazard Ratio of Onset over 8 Years of High Biological Risk among Those with Normal Levels at Baseline
| Total Cholesterol | HDL Cholesterol | HbA1c | CRP | Cystatin C | |
|---|---|---|---|---|---|
| Non-Hispanic White (ref) | |||||
| Non-Hispanic Black | 1.02* | 1.03* | 1.06* | 0.96* | 1.02* |
| Hispanic | 1.04* | 1.01* | 1.01* | 1.03* | 1.01* |
| N | 3,088 | 3,181 | 3,415 | 2,403 | 3,635 |
Age and gender controlled
p<.001
DISCUSSION
The Health and Retirement Study (HRS) can be very useful in advancing our understanding of health and aging by offering longitudinal biomarker data collected through dried blood spots (DBS) from a nationally representative sample of Americans over age 50. The HRS played a pioneering role as an early adopter of dried blood spot (DBS) sampling in population representative surveys, which has now been used in numerous surveys and become a relatively standard part of the toolkit available to population-based surveys on health. HRS initially chose to use DBS blood samples because they offer several advantages over venous blood sampling (VBS). DBS sampling is non-invasive and convenient, involving a simple finger prick, which reduces participant burden and increases adherence in large-scale studies like the HRS. It is also cost-effective and time-efficient, requiring minimal training of interviewers and minimal equipment. The collection process can be done in a short time in the home by a non-medical professional. Additionally, DBS samples do not require immediate processing and have long-term stability.
While DBS sampling offers numerous benefits, it also presents certain challenges. The limited sample volume produced with DBS limits the number of biomarkers that can be analyzed; and the validated assays available for use with DBS are limited. Proper collection including maintaining the quality of the spots and cards, handling (e.g., time before freezing), shipping, storage, and temperature and humidity in preanalytic phase are all crucial for reliable and valid results based on DBS (Borsch-Supan et al., 2021; Crimmins et al., 2020). Assaying biomarkers from DBS samples is not routinely done by labs, so the quality and availability of laboratories for assay is an issue. Additionally, the lack of direct comparability with studies using VBS is limited and studies need to be designed to assess this and convert DBS to more conventional measures to be most useful in generalizing the scientific literature. Because of the issues of limited sample and assay availability, HRS is no longer collecting DBS on a routine basis.
Our results of the changes over time and age distributions reveal important insights. Total cholesterol levels decreased across waves while HbA1c levels increased over time. The decline in high total cholesterol levels may be due to improved cholesterol management and increased awareness of cardiovascular health, leading to better control of cholesterol levels through lifestyle modifications or medical interventions; an increase in high HbA1c levels may indicate a growing burden of diabetes and increasing obesity. We clarify that the HRS data should not be used to examine time trends in some biomarkers because either assays changed or no appropriate gold standard exists or both.
Examining the differences in biomarker levels by age and over time can provide valuable insights into the physiological changes associated with aging and time change in population health for age groups. Our analysis on individual biomarker change over eight years provides evidence that recent onset of high biological risk is greater among race/ethnic minorities. This demonstrates the value of data that can be used to assess current health conditions rather than lifetime circumstances. These data offer valuable opportunity for those from many fields to investigate health change in the older population and individuals in that population.
Data resource access
The Health and Retirement Study Biomarker data have been funded by the National Institute on Aging (NIA U01AG009740) and are publicly available. Researchers who want to use biomarker data are required to submit a Sensitive Data Access Use Agreement (https://hrsdata.isr.umich.edu/data-products/sensitive-health/order-form). Users must agree to make no attempt to identify individuals in the dataset, to adhere to the guidelines that protect personal identity when analyzing data and producing tabulations for distribution, and to store the data in a secure place. (https://hrs.isr.umich.edu/sites/default/files/HRS-Sensitive-Data-Access-Use-Agreement.pdf?_ga=2.248918979.551272444.1632174039-373346087.1623772278).
Supplementary Material
Footnotes
Ethics statement
Not applicable
Supplementary data
Supplementary data are available online.
Conflict of interest
The authors declare no potential conflict of interest.
References
- 1.Bloom DE, Hu P, Arokiasamy P, Risbud A, Sekher TV, Mohanty SK, Kale V, O’Brien J, Chien CS, & Lee J. LongitudinalAging Study in India: Biomarker Data Documentation. PGDA Working Paper. 2014;114. [Google Scholar]
- 2.Börsch-Supan A, Brandt M, Hunkler C, Kneip T, Korbmacher J, Malter F, Schaan B, Stuck S, & Zuber S. (2013). Data Resource Profile: the Survey of Health, Ageing and Retirement in Europe (SHARE), International Journal of Epidemiology, 42(4), 992–1001. 10.1093/ije/dyt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Börsch-Supan M, Horton H, Sun A, Weiss L, Groh R, Schmidutz D, Börsch-Supan A, Andersen-Ranberg K, Borbye-Lorenzen N, Skogstrand K, Cofferen J, Kerschner E, Kha T, Potter A, & Wener M. (forthcoming). Biomarkers in SHARE: Documentation of implementation, collection, and analysis of dried blood spot (DBS) samples 2015 – 2023.
- 4.Börsch-Supan A, Weiss LM, Börsch-Supan M, Potter Al, Cofferen J, & Kerschner E. (2021). Dried blood spot collection, sample quality, and fieldwork conditions: Structural validations for conversion into standard values. American Journal of Human Biology, 33, e23517. 10.1002/ajhb.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M, Gilbert E, Calderwood L, Taylor K, & Morgan H. (2019). Collecting biomedical and social data in a longitudinal survey: A comparison of two approaches. Longitudinal and Life Course Studies, 10(4), 453–469. 10.1332/175795919X15694156772013 [DOI] [Google Scholar]
- 6.Chen X, Crimmins E, Hu P, Kim JK, Meng Q, Strauss J, Wang Y, Zeng J, Zhang Y, & Zhao Y. (2019). Venous blood-based biomarkers in the China Health and Retirement Longitudinal Study (CHARLS): Rational, design, and results of the 2015 wave. American Journal of Epidemiology, 188(11), 1871–1877. 10.1093/aje/kwz170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen K. (2004). Human biodemography: Some challenges and possibilities for aging research. Demographic Research, 19, 1575–1586. [Google Scholar]
- 8.Crimmins E, Faul J, Kim JK, Guyer H, Langa K, Ofstedal MB, Sonnega A, Wallace R, & Weir D. (2013). Documentation of biomarkers in the 2006 and 2008 health and retirement study. Ann Arbor, MI: Survey Research Center, University of Michigan. https://hrs.isr.umich.edu/sites/default/files/biblio/Biomarker2006and2008.pdf [Google Scholar]
- 9.Crimmins E, Faul J, Kim JK, & Weir D. (2015). Documentation of biomarkers in the 2010 and 2012 Health and Retirement Study. Ann Arbor, MI: Survey Research Center, University of Michigan. https://hrs.isr.umich.edu/sites/default/files/biblio/Biomarker2010and2012.pdf [Google Scholar]
- 10.Crimmins E, Faul J, Kim JK, & Weir D. (2017). Documentation of blood-based biomarkers in the 2014 Health and Retirement Study. Ann Arbor, MI: Survey Research Center, University of Michigan. https://hrs.isr.umich.edu/sites/default/files/biblio/Biomarker%202014_Dec2017.pdf [Google Scholar]
- 11.Crimmins E, Faul J, Kim JK, & Weir D. (2020). Documentation of DBS blood-based biomarkers in the 2016 Health and Retirement Study. Ann Arbor, MI: Survey Research Center, University of Michigan. https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/HRS%20Data%20Documentation%20for%202016%20DBS%20Release.pdf [Google Scholar]
- 12.Crimmins EM, Hu J, Hu P, Huang W, Kim JK, Shi Y, Strauss J, Zhang L, Zhao XZ, & Zhao Y. (2011). CHARLS Pilots: Blood-based Biomarker Documentation. https://charls.pku.edu.cn/wenjian/ganxuebanyonghushouce.pdf
- 13.Crimmins EM, Kim JK, Faul J. & Weir D. (forthcoming). HRS interleukin-6 (IL-6) 2014 and 2016: Assays from dried blood spots. Ann Arbor, MI: Survey Research Center, University of Michigan. [Google Scholar]
- 14.Crimmins EM, Kim JK, McCreath H, Faul J, Weir D, & Seeman T. (2014). Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography and Social Biology, 60, 38–48. 10.1080/19485565.2014.901885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crimmins EM, Zhang YS, Kim JK, Frochen S, Kang H, Shim H, Ailshire J, Potter A, Cofferen J, & Faul J. (2020). Dried blood spots: Effects of less than optimal collection, shipping time, heat, and humidity. American Journal of Human Biology, 32, e23390. 10.1002/ajhb.23390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman N, Turra C, Rosero-Bixby L, Weir D, & Crimmins E. (2010). Do biological measures mediate the relationship between education and health: A comparative study. Social Science and Medicine, 72, 307–315. 10.1016/j.socscimed.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groh R, Weiss LM, Börsch-Supan M, & Börsch-Supan A. (2022). Effects of spot size on biomarker levels of field-collected dried blood spots: A new algorithm for exact dried blood spot size measurement. American Journal of Human Biology, 34(10), e23777. 10.1002/ajhb.23777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris KM, & Schorpp KM (2018). Integrating biomarkers in social stratification and health research. Annual Review of Sociology, 44, 361–386. 10.1146/annurev-soc-060116-053339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herningtyas E, Hu P, Edenfield M, Strauss J, Crimmins E, Witoelar F, Zhang Y, Kim JK, & Sikoki B. (2017). IFLS Wave 5 Dried Blood Spot Data User Guide. https://www.researchgate.net/profile/Elizabeth-Henny-Herningtyas/publication/322306932_IFLS_Wave_5_Dried_Blood_Spot_Data_User_Guide/links/5a53061aaca2725638c7d55b/IFLS-Wave-5-Dried-Blood-Spot-Data-User-Guide.pdf
- 20.Kim JK, Ailshire JA, & Crimmins EM (2019). Twenty-year trends in cardiovascular risk among men and women in the United States. Aging Clinical and Experimental Research, 31, 135–143. 10.1007/s40520-018-0932-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumari M, & Benzeval M. (2021). Collecting biomarkers data in longitudinal surveys. In Lynn P. (Ed.), Advances in Longitudinal Survey Methodology (pp. 26–46). Hoboken NJ: John Wiley & Sons Ltd. [Google Scholar]
- 22.Mayeux R. (2004). Biomarkers: Potential uses and limitations. NeuroRx, 1, 182–188. 10.1602/neurorx.1.2.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDade TW (2014). Development and validation of assay protocols for use with dried blood spot samples. American Journal of Human Biology, 26, 1–9. 10.1002/ajhb.22463 [DOI] [PubMed] [Google Scholar]
- 24.McDade TW, Williams SR, & Snoddgrass JJ (2007). What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography, 44, 899–925. 10.1353/dem.2007.0038 [DOI] [PubMed] [Google Scholar]
- 25.McLawhon RW (2011). Patient safety and clinical effectiveness as imperatives for achieving harmonization inside and outside the clinical laboratory. Clinical Chemistry, 57, 936–938. 10.1373/clinchem.2011.166041 [DOI] [PubMed] [Google Scholar]
- 26.Plebani M. (2013). Harmonization in laboratory medicine: the complete picture. Clinical Chemistry and Laboratory Medicine, 51(4), 741–751. 10.1515/cclm-2013-0075 [DOI] [PubMed] [Google Scholar]
- 27.Rosero-Bixby L, & Dow WH (2012). Predicting mortality with biomarkers: a population-based prospective cohort study for elderly Costa Ricans. Population Health Metrics, 10, 11. 10.1186/1478-7954-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak MG, Katz R, Kestenbaum B, Fried L, Newman A, Siscovick D, Stevens L, & Sarnak M. (2009). Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. American Journal of Nephrology, 30(3), 171–178. 10.1159/000212381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnega A, Faul JD, Ofstedal MB, Langa K, Phillips J, Weir D. (2014). Cohort Profile: the Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepman HC, Tiikkainen U, Stöckl D, Vesper H, Edwards S, Laitinen H, Pelanti J, & Thienpont L. (2014). Measurements for 8 common analytes in native sera identify inadequate standardization among 6 routine laboratory assays. Clinical Chemistry, 60(6), 855–863. 10.1373/clinchem.2013.220376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabák A, Kivimäki M, Brunner E, Lowe GD, Jokela M, Akbaraly TN, Singh-Manoux A, Ferrie JE, & Witte DR (2010). Changes in C-reactive protein levels before type 2 diabetes and cardiovascular death: the Whitehall II study. European Journal of Endocrinology, 163, 89–95. 10.1530/EJE-10-0277 [DOI] [PubMed] [Google Scholar]
- 32.Warnick GR, Kimberly MM, Waymack PP, & Leary ET (2008). Standardization of measurements for cholesterol, triglycerides, and major lipoproteins. Laboratory Medicine, 39(8), 481–490. 10.1309/6UL9RHJH1JFFU4PY [DOI] [Google Scholar]
- 33.Weir DR (2007). Elastic powers: The integration of biomarkers into the Health and Retirement Study. In Weinstein M, Vaupel JW, & Wachter K. (Eds.). Bio-Social Surveys: Current insight and Future Promise (pp. 78–95). Washington DC: The National Academies Press. [Google Scholar]
- 34.Weiss L, Börsch-Supan M, Myck M, Nocoń K, Oczkowska M, Topór-Mądry R, Andersen-Ranberg K, & Börsch-Supan A. (2019). Blood collection in the field – results and lessons from the polish test study. In Börsch-Supan A, Bristle J, Andersen-Ranberg K, Brugiavini A, Jusot F, H Litwin, & b G. (Eds.). Health and Socio-economic Status Over the Life Course; (pp. 367–374), Berlin, Boston: De Gruyter Oldenbourg. [Google Scholar]
- 35.Wu Q, Ailshire J, Kim JK, & Crimmins EM (2021). Cardiometabolic risk trajectory among older Americans: Findings from the Health and Retirement Study. Journal of Gerontology A Biological Sciences and Medical Science, 76(12), 2265–2274. 10.1093/gerona/glab205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao E, Ailshire J, Kim JK, Wu Q, & Crimmins EM (in review). Associations between change in kidney functioning, age, race/ethnicity and health indicators in the Health and Retirement Study. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.