Abstract
Background.
Epidemiologic studies have established obesity as a risk factor for multiple sclerosis (MS). These studies relied on body-mass index (BMI) and body size silhouettes as the primary measures of obesity. Unfortunately, the causal mechanisms through which obesity confers MS risk are not yet known.
Objectives.
To investigate the causal effects of multiple specific measures of body fat on MS risk in populations of European descent, using Mendelian randomization (MR).
Methods.
MR is a genetic instrumental variable analysis utilizing genome-wide association (GWA) summary statistics to infer causality between phenotypes. MR analyses were performed to investigate the relationships between seven measures of body fat (BMI, waist-hip ratio, visceral adipose tissue [VAT], subcutaneous adipose tissue, and arm-, leg-, and trunk-fat to total body fat ratio) and MS risk.
Results.
Only BMI and VAT were significantly associated with MS risk in separate MR analyses (βBMI=0,27, pBMI<0.001; βVAT=0.28, pVAT=0.006). High correlation between BMI and VAT instruments suggest that two-sample MR associations for BMI and VAT likely capture the same causal mechanisms.
Conclusions.
BMI and VAT were causally associated with MS risk in European populations, though their effects do not appear independent, suggesting overlap in the role of overall body mass and visceral obesity in MS pathogenesis.
Keywords: Multiple sclerosis, Mendelian randomization, Body fat, Risk
1. Introduction
Childhood and early adulthood obesity are established risk factors for adult-onset MS, with excessive body fat accumulation conferring increased risk 1-3. Considering the global obesity epidemic, it continues to be important to unravel the mechanisms through which obesity predisposes individuals for MS. Epidemiological studies of obesity and MS risk have utilized body mass index (BMI), and a few have used body silhouettes, reporting that larger body sizes are associated with increased MS risk 2,4 Mendelian randomization (MR) studies have confirmed causal associations with MS risk for childhood and adulthood BMI and highly correlated measures of body fat mass (i.e., whole body and region-specific fat mass and percentage) 5-9 but not for adiposity-related hormones adiponectin and leptin 10-12. Together, these findings implicate excessive body fat as a risk factor for MS. Yet, there are opportunities to add further resolution by examining the role of specific fat tissues and the relative anthropometric distribution of body fat in relation to MS risk.
The adipose organ is comprised of multiple compartments of cells with differing functions that are likely derived from several developmental lineages 37. Body fat is mostly subcutaneous adipose tissue (SAT; 80-90%) or visceral adipose tissue (VAT; <20%) 38. SAT is primarily deposited beneath the skin in the abdominal (trunk), upper back, gluteal, and femoral (thigh) regions, and VAT is deposited within the abdominal cavity near internal organs where it is drained by the portal vein allowing its products to preferentially impact metabolism 37, 38. Thus, SAT and VAT have varied biological properties, for example: both are correlated with fasting glucose and triglyceride levels; they have different gene expression profiles; VAT confers risk for cardiovascular disease, metabolic syndrome, and mortality, and it has been associated with systemic oxidative stress, dysregulated glucose homeostasis, and fatty liver; abdominal SAT has been associated with adverse lipid, insulin, and C-reactive protein; and gluteofemoral SAT confers little metabolic disease risk and may indeed confer lower risk of type 2 diabetes, cardiovascular diseases, and adverse lipid levels 37, 38. To add further complexity, the distribution of body fat is sexually dimorphic, and aging and sex hormones are prominent determinants 13-15.
BMI and regional body fat mass are highly correlated traits with substantial overlap in their genetic architecture (many r2>80%), and MR studies of these traits on MS risk have been illuminating 13. However, it is not known if VAT and/or SAT specifically confer MS risk, or if body shape matters, such as waist to hip ratio (WHR) which measures fat deposition in the abdomen relative to the gluteofemoral regions and it is moderately correlated with overall and regional fat mass (r2: 0.35-0.65) 13. Also, other measures of body shape have not been studied in relation to MS, and include arm fat to total body fat ratio (AFR), trunk fat to total body fat ratio (TFR), and leg fat to total body fat ratio (LFR) – characterizing the causal relationships between these anthropometric attributes would provide insights to the extent to which imbalances in fat deposition may influence MS risk.
In the current study, we performed multiple MR analyses, characterizing the causal relationships between VAT, SAT, WHR, AFR, TFR and LFR and MS risk in order to further deconstruct the established risk relationship between obesity and MS.
2. Materials and Methods
2.1. Mendelian Randomization
MR is a genetic instrumental variable analysis where genetic associations for a predictor of interest serve as proxies for the predictor, and it is exceptional useful when direct measures of the predictor and the outcome are not jointly available or when the relationship of interest is likely to be subject to unmeasured confounding. Three assumptions are necessary for a robust MR analysis: first, that the genetic variants are truly associated with the predictor of interest, second, that the genetic variants do not affect the outcome except through the potential effect of the predictor on the outcome, and third, that there are no upstream factors that causally influence both the genetic variants in the instrument and the outcome. 2-sample (2S) MR is a form of MR where genome-wide association summary statistics for both the predictor and the outcome of interest are used to infer causal relationships, based on the assumptions listed. 2SMR analysis is performed by creating a ratio of the effect estimate of the effect SNP on the outcome, divided by the effect estimate of the effect SNP on the exposure; known as a Wald ratio. This is repeated for each SNP in the genetic instrument. The ratios are then interrogated for overall effect by conducting inverse-variance weighted (IVW) meta-analysis. However, other methods are frequently employed to account for issues of horizontal pleiotropy, such as the MR-Egger approach (see section 2.3 Statistical Analysis).
2.2. Instrument Selection
Alongside BMI 16, predictors of interests included VAT 17, SAT 18, WHR 19, and AFR, LFR, and TFR 20. Summary statistics from large-scale consortium-driven genome-wide association (GWA) studies in European populations were utilized to identify variants significantly associated (p<5x10−8) with each predictor of interest (Table 1). Additional descriptions of how the exposures were measured in their respective GWAS can be found in the Supplementary Methods. The number of variants in each MR instrument before harmonization and linkage disequilibrium (LD) clumping are noted in Table 1. The GWA summary statistics for MS risk were obtained from the International Multiple Sclerosis Genetics Consortium (IMSGC) using a cohort of 14,802 cases and 26,703 controls in the discovery stage 21 (Table 1). Variants in each instrument were excluded if the alleles were palindromic and the forward strand could not be inferred by minor allele frequency (MAF > 0.42). The variants were then clumped at a LD threshold of r2<0.05 using the 1000 Genomes European Ancestry (1000G EUR) reference panel (Supplementary Table 1A).
Table 1.
Genome-wide association datasets used in Mendelian randomization analyses.
| Phenotype | Consortium | Sample Size | Authors | nSNP* (trait) |
h2** (trait) |
h2 (instrument) |
|---|---|---|---|---|---|---|
| Body-mass index | GIANT, UK Biobank | 681,275 | Yengo et al. 2018 | 656 | 0.35 | 0.25 |
| Waist-hip ratio | GIANT, UK Biobank | 694,649 | Pulit et al. 2019 | 316 | 0.19 | 0.03 |
| Visceral adipose tissue | UK Biobank | 325,123 | Karlsson et al. 2019 | 205 | (F) 0.37 (M) 0.39 |
Not reported |
| Subcutaneous Adipose Tissue | UK Biobank | 32,860 | Liu et al. 2021 | 1 | 0.35 | Not Reported |
| Arm to total body fat ratio | UK Biobank | 362,499 | Rask-Andersen et al. 2019 | 23 | (F) 0.25 (M) 0.15 |
Not reported |
| Leg to total body fat ratio | 35 | (F) 0.21 (M) 0.13 |
Not reported | |||
| Trunk to total body fat ratio | 38 | (F) 0.23 (M) 0.11 |
Not reported | |||
| Multiple sclerosis | IMSGC | 14,802 cases 26,703 controls | Patsopoulos et al. 2019 | NA | 0.22 | NA |
nSNP, the number of SNPs included in the genetic instrument prior to quality control; identified as the independent significant signals identified in the corresponding GWAS
h2, the measure of narrow-sense heritability for the full trait and for the select SNPs included in the MR genetic instrument prior to instrument quality control; (F) h2 amongst females in the sample; (M) h2 amongst males in the sample
Instruments were also created using non-overlapping genetic variants for each of the seven exposure phenotypes in order to investigate effects driven by individual obesity-related exposures, as opposed to obesity in general. If a variant was originally included in the instruments of two or more of the seven obesity-related exposures, it was subsequently removed from each relevant instrument (Supplementary Table 2). Once a list of non-overlapping variants was obtained for each obesity-related exposure, the variants in each instrument were excluded if they were palindromic, and then clumped for LD (Supplementary Table 1B).
2.3. Statistical Analysis
2SMR analyses were performed for each obesity-related trait and MS risk using the R package TwoSampleMR 22. IVW meta-analysis was performed for the Wald ratios of each obesity-related trait on MS risk. MR-PRESSO (Mendelian Randomization Residual Sum and Outlier) tests were performed for each instrument on MS risk to identify overall horizontal pleiotropy in the meta-analyzed effect using the MRPRESSO R package 23. 2SMR and MR-PRESSO were repeated for MR instruments that excluded overlapping variants for each of the seven obesity-related traits and MS risk. Multivariable (MV) MR was conducted to estimate the independent direct effects of multiple exposures simultaneously on MS risk. Obesity-related traits were included in the MVMR model if they demonstrated a significant association with risk for MS in the 2SMR stage under the IVW approach, with support from the MR-PRESSO results. Regression-based MVMR 24 was conducted with and without the inclusion of an intercept term. For the MVMR instrument, variants were selected if they were present in at least one of the original 2SMR instruments for the obesity-related traits included in the model and were present in all relevant sets of GWA summary statistics. Palindromic variant exclusion and clumping techniques were applied to the MVMR instrument similarly to the 2SMR instruments (see 2.2 Instrument Selection).
2.4. Data Availability and Ethical Board Review
Summary-level data used to construct the MR instruments is available per request to the authors, but can be readily requested from the authors of the originating studies. This study was deemed non-human subjects by the Case Western Reserve University’s institutional review board as all data are aggregate summary statistics (STUDY20210195). Study protocol and details were not pre-registered.
3. Results
3.1. Two-sample Mendelian Randomization
Two-sample MR was conducted for each of the seven obesity-related traits and risk for MS (Table 2). Genetic variants comprising each of the seven MR instruments can be found in Supplementary Tables 3-9. Only BMI and VAT were found to be significantly associated with risk for MS under the IVW meta-analysis (βBMI=0.269, pBMI<0.001; βVAT=0.282, pVAT=0.006) (Figure 1). A single Wald ratio analysis investigated the association between SAT and MS risk as there was only one genetic instrumental variable, and the association was suggestive of a positive relationship with MS risk βSAT=0.451, pSAT=0.055).
Table 2.
Results for 2-Sample MR Univariate Analyses
| Exposure | Number of Variants |
Method | Beta | SE | p-value | MR-PRESSO | ||
|---|---|---|---|---|---|---|---|---|
| p(Global) | p(Outlier) | p(Distortion) | ||||||
| BMI | 530 | Inverse-variance Weighted | 0.269 | 0.067 | <0.001 | <0.001 | <0.001 | 0.984 |
| WHR | 254 | Inverse-variance Weighted | 0.029 | 0.127 | 0.822 | <0.001 | 0.036 | 0.488 |
| VAT | 144 | Inverse-variance Weighted | 0.282 | 0.102 | 0.006 | <0.001 | <0.001 | 0.537 |
| SAT | 1 | Wald Ratio | 0.451 | 0.235 | 0.055 | NA | NA | NA |
| AFR | 11 | Inverse-variance Weighted | 0.225 | 0.318 | 0.480 | 0.002 | 0.072 | 0.525 |
| LFR | 28 | Inverse-variance Weighted | −0.229 | 0.180 | 0.203 | 0.012 | 0.038 | 0.764 |
| TFR | 32 | Inverse-variance Weighted | 0.164 | 0.163 | 0.315 | 0.007 | 0.137 | 0.852 |
Figure 1.
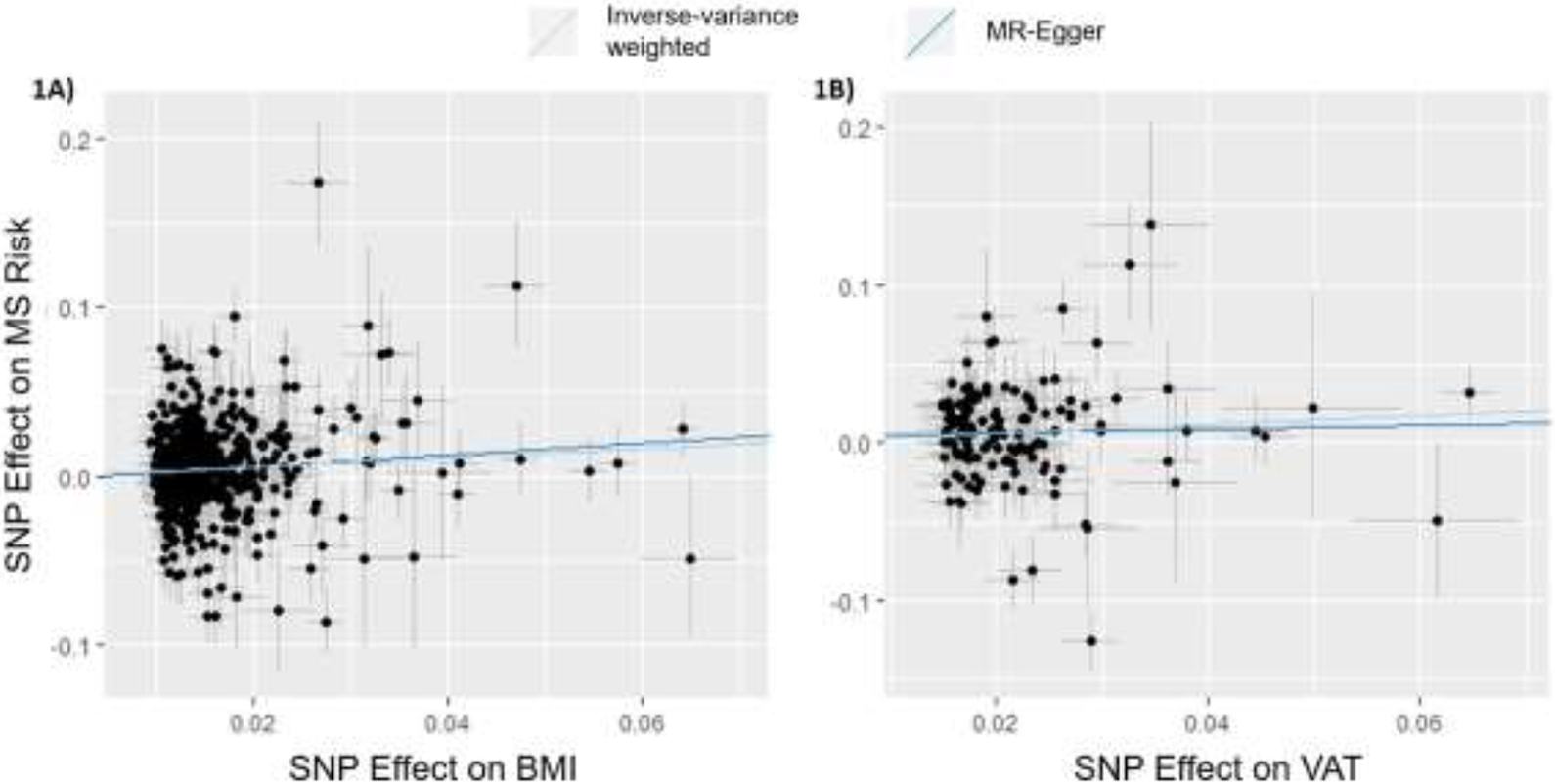
Scatterplots of MR effects for BMI and VAT Original Instruments.
Using MR-PRESSO, global horizontal pleiotropic effects were found for all six qualifying obesity-related traits and MS Risk (Table 2). MR-PRESSO could not be conducted for SAT. In the BMI-MS Risk and VAT-MS Risk relationships, 15 and 5 variants were identified as exhibiting substantial horizontal pleiotropy, respectively. IVW meta-analysis was repeated with these variants removed from their respective instruments, demonstrating a persistent relationship between BMI and MS Risk (pBMI<0.001) and VAT and MS Risk (pVAT<0.001) (Table 2). Lastly, the effect estimates for the original and outlier-removed IVW analyses were tested for significant difference using the MR-PRESSO distortion test. Neither BMI nor VAT showed significant differences in their effects on MS risk before and after the removal of horizontally pleiotropic outliers (pBMI=0.984, pVAT=0.537) (Table 2), indicating that the effects of the pleiotropic variants do not significantly influence the association between BMI and MS risk, or VAT and MS risk. Supplementary Figures 1A-1D graphically represent the null associations between WHR, AFR, LFR, and TFR, and MS risk, respectively. These results were further supported by tests of the Egger intercept term for significance in all IVW models (Supplementary Table 10). Collectively, these results robustly demonstrate that aspects of obesity captured by BMI and by VAT are causally associated with MS.
3.2. Two-sample Mendelian Randomization for Instruments with Non-Overlapping Variants
Two-sample MR was conducted for each of the MR instruments of non-overlapping variants for BMI, WHR, VAT, AFR, LFR, and TFR. Genetic variants in each MR instrument can be found in Supplementary Tables 11-16. Results were similar to the previous 2SMR analyses (Table 3, Figure 2). Only BMI and VAT demonstrated significant causal associations with risk for MS in the IVW meta-analysis (βBMI=0.283, pBMI<0.001; βVAT=0.282, pVAT=0.007). Global horizontal pleiotropic effects were found in the relationships between all seven obesity-related traits and MS Risk (MR-PRESSO poutlier<0.05). Outlier-corrected instruments remained significantly associated with MS Risk for both BMI (p<0.001) and VAT (p<0.001). The MR-PRESSO distortion test detected no substantial difference between the BMI IVW and outlier-corrected analyses (p=0.527) or the VAT IVW and outlier-corrected analyses (p=0.562). Notably, LFR only had one non-overlapping variant in the MR analysis, and so a single Wald ratio was used to calculate the effects of LFR on risk for MS. Supplementary Figures 2A-2C graphically represent the null associations between WHR, AFR, and TFR, and risk for MS, respectively. MR-PRESSO could not be conducted for LFR and MS Risk with only one available variant for analysis. Of the conducted IVW models, testing of the Egger intercept term further supported these results (Supplementary Table 10), demonstrating that the genetically-driven aspects of obesity captured by BMI and by VAT are causally associated with MS risk.
Table 3.
Results for 2-Sample MR Univariate Analyses of Non-Overlapping Instruments
| Exposure | Number of Variants |
Method | Beta | SE | p-value | MR-PRESSO | ||
|---|---|---|---|---|---|---|---|---|
| p(Global) | p(Outlier) | p(Distortion) | ||||||
| BMI | 502 | Inverse-variance Weighted | 0.283 | 0.070 | <0.001 | <0.001 | <0.001 | 0.527 |
| WHR | 227 | Inverse-variance Weighted | −0.016 | 0.137 | 0.907 | <0.001 | 0.313 | 0.336 |
| VAT | 130 | Inverse-variance Weighted | 0.282 | 0.104 | 0.007 | <0.001 | <0.001 | 0.562 |
| AFR | 7 | Inverse-variance Weighted | 0.238 | 0.422 | 0.573 | 0.006 | 0.067 | 0.473 |
| LFR | 1 | Wald Ratio | −0.197 | 0.663 | 0.766 | NA | NA | NA |
| TFR | 6 | Inverse-variance Weighted | 0.127 | 0.475 | 0.505 | 0.048 | NA | NA |
Figure 2.
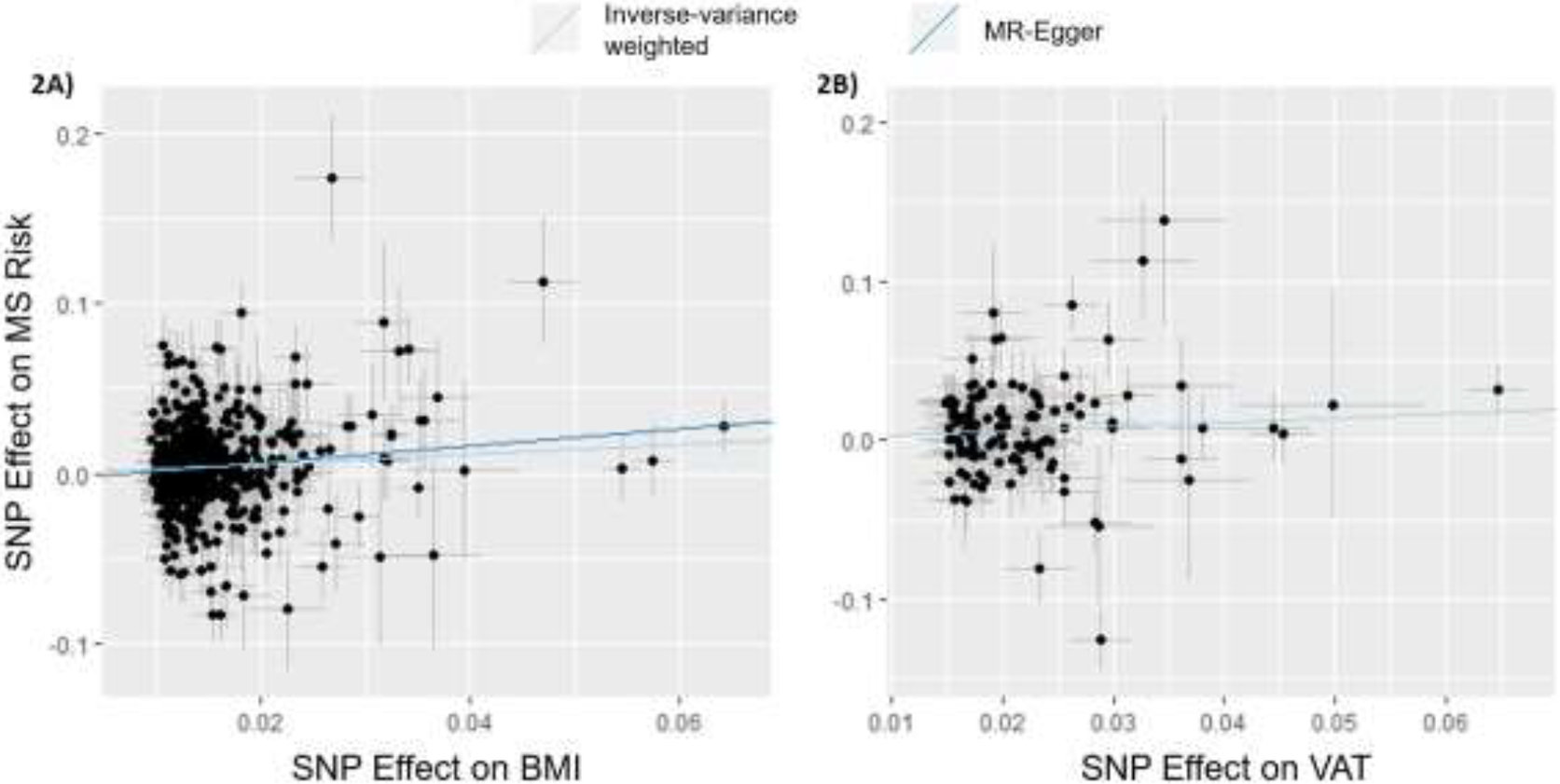
Scatterplot of MR Effects for BMI and VAT Non-overlapping Variant Instruments.
3.3. Multivariable Mendelian Randomization.
MVMR was conducted to investigate the simultaneous effects of BMI and VAT on MS Risk (Figure 3). Summary statistics for BMI were taken from the GIANT consortium only 25, to avoid sample overlap with the UK Biobank and the IMSGC (for the VAT and MS Risk phenotypes, respectively). Genetic variants used in MVMR analysis can be found in Supplementary Table 17. MVMR determined that upon adjustment for VAT, BMI was not associated with risk for MS (βBMI=0.133, pBMI=0.500). Similarly, when adjusted for BMI, VAT did not demonstrate an association with risk for MS (βVAT=0.068, pVAT=0.746). Upon closer inspection, we observed high correlation in effect estimates between BMI and VAT instruments (r2<0.95; data not shown) suggesting multicollinearity likely resulted in the null MVMR associations. Thus, these MVMR results indicate that BMI and VAT do not exhibit effects on MS risk independently of each other.
Figure 3.
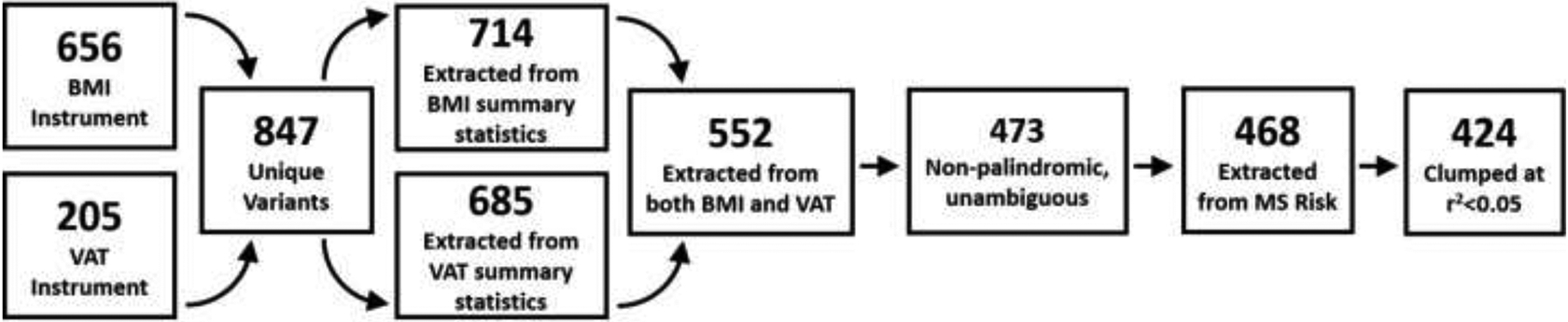
Flow chart of construction of MVMR instrument.
4. Discussion
This is the first study to demonstrate that increased VAT is causally associated with risk for MS in populations of European descent. We also demonstrate that these effects for VAT are related to those demonstrated for BMI on risk for MS. 2SMR was used to investigate the relationship between obesity and MS risk for seven specific obesity-related traits that measured fat accumulation and distribution. Persistent associations between BMI and MS risk and VAT and MS risk were found in multiple analyses. Interestingly, no significant associations were found for WHR, AFR, LFR, or TFR and risk for MS in any 2SMR analysis. The association for SAT was marginally significant but it was based on only one instrument. In MVMR analysis, the effects of BMI and VAT were highly correlated, indicating that their effects on MS risk are not independent.
Previous MR studies of the obesity-MS Risk relationship have shown similar associations between adult BMI and risk for MS. Two recent MR studies of adult BMI and MS risk demonstrated a causal association between BMI and MS using GWA summary statistics from the GIANT consortium data collected and processed in 2015. Both studies reported increased odds of MS with increasing BMI under a 2SMR approach 10, 26. Studies using the GWA summary statistics from the meta-analysis of adult BMI summary statistics from both the GIANT consortium and UK Biobank report similar findings 7, 8. Our work is in alignment with these results, demonstrating that increasing BMI also increases risk for MS (OR=1.31, 95% CI 1.18-1.41). Similarly, our 2SMR analyses of VAT show that as VAT mass increases, so does risk for MS (OR: 1.33, 95% CI 1.13-1.53). However, upon additional investigation, BMI and VAT effect estimates were highly correlated, with an r2 of 0.93.
From the MVMR analysis it became evident that VAT does not exhibit effects of MS risk independent of BMI, or it may and we were unable to detect such a relationship due to the high correlation between the BMI and VAT genetic instruments. BMI is a measure of overall mass; while it can be substantially influenced by obesity or excess adipose fat, it does not differentiate between tissue types. Adipose tissue dysfunction plays several key roles in metabolically-unhealthy obesity, including promoting systemic inflammation via abnormal T-cell activation and the accumulation of B cells and pro-inflammatory macrophages 27. Given the role of autoimmune activity and chronic inflammation in MS pathogenesis, activity, and progression, it is plausible that the link between high BMI and risk for MS is due in part to the inclusion of adipose fat mass, specifically VAT, in the measure of total body mass captured by BMI.
BMI has also been varyingly associated with different measures of brain volume 28. Amongst persons with MS, there is evidence suggestive of an association with obesity and brain volume 29, where as BMI increases, decreases are seen in total and grey matter volume. However, some longitudinal studies have indicated that overweight and obese status did not appear to impact brain volume over time 30. Brain atrophy 31 and white and grey matter lesions 32 are hallmarks of MS. Cardiometabolic conditions like hypertension and diabetes mellitus are also thought to influence brain atrophy 33, 34, both of which have inflammatory components that overlap with those of obesity 35. Visceral adiposity is associated with several cardiometabolic risk factors that fall into the sphere of metabolic syndrome, including dyslipidemia, both local and systemic inflammation, hypertension, and dysglycemia 36 VAT is deposited within the abdominal cavity near internal organs where it is drained by the portal vein allowing its products to preferentially impact metabolism, whereas SAT is primarily deposited beneath the skin in the abdominal (trunk), upper back, gluteal, and femoral regions. As a result, SAT and VAT have varied biological properties, such that both are correlated with fasting glucose and triglyceride levels; however, they have different gene and cytokine expression profiles 37, 38. Inflammation and dysfunctional immune response are some of the key drivers of early MS, and it is likely that obesity confers risk for MS through overlapping inflammatory pathways. As BMI and VAT measure different but potentially overlapping measures of body fat (i.e. BMI is a non-specific measure of total body fat and VAT as a specific measure of body fat stored around internal organs), it is possible that they both confer risk for MS through overlapping biological pathways.
4.1. Measures of Horizontal Pleiotropy.
Horizontal pleiotropy is a potential violation of the second assumption of MR: that the genetic instrument is only associated with the outcome of interest through the exposure-outcome pathway. The MR-PRESSO method was applied to the 2SMR analyses to investigate the effects of horizontal pleiotropy. MR-PRESSO was prioritized as a measure of pleiotropic influence; the MR-Egger adjustment is sensitive to individual genetic variants demonstrating particularly strong effects on the overall association between exposure and outcome 39 whereas MR-PRESSO investigates each variant in the instrument individually for horizontal pleiotropy 23. Across all 2SMR analyses, the MR-PRESSO distortion test demonstrated that horizontal pleiotropy did not significantly impact any of the exposure-outcome relationships.
4.2. Strengths and Limitations
This study is the first to assess the relationship between obesity and MS risk using adipose-tissue and anthropomorphic specific measures of obesity. MR analyses that incorporate summary-level data, such as 2SMR, forgo the need to have individual-level phenotype data for each participant in the study. This allowed us to use cohorts ranging in sample size from 41,515 (MS cases and controls as sampled by the IMSGC) to 694,649 (BMI measures taken from the GIANT consortium) in well-defined European cohorts. The large sample sizes are a key aspect of MR study designs utilizing summary-level data, as they lead to increased power for statistical analysis.
A key limitation of this study is weak instrument bias. Weak instrument bias is a potential violation of the first assumption of MR, which declares that there is a strong association between the genetic instrument and the exposure of interest. Weak instrument bias may occur when the association between the genetic instruments and the exposure is small. While narrow-sense heritability was noted for each of the exposure phenotypes under investigation, future work will also incorporate other assessments of instrument strength, such as the F-test. In addition, the summary statistics for VAT were originally calculated in Karlsson et al. 2019 17 using a measure of predicted VAT mass (kg), calculated from several variables available in the UK Biobank including weight, height, and waist and hip circumference. Use of a predicted measure (as opposed to a direct measure of the trait) may introduce bias if the model is overfitted to the training dataset. However, out-of-sample validation in the original manuscript sufficiently demonstrated a lack of overfitting. Secondly, only one instrument was available for SAT, which generated a suggestive association with MS risk and may also have been influenced by weak instrument bias – as novel genetic instrument variables are identified, these analyses should be revisited. Lastly, obesity may exhibit time-dependent effects on risk for MS. This study only investigated measures of adiposity in adults (i.e. all GWAS samples were ≥18 years old). The effects of childhood obesity on MS risk were not evaluated in this paper, nor were the effects of fluctuation in obesity over time.
5. Conclusions
Here, we demonstrate that both BMI and VAT are significantly associated with risk for MS in European populations. However, the close correlation between the BMI and VAT genetic instruments, combined with null results demonstrated by MVMR analysis of the simultaneous effects of BMI and VAT on MS risk, suggests that both phenotypes influence MS through some degree of overlapping biological pathways.
Supplementary Material
Table 4.
Multivariable Mendelian Randomization Results
| Method | Number of Variants |
Exposure | Beta | SE | p-value |
|---|---|---|---|---|---|
| Inverse-variance Weighted | 424 | BMI | 0.133 | 0.197 | 0.500 |
| VAT | 0.068 | 0.210 | 0.746 |
Highlights.
Body-mass index and visceral adipose tissue are associated with MS risk.
Body-mass index and visceral adipose tissue do not appear to confer risk of MS independently from each other.
Waist-hip ratio, subcutaneous adipose tissue, and arm-, leg-, and trunk-fat to total body fat ratios are not associated with MS risk.
Acknowledgements
The authors appreciate the contributions of Dr. Dana Crawford, Dr. Marijne Vandebergh, Dr. Milena Gianfrancesco, and Dr. Adil Harroud for their constructive scientific advice and methodological insights.
Funding
EM was supported by the Case Western Reserve University Biometric Genetic Analysis of Cardiopulmonary Disease Fellowship (5T32HL007567-35).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
The authors declare that there are no conflicts of interest.
Credit Author Statement
All authors were involved in the design of this study. EM and FB drafted and finalized the manuscript. All authors reviewed and approved the manuscript.
References
- 1.Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler 2013;19:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianfrancesco MA, Acuna B, Shen L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract 2014;8:e435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wesnes K, Riise T, Casetta I, et al. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult Scler 2015;21:388–395. [DOI] [PubMed] [Google Scholar]
- 5.Gianfrancesco MA, Glymour MM, Walter S, et al. Causal Effect of Genetic Variants Associated With Body Mass Index on Multiple Sclerosis Susceptibility. Am J Epidemiol 2017;185:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harroud A, Mitchell RE, Richardson TG, et al. Childhood obesity and multiple sclerosis: A Mendelian randomization study. Mult Scler 2021;27:2150–2158. [DOI] [PubMed] [Google Scholar]
- 7.Vandebergh M, Goris A. Smoking and multiple sclerosis risk: a Mendelian randomization study. J Neurol 2020;267:3083–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs BM, Noyce AJ, Giovannoni G, Dobson R. BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian Randomization study. Neurol Neuroimmunol Neuroinflamm 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almramhi MM, Storm CS, Kia DA, Coneys R, Chhatwal BK, Wood NW. The role of body fat in multiple sclerosis susceptibility and severity: A Mendelian randomisation study. Mult Scler 2022:13524585221092644. [DOI] [PubMed] [Google Scholar]
- 10.Harroud A, Manousaki D, Butler-Laporte G, et al. The relative contributions of obesity, vitamin D, leptin, and adiponectin to multiple sclerosis risk: A Mendelian randomization mediation analysis. Mult Scler 2021;27:1994–2000. [DOI] [PubMed] [Google Scholar]
- 11.Devorak J, Mokry LE, Morris JA, et al. Large differences in adiponectin levels have no clear effect on multiple sclerosis risk: A Mendelian randomization study. Mult Scler 2017;23:1461–1468. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Li R, Zhang W, Lu H. Mendelian randomization analysis does not support a role for leptin in multiple sclerosis. Mult Scler 2021;27:160–161. [DOI] [PubMed] [Google Scholar]
- 13.Poddighe S, Murgia F, Lorefice L, et al. Metabolomic analysis identifies altered metabolic pathways in Multiple Sclerosis. Int J Biochem Cell Biol 2017;93:148–155. [DOI] [PubMed] [Google Scholar]
- 14.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009;8:339–348. [DOI] [PubMed] [Google Scholar]
- 15.Bredella MA. Sex Differences in Body Composition. Adv Exp Med Biol 2017;1043:9–27. [DOI] [PubMed] [Google Scholar]
- 16.Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet 2018;27:3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson T, Rask-Andersen M, Pan G, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med 2019;25:1390–1395. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Basty N, Whitcher B, et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulit SL, Stoneman C, Morris AP, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet 2019;28:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rask-Andersen M, Karlsson T, Ek WE, Johansson A. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun 2019;10:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Multiple Sclerosis Genetics C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 2018;27:R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokry LE, Ross S, Timpson NJ, Sawcer S, Davey Smith G, Richards JB. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med 2016;13:e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 2020;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YP, Tang X, Han M, et al. Relationship between obesity and structural brain abnormality: Accumulated evidence from observational studies. Ageing Res Rev 2021;71:101445. [DOI] [PubMed] [Google Scholar]
- 29.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:181–187. [DOI] [PubMed] [Google Scholar]
- 30.Jakimovski D, Gandhi S, Paunkoski I, et al. Hypertension and heart disease are associated with development of brain atrophy in multiple sclerosis: a 5-year longitudinal study. Eur J Neurol 2019;26:87–e88. [DOI] [PubMed] [Google Scholar]
- 31.Andravizou A, Dardiotis E, Artemiadis A, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights 2019;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klineova S, Lublin FD. Clinical Course of Multiple Sclerosis. Cold Spring Harb Perspect Med 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604–1610. [DOI] [PubMed] [Google Scholar]
- 34.Korf ES, van Straaten EC, de Leeuw FE, et al. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med 2007;24:166–171. [DOI] [PubMed] [Google Scholar]
- 35.Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2:901–910. [DOI] [PubMed] [Google Scholar]
- 37.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007. Oct 19;131(2):242–56. doi: 10.1016/j.cell.2007.10.004. Erratum in: Cell. 2008 Oct 17;135(2):366. [DOI] [PubMed] [Google Scholar]
- 38.Frank AP, de Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019. Oct;60(10):1710–1719. doi: 10.1194/jlr.R086975. Epub 2018 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary-level data used to construct the MR instruments is available per request to the authors, but can be readily requested from the authors of the originating studies. This study was deemed non-human subjects by the Case Western Reserve University’s institutional review board as all data are aggregate summary statistics (STUDY20210195). Study protocol and details were not pre-registered.


