Abstract
Background:
As the largest organ in the human body, skin is continuously exposed to intrinsic and extrinsic stimuli that impact its functionality and morphology with aging. Skin aging entails dysregulation of skin cells and loss, fragmentation, or fragility of extracellular matrix fibers that are manifested macroscopically by wrinkling, laxity, and pigmentary abnormalities. Age-related skin changes are the focus of many surgical and non-surgical treatments aimed at improving overall skin appearance and health.
Summary:
As a hallmark of aging, cellular senescence, an essentially irreversible cell cycle arrest with apoptosis resistance and a secretory phenotype, manifests across skin layers by affecting epidermal and dermal cells. Knowledge of skin-specific senescent cells, such as melanocytes (epidermal aging) and fibroblasts (dermal aging), will promote our understanding of age-related skin changes and how to optimize patient outcomes in aesthetic procedures.
Key Messages:
This review provides an overview of skin aging in the context of cellular senescence and discusses senolytic intervention strategies to selectively target skin senescent cells that contribute to premature skin aging.
Keywords: Cellular senescence, skin aging, age-related changes, epidermal senescence, dermal senescence
Introduction
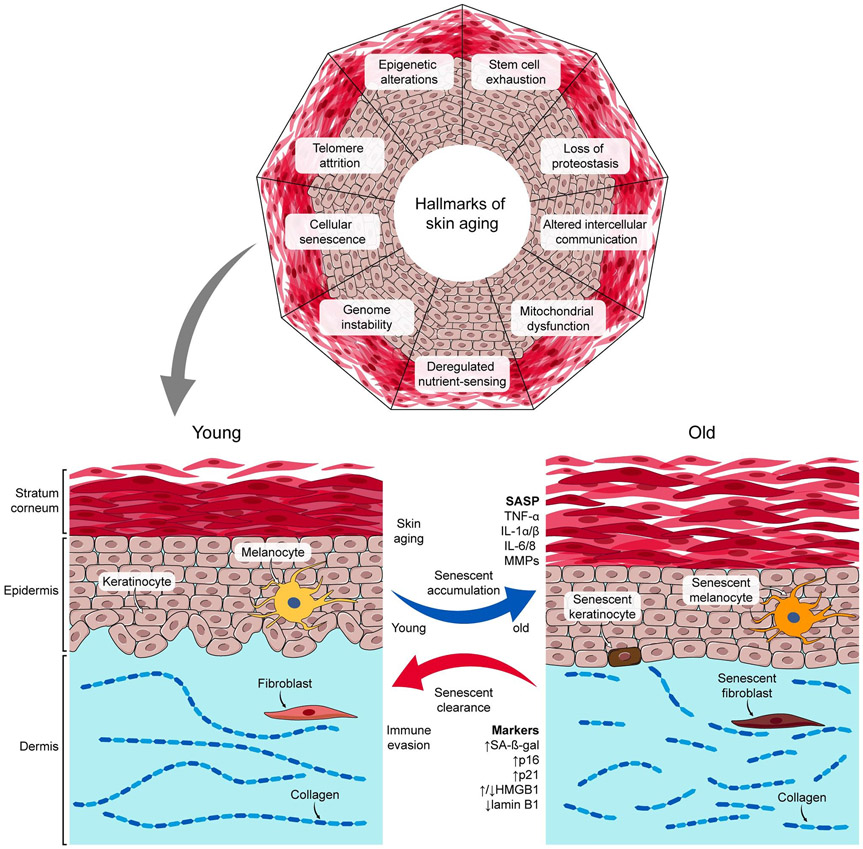
Aging, associated with a time-dependent functional decline in most living organisms, has piqued the quest to slow or reverse biological aging throughout the history of humankind [1, 2]. Skin aging, akin to organismal whole-body aging, is characterized by gradual loss of function and regenerative capacity [3]. The human epidermis has the innate capacity to renew approximately every 40-56 days but slows with aging [4]. Intrinsic and extrinsic insults drive the skin aging process [5]. Intrinsic aging primarily reflects genetic background, whereas extrinsic aging reflects environmental triggers, such as ultraviolet (UV) exposure, air pollution, smoking, alcohol intake, and poor nutrition, among others [6], resulting in reduced regenerative potential. Clinically, skin aging is linked to reduced barrier protection, poor wound healing [7], increased inflammation [8], deficient water and thermal homeostasis [9], and susceptibility to skin disorders, including skin cancers [10]. Indeed, the interlinked hallmarks of whole-body aging, characterized by a progressive loss of physiological integrity, include genomic instability [11], telomere attrition [12], epigenetic alterations [13], loss of proteostasis [14], deregulated nutrient-sensing [15], mitochondrial dysfunction [16], cellular senescence [17], stem cell exhaustion [18], and altered intercellular communication [19] (Figure 1). In this review, we primarily focus on the role of cellular senescence in skin aging and regeneration.
Fig. 1. Hallmarks of Skin Aging.
An illustration of skin senescent cell accumulation and corresponding senescence associated secretory phenotype (SASP) factor release in young vs. old human skin models.
Cellular senescence is an essentially permanent state of cell cycle arrest with both beneficial and detrimental effects in development and aging. Leonard Hayflick and Paul Moorhead originally hypothesized the connection between aging and senescence in 1961 after noticing limited proliferative capacity in serially-subcultured human primary fibroblasts [20]. While cellular senescence has an evolutionarily advantageous role in facilitating tissue remodeling during development and after injury, it can also play a damaging role in the aging process by impairing tissue regeneration, causing inflammation and fibrosis, and promoting tumor growth [21]. Senescent cells exhibit extensive alterations in chromatin architecture and gene expression in addition to growth arrest [22]. The senescence-associated secretory phenotype (SASP) is a prominent characteristic of senescent cells that can includes the secretion of several pro-inflammatory cytokines, chemokines, growth factors, proteases, bioactive lipids (bradykines, ceramides, prostenoids), non-coding nucleotides (e.g., microRNA’s and mitochondrial DNA), and other factors [23, 24, 25, 26, 27, 28]. The SASP portfolio, which includes factors that modulate immune cell proliferation and migration, allows senescent cells to activate, suppress, modulate, and/or evade the immune system [29] (shown in Fig. 1). Indeed, various types of cellular stressors can trigger cellular senescence in vitro [30]; yet, the identification of unique senescence markers, particularly in vivo, is still under investigation. Therefore, the field of translational geroscience continues to define the senescent phenotype in specific tissues and identify new pathways for therapeutic removal of senescent cells directly relevant to the skin.
Histology of Skin Aging
Skin consists of three layers: the epidermis, dermis, and subcutaneous tissue. The epidermis, comprised of multiple cell types including keratinocytes, melanocytes, Langerhans cells, and Merkel cells, is a stratified squamous epithelium that undergoes continuous renewal [31]. Histologically, skin aging results in epidermal thinning with flattening of the dermal-epidermal junction [32]. This manifests as increased skin fragility and reduced nutrient transfer between dermis and epidermis, attributed to the loss of surface area of the dermal-epidermal interface. Furthermore, epidermal cell turnover decreases with age [33], which accounts for less effective desquamation and reduced wound healing.
Beyond the epidermis, the dermis experiences the most significant ultrastructural change with age [34]. The dermis, divided into the more superficial papillary dermis and the deeper reticular dermis, consists of extracellular matrix (ECM) fibers, which are crucial for maintaining skin's structural integrity [35]. Deterioration causes the dermis to separate from the epidermis, resulting in skin laxity and decreased epidermal stem cell renewal [36]. Fibroblasts, the most prevalent cells in the dermis, deposit the collagen and elastic fibers of the ECM [37]. Throughout the aging process, fibroblasts synchronously decrease in number and function [38]. Young dermal fibroblasts produce glycosaminoglycans and extracellular matrix fibers, including elastin and type I collagen, which make up approximately 90% of the extracellular matrix [39]. As the number and diameter of collagen fibers decrease with age, the ratio of type III collagen to type I collagen increases [40]. Furthermore, aged skin is associated with dermal collagen and elastin fragmentation, which presents as decreased skin elasticity and turgor [41]. Together, these age-dependent ultrastructural changes account for the physical manifestations of cutaneous aging [42, 43].
Molecular Biomarkers of Skin Aging
Markers of cellular senescence in skin, including nuclear and SASP markers, have been used to detect senescent cells in aging and disease. Upregulation or downregulation of various cellular senescence markers have been used to characterize cellular senescence burden in skin. Increase in SA-β-galactosidase has been applied extensively as a marker of cellular senescence [44, 45]. Similarly, the cell cycle markers p16INK4a and p21CIP1/WAF1 have been used to study senescent fibroblasts and melanocytes in skin [46, 47, 48]. Alterations in the level of lamin B1 have been implicated as an early senescence marker in multiple tissues, including skin [49, 50, 51, 52]. Particularly, reductions in lamin B1 were found in dermal fibroblasts and keratinocytes from older donors [53], keratinocytes in photoaged skin [52], and melanocytes in melanocytic nevi [51]. Senescent fibroblasts have also been demonstrated to secrete HMGB1 before developing a SASP [54]. On the other hand, melanocytes and keratinocytes from older donors expressed reduced HMGB1 [55]. In addition to these, numerous biomarkers have been developed for skin aging, such as telomere-associated foci [48].
Epidermal Aging: Role of Keratinocytes
Keratinocytes are the most abundant cells in the epidermis and directly contribute to the skin barrier. As skin ages, there is a shift in keratinocyte morphology that contributes to epidermal thinning. Basal keratinocytes become shorter and larger, and corneocytes, which are terminally differentiated keratinocytes, also grow larger due to reduced epidermal turnover [56]. The notion of whether keratinocytes can acquire senescent phenotypes has been questioned given their highly proliferative state. p16INK4a, a marker of cellular senescence, was detected in human skin biopsies of sun-exposed areas [57, 58, 59]. Skin tissues from photoprotected areas of young and old donors showed that p16INK4a-positive cells were predominantly melanocytes and not keratinocytes in the epidermal layer, highlighting the differences in senescence phenotypes given sun exposure [55, 60]. However, senescent cell markers were detected in keratinocytes from actinic keratoses, UV-associated lesions. Specifically, actinic keratosis was associated with increased p16INK4a and reduced lamin B1 and HMGB1, and p16INK4a expression was associated with development of squamous cell carcinoma [61, 62].
Epidermal Aging: Role of Melanocytes
Melanocytes, or pigment-producing cells derived from the neural crest, are in spatial proximity to keratinocytes in the epidermal layer. It has been postulated that cellular senescence may provide an evolutionary protection against malignant transformation of melanocytes, as pigmentation is a strong defense against melanoma [63]. As such, melanin accumulation in the epidermis, through α-melanocyte stimulating hormone or cholera toxin, can induce melanocyte senescence through the p16/CDK4/pRB pathway [64]. Studies have also shown that p16INK4a-positive melanocytes accumulate in aged human epidermis. A correlation between increased numbers of p16INK4a-positive melanocytes and facial aging phenotypes, such as wrinkles, morphological changes in elastic fibers, and dysfunctional telomeres, has been reported [55, 65, 66, 67]. In addition, UV-irradiated melanocytes enter premature senescence with downregulation of DNA repair programs such as nucleotide excision repair (NER) pathway genes, especially genes involved in DNA damage recognition (RAD23B, XPC, ERCC3, ERCC8, and RPA1) [68]. Moreover, senescent melanocytes could result in tissue-level disruption. p16-positive melanocytes induce gamma-H2A-X foci in neighboring keratinocytes, indicating telomere dysfunction, and exposure to senescent-melanocyte-conditioned media induced telomere damage in fibroblasts [55]. Interestingly, clearance of senescent melanocytes with ABT-737, a BCL-2 inhibitor, or MitoQ, a mitochondrial-targeted antioxidant, attenuated telomere dysfunction [55]. Yet, caution must be utilized in its clearance as p16 has a function in suppressing or limiting growth of melanocytic nevi (moles) and germline mutations in p16 are often associated with dysplastic nevi and even melanomas [69].
Dermal Aging: Role of Fibroblasts
Fibroblasts, as the most abundant cell type that resides in the dermis, largely contribute to hallmarks of skin aging [70]. Dermal fibroblasts subjected to in vitro aging protocols accumulate double-strand breaks [71], oxidative DNA damage, chromosomal and epigenetic abnormalities, telomere shortening or oxidation, and impaired DNA repair mechanisms [72]. Senescent fibroblasts garner defects in protein synthesis, folding, and degradation, in addition to defects in post-translational modifications such as oxidation and cross-linking, which affect protein homeostasis (quantitative and qualitative of the cellular proteome). These changes cause senescent fibroblasts to display biomarkers such as increased senescence-associated-beta-galactosidase (SA-β-gal), p16INK4a, and p21CIP1/WAF1 [73, 74, 75].
Indeed, senescent fibroblasts in the skin can cause harmful effects through different mechanisms. UV-induced dermal senescence can alter the extracellular matrix as well as the function of adjacent cells, increasing the risk of carcinogenesis. For example, the cytokines IL-1α, IL-1β, IL-6, and TNF-α, are highly secreted by senescent cells and have been reported to induce skin carcinogenesis. Furthermore, the secretion of MMPs as a consequence of photodamage leads to collagen degradation, epithelial-mesenchymal conversion, angiogenesis, and inflammation [76, 77]. In cultured fibroblasts, UVA and/or a combination of UVA and UVB upregulate MMP-1 [78, 79], leading to skin aging phenotypes.
It has also been reported that an age-dependent increase in human fibroblast senescence occurs as indicated by p16INK4a and SA-β-gal expression in skin biopsies from donors across the age groups of 0-20 years, 21-70 years, and 71-95 years [80]. Analysis of primary human dermal fibroblasts in multiple in vitro aging models, including UVB irradiation and accelerated proliferation of human dermal fibroblasts in young vs. elderly donors, revealed reduced cell growth rate and premature senescence [81]. Further reports indicate that young skin is more resilient to wound healing, particularly in the context of chronic wounds that accumulate senescent cell phenotypes [82, 83]. However, a transient induction of senescent cells occurs in normal acute wound healing and could be beneficial [84]. These findings implicate senescent fibroblasts as a potential target for reducing the negative effects on extracellular matrix due to SASP factors and for enhancing dermal skin rejuvenation.
Targeting Cellular Senescence in Skin Aging
Initial reports that conveyed an inverse association between senescent cell burden and healthspan led to the advent of senolytics – a class of drugs that selectively clears senescent cells [85, 86, 87]. The impact of senescent cell accumulation was demonstrated when killing senescent cells via a suicide gene in a mouse model of premature aging reduced age-related diseases, such as sarcopenia, cataracts, and loss of subdermal adipose tissue in progeroid mice [88] and adipose and metabolic dysfunction in naturally-aged mice [89]. Therapeutic interventions that target senescent cells are categorized as senotherapeutics. Specifically, modulation of cellular senescence can be achieved by selective induction of cell death (senolytics) or SASP inhibition (senomorphics). Skin presents as an ideal site for senotherapeutic testing due to its accessibility and established characterization. However, translation of senotherapeutics has been limited by the need for better in vitro models of skin aging for testing. Few models of skin aging have been described, and they are limited by cell type [90, 91, 92].
A hypothesis-driven, mechanism-based drug discovery approach, stemming from the observation that senescent cells resist apoptosis, led to the development of the first senolytic drugs [85]. In particular, agents that transiently decrease anti-apoptotic regulators, such as Src kinases or Bcl-xL or other BCL-2 family members, were effective in disabling defenses of senescent cells against their own pro-apoptotic SASP, causing them to undergo apoptosis [87, 93]. Next, bioinformatics approaches were utilized to find compounds whose mechanisms of action targeted these senescent cell anti-apoptotic pathways (SCAPs). These agents included Dasatinib (D), the Src tyrosine kinase inhibitor, and Quercetin (Q), a naturally occurring flavonoid found in apple peels that targets other SCAP pathways. First-generation senolytics also include fisetin, luteolin, curcumin, navitoclax (ABT263), and procyanidin C1 among others [85, 87, 94, 95, 96, 97](PMID: 34873338). Second generation senolytic agents are being identified through other drug discovery methods, including random high-throughput drug library screens, vaccines, toxin-loaded nanoparticles preferentially lysed by senescent cells, and immunomodulators [45, 98, 99, 100]. In particular, the first-generation senolytic Dasatanib and Quercetin (D+Q) showed trends of reducing p16 and p21 expression in human epidermis, suggesting its potential efficacy [101].
Senomodifiers or senomorphics are drugs that suppress the adverse effects of senescent cells without directly clearing them, such as JAK inhibitors [102] or rapamycin [103]. Rapamycin, which targets the mTOR pathway regulating cell growth, metabolism, protein synthesis, and autophagy, has been found to reduce senescent cells in human skin, specifically dermal fibroblasts [57, 104], possibly because the SASP spreads senescence [93] and, hence, inhibiting the SASP could reduce senescent cell burden. Rapamycin inhibits the upregulation of IL-1α in senescent fibroblasts, which subsequently blocks IL-1α-induced secretion of other pro-SASP factors [105, 106, 107]. In accordance, rapamycin reduced signs of cellular skin aging in murine skin fibroblasts following UVB irradiation [106]. Indeed, 5 μM rapamycin significantly decreased SA-β-gal-positive cells, preserved elongated fibroblast morphology, and attenuated irradiation-induced reactive oxygen species release [106]. This was also described with the senomorphic and mTOR inhibitor, AZD8055, in foreskin fibroblasts [108]. Taken together, these observations suggest that targeting cellular senescence, in part, may contribute to skin rejuvenation and overall skin health. In addition, senotherapeutics could potentially block cancer pathways associated with cellular senescence, making them candidates to treat or prevent precancerous lesions, such as actinic keratoses.
Conclusions
This review highlights the importance of understanding cellular mechanisms of skin aging, especially, cellular senescence. Age-dependent physiological consequences of epidermal (keratinocytes and melanocytes) aging and dermal (fibroblasts) aging considerably affect skin health in the elderly population. Targeting cellular senescence as a driver of biological aging may allow modulation of age-related dysfunction to alleviate multimorbidity. However, there is need for future studies to evaluate senescent cell types and interactions in skin using large scale datasets and bioinformatics. With deeper understanding of cellular senescence in skin aging, applications of senotherapeutics for skin aging raise many possibilities. Can senotherapeutics reverse skin aging phenotypes resulting from premature senescence and/or photoaging? How can we selectively target senescent cells that compromise skin tissue functionality while retaining the evolutionary benefit of senescence as a barrier to tumorigenesis? These questions warrant further research into testing senotherapeutics in the context of human skin aging.
Acknowledgements
We thank Ms. Traci Paulson for her contribution to formatting the manuscript.
Funding Sources
This work was supported by Robert and Arlene Kogod Center on Aging (S.P.W.), National Institute of Health grants AG013925 (J.L.K.), AG062413 (J.L.K.) and AR076347 (A.G.), the Translational Geroscience Network (AG061456: J.L.K.), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), the Noaber Foundation (J.L.K.), and the Mayo Clinic Medical Scientist Training Program institutional training grant (T32 GM065841, G.T.Y.).
Footnotes
Conflict of Interest Statement
Patents on senolytic drugs to J.L.K. and T.T. are held by Mayo Clinic. S.P.W. has a nonrelevant financial interest in Rion LLC. The authors have no financial interest to declare in relation to the content of this article. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies.
References
- 1.Dodig S, Cepelak I, Pavic I. Hallmarks of senescence and aging. Biochem Med (Zagreb). 2019;29(3):030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber F, Kremslehner C, Eckhart L, Tschachler E. Cell aging and cellular senescence in skin aging - Recent advances in fibroblast and keratinocyte biology. Exp Gerontol. 2020;130:110780. [DOI] [PubMed] [Google Scholar]
- 4.Halprin KM. Epidermal "turnover time"--a re-examination. Br J Dermatol. 1972;86(1):14–9. [DOI] [PubMed] [Google Scholar]
- 5.Ho CY, Dreesen O. Faces of cellular senescence in skin aging. Mech Ageing Dev. 2021;198:111525. [DOI] [PubMed] [Google Scholar]
- 6.Puizina-Ivic N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17(2):47–54. [PubMed] [Google Scholar]
- 7.Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, et al. Impaired epidermal to endritic T cell signaling slows wound repair in aged skin. Cell. 2016;167(5):1323–38 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilkington SM, Bulfone-Paus S, Griffiths CEM, Watson REB. Inflammaging and the skin. J Invest Dermatol. 2021;141(4S):1087–95. [DOI] [PubMed] [Google Scholar]
- 9.Sreedhar A, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11(6):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Y, Wang XJ. TGFbeta signaling in photoaging and UV-induced skin cancer. J Invest Dermatol. 2021;141(4S):1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedernhofer LJ, Gurkar AU, Wang Y, Vijg J, Hoeijmakers JHJ, Robbins PD. Nuclear genomic instability and aging. Annu Rev Biochem. 2018;87:295–322. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen P, Shah PP, Nativio R, Berger SL. Epigenetic mechanisms of longevity and aging. Cell. 2016;166(4):822–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aunan JR, Watson MM, Hagland HR, Soreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103(2):e29–46. [DOI] [PubMed] [Google Scholar]
- 16.Picca A, Guerra F, Calvani R, Bucci C, Lo Monaco MR, Bentivoglio AR, et al. Mitochondrial dysfunction and aging: Insights from the analysis of extracellular vesicles. Int J Mol Sci. 2019;20(4):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regulski MJ. Cellular senescence: What, why, and how. Wounds. 2017;29(6):168–74. [PubMed] [Google Scholar]
- 18.Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC. Regulation of stem cellaAging by metabolism and epigenetics. Cell Metab. 2017;26(3):460–74. [DOI] [PubMed] [Google Scholar]
- 19.Schaum N, Lehallier B, Hahn O, Palovics R, Hosseinzadeh S, Lee SE, et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 2020;583(7817):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–53. [DOI] [PubMed] [Google Scholar]
- 22.Pathak RU, Soujanya M, Mishra RK. Deterioration of nuclear morphology and architecture: A hallmark of senescence and aging. Ageing Res Rev. 2021;67:101264. [DOI] [PubMed] [Google Scholar]
- 23.Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28(8):1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iske J, Seyda M, Heinbokel T, Maenosono R, Minami K, Nian Y, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11(1):4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiley CD, Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nature Metabolism. 2021;3(10):1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamsanathan S, Gurkar AU. Lipids as Regulators of Cellular Senescence. Front Physiol. 2022;13:796850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelmohsen K, Panda A, Kang M-J, Xu J, Selimyan R, Yoon J-H, et al. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell. 2013;12(5):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prata L, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol. 2018;40:101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohn A, Weber D, Jung T, Ott C, Hugo M, Kochlik B, et al. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khavkin J, Ellis DA. Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin North Am. 2011;19(2):229–34. [DOI] [PubMed] [Google Scholar]
- 32.Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Dermatol. 1979;73(1):47–53. [DOI] [PubMed] [Google Scholar]
- 33.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241–51. [DOI] [PubMed] [Google Scholar]
- 34.Shin JW, Kwon SH, Choi JY, Na JI, Huh CH, Choi HR, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20(9):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3(4):a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann L, Bernstein EF, Weiss AS, Bates D, Humphrey S, Silberberg M, et al. Clinical relevance of elastin in the structure and function of skin. Aesthet Surg J Open Forum. 2021;3(3):ojab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H, Hong Y, Kim M. Structural and functional changes and possible molecular mechanisms in aged skin. Int J Mol Sci. 2021;22(22):12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge B, Wang H, Li J, Liu H, Yin Y, Zhang N, et al. Comprehensive assessment of Nile Tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Mar Drugs. 2020;18(4):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovell CR, Smolenski KA, Duance VC, Light ND, Young S, Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br J Dermatol. 1987;117(4):419–28. [DOI] [PubMed] [Google Scholar]
- 41.Asserin J, Lati E, Shioya T, Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14(4):291–301. [DOI] [PubMed] [Google Scholar]
- 42.Balansin Rigon R, Kaessmeyer S, Wolff C, Hausmann C, Zhang N, Sochorova M, et al. Ultrastructural and molecular analysis of ribose-induced glycated reconstructed human skin. Int J Mol Sci. 2018;19(11):3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breitenbach JS, Rinnerthaler M, Trost A, Weber M, Klausegger A, Gruber C, et al. Transcriptome and ultrastructural changes in dystrophic Epidermolysis bullosa resemble skin aging. Aging (Albany NY). 2015;7(6):389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itahana K, Campisi J, Dimri GP. Methods to Detect Biomarkers of Cellular Senescence. In: Tollefsbol TO, editor. Biological Aging: Methods and Protocols. Totowa, NJ: Humana Press; 2007. p. 21–31. [DOI] [PubMed] [Google Scholar]
- 45.Fuhrmann-Stroissnigg H, Santiago FE, Grassi D, Ling Y, Niedernhofer LJ, Robbins PD. SA-β-Galactosidase-based screening assay for the identification of senotherapeutic drugs. J Vis Exp. 2019(148):e58133. [DOI] [PubMed] [Google Scholar]
- 46.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23(1):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein GH, Drullinger LF, Soulard A, Dulić V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19(3):2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. The EMBO Journal. 2019;38(23):e101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23(11):2066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freund A, Orjalo AV, Desprez P-Y, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends in Molecular Medicine. 2010;16(5):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson DM, Rai TS, et al. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202(1):129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang AS, Ong PF, Chojnowski A, Clavel C, Dreesen O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci Rep. 2017;7(1):15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200(5):605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201(4):613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019;38(23):e101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care (New Rochelle). 2013;2(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung CL, Lawrence I, Hoffman M, Elgindi D, Nadhan K, Potnis M, et al. Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience. 2019;41(6):861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon JE, Kim Y, Kwon S, Kim M, Kim YH, Kim JH, et al. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics. 2018;8(17):4620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitsiou E, Pulido T, Campisi J, Alimirah F, Demaria M. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J Invest Dermatol. 2021;141(4s):1119–26. [DOI] [PubMed] [Google Scholar]
- 60.Waaijer MEC, Gunn DA, van Heemst D, Slagboom PE, Sedivy JM, Dirks RW, et al. Do senescence markers correlate in vitro and in situ within individual human donors? Aging (Albany NY). 2018;10(2):278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodges A, Smoller BR. Immunohistochemical comparison of p16 expression in actinic keratoses and squamous cell carcinomas of the skin. Mod Pathol. 2002;15(11):1121–5. [DOI] [PubMed] [Google Scholar]
- 62.Wang AS, Nakamizo S, Ishida Y, Klassen G, Chong P, Wada A, et al. Identification and quantification of senescent cell types by lamin B1 and HMGB1 in Actinic keratosis lesions. Journal of Dermatological Science. 2022;105(1):61–4. [DOI] [PubMed] [Google Scholar]
- 63.Bennett DC, Medrano EE. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002;15(4):242–50. [DOI] [PubMed] [Google Scholar]
- 64.Bandyopadhyay D, Medrano EE. Melanin accumulation accelerates melanocyte senescence by a mechanism involving p16INK4a/CDK4/pRB and E2F1. Ann N Y Acad Sci. 2000;908:71–84. [DOI] [PubMed] [Google Scholar]
- 65.Pawlikowski JS, McBryan T, van Tuyn J, Drotar ME, Hewitt RN, Maier AB, et al. Wnt signaling potentiates nevogenesis. Proc Natl Acad Sci U S A. 2013;110(40):16009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waaijer ME, Gunn DA, Adams PD, Pawlikowski JS, Griffiths CE, van Heemst D, et al. P16INK4a positive cells in human skin are indicative of local elastic fiber morphology, facial wrinkling, and perceived age. J Gerontol A Biol Sci Med Sci. 2016;71(8):1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11(4):722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sha J, Arbesman J, Harter ML. Premature senescence in human melanocytes after exposure to solar UVR: An exosome and UV-miRNA connection. Pigment Cell Melanoma Res. 2020;33(5):671–84. [DOI] [PubMed] [Google Scholar]
- 69.Hayward N. New developments in melanoma genetics. Curr Oncol Rep. 2000;2(4):300–6. [DOI] [PubMed] [Google Scholar]
- 70.Wlaschek M, Maity P, Makrantonaki E, Scharffetter-Kochanek K. Connective Tissue and Fibroblast Senescence in Skin Aging. J Invest Dermatol. 2021;141(4S):985–92. [DOI] [PubMed] [Google Scholar]
- 71.Nowotny K, Jung T, Grune T, Hohn A. Accumulation of modified proteins and aggregate formation in aging. Exp Gerontol. 2014;57:122–31. [DOI] [PubMed] [Google Scholar]
- 72.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18(10):611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohamad Kamal NS, Safuan S, Shamsuddin S, Foroozandeh P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur J Cell Biol. 2020;99(6):151108. [DOI] [PubMed] [Google Scholar]
- 76.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. [DOI] [PubMed] [Google Scholar]
- 77.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293(11):576–83. [DOI] [PubMed] [Google Scholar]
- 79.Fagot D, Asselineau D, Bernerd F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem Photobiol. 2004;79(6):499–505. [DOI] [PubMed] [Google Scholar]
- 80.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379–89. [DOI] [PubMed] [Google Scholar]
- 81.Lago JC, Puzzi MB. The effect of aging in primary human dermal fibroblasts. PLoS One. 2019;14(7):e0219165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ. 2005;12(7):695–8. [DOI] [PubMed] [Google Scholar]
- 83.Wilkinson HN, Hardman MJ. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front Cell Dev Biol. 2020;8:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. 2020;287(12):2418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adamus J, Aho S, Meldrum H, Bosko C, Lee JM. p16INK4A influences the aging phenotype in the living skin equivalent. J Invest Dermatol. 2014;134(4):1131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diekmann J, Alili L, Scholz O, Giesen M, Holtkötter O, Brenneisen P. A three-dimensional skin equivalent reflecting some aspects of in vivo aged skin. Experimental Dermatology. 2016;25(1):56–61. [DOI] [PubMed] [Google Scholar]
- 92.Weinmüllner R, Zbiral B, Becirovic A, Stelzer EM, Nagelreiter F, Schosserer M, et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech Dis. 2020;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. 2021;3(12):1706–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY). 2017;9(3):955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Z, Hu K, Feng L, Su R, Lai N, Yang Z, et al. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. 2018;109(6):1753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10(9):e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakagami H. Cellular senescence and senescence-associated T cells as a potential therapeutic target. Geriatr Gerontol Int. 2020;20(2):97–100. [DOI] [PubMed] [Google Scholar]
- 101.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17(9):1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thapa RK, Nguyen HT, Jeong JH, Kim JR, Choi HG, Yong CS, et al. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep. 2017;7:43299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(8):1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin D, Ren R, Jia C, Lu Y, Yang Q, Chen L, et al. Rapamycin protects skin fibroblasts from ultraviolet B-Induced photoaging by suppressing the production of reactive oxygen species. Cell Physiol Biochem. 2018;46(5):1849–60. [DOI] [PubMed] [Google Scholar]
- 107.Tomimatsu K, Narita M. Translating the effects of mTOR on secretory senescence. Nat Cell Biol. 2015;17(10):1230–2. [DOI] [PubMed] [Google Scholar]
- 108.Walters HE, Deneka-Hannemann S, Cox LS. Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging (Albany NY). 2016;8(2):231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]