Abstract
We reported previously that a monosynaptic pathway connects the substantia nigra pars compacta (SNpc) to neurons of the dorsal motor nucleus of the vagus (DMV). This monosynaptic pathway modulates the vagal control of gastric motility. It is not known, however, whether this nigro-vagal pathway also modulates the tone and motility of the proximal colon. In rats, microinjection of retrograde tracers in the proximal colon and of anterograde tracers in SNpc showed that bilaterally labelled colonic-projecting neurons in the DMV received inputs from SNpc neurons. Microinjections of the ionotropic glutamate receptor agonist, NMDA, in the SNpc increased proximal colonic motility and tone as measured via a strain gauge aligned with the colonic circular smooth muscle; the motility increase was inhibited by acute subdiaphragmatic vagotomy. Upon transfection of SNpc with pAAV-hSyn-hM3D(Gq)-mCherry, chemogenetic activation of nigro-vagal nerve terminals by brainstem application of CNO increased the firing rate of DMV neurons and proximal colon motility; both responses were abolished by brainstem pretreatment with the dopaminergic D1-like antagonist SCH23390. Chemogenetic inhibition of nigro-vagal nerve terminals following SNpc transfection with pAAV-hSyn-hM4D(Gi)-mCherry, decreased the firing rate of DMV neurons and inhibited proximal colon motility. These data suggest that a nigro-vagal pathway modulates activity of the proximal colon motility tonically via a discrete dopaminergic synapse in a manner dependent on vagal efferent nerve activity. Impairment of this nigro-vagal pathway may contribute to the severely reduced colonic transit and prominent constipation observed both in patients and in animal models of Parkinsonism.
Keywords: Substantia nigra pars compacta, brain stem, dorsal motor nucleus of the vagus, proximal colon motility
Graphical Abstract
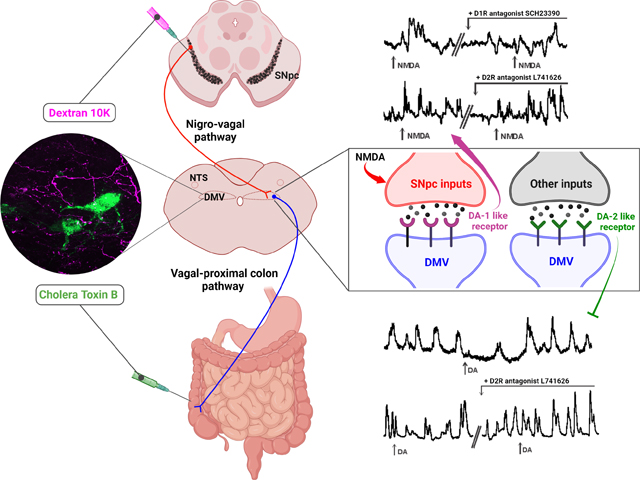
The dorsal vagal complex (DVC) receives inputs from the substantia nigra pars compacta (SNpc) via a nigro-vagal pathway. In this study, we examined whether this nigro-vagal pathway extends its influence to the proximal colon. In rats, colonic-projecting neurons in the dorsal motor nucleus of the vagus (DMV) receive inputs from SNpc neurons, as demonstrated by simultaneous anterograde (SNpc) and retrograde (proximal colon) neuronal tracing. Activation of SNpc neurons via the ionotropic glutamate selective agonist, NMDA, increases proximal colonic motility and tone in a manner that was reduced by brainstem pretreatment with the dopaminergic D1-like antagonist SCH23390, but not by the D2-like antagonist L741646. These studies suggest that the nigro-vagal pathway tonically modulates the tone and motility of the proximal colon and excites vagal efferent motoneurons via activation of dopaminergic D1 receptors.
Introduction
The gastrointestinal (GI) tract from the lower third of the esophagus to the transverse colon undergoes robust modulation by vagal inputs (Browning & Travagli, 2014; Travagli & Anselmi, 2016). The vagal motor inputs to the GI tract arise from preganglionic cholinergic parasympathetic motoneurons of the dorsal motor nucleus of the vagus (DMV) which project to postganglionic myenteric neurons of the enteric nervous system (ENS) that, ultimately, modulate, motility amongst other responses (Browning & Travagli, 2014). One of the remarkable, and fundamental, properties of GI-projecting DMV motoneurons is their spontaneous, slow pacemaker-like activity, the rate of which is modulated by synaptic inputs (Browning & Travagli, 2014). One immediate implication is that the activity of the GI tract, even at rest, is modulated by DMV neurons and vagal efferent outflow, the activity of which is continuously sculpted by a large array of synaptic inputs from brainstem, midbrain, and higher CNS centers (Browning & Travagli, 2014). Among the several physiologically relevant inputs to DMV neurons, we have shown that a nigro-vagal pathway, originating from dopaminergic neurons of the substantia nigra pars compacta (SNpc), exerts a tonic modulation over gastric motor activity (Anselmi et al., 2017a).
Degeneration of dopaminergic neurons in the SNpc induces uncontrolled tremors at rest, bradykinesia, postural instability, and rigidity, which are the movement disorders commonly associated with Parkinson’s disease (PD) (Goedert et al., 2013; Del Rey et al., 2018; Dickson, 2018; Goedert & Compston, 2018; Johnson et al., 2019). There are, however, several non-motor symptoms, including autonomic dysfunctions, that increase the overall morbidity of parkinsonian patients significantly (Cersosimo & Benarroch, 2012; Goldstein, 2014; Postuma & Berg, 2016; Schapira et al., 2017; Coon et al., 2018). GI dysfunctions, including delayed gastric emptying and severe constipation, are amongst the most prominent non-motor manifestations of PD (Travagli et al., 2020); these GI issues can precede the onset of motor symptoms by several decades and reduce significantly the patients’ quality of life. Specifically, the delayed colonic transit observed in PD patients is a major complication that may evolve in chronic constipation unresponsive to first-line treatments (Giancola et al., 2017; Travagli et al., 2020).
Among various hypotheses for the etiology of PD (Surmeier et al., 2017; Liddle, 2018; Johnson et al., 2019; Marras et al., 2019; Wichmann, 2019), a notable hypothesis has been put forward by Braak’s group who postulated that the infiltration and ENS absorption of ingested toxicants and the spread of the resulting synucleinopathy via retrograde transport through vagal pathways to the DMV and, ultimately, to the SNpc, basal ganglia and cerebral cortex (Braak et al., 2003b; Goedert et al., 2013; Anselmi et al., 2018) induces neuronal loss.
The functions of the colon include the absorption of water and electrolytes as well as peristalsis to move intestinal contents aborally (Furness, 2012). Vagal efferent innervation of the proximal colon, although more sparse than that projecting to the stomach and upper small intestine, may represent another area of the GI tract that is implicated in the infiltration of soluble ingested toxicants, their retrograde transport to the CNS and, ultimately, to SNpc, basal ganglia and cerebral cortex. The synucleinopathy observed in the proximal colon (Anselmi et al., 2018) may impair motility, however, little is known about the extent to which or the mechanism of action of vagal inputs from the SNpc modulate the function of the proximal colon. Indeed, the anatomical evidence and the physiological significance of a potential link between the SNpc and the proximal colon has not been reported.
The aim of our study was to demonstrate the existence of a nigro-vagal-proximal colon pathway, to characterize this connection, and to assess if this pathway regulates the tone and motility of the proximal colon.
Preliminary accounts of the present work have been presented at the 2020 Digestive Disease Week (Nanni & Travagli, 2020).
Material and Methods
Ethical Approval
Male Sprague-Dawley rats (N=87); Charles River, Wilmington, MA, USA; 150–200g at the beginning of the experiments) were housed in an AAALAC accredited animal care facility at 24°C on a 12:12 hour light cycle with food and water ad libitum. Surgical procedures were performed using aseptic techniques and were conducted in accordance with NIH guidelines, with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee (protocol #47048), and according to the ARRIVE guidelines for animal care and use.
While we acknowledge the potential limitations in restricting the present study to male rats only, recent studies have shown that estrogens act both peripherally (Li et al., 2016; Liu et al., 2019; Balasuriya et al., 2021) and centrally (Jiang et al., 2019; Meister et al., 2019) to modulate GI functions rendering data interpretation problematic unless experiments are conducted in an estrogen-controlled environment which was beyond the scope of the present study.
Neuronal Tracing
Rats (N=7) were anaesthetized with a solution of ketamine/xyalzine/acepromazine (80/1.6/5mg ml−1kg−1 i.p.). The anesthesia level was monitored continuously throughout the experiment, and core temperature was kept at 37°C with a heating pad. Once a deep plane of anesthesia was achieved (absence of palpebral reflex), an abdominal laparotomy was performed to expose the colon. Five-10 microinjections of the retrograde tracer cholera toxin-B (CTB;5–10nl each injection, 0.5% w/v; List Biological Laboratories, Campbell, CA) were made into the proximal colon ~2–3 cm from the ileocecal valve; the wound was then closed with 5:0 vicryl suture. The rats were then placed on a stereotaxic frame (Kopf Instruments, Tujunga CA), the head was shaved, the skin retracted and the skull was exposed. Two-3mm wide holes were drilled bilaterally to allow microinjection of the anterograde tracers biotinylated or fluorescein dextran 10,000MW (5% of 6.5% w/v, respectively, dissolved in sterile saline; Life Technologies, Grand Island, NY) into the SNpc. Four microinjections of tracer (600nL each location) were made bilaterally into the SNpc at sites RC: −5.0mm from bregma, ML: ±2.4 mm from midline, dorso-ventral (DV): −7.6 mm from the surface of the dura mater and RC: −5.6 mm, ML: ±1.6 mm, and DV: −7.8 mm. The scalp was sutured (5/0 vicryl) and rats allowed to recover. Rats received carprofen (5mg/kg s.c.) peri-operatively and at 24hr intervals post-operatively for analgesia, and Baytril® (enrofloxacin, 5mg/kg s.c.) for 5 days as a prophylactic antibiotic. After 10–15 days, rats were anesthetized with Inactin® (sodium thiobutabarbitol, Sigma, St Louis, MO, 100–150mg/kg i.p.) and euthanized via administration of a bilateral pneumothorax (laparotomy and diaphragm penetration), before being perfused transcardially with 0.9% saline followed by 4% paraformaldehyde solution (PFA).
In another set of neuronal tracing experiments (N=4), dextran 10,000MW (in either the biotinylated (5% w/v) or the fluorescein (7.5% w/v) formulation, Life Technologies) was injected in the dorsal vagal complex (DVC; RC: 0.0–0.6 mm from calamus scriptorius; ML: ±0.5–0.7 mm from midline; DV: 0.55–0.7 mm from the brainstem surface); after 15–30 days of recovery, rats were anesthetized with Inactin® (100–150 mg kg−1 i.p.; abolition of the foot pinch withdrawal reflex) and euthanized via administration of a bilateral pneumothorax (laparotomy and diaphragm penetration), before being perfused transcardially with 0.9% saline followed by 4% paraformaldehyde solution (PFA).
Tissue Processing
After transcardial perfusion, brains were removed and post-fixed for 4 days at 4 °C with 4% PFA containing 20% sucrose before being transferred to a solution containing PBS and 20% sucrose for at least 1 day. Transverse slices (50μm) were made throughout the entire rostrocaudal extent of SNpc at as well as the DVC using a freezing sledge microtome. Slices were cut into sets of 4 and preserved in long term storage buffer (Phosphate buffer, PBS, 0.1M, sucrose 30%, ethylene glycol 30%) at −20°C.
In the rats which received microinjection of anterograde tracer in the DVC, the proximal colon was extracted prior to PFA perfusion and immersed in PBS. The colon was opened along the mesenteric border, washed and pinned under tension to the bottom of silicon-coated dishes. Specimens were fixed 1–2 days in 4% PFA at 4°C, washed in PBS and stored in PBS + sodium azide 0.05% until dissection within 2–5 days. Specimens were then dissected under magnification to produce longitudinal muscle-myenteric plexus whole mount preparations by peeling away the mucosa, submucosa, and circular muscle.
Immunohistochemical analyses
All immunohistochemistry steps were performed at room temperature on a shaker. After being washed in Tris-PBS containing 0.03% Triton-X (TPBS) and incubation in 1% normal donkey serum (NDS), specimens were incubated in primary antibodies diluted in TPBS containing 10% NDS for 3 days. After washing in PBS, the sections were incubated overnight in secondary antibodies diluted in TPBS containing 1% NDS. After several washes, tissues were mounted on gelatin-subbed slides and coverslipped using Fluoromount-G (Southern Biotechnology Associated, Birmingham, AL). The primary antibodies were: goat α-CTB (List Biological Labs, Campbell, CA; 1:100,000); rabbit-α-PGP 9.5 (Millipore, Billerica, MA; 1:500); rabbit-α-choline acetyltransferase (Sigma, Burlington, MA; 1:1000); mouse α-tyrosine hydroxylase (TH; ImmunoStar, Hudson, Wisconsin; 1:1000) and rabbit anti-c-fos (EnCor Biotechnology Inc; 1:500) and their dilutions were determined by titration in tissue fixed and processed in the same ways as the experimental tissue. The secondary antibodies used were: donkey anti-mouse/rabbit/goat Alexa Fluor 488, 568 or 647; all fluorescent secondary antibodies were purchased from Life Technologies (Grand Island, NY) and were used at 1:500 dilution.
Immunofluorescent images were captured with a Zeiss AXIO Observer Z1 confocal laser scanning microscope. In rats (N=4) that received microinjections of anterograde tracers in the DVC, a minimum of 200 proximal colon PGP9.5-immunoreactive (-IR) myenteric neurons were analysed for their close association with labelled efferent vagal fibers. Data are reported as percentage of PGP9.5-IR neurons that received close contacts from vagal fibers. In a different group of rats (N=5) which received microinjections of anterograde tracers in the SNpc and retrograde tracers in the proximal colon, every fourth DVC slice was analysed to assess the percentage of labelled DMV neurons receiving close contacts from SNpc-labelled fibers.
Motility and tone studies
Rats (N=69) were fasted overnight (water ad libitum) and anesthetized with Inactin® (100–150 mg kg−1 i.p.). The anesthesia level was monitored continuously throughout the experiment, and core temperature was kept at 37°C with a heating pad. Once a deep plane of anesthesia was achieved (absence of palpebral reflex), rats were intubated with a tracheal catheter, a midline laparotomy was performed to expose the proximal colonic wall, and a miniature strain gauge (MSR Neurobiology, Roaring Spring, PA) was sutured to the serosal surface of the circular smooth muscle of the proximal colon approximately 2–3 cm from the ileo-cecal valve, and the leads were exteriorized prior to abdominal closure. Rats were then placed on a stereotaxic frame, neck muscles were blunt dissected, and the brainstem was exposed following removal of meningeal membranes above the fourth ventricle. The skull was then exposed and 2–3mm wide holes were drilled bilaterally at (from Bregma) RC: 5.6 mm and ML: ±1.6 mm to allow subsequent microinjection of drugs into the SNpc. Injections into the DVC were made at RC: 0.0−0.6 mm from calamus scriptorius; ML: ±0.5−0.7 mm from midline; DV: 0.55−0.7 mm from the brainstem surface. Rats were allowed to stabilize for at least 45min before beginning the tone/motility experiment and supplemented with 5ml of pre-warmed saline (s.c.) prior to beginning the experiment. The depth of anesthesia was assessed every 15–30min throughout the duration of the experiment.
NMDA (5nmoles/210nl; N=21) or AMPA (5nmoles/210nl; N=4) were microinjected into the SNpc over the course of 2min and the resulting change in colonic tone and motility noted. After 30min of recovery, 2μl of a PBS solution containing the D1- or the D2-like receptor antagonists SCH23390 or L741626, respectively, (45nmoles for each) was applied to the surface of the 4th ventricle using an 5μl Hamilton syringe, taking care to not touch the floor of the fourth ventricle or the adjacent area postrema, followed 2–5min later by a second NMDA microinjection. All drugs were dissolved in isotonic PBS. In the experiments in which the effects of antagonist application were assessed, their effects on motility and tone were monitored for ~5min.
In different group of rats (N=5) the subdiaphragmatic right vagus was sectioned prior to strain gauge apposition and the left cervical vagus was exposed and loosely ligated with a thread which was exteriorized for easy access later in the experiment. NMDA was microinjected in the left SNpc. Following a recovery period of at least 30min, the left cervical vagus was severed by pulling the exteriorized thread, thus attaining a complete vagotomy. After a 45min stabilization period, the NMDA microinjection into the left SNpc was repeated.
At the conclusion of the experiments, rats were euthanized via administration of a bilateral pneumothorax (laparotomy and diaphragm penetration) and perfused transcardially with saline (0.9%) following by 4% paraformaldehyde. The brain was post-fixed as above for subsequent immunohistochemical localization of the microinjection sites.
Colonic tone was measured as absolute tone variation (in mg) from baseline. Colonic motility was calculated using the following formula, as described previously (Anselmi et al., 2017b):
where equals the number of peaks in a particular force range (, , , ) and equals the time interval over which the colonic motility was measured. The effect of drugs on colonic motility was measured relative to the averaged value (expressed as arbitrary units, a.u.) of motility before microinjection (baseline=100%).
Chemogenetic studies
Following induction of anesthesia with ketamine/xyalzine/acepromazine (80/1.6/5mg ml−1kg−1 i.p.), and, once a deep plane of anesthesia was obtained (abolition of the foot pinch withdrawal reflex), rats (N=27) were laid on a homeothermic heating pad that maintained their core body temperature at 37°C. pAAV-hSyn-hM3D(Gq)-mCherry [“hM3D(Gq)”] (2×1012 vg/ml, Addgene Plasmid #50474, Watertown, MA) or pAAV-hSyn-DIO-hM4D(Gi)-mCherry [“hM4D(Gq)”] ( 2×1012 vg/ml, Addgene Plasmid #44362, Watertown, MA) or rAAV2/hsyn-EYFP (“empty vector”) (3.4 ×1012 vg/ml, UNC GTC vector, Chapel Hill, NC) were injected bilaterally into the SNpc at sites RC: −5.0mm from bregma, ML: ±2.4 mm from midline, DV: −7.6 mm from the surface of the dura mater and RC: −5.6 mm, ML: ±1.6 mm, and DV: −7.8 mm. The scalp was sutured (5/0 vicryl) and rats allowed to recover; rats received carprofen (5mg/kg s.c.) peri-operatively and at 24hr intervals post-operatively for analgesia, and Baytril® (enrofloxacin, 5mg/kg s.c.) for 5 days as a prophylactic antibiotic. After allowing sufficient time for recovery from surgery and expression of the microinjected viral vectors (3–4 weeks), a group of rats (N=18) was then anesthetized with Inactin® (100–150 mg kg−1 i.p.). The anesthesia level was monitored continuously throughout the experiment, and core temperature was kept at 37°C with a heating pad. Once a deep plane of anesthesia was achieved (absence of the palpebral reflex), rats were instrumented for in vivo colonic recordings as described above, and chemostimulation of the DVC was performed using clozapine-N-oxide (CNO, Sigma, St. Louis, MO, 2μl/200nmole) applied to the floor of the IV ventricle. The depth of anesthesia was assessed every 15–30min throughout the duration of the experiment. At the conclusion of the experiments, rats were euthanized via administration of a bilateral pneumothorax (laparotomy and diaphragm penetration) and perfused transcardially with saline (0.9%) following by 4% paraformaldehyde. The brain was post-fixed as above for subsequent immunohistochemical localization of the microinjection sites.
A subgroup of virally-transfected rats (N=9) was used for whole cell patch clamp recordings from DMV neurons in thin brainstem slices. Rats were anesthetized with isoflurane (5% in air) until a deep place of anesthesia was induced (abolition foot pinch withdrawal reflex), before administration of a bilateral pneumothorax (laparotomy and diaphragm penetration). The brainstem was excised quickly, submerged in cold (4°C) oxygenated Krebs’ solution, and cut at 300μm-thick sections which were placed in warm (30°C) oxygenated Krebs’ solution for 90 minutes before recording.
A brainstem slice was placed in a perfusion chamber (volume 500μl; MSR Neurobiology, Roaring Springs, PA, USA) fitted on the stage of a Nikon E600FN microscope and perfused with warmed (32°C) Krebs’ solution (in mM: 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 10 D-glucose, kept at pH 7.4 by bubbling with 95%O2/5% CO2). Electrophysiological recordings of DMV neurons were made using 2–4MΩ patch pipettes filled with a potassium gluconate (in mM: 128 Kgluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 1 NaATP, and 0.25 NaGTP adjusted to pH 7.35) and a single electrode voltage clamp amplifier (Axopatch 200B; Molecular Devices, Union City, CA). Only one cell per brainstem slice was used to avoid potential confounding results from multiple drug applications. Data were filtered at 2kHz digitized via a Digidata 1440 Interface and stored and analyzed on a PC with pClamp 10 software (Molecular Devices). Recordings with a series resistance of >20MΩ were eliminated from the study. DMV neurons were current clamped at a membrane potential that allowed spontaneous action potential firing of approximately ~1 event/sec. Freshly prepared CNO (10μM diluted in Krebs’ solution) was perfused until a stable response was observed or for 5min if no response was observed. Each neuron served as its own control, with responses assessed before and after CNO application. A neuron was considered responsive if CNO altered its firing rate by >25% relative to baseline.
Data Collection, Analysis and Preparation of Figures
Immunofluorescent images were captured with a Zeiss AXIO Observer Z1 confocal laser scanning microscope with the Zen 3.3 (blue edition) software.
Motility and/or tone data were collected from all animals; 6 rats in which the motility and tone response were assessed following NMDA injection into the SNpc were excluded from the final analysis. Of these 6 rats, 4 were excluded because they did not show a measurable change in tone or motility following microinjection; subsequent post hoc verification confirmed the injection site was off-target. The remaining 2 rats were excluded from analysis because of failure of the strain gauge during the recording. Colonic tone and motility traces were analyzed with Axoscope® software (Molecular Devices). All data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test, or t-test with GraphPad Prism9 (GraphPad Software Inc., LaJolla, CA). Data are expressed as Mean ±SD, with significance set at p<0.05.
Results
Anatomical characterization of the nigro-vagal-proximal colon connection.
To confirm that brainstem vagal neurons innervate the proximal colon, vagal efferent neurons were labelled with microinjections of an anterograde tracer in the DMV (N=4). Subsequent immunohistochemical analyses of the proximal colon showed that out of 220±14.6 PGP 9.5-IR myenteric neurons, 105±5.5 (i.e. 47.9±2.81%) received close appositions from, and were occasionally encircled by, vagal efferent fibers (Fig.1A).
Figure 1. SNpc provides an excitatory input to neurons of the dorsal motor nucleus of the vagus that innervate the proximal colon.

A. Representative micrograph of vagal efferent fibers apposing myenteric neurons of the proximal colon. Following microinjection of an anterograde tracer (magenta) in the dorsal vagal complex (DVC; N=4), labeled fibers are observed in PGP9.5-IR myenteric neurons of the proximal colon (magenta arrows). Some PGP9.5-IR neurons appeared to receive close contacts from vagal efferent fibers and were encircled with dextran labeled fibers (white arrows).
B. Representative micrograph of neurons in the DMV. Following microinjection of an anterograde tracer in the SNpc, labeled fibers (white arrows) can be seen apposing DMV neurons labelled following injections of the retrograde tracer CTB in the proximal colon (N=5).
C. Representative micrographs of ChAT-IR neurons (brown) and c-fos (blue-black) within the within the DMV. Black arrows indicate c-fos-IR neurons within the lateral DMV following NMDA microinjection into the DMV.
Scale bars: 100μm in all panels.
DMV: dorsal motor nucleus of the vagus; NTS: nucleus tractus solitarius; 4V: fourth ventricle
To investigate whether colon-projecting vagal efferent motoneurons receive projections from the SNpc, the retrograde tracer, CTB, was injected in the proximal colon and the anterograde tracer, dextran 10,000MW was microinjected into the SNpc (N=7). Subsequent analysis of every fourth vagal brainstem slice showed 61 CTB-positive DMV neurons in the lateral tips of the DMV throughout the rostro-caudal extent of the DVC; these neurons were located in the rostral (Bregma −13.3mm; N=22), intermediate (Bregma −13.9mm; N=32), and caudal (Bregma −14.3mm; N=7) DMV. Forty-two (i.e. 68.8%) of these retrogradely labeled neurons received close contacts from dextran labelled SNpc fiber projections (17 of 22 neurons in the rostral, 19 of 32 neurons in the intermediate, and 6 of 7 neurons in the caudal DMV, respectively) (Fig.1B).
To confirm that the nigro-vagal pathway modulates the activity of DMV neurons, brainstem slices from an additional 4 rats that had undergone NMDA microinjection into the SNpc (see below) were assessed for the co-localization of c-fos and ChAT-IR. SNpc stimulation induced c-fos expression in DMV neurons throughout the rostro-caudal extent of the DVC, including in the lateral third of the DMV which contains presumed colon-projecting neurons (Fig. 1C). These neurons were located in the rostral (Bregma −13.3mm; N=12 laterally located of 21 total DMV c-fos-IR neurons), intermediate (Bregma −13.9mm, N=14 of 60 neurons), and caudal (Bregma −14.2mm, N=6 of 16 neurons).
These data provide anatomical proof of a discrete nigro-vagal pathway through which SNpc neurons are presumed to innervate proximal colon-projecting DMV neurons.
The nigro-vagal pathway modulates tone and motility of the proximal colon.
To investigate whether SNpc neurons modulate proximal colon tone and motility, NMDA was microinjected into the left SNpc (injection locations are shown in Fig.2Ab) while colon tone and motility were assessed (N=21). Microinjection of NMDA increased both the motility 157±117.5 to 255±130.3 a.u. (i.e. 190±98.9% of baseline; t20=4.189, p<0.0001 vs baseline, one tailed paired t test) and the tone (399±330.4mg) of the proximal colon (Fig.2B, C). Since the baseline motility was similar for all the experiments, the results of the experiments described below are expressed as % of baseline.
Figure 2. NMDA microinjection in the SNpc increases the tone and motility of the proximal colon.

A. Representative micrograph showing the location of a NMDA injection (arrow) in SNpc (Aa, scale bar: 500μm). Schematic image summarizing the location of the injections in SNpc (Ab, N=21).
B. Representative trace showing that the NMDA (5nmoles/210nl) microinjection in the left SNpc (arrow) increases both tone and motility of the proximal colon.
C. Summary graphic showing the increase in motility (N=21, p<0.0001, one tailed paired t test) and tone (N=21) of the proximal colon upon NMDA microinjection in the left SNpc.
D. Representative trace showing that the AMPA (5nmoles/210nl) microinjection in the left SNpc (arrow) increases both tone and motility of the proximal colon.
E. Summary graphic showing the increase in motility (N=4, p=0.0463, one tailed paired t test) and tone (N=4) of the proximal colon upon AMPA microinjection in the left SNpc.
To confirm that the response to NMDA microinjection was due to physiological rather than excitotoxic activation of SNpc neurons, microinjections of AMPA were made into the SNpc of 4 rats, approximately 60min after recovery from NMDA injection. In these rats, AMPA increased both the motility (158±48.4% of baseline; t3=2.438, p=0.0463 vs baseline, one tailed paired t test) and tone (273±111.1mg) of the proximal colon.
To investigate whether the increase in proximal colon tone and motility were vagally-dependent, NMDA was microinjected in the left SNpc after posterior subdiaphragmatic vagotomy. NMDA microinjection increased tone by 558±264.9mg and motility to 207±63.7% of baseline (p= 0.0097 vs baseline; N=5; one tailed paired t test). In the same rats, after complete subdiaphragmatic vagotomy, subsequent NMDA microinjection had no effect on tone (9±30mg; N=5) or motility (79.33±41.35% of baseline; N=5; t4=1.118, p= 0.3263, two tailed paired t test) of the proximal colon (Fig. 3).
Figure 3. Vagotomy prevents the increase in tone and motility of the proximal colon following SNpc microinjection of NMDA microinjection.

A. Representative traces showing that the increase in tone and motility of the proximal colon induced by NMDA (5nmoles/210nl, arrow) microinjection in the left SNpc (upper panel) is prevented by vagotomy (lower panel).
B. Summary graphic showing the increase in motility and tone observed in response to NMDA before (N=5; p=0.0097 NMDA vs baseline, one tailed paired t test) and after vagotomy (ns: p=0.3263 Vagotomy+NMDA vs baseline, two tailed paired t test; tone: p=0.0097 NMDA vs. Vagotomy +NMDA, one-tailed paired t-test).
ns: not significant.
These data indicate that the SNpc modulates the tone and motility of the proximal colon in a vagally-dependent manner.
Chemogenetic stimulation of DVC confirms SNpc inputs modulate DMV neuronal activity
To confirm that the nigro-vagal pathway exerts modulatory control over proximal colon activity, the effects of chemogenetic modulation of nigro-vagal terminals on the activity of DMV neurons and proximal colon tone and motility were assessed. Post-hoc verification of transfection location and efficacy in hM3D(Gq) (N=7), hM4D(Gi) (N=5), or empty (control) vector (N=6) rats in the SNpc (Fig.4A), showed co-localization of vector in tyrosine hydroxylase-IR SNpc neurons (TH-IR, blue; Fig.4B upper panels) and mCherry and YFP labelled fibers scattered bilaterally throughout the DMV (Fig. 4B lower panel).
Figure 4. Chemogenetic activation of nigro-vagal projections increases the tone and motility of the proximal colon.

A. Schematic diagram illustrating the experimental protocol used for chemogenetic manipulation of the nigro-vagal pathway.
B. Representative micrograph showing the location of hM3D(Gq) (upper left; red) and empty vector (upper right, yellow) microinjection. SNpc neurons are labeled with tyrosine hydroxylase. Representative micrograph showing the location of the mCherry and EYFP labelled fibers in the DMV following SNpc transfection, and apposing choline acetyl transferase-IR neurons (lower panels).
C. Recording of proximal colon function in an empty vector transfected rat following application of CNO to the 4th ventricle.
D. Graphical summary of the change in proximal colon motility (left, N=6; p=0.5114, two tailed paired t test) and tone (right) in empty vector transfected rats in response to brainstem application of CNO.
E. Recording of proximal colon function in an hM3D(Gq) transfected rat following application of CNO to the 4th ventricle.
F. Graphical summary of the change in proximal colon motility (left, N=7; p=0.0434, two tailed paired t test) and tone (right) in hM3D(Gq) transfected rats following brainstem application of CNO.
G. Recording of proximal colon function in an hM4D(Gi) transfected rat following application of CNO to the 4th ventricle.
H. Graphical summary of the change in proximal colon motility (left, N=5; p=0.0051, two tailed paired t test) and tone (right) in hM4D(gi) transfected rats following brainstem application of CNO.
Scale bars: 100μm; ns: not significant.
Fourth ventricular application of CNO (2μl/200nmole) had no effect on either proximal colon motility (93±23.9% of baseline; N=6; t5=0.7066, p=0.5114, two tailed paired t test) or tone (8±41.2mg) in any of the 6 rats that were transfected with the empty vector Fig.4C,D). In contrast, CNO application to hM3D(Gq) transfected rats increased motility (192±95.3% of baseline; N=7; t6=2.552, p=0.0434, two tailed paired t test ) and tone (137±136mg, N=7) of the proximal colon (Fig.4E,F) while CNO application to hM4D(Gi) transfected rats decreased tone (−71±25.6 mg, N=6) and motility (50±20.1% of baseline; N=5; t4=5.564, p=0.0051, two tailed paired t test) of the proximal colon (Fig.4G,H).
In a subgroup of rats in which the SNpc was transfected with hM3D(Gq), hM4D(Gi), or empty (control) vector in the SNpc, whole cell patch clamp recordings were made from DMV neurons and the effects of CNO (10 μM) to modulate action potential firing rate were assessed. In 7 of 8 neurons from 3 hM3D(Gq) rats, CNO increased the firing rate of DMV neurons from 1.2±0.26 to 1.9±0.55 events/sec. This increase in firing rate was prevented by pretreatment with the D1-like antagonist SCH23390 (1.07±0.21 events/sec with SCH23390+CNO; p=0.0255; F(DFn, DFd=0.9080, 4,994)=10.17, Geissner-Greenhouse epsilon=0.4540; one-way ANOVA, mixed effects model) (Fig.5C,D). Conversely, in 7 out of 8 neurons from 3 hM4D(Gi) rats, CNO decreased the firing rate of DMV neurons from 1.1±0.33 to 0.6±0.52 events/sec (t6=2.949, p=0.0257; two-tailed paired t-test) (Fig.5E,F). Perfusion with CNO had no effect on the firing rate in 7 out of 8 DMV neurons from 3 empty vector rats (1.2±0.35 and 1.1±0.57 events/sec at baseline and after CNO perfusion, respectively (t7=0.6608, p=0.5300; two-tailed paired t-test) (Fig.5A, B).
Figure 5. Chemogenetic activation of nigro-vagal projections modulates DMV neuronal activity.

Whole cell patch clamp recordings from a DMV neuron current clamped at a potential to allow spontaneous firing at approximately 1 event/sec.
A. In an empty vector transfected rat, superfusion with CNO had no effect on action potential firing rate.
B. Graphical summary of the effects of CNO on action potential firing rate in DMV neurons from empty vector transfected rats (N=7, p=0.5300; two-tailed paired t-test).
C. In an hM3D(Gq) transfected rat, superfusion with CNO increased action potential firing in a manner that was reversed following superfusion with the D1-like receptor antagonist, SCH23390.
D. Graphical summary of the effects of CNO to alter action potential firing rate in DMV neurons from hM3D(Gq) transfected rats (N=7; p=0.0454 baseline vs CNO, p=0.0112 CNO vs SCH, p=0.4204 baseline vs SCH, one-way ANOVA, mixed effects model).
E. In an hM4D(Gi) transfected rat, superfusion with CNO decreased action potential firing in a reversible manner.
F. Graphical summary of the effects of CNO to alter action potential firing rate in DMV neurons from hM4D(Gq) transfected rats (N=7; p=0.0257; two-tailed paired t-test).
Bars indicate a 2min interval. ns: not significant.
These data indicate that the nigro-vagal pathway exerts a tonically active and physiological relevant modulatory control over DMV neuronal activity as well as tone and motility of the proximal colon.
The nigro-vagal pathway modulates tone and motility of the proximal colon via D1-like receptors in the DMV.
To investigate the neurotransmitter(s) used by nigro-vagal pathway to increase the tone and motility of the proximal colon, the effects of NMDA stimulation of the SNpc were carried out before and after application of the D1-like antagonist SCH23390 or the D2-like antagonist L741646 (both at 45nmoles/2μl) applied to the floor of the IV ventricle.
In a subgroup of rats (N=5), 4th ventricular application of SCH23390 had no effect on motility (108±10.8% of baseline; t4=1.784, p=0.1491 vs baseline, two-tailed paired t test) or tone (−3±10.9mg) (Fig. 6B) of the proximal colon. In the presence of SCH23390, the NMDA-induced increase in tone was reduced from 692±565.4mg to 327±286.1mg (N=5; t4=2.716, p=0.0266 vs NMDA alone, one-tailed paired t test). Similarly, NMDA microinjection in the SNpc increased the motility of the proximal colon to 219±68.7% of baseline (n=4; t3=3.456, p=0.0204, one-tailed paired t test); this increase was reduced significantly to 94±13.1% of baseline in the presence of SCH23390 (n=4; t3=3.085, p=0.0270, one-tailed paired t test) (Fig. 6A, B).
Figure 6. Pretreatment with the dopamine D1-like antagonist SCH23390 prevents the increase in tone and motility of the proximal colon following NMDA microinjection in the left SNpc.

A. Representative traces showing that the increase in tone and motility of the proximal colon induced by NMDA (5nmoles/210nl, arrow) microinjection in the left SNpc (A, left) is prevented by 4th ventricular application of the dopamine D1-like antagonist SCH23,390 (45nmoles/2μl) (A, right).
B. Summary graphic showing that the increase in motility (N=4; p=0.0204, one-tailed paired t-test) and tone (N=5) of the proximal colon observed in response to SNpc microinjection of NMDA is prevented by pretreatment with SCH23390 in the IV ventricle (motility: p=0.3974 vs baseline, one-tailed paired t-test; tone: p=0.0266, one-tailed paired t test). 4th ventricular application of SCH23390 had no effect on motility (N=5; p=0.1491 vs baseline, two-tailed paired t test) or tone of the proximal colon.
C. Representative traces showing that the increase in tone and motility of the proximal colon induced by NMDA (5nmoles/210nl, arrow) microinjection in the left SNpc (left) is not affected by pretreatment with the dopamine D2-like antagonist L741646 (45nmoles/2μl) on the floor of the IV ventricle (right).
D. Summary graphic showing that the NMDA-induced increase in motility (N=5; p=0.0018, one-tailed paired t-test) and tone (N=5) of the proximal colon is not altered by pretreatment with L741646 (motility: p=0.3996 NMDA vs L741646+NMDA, two-tailed paired t test; tone p=0.8051, two-tailed paired t test). 4th ventricular application of the D2-like antagonist L741646 increased both the motility (N=5; p=0.0088 vs baseline, one-tailed paired t test) and the tone.
Bars indicate a 40min interval. ns: not significant.
In a subgroup of rats (N=5), 4th ventricular application of the D2-like antagonist L741646 increased both the motility (190±51.9% of baseline; t4=3.898, p=0.0088 vs baseline, one-tailed paired t test) and the tone (325±108.2mg) (Fig. 6D). In the presence of L741646, however, the increase in tone or the motility of the proximal colon following microinjection of NMDA into the SNpc were unaltered (tone: NMDA 221±130.8mg vs L741646+NMDA 212±90.3mg; N=5; t4=0.2636, p=0.8051, two-tailed paired t test; motility: 199±36.2% vs 227±57.9% of baseline in NMDA vs L741646+NMDA, respectively; N=5; t4=0.9419, p=0.3996, two-tailed paired t test) (Fig. 6C, D).
Overall these data indicate that excitation of the nigro-vagal pathway increases tone and motility of the proximal colon via activation of DMV D1-like receptors, but that this nigro-vagal pathway is distinct from that which results in tonic activation of D2-like receptors to inhibit activity of the proximal colon.
To confirm the distinct and different effects of DA to modulate vagal control of the proximal colon, dopamine (DA; 100nmoles/60nl) was microinjected in the left DMV while proximal colon tone and motility were recorded. In contrast to the D1-like receptor dependent excitation observed following SNpc stimulation, microinjection of DA decreased both the tone (−89±41.7mg, N=10) and motility (46±23.7% of baseline; N=10; t9=7.163, p <0.0001, one-tailed paired t test) of the proximal colon (Fig.7B, C). These inhibitory effects were attenuated significantly by application of the D2-like antagonist L741646, indeed, the response to exogenous application of DA became excitatory (tone: −99.5±58.9mg vs 99±100.1mg; N=4; t3=2.567, p=0.0414, one-tailed paired t test; motility: 62±16.7% and 137±40.9% of baseline in the absence and presence of L741646 respectively; N=4; t3=2.725, p=0.0.0361, one-tailed paired t test) (Fig.7D, E)
Figure 7. Pretreatment with the dopamine D2-like antagonist L741646 attenuates the decrease in motility of the proximal colon following DA microinjection in the DVC.

A. Representative micrograph showing the location of a dopamine (DA) injection (arrow) in the DMV (Aa, scale bar: 100μm). Schematic image summarizing the location of the injection of DA in the DMV (Ab, N=5, other microinjection locations were omitted for clarity).
DMV: dorsal motor nucleus of the vagus; NTS: nucleus tractus solitarius; cc: central canal; AP area postrema
B. Representative trace showing that DVC microinjection of DA (100nmoles/60nl; arrow) decreases both the tone and motility of the proximal colon.
C. Summary graphic showing the decrease in motility (left; N=10; p<0.0001, one-tailed paired t test) and tone (right; N=10) of the proximal colon upon DA microinjection in the left DMV.
D. Representative traces showing that the decrease in motility and tone of the proximal colon induced by DVC microinjection of DA (100nmoles/60nl; left trace) attenuated by 4th ventricular application of the dopamine D2-like antagonist L741646 (45nmoles/2μl; right trace). Bars indicate a 40min.interval.
E. Summary graphic showing that the decrease in proximal colon motility (N=5; p=0.01, one-tailed paired t-test) and tone (N=4) observed after DVC microinjection of DA is attenuated by IV ventricle application of L741646 (motility: p=0.2386 baseline vs. L741626+DA, two-tailed paired t-test, p=0.0361 DA vs. L741626+DA one-tailed paired t-test; tone: p=0.0414 DA vs. L741626+DA, one-tailed paired t-test).
Bars indicate a 40min interval. ns: not significant.
These data indicate that the SNpc modulates the tone and motility of the proximal colon via a nigro-vagal neurocircuit which activates D1 receptors on DMV neurons. These data also suggest that the DMV neurons that respond to SNpc stimulation form a discrete neurocircuit which can be differentiated from the other DMV neurons which receive different dopaminergic inputs.
Discussion
In this study we have demonstrated: 1) the existence of an anatomically defined pathway that connects the SNpc to brainstem vagal motoneurons that innervate the proximal colon; 2) nigral fibers form close anatomically connections with a discrete subpopulation of vagal proximal-colon projecting motoneurons and modulate proximal colon tone and motility; and, 3) this nigro-vagal pathway is dopaminergic and excitatory. These novel observations suggest the possibility that the impairment of this nigro-vagal dopaminergic pathway may be involved in the severely reduced colonic transit and prominent constipation observed in parkinsonism regardless of the etiology (central or peripheral) of Parkinson’s Disease.
Traditionally, the vagal innervation of the distal intestine was considered extremely sparse, despite tracing studies having revealed vagal projections that extend to the transverse colon (Berthoud et al., 1991; Powley, 2021). Furthermore, mapping of the peripheral projections of brainstem vagal motoneurons identified clusters of intestinal-projecting neurons in the lateral tips of the DMV (Fox & Powley, 1985; Norgren & Smith, 1988; Altschuler et al., 1993; Browning et al., 1999), in the area we report here as receiving projections from SNpc fibers. In the present study we used a combination of anterograde tracing from the SNpc, and retrograde tracing from the proximal colon to confirm the location of colonic-projecting DMV neurons, and to demonstrate that these vagal motoneurons receive close anatomical connections from the SNpc. Indeed, neither the neuronal tracers (dextran or CTB) or the viral constructs (hM3D(Gq) or hM4D(Gi)) used in the present study cross synapses, and both the anatomical and functional studies described herein suggest that the nigro-vagal pathway may be monosynaptic.
The physiological relevance of the nigro-vagal pathway and its modulation of proximal colonic function is provided by the observation that both pharmacological and chemogenetic stimulation of this pathway increase the tone and motility of the proximal colon in a vagally-dependent manner. As reported previously relative to the control of gastric tone and motility (Anselmi et al., 2017a), the nigro-vagal pathway described in the present study also appears to use dopamine (DA) as the main neurotransmitter that activates proximal colon-projecting DMV neurons. In fact, the excitatory effects of SNpc stimulation were attenuated significantly by brainstem application of the D1-like antagonist SCH 23390, but not by the D2-like antagonist L741646 implying the nigrovagal pathway excites vagal efferent motoneurons via activation of dopaminergic D1 receptors.
Interestingly, the excitatory D1 receptor mediated effects on proximal colon function following nigro-vagal stimulation is in sharp contrast with the inhibitory effects observed upon exogenous application of DA to the DVC. It should be noted, however, that this inhibitory effect was reversed by the application of a D2 receptor antagonist, implying that the physiological response to brainstem application of DA is a balance between excitatory D1 and inhibitory D2 receptor activation. Notably, the observation that pharmacological and chemogenetic stimulation of the nigro-vagal pathway elicit only excitatory effects on proximal colon function provides further support to the selectivity and specificity of brainstem vagal neurocircuit organization, as put forward previously by both our group and others (Browning et al., 1999; Evans et al., 2003; Grabauskas & Moises, 2003; Davis et al., 2004; Browning et al., 2005; Gao et al., 2009; Babic et al., 2011; Browning & Travagli, 2014; Anselmi et al., 2017b),
The enteric nervous system (ENS) comprises a large number of neurons whose density increases gradually from the proximal toward the distal portions of the GI tract (Wood, 1987; Grundy et al., 2006; Furness, 2012). Myenteric neurons of the ENS play a fundamental role in the control of small and large intestine functions, including the organization of appropriate behavior patterns that direct effective secretion, absorption, and transit. The present study provides the first experimental evidence, both anatomical and physiological, that a nigro-vagal neurocircuit regulates proximal colonic functions in a vagally dependent manner. Furthermore, the anatomical association between the proximal colon, vagal efferent motor neurons within the DMV, and SNpc delineates a neural pathway by which colonic dysfunction may be explained in both “top down” (i.e., PD pathology is initiated centrally in the SNpc) as well as “bottom up” (i.e, PD pathology is initiated in the gut and travels centrally as per Braak’s hypothesis) forms of parkinsonism.
While the etiology of PD is still open to debate, with evidence for both central and peripheral disease origins (Surmeier et al., 2017; Liddle, 2018; Johnson et al., 2019; Marras et al., 2019; Wichmann, 2019), it is also clear that the majority of Parkinsonian patients experience prodromal GI issues that comprise, among other pathologies, severe constipation (Edwards et al., 1992; Pfeiffer, 1998; Cersosimo et al., 2013; Fasano et al., 2015; Giancola et al., 2017; Knudsen et al., 2017; Liddle, 2018; Ramprasad et al., 2018; De Pablo-Fernandez et al., 2019; Travagli et al., 2020). Indeed misfolded α-synuclein, the histological hallmark of Parkinson’s disease (Spillantini et al., 1997), has been described in both enteric and DMV neurons (Goedert et al., 2013; Goedert, 2015) which led Braak’s group to hypothesize that, in some instances, Parkinson’s disease may originate in the GI tract with the misfolding of α-synuclein originating in myenteric neurons of the GI tract before being transported retrogradely, initially to vagal motoneurons of the DMV and then onto higher centers such as the SNpc) (Braak et al., 2003a; Hawkes et al., 2010; Goedert et al., 2013; Braak & Del Tredici, 2017). The initial involvement of the GI tract may not only account for the prodromal GI-related motor dysfunctions experienced by Parkinsonian patients but also highlight the relevance and importance of vagal neurocircuitry in disease pathology and pathogenesis. Indeed, analysis of Parkinsonian patients has shown that their gastric myoelectric activity is altered (Lu et al., 2004; Naftali et al., 2005) and is similar to that recorded in patients who received a recent vagotomy (Soykan et al., 1999). Furthermore, a large Scandinavian retrospective study suggested that truncal vagotomy reduces the incidence of Parkinson’s disease (Kalaitzakis et al., 2008; Svensson et al., 2015). Notably, our previous studies using an environmental model of parkinsonism demonstrated that motor deficits were preceded by gastric dysmotility, that vagotomy prevented the development of parkinsonian motor dysfunctions as well as the loss of SNpc neurons, and that vagotomy constrained the location of α-synuclein aggregates to the enteric nervous system (Anselmi et al., 2018).
The present study, therefore, which demonstrates that proximal colon motility is under similar tonic modulation by a nigro-vagal pathway, provides further support for a gut-brain etiology of PD as well as a functional and anatomical support for Braak’s staging hypothesis. It is important to note, however, that while Braak’s hypothesis may explain the pathology of some instances of PD, it does not provide explanations for all instances of idiopathic PD. The current study, by demonstrating that the nigro-vagal pathway we described previously (Anselmi et al., 2017a) modulates not only gastric tone and motility, but also extends its influence distally to the proximal colon, delineates a neural pathway by which GI dysfunctions in PD may be explained independently of central or peripheral etiology.
Supplementary Material
Authors’ Translational Perspective.
The current study provides the first anatomical and physiological experimental evidence that a nigro-vagal neurocircuit regulates proximal colonic functions in a vagally dependent manner. Furthermore, the close anatomical association between SNpc neuronal projections and proximal colon-projecting vagal efferent motor neurons within the DMV provides a synaptically-connected conduit that might be responsible for the prodromal gastrointestinal dysfunctions, including severe constipation, observed in most PD patients. Future studies will define whether this nigro-vagal-proximal colon pathway represents a direct route by which environmental toxins or synucleinopathies can travel retrogradely to CNS centers and promote the degeneration of dopaminergic neurons of the SNpc which would then trigger the prodromal severe constipation observed in most PD patients.
Key points.
Substantia nigra pars compacta (SNpc) neurons are connected to the dorsal motor nucleus of the vagus (DMV) neurons via a presumed direct pathway.
Brainstem neurons in the lateral DMV innervate the proximal colon. Colonic-projecting DMV neurons receive inputs from neurons of the SNpc.
The nigro-vagal pathway modulates tone and motility of the proximal colon via D1-like receptors in the DMV.
This study provides the mechanistic basis for explaining how SNpc alterations may lead to a high rate of constipation in patients with Parkinson disease.
Acknowledgments:
We thank Cesare M. and Zoraide Travagli, and W. Nairn Browning for support and encouragement.
Additional Information Section:
This work was supported by National Institute of Health grants DK124098 and DK55530, and DoD grant W81XWH2110915.
The work was performed at Penn State College of Medicine.
References
- Altschuler SM, Escardo J, Lynn RB & Miselis RR. (1993). The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104, 502–509. [DOI] [PubMed] [Google Scholar]
- Anselmi L, Bove C, Coleman FH, Le K, Subramanian MP, Venkiteswaran K, Subramanian T & Travagli RA. (2018). Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. Naturepj Parkinson’s Disease 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi L, Toti L, Bove C, Hampton J & Travagli RA. (2017a). A Nigro-Vagal Pathway Controls Gastric Motility and is Affected in a Rat Model of Parkinsonism. Gastroenterology 153, 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi L, Toti L, Bove C & Travagli RA. (2017b). Vagally mediated effects of brain stem dopamine on gastric tone and phasic contractions of the rat. Am J Physiol Gastrointest Liver Physiol 313, G434–G441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic T, Browning KN & Travagli RA. (2011). Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300, G21–G32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasuriya GK, Nugapitiya SS, Hill-Yardin EL & Bornstein JC. (2021). Nitric Oxide Regulates Estrus Cycle Dependent Colonic Motility in Mice. Front Neurosci 15, 647555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR & Powley TL. (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260, R200–R207. [DOI] [PubMed] [Google Scholar]
- Braak H, Del TK, Rub U, De Vos RA, Jansen Steur EN & Braak E. (2003a). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H & Del Tredici K. (2017). Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J Parkinsons Dis 7, S71–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP & Del Tredici K. (2003b). Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110, 517–536. [DOI] [PubMed] [Google Scholar]
- Browning KN, Coleman FH & Travagli RA. (2005). Characterization of Pancreas-Projecting Rat Dorsal Motor Nucleus Of The Vagus Neurons. Am J Physiol Gastrointest Liver Physiol 288, G950–G955. [DOI] [PubMed] [Google Scholar]
- Browning KN, Renehan WE & Travagli RA. (1999). Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. Journal of Physiology 517, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN & Travagli RA. (2014). Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 4, 1339–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG & Benarroch EE. (2012). Autonomic involvement in Parkinson’s disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci 313, 57–63. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutierrez C, Micheli FE & Benarroch EE. (2013). Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Coon EA, Cutsforth-Gregory JK & Benarroch EE. (2018). Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord. [DOI] [PubMed] [Google Scholar]
- Davis SF, Derbenev AV, Williams KW, Glatzer NR & Smith BN. (2004). Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo-Fernandez E, Passananti V, Zarate-Lopez N, Emmanuel A & Warner T. (2019). Colonic transit, high-resolution anorectal manometry and MRI defecography study of constipation in Parkinson’s disease. Parkinsonism Relat Disord 66, 195–201. [DOI] [PubMed] [Google Scholar]
- Del Rey NL, Quiroga-Varela A, Garbayo E, Carballo-Carbajal I, Fernandez-Santiago R, Monje MHG, Trigo-Damas I, Blanco-Prieto MJ & Blesa J. (2018). Advances in Parkinson’s Disease: 200 Years Later. Front Neuroanat 12, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. (2018). Neuropathology of Parkinson disease. Parkinsonism Relat Disord 46 Suppl 1, S30–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LL, Quigley EM & Pfeiffer RF. (1992). Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 42, 726–732. [DOI] [PubMed] [Google Scholar]
- Evans C, Baxi S, Neff R, Venkatesan P & Mendelowitz D. (2003). Synaptic activation of cardiac vagal neurons by capsaicin sensitive and insensitive sensory neurons. Brain Res 979, 210–215. [DOI] [PubMed] [Google Scholar]
- Fasano A, Visanji NP, Liu LW, Lang AE & Pfeiffer RF. (2015). Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14, 625–639. [DOI] [PubMed] [Google Scholar]
- Fox EA & Powley TL. (1985). Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res 341, 269–282. [DOI] [PubMed] [Google Scholar]
- Furness JB. (2012). The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D & Smith BN. (2009). Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res 1291, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola F, Torresan F, Repossi R, Bianco F, Latorre R, Ioannou A, Guarino M, Volta U, Clavenzani P, Mazzoni M, Chiocchetti R, Bazzoli F, Travagli RA, Sternini C & De Giorgio R. (2017). Downregulation of neuronal vasoactive intestinal polypeptide in Parkinson’s disease and chronic constipation. Neurogastroenterol Motil 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M (2015). NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 349, 1255555. [DOI] [PubMed] [Google Scholar]
- Goedert M & Compston A. (2018). Parkinson’s disease - the story of an eponym. Nat Rev Neurol 14, 57–62. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del TK & Braak H. (2013). 100 years of Lewy pathology. Nat Rev Neurol 9, 13–24. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. (2014). Dysautonomia in Parkinson disease. Compr Physiol 4, 805–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskas G & Moises HC. (2003). Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol 549, 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Tache Y & Wood JD. (2006). Fundamentals of neurogastroenterology: basic science. Gastroenterology 130, 1391–1411. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del TK & Braak H. (2007). Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K & Braak H. (2010). A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16, 79–84. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Babic T & Travagli RA. (2019). Sex differences in GABAergic neurotransmission to rat DMV neurons. Am J Physiol Gastrointest Liver Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Stecher B, Labrie V, Brundin L & Brundin P. (2019). Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci 42, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzakis ME, Graeber MB, Gentleman SM & Pearce RK. (2008). The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol 34, 284–295. [DOI] [PubMed] [Google Scholar]
- Knudsen K, Krogh K, Ostergaard K & Borghammer P. (2017). Constipation in parkinson’s disease: Subjective symptoms, objective markers, and new perspectives. Mov Disord 32, 94–105. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu J, Jiang F, Jiang Z, Liu C, Li L, Luo Y, Lu R, Mu Y, Liu Y & Xue B. (2016). G protein-coupled estrogen receptor is involved in modulating colonic motor function via nitric oxide release in C57BL/6 female mice. Neurogastroenterol Motil 28, 432–442. [DOI] [PubMed] [Google Scholar]
- Liddle RA. (2018). Parkinson’s disease from the gut. Brain Res 1693, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JYH, Lin G, Fang M & Rudd JA. (2019). Localization of estrogen receptor ERalpha, ERbeta and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen Comp Endocrinol 272, 63–75. [DOI] [PubMed] [Google Scholar]
- Lu CL, Shan DE, Chen CY, Luo JC, Chang FY, Lee SD, Wu HC & Chen JD. (2004). Impaired gastric myoelectrical activity in patients with Parkinson’s disease and effect of levodopa treatment. Dig Dis Sci 49, 744–749. [DOI] [PubMed] [Google Scholar]
- Marras C, Canning CG & Goldman SM. (2019). Environment, lifestyle, and Parkinson’s disease: Implications for prevention in the next decade. Mov Disord 34, 801–811. [DOI] [PubMed] [Google Scholar]
- Meister AL, Jiang Y, Doheny KK & Travagli RA. (2019). Correlation between the motility of the proximal antrum and the high-frequency power of heart rate variability in freely moving rats. Neurogastroenterol Motil 31, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftali T, Gadoth N, Huberman M & Novis B. (2005). Electrogastrography in patients with Parkinson’s disease. Can J Neurol Sci 32, 82–86. [DOI] [PubMed] [Google Scholar]
- Nanni G & Travagli RA. (2020). A nigro-vagal pathway controls colonic motility and may be impaired in a model of envoronmental Parkinson’s disease. In Digestive Disease Week. Virtual conference. [Google Scholar]
- Norgren R & Smith GP. (1988). Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273, 207–223. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RF. (1998). Gastrointestinal dysfunction in Parkinson’s disease. Clin Neurosci 5, 136–146. [PubMed] [Google Scholar]
- Postuma RB & Berg D. (2016). Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12, 622–634. [DOI] [PubMed] [Google Scholar]
- Powley TL. (2021). Brain-gut communication: vagovagal reflexes interconnect the two “brains”. Am J Physiol Gastrointest Liver Physiol 321, G576–G587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasad C, Douglas JY & Moshiree B. (2018). Parkinson’s Disease and Current Treatments for Its Gastrointestinal Neurogastromotility Effects. Curr Treat Options Gastroenterol 16, 489–510. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR & Jenner P. (2017). Non-motor features of Parkinson disease. Nat Rev Neurosci 18, 509. [DOI] [PubMed] [Google Scholar]
- Soykan I, Lin Z, Bennett JP & McCallum RW. (1999). Gastric myoelectrical activity in patients with Parkinson’s disease: evidence of a primary gastric abnormality. Dig Dis Sci 44, 927–931. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R & Goedert M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA & Halliday GM. (2017). Parkinson’s Disease Is Not Simply a Prion Disorder. J Neurosci 37, 9799–9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P & Sorensen HT. (2015). Vagotomy and Subsequent Risk of Parkinson’s Disease. Ann Neurol 78, 522–529. [DOI] [PubMed] [Google Scholar]
- Travagli RA & Anselmi L. (2016). Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Browning KN & Camilleri M. (2020). Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 17, 673. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Brooks PL, Hazrati LN & Lang AE. (2013). The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T (2019). Changing views of the pathophysiology of Parkinsonism. Mov Disord 34, 1130–1143. [DOI] [PubMed] [Google Scholar]
- Wood JD. (1987). Physiology of the enteric nervous system. In Physiology of the gatsrointestinal tract, 2nd edn, ed. Johnson LR, pp. 67–110. Raven Press, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


