Abstract
To study the influence of Helicobacter pylori on epithelial barrier function, bacteria, bacterial sonicates, or broth culture supernatants were incubated for 24 h with HT29-19A intestinal cells grown as monolayers. Subsequently, the monolayers were mounted in Ussing chambers, and electrical resistance (R), fluxes of 22Na (JNa) and 14C-mannitol (JMan) (markers of the paracellular pathway), and fluxes of horseradish peroxidase (HRP) in total (J3H-HRP), intact (JHRPi), and degraded forms were measured. H. pylori did not induce any modification of the paracellular pathway (R = 148 ± 10 versus 174 ± 16 Ω · cm2; JNa = 4.16 ± 0.44 versus 3.51 ± 0.41 μEq/h · cm2; JMan = 0.081 ± 0.01 versus 0.058 ± 0.009 μmol/h · cm2), nor did it modify J3H-HRP (2,201 ± 255 versus 2,110 ± 210 ng/h · cm2 for H. pylori-infected and control cells, respectively). However, in the presence of H. pylori, we observed a significant increase in JHRPi (520 ± 146 versus 171 ± 88 ng/h · cm2). This effect was not dependent of the cag status of the strain and was not reproduced by the sonicates or the culture supernatants. It was related to the presence of urease, since a urease-negative mutant of H. pylori did not induce this effect. Ammonia and bafilomycin A1, two agents known to increase the endolysosomal pH, reproduced the increase in JHRPi. In conclusion, H. pylori does not affect directly the integrity of intercellular junctions of epithelial cells in vitro, but it increases the passage of intact HRP, probably by inhibition of the intralysosomal degradation due to the release of ammonia. The increased transport of intact macromolecules may contribute to the induction and maintenance of gastric inflammation by H. pylori.
Helicobacter pylori is now recognized as the main cause of chronic gastritis and intimately associated with the development of peptic ulcer disease (11, 18, 32) and in some cases with a gastric carcinogenesis (15). However, the exact mechanism of its noxious effect remains poorly understood. Particularly, it is not known why gastric inflammation, which develops in the presence of the bacteria, persists long after bacteria have been eliminated. In fact, follow-up studies have shown that months and even years after eradication of bacteria, chronic gastric inflammation is present (7, 16, 44).
On the other hand, the possibility of the development in H. pylori-infected patients of allergic manifestations such as food allergy and other allergic diseases has been suggested (14, 34), but the pathogenic mechanism has not been elucidated. The first step leading to food sensitization in predisposed subjects is the absorption of food antigens across the gastrointestinal epithelium. Possibly H. pylori, by altering the gastric permeability to different antigens, activates abnormally the local immune system, leading to the development of gastric inflammation and, in some cases, to food protein sensitization. The aim of this study was to assess the effect of H. pylori on digestive epithelial permeability in vitro and, in order to gain some insight into the possible mechanisms involved in the action of H. pylori on epithelial function, to determine the possible role of different pathogenicity factors such as cag status, presence of the VacA cytotoxin, and production of urease, in this effect.
MATERIALS AND METHODS
Bacterial strains; preparation of bacterial extract and broth culture supernatants.
Three wild-type strains of H. pylori, isolated from two patients with duodenal ulcer and from one patient with only chronic gastritis, were studied. Two of these strains possessed the pathogenicity marker cagA [cagA(+) strains], which indicates that they possess the cag pathogenicity island, while the third one, designated cagA(−), lacked the cag marker as assessed by PCR performed on bacterial cultures. Additionally, an urease-negative H. pylori mutant, which had lost its urease activity after multiple passages, was studied.
The bacteria were grown on Vitox chocolate agar (Oxoid) under microaerobic conditions (GaspaK in jar) for 24 to 36 h at 37°C. A suspension of each strain, containing 6 × 107 to 9 × 107 of CFU/ml, was prepared and used to infect epithelial cells. To obtain the bacterial extract of the cagA(+) H. pylori strain used in some experiments, 2 ml of the bacterial suspension obtained as described above was sonicated (Vibra cell; Bioblock Scientific) and centrifuged for 30 min at 48,400 × g (Beckman J2-21 M/E; JA - 20 rotor). In another set of experiments, the broth culture supernatant of the cagA(+) strain, containing the VacA cytotoxin and other products secreted by H. pylori, was prepared. It was obtained by growing the bacteria in Brucella broth supplemented with 5% fetal calf serum for 24 to 36 h at 37°C in a thermostatic shaker under microaerophilic conditions. Culture supernatant was separated from bacteria by centrifugation. The VacA content of the supernatant was controlled by testing for cytotoxicity as described by Figura (13). The bacterial extracts and broth culture supernatants were frozen at −80°C until used.
Culture of the intestinal cell line HT29-19A.
The human differentiated colon carcinoma cell line HT29-19A (4) was used as an epithelial model. This epithelial cell line was chosen because gastric cell lines growing in monolayers and forming a polarized epithelium with tight junctions are not available. Moreover, H. pylori has been shown to adhere to various intestinal cell lines (8, 12, 35). Between passages 10 and 35, HT29-19A cells were cultured at 37°C in Dulbecco’s modified Eagle medium (Eurobio, Les Ulis, France) supplemented with 10% heat-inactivated fetal calf serum (Boehringer Mannheim, Grenoble, France) and 4 mmol of glutamine (Sigma-Aldrich Chimie S.A.R.L., Saint-Quentin Fallavier, France) per liter. Cells were passaged every week and seeded at 106 cells per 25 cm2 in the absence of antibiotics. For experimental purposes, cells were seeded at a density of 0.8 × 106 per cm2 in Dulbecco’s modified Eagle medium containing gentamicin (50 μg/ml; Sigma) on polyethylene terephthalate filters with a pore diameter of 0.4 μm and a surface area of 0.9 cm2 (Falcon; Becton Dickinson Labware, N.J.). Upon reaching confluency, these cells progressively formed a monolayer with apical and basolateral domains separated by tight junctions. They were studied after 21 days of culture or later, when the electrical resistance (R) had reached a stable value of about 150 Ω/cm2.
Infection of intestinal monolayers HT29-19A with H. pylori.
Gentamicin was eliminated from the culture medium bathing epithelial cells 3 days before infection. Fifty microliters of bacterial suspension of each H. pylori strain, or of bacterial extract of a cagA(+) H. pylori strain, was placed in the apical compartment (total volume, 500 μl) of the insert, where the epithelial cells were grown on filters. The broth culture supernatant was introduced on the apical side of the filter-grown cells without dilution. Noninfected cells served as controls. After 24 h of incubation, the filter-grown monolayers were washed three times with Ringer solution (to remove the nonadherent bacteria), gently cut out with a sharp razor blade, and placed between two half-chambers of a small Ussing chamber (exposed area, 0.15 cm2) to measure epithelial function.
In some experiments, control and H. pylori-treated filter-grown cell monolayers were used for light microscopy and histological control of infection. After three extensive washings, monolayers were submerged in Bouin’s solution, embedded in paraffin, and stained with Giemsa reagent for assessment of the epithelial monolayer integrity and detection of H. pylori by light microscopy.
To assess the impact of H. pylori on HT29-19A cell viability, lactate dehydrogenase (LDH) activity and the release of lactic acid were measured. After incubation for 24 h, the apical and basal compartment media from control and H. pylori-infected cells were collected and analyzed for LDH activity and lactic acid concentration (Olympus AU 800 for LDH assay and Ortho Clinical Diagnostics VITROS 700 for lactic acid assay).
Measurement of epithelial function in Ussing chambers. (i) Electrical parameters.
Each side of the exposed cell monolayer was bathed with 1.5 ml of Ringer solution (containing the following, in millimoles per liter): Na+, 140; K+, 5.2; Ca2+, 1.2; Mg2+, 1.2; Cl−, 120; HCO3, 25; HPO4, 2.4; and H2PO4, 0.4) and was oxygenated and circulated with a carbogen lift (95% O2–5% CO2). Cell monolayers were short-circuited by using an automatic voltage clamp after appropriate correction for fluid and system resistance. Potential difference (PD) was checked at 30-min intervals, and R was calculated by clamping the monolayer at an imposed potential of 5 mV and applying Ohm’s law according to the current deflection. R was considered an index of epithelial integrity, and short-circuit current (Isc) was considered an index of electrogenic ion movement.
(ii) Measurements of Na+, mannitol, and HRP fluxes across the epithelial monolayers.
Electrical parameters were equilibrated for 15 min, and the permeability markers Na+, mannitol, and horseradish peroxidase (HRP) were added to the apical compartment. In our conditions, Na+ ions and mannitol were markers of the paracellular pathway and can be considered markers of the tightness of intercellular junctions. HRP (molecular weight, 40,000) was used as a food protein tracer, capable of crossing the epithelium across the epithelial cells by transcytosis and considered a marker of the transcellular pathway.
All markers were applied simultaneously in the same apical compartment, and their appearance on the basal side was monitored by sampling 800 μl of the basal compartment at 30-min intervals for 100 min. HRP was added at a final concentration of 0.4 mg/ml (10 μmol/liter) with a tracer dose of 3H-HRP (37 kBq/ml). Mannitol (5 mM) was introduced on both sides of the monolayer and 14C-mannitol (12.2 kBq/ml; NEN Life Sciences Products, Boston, Mass.) was introduced on the apical side only. Finally, 22Na (NEN) was added apically to obtain activity of 14.8 kBq/ml.
Radioactivities were analyzed on 500-μl samples by liquid scintillation photometry, using three appropriate channels and triple-label correction. HRP was immediately assayed on 200-μl aliquots, and intact HRP fluxes (JHRPi) were calculated. 3H-HRP-equivalent fluxes (J3H-HRP) were calculated by using 3H counts representing the total amount of HRP transported in either intact or degraded form. Degraded HRP fluxes (JD) were then calculated as J3H-HRP minus JHRPi. JHRPi and JD, as well as mannitol and sodium fluxes (JMan and JNa), calculated according to specific activity in the medium, were expressed as shown in the tables. (The term “flux” as used here expresses unidirectional [mucosal-serosal] absorption of the permeability markers used. Since this term is widely accepted and used with respect to the transepithelial passage of different molecules measured in an Ussing chamber, it was employed in this study.)
In some experiments, the effects of bafilomycin A1 and ammonium chloride on the epithelial transport and processing of HRP were tested. Bafilomycin A1 is a potent inhibitor of the V-type H+-ATPase, which inhibits the acidification of the endosomal compartment (45). Ammonium chloride is a weak base which concentrates within the lysosomal compartment, increasing the pH (41). Bafilomycin A1 was added to the apical compartment of filter-grown HT29-19A cells at a final concentration of 0.5 μM 30 min before the cell monolayers were mounted in Ussing chambers; it was added at the same concentration to the apical compartment of each Ussing chamber. Ammonium chloride was introduced to the apical compartment of the culture insert, bathing HT29-19A cells at a final concentration of 20 mM, 12 h before the experiment; it was also added at the same concentration to the apical compartment of each Ussing chamber.
(iii) HPLC analysis of 3H-HRP transport and processing across HT29-19A intestinal cell monolayers.
In another set of experiments, using high-performance liquid chromatography (HPLC) analysis of the basal compartment at the end of experiment, we characterized the metabolites formed during transepithelial transport of 3H-HRP across control and H. pylori-infected cell monolayers. For this purpose, the filter-grown intestinal cell monolayers were infected for 24 h with the cagA(+) strain of H. pylori or left untreated. Since transcytosis allows the transport of a very small amount of HRP, in order to obtain improved sensitivity, we used an exposed surface area in Ussing chambers larger than that used in the transport experiments (3.5 instead of 0.15 cm2) and a longer incubation time with 3H-HRP (4.5 instead of 2 h). Accordingly, the intestinal monolayers were mounted in adapted Ussing chambers exposed on each side to 12 ml of oxygenated Ringer solution containing HRP at a final concentration of 0.4 mg/ml and 333 kBq of 3H-HRP. The basal compartments from six Ussing chambers containing control or H. pylori-treated cells (12 ml in each) were then pooled and concentrated by passage through a solid-phase extraction microcolumn (SepPak C18 cartridge; Waters Chromatography, Millipore, Milford, Mass.), which retained the proteins and peptides but not the amino acids (42). Briefly, the column was equilibrated with 10 ml of acetonitrile and washed with 10 ml water containing 1% trifluoroacetic acid (TFA). The sample was acidified with TFA (final concentration, 1% [vol/vol]) and passed through the microcolumn at a flow rate of 10 ml/min. Next, the column was washed with 0.1% TFA, and salts and amino acids were recovered in the eluate. Finally, the proteins and peptides retained were eluted from the column with 4 ml of 80% acetonitrile–20% water–0.1% TFA solution at a flow rate of 2.5 ml/min. The protein and peptide fraction was concentrated in a Speed-Vac system at a low temperature and adjusted to 200 μl before injection in the steric exclusion HPLC column.
The 3H-HRP and 3H-metabolites were separated by steric exclusion HPLC to determine their relative amounts. A Superdex Peptide PE 7.5 (300) column was eluted at 30°C for 45 min with 0.1% TFA in 30% acetonitrile. The percentages of amino acids, peptides, and intact HRP were calculated. Briefly, the total radioactivity of the basal compartments of Ussing chambers representing 100%, the percentage of amino acids was calculated by counting the radioactivity of the first microcolumn eluate and washing. The percentages of peptides and intact protein were estimated on the basis of the steric exclusion HPLC profile, after integration of the peaks by using Borwin software (42).
Statistical analysis.
Data were analyzed by using the SAS package (SAS Institute, Cary, N.C.). The results are expressed as means ± standard errors (SE). Comparisons of means were performed by analysis of variance and Student’s t test.
RESULTS
Effect of H. pylori on the epithelial barrier. (i) Viability of HT29-19A epithelial cells in the presence of H. pylori.
Possible acidification of the culture medium could alter the viability or function of epithelial cells infected by H. pylori. However, the lactic acid concentration was not higher in H. pylori-infected cells than in noninfected cells (14.9 ± 0.1 versus 16.4 ± 0.3 mmol/liter [mean ± SE] in the apical compartment medium and 17.1 ± 0.4 versus 17.8 ± 0.3 mmol/liter) in the basal compartment medium).
Moreover, LDH activity in the medium obtained from H. pylori-infected cells was not significantly different from that of a control medium (105.8 ± 22.6 versus 130.5 ± 30.5 IU/liter in the apical compartment medium and 67.0 ± 1.4 versus 65.5 ± 1.2 IU/liter in the basal compartment medium for H. pylori-infected and control medium, respectively). These results indicate that H. pylori infection does not alter the viability of HT29-19A cells and that it is not the acidification of cells which is responsible for the effect observed.
For some of the epithelial layers, morphological control was done by light microscopy, using Giemsa staining to allow visualization of the bacteria. In the specimens infected with H. pylori, the monolayers were intact and bacteria were found near the epithelial cells (not shown).
(ii) Effect of cagA(+) strains of H. pylori on the epithelial barrier.
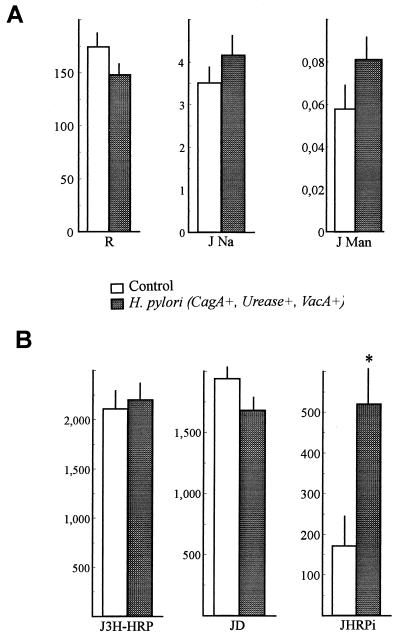
The results obtained with two cagA(+) strains of H. pylori were analyzed together. Forty filters with H. pylori-infected cell monolayers (20 filters for each strain) and 40 filters with control, noninfected cells were studied. Infection with H. pylori did not induce modifications of the electrical parameters compared to the control monolayers (PD = 0.94 ± 0.08 versus 1.1 ± 0.1 mV and Isc = 6.4 ± 0.4 versus 7.4 ± 0.6 μA/cm2, respectively). As shown in Fig. 1A, the parameters reflecting paracellular permeability, namely, R (174 ± 16 versus 148 ± 10 Ω/cm2), JNa (3.51 ± 0.41 versus 4.16 ± 0.44 μEq/h · cm2), and JMan (0.058 ± 0.009 versus 0.081 ± 0.010 μmol/h · cm2) for control versus H. pylori-infected monolayers, respectively, were not modified by the bacteria. These results indicate that the tight junctions and epithelial integrity are not altered by H. pylori.
FIG. 1.
Effect of infection of HT29-19A epithelial cell monolayers with H. pylori cagA(+) strains on epithelial barrier function in an Ussing chamber. (A) Parameters reflecting paracellular permeability. No significant difference between the H. pylori-treated and control cells was found, indicating that H. pylori does not alter the paracellular permeability of the epithelium. (B) Parameters reflecting the transcellular transport of HRP by transcytosis. ∗, Significantly different from control value (P < 0.04). Results are expressed (for units of measure, see Table 1) as means ± SE of control (n = 20) or H. pylori-infected (n = 20) cell monolayers.
Figure 1B shows the transport and processing of HRP across the HT29-19A cell monolayers. J3H-HRP was not modified by H. pylori (2,110 ± 210 versus 2,201 ± 255 ng/h · cm2 for control versus H. pylori infection condition, respectively).
As HRP is processed during transepithelial transport, the 3H-HRP flux is composed of intact and degraded HRP. Intact HRP fluxes across the H. pylori-infected cells were significantly greater (520 ± 146 ng/h · cm2) than for the control cells (172 ± 88 ng/h · cm2, P < 0.04). In contrast, degraded HRP fluxes were not significantly modified by the bacteria, although a small decrease was observed (1,681 ± 155 versus 1,938 ± 160 ng/h · cm2 for infected versus control cells, respectively).
(iii) Effect of cagA(−) strains of H. pylori on the epithelial barrier.
Like the cagA(+) strains, the cagA(−) strain did not induce modifications of the parameters reflecting the paracellular permeability pathway, i.e., R, JNa, and JMan. As for the cagA(+) strains, infection with the cagA(−) strain of H. pylori increased the flux of intact HRP (P < 0.04), without modifying the flux of total or degraded HRP (Table 1).
TABLE 1.
Effect of a cagA(−) strain of H. pylori on the epithelial function of HT29-19A cell measured in vitro in an Ussing chamber
| Cells (no. of filter-grown HT29-19A cell mono-layers analyzed) | Mean ± SE
|
|||||
|---|---|---|---|---|---|---|
| R (Ω/cm2) | JNa (μEq/h · cm2) | JMan (μmol/h · cm2) | J3H-HRP (ng/h · cm2) | JD (ng/h · cm2) | JHRPi (ng/h · cm2) | |
| Control (20) | 127 ± 9 | 3.8 ± 0.3 | 0.06 ± 0.0 | 1,004 ± 124 | 699 ± 124 | 153 ± 38 |
| H. pylori (20) | 160 ± 11 | 3.2 ± 0.2 | 0.05 ± 0.0 | 893 ± 110 | 740 ± 103 | 305 ± 71a |
Significantly higher than control value (P < 0.04).
HPLC analysis of HRP processing during transepithelial transport: effect of a cagA(+) strain of H. pylori.
HPLC analysis of basal compartments of Ussing chambers after 3H-HRP transport across the epithelial layer was performed to assess the nature of the 3H-metabolites formed during 3H-HRP transport.
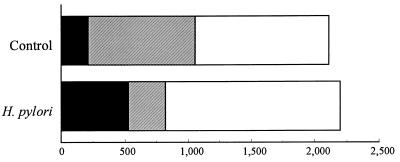
Transport studies using Ussing chambers have shown that infection of the epithelial layers with H. pylori increases 2.4-fold the amount of HRP transported in intact form (24% versus 10% of intact HRP) compared to controls. As shown in Fig. 2, HPLC analysis demonstrated that the increase of the passage of intact HRP was mainly due to the decreased degradation of this protein into peptides in the enterocyte (13% versus 40% of peptides), whereas the total amount of HRP transported remained unchanged.
FIG. 2.
Effect of infection of HT29-19A epithelial cell monolayers with a cagA-positive, urease-positive, VacA-positive H. pylori strain on HRP transcytosis and processing. An increase in HRP transport in intact form, representing 10 and 25% of the total transport in control and H. pylori infection conditions, respectively, is observed. ▪, intact HRP; ▨, peptides; □, amino acids.
Effect of the bacterial extract and the VacA cytotoxin-containing broth culture supernatant of a cagA(+) H. pylori strain on the epithelial barrier.
Table 2 indicates that the bacterial extract of a cagA(+) strain of H. pylori did not induce significant changes in electrical parameters of the epithelial monolayers. The fluxes of Na+ and mannitol across the monolayers treated with bacterial extract (n = 12) were not significantly different from those across the control monolayers (n = 12). Similarly, the fluxes of total, intact, and degraded HRP across the epithelium were not modified by the bacterial extract.
TABLE 2.
Effects of bacterial extract (supernatant of sonicate) and VacA cytotoxin-containing broth culture supernatant of a cagA(+) H. pylori strain on HT29-19A epithelial function in an Ussing chamber
| Prepn (no. of filters examined) | Mean ± SE
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Isc (μA/cm2) | PD (mV) | R (Ω/cm2) | JNa (μEq/h · cm2) | JMan (μmol/h · cm2) | J3H-HRP (ng/h · cm2) | JD (ng/h · cm2) | JHRPi (ng/h · cm2) | |
| Control (12) | 3.8 ± 0.7 | 0.4 ± 0.1 | 114 ± 12 | 5.1 ± 0.3 | 0.04 ± 0.00 | 1,947 ± 131 | 1,878 ± 137 | 78 ± 22 |
| Bacterial extract (12) | 4.9 ± 1.1 | 0.4 ± 0.0 | 104 ± 6 | 5.5 ± 0.3 | 0.04 ± 0.00 | 1,993 ± 162 | 1,928 ± 162 | 65 ± 4 |
| Control (21) | 2.0 ± 0.4 | 0.3 ± 0.1 | 143 ± 18 | 3.5 ± 0.3 | 0.04 ± 0.00 | 2,117 ± 139 | 2,050 ± 130 | 68 ± 12 |
| VacA supernatant (21) | 6.2 ± 0.4a | 1.1 ± 0.1a | 184 ± 13 | 2.6 ± 0.2 | 0.04 ± 0.00 | 2,031 ± 181 | 1,963 ± 181 | 68 ± 11 |
Significantly different from control monolayer value (P < 0.0001).
Table 2 also shows that the broth culture supernatant containing the VacA cytotoxin modified neither the integrity of the epithelial barrier, as evidenced by the R, JNa, and JMan values, which were not different from those of the controls, nor the macromolecular transport of HRP across the monolayers. These results suggest that in our model, the VacA cytotoxin-containing supernatant was probably not responsible for the effect observed.
It is noticeable that the Isc and PD values were significantly higher in epithelial cells that had been treated with the broth culture supernatant than in the nontreated cells.
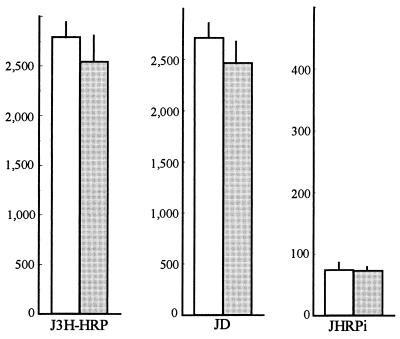
Effect of a urease-negative H. pylori mutant on the epithelial barrier.
To examine the importance of the presence of urease in the increase in intact HRP transport induced by H. pylori, we tested the effect of a urease-negative strain. This mutant did not induce any increase in intact JHRPi (Fig. 3), which strongly suggests that the urease activity of H. pylori is required for the observed effect on HRP transport and processing across the intestinal epithelial layer.
FIG. 3.
Effect of a urease-negative mutant of H. pylori ( ) on HRP transport and processing across HT29-19A epithelial cell monolayers (n = 12). □, control.
) on HRP transport and processing across HT29-19A epithelial cell monolayers (n = 12). □, control.
Effects of bafilomycin A1 and ammonium chloride on the epithelial barrier.
Bafilomycin A1 significantly increased (P < 0.04) the fluxes of intact HRP across the HT29-19A epithelial cells without changing the total fluxes of HRP (Table 3). The parameters reflecting the paracellular permeability were not modified by bafilomycin: R = 178 ± 15 versus 174 ± 13 Ω/cm2; JNa = 2.9 ± 0.2 versus 2.9 ± 0.2 μEq/h · cm2, and JMan = 0.03 ± 0.00 versus 0.04 ± 0.00 μmol/h · cm2 for control versus bafilomycin-treated cells, respectively.
TABLE 3.
Effects of bafilomycin A1 and ammonium chloride on HRP transport across HT29-19A epithelial cells in an Ussing chamber
| Treatment (no. of filters used) | Mean flux (ng/h · cm2) ± SE
|
||
|---|---|---|---|
| J3H-HRP | JHRPi | JD | |
| Control | 2,999 ± 232 | 61.7 ± 11.6 | 2,937 ± 234 |
| 0.5 μM bafilomycin A1 (16) | 2,660 ± 176 | 137.0 ± 34.4a | 2,523 ± 182 |
| 20 mM ammonium chloride (13) | 3,423 ± 395 | 116 ± 21a | 3,307 ± 396 |
Significantly different from control value (P < 0.04).
Treatment of the epithelial cells with ammonium chloride gave results similar to those seen after treatment with bafilomycin, namely, an increase of intact HRP fluxes across the cell monolayer, without significant modification of the total and degraded HRP fluxes (Table 3). Paracellular permeability was not altered by ammonium, as evidenced by the finding that the values of R, JNa, and JMan were not significantly different from those for the control, untreated cells (R = 178 ± 15 versus 140±9 Ω/cm2, JNa = 2.9 ± 0.2 versus 3.2 ± 0.2 μEq/h · cm2, and JMan = 0.03±0.00 versus 0.04 ± 0.00 μmol/h · cm2 for control versus ammonium-treated cells, respectively).
DISCUSSION
The results presented above indicate that infection of the digestive tract epithelium with H. pylori alters the epithelial barrier capacity to macromolecules by inhibiting the degradative process leading to the hydrolysis of exogenous luminal antigens during transcytosis. They further suggest that the presence of urease may be responsible for this effect, probably by increasing the endolysosomal pH.
Since gastric polarized epithelial cell lines do not exist, we used an intestinal epithelial cell line as a model for studying the gastric epithelial barrier function. Although it is not an ideal model, it has many advantages. It is an easily available and well-established model of the digestive tract epithelium constituted of polarized cells developing tight junctions which are required for studying the function of the epithelial barrier. This model has been widely used in studies on the absorption of different permeability markers across the epithelial barrier (21, 23, 28), allowing comparison of the results obtained. Moreover, H. pylori has been shown to adhere easily to different intestinal cell lines (8, 12, 35), and this model has been found to be useful in different physiopathological studies (5, 31, 33, 40). However, the intestinal epithelial cell model does not reproduce all properties of the gastric epithelium. Particularly, HT-29 intestinal cells do not produce mucus whereas the gastric epithelium is covered by a mucus layer. Despite these drawbacks, we believe that this model is suitable for studies of the effect of H. pylori on epithelial barrier function as the initial model, preceding the in vivo studies.
The stomach is traditionally considered an organ of primarily secretory function. However, it also represents a continuous barrier to the exogenous antigens, such as food antigens, which circulate in large quantity in the digestive tract. It has been shown that in the rat, the small intestine epithelium, like the gastric epithelium, is able to take up, degrade, and transport small amounts of macromolecules (6, 9). Here we show that H. pylori alters such macromolecular transport and processing. The alteration of the epithelial barrier function by H. pylori, by increasing the load of intact proteins entering the digestive mucosa, could be a starting point for a sensitization process which may lead ultimately to the development of allergy.
There are two major pathways of transepithelial transport in the digestive tract, paracellular and transcellular (22, 24). In physiological conditions, epithelial cells are closely connected with each other by tight intercellular junctions, and they form a confluent monolayer. The paracellular pathway corresponds to passage through the narrow intercellular spaces and tight junctions, mainly permeable to small molecules. Different factors, including toxins and bacteria, which alter tight junctions may decrease the integrity of the epithelial layer. In vitro, the epithelial barrier function can be assessed by the fluxes of small molecules (mannitol) and ions (Na+) and by modifications of electrical resistance, considered a good index of paracellular permeability in tight epithelia (27). Exogenous proteins that have escaped luminal hydrolysis can then pass the epithelial barrier via a transcellular pathway, using transcytosis. During this passage, in physiological conditions, the majority (90%) of the protein is degraded in the lysosomal system and only a small portion (10%) passes in intact form (22, 23). When the epithelium is damaged, the passage of intact macromolecules may increase, due either to leakage through the intercellular junctions or to an increase of transcellular passage (transcytosis). In this study, the second mechanism is activated and multiple factors may play a role in the effect observed. This effect is not related to the cag status of the strain but seems to depend on the presence of the whole bacteria since it is not seen when bacterial extract is used instead of whole bacteria. Therefore, it cannot be excluded that bacterial adherence to the epithelial cells is necessary to induce increased transport of the intact HRP by these cells. In fact, it has been demonstrated that adherence of H. pylori to Caco-2 cells induces reorganization of intracellular actin in vitro (40), although the subsequent effect on intracellular macromolecular traffic has not been studied.
It is important to note that paracellular transport is not modified by H. pylori since the parameters reflecting the integrity of the epithelial layer (R, JNa, and JMan) are the same as for controls, indicating that tight junctions are not altered by H. pylori. Consequently, the increased passage of intact HRP across the epithelial layer must follow a transcellular pathway. This does not exclude the possibility of an alteration of the tight junctions in the presence of bacteria taking place in vivo, due to the action of mediators of inflammation released in the digestive mucosa in response to bacterial colonization (28). In our study, we used a single epithelial monolayer, without immune cells of the mucosa, which indicates that the effect observed could be one of the direct effects of the bacteria on epithelial cell function.
It is conceivable that some bacterial products released into the extracellular space can stimulate the endocytic process in epithelial cells. For example, one of the earliest effects of Clostridium difficile toxins is to increase the rate of transcytosis of HRP in mouse ceca mounted in Ussing chambers (19). Similarly, in mice, Escherichia coli heat-stable and heat-labile enterotoxins increase the intestinal absorption of intact gliadin and β-lactoglobulin in vivo (43). Enteropathogenic strains of E. coli have been also shown to decrease the barrier to macromolecules, both in vitro (in T84 monolayers and rabbit ileum [26, 36]) and in vivo (in rabbits infected with strain RDEC-1 [17]). However, an increase in transepithelial fluxes of protein in both intact and degraded forms was observed, suggesting an effect on both paracellular and transcellular permeability. In this study, it was rather the intracellular processing of the protein that was modified by H. pylori infection. The increased transport of intact protein could be related to the presence of cytoplasmic vacuoles, observed in cell lines infected with VacA cytotoxin-producing strains of H. pylori. Indeed, a recent study indicated that the vacuolating cytotoxin induces a partial neutralization of the lysosomes, mistargeting cathepsins migration and decreasing the activity of lysosomal proteases (39). However, this mechanism is unlikely in our model since (i) all three strains studied had the same effect on epithelial permeability, but it had been confirmed by PCR that one of them had a genotype that does not produce cytotoxin, and (ii) broth culture supernatant of the VacA-positive H. pylori strain gave negative results. It should be emphasized, however, that the VacA cytotoxin of H. pylori has been shown to be active only at low pH (10), while in our model the pH of the medium was neutral. On the other hand, it is interesting that VacA cytotoxin-containing broth culture supernatant of H. pylori induced an increase of transepithelial PD and Isc of the epithelial layer. The increased Isc might reflect the increased secretion of Cl− ions by the monolayer, although measurements of Cl− transport would be necessary to confirm this. The factor responsible for this phenomenon has not been identified, but it is conceivable that some component of the enterotoxin-like properties is involved in this action. In fact, it has been demonstrated that stimulation of Cl− secretion of the epithelial cell layer by the diarrhea-inducing enterotoxins (i.e., heat-stable enterotoxin of E. coli) is accompanied by an increased electrogenic activity of this layer reflected by the increased Isc (25).
It is most likely that the increase in intact protein absorption is linked to the capacity of H. pylori to secrete a large amount of urease (up to 10% of total proteins secreted by the organism). Indeed, it is known that protein degradation is inhibited by an increase of the endolysosomal pH, which inhibits cathepsin activity, normally requiring a low acidic pH. An increase in lysosomal pH is possible in the course of infection with H. pylori, since the release of urease in the vicinity of the enterocyte membrane leads to the production of ammonia, a weak base well known to concentrate within acidic organelles and to inhibit acid proteases (41). In fact, this hypothesis is strongly supported by the observation that the urease-negative mutant of H. pylori does not induce modification of the transepithelial transport of intact HRP. Moreover, treatment of cells with ammonia and with bafilomycin A1, two agents known to decrease the endolysosomal pH (41, 45), led to the increased transport of intact HRP. However, the urease-containing bacterial extract did not induce any modifications of macromolecular transport. It seems, therefore, that whereas the urease continuously produced by living bacteria exerts an effect on protein degradation, urease added to the culture medium does not. It is possible that the ultimate intracellular urease concentration is determinative. This concentration may be higher when living bacteria are producing urease near the cells than when urease-containing bacterial extract is added to cell culture medium (because of degradation of enzyme and/or weaker access to the epithelial cells).
If H. pylori modifies the passage of other proteins (for example, H. pylori antigens) through the epithelium, this could contribute to the development of inflammation. Indeed, H. pylori antigens have been found in the deeper layer of the gastric mucosa (29), probably contributing to the recruitment of inflammatory cells, which, in turn, via the cytokines produced, can also contribute to an increase of transmucosal permeability. Such an increase in gastric permeability in vivo during H. pylori infection could explain the persistence of gastric inflammation, even after eradication of bacteria, due to the sustained stimulation of the local immune system by the bystander antigens. This antigenic stimulation could also contribute to the development of allergic conditions more frequently in H. pylori-infected patients than in noninfected control subjects. Indeed, the greater susceptibility to the development of food allergy of H. pylori-infected subjects than of H. pylori-negative controls was suggested. In fact, it has been demonstrated in a case control study including 38 adult patients with symptomatic food allergy and 53 age-matched controls (including 31 patients with respiratory allergy) that food allergy, but not respiratory allergy, is associated with a significantly higher rate of infection with CagA-positive H. pylori strains (14). In the same way, it has been proposed that microbial infections of the intestine, during which increased antigen permeability is observed (20), could trigger allergic sensitization. There is some evidence for an implication of H. pylori infection in atopic dermatitis (34). Furthermore, the features of an atopic condition have been observed in several patients with chronic gastritis and peptic ulceration (38). The histological picture of H. pylori-associated gastritis is quite consistent with that observed in allergic reactions. Indeed, biopsy specimens containing the bacterium are characterized by the presence of a neutrophilic infiltrate and eosinophil leukocytes (2, 30). Moreover, immunoglobulin E (IgE)-mediated reactions in patients with erosive gastritis and peptic ulcer have been demonstrated (3). In addition, IgE directed against cockroach antigens has been detected in serum and gastric mucosa samples of patients with peptic ulcer and chronic gastritis (38). The ability of H. pylori to induce a specific IgE immune response has been demonstrated in H. pylori-infected patients (1). Finally, a significant association between positivity for anti-cow milk protein and anti-H. pylori antibodies in diabetic subjects was found (37).
Taken together, our results suggest that H. pylori, by interfering with the intracellular metabolism of protein in epithelial cells, can modify the immune response of the host to bystander luminal antigens.
ACKNOWLEDGMENTS
This study was partly supported by a grant from Sanofi Winthrop, Gentilly, France.
We thank Natacha Patey for performing the histopathological analysis of cell monolayers, Marie-France Renard for performing the LDH and lactic acid assays, Béatrice Vigneron and Evelyne Seyler for excellent assistance in bacteriological examinations, and Marie-Agnès Blaton and Céline Candalh for valuable daily technical assistance.
REFERENCES
- 1.Aceti A, Celestino D, Caferro M, Casale V, Citarda F, Conti E, Grassi A, Grilli A, Pennica A, Sciarretta F, Leri O, Ameglio F, Sebastiani A. Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology. 1991;101:131–137. doi: 10.1016/0016-5085(91)90469-2. [DOI] [PubMed] [Google Scholar]
- 2.Andersen L, Holck S, Povlsen C, Elseborg L, Justesen T. Campylobacter pyloridis in peptic ulcer disease. I. Gastric and duodenal infection caused by C. pyloridis: histopathologic and microbiologic findings. Scand J Gastroenterol. 1987;22:219–224. doi: 10.3109/00365528708991883. [DOI] [PubMed] [Google Scholar]
- 3.Andrè C, Moulinier B, Andrè F, Danière S. Evidence for anaphylactic reaction in peptic ulcer and varioliform gastritis. Ann Allergy. 1983;51:325–331. [PubMed] [Google Scholar]
- 4.Augeron C, Laboisse C L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–3969. [PubMed] [Google Scholar]
- 5.Brännström J, Zachrisson K, Hultén K, Engstrand L, Uribe A. Helicobacter pylori stimulates DNA synthesis in a small intestinal cell line in vitro. Digestion. 1998;59:33–39. doi: 10.1159/000007464. [DOI] [PubMed] [Google Scholar]
- 6.Catto-Smith A G, Patrick M K, Scott R B, Davison J S, Gall D G. Gastric response to mucosal IgE-mediated reactions. Am J Physiol. 1989;257:G704–G708. doi: 10.1152/ajpgi.1989.257.5.G704. [DOI] [PubMed] [Google Scholar]
- 7.Cayla R, Carles B, de Mascarel A, Zerbib F, Lamouliatte H. Long term follow-up of chronic gastritis after persistent Helicobacter pylori eradication in duodenal ulcer patients. Gastroenterology. 1995;108:A68. doi: 10.1097/00042737-200012070-00001. . (Abstract.) [DOI] [PubMed] [Google Scholar]
- 8.Corthésy-Theulaz I, Porta N, Pringault E, Racine L, Bogdanova A, Kraehenbuhl J, Blum A L, Michetti P, Philipott D. Adhesion of Helicobacter pylori to polarized T84 human intestinal cell monolayers is pH dependent. Infect Immun. 1996;64:3827–3832. doi: 10.1128/iai.64.9.3827-3832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis G H, Gall D G. Macromolecular transport by rat gastric mucosa. Am J Physiol. 1992;262:G1033–G1040. doi: 10.1152/ajpgi.1992.262.6.G1033. [DOI] [PubMed] [Google Scholar]
- 10.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 11.Dixon M. Helicobacter pylori and chronic gastritis. In: Rathbone B, Heatley R, editors. Helicobacter pylori and gastroduodenal disease. London, England: Blackwell Scientific Publications; 1992. pp. 124–139. [Google Scholar]
- 12.Evans D G, Evans D J, Jr, Graham D Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 13.Figura N. Culture of Helicobacter pylori in broth, determination of vacuolising activity of bacterial broth cultures and neutralisation of the vacuolising activity. In: Lee A, Mégraud F, editors. Helicobacter pylori: techniques for clinical diagnosis & basic research. W. B. London, England: Saunders Company Ltd.; 1996. pp. 224–234. [Google Scholar]
- 14.Figura N, Perrone A, Gennari C, Vagliasindi M, Rottoli P. Food allergy and H. pylori infection. Gut. 1996;39:A93. . (Abstract.) [PubMed] [Google Scholar]
- 15.Forman D, Webb P. Helicobacter pylori and gastric cancer: the significance of the problem. In: Hunt R, Tytgat G, editors. Helicobacter pylori: basic research to clinical cure. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 461–468. [Google Scholar]
- 16.Genta R, Lew G, Graham D. Changes in the gastric mucosa following eradication of Helicobacter pylori. Mod Pathol. 1993;6:281–289. [PubMed] [Google Scholar]
- 17.Gotteland M, Isolauri E, Heyman M, Tome D, Desjeux J F. Antigen absorption in bacterial diarrhea: in vivo intestinal transport of beta-lactoglobulin in rabbits infected with the entero-adherent Escherichia coli strain RDEC-1. Pediatr Res. 1989;26:237–240. doi: 10.1203/00006450-198909000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Graham D Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- 19.Heyman M, Corthier G, Lucas F, Meslin J C, Desjeux J F. Evolution of the caecal epithelial barrier during Clostridium difficile infection in the mouse. Gut. 1989;30:1087–1093. doi: 10.1136/gut.30.8.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyman M, Corthier G, Petit A, Meslin J C, Moreau C, Desjeux J F. Intestinal absorption of macromolecules during viral enteritis: an experimental study on rotavirus-infected conventional and germ-free mice. Pediatr Res. 1987;22:72–78. doi: 10.1203/00006450-198707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyman M, Darmon N, Dupont C, Dugas B, Hirribaren A, Blaton M A, Desjeux J F. Mononuclear cells from infants allergic to cow’s milk secrete tumor necrosis factor alpha, altering intestinal function. Gastroenterology. 1994;106:1514–1523. doi: 10.1016/0016-5085(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 22.Heyman M, Desjeux J F. Significance of intestinal food protein transport. J Pediatr Gastroenterol Nutr. 1992;15:48–57. [PubMed] [Google Scholar]
- 23.Heyman M, Desjeux J. Antigen handling by intestinal epithelial cells. In: Kaiserlian D, editor. Antigen presentation by intestinal epithelial cells. R. G. Austin, Tex: Landes Company; 1996. pp. 1–20. [Google Scholar]
- 24.Heyman M, Ducroc R, Desjeux J F, Morgat J L. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am J Physiol. 1982;242:G558–G564. doi: 10.1152/ajpgi.1982.242.6.G558. [DOI] [PubMed] [Google Scholar]
- 25.Huott P, Liu W, McRoberts J, Giannella R, Dharmsathaphorn K. Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Investig. 1998;82:514–523. doi: 10.1172/JCI113626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isolauri E, Gotteland M, Heyman M, Pochart P, Desjeux J F. Antigen absorption in rabbit bacterial diarrhea (RDEC-1). In vitro modifications in ileum and Peyer’s patches. Dig Dis Sci. 1990;35:360–366. doi: 10.1007/BF01537415. [DOI] [PubMed] [Google Scholar]
- 27.Madara J L. Loosening tight junctions. Lessons from the intestine. J Clin Investig. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madara J L, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Investig. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mai U E, Perez-Perez G I, Allen J B, Wahl S M, Blaser M J, Smith P D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGovern, T., G. Kephart, G. Gleich, and N. Talley. 1989. Eosinophil infiltration and degranulation in Campylobacter pylori gastritis. Klin. Wochenschr. 67(Suppl. 18):46.
- 31.Megraud F, Neman-Simha V, Brugmann D. Further evidence of the toxic effect of ammonia produced by Helicobacter pylori urease on human epithelial cells. Infect Immun. 1992;60:1858–1863. doi: 10.1128/iai.60.5.1858-1863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mégraud F, Lamouliatte H. Helicobacter pylori and duodenal ulcer. Evidence suggesting causation. Dig Dis Sci. 1992;37:769–772. doi: 10.1007/BF01296437. [DOI] [PubMed] [Google Scholar]
- 33.Micots I, Augeron C, Laboisse C L, Muzeau F, Mégraud F. Mucin exocytosis: a major target for Helicobacter pylori. J Clin Pathol. 1993;46:241–245. doi: 10.1136/jcp.46.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami K, Fujioka T, Nishizono A, Nagai J, Tokieda M, Kodama R, Kubota T, Nasu M. Atopic dermatitis successfully treated by eradication of Helicobacter pylori. J Gastroenterol. 1996;31:77–82. [PubMed] [Google Scholar]
- 35.Neman-Simha V, Mégraud F, Corthésy-Theulaz I. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988;56:3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 37.Pocecco M, Buratti E, Tommasini A, Torre G, Not T. High risk of Helicobacter pylori infection associated with cow’s milk antibodies in young diabetics. Acta Pediatr. 1997;86:700–703. doi: 10.1111/j.1651-2227.1997.tb08571.x. [DOI] [PubMed] [Google Scholar]
- 38.Romanski B, Bartuzi Z, Zbikowska-Gotz M, Staszynska M, Korenkiewicz J. Allergy to cockroach antigens in patients with peptic ulcers and chronic gastritis. Allergol Immunopathol. 1988;16:219–224. [PubMed] [Google Scholar]
- 39.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 40.Segal E D, Shon J, Tompkins L S. Characterization of Helicobacter pylori urease mutants. Infect Immun. 1992;60:1883–1889. doi: 10.1128/iai.60.5.1883-1889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seglen P O, Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976;100:276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- 42.Terpend K, Boisgerault F, Blaton M, Desjeux J, Heyman M. Protein transport and processing by human HT29-19A intestinal cells: effect of IFN gamma. Gut. 1998;42:538–545. doi: 10.1136/gut.42.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma M, Majumdar S, Ganguly N K, Walia B N. Effect of Escherichia coli enterotoxins on macromolecular absorption. Gut. 1994;35:1613–1616. doi: 10.1136/gut.35.11.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witteman E, Mravunac M, Becx M, Hopman W, Verschoor J, Tytgat G, de Koning R. Improvement of gastric inflammation and resolution of epithelial damage one year after eradication of Helicobacter pylori. J Clin Pathol. 1995;48:250–256. doi: 10.1136/jcp.48.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]