Abstract
Low-dose radiotherapy (LDRT), defined in this study as 2 fractions of 4 Gy delivered on consecutive days, is an effective option for local palliation of mycosis fungoides (MF), but its efficacy for tumoral lesions (TL) needs investigation. We assessed response and local control (LC) rates for patients treated with LDRT for MF and compared these outcomes between TL and non-TL. A total of 73 lesions in 18 patients treated with LDRT between 2013–2020 were analyzed. Response was defined as complete response (CR), partial response (PR), or no response (NR). In the non-TL versus TL groups, CR was observed in 16.7% v. 4.0%, PR in 81.2% v. 80.0%, NR in 2.1% v. 16.0%, respectively. 2-year LC was 100% for non-TL and 61% for TLs (p < 0.01). LDRT yields excellent response and lesion control for non-TLs and is associated with lower response rates and LC for TLs.
Keywords: Cutaneous t-cell lymphoma, dermatopathology, oncology, radiation therapy, tumor
1. Introduction
Cutaneous lymphomas are a diverse group of non-Hodgkin lymphomas arising from the cutaneous tissues and is comprised of cutaneous T-cell lymphoma (CTCL) and cutaneous B-cell lymphoma. CTCL, which makes up approximately 4% of all non-Hodgkin lymphomas, has as many as 12 subtypes that have been identified [1,2]. This is a relatively rare cancer because, as a class, non-Hodgkin lymphoma accounts for about 4% of the total cancer burden in the United States [3]. The most common subtype of CTCL is mycosis fungoides (MF) [2]. Patients between the ages of 50 and 60 years are the demographic most likely to be affected, but this disease can rarely be found in children as well [4]. CTCL has a higher occurrence in males in comparison to females, African Americans are more likely to be affected than Caucasians and Asians, and males are more likely to present with tumoral disease than females [5]. The aetiology of these cancers is uncertain, but it has been hypothesized that this disease may be caused by genetic factors, chemical exposures, human T-lymphotropic virus (Type 1), and immunologic phenomena [6].
There is a varying degree of disease burden among CTCL patients of the MF subtype. As the disease progresses, patients may progress to a tumoral stage of disease and develop a mass rather than cutaneous patches and/or plaques [7,8]. These tumoral lesions (TLs) can lead to disfigurement that can be much more impactful to the patient in comparison to non-tumoral lesions (non-TLs). In 20–55% of cases of MF, the lymphocytes involved may develop genetic changes that make them begin to exhibit a large cell phenotype known as large cell transformation (LCT). LCT is associated with a poor prognosis, which is associated with higher rates of relapse and tumoral stage disease [9]. In addition to this, LCT has a low survivability with a mean 5-year survival rate of less than 20% [9]. Patients with TL disease are more likely to present with LCT, which can alter the treatment course [10].
CTCL is often managed with a combination of therapies including phototherapy (with or without psoralen-based agents), topical therapy, steroids, radiation therapy (RT), and/or systemic agents including but not limited to cytotoxic chemotherapy [6]. While some of the available treatments can cure local disease presenting as a single lesion, CTCL is considered incurable due to the recurrent nature of the disease. When patients with single lesions of MF that are non-tumoral are treated with curative intent RT, complete response (CR) rates can be as high as 100% with no recurrences at treated sites of localize disease [11]. Historically, MF was treated with higher doses of RT (above 30 Gy) with excellent local control, primarily in patients with limited extent of skin involvement or single lesions [12, 13]. However, a prior study of low-dose RT (LDRT) showed a high response rate for CTCL, which has led to LDRT becoming the standard palliative treatment [14]. LDRT is often delivered using either single-fraction or two-fraction regimens [14–16]. Superficial electron beams are most commonly used, but for thicker or deeply invasive tumors, the use of photons may allow for treatment of deeper targets [17]. For patients with extensive cutaneous disease or multi-organ disease (lymph node, bone marrow, liver, spleen, etc.), the use of RT is limited to local palliation of disease in strategic areas. Patients receiving RT for MF may experience acute effects such as itching, erythema, pigmentation changes, and/or radiation dermatitis, but toxicity is very rare using LDRT [14–16].
When considering the treatment dosage of radiotherapy, the presence of TL disease may require the use of higher doses of radiation therapy [17]. Despite the fact that LDRT is an effective palliative treatment for most forms of MF, prior retrospective analyses identified tumoral stage to be associated with reduced response rates [18]. Treatment response and local control rates after LDRT for tumoral MF have not been explored thoroughly in literature. In this analysis, we sought to inform outcomes of LDRT for mycosis fungoides, focusing on outcomes for patients with TL versus non-TL.
2. Materials and methods
In this Institutional Review Board-approved retrospective review, after waiver of informed consent, we identified patients treated with LDRT for mycosis fungoides between 2013–2020 at our institution through query of the radiation oncology electronic medical record. Inclusion criteria was clinical or pathologic diagnosis of cutaneous lymphoma that were treated with RT. Patients with other cutaneous lymphomas (i.e. cutaneous B-cell lymphoma) were excluded, as were patients with lesions treated to RT doses higher than 8 Gy and those without follow-up data. Within the study period, only 2 patients were identified as having been treated with higher doses of RT that were excluded for this reason. One patient received 24 Gy in 12 fractions to one lesion, and one patient received 30 Gy in 15 fractions to 4 lesions.
LDRT was defined as 2 fractions of 4 Gy delivered on two consecutive days using either superficial electron beam or photon beam therapy, with bolus to ensure adequate coverage of the cutaneous disease. Patient health and demographic factors, disease-related factors, workup, stage of disease, treatment details and the location of each lesion recorded. The outcomes of interest included response, strength of response, durability of response, occurrence of local failure, local control and patient survival. Response to LDRT was determined based on the first follow-up visit after documentation in the medical record, including patient notes, images, radiographic imaging, and pathologic information where applicable. Response was defined according to Neelis et al.: complete response (CR), 100% reduction/disappearance of skin lesions and symptoms involved; partial response (PR), >50% but <100% reduction; no response (NR), <50% reduction. Overall response (OR) was a composite outcome of CR/PR versus NR. Local control (LC) was defined for each individual lesion treated as the duration of time from the end of LDRT to recurrence at that site or last follow-up (right censor) [18].
Descriptive analyses were performed using count (frequency) and median (interquartile range) and compared between groups using the Fisher’s exact or chi square test and t-test or Kruskal–Wallis test as appropriate for categorical and continuous variables, respectively. LC was estimated using the Kaplan-Meier method; local failure at the treated site was the event of interest with right-censoring at the date of last follow-up. LC was compared between groups using the log-rank test. Statistics were performed using R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
In total, 73 lesions in 18 patients were treated with LDRT using superficial electrons (90%) or photons (10%) of beam energies of 6–12 MeV or 6–10 MV, respectively. Mean age was 63 (±14) and 85% of the patients identified as male. The most common self-identified race was Caucasian (64%), followed by Black/African American (34%), and other (1%). Most common lesion sites were on the lower extremity and upper extremity (34 and 30% respectively). Median follow-up of individual lesions was 9.3 months (95% CI 1.2–68.3). Patient characteristics and response rates are summarized in Table 1.
Table 1.
Characteristics and outcomes of low-dose RT for CTCL.
| Characteristic or outcome | N (%) | |
|---|---|---|
|
| ||
| Total | 73 | |
| Age | ||
| Mean age ± SD | 63 ± 14 | |
| Range | 40–89 | |
| Gender | ||
| Female | 11 (15%) | |
| Male | 62 (85%) | |
| Race | ||
| Black or African American | 25 (34.2%) | |
| White or Caucasian | 47 (64.4%) | |
| other | 1 (1.4%) | |
| Lesion type | ||
| TL | 25 (34.2%) | |
| Non-TL | 48 (65.7%) | |
| Location of lesion | ||
| Head/neck | 12 (16%) | |
| Chest, abdomen, or trunk | 14 (19%) | |
| Upper extremity | 22 (30%) | |
| Lower extremity | 25 (34%) | |
| Large cell transformation? | ||
| Yes | 5 (7%) | |
| No | 66 (93%) | |
| Response | TL | Non-TL |
|
| ||
| CR | 1 (4%) | 8 (17%) |
| PR | 20 (80%) | 39 (81%) |
| NR | 4 (16%) | 1 (2%) |
| OR | 21 (84%) | 47 (98%) |
Of the 73 lesions, 25 were TL and 48 were non-TL. A greater proportion of B/AA race was observed in the TL group (52% TL v. 25% non-TL, p = 0.05). Five of the 73 lesions demonstrated histologic large cell transformation prior to LDRT. All patients with large cell transformations were in the TL group.
The response assessment visit at first follow-up appointment occurred at a median of 7 weeks. Among the 18 patients, there were 40 separate treatment courses (patients were treated with multiple treatment courses in some cases, and multiple sites could be treated within each synchronous treatment course): the worst response observed was CR in 3 courses, PR in 34 courses, and NR in 3 courses. OR was 93.2% overall and was significantly different between non-TL (97.9%) and TL (84%, p = 0.04). In the non-TL versus TL groups, CR was observed in 16.7% v. 4.0%, PR in 81.2% v. 80.0%, NR in 2.1% v. 16.0%, respectively. Overall, 6-, 12-, and 24-month LC was 94.9% (95% CI 89.3–100), 92.7% (86.0–99.9), and 85.8% (75.3–97.8) (Figure 1(A)). Local recurrence was noted in 6/25 TL and 0/48 non-TL. LC at 6, 12, and 24 months was 100% for non-TLs compared with 86.6% (95% CI 73.4–100), 80.8% (65.3–100), and 60.6% (38.5–95.4) for TLs (p < 0.01) (Figure 1(B)). In total, 2 of 18 patients experienced a local failure at one or more sites. Median time-to-progression in the 6 TL that failed was 5.5 months (95% CI 3.2–17.3).
Figure 1.
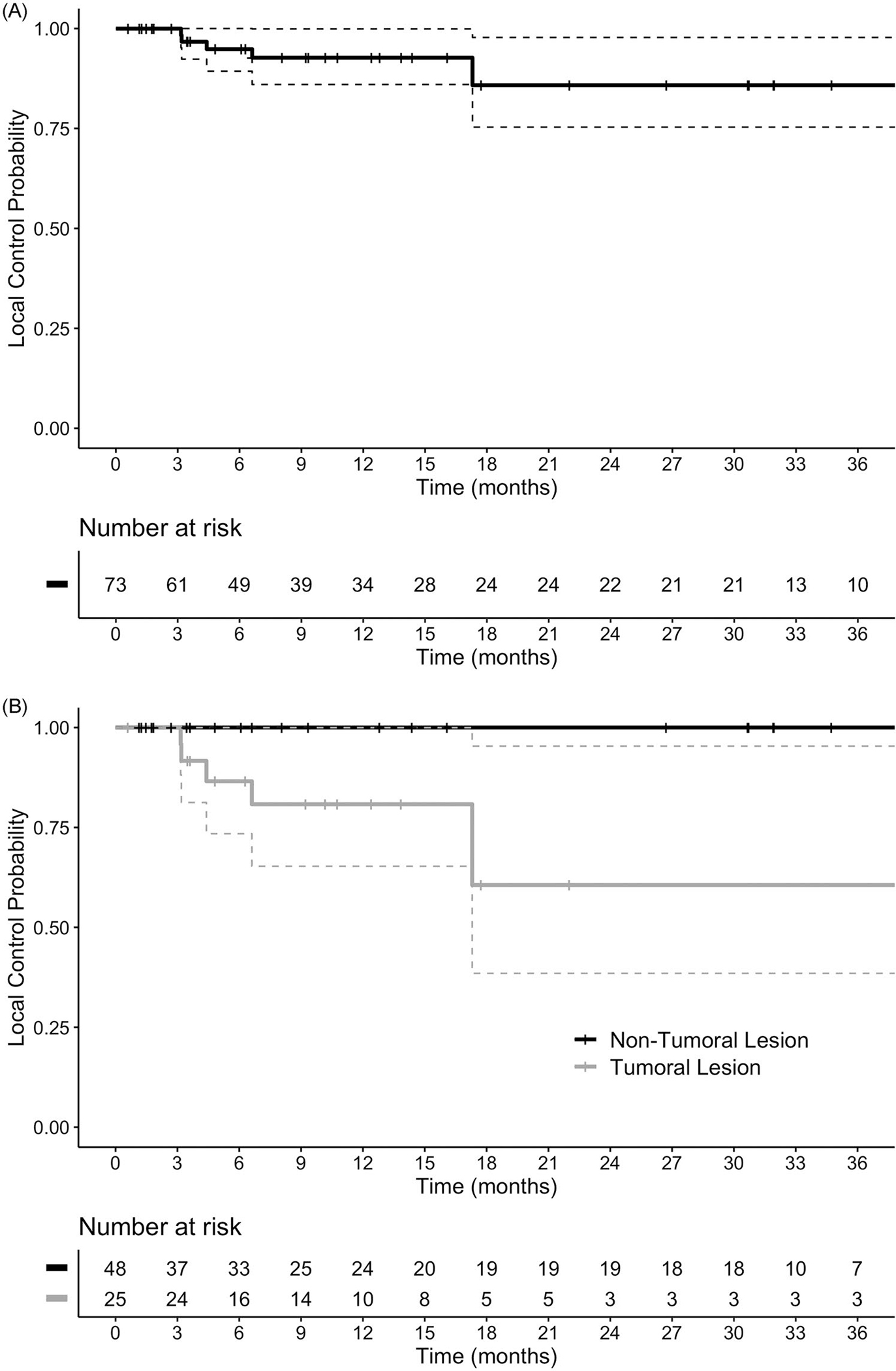
kaplan–meier plot of local control for all lesions (a), and for lesions stratified by tumoral status (B).
4. Discussion
In the literature, response rates for CTCL treated with radiation therapy have been excellent. (CITE). Previous case reports on LDRT for TLs have shown favorable results demonstrating both partial and complete resolution [19]. With evidence of a favorable treatment profile in these case studies, expanding this treatment to a larger population of patients suffering from this disease could be beneficial. Our results provided evidence that TLs treated with LDRT had a very favorable response rates with an OR in 84% of the lesions. This would indicate that there is a benefit of LDRT for use clinically in the palliation of TLs, which has the potential to help many patients who are suffering with this disease. However, only 4% of the TLs had a complete resolution of disease, and long-term local control was significantly worse than that observed in non-TLs. This suggests that a longer course may be reasonable to consider for TLs, particularly when the goal of therapy is long-term LC at that site. For the majority of patients that experience clinical response, the duration of response after LDRT described in this study is substantial. Two-year estimates in this analysis identified high rates of local control at the treated sites, suggesting this treatment with minimal expected morbidity provides patients with prolonged disease control at the treated site. For patients with problematic lesions where local control is closely intertwined with quality of life, LDRT represents an excellent local therapeutic option, particularly for non-TLs.
In all measured outcomes, non-TLs were more responsive to treatment than TLs with a significant difference of 14% for OR. This may infer that more aggressive measures may be necessary for TLs, considering their apparently more aggressive clinical course and resistance to treatment. A higher dose of RT is often considered for TL or for those with LCT, which often present as TLs. In the appropriate clinical context, such as TL with or without pathologic findings of large cell transformation, higher doses such as 24 Gy in 12 fractions may be more likely to lead to a more durable local control [17]. However, an OR rate of 84% with LDRT for TLs remains a very favorable outcome considering the minimal morbidity of treatment that has been previously reported with this regimen [14]. These findings are consistent with a prior study of patients treated with single-fraction radiotherapy of at least 7 Gy which demonstrated high response rates overall with decrements in response for patients with tumoral and LCT disease [16]. With a median time-to-local recurrence of 5.5 months, this regimen remains a reasonable option for palliation of TLs, particularly in patients who are either unable or unwilling to undergo a longer treatment course.
Another important finding in this study is the increased rates of TLs in B/AA patients. While trends toward younger age at diagnosis and more advanced disease at diagnosis have been reported, is unclear if this is related to biological factors or other factors [20]. Future studies should be conducted to establish if the greater proportion of B/AA individuals affect was due to a type 1 error or a true difference. If this is established, other factors should be considered such as time to treatment initiation, healthcare access, and patient-physician biases. In addition to this, it is important to consider increased radiation dosages as salvage therapy when disease recurs after treatment. Particularly in the case of more advanced disease at diagnosis, this may be an important consideration for salvage therapy.
Our retrospective study had some limitations. First, scheduling for follow-up appointments varied based on many factors and often occurred at non-standardized intervals. This decreased our ability to properly assess the axis of time for treatment response and recurrence. While we collected data on non-radiotherapeutic treatments, many of the patients had an incomplete profile regarding the treatments received prior to and during LDRT. There may have been some synergistic or opposing effects with the use of these other agents. In this retrospective analysis, toxicity data were lacking, and thus we cannot make firm conclusions with regard to toxicity profile of LDRT, though prior reports have shown virtually no toxicity with 1–2 fraction LDRT [14–16]. Considering the majority of the available literature on LDRT for MF is limited to retrospective studies, a multicenter prospective trial would be optimal to define response rates, rates of toxicity (though expectedly low), and evaluate for differences in response between various disease factors (e.g. patch and plaque disease, TL versus non-TL, large cell transformation). While this is logistically challenging and unfortunately difficult to achieve funding to support, it would provide the most standardized data possible to guide management decisions and patient counseling and should be pursued. Assessing skin-related quality of life with a validated patient-reported outcome measure would also provide valuable evidence to the utility of LDRT in this clinical scenario. Additionally, the lack of a comparator arm utilizing a more protracted RT course (>20 Gy) limits our ability to compare outcomes between low-dose and more conventional RT courses.
In this study, we identified excellent response rates and clinically meaningful local control at mycosis fungoides sites treated with LDRT. Tumoral lesions treated with LDRT exhibit significantly lower overall response and local control rates compared with non-tumoral lesions. Consideration of a higher RT dose for tumoral lesions might be reasonable, but if local palliation with a short course of treatment is clinically indicated, LDRT remains a reasonable approach. Further prospective study is needed to determine the interaction between RT dose, tumoral characteristics, and high-risk features such as large-cell transformation.
KEY MESSAGE.
Low-dose radiation therapy yields excellent response and lesion control for non-tumoral lesions.
Funding
We would like to acknowledge the data management services of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. Dr. Lindsay Strowd has received consulting fees from Sanofi, Galderma, Lilly Personal, and Incyte.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10428194.2023.2254435.
References
- [1].Geller S, Myskowski PL, Pulitzer M, et al. Cutaneous T-cell lymphoma (CTCL), rare subtypes: five case presentations and review of the literature. Chin Clin Oncol. 2019;8(1):5–5. doi: 10.21037/cco.2018.11.01 [DOI] [PubMed] [Google Scholar]
- [2].Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–1714. doi: 10.1182/blood-2018-11-881268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].American Cancer Society. Cancer Facts & Figures 2022. 2022.
- [4].Hristov AC, Tejasvi T, Wilcox RA. Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management. Am J Hematol. 2019; 94(9):1027–1041. doi: 10.1002/ajh.25577 [DOI] [PubMed] [Google Scholar]
- [5].Wilson LD, Hinds GA, Yu JB. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin Lymphoma Myeloma Leuk. 2012;12(5):291–296. doi: 10.1016/j.clml.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vaidya T, Baldri T. Mycosis Fungoides. 2022. In StatPearls, StatPearls Publishing. Accessed Nov 12, 2022. [PubMed] [Google Scholar]
- [7].Denis D, Beneton N, Laribi K, et al. Management of mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL): focus on chlormethine gel. Cancer Manag Res. 2019;11:2241–2251. doi: 10.2147/CMAR.S138661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cerroni L Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):2–10. doi: 10.12788/j.sder.2018.002 [DOI] [PubMed] [Google Scholar]
- [9].Pulitzer M, Myskowski PL, Horwitz SM, et al. Mycosis fungoides with large cell transformation: clinicopathological features and prognostic factors. Pathology. 2014;46(7):610–616. doi: 10.1097/PAT.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wong HK, Mishra A, Hake T, et al. Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome). Br J Haematol. 2011;155(2):150–166. doi: 10.1111/j.1365-2141.2011.08852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Micaily B, Miyamoto C, Kantor G, et al. Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncol Biol Phys. 1998;42(2):361–364. doi: 10.1016/s0360-3016(98)00218-1 [DOI] [PubMed] [Google Scholar]
- [12].Hoppe RT, Fuks Z, Bagshaw MA. The rationale for curative radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 1977;2(9–10):843–851. doi: 10.1016/0360-3016(77)90182-1 [DOI] [PubMed] [Google Scholar]
- [13].Wilson LD, Kacinski BM, Jones GW. Local superficial radiotherapy in the management of minimal stage IA cutaneous T-cell lymphoma (mycosis fungoides). Int J Radiat Oncol Biol Phys. 1998;40(1):109–115. doi: 10.1016/s0360-3016(97)00553-1 [DOI] [PubMed] [Google Scholar]
- [14].Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. 2009;74(1):154–158. doi: 10.1016/j.ijrobp.2008.06.1918 [DOI] [PubMed] [Google Scholar]
- [15].Wang P, Gilbert M, Lim HW, et al. Single-fraction radiation therapy for localized cutaneous T-cell lymphoma. Pract Radiat Oncol. 2023;13(4):346–350. doi: 10.1016/j.prro.2023.03.015 [DOI] [PubMed] [Google Scholar]
- [16].Thomas TO, Agrawal P, Guitart J, et al. Outcome of patients treated with a single-fraction dose of palliative radiation for cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2013;85(3):747–753. doi: 10.1016/j.ijrobp.2012.05.034 [DOI] [PubMed] [Google Scholar]
- [17].Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):32–39. doi: 10.1016/j.ijrobp.2015.01.008 [DOI] [PubMed] [Google Scholar]
- [18].Patel AM, West L, Atluri PS, et al. Optimizing palliative focal radiation therapy dose in primary cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2020;108(3):S166–S167. doi: 10.1016/j.ijrobp.2020.07.936 [DOI] [Google Scholar]
- [19].Masters AH, Hughes RT, Strowd L, et al. Efficacy of low-dose radiotherapy for refractory mycosis fungoides of the face. JAAD Case Rep. 2019;5(4):348–351. doi: 10.1016/j.jdcr.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sezary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72(2):276–285. doi: 10.1016/j.jaad.2014.10.019 [DOI] [PubMed] [Google Scholar]


