Monocytes restrict Yersinia infection within intestinal pyogranulomas. Here, we report that monocyte-intrinsic TNF signaling drives the production of IL-1 that signals non-hematopoietic cells to control intestinal Yersinia infection within pyogranulomas.
Abstract
Tumor necrosis factor (TNF) is a pleiotropic inflammatory cytokine that mediates antimicrobial defense and granuloma formation in response to infection by numerous pathogens. We previously reported that Yersinia pseudotuberculosis colonizes the intestinal mucosa and induces the recruitment of neutrophils and inflammatory monocytes into organized immune structures termed pyogranulomas (PG) that control Yersinia infection. Inflammatory monocytes are essential for the control and clearance of Yersinia within intestinal PG, but how monocytes mediate Yersinia restriction is poorly understood. Here, we demonstrate that TNF signaling in monocytes is required for bacterial containment following enteric Yersinia infection. We further show that monocyte-intrinsic TNFR1 signaling drives the production of monocyte-derived interleukin-1 (IL-1), which signals through IL-1 receptors on non-hematopoietic cells to enable PG-mediated control of intestinal Yersinia infection. Altogether, our work reveals a monocyte-intrinsic TNF-IL-1 collaborative inflammatory circuit that restricts intestinal Yersinia infection.
Introduction
Granulomas form in response to a wide variety of infections, acting as barriers to pathogen dissemination (Petersen and Smith, 2013; Pagán and Ramakrishnan, 2018). Although generally considered protective, granulomas can also provide a replicative niche from which pathogens can spread, such as in immune-compromised patients who experience reactivation of latent Mycobacterium tuberculosis (Davis and Ramakrishnan, 2009; Diedrich et al., 2016). Moreover, pathogens within granulomas often exist in an antibiotic-resistant state and can pose a significant therapeutic challenge (Adams et al., 2011). Granulomas thus represent a localized niche where pathogens can be contained but can also resist immune clearance. Understanding how immune cells communicate within chronic granulomatous lesions to control pathogens remains an important question that could enable the development of immunomodulatory treatments against infectious agents that persist within this niche.
Pyogranulomas and pyogranulomatous lesions (PGs) are a subset of chronic granulomatous inflammation characterized by a predominance of neutrophils and macrophages, often in combination with plasma cells and giant cells (Raskin, 2010). The enteropathogenic Yersiniae, which include Yersinia pseudotuberculosis (Yp) and Yersinia enterocolitica, colonize the intestinal mucosa and lymphoid tissues of both mice and humans, triggering formation of PGs that are composed of extracellular bacterial colonies in close association with neutrophils, bordered closely by monocytes and macrophages (El-Maraghi and Mair, 1979; Lamps et al., 2001; Kojima et al., 2007; Rohena et al., 2014; Peterson et al., 2017; Zhang et al., 2018; Sorobetea et al., 2023).
We previously demonstrated that organized PGs, characterized by central cores of cell debris surrounded by dense cuffs of neutrophils and epithelioid macrophages, form by 5 days after Yp infection within the mesenteric lymph nodes (MLN) (Peterson et al., 2017). We recently also described organized PGs containing viable bacteria, a central neutrophil core, inflammatory monocytes, and epithelioid macrophages along the length of the gastrointestinal tract following oral Yp infection (Sorobetea et al., 2023). PGs formed in response to the activity of Yp outer proteins (Yops), which are injected into host cells through the Yp type III secretion system and block essential antimicrobial functions (Atkinson and Williams, 2016; Bliska et al., 2021; Sorobetea et al., 2023). We further demonstrated that inflammatory monocytes were critical for the maintenance of organized PGs and enabling neutrophils to overcome the activity of Yp virulence factors that block host phagocytosis and oxidative burst (Sorobetea et al., 2023). However, the mechanisms by which inflammatory monocytes mediate anti-Yp host defense are unclear.
Tumor necrosis factor (TNF) is a pleiotropic inflammatory cytokine associated with protection during infectious granulomatous disease, notably tuberculosis (Kindler et al., 1989; Flynn et al., 1995; Bean et al., 1999; Roach et al., 2002; Algood et al., 2005; Chakravarty et al., 2008; Clay et al., 2008; Lin et al., 2010). While the role of TNF in maintaining intact granulomas is well-appreciated, its precise cellular targets and mechanisms of action remain elusive due to the broad expression of its main receptor, TNFR1, and its numerous downstream signaling functions, including cell-extrinsic apoptosis, promotion of cell survival, and control of proinflammatory gene expression (Chen and Goeddel, 2002; Takeda and Akira, 2004; Kusnadi et al., 2019). TNF plays a critical role in protection against infection by intracellular pathogens, as the extensive clinical use of anti-TNF blockade in the setting of autoinflammatory disease is associated with an increased risk of severe infections (Ali et al., 2013; Kusnadi et al., 2019).
Although Yp is primarily an extracellular pathogen, TNF is required for host protection against Yp (Autenrieth and Heesemann, 1992; Parent et al., 2006; Peterson et al., 2016). However, the mechanisms by which TNF controls Yp infection are not completely understood. Here, we demonstrate that monocytes serve as an essential cellular source of TNF during Yp infection. We find that signaling through both the TNF and IL-1 receptors is required to control Yp replication in PGs. Intriguingly, monocyte-intrinsic TNF production and receptor signaling are required for monocytes within PGs to produce IL-1. IL-1R signaling is required for the production of the chemokine CXCL1, and we further find that IL-1 receptor is required on non-hematopoietic cells to mediate control of intestinal Yp infection. Altogether, our study demonstrates that a monocyte-driven TNF-IL-1 feedforward circuit mediates the control of Yp infection within systemic and intestinal sites.
Results
TNFR1 is required for organized PG formation and Yp control
We recently identified the formation of PGs in the murine intestinal mucosa during acute Yp infection, wherein inflammatory monocytes were required for neutrophil activation, maintenance of organized PG architecture, and bacterial clearance (Sorobetea et al., 2023). TNF is critical for granuloma maintenance and bacterial control in the lung during tuberculosis infection (Kindler et al., 1989; Flynn et al., 1995; Bean et al., 1999; Roach et al., 2002; Algood et al., 2005; Chakravarty et al., 2008; Clay et al., 2008; Lin et al., 2010), and we previously reported that TNFR1 is required to control systemic bacterial burdens following oral Yp infection (Peterson et al., 2016). Notably, while Tnfr1−/− mice formed similar numbers of macroscopic intestinal lesions as wild-type (WT) mice at day 5 after infection (Fig. S1 A), histopathologic analyses revealed that intestinal lesions in Tnfr1−/− mice were strikingly similar to lesions that we recently described in Yp-infected CCR2-deficient mice lacking circulating monocytes (Sorobetea et al., 2023) (Fig. 1 A). In contrast to WT PG, which had robust immune cell aggregation and a small central Yp microcolony, Tnfr1−/− intestinal lesions contained an area of central necrosis surrounded by a greatly expanded Yp colony, ringed by limited immune cell infiltrates (Fig. 1, A and B). In line with these histopathological findings, bacterial burdens in both PG-containing (PG+) intestinal punch biopsies and adjacent non-PG (PG−) biopsies were elevated in Tnfr1−/− mice (Fig. 1 C). Furthermore, Tnfr1−/− lesions contained fewer monocytes, macrophages, and neutrophils, as determined by flow cytometry (Fig. 1 D and Fig. S1, B and C). In line with these findings, whole-mount immunofluorescence microscopy revealed that in contrast to WT PG, where Yp bacteria are entirely contained within the CD11b+ and Ly-6G+ myeloid cell infiltrate, Tnfr1−/− PG had limited recruitment of CD11b+ myeloid cells or Ly-6G+ neutrophils and were unable to contain the dramatically expanded bacterial colony, similar to PG we previously described in CCR2-deficient animals (Sorobetea et al., 2023) (Fig. 1 E and Fig. S1 D). Surface expression of the integrin CD11b, a marker of neutrophil activation (Borjesson et al., 2002; Mann and Chung, 2006; Yoon et al., 2007), was also significantly reduced in PG of Tnfr1−/− mice compared with WT controls (Fig. 1 F), suggesting a defect in neutrophil activation in the absence of TNFR1 signaling, consistent with our recent finding that monocytes are required for maximal PG neutrophil activation (Sorobetea et al., 2023). Also consistent with our previous findings (Peterson et al., 2016), Tnfr1−/− mice exhibited elevated bacterial burdens in the spleen and liver (Fig. 1 G). Moreover, Tnfr1−/− mice succumbed to infection around day 8, while most WT mice survived (Fig. 1 H). Notably, while TNFR2 can also signal in response to TNF, TNFR2-deficient mice generally had no defect in intestinal or systemic control of Yp infection, with the exception of the liver, which showed only a relatively small increase in bacterial burdens at day 5 after infection (Fig. S1, E and F). Overall, these data suggest that signaling through TNFR1 rather than TNFR2 limits necrotic core formation in PGs during Yp infection and controls bacterial growth.
Figure S1.
Effects of TNFR1 deficiency on PG formation in intestinal and lymphatic tissue during Yp infection. (A) Total number of intestinal lesions at day 5 after infection with Yp. Each circle represents one mouse. Lines represent the median. Pooled data from four independent experiments. (B) Total numbers of CD45+ hematopoietic cells in PG+ small intestinal tissue isolated 5 days after infection. Each circle represents the mean of 3–20 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (C) Flow cytometry plots depicting the gating strategy employed to identify neutrophils (CD11b+ Ly-6G+), monocytes (CD64+ Ly-6Chi), and macrophages (CD64+ Ly-6Clo MHC-IIhi) in small intestinal PG+ tissue. Plots representative of three independent experiments. (D) Fluorescently labeled whole-mount small intestinal PG+ tissue from Yp-infected Ccr2gfp/gfp mouse at day 5 after infection. Red (Yp-mCherry), yellow (Ly-6G-AF647), and blue (CD11b-AF700). Scale bar = 100 μm. Representative image of two independent experiments. (E) Total number of intestinal lesions at day 5 after infection with Yp. Each circle represents one mouse. Lines represent the median. Pooled data from two independent experiments. (F) Bacterial burdens in small intestinal PG− and PG+ tissue (left) and indicated organs (right) isolated day 5 after infection. Each circle represents the mean CFU of three to five pooled punch biopsies from one mouse (left) or individual mice (right). Lines represent the geometric mean. Pooled data from two independent experiments. (G) Flow cytometry plots depicting chimerism (left) and frequencies of indicated cell types (right) in the blood of uninfected chimeric mice. Graphs represent pooled data from two independent experiments. All statistical analyses by Mann–Whitney U test. *P < 0.05, ns = not significant.
Figure 1.
TNFR1 is required for organized PG formation and Yp control. (A) H&E-stained paraffin-embedded small intestinal sections from Yp-infected WT (left) and Tnfr1−/− (right) mice at day 5 after infection; a (black dashed lines) = immune cell infiltrate, b = bacterial colony, and c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular colonies. Images representative of two independent experiments. Scale bars = 100 μm. (B) Histopathological scores of small intestinal tissue from Yp-infected WT and Tnfr1−/− mice at day 5 after infection. Each mouse was scored between 0 and 4 (minimal to extensive) for the size of the bacterial colony. Each circle represents one mouse. Lines represent the median. Pooled data from two independent experiments. (C) Bacterial burdens in small intestinal PG− and PG+ tissue isolated on day 5 after infection. Each circle represents the mean CFU of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (D) Total numbers (top) and frequencies (bottom) of neutrophils, monocytes, and macrophages in PG+ small intestinal tissue isolated 5 days after infection. Each circle represents the mean of 3–20 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (E) Fluorescently labeled whole-mount small intestinal PG+ tissue from Yp-infected WT (left) and Tnfr1−/− (right) mice at day 5 after infection. Red (Yp-mCherry), yellow (Ly-6G-AF647), and blue (CD11b-AF700). Scale bars = 100 μm. Representative image of two independent experiments. (F) CD11b surface expression on neutrophils in PG+ tissue at day 5 after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Data are representative of three independent experiments. (G) Bacterial burdens in indicated organs at day 5 after infection. Each circle represents one mouse. Lines represent the geometric mean. Pooled data from four independent experiments. (H) Survival of infected WT (n = 9) and Tnfr1−/− (n = 21) mice. Pooled data from two independent experiments. (I) Bacterial burdens in small intestinal PG− and PG+ tissue at day 5 after infection of indicated chimeric mice. Each symbol represents the mean Yp CFU of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (J) Bacterial burdens in indicated organs at day 5 after infection of indicated chimeric mice. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. Statistical analysis by Mann–Whitney U test (B–D, F, and G), Mantel–Cox test (H), and Kruskal–Wallis test with Dunn’s multiple comparisons correction (I and J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
TNFR1 signaling can enhance the ability of both hematopoietic (immune) and non-hematopoietic (stromal and epithelial) cells to control pathogens (Flynn et al., 1995; Bean et al., 1999; Clay et al., 2008; Peterson et al., 2016; Pham et al., 2020). To test which compartment requires TNFR1 signaling to control Yp, we generated bone marrow (BM) chimeras in which TNFR1 expression was ablated in either the hematopoietic or non-hematopoietic compartment (Fig. S1 G). Mice lacking TNFR1 in either the hematopoietic or non-hematopoietic compartment had elevated bacterial burdens within PG compared with WT control chimeras, indicating that TNFR1 signaling is required non-redundantly in both hematopoietic and non-hematopoietic cells to mediate control of intestinal Yp (Fig. 1 I). In contrast, mice lacking TNFR1 in hematopoietic cells had highly elevated bacterial burdens in the systemic tissues, while mice lacking TNFR1 in non-hematopoietic cells had a significant but smaller increase in systemic bacterial burdens compared with WT controls (Fig. 1 J). Taken together, these results demonstrate that TNFR1 signaling in both hematopoietic and non-hematopoietic cells contribute to bacterial control in the intestine, while TNFR1 signaling in hematopoietic cells plays a dominant role in bacterial control in systemic tissues during acute Yp infection.
Autocrine TNF signaling in monocytes is required for control of Yp
TNF receptor expression is widespread in both hematopoietic and non-hematopoietic cells, raising the question of which cells require TNF signaling for the control of Yp infection. CCR2-deficient mice lacking circulating monocytes fail to form functional intestinal PG, are unable to control Yp burdens, and rapidly succumb to infection (Sorobetea et al., 2023). Given the similar outcomes of infection and histopathological appearance of PG in TNFR1- and CCR2-deficient mice, we sought to test the hypothesis that TNF is produced and/or detected by monocytes. We generated mice in which TNFR1 was specifically deleted on inflammatory monocytes by means of mixed BM chimera where irradiated WT recipient mice were reconstituted with a 1:1 ratio of Ccr2gfp/gfp:Tnfr1−/− BM cells. Since CCR2-deficient cells are unable to egress from the BM to enter circulation (Serbina and Pamer, 2006), this approach generates mice in which circulating CCR2+ monocytes are derived only from the Tnfr1−/− cells, whereas other hematopoietic cell types arise from both genotypes and are thus sufficient for TNFR1 signaling (Fig. 2 A and Fig. S2 A). Intriguingly, compared with WT chimeric mice, mice lacking TNFR1 specifically on CCR2+ monocytes formed intestinal PG lesions with expanded bacterial colonies and had elevated bacterial burdens in PG− and systemic tissues, indicating a failure to control Yp infection and largely recapitulating the phenotype of mice lacking CCR2 in the entire hematopoietic system (Fig. 2, B–D). Despite apparent gross histological differences, bulk PG bacterial burdens as measured by colony-forming units (CFUs) were comparable across chimeric genotypes, potentially due to the general increased susceptibility of irradiated chimeric mice to infection (Fig. 2 C). Importantly, the defect in bacterial control was not due to the absence of TNFR1 expression on 50% of immune cells, as mixed chimeras from Tnfr1−/−:WT chimeric mice still had significantly lower bacterial burdens relative to Tnfr1−/−:Ccr2gfp/gfp mice, notably in systemic tissues (Fig. S2, B–D). Notably, mice lacking TNFR1 expression on all hematopoietic cells had significantly higher burdens than mice lacking TNFR1 on monocytes alone (Fig. S2 C). Altogether, these data suggest that while TNFR1 has additional important roles in other cell types beyond monocytes, TNFR1 signaling in monocytes is nonetheless essential for their protective role against Yp infection.
Figure 2.
Autocrine TNF signaling in monocytes is required for control of Yp. (A) Schematic of mixed BM chimeras. Lethally irradiated WT mice reconstituted with BM cells from indicated donor mice yield either a TNFR1-sufficient immune system (left), an immune system in which all monocytes lack TNFR1 whereas half of the remaining immune cells are TNFR1-proficient (middle), or a TNFR1-sufficient immune system devoid of monocytes (right). (B) H&E-stained paraffin-embedded small intestinal sections from chimeric WT mice reconstituted with Ccr2gfp/gfp + WT (left), Ccr2gfp/gfp + Tnfr1−/− (middle), or Ccr2gfp/gfp (right) BM at day 5 after infection; a (black dashed lines) = immune cell infiltrate, b = bacterial colony, c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular bacterial colonies. Scale bars = 100 μm. Images representative of two independent experiments. (C) Bacterial burdens in small intestinal PG− and PG+ tissue of chimeric WT mice reconstituted with either Ccr2gfp/gfp:WT (circles), Ccr2gfp/gfp:Tnfr1−/− (squares), or Ccr2gfp/gfp (diamonds) at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (D) Bacterial burdens in indicated organs at day 5 after infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (E) Bacterial burdens in small intestinal PG− and PG+ tissue of chimeric WT mice reconstituted with either Tnf−/−:WT (circles), Tnf−/−:Ccr2gfp/gfp (squares), or Tnf−/− (diamonds) at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (F) Bacterial burdens in indicated organs at day 5 after infection. Each symbol represents one mouse. Lines the represent geometric mean. Pooled data from three independent experiments. All statistical analyses by Kruskal–Wallis test with Dunn’s multiple comparisons correction. **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Figure S2.
Autocrine TNF signaling in monocytes is required for systemic control of Yp. (A) Flow cytometry plots (top) and graphs (bottom) depicting the percent chimerism of hematopoietic cells in the blood of uninfected chimeric mice. (B) Flow cytometry plots (top) and graphs (bottom) depicting the percent chimerism of hematopoietic cells in the spleens of chimeric mice. (C) Bacterial burdens in indicated organs at day 5 after infection. Each symbol represents one mouse. Lines represent the geometric mean. (D) Bacterial burdens in small intestinal PG− and PG+ tissue at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. (E) Flow cytometry plots (top) and graphs (bottom) depicting the percent chimerism of hematopoietic cells in PG of chimeric mice. All data were pooled from two independent experiments. All statistical analyses by Kruskal–Wallis test with Dunn’s multiple comparisons correction. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Multiple immune cell types produce TNF in response to inflammatory signals, including monocytes, which are a major source of TNF during Yp infection (Peterson et al., 2017). To test whether monocyte-derived TNF is important for control of Yp infection, we reconstituted irradiated WT recipient mice with a 1:1 ratio of Ccr2gfp/gfp:Tnf−/− BM cells, thereby generating cohorts of mice in which circulating CCR2+ monocytes lacked the ability to produce TNF, with other hematopoietic cell types being comprised of a 1:1 mixture of WT and Tnf−/− cells (Fig. S2 E). Strikingly, mice lacking TNF specifically in monocytes failed to control Yp infection in the spleen and liver and had bacterial burdens comparable with mice lacking TNF production in all hematopoietic cells (Fig. 2, E and F). Altogether, our findings demonstrate that autocrine TNF signaling in monocytes is required to control enteric Yp infection.
TNFR1 signaling in monocytes controls Yp independently of RIPK1 kinase–induced cell death
TNFR1 can mediate inflammatory gene expression or promote cell-extrinsic apoptosis in response to infection by pathogens, including Yp (Ea et al., 2006; Ofengeim and Yuan, 2013; Christofferson et al., 2014; Weinlich and Green, 2014; Peterson et al., 2016; Delanghe et al., 2020; Yeap and Chen, 2022). Yp-induced cell death is triggered by YopJ-mediated blockade of IκB kinase signaling and involves contributions from both TLR4/TRIF and TNFR1 signaling through the kinase activity of the adapter RIPK1 (Monack et al., 1997; Palmer et al., 1998; Orth et al., 1999; Yoon et al., 2003; Mukherjee et al., 2006; Philip et al., 2014; Peterson et al., 2017). We previously demonstrated that mice lacking RIPK1 kinase activity (Ripk1K45A) in hematopoietic cells fail to form intact MLN PG and rapidly succumb to Yp infection (Peterson et al., 2017). Furthermore, activation of gasdermin D and gasdermin E in macrophages and neutrophils, respectively, downstream of RIPK1 kinase activity promotes control of Yp infection (Chen et al., 2021). We hypothesized that RIPK1 activation downstream of TNFR1 signaling in monocyte-lineage cells mediates control of Yp within intestinal PGs. Notably, Ripk1K45A mice formed necrotic intestinal lesions and were deficient in restricting Yp burdens (Fig. 3, A and B), consistent with prior findings (Peterson et al., 2017). To test whether monocyte-intrinsic TNFR1 signaling promotes anti-Yp host defense through activation of RIPK1-induced monocyte cell death, we generated mixed BM chimeras in which irradiated WT recipient mice were reconstituted with a 1:1 ratio of Ccr2gfp/gfp:Ripk1K45A BM cells. Reconstituted mice contained CCR2+ monocytes that lacked RIPK1 kinase activity, with other hematopoietic cells being equally reconstituted by both donor BM progenitors (Fig. S3, A and B). Surprisingly, monocyte-specific ablation of RIPK1 kinase activity did not impact the ability of mice to form intact intestinal PG or control enteric Yp infection (Fig. 3, C–E), indicating that RIPK1 kinase activity in monocytes is dispensable to control Yp infection downstream of TNF signaling. We previously found that the acute susceptibility of Ripk1K45A mice is reversed in the setting of infection with YopJ-deficient bacteria, illustrating that RIPK1 kinase–induced cell death is necessary to counteract the blockade of immune signaling by YopJ (Peterson et al., 2017). However, Tnfr1−/− mice still formed necrotic intestinal PG, failed to control bacterial burdens in systemic tissues, and succumbed to infection by YopJ-deficient bacteria (Fig. 3, F–H). Collectively, these data indicate that monocyte-dependent TNFR1 signaling mediates anti-Yp host defense via a mechanism distinct from YopJ-dependent, RIPK1-induced cell death.
Figure 3.
TNFR1 signaling in monocytes controls Yp independently of RIPK1 kinase–induced cell death. (A) H&E-stained paraffin-embedded small intestinal sections from WT (left) and Ripk1K45A (right) mice at day 5 after Yp infection; a (black dashed lines) = immune cell infiltrate, b = bacterial colony, c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular bacterial colonies. Scale bars = 100 μm. Images representative of two independent experiments. (B) Bacterial burdens in small intestinal PG− and PG+ tissue (left) and indicated organs (right) of WT and Ripk1K45A mice at day 5 after Yp infection. Each circle represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (C) H&E-stained paraffin-embedded small intestinal sections from chimeric WT mice reconstituted with either Ccr2gfp/gfp:WT (left), Ccr2gfp/gfp:Ripk1K45A (middle), or Ccr2gfp/gfp (right) BM at day 5 after Yp infection. a (black dashed lines) = immune cell infiltrate, b = bacterial colony, c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular bacterial colonies. Scale bars = 100 μm. Representative images of two independent experiments. (D) Bacterial burdens in small intestinal PG− and PG+ tissue of chimeric WT mice reconstituted with either Ccr2gfp/gfp:WT (circles), Ccr2gfp/gfp:Ripk1K45A (squares), or Ccr2gfp/gfp (diamonds) at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (E) Bacterial burdens in indicated organs of chimeric WT mice reconstituted with either Ccr2gfp/gfp:WT (circles), Ccr2gfp/gfp:Ripk1K45A (squares), or Ccr2gfp/gfp (diamonds) at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. (F) Bacterial burdens in indicated organs of WT (gray) and Tnfr1−/− (red) mice infected with WT (circles) or ΔyopJ (squares) Yp at day 5 after infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from four independent experiments. (G) H&E-stained paraffin-embedded small intestinal sections from WT and Tnfr1−/− mice infected with either WT or ΔyopJ Yp at day 5 after infection. a (black dashed lines) = immune cell infiltrate, b = bacterial colony, c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular bacterial colonies. Scale bars = 100 μm. Representative images of three independent experiments. (H) Survival of WT (gray) and Tnfr1−/− (red) mice infected with WT (circles) or ΔyopJ (squares) Yp. n = 9–12 mice per group. Pooled data from two independent experiments. (I) Survival of WT (gray) or Tnfr1−/− (red) mice infected with WT (circles) or yopEH (squares) Yp. n = 11–15 mice per group. Pooled data from two independent experiments. Statistical analysis by Mann–Whitney U test (B), Kruskal–Wallis test with Dunn’s multiple comparisons correction (D–F), and Mantel–Cox test (H and I). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Figure S3.
TNFR1 signaling in monocytes is independent of YopJ-induced RIPK1 kinase activity. (A) Schematic of mixed BM chimeras. Lethally irradiated WT mice reconstituted with BM cells from indicated donor mice yield either a RIPK1 kinase–sufficient immune system (left), one in which all monocytes lack RIPK1 kinase activity whereas half of the remaining immune cells are RIPK1 kinase–sufficient (middle), or a RIPK1 kinase–sufficient immune system devoid of monocytes (right). (B) Flow cytometry plots depicting chimerism (top) and graphs depicting frequencies of indicated cell types (bottom) in the blood of uninfected chimeric mice. (C) Survival of WT (left) and Tnfr1−/− (right) mice infected with WT (circles), ΔyopE (white squares), or YopHR409A (gray squares) Yp. n = 5–32 (WT) and 13–20 (Tnfr1−/−) mice per group. Pooled data from two to four independent experiments. All statistical analyses by Mantel–Cox test. ***P < 0.001, ns = not significant.
We recently reported that intestinal PGs form in response to the activities of the Yp effectors YopE and YopH, and that monocytes counteract YopH-mediated blockade of innate immunity (Sorobetea et al., 2023). YopE and YopH both block phagocytosis and the oxidative burst through actin cytoskeleton disruption (Rosqvist et al., 1988, 1991; Bliska et al., 1991; Galyov et al., 1993; Bliska and Black, 1995; Green et al., 1995; Mecsas et al., 1998; Black and Bliska, 2000; Grosdent et al., 2002; Taheri et al., 2016). However, whether TNFR1 is required to overcome the immune blockade posed by YopE and YopH is unknown. Strikingly, TNFR1-deficient mice largely survived infection with yopEH mutant Yp (Fig. 3 I), indicating that TNFR1 signaling counteracts the activity of YopE and YopH. However, in contrast to our previous findings with CCR2-deficient mice (Sorobetea et al., 2023), Tnfr1−/− mice were not able to control either single Yp mutant, although there was a modest but significant decrease in mortality in response to infection with yopH mutant bacteria (Fig. S3 C). Together, these findings demonstrate that TNFR1 signaling controls enteric Yp infection by counteracting the virulence activities of YopE and YopH.
Cell-intrinsic TNFR1 signaling is required for maximal IL-1 production within intestinal PGs during Yp infection
Our findings collectively indicate that monocyte-intrinsic TNFR1 signaling promotes intestinal PG formation and control of Yp infection via RIPK1 kinase–independent mechanisms. We therefore considered that TNFR1 signaling in monocytes contributes to the control of Yp infection by promoting inflammatory cytokine production. Multiplex cytokine profiling of intestinal PG+ and PG− from mixed BM chimeric mice with monocyte-intrinsic TNFR1 deficiency revealed that IL-1α levels were significantly decreased in the PG lesions of these mice (Fig. 4 A), in contrast to other proinflammatory cytokines such as IL-6 (Fig. S4 A). Neither IL-1α nor IL-1β were detected in the sera of these mice at day 5 after Yp infection, suggesting that IL-1 production in response to TNFR1 signaling is localized to intestinal PG (Fig. S4 B). Since TNFR1 expression on monocytes is required for restriction of bacterial burdens, we hypothesized that TNFR1 signaling promotes monocyte production of IL-1 within intestinal PG. Indeed, Tnfr1−/− monocytes and neutrophils exhibited reduced levels of IL-1α and IL-1β expression as determined by intracellular cytokine staining, indicating that TNFR1 signaling is necessary for maximal IL-1 production in both monocytes and neutrophils within intestinal PG (Fig. 4 B). To test whether TNFR1 is required in a cell-intrinsic or -extrinsic manner to promote IL-1 expression, we generated mixed BM chimeras in which lethally irradiated WT recipients were reconstituted with a 1:1 mixture of WT:Tnfr1−/− BM or entirely with Tnfr1−/− BM as a positive control. Importantly, there was no competitive defect in the reconstitution of Tnfr1−/− cells in the mixed chimera setting, nor a competitive defect in the recruitment of Tnfr1−/− cells to intestinal PG, as these mice contained a 1:1 ratio of WT and Tnfr1−/− immune cells within both the spleen and PG (Fig. S4 C). Strikingly, IL-1 production was reduced in both monocytes and neutrophils lacking TNFR1 relative to WT cells within the same mice, demonstrating that cell-intrinsic TNFR1 signaling mediates optimal production of IL-1 in monocytes and neutrophils (Fig. 4, C and D; and Fig. S4 D). Moreover, TNF levels were reduced in both monocytes and neutrophils lacking TNFR1 relative to WT cells isolated from the same intestinal PG, demonstrating that TNFR1 signals in a feedforward loop to promote TNF production in a cell-intrinsic fashion (Fig. S4 E). Notably, Toll-like receptor 4 (TLR4) was not required for enhanced expression of TNF or IL-1 by monocytes in response to Yp infection or control of bacterial burdens in PG− or PG+ intestinal tissues, indicating that other TLRs or pattern recognition receptors detect Yp to promote inflammatory cytokine production and bacterial restriction (Fig. S4, F and G). Overall, these data show that cell-intrinsic TNFR1 signaling promotes optimal IL-1 production in myeloid cells within intestinal PG, raising the question of whether IL-1 production downstream of TNFR1 signaling in monocytes contributes to control of Yp during early intestinal infection.
Figure 4.
Cell-intrinsic TNFR1 signaling is required for maximal IL-1 production within intestinal PGs during Yp infection. (A) Cytokine levels in tissue punch biopsy homogenates isolated 5 days after infection of chimeric WT mice reconstituted with indicated donor cells. Each symbol represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from two independent experiments. (B) Frequency of IL-1α– or IL-1β–producing monocytes and neutrophils isolated from small intestinal PG+ tissue 5 days after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (C) Flow cytometry plots of intracellular IL-1 staining in monocytes (CD64+ Ly-6Chi) from small intestinal PG+ tissue at day 5 after infection. Plots representative of two independent experiments. (D) Intracellular IL-1 staining in monocytes and neutrophils in small intestinal PG+ tissue at day 5 after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines connect congenic cell populations within individual mice. Pooled data from two independent experiments. Statistical analysis by Kruskal–Wallis test with Dunn’s multiple comparisons correction (A), Mann–Whitney U test (B), and for congenically marked cells within mice: Wilcoxon test; across groups: Mann–Whitney U test (D). *P < 0.05, **P < 0.01, ****P < 0.0001, ns = not significant.
Figure S4.
Cytokine production downstream of TNFR1 expression on monocytes is specific to IL-1 in intestinal PGs. (A) Cytokine levels in homogenates of PG− and PG+ tissue punch biopsies at day 5 after infection of chimeric WT mice reconstituted with indicated cells. Each symbol represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from two independent experiments. (B) Cytokine levels in serum at day 5 after infection of chimeric WT mice reconstituted with indicated cells. Lines represent the median. Pooled data from two independent experiments. (C) Flow cytometric plots and graphs depicting frequencies of indicated cell types in small intestinal PG+ tissue or spleen at day 5 after infection of WT chimeric mice reconstituted with the indicated cells. Plots representative of two independent experiments. Pooled data from two independent experiments. (D) Flow cytometry plots of intracellular IL-1 in monocytes (CD64+ Ly-6Chi) from small intestinal PG+ tissue in WT and Il1b−/− mice at day 5 after infection. Plots representative of two independent experiments. (E) Frequency of monocytes and neutrophils producing TNF in small intestinal PG+ tissue at day 5 after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines connect congenic cell populations within individual mice. Pooled data from two independent experiments. (F) Frequency of monocytes producing the indicated cytokines (left) and flow cytometric plots depicting intracellular cytokine staining (right) in monocytes isolated from small intestinal PG+ tissue 5 days after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (G) Bacterial burdens in small intestinal PG− and PG+ tissue (left) and indicated organs (right) of WT and Tlr4−/− mice at day 5 after Yp infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from two independent experiments. Statistical analyses by Kruskal–Wallis test with Dunn’s multiple comparisons correction (A and B). For congenically marked cells within mice, Wilcoxon test; across groups, Mann–Whitney U test (E), and Mann–Whitney U test (F and G). ****P < 0.0001, ns = not significant.
IL-1 signaling is required for PG formation and intestinal control of Yp
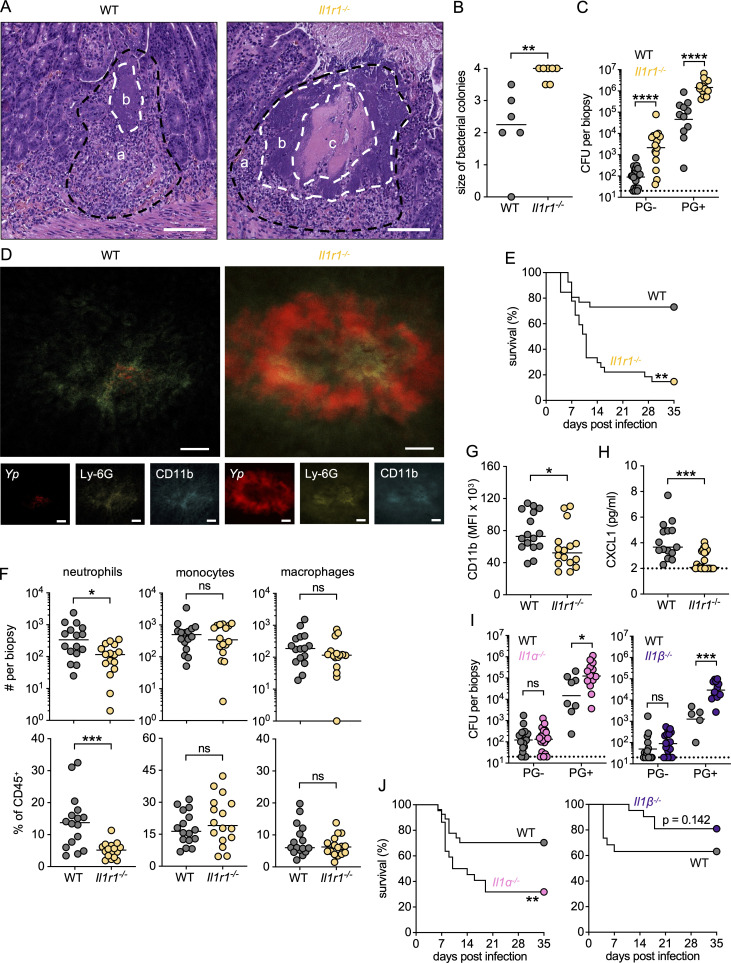
IL-1 plays a critical role in host defense against other pathogens by promoting immune cell recruitment and activation, cytokine production, angiogenesis, and vascular permeability (Franchi et al., 2012; Barry et al., 2013; Copenhaver et al., 2015; Lee et al., 2015; Fahey and Doyle, 2019; Liu et al., 2020). Mice lacking IL-1 signaling are more susceptible to systemic Yp infection (Vladimer et al., 2012; Ratner et al., 2016; Chen et al., 2021). However, IL-1β can also promote increased intestinal permeability and barrier dysfunction during Yp infection (Meinzer et al., 2012), suggesting multifaceted roles for IL-1 signaling within specific compartments and stages of infection. To test the contribution of IL-1 signaling in the control of enteric Yp infection within PGs, we performed oral Yp infections in Il1r1−/− mice, which lack IL-1R and thus cannot respond to IL-1 cytokines. Notably, the intestinal lesions in Il1r1−/− mice contained a central area of tissue necrosis surrounded by expanded bacterial microcolonies (Fig. 5, A and B), remarkably similar to the necrosuppurative intestinal lesions seen in Ccr2gfp/gfp mice (Sorobetea et al., 2023) and Tnfr1−/− mice (Fig. 1 A and Fig. 5 A). Further, at day 5 after infection, Il1r1−/− mice had significantly higher intestinal bacterial burdens than WT mice, specifically in PG+ and PG− tissue and Peyer’s patches (Fig. 5 C and Fig. S5 A). Myeloid cells in IL-1R–deficient mice also failed to contain Yp microcolonies as determined by immunofluorescence microscopy (Fig. 5 D). This failure to contain bacteria within organized PGs was reflected in decreased survival of Il1r1−/− mice relative to WT mice (Fig. 5 E). However, at day 5 after infection, bacterial burdens in systemic organs were only moderately increased compared with those of WT mice (Fig. S5 B), suggesting that IL-1–independent mechanisms of systemic bacterial control operate downstream of TNF signaling during acute infection.
Figure 5.
IL-1 signaling is required for PG formation and intestinal control of Yp. (A) H&E-stained paraffin-embedded small intestinal sections from Yp-infected mice at day 5 after infection; a (black dashed lines) = immune cell infiltrate, b = bacterial colony, c = necrosis. White dashed lines delineate the edges of central necrosis and extracellular bacterial colonies. Representative images of one experiment. Scale bars = 250 µm. (B) Histopathological scores of small intestinal tissue from uninfected or Yp-infected mice at day 5 after infection. Each mouse was scored between 0 and 4 (minimal to extensive) for the size of the bacterial colony. Each circle represents one mouse. Lines represent the median. Pooled data from two experiments. (C) Bacterial burdens in small intestinal PG− and PG+ tissues isolated 5 days after infection. Each circle represents the mean of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (D) Fluorescently labeled whole-mount small intestinal PG+ tissue from Yp-infected WT (left) and Il1r1−/− (right) mice at day 5 after infection. Red (Yp-mCherry), yellow (Ly-6G-AF647), blue (CD11b-AF700). Scale bars = 100 μm. Representative image of two independent experiments. (E) Survival of infected WT (n = 26) and Il1r1−/− (n = 20) mice. Pooled data from two independent experiments. (F) Total numbers (top) and frequencies (bottom) of neutrophils, monocytes, and macrophages in PG+ small intestinal tissue isolated 5 d after infection. Each circle represents the mean of 3–20 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (G) CD11b surface expression on neutrophils in PG+ tissue at day 5 after infection. Each circle represents the mean of 3–10 pooled punch biopsies from one mouse. Lines represent the median. Data representative of three independent experiments. (H) CXCL1 levels in PG+ tissue punch biopsy homogenates isolated 5 days after infection. Each datapoint represents the mean of 3–20 pooled punch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (I) Bacterial burdens in small intestinal PG− and PG+ tissues at day 5 after infection of indicated genotypes. Each circle represents the mean of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (J) Survival of infected WT (left: n = 27; right: n = 19), Il1a−/− (n = 22), and Il1b−/− (n = 21) mice. Pooled data from (left) three and (right) two independent experiments. Statistical analysis by Mann–Whitney U test (B, C, and F–I) and Mantel–Cox test (E and J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Figure S5.
Monocyte-derived IL-1 signals to non-hematopoietic cells to restrict Yp infection. (A) Bacterial burdens in Peyer’s patches (PP) at day 5 after infection. Each circle represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (B) Bacterial burdens in indicated organs at day 5 after infection. Each circle represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (C) Cytokine levels in PG+ tissue punch biopsy homogenates isolated 5 days after infection. Each datapoint represents the mean of 3–20 pooled bunch biopsies from one mouse. Lines represent the median. Pooled data from three independent experiments. (D and E) Bacterial burdens in indicated organs at day 5 after infection. Each circle represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (F) Flow cytometric plots and graphs depicting the percent chimerism in the blood of chimeric mice. Flow cytometric plots are representative of two independent experiments. Pooled data from two independent experiments. (G) Bacterial burdens in Peyer’s patches isolated 5 days after infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (H) Frequencies of indicated cell types in small intestinal PG+ tissue at day 5 after infection of indicated chimeric mouse. Pooled data from three independent experiments. (I) Bacterial burdens in Peyer’s patches isolated 5 days after infection. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. Statistical analysis by Mann–Whitney U test (A–E), and Kruskal–Wallis test with Dunn’s multiple comparisons correction (G and I). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
Given that mice defective in TNFR1 signaling had impaired neutrophil activation and reduced myeloid cell numbers within PGs, we considered the possibility that IL-1R signaling also plays a role in neutrophil recruitment and activation during Yp infection. Intriguingly, while Il1r1−/− PG contained similar numbers of monocytes and macrophages as WT PG, they had significantly fewer neutrophils (Fig. 5 F). Surface expression of the neutrophil activation marker CD11b was also significantly reduced in Il1r1−/− PG compared with WT PG (Fig. 5 G). Furthermore, levels of the neutrophil-recruiting chemokine CXCL1 were also significantly reduced in Il1r1−/− PG compared with WT PG (Fig. 5 H). Interestingly, CCL2, CCL4, and IL-6 levels were similar between Il1r1−/− and WT PG, indicating that this effect was specific to CXCL1 (Fig. S5 C). IL-1R is activated by both IL-1α and IL-1β, which have unique and shared functions (Dinarello, 2018). Notably, compared with WT mice, both Il1a−/− and Il1b−/− mice had elevated bacterial burdens in PG+ tissue and PP but not in PG− tissue (Fig. 5 I and Fig. S5 A), in contrast to Il1r1−/− mice, which had elevated bacterial burdens in all three intestinal compartments. Moreover, similar to Il1r1−/− mice, mice lacking either IL-1α or IL-1β had relatively mild increases in bacterial burdens in systemic organs as compared with WT mice on day 5 after infection (Fig. S5, D and E). Intriguingly, Il1a−/− mice had a survival defect comparable with that of Il1r1−/− mice, whereas Il1b−/− mice had similar survival to WT mice following Yp infection (Fig. 5 J). These data suggest that while IL-1α and IL-1β contribute non-redundantly to early control of enteric Yp infection in PGs, IL-1α is more important than IL-1β in promoting host survival. Collectively, these results indicate that IL-1R signaling is important for the control of enteric Yp infection in intestinal PGs and contributes to intestinal neutrophil recruitment and activation.
Monocyte-derived IL-1 signals to non-hematopoietic cells to restrict Yp in intestinal PGs
Our findings demonstrate that autocrine TNF signaling in inflammatory monocytes promotes cell-intrinsic IL-1 production and subsequent IL-1R signaling promotes anti-Yp immune defense. However, whether monocyte-derived IL-1 is specifically required for the control of intestinal Yp has not been tested. We therefore infected mixed BM chimeras in which irradiated WT recipient mice were reconstituted with a 1:1 ratio of Il1ab−/−:Ccr2gfp/gfp BM cells to generate cohorts of mice lacking IL-1α and IL-1β production in monocytes (Fig. S5 F). Critically, these mice had significantly elevated bacterial burdens in PG, Peyer’s patches, spleen, and liver compared with control mice (Fig. 6, A and B; and Fig. S5 G). Bacterial burdens in PG− biopsies were elevated in mice entirely lacking hematopoietic IL-1 but not in mice with monocyte-specific IL-1 deficiency. Together, these results suggest that IL-1 production from monocytes is important for bacterial restriction in intestinal compartments and systemic organs, although IL-1 production from non-monocytic cell types also contributes to restriction of intestinal infection within PG.
Figure 6.
Monocyte-derived IL-1 signals to non-hematopoietic cells to restrict Yp in intestinal PGs. (A) Bacterial burdens in small intestinal PG− and PG+ tissues at day 5 after infection of indicated chimeric mice. Each symbol represents the mean of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Data were pooled from four independent experiments. (B) Bacterial burdens in the indicated organ at day 5 after infection of indicated chimeric mouse. Each symbol represents one mouse. Lines represent the geometric mean. Pooled data from four independent experiments. (C) Bacterial burdens in small intestinal PG− and PG+ tissues at day 5 after infection of indicated chimeric mice. Each symbol represents the mean of three to five pooled punch biopsies from one mouse. Lines represent the geometric mean. Pooled data from three independent experiments. (D) Bacterial burdens in indicated organs at day 5 after infection of the indicated chimeric mouse. Each symbol represents one mouse. Lines represent the geometric mean. Data were pooled from three independent experiments. (E) Model of TNF-IL-1 circuit mediated by monocytes and non-hematopoietic cells to restrict Yp infection within intestinal PGs. All statistical analyses by Kruskal–Wallis test with Dunn’s multiple comparisons correction. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant.
IL-1R signaling within the non-hematopoietic compartment is critical for antibacterial defense against other bacterial pathogens (Homaidan et al., 2001; Moon et al., 2014; Lee et al., 2015; Bohrer et al., 2018; Liu et al., 2020; Barnett et al., 2022). To test whether IL-1R in the non-hematopoietic compartment might be important for the formation of intestinal PG and restriction of Yp, we reconstituted lethally irradiated Il1r1−/− mice with WT BM (Fig. S5 H). Notably, mice lacking IL-1R in non-hematopoietic cells had elevated bacterial burdens in both intestinal (PP, PG) and systemic (liver and spleen) tissues following oral Yp infection compared with WT control chimeras, while mice lacking IL-1R in the hematopoietic compartment had bacterial burdens similar to those observed in the WT control chimeras (Fig. 6, C and D; and Fig. S5 I). Altogether, our findings demonstrate that IL-1R signaling in non-immune cells restricts Yp infection in intestinal and systemic tissues and that this restriction is driven by a monocyte-intrinsic TNF-IL-1 circuit (Fig. 6 E).
Discussion
The enteric pathogen Yp induces the formation of PGs in both the human and murine intestine (El-Maraghi and Mair, 1979; Lamps et al., 2001; Kojima et al., 2007; Rohena et al., 2014; Davis et al., 2015; Richardson et al., 2018; Zhang et al., 2018; Sorobetea et al., 2023). We previously described PGs histopathologically within MLN (Peterson et al., 2017), as well as within the lamina propria of the gastrointestinal tract (Sorobetea et al., 2023), based on the presence of a central core of cell debris surrounded by dense cuffs of neutrophils and epithelioid macrophages within these structures (Peterson et al., 2017). Here, we uncover a crucial myeloid TNF/IL-1 signaling circuit that restricts Yp replication within intestinal PGs. Notably, in the absence of protective TNFR1 and IL-1R1 signals, mice exhibited defective control of Yp and had PGs with central areas of necrosis and expanded bacterial colonies not seen in WT mice.
TNF plays an important role in bacterial granuloma formation and maintenance (Kindler et al., 1989; Flynn et al., 1995; Bean et al., 1999; Roach et al., 2002; Algood et al., 2005; Chakravarty et al., 2008; Clay et al., 2008; Lin et al., 2010). Anti-TNF therapy triggers the reactivation of dormant Mycobacterium tuberculosis infection (Botha and Ryffel, 2003; Chakravarty et al., 2008; Matty et al., 2015). TNF also promotes macrophage-dependent control of bacterial replication within granulomas during chronic Salmonella infection (Pham et al., 2020). TNF promotes multiple antimicrobial activities of macrophages that are critical for granuloma formation and control of tuberculosis (Flynn et al., 1995; Bean et al., 1999; Clay et al., 2008; Tobin et al., 2010). However, excessive TNF causes macrophage cell death that can be detrimental to bacterial control (Tobin et al., 2012). Here, we find that autocrine TNF signaling on monocyte-lineage cells promotes cell-intrinsic production of IL-1, which signals through non-hematopoietic IL-1R to control Yp bacterial burdens within intestinal PGs. Our findings highlight a key role for TNF signaling in the control of Yp infection within intestinal PGs. However, Yp YopJ suppresses cell-intrinsic inflammatory cytokine expression (Palmer et al., 1998), raising the question of how anti-Yp immune responses are initiated. During Legionella pneumophila infection, IL-1 signaling induces TNF production in uninfected bystander cells, thus bypassing Legionella virulence factor–induced blockade of host protein synthesis (Copenhaver et al., 2015; Liu et al., 2020). Bystander cells not injected with YopJ are thus a potential source of TNF or IL-1 during Yp infection (Peterson et al., 2017).
TNFR1 signaling promotes Yp-induced cell death via RIPK1 activity, and RIPK1 kinase activity is necessary for the control of Yp infection (Peterson et al., 2017). However, while RIPK1 kinase activity was essential for intestinal PG architecture, inflammatory monocytes did not require RIPK1 kinase activity for PG formation and Yp restriction. These findings suggest that two distinct pathways mediate protection against enteric Yp infection: a monocyte-intrinsic TNFR1 pathway that amplifies IL-1 cytokine production, and a separate pathway involving RIPK1 kinase–mediated cell death of non-monocytic cells in response to YopJ-induced blockade of immune signaling. Notably, neutrophils undergo gasdermin E–dependent pyroptosis downstream of RIPK1 kinase activity, which contributes to the restriction of Yp infection in vivo (Chen et al., 2021). Future studies will elucidate whether RIPK1 kinase–induced neutrophil cell death enables control of Yp.
TNF promotes IL-1β expression in dendritic cells (Jain et al., 2020) and tumor-associated macrophages (Caronni et al., 2023). In human macrophages, TNF promotes IL-1β expression via sterol response element binding protein (Kusnadi et al., 2019). Our findings indicate that monocyte-derived IL-1 controls Yp infection independent of RIPK1 kinase–mediated monocyte cell death. Monocytes may release IL-1 through inflammasome-dependent pyroptosis or in a “hyperactivated” state, whereby cells release IL-1 in a gasdermin D–dependent manner in the absence of pyroptosis (Chen et al., 2014; Gaidt et al., 2016; Evavold et al., 2018; Aizawa et al., 2020; Zhivaki et al., 2020). Future studies will clarify mechanisms of IL-1 expression and release downstream of TNF during Yp infection.
IL-1R signaling is critical for bacterial control during tuberculosis infection, and the loss of IL-1R signaling leads to necrotic lesions in the lung (Yamada et al., 2000; Sugawara et al., 2001; Mayer-Barber et al., 2010, 2011; Di Paolo et al., 2015; Ji et al., 2019; Silvério et al., 2021). Here, we observed that in the absence of IL-1R, intestinal PGs exhibited a necrotic pathology associated with elevated intestinal Yp burdens. Unexpectedly, systemic bacterial burdens were similar between WT and Il1r1−/− mice, suggesting that TNF engages IL-1–independent pathways to restrict systemic bacterial replication. Mice lacking IL-1R signaling were more susceptible than mice lacking IL-1α or IL-1β alone, indicating that both IL-1 cytokines contribute non-redundantly to bacterial control. While mice deficient in IL-1α exhibited elevated mortality, IL-1β–deficient mice did not. During the early intestinal stage of infection, IL-1α and IL-1β may mediate overlapping mechanisms of Yp restriction, while at later stages, IL-1α plays a unique role in Yp control. IL-1α mediates intestinal inflammation and inflammatory cell recruitment during Yersinia enterocolitica infection (Dube et al., 2001). In contrast, IL-1β contributes to Yp restriction (Chen et al., 2021), but also promotes intestinal barrier permeability and translocation of intestinal bacteria downstream of YopJ activity (Jung et al., 2012). Tight regulation of intestinal IL-1 signaling may therefore be important to combat Yp infection while avoiding excessive tissue damage.
While IL-1β is released from hematopoietic cells downstream of inflammasome activation, IL-1α is more broadly expressed across cell types and can function within the nucleus as a cell surface–associated cytokine or as an alarmin released from dying cells (Dinarello, 2018). Monocyte-specific loss of both IL-1α and IL-1β led to defective control of Yp within intestinal PG. However, IL-1α and IL-1β production from monocytes was dispensable for control of Yp burdens in PG− tissue and systemic organs, suggesting that other cell types contribute to IL-1–mediated bacterial restriction in these compartments. TNFR1 signaling also promoted neutrophil IL-1 production during enteric Yp infection, in line with prior studies identifying a role for GSDME-dependent IL-1β production by neutrophils in the control of enteric Yp infection (Chen et al., 2021). Additionally, epithelial cells are a major source of IL-1α that drives inflammation during intestinal injury (Bersudsky et al., 2014; Scarpa et al., 2015). Whether neutrophils, epithelial cells, or other cell types produce IL-1 to control intestinal Yp remains to be investigated. Interestingly, several other IL-1 family members, including IL-18 and IL-33, have been linked to granuloma formation in the setting of sarcoidosis or schistosomiasis, and IL-18 also contributes to host defense against Yersinia pestis (Greene et al., 2000; Townsend et al., 2000; Vladimer et al., 2012; Ratner et al., 2016). Future studies will investigate the potential role of other IL-1 family cytokines within PGs during intestinal Yp infection.
Our observations that IL-1R on stromal cells is critical for the control of Yp infection highlights a recurring theme of IL-1–mediated crosstalk between immune and non-immune cells during infection (Lee et al., 2015; Bohrer et al., 2018; Orzalli et al., 2018; Liu et al., 2020; Overcast et al., 2023). IL-1R is expressed in non-lymphoid tissues, including epithelial and endothelial cells (Deyerle et al., 1992), suggesting a general mechanism by which non-hematopoietic cells amplify and coordinate antipathogen defense. The cellular subpopulations within the intestine that respond to IL-1 and their downstream effector functions that restrict Yp remain unknown. IL-1R signaling upregulates antimicrobial peptide expression, promotes neutrophil recruitment, and modulates intestinal permeability (Homaidan et al., 2001; Yan et al., 2006; Al-Sadi and Ma, 2007; Franchi et al., 2012; Moon et al., 2014; Lee et al., 2015; Overcast et al., 2023). We observed decreased neutrophil recruitment in mice lacking monocytes (Sorobetea et al., 2023), TNFR1, or IL-1R, and IL-1R deficiency was associated with decreased CXCL1 levels within PGs. Altogether, our work uncovers a monocyte-intrinsic TNF/IL-1 circuit that signals to IL-1R on stromal cells to control Yp infection, providing new insight into the cytokine networks within enteric PG that mediate the control of bacterial pathogens.
Materials and methods
Mice
C57BL/6J (CD45.2), C57BL/6.SJL (CD45.1), Tnfr2−/− (Erickson et al., 1994), and Ccr2gfp/gfp mice (Satpathy et al., 2013) were obtained from the Jackson Laboratory. Tnfr1−/− (Pfeffer et al., 1993), Ripk1K45A (Berger et al., 2014), Il1r1−/− (Glaccum et al., 1997), Tlr4−/− (Hoshino et al., 1999), Il1a−/− (Horai et al., 1998), Il1b−/− (Horai et al., 1998), and Il1a−/−Il1b−/− (Horai et al., 1998) mice were previously described. All mice were bred at the University of Pennsylvania by homozygous mating and housed separately by genotype. Mice of either sex between 8 and 12 wk of age were used for all experiments. All animal studies were performed in strict accordance with University of Pennsylvania Institutional Animal Care and Use Committee–approved protocols (protocol #804523).
Bacteria
WT Yp (clinical isolate strain 32777, serogroup O1) (Simonet and Falkow, 1992) and isogenic YopJ-deficient mutant were provided by Dr. James Bliska (Dartmouth College, Hanover, NH, USA) and previously described (Philip et al., 2014). Mutants lacking YopE (ΔyopE), YopH enzymatic activity (YopHR409A), or both (denoted yopEH) were previously described (Sorobetea et al., 2023).
BM chimeras
WT B6.SJL mice (CD45.1 background) or knockout mice (Tnfr1−/− or Il1r1−/−, CD45.2 background) were lethally irradiated (1,096 rads). B6.SJL mice were obtained from the Jackson Laboratory and therefore microbiota-matched. 6 h later, mice were injected retro-orbitally with freshly isolated BM cells (5 × 106 total cells, 2.5 × 106 cells per donor in mixed groups) from isogenic donors of the indicated genotypes. All chimeras were provided with antibiotic-containing acidified water (40 mg trimethoprim and 200 mg sulfamethoxazole per 500 ml drinking water) for 4 wk after irradiation and subsequently provided acidified water without antibiotics for a total of at least 10 wk. The reconstitution of hematopoietic cells (proportion of donor CD45+ cells among total CD45+ cells) in the blood, spleen, or intestine was analyzed by flow cytometry.
Mouse infections
Yp was cultured to stationary phase at 28°C and 250 rpm shaking for 16 h in 2xYT broth supplemented with 2 μg/ml triclosan (Millipore Sigma). Mice were fasted for 16 h and subsequently inoculated by oral gavage with 200 μl phosphate-buffered saline (PBS) as previously described (Sorobetea et al., 2023) All bacterial strains were administered at 2 × 108 CFU per mouse.
Bacterial CFU quantifications
Tissues were collected in sterile PBS, weighed, and homogenized for 40 s with 6.35-mm ceramic spheres (MP Biomedical) using a FastPrep-24 bead beater (MP Biomedical). Samples were serially diluted 10-fold in PBS, plated on LB agar supplemented with 2 μg/ml triclosan, and incubated for 2 days at room temperature. Dilutions of each sample were plated in triplicate and expressed as the mean CFU per gram or per biopsy.
Cytokine quantification
Cytokines were measured in homogenized tissue supernatants using a Cytometric Bead Array (BD Biosciences) according to the manufacturer’s instructions with the following modification: the amounts of capture beads, detection reagents, and sample volumes were scaled down 10-fold. Data were collected on an LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo v10 (BD Biosciences).
Tissue preparation and cell isolation
Blood was harvested by cardiac puncture upon euthanasia and collected in 250 U/ml Heparin solution (Millipore Sigma). Erythrocytes were lysed with a red blood cell lysing buffer (Millipore Sigma).
Spleens were homogenized through a 70-μm cell strainer (Thermo Fisher Scientific), then flushed with R10 buffer consisting of RPMI 1640 (Millipore Sigma) supplemented with 10 mM HEPES (Millipore Sigma), 10% fetal bovine serum (Omega Scientific), 1 mM sodium pyruvate (Thermo Fisher Scientific), and 100 U/ml penicillin + 100 μg/ml streptomycin (Thermo Fisher Scientific). Erythrocytes were lysed with a red blood cell lysing buffer (Millipore Sigma).
Intestines were excised, flushed luminally with sterile PBS to remove the feces, opened longitudinally along the mesenteric side, and placed luminal side down on cutting boards (Epicurean). Small intestinal tissue containing macroscopically visible PGs (PG+), adjacent non-granulomatous areas (PG−), and uninfected control tissue were excised using a 2-mm-diameter dermal punch-biopsy tool (Keyes). Biopsies within each mouse were pooled groupwise, suspended in epithelial dissociation buffer consisting of calcium and magnesium-free HBSS (Thermo Fisher Scientific) supplemented with 15 mM HEPES, 10 mg/ml bovine serum albumin (Millipore Sigma), 5 mM EDTA (Millipore Sigma), and 100 U/ml penicillin + 100 μg/ml streptomycin, and then incubated for 30 mins at 37°C under continuous agitation at 300 RPM. To isolate immune cells from the lamina propria, the tissue was enzymatically digested in R10 buffer, along with 0.5 Wünsch units/ml liberase TM (Roche), 30 μg/ml DNase I (Roche), and 5 mM CaCl2 for 20 min at 37°C under continuous agitation. The resulting cell suspensions were filtered through 100-μm cell strainers (Thermo Fisher Scientific) and subjected to density gradient centrifugation using Percoll (GE Healthcare). Briefly, cells were suspended in 40% Percoll and centrifuged over a 70% Percoll layer for 20 min at 600 × g with the lowest brake at room temperature. Cells collected between the layers were washed with R10 buffer for downstream analysis.
Flow cytometry
Non-specific Fc binding was blocked for 10 mins on ice with unconjugated anti-CD16/CD32 (93; Thermo Fisher Scientific). Cells were subsequently labeled for 30 mins on ice with the following antibodies and reagents: PE-conjugated rat anti-mouse Siglec-F (E50-2440; BD Biosciences), PE-TxR or PE-Cy5–conjugated rat anti-mouse CD11b (M1/70.15; Thermo Fisher Scientific), PE-Cy5.5 or PE-Cy7-conjugated rat anti-mouse CD4 (RM4-5; Thermo Fisher Scientific), BV510-conjugated rat anti-mouse CD3e (145-2C11; BioLegend), AF700 or PerCP-Cy5.5-conjugated rat anti-mouse Ly-6C (HK1.4; Thermo Fisher Scientific), BV605-conjugated Armenian hamster anti-mouse TCRβ (H57-597; BD Biosciences), BV650-conjugated rat anti-mouse I-A/I-E (M5/114.15.2; BD Biosciences), BV711-conjugated rat anti-mouse CD8α (53-6.7; BD Biosciences), BV785-conjugated rat anti-mouse Ly-6G (1A8; Thermo Fisher Scientific), PE-Cy7 or AF647-conjugated mouse anti-mouse CD64 (X54-5/7.1; BD Biosciences), AF700-conjugated mouse anti-mouse CD45.2 (104; BioLegend), PE-Cy5-conjugated mouse anti-mouse CD45.1 (A20; Thermo Fisher), and PE-Cy5 or PE-CF594-conjugated rat anti-mouse CD45R/B220 (RA3-6B2; BD Biosciences) along with eF780 viability dye (BioLegend) diluted in PBS. Antibodies were used at 1:200 dilution and viability dye at 1:1,500 dilution.
For intracellular staining, cells isolated from indicated tissues were incubated ex vivo for 3 h at 37°C with 5% CO2 in R10 buffer supplemented with 0.33 μl/ml GolgiStop (BD Biosciences) and 15 μg/ml DNase I. Surface proteins were stained as above, and cells were fixed for 20 min on ice with Cytofix/Cytoperm Fixation/Permeabilization solution (BD Biosciences). Intracellular cytokines were stained at 4°C overnight with FITC or PerCP-e710-conjugated rat anti-mouse IL-1β (NJTEN3; Thermo Fisher Scientific) and PE-conjugated Armenian hamster anti-mouse IL-1α (ALF-161; BioLegend). All antibodies were diluted 1:200 in Perm/Wash Buffer (BD Biosciences). Cells were acquired on an LSRFortessa flow cytometer, and data were analyzed with FlowJo v10. Cells were gated on live singlets prior to downstream analyses.
Histology
Tissues were fixed in 10% neutral-buffered formalin (Thermo Fisher Scientific) and stored at 4°C until further processed. Tissue pieces were embedded in paraffin, sectioned by standard histological techniques, and stained with hematoxylin and eosin (H&E). Slides were scanned on an Aperio VERSA using brightfield at 20× magnification. Histopathological disease scoring was performed by blinded board-certified pathologists. Tissue sections were given a score from 0 to 4 (healthy to severe) for multiple parameters, including degree of inflammatory cell infiltration, necrosis, and free bacterial colonies, along with tissue-specific parameters such as villus blunting and crypt hyperplasia.
Immunofluorescence microscopy
Small intestinal tissue was dissected and flushed with PBS. Intestines were opened longitudinally and ∼0.5 cm tissue pieces containing macroscopically visible lesions were excised. Tissues were fixed in 1% paraformaldehyde overnight, then blocked overnight at 4°C in a blocking solution containing 10% bovine serum albumin, 1 μg/ml anti-CD16/32, and 1% normal rat serum in PBS. AF647-conjugated anti-Ly-6G antibody (1A8; BioLegend) and AF700-conjugated anti-CD11b antibody (M1/80; BioLegend) were added at 0.01 mg/ml in 100 μl PBS per sample and then whole tissue was stained for 24 h at 4°C. Samples were washed three times with PBS, mounted whole onto slides in Prolong Glass Antifade Mountant (Thermo Fisher Scientific), and cured for 2 days at room temperature. Images were acquired on a DMI 6000 laser-scanning confocal microscope (Leica) with a 10× objective. The center of the sample was determined in the Z direction and then imaged. Images were analyzed using ImageJ v2.1. Adjustments for brightness and contrast were applied to the entire image. No threshold manipulation was performed.
Statistics
Statistical analyses were performed using Prism v9.0 (GraphPad Software). Independent groups were compared by Mann–Whitney U test or Kruskal–Wallis test with Dunn’s multiple comparisons test. Survival curves were compared by Mantel–Cox test. Statistical significance is denoted as * (P < 0.05), ** (P < 0.01), *** (P < 0.001), **** (P < 0.0001), or ns (not significant).
Online supplemental material
Fig. S1 further characterizes Yp-infected Tnfr1−/− and Tnfr2−/− mice. Fig. S2 details the chimerism of BM chimeras associated with Fig. 2. Fig. S3 details the chimerism of BM chimeras associated with Fig. 3, as well as depicting survival data of mutant Yp infections. Fig. S4 depicts cytokine analyses in mixed BM chimeras associated with Fig. 4, as well as cytokine data of Tlr4−/− mice. Fig. S5 details additional data from IL-1 chimeras associated with Figs. 5 and 6.
Acknowledgments
We thank Enrico Radaelli for constructive input and the staff at the PennVet Comparative Pathology Core for their help in preparing and analyzing the histological samples. We thank members of the Brodsky and Shin Labs for the scientific discussion.
This work was supported by National Institutes of Health (NIH) awards R01AI128530 (I.E. Brodsky), R01AI1139102A1 (I.E. Brodsky), and R01DK123528 (I.E. Brodsky); a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award (I.E. Brodsky and S. Shin); Mark Foundation grant 19-011MIA (I.E. Brodsky), the Foundation Blanceflor Postdoctoral Scholarship (D. Sorobetea), the Swedish Society for Medical Research postdoctoral fellowship (D. Sorobetea), and the Sweden-America Foundation J. Sigfrid Edström award (D. Sorobetea); NIH National Research Service Award (NRSA) F31AI160741-01 (R. Matsuda); NIH T32 AI141393-2 (R. Matsuda); F32 AI164655 (J.P. Grayczyk); NIH NRSA F31AI161319 (B. Herrmann); and National Science Foundation Graduate Research Fellowships Program Award (S.T. Peterson); NIH T32 AI141393-03 (J. Zhang); NIH awards R21AI151476 (S. Shin), R01AI118861 (S. Shin), R01AI123243 (S. Shin); and in part by the intramural research program of the National Institute of Allergy and Infectious Diseases (K.D. Mayer-Barber, A.C. Bohrer). The veterinary pathologists performing the histopathological analysis are supported by the Abramson Cancer Center Support Grant (P30 CA016520). The scanner used for whole-slide imaging and the image visualization software was supported by an NIH Shared Instrumentation Grant (S10 OD023465-01A1).
Author contributions: R. Matsuda, D. Sorobetea, and J. Zhang conceived the study, devised and performed experiments, and analyzed data. S.T. Peterson, J.P. Grayczyk, W. Yost, N. Apenes, M.E. Kovalik, B. Herrmann, and R.J. O’Neill performed experiments. M. Lanza and C.-A. Assenmacher performed the histology and histopathological scoring. S. Shin and I.E. Brodsky acquired funding and conceived and directed the study. A.C. Bohrer and K.D. Mayer-Barber provided key reagents and expertise. R. Matsuda, D. Sorobetea, J. Zhang, S. Shin, and I.E. Brodsky wrote the original draft. All authors reviewed and edited the manuscript.
Data availability
Data supporting the findings of this research article are available upon request to the corresponding author.
References
- Adams, K.N., Takaki K., Connolly L.E., Wiedenhoft H., Winglee K., Humbert O., Edelstein P.H., Cosma C.L., and Ramakrishnan L.. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 145:39–53. 10.1016/j.cell.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa, E., Karasawa T., Watanabe S., Komada T., Kimura H., Kamata R., Ito H., Hishida E., Yamada N., Kasahara T., et al. 2020. GSDME-dependent incomplete pyroptosis permits selective IL-1α release under caspase-1 inhibition. iScience. 23:101070. 10.1016/j.isci.2020.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi, R.M., and Ma T.Y.. 2007. IL-1β causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 178:4641–4649. 10.4049/jimmunol.178.7.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algood, H.M.S., Lin P.L., and Flynn J.L.. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41:S189–S193. 10.1086/429994 [DOI] [PubMed] [Google Scholar]
- Ali, T., Kaitha S., Mahmood S., Ftesi A., Stone J., and Bronze M.S.. 2013. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient Saf. 5:79–99. 10.2147/DHPS.S28801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, S., and Williams P.. 2016. Yersinia virulence factors - A sophisticated arsenal for combating host defences. F1000Res. 5:F1000 Faculty Rev-1370. 10.12688/f1000research.8466.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth, I.B., and Heesemann J.. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. 181:333–338. 10.1007/BF00191545 [DOI] [PubMed] [Google Scholar]
- Barnett, K.C., Xie Y., Asakura T., Song D., Liang K., Taft-Benz S.A., Guo H., Yang S., Okuda K., Gilmore R.C., et al. 2022. An epithelial-immune circuit amplifies inflammasome and IL-6 responses to SARS-CoV-2. Cell Host Microbe. 31:243–259.e6. 10.1016/j.chom.2022.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, K.C., Fontana M.F., Portman J.L., Dugan A.S., and Vance R.E.. 2013. IL-1α signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J. Immunol. 190:6329–6339. 10.4049/jimmunol.1300100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean, A.G., Roach D.R., Briscoe H., France M.P., Korner H., Sedgwick J.D., and Britton W.J.. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504–3511. 10.4049/jimmunol.162.6.3504 [DOI] [PubMed] [Google Scholar]
- Berger, S.B., Kasparcova V., Hoffman S., Swift B., Dare L., Schaeffer M., Capriotti C., Cook M., Finger J., Hughes-Earle A., et al. 2014. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192:5476–5480. 10.4049/jimmunol.1400499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky, M., Luski L., Fishman D., White R.M., Ziv-Sokolovskaya N., Dotan S., Rider P., Kaplanov I., Aychek T., Dinarello C.A., et al. 2014. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 63:598–609. 10.1136/gutjnl-2012-303329 [DOI] [PubMed] [Google Scholar]
- Black, D.S., and Bliska J.B.. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515–527. 10.1046/j.1365-2958.2000.02021.x [DOI] [PubMed] [Google Scholar]
- Bliska, J.B., Guan K.L., Dixon J.E., and Falkow S.. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA. 88:1187–1191. 10.1073/pnas.88.4.1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska, J.B., and Black D.S.. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681–685. 10.1128/iai.63.2.681-685.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska, J.B., Brodsky I.E., and Mecsas J.. 2021. Role of the Yersinia pseudotuberculosis virulence plasmid in pathogen-phagocyte interactions in mesenteric lymph nodes. Ecosal Plus. 9:eESP00142021. 10.1128/ecosalplus.ESP-0014-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer, A.C., Tocheny C., Assmann M., Ganusov V.V., and Mayer-Barber K.D.. 2018. Cutting edge: IL-1R1 mediates host resistance to Mycobacterium tuberculosis by trans-protection of infected cells. J. Immunol. 201:1645–1650. 10.4049/jimmunol.1800438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson, D.L., Simon S.I., Hodzic E., Ballantyne C.M., and Barthold S.W.. 2002. Kinetics of CD11b/CD18 up-regulation during infection with the agent of human granulocytic ehrlichiosis in mice. Lab. Invest. 82:303–311. 10.1038/labinvest.3780424 [DOI] [PubMed] [Google Scholar]
- Botha, T., and Ryffel B.. 2003. Reactivation of latent tuberculosis infection in TNF-deficient mice. J. Immunol. 171:3110–3118. 10.4049/jimmunol.171.6.3110 [DOI] [PubMed] [Google Scholar]
- Caronni, N., La Terza F., Vittoria F.M., Barbiera G., Mezzanzanica L., Cuzzola V., Barresi S., Pellegatta M., Canevazzi P., Dunsmore G., et al. 2023. IL-1β+ macrophages fuel pathogenic inflammation in pancreatic cancer. Nature. 623:415–422. 10.1038/s41586-023-06685-2 [DOI] [PubMed] [Google Scholar]
- Chakravarty, S.D., Zhu G., Tsai M.C., Mohan V.P., Marino S., Kirschner D.E., Huang L., Flynn J., and Chan J.. 2008. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect. Immun. 76:916–926. 10.1128/IAI.01011-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G., and Goeddel D.V.. 2002. TNF-R1 signaling: A beautiful pathway. Science. 296:1634–1635. 10.1126/science.1071924 [DOI] [PubMed] [Google Scholar]
- Chen, K.W., Groß C.J., Sotomayor F.V., Stacey K.J., Tschopp J., Sweet M.J., and Schroder K.. 2014. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8:570–582. 10.1016/j.celrep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- Chen, K.W., Demarco B., Ramos S., Heilig R., Goris M., Grayczyk J.P., Assenmacher C.A., Radaelli E., Joannas L.D., Henao-Mejia J., et al. 2021. RIPK1 activates distinct gasdermins in macrophages and neutrophils upon pathogen blockade of innate immune signaling. Proc. Natl. Acad. Sci. USA. 118:e2101189118. 10.1073/pnas.2101189118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson, D.E., Li Y., and Yuan J.. 2014. Control of life-or-death decisions by RIP1 kinase. Annu. Rev. Physiol. 76:129–150. 10.1146/annurev-physiol-021113-170259 [DOI] [PubMed] [Google Scholar]
- Clay, H., Volkman H.E., and Ramakrishnan L.. 2008. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 29:283–294. 10.1016/j.immuni.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver, A.M., Casson C.N., Nguyen H.T., Duda M.M., and Shin S.. 2015. IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis. Proc. Natl. Acad. Sci. USA. 112:7557–7562. 10.1073/pnas.1501289112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J.M., and Ramakrishnan L.. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 136:37–49. 10.1016/j.cell.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, K.M., Mohammadi S., and Isberg R.R.. 2015. Community behavior and spatial regulation within a bacterial microcolony in deep tissue sites serves to protect against host attack. Cell Host Microbe. 17:21–31. 10.1016/j.chom.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanghe, T., Dondelinger Y., and Bertrand M.J.M.. 2020. RIPK1 kinase-dependent death: A symphony of phosphorylation events. Trends Cell Biol. 30:189–200. 10.1016/j.tcb.2019.12.009 [DOI] [PubMed] [Google Scholar]
- Deyerle, K.L., Sims J.E., Dower S.K., and Bothwell M.A.. 1992. Pattern of IL-1 receptor gene expression suggests role in noninflammatory processes. J. Immunol. 149:1657–1665. 10.4049/jimmunol.149.5.1657 [DOI] [PubMed] [Google Scholar]
- Diedrich, C.R., O’Hern J., and Wilkinson R.J.. 2016. HIV-1 and the Mycobacterium tuberculosis granuloma: A systematic review and meta-analysis. Tuberculosis. 98:62–76. 10.1016/j.tube.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Dinarello, C.A. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281:8–27. 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube, P.H., Revell P.A., Chaplin D.D., Lorenz R.G., and Miller V.L.. 2001. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc. Natl. Acad. Sci. USA. 98:10880–10885. 10.1073/pnas.191214498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ea, C.K., Deng L., Xia Z.P., Pineda G., and Chen Z.J.. 2006. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell. 22:245–257. 10.1016/j.molcel.2006.03.026 [DOI] [PubMed] [Google Scholar]
- El-Maraghi, N.R.H., and Mair N.S.. 1979. The histopathology of enteric infection with Yersinia pseudotuberculosis. Am. J. Clin. Pathol. 71:631–639. 10.1093/ajcp/71.6.631 [DOI] [PubMed] [Google Scholar]
- Erickson, S.L., de Sauvage F.J., Kikly K., Carver-Moore K., Pitts-Meek S., Gillett N., Sheehan K.C., Schreiber R.D., Goeddel D.V., and Moore M.W.. 1994. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 372:560–563. 10.1038/372560a0 [DOI] [PubMed] [Google Scholar]
- Evavold, C.L., Ruan J., Tan Y., Xia S., Wu H., and Kagan J.C.. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 48:35–44.e6. 10.1016/j.immuni.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey, E., and Doyle S.L.. 2019. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front. Immunol. 10:1426. 10.3389/fimmu.2019.01426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, J.L., Goldstein M.M., Chan J., Triebold K.J., Pfeffer K., Lowenstein C.J., Schreiber R., Mak T.W., and Bloom B.R.. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 2:561–572. 10.1016/1074-7613(95)90001-2 [DOI] [PubMed] [Google Scholar]
- Franchi, L., Kamada N., Nakamura Y., Burberry A., Kuffa P., Suzuki S., Shaw M.H., Kim Y.G., and Núñez G.. 2012. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 13:449–456. 10.1038/ni.2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt, M.M., Ebert T.S., Chauhan D., Schmidt T., Schmid-Burgk J.L., Rapino F., Robertson A.A., Cooper M.A., Graf T., and Hornung V.. 2016. Human monocytes engage an alternative inflammasome pathway. Immunity. 44:833–846. 10.1016/j.immuni.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Galyov, E.E., Håkansson S., Forsberg A., and Wolf-Watz H.. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 361:730–732. 10.1038/361730a0 [DOI] [PubMed] [Google Scholar]
- Glaccum, M.B., Stocking K.L., Charrier K., Smith J.L., Willis C.R., Maliszewski C., Livingston D.J., Peschon J.J., and Morrissey P.J.. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159:3364–3371. 10.4049/jimmunol.159.7.3364 [DOI] [PubMed] [Google Scholar]
- Green, S.P., Hartland E.L., Robins-Browne R.M., and Phillips W.A.. 1995. Role of YopH in the suppression of tyrosine phosphorylation and respiratory burst activity in murine macrophages infected with Yersinia enterocolitica. J. Leukoc. Biol. 57:972–977. 10.1002/jlb.57.6.972 [DOI] [PubMed] [Google Scholar]
- Greene, C.M., Meachery G., Taggart C.C., Rooney C.P., Coakley R., O’Neill S.J., and McElvaney N.G.. 2000. Role of IL-18 in CD4+ T lymphocyte activation in sarcoidosis. J. Immunol. 165:4718–4724. 10.4049/jimmunol.165.8.4718 [DOI] [PubMed] [Google Scholar]
- Grosdent, N., Maridonneau-Parini I., Sory M.P., and Cornelis G.R.. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165–4176. 10.1128/IAI.70.8.4165-4176.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaidan, F.R., Chakroun G.S., Dbaibo W., El-Assaad W., and El-Sabban M.E.. 2001. IL-1 activates two phospholipid signaling pathways in intestinal epithelial cells. Inflamm. Res. 50:375–381. 10.1007/PL00000259 [DOI] [PubMed] [Google Scholar]
- Horai, R., Asano M., Sudo K., Kanuka H., Suzuki M., Nishihara M., Takahashi M., and Iwakura Y.. 1998. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 187:1463–1475. 10.1084/jem.187.9.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., and Akira S.. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752. 10.4049/jimmunol.162.7.3749 [DOI] [PubMed] [Google Scholar]
- Jain, A., Irizarry-Caro R.A., McDaniel M.M., Chawla A.S., Carroll K.R., Overcast G.R., Philip N.H., Oberst A., Chervonsky A.V., Katz J.D., and Pasare C.. 2020. T cells instruct myeloid cells to produce inflammasome-independent IL-1β and cause autoimmunity. Nat. Immunol. 21:65–74. 10.1038/s41590-019-0559-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, D.X., Yamashiro L.H., Chen K.J., Mukaida N., Kramnik I., Darwin K.H., and Vance R.E.. 2019. Type I interferon-driven susceptibility to Mycobacterium tuberculosis is mediated by IL-1Ra. Nat. Microbiol. 4:2128–2135. 10.1038/s41564-019-0578-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, C., Meinzer U., Montcuquet N., Thachil E., Château D., Thiébaut R., Roy M., Alnabhani Z., Berrebi D., Dussaillant M., et al. 2012. Yersinia pseudotuberculosis disrupts intestinal barrier integrity through hematopoietic TLR-2 signaling. J. Clin. Invest. 122:2239–2251. 10.1172/JCI58147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler, V., Sappino A.P., Grau G.E., Piguet P.F., and Vassalli P.. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 56:731–740. 10.1016/0092-8674(89)90676-4 [DOI] [PubMed] [Google Scholar]
- Kojima, M., Morita Y., Shimizu K., Yoshida T., Yamada I., Togo T., and Johshita T.. 2007. Immunohistological findings of suppurative granulomas of Yersinia enterocolitica appendicitis: A report of two cases. Pathol. Res. Pract. 203:115–119. 10.1016/j.prp.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Kusnadi, A., Park S.H., Yuan R., Pannellini T., Giannopoulou E., Oliver D., Lu T., Park-Min K.H., and Ivashkiv L.B.. 2019. The cytokine TNF promotes transcription factor SREBP activity and binding to inflammatory genes to activate macrophages and limit tissue repair. Immunity. 51:241–257.e9. 10.1016/j.immuni.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamps, L.W., Madhusudhan K.T., Greenson J.K., Pierce R.H., Massoll N.A., Chiles M.C., Dean P.J., and Scott M.A.. 2001. The role of Yersinia enterocolitica and Yersinia pseudotuberculosis in granulomatous appendicitis: A histologic and molecular study. Am. J. Surg. Pathol. 25:508–515. 10.1097/00000478-200104000-00011 [DOI] [PubMed] [Google Scholar]
- Lee, Y.-S., Yang H., Yang J.Y., Kim Y., Lee S.H., Kim J.H., Jang Y.J., Vallance B.A., and Kweon M.N.. 2015. Interleukin-1 (IL-1) signaling in intestinal stromal cells controls KC/CXCL1 secretion, which correlates with recruitment of IL-22- secreting neutrophils at early stages of Citrobacter rodentium infection. Infect. Immun. 83:3257–3267. 10.1128/IAI.00670-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, P.L., Myers A., Smith L., Bigbee C., Bigbee M., Fuhrman C., Grieser H., Chiosea I., Voitenek N.N., Capuano S.V., et al. 2010. TNF neutralization results in disseminated disease during acute and latent M. tuberculosis infection with normal granuloma structure. Arthritis Rheum. 62:340–350. 10.1002/art.27271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Boyer M.A., Holmgren A.M., and Shin S.. 2020. Legionella-infected macrophages engage the alveolar epithelium to metabolically reprogram myeloid cells and promote antibacterial inflammation. Cell Host Microbe. 28:683–698.e6. 10.1016/j.chom.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, B.S., and Chung K.F.. 2006. Blood neutrophil activation markers in severe asthma: Lack of inhibition by prednisolone therapy. Respir. Res. 7:59. 10.1186/1465-9921-7-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matty, M.A., Roca F.J., Cronan M.R., and Tobin D.M.. 2015. Adventures within the speckled band: Heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunol. Rev. 264:276–287. 10.1111/imr.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber, K.D., Barber D.L., Shenderov K., White S.D., Wilson M.S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., et al. 2010. Caspase-1 independent IL-1β production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184:3326–3330. 10.4049/jimmunol.0904189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber, K.D., Andrade B.B., Barber D.L., Hieny S., Feng C.G., Caspar P., Oland S., Gordon S., and Sher A.. 2011. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 35:1023–1034. 10.1016/j.immuni.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecsas, J., Raupach B., and Falkow S.. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269–1281. 10.1046/j.1365-2958.1998.00891.x [DOI] [PubMed] [Google Scholar]
- Meinzer, U., Barreau F., Esmiol-Welterlin S., Jung C., Villard C., Léger T., Ben-Mkaddem S., Berrebi D., Dussaillant M., Alnabhani Z., et al. 2012. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 11:337–351. 10.1016/j.chom.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Monack, D.M., Mecsas J., Ghori N., and Falkow S.. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA. 94:10385–10390. 10.1073/pnas.94.19.10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, C., VanDussen K.L., Miyoshi H., and Stappenbeck T.S.. 2014. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 7:818–828. 10.1038/mi.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, S., Keitany G., Li Y., Wang Y., Ball H.L., Goldsmith E.J., and Orth K.. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 312:1211–1214. 10.1126/science.1126867 [DOI] [PubMed] [Google Scholar]
- Ofengeim, D., and Yuan J.. 2013. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 14:727–736. 10.1038/nrm3683 [DOI] [PubMed] [Google Scholar]
- Orth, K., Palmer L.E., Bao Z.Q., Stewart S., Rudolph A.E., Bliska J.B., and Dixon J.E.. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 285:1920–1923. 10.1126/science.285.5435.1920 [DOI] [PubMed] [Google Scholar]
- Orzalli, M.H., Smith A., Jurado K.A., Iwasaki A., Garlick J.A., and Kagan J.C.. 2018. An antiviral branch of the IL-1 signaling pathway restricts immune-evasive virus replication. Mol. Cell. 71:825–840.e6. 10.1016/j.molcel.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overcast, G.R., Meibers H.E., Eshleman E.M., Saha I., Waggoner L., Patel K.N., Jain V.G., Haslam D.B., Alenghat T., VanDussen K.L., and Pasare C.. 2023. IEC-intrinsic IL-1R signaling holds dual roles in regulating intestinal homeostasis and inflammation. J. Exp. Med. 220:e20212523. 10.1084/jem.20212523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán, A.J., and Ramakrishnan L.. 2018. The Formation and function of granulomas. Annu. Rev. Immunol. 36:639–665. 10.1146/annurev-immunol-032712-100022 [DOI] [PubMed] [Google Scholar]
- Palmer, L.E., Hobbie S., Galán J.E., and Bliska J.B.. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953–965. 10.1046/j.1365-2958.1998.00740.x [DOI] [PubMed] [Google Scholar]
- Di Paolo, N.C., Shafiani S., Day T., Papayannopoulou T., Russell D.W., Iwakura Y., Sherman D., Urdahl K., and Shayakhmetov D.M.. 2015. Interdependence between interleukin-1 and tumor necrosis factor regulates TNF-dependent control of Mycobacterium tuberculosis infection. Immunity. 43:1125–1136. 10.1016/j.immuni.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, M.A., Wilhelm L.B., Kummer L.W., Szaba F.M., Mullarky I.K., and Smiley S.T.. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun. 74:3381–3386. 10.1128/IAI.00185-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, H.J. and Smith A.M.. 2013. The role of the innate immune system in granulomatous disorders. Front. Immunol. 4:120. 10.3389/fimmu.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, L.W., Philip N.H., Dillon C.P., Bertin J., Gough P.J., Green D.R., and Brodsky I.E.. 2016. Cell-extrinsic TNF collaborates with TRIF signaling to promote yersinia-induced apoptosis. J. Immunol. 197:4110–4117. 10.4049/jimmunol.1601294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, L.W., Philip N.H., DeLaney A., Wynosky-Dolfi M.A., Asklof K., Gray F., Choa R., Bjanes E., Buza E.L., Hu B., et al. 2017. RIPK1-dependent apoptosis bypasses pathogen blockade of innate signaling to promote immune defense. J. Exp. Med. 214:3171–3182. 10.1084/jem.20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, K., Matsuyama T., Kündig T.M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P.S., Krönke M., and Mak T.W.. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73:457–467. 10.1016/0092-8674(93)90134-C [DOI] [PubMed] [Google Scholar]
- Pham, T.H.M., Brewer S.M., Thurston T., Massis L.M., Honeycutt J., Lugo K., Jacobson A.R., Vilches-Moure J.G., Hamblin M., Helaine S., and Monack D.M.. 2020. Salmonella-driven polarization of granuloma macrophages antagonizes TNF-mediated pathogen restriction during persistent infection. Cell Host Microbe. 27:54–67.e5. 10.1016/j.chom.2019.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N.H., Dillon C.P., Snyder A.G., Fitzgerald P., Wynosky-Dolfi M.A., Zwack E.E., Hu B., Fitzgerald L., Mauldin E.A., Copenhaver A.M., et al. 2014. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl. Acad. Sci. USA. 111:7385–7390. 10.1073/pnas.1403252111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin, R.E. 2010. General Categories of Cytologic Interpretation. In Canine and Feline Cytology: A Color Atlas and Interpretation Guide. Saunders W.B., editor. Second edition. Elsevier, St. Louis, MO. pp. 15–25. 10.1016/B978-141604985-2.50001-3. [Google Scholar]
- Ratner, D., Orning M.P., Starheim K.K., Marty-Roix R., Proulx M.K., Goguen J.D., and Lien E.. 2016. Manipulation of interleukin-1β and interleukin-18 production by yersinia pestis effectors YopJ and YopM and redundant impact on virulence. J. Biol. Chem. 291:9894–9905. 10.1074/jbc.M115.697698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, T., Jones M., Akhtar Y., and Pollard J.. 2018. Suspicious Yersinia granulomatous enterocolitis mimicking appendicitis. BMJ Case Rep. 2018:bcr2018224177. 10.1136/bcr-2018-224177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach, D.R., Bean A.G., Demangel C., France M.P., Briscoe H., and Britton W.J.. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620–4627. 10.4049/jimmunol.168.9.4620 [DOI] [PubMed] [Google Scholar]
- Rohena, F.J., Almira-Suárez M.I., and González-Keelan C.. 2014. Granulomatous enterocolitis secondary to Yersinia in an 11-year-old boy from Puerto Rico, confirmed by PCR: A case report. P. R. Health Sci. J. 33:27–30. [PubMed] [Google Scholar]
- Rosqvist, R., Bölin I., and Wolf-Watz H.. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: A virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139–2143. 10.1128/iai.56.8.2139-2143.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist, R., Forsberg A., and Wolf-Watz H.. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562–4569. 10.1128/iai.59.12.4562-4569.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy, A.T., Briseño C.G., Lee J.S., Ng D., Manieri N.A., Kc W., Wu X., Thomas S.R., Lee W.L., Turkoz M., et al. 2013. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 14:937–948. 10.1038/ni.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa, M., Kessler S., Sadler T., West G., Homer C., McDonald C., de la Motte C., Fiocchi C., and Stylianou E.. 2015. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am. J. Pathol. 185:1624–1637. 10.1016/j.ajpath.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina, N.V., and Pamer E.G.. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311–317. 10.1038/ni1309 [DOI] [PubMed] [Google Scholar]
- Silvério, D., Gonçalves R., Appelberg R., and Saraiva M.. 2021. Advances on the role and applications of interleukin-1 in tuberculosis. MBio. 12:e0313421. 10.1128/mBio.03134-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet, M., and Falkow S.. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414–4417. 10.1128/iai.60.10.4414-4417.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorobetea, D., Matsuda R., Peterson S.T., Grayczyk J.P., Rao I., Krespan E., Lanza M., Assenmacher C.A., Mack M., Beiting D.P., et al. 2023. Inflammatory monocytes promote granuloma control of Yersinia infection. Nat. Microbiol. 8:666–678. 10.1038/s41564-023-01338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, I., Yamada H., Hua S., and Mizuno S.. 2001. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol. Immunol. 45:743–750. 10.1111/j.1348-0421.2001.tb01310.x [DOI] [PubMed] [Google Scholar]
- Taheri, N., Fahlgren A., and Fällman M.. 2016. Yersinia pseudotuberculosis blocks neutrophil degranulation. Infect. Immun. 84:3369–3378. 10.1128/IAI.00760-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, K., and Akira S.. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9. 10.1016/j.smim.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Tobin, D.M., Vary J.C. Jr., Ray J.P., Walsh G.S., Dunstan S.J., Bang N.D., Hagge D.A., Khadge S., King M.C., Hawn T.R., et al. 2010. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 140:717–730. 10.1016/j.cell.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, D.M., Roca F.J., Oh S.F., McFarland R., Vickery T.W., Ray J.P., Ko D.C., Zou Y., Bang N.D., Chau T.T., et al. 2012. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 148:434–446. 10.1016/j.cell.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, M.J., Fallon P.G., Matthews D.J., Jolin H.E., and McKenzie A.N.. 2000. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191:1069–1076. 10.1084/jem.191.6.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimer, G.I., Weng D., Paquette S.W., Vanaja S.K., Rathinam V.A., Aune M.H., Conlon J.E., Burbage J.J., Proulx M.K., Liu Q., et al. 2012. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 37:96–107. 10.1016/j.immuni.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich, R., and Green D.R.. 2014. The two faces of receptor interacting protein kinase-1. Mol. Cell. 56:469–480. 10.1016/j.molcel.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, H., Mizumo S., Horai R., Iwakura Y., and Sugawara I.. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1 α/β double-knockout mice. Lab. Invest. 80:759–767. 10.1038/labinvest.3780079 [DOI] [PubMed] [Google Scholar]
- Yan, S.R., Joseph R.R., Wang J., and Stadnyk A.W.. 2006. Differential pattern of inflammatory molecule regulation in intestinal epithelial cells stimulated with IL-1. J. Immunol. 177:5604–5611. 10.4049/jimmunol.177.8.5604 [DOI] [PubMed] [Google Scholar]
- Yeap, H.W., and Chen K.W.. 2022. RIPK1 and RIPK3 in antibacterial defence. Biochem. Soc. Trans. 50:1583–1594. 10.1042/BST20211242 [DOI] [PubMed] [Google Scholar]
- Yoon, J.W., Pahl M.V., and Vaziri N.D.. 2007. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 71:167–172. 10.1038/sj.ki.5002019 [DOI] [PubMed] [Google Scholar]
- Yoon, S., Liu Z., Eyobo Y., and Orth K.. 2003. Yersinia effector YopJ inhibits yeast MAPK signaling pathways by an evolutionarily conserved mechanism. J. Biol. Chem. 278:2131–2135. 10.1074/jbc.M209905200 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Khairallah C., Sheridan B.S., van der Velden A.W.M., and Bliska J.B.. 2018. CCR2+ inflammatory monocytes are recruited to Yersinia pseudotuberculosis pyogranulomas and dictate adaptive responses at the expense of innate immunity during oral infection. Infect. Immun. 86:e00782-17. 10.1128/IAI.00782-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivaki, D., Borriello F., Chow O.A., Doran B., Fleming I., Theisen D.J., Pallis P., Shalek A.K., Sokol C.L., Zanoni I., and Kagan J.C.. 2020. Inflammasomes within hyperactive murine dendritic cells stimulate long-lived T cell-mediated anti-tumor immunity. Cell Rep. 33:108381. 10.1016/j.celrep.2020.108381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this research article are available upon request to the corresponding author.