Abstract
Background
To investigate evidence of residual viral infection, intrathecal immune activation, central nervous system (CNS) injury, and humoral responses in cerebrospinal fluid (CSF) and plasma in patients recovering from coronavirus disease 2019 (COVID-19), with or without neurocognitive post-COVID condition (PCC).
Methods
Thirty-one participants (25 with neurocognitive PCC) underwent clinical examination, lumbar puncture, and venipuncture ≥3 months after COVID-19 symptom onset. Healthy volunteers were included. CSF and plasma severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid and spike antigen (N-Ag, S-Ag), and CSF biomarkers of immune activation and neuronal injury were analyzed.
Results
SARS-CoV-2 N-Ag or S-Ag were undetectable in all samples and no participant had pleocytosis. We detected no significant differences in CSF and plasma cytokine concentrations, albumin ratio, IgG index, neopterin, β2M, or in CSF biomarkers of neuronal injury and astrocytic damage. Furthermore, principal component analysis (PCA1) analysis did not indicate any significant differences between the study groups in the marker sets cytokines, neuronal markers, or anti-cytokine autoantibodies.
Conclusions
We found no evidence of ongoing viral replication, immune activation, or CNS injury in plasma or CSF in patients with neurocognitive PCC compared with COVID-19 controls or healthy volunteers, suggesting that neurocognitive PCC is a consequence of events suffered during acute COVID-19 rather than persistent viral CNS infection or residual CNS inflammation.
Keywords: SARS-CoV-2, COVID-19, central nervous system, cerebrospinal fluid, post-COVID condition
We found no evidence of ongoing viral replication, immune activation, or CNS injury in plasma or CSF in patients with neurocognitive post-COVID condition compared with COVID-19 controls or healthy volunteers.
Following the initial wave of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, reports indicated that a number of heterogeneous symptoms persisted or emerged weeks to months after the acute phase of the infection in a subset of individuals recovering from coronavirus disease 2019 (COVID-19) [1]. Residual symptoms such as dyspnea, chest pain, palpitations, anosmia, dysgeusia, paresthesia, and cognitive impairment, collectively referred to as long-COVID, has subsequently been named post-COVID condition (PCC) and has emerged as a major public health concern [2, 3].
Epidemiological studies have estimated that up to one-third of patients may present with symptoms of PCC as they recuperate from their primary SARS-CoV-2 infection. Neurological or neurocognitive symptoms such as fatigue, “brain fog,” headaches, and cognitive changes, including memory or concentration, are integral parts of PCC and have been reported in up to two-thirds of patients [4–6].
The specific risk factors for developing PCC are still largely unknown [7]. Although postinfectious outcomes are commonly observed in patients requiring intensive care due to other acute infections or other conditions, PCC is not limited to individuals with severe COVID-19 and has been described in patients with mild as well as moderate COVID-19 [7, 8].
The underlying pathogenesis of PCC is under intense investigation but to a large extent is still not understood. Consequently, it is unclear whether PCC is attributable to processes uniquely associated with SARS-CoV-2 infection or from host responses to infection of a more universal character. The heterogeneity of PCC symptoms suggests that multiple mechanisms are separately or collaboratively involved in different individuals.
Various factors, such as direct central nervous system (CNS) damage caused by viral neuroinvasion, indirect outcomes of systemic or intrathecal inflammatory responses, microvascular injuries and/or thromboembolic events, and misguided host immunological response, have been identified as potential contributors to CNS pathogenesis during acute SARS-CoV-2 infection [9, 10]. However, it remains unclear whether neurological or neurocognitive sequelae in PCC represent an ongoing infectious or inflammatory process within the CNS or are consequences of earlier events triggered by SARS-CoV-2 infection.
Using plasma biomarkers of CNS injury, we have previously shown that neurofilament light-chain (NfL) and glial fibrillary acidic protein (GFAp) normalized in all patients from acute infection to postinfection follow-up [11]. Furthermore, we found no significant correlations between biomarkers of brain injury and persisting neurocognitive symptoms postinfection, suggesting the absence of brain damage during follow-up despite cognitive impairment [11].
However, while plasma analysis has the advantage of accessibility and can measure CNS injury via biomarker leakage through blood-brain barrier [12], cerebrospinal fluid (CSF) is usually considered more closely reflective of biochemical changes in the brain as it communicates with brain interstitial fluid [13]. In our previous investigations we observed the presence of SARS-CoV-2 nucleocapsid antigen (N-Ag) in the CSF in the majority of patients during the acute phase of the infection, in concentrations closely correlated to plasma levels. Furthermore, we found elevated CSF biomarkers of immune activation in most individuals, with patients exhibiting CNS symptoms displaying a more pronounced inflammatory biomarker profile. This suggests an association between viral antigen, inflammation, and CNS dysfunction during acute COVID-19 [14]. However, the detection of viral RNA in CSF is uncommon, and the extent of viral neuroinvasion during SARS-CoV-2 infection (if it occurs at all) as well as the possibility of viral persistence within the CNS remain contentious [14–17].
The objective of this longitudinal cohort study was to comprehensively investigate any evidence of residual viral infection, intrathecal immune activation, CNS injury, and humoral responses in CSF and plasma in patients recovering from COVID-19, with or without neurocognitive PCC, as well as in healthy volunteers.
METHODS
Study Population
In this single-center, cross-sectional study, we identified participants aged ≥18 years with confirmed SARS-CoV-2 infection who had been prospectively included in a longitudinal research cohort [14, 18] and had undergone clinical examination, lumbar puncture, and venipuncture ≥3 months after COVID-19 symptom onset. Patients were either monitored longitudinally from initial admission due to COVID-19, or by outpatient referral due to persisting neurocognitive symptoms of PCC, at the Department of Infectious Diseases, Sahlgrenska University Hospital, Gothenburg, Sweden. Participants were diagnosed with COVID-19 between March 2020 and May 2021. Patients with active neurological or neurocognitive diseases before COVID-19 were not included. COVID-19 disease severity was classified based on the World Health Organization clinical progression scale [19], with mild disease indicating ambulatory patients, moderate disease indicating hospitalized patients receiving oxygen therapy, and severe disease indicating hospitalized patients requiring high-flow nasal oxygen, admission to intensive care unit, and/or on mechanical ventilation.
From February 2021 to November 2021, we conducted study visits that involved clinical neurological examination, lumbar punctures, and venipunctures. An infectious disease physician and specialist nurse evaluated PCC symptoms by conducting patient interviews, and medical history and physical status were recorded in an electronic medical database. In addition, patients completed a self-report symptom questionnaire as previously reported [11], with symptoms subjectively graded from 1 (mild) to 5 (severe). To be included in the PCC group, patients were required to have at least 2 points on the subjective symptom grading. Information on specific symptoms, disease severity, and recovery was collected, and study participants were categorized into 2 groups based on persisting neurocognitive symptoms at follow-up: PCC (ongoing neurocognitive sequelae) and COVID-controls (reporting full recovery). Using an advertisement on newspaper platforms, we recruited a control group of healthy, age-matched volunteers without known history of COVID-19 and with negative real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 RNA and serology if unvaccinated.
The study was conducted in accordance with the ethical principles set out in the Declaration of Helsinki. The study was approved by the Swedish Ethical Review Authority (2020-05050). All participants provided written informed consent.
Virus Detection
SARS-CoV-2 infection was confirmed with RT-PCR analysis of nasal and throat swab specimens as previously reported [20]. SARS-CoV-2 RNA was analyzed by RT-PCR in CSF and plasma samples as previously described [17].
Detection of SARS-CoV-2 nucleocapsid and spike proteins was performed using MSD S-PLEX CoV-2 N and MSD S-PLEX CoV-2 assay kits (Meso Scale Discovery). The assays were run according to protocols in the kit package inserts (Supplementary Methods). The CSF and plasma samples were run undiluted (25 μL per well). Sample quantitation was achieved using a calibration curve generated using a recombinant antigen standard. For graphing and analyses, any concentrations below the limit of detection (LOD) were assigned the LOD value, and any concentrations above the highest calibration standard were assigned its value. LOD values and assay cutoffs (concentrations used for classifying samples as antigen positive) were previously established [21]: N, LOD = 0.16 pg/mL, cutoff = 0.32 pg/mL; S, LOD = 0.28 pg/mL, cutoff = 0.41 pg/mL.
Biomarker Analyses
Plasma and CSF were measured in single replicates on 19 MSD (Meso Scale Discovery) MULTI-ARRAY panels; 1 mL of plasma and 1 mL of CSF was available for the study. Each plate included an 8-point calibration curve in duplicates and quality control samples. Panels included commercially available S-PLEX, V-PLEX, U-PLEX, and R-PLEX panels and selected panels that are currently under development (see Supplementary Table 1, which also includes sample dilution factors). Assays were carried out according to the protocols in the kit packages (www.mesoscale.com) (Supplementary Methods). Autoantibodies to cytokines were measured by immobilizing cytokines on the carbon surface of 10-spot MULTI-ARRAY plates and detecting autoantibodies with a SULFO-TAG labeled anti-human Ig antibody.
C-reactive protein (mg/L) and lymphocyte count (×109/L) were measured by routine clinical methods in plasma. CSF analyses of white blood count, immunoglobulin G (IgG), and albumin concentrations were performed as previously described [17]. CSF β-2 microglobulin (β2M) and neopterin concentrations were measured with the N Latex β2M kit on the Atellica NEPH 630 System (Siemens Healthcare) and commercially available immunoassay (BRAHMS), respectively.
Statistical Analyses
All biomarkers were analyzed on a log scale and PCC, COVID-19 controls, and healthy controls were compared using ANCOVA adjusting for sex and age. Principal component analysis (PCA) was performed on 3 sets of markers in CSF: cytokines (n = 18), neuronal (n = 5), and anti-cytokine autoantibodies (n = 8). In each of these sets, ANCOVA adjusting for sex and age was performed on the first principal component (PCA1). All eligible individuals were included in the analysis; no statistical power calculation was performed.
RESULTS
Patient Characteristics
We recruited a total of 31 participants with confirmed COVID-19, comprising 17 (55%) men and 14 (45%) women. In addition, 17 healthy COVID-negative controls were included, of which 6 (35%) were men and 11 (65%) were women. PCC symptoms were observed in 25 of the participants (PCC group), while the remaining 6 participants had fully recovered from COVID-19 (COVID-19 controls). Table 1 shows the baseline characteristics of all study participants. Median age in the PCC groups was 50.0 years (interquartile range [IQR], 40.6–56.4 years), while in the COVID-19 control group it was 60.0 years (IQR, 54.6–64.7 years). Median age in the healthy control group was 54.7 years (IQR, 48.5–59.0 years). Time from COVID-19 symptom onset to follow-up study visit was 134 days (IQR, 104–268 days) for patients with PCC and 110 days (IQR, 110–112.5 days) for COVID-19 controls. Compared to the COVID-19 control group, patients in the PCC group (n = 25) were younger, had more underlying comorbidities, and were mostly women.
Table 1.
Patients' Characteristics (n = 48)
| Characteristic | PCC (n = 25) | COVID-19 Controls (n = 6) | Healthy Controls (n = 17) |
|---|---|---|---|
| Age, y, median (IQR) | 50.2 (40.6–56.4) | 59.9 (54.6–64.8) | 54.7 (48.5–59) |
| Disease severity, No. (%) | |||
| Mild | 5 (20) | 0 (0) | NA |
| Moderate | 11 (44) | 3 (50) | NA |
| Severe | 9 (36) | 3 (50) | NA |
| Sex, No. (%) | |||
| Female | 14 (56) | 0 (0) | 11 (65) |
| Male | 11 (44) | 6 (100) | 6 (35) |
| Comorbidities, No. (%) | |||
| Hypertension | 5 (20) | 2 (33) | 1 (6) |
| Overweight/obesity | 11 (44) | 6 (100) | NA |
| BMI, median (IQR) | 26.3 (24.3–31)a | 29.6 (26.4–30.8)b | NA |
| Diabetes | 3 (12) | 1 (17) | 0 (0) |
| Blood analysis, median (IQR) | |||
| CRP, mg/L | 98.5 (39–190)c | 160 (152.5–167.5) | NA |
| Lymphocyte count 109/L | 0.8 (0.7–1.4)d | 0.7 (0.5–1.0) | NA |
| Vaccinated, No. (%) | 14 (56)e | 3 (50)e | 9 (53)e |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IQR, interquartile range; NA, not applicable/unknown; PCC, post-COVID condition.
aBMI available in only 1/5 mild, 10/11 moderate, and in 7/7 severely ill participants.
bBMI available in 2/3 moderate and 3/3 severely ill participants.
cCRP not available in mild disease severity group.
dLymphocyte count available in 1/5 mild, and all moderate and severely ill.
eVaccination status not known in 9 participants in PCC group, 3 in COVID-19 controls, and 6 in healthy controls.
Supplementary Table 2 shows the neurocognitive symptoms reported by participants in the PCC group during follow-up. Fatigue was the most frequently reported symptom, observed in 20 participants (80%). Changes in cognition, defined as memory loss or changes in concentration, were reported by 16 participants (64%), and 7 (28%) reported experiencing brain fog. No participant reported hyposmia or dysgeusia during follow-up.
Table 1 displays the vaccination status of the participants. Among the PCC participants, 14 (56%) reported having received at least 1 dose of vaccine prior to sampling. The corresponding numbers for COVID-19 controls and healthy controls were 3 (50%) and 9 (53%), respectively. Whether participants were vaccinated against COVID-19 before symptom onset is unknown, but it is possible that they had not been vaccinated as the vaccine was introduced for their age group later in 2021.
Biomarker Concentrations
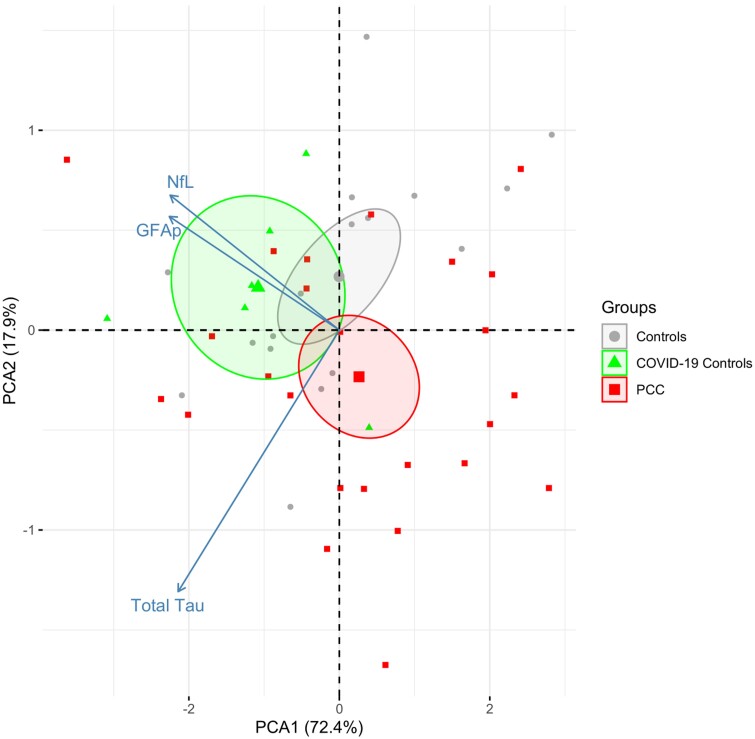
CSF testing did not detect SARS-CoV-2 N-Ag or spike antigen (S-Ag) in any of the groups during follow-up (Supplementary Table 3). In plasma, median concentrations of SARS-CoV-2 N-Ag were slightly above the limit of detection (0.16 pg/mL) in all groups, but the levels were below the diagnostic positive/negative cutoff. No participant had pleocytosis, as demonstrated in Supplementary Table 3. Moreover, there were no significant differences in CSF/plasma albumin ratio, IgG index, neopterin, β2M, or in CSF biomarkers of neuronal injury (NfL) and astrocytic damage (GFAp).
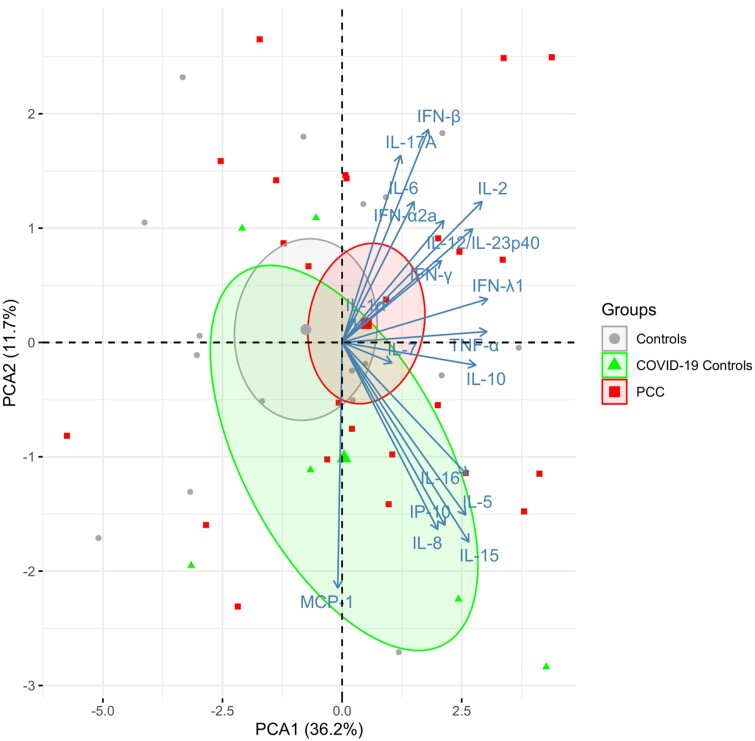
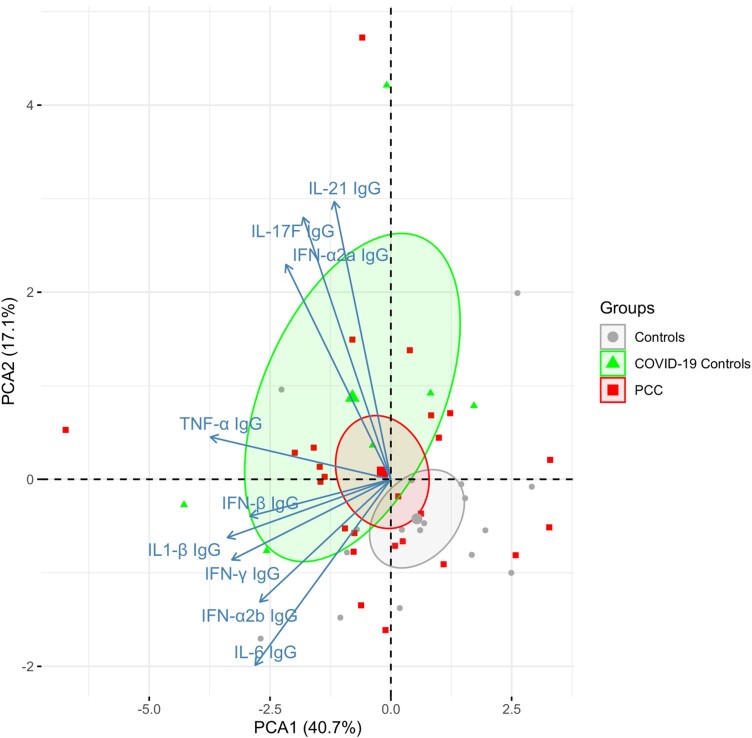
Plasma concentrations of interleukin 10 (IL-10), CSF and plasma IL-17A, plasma IL-16, plasma interferon-α2a (IFN-α2a) IgG, CSF IL-17F IgG, and plasma IL-17F IgG were significantly higher in the PCC group than in the healthy control group (nominal P < .05). Plasma concentration of IL-8 and IFN-β were significantly higher in the healthy control group than in the PCC group (nominal P < .05). Except for these biomarkers, there were no significant differences in cytokine concentrations analyzed in plasma or CSF concentrations between the study groups (Supplementary Table 3). Furthermore, the PCA1 analysis did not indicate any significant differences between the study groups in the CSF cytokine marker sets (Figure 1), neuronal markers (Figure 2), or anti-cytokine autoantibodies (Figure 3).
Figure 1.
Principal component analysis plot of 18 cytokines in CSF. The centroids for each group are indicated and surrounded with ellipses indicating 95% confidence intervals. No difference between PCC and controls for PCA1 (P = .24). The percentages captured by principal components 1 and 2 are indicated. Abbreviations: COVID-19, coronavirus disease 2019; IFN, interferon; IL, interleukin; IP-10, inducible protein-10; MCP-1, monocyte chemoattractant protein-1; PCA, principal component analysis; PCC, post-COVID condition; TNF-α, tumor necrosis factor-α.
Figure 2.
Principal component analysis plot of 5 neurobiomarkers in CSF. The centroids for each group are indicated and surrounded with ellipses indicating 95% confidence intervals. No difference between PCC and controls for PCA1 (P = .61). The percentages captured by principal components 1 and 2 are indicated. Abbreviations: COVID-19, coronavirus disease 2019; GFAp, glial fibrillary acidic protein; NfL, neurofilament light-chain; PCA, principal component analysis; PCC, post-COVID condition.
Figure 3.
Principal component analysis plot of 8 anti-cytokine autoantibodies in CSF. The centroids for each group are indicated and surrounded with ellipses indicating 95% confidence intervals. No difference between PCC and controls for PCA1 (P = .22). The percentages captured by principal components 1 and 2 are indicated. Abbreviations: COVID-19, coronavirus disease 2019; IFN, interferon; IgG, immunoglobulin G; IL, interleukin; PCA, principal component analysis; PCC, post-COVID condition; TNF-α, tumor necrosis factor-α.
DISCUSSION
In this study, we have investigated COVID-19 patients with and without neurological/neurocognitive sequelae at ≥3 months follow-up and compared them to healthy controls. We did not detect any signs of residual viral infection, intrathecal immune activation, CNS injury, or humoral response in either plasma or CSF in any of the groups, including patients with neurological sequelae.
Studies have identified SARS-CoV-2 N-Ag in CSF during the acute phase of COVID-19 infection, even in the absence of SARS-CoV-2 RNA with an ongoing intrathecal immune activation [14, 17, 18, 22]. While a recent brain autopsy study detected SARS-CoV-2 RNA in 10 out of 11 cases [16], the detection of viral RNA in other studies has been infrequent and subject to debate [16, 23–25]. Additionally, immune cell activation, which induces a proinflammatory state in the CNS, is believed to contribute to neuroaxonal damage during the acute phase of COVID-19. This is suggested by the concurrent increase in CSF cytokines and NfL levels. Importantly, such findings are not exclusive to COVID-19 infection and have been reported in other infections [26].
PCC refers to the persistence of symptoms or new symptoms that last for more than 30 days after SARS-CoV-2 infection and can continue for months beyond the acute phase [27]. Our group of PCC patients exhibited 1 or more self-reported neurological or neurocognitive symptoms, such as fatigue, brain fog, and tiredness, 4 months after the acute infection. However, no indication of increased biomarkers of neurological damage or cytokines/chemokines were identified despite the presence of neurological/cognitive sequelae. Additionally, no detectable SARS-CoV-2 N-Ag or S-Ag were found in CSF or plasma during follow-up in any of the groups, indicating the absence of ongoing viral infection. These results are consistent with a recent autopsy study that did not detect SARS-CoV-2 RNA in the brain tissue of deceased COVID-19 patients [28]; however, these findings are still a matter of debate. In a recent study, SARS-CoV-2 S-Ag was found in the plasma of 31 PCC patients 12 months after acute infection and SARS-CoV-2 N-Ag was only found in a single patient at multiple time points [29]; importantly, the study did not include CSF analyses or COVID-19–negative controls. Thus, the importance of detecting viral antigens in this context remains uncertain and warrants further investigation in larger cohorts and demographically matched controls.
In our analysis, no biomarker abnormalities were detected in either plasma or CSF for any of the 3 groups, including the PCC group. These results stand in contrast to recent reports that have indicated signs of immune activation in serum during follow-up of individuals with prior COVID-19 [30–33]. In these studies, proinflammatory markers such as IFN-β, IFN-γ, C-X-C motif chemokine ligand 9 (CXCL9), CXCL19, and IL-8 were examined at 4 months follow-up, and both the PCC and COVID-19 control groups demonstrated higher inflammatory markers compared to healthy controls [30]. Conversely, earlier research has shown high levels of proinflammatory markers in the CSF of COVID-19 patients with neurological symptoms up to 2 months following the acute infection, including increased levels of IL-6, IL-8, IL-10, and IFN-γ [14, 22, 34, 35].
There are several potential explanations for the differences in our findings, including methodological variations in serum biomarker analyses across cohorts. Additionally, one of the studies demonstrating increased proinflammatory markers in plasma included a cohort with ongoing cancer that had metastasized to the brain, which is known to increase the systemic proinflammatory burden [34]. On the other hand, successful clearance of crucial proinflammatory markers in the CSF may also account for these discrepancies. Notably, no increase in concentrations of neuronal and astrocytic injury biomarkers (NfL and GFAp) were found in the CSF during follow-up, which is consistent with our prior results from plasma analyses in a partly overlapping cohort [11].
Our study has several noteworthy strengths. We recruited patients with varying degrees of initial COVID-19 severity, ranging from mild to critical disease. Notably, we performed both CSF and serum analyses in all study participants, providing us with a comprehensive assessment of biomarker profiles across patient groups. However, the study also has important limitations. First and foremost, despite having a relatively large sample size for a study involving CSF analyses, our sample size was limited, particularly for the COVID-19 control group, which did not include any female participants, limiting the generalizability of our findings. Second, the study was cross-sectional, and lacked longitudinal sampling. Third, the assessment of neurocognitive sequelae relied on interviews and nonvalidated self-reported questionnaires, preventing a reliable grading of PCC symptoms severity. Finally, we did not include any subjects who exhibited indications of severe neurocognitive sequelae.
In conclusion, our study did not find any evidence of ongoing viral replication, immune, or inflammatory activation in the plasma or CSF in patients with PCC, irrespective of their acute COVID-19 infection severity, when compared to COVID-19 controls without PCC or healthy volunteers. Our results suggest that the pathogenesis of neurocognitive PCC may be related to events that occurred during acute phase of SARS-CoV-2 infection (including the presence of CNS inflammation during acute infection described previously), rather than a consequence of persistent viral CNS infection or residual CNS immune activation. These observations have important potential implications for future studies of pathogenesis as well as potential therapeutic interventions in relation to neurocognitive sequelae after COVID-19. However, further studies are needed to investigate pathogenetic mechanisms involved in PCC, whether specific to SARS-CoV-2 infection or infectious diseases in general.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Nelly Kanberg, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Anna Grahn, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Erika Stentoft, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Daniel Bremell, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Aylin Yilmaz, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Marie Studahl, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Staffan Nilsson, Department of Laboratory Medicine, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Michael Schöll, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Gothenburg, Sweden; Department of Neurodegenerative Disease, Dementia Research Centre, Institute of Neurology, University College London, London, United Kingdom.
Johanna M Gostner, Institute of Medical Biochemistry, Biocenter, Medical University of Innsbruck, Innsbruck, Austria.
Kaj Blennow, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden.
Henrik Zetterberg, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Neurodegenerative Disease, Dementia Research Centre, Institute of Neurology, University College London, London, United Kingdom; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden; UK Dementia Research Institute, University College London, London, United Kingdom; Hong Kong Center for Neurodegenerative Diseases, Hong Kong, China; Wisconsin Alzheimer's Disease Research Center, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Nikhil Padmanabhan, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Rachel Cohen, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Salvia Misaghian, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Daniel Romero, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Christopher Campbell, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Anu Mathew, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Mingyue Wang, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
George Sigal, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Martin Stengelin, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Arvid Edén, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Magnus Gisslén, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Infectious Diseases, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Notes
Financial support . This work was supported by the Government of Swedish, under an agreement between the Swedish government and the county councils (grant numbers ALFGBG-965885, ALFGBG-71320, and ALFGBG-966347); Knut and Alice Wallenberg Foundation (grant numbers 2020.0182 and 2020.0241 to SciLifeLab); the Swedish Research Council (grant number 2021-06545); and the King Gustaf V’s and Queen Victoria´s Foundation.
References
- 1. Centers for Disease Control and Prevention . Post-COVID conditions: information for healthcare providers.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed 22 September 2022.
- 2. Briggs A, Vassall A. Count the cost of disability caused by COVID-19. Nature 2021; 593:502–5. [DOI] [PubMed] [Google Scholar]
- 3. Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol 2021; 8:1073–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shanley JE, Valenciano AF, Timmons G, et al. Longitudinal evaluation of neurologic-post acute sequelae SARS-CoV-2 infection symptoms. Ann Clin Transl Neurol 2022; 9:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022; 400:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliddal S, Banasik K, Pedersen OB, et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep 2021; 11:13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goertz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6:00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol 2020; 16:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985) 2020; 129:864–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanberg N, Simrén J, Edén A, et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine 2021; 70:103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol 2016; 12:563–74. [DOI] [PubMed] [Google Scholar]
- 14. Edén A, Grahn A, Bremell D, et al. Viral antigen and inflammatory biomarkers in cerebrospinal fluid in patients with COVID-19 infection and neurologic symptoms compared with control participants without infection or neurologic symptoms. JAMA Netw Open 2022; 5:e2213253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022; 612:758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021; 144:2696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edén A, Kanberg N, Gostner J, et al. CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology 2021; 96:e294–300. [DOI] [PubMed] [Google Scholar]
- 18. Edén A, Simrén J, Price RW, Zetterberg H, Gisslén M. Neurochemical biomarkers to study CNS effects of COVID-19: a narrative review and synthesis. J Neurochem 2021; 159:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yilmaz A, Marklund E, Andersson M, et al. Upper respiratory tract levels of severe acute respiratory syndrome coronavirus 2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical coronavirus disease 2019. J Infect Dis 2021; 223:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sigal GB, Novak T, Mathew A, et al. Measurement of severe acute respiratory syndrome coronavirus 2 antigens in plasma of pediatric patients with acute coronavirus disease 2019 or multisystem inflammatory syndrome in children using an ultrasensitive and quantitative immunoassay. Clin Infect Dis 2022; 75:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pilotto A, Masciocchi S, Volonghi I, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis 2021; 73:e3019–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci 2021; 421:117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383:989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020; 19:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gisslen M, Keating SM, Spudich S, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One 2021; 16:e0250987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horberg MA, Watson E, Bhatia M, et al. Post-acute sequelae of SARS-CoV-2 with clinical condition definitions and comparison in a matched cohort. Nat Commun 2022; 13:5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 2021; 384:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis 2023; 76:e487–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23:210–6. [DOI] [PubMed] [Google Scholar]
- 31. Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021; 21:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 224:1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Queiroz MAF, Neves P, Lima SS, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol 2022; 12:922422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Remsik J, Wilcox JA, Babady NE, et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell 2021; 39:276–283.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benameur K, Agarwal A, Auld SC, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 2020; 26:2016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.