Abstract
Background
Previously, we showed that children with asymptomatic Plasmodium falciparum (Pf) malaria infection had higher Kaposi sarcoma–associated herpesvirus (KSHV) viral load, increased risk of KSHV seropositivity, and higher KSHV antibody levels. We hypothesize that clinical malaria has an even larger association with KSHV seropositivity. In the current study, we investigated the association between clinical malaria and KSHV seropositivity and antibody levels.
Methods
Between December 2020 and March 2022, sick children (aged 5–10 years) presenting at a clinic in Uganda were enrolled in a case-control study. Pf was detected using malaria rapid diagnostic tests (RDTs) and subsequently with quantitative real-time polymerase chain reaction (qPCR). Children with malaria were categorized into 2 groups: RDT+/PfPCR+ and RDT–/PfPCR+.
Results
The seropositivity of KSHV was 60% (47/78) among Pf-uninfected children, 79% (61/77) among children who were RDT–/PfPCR+ (odds ratio [OR], 2.41 [95% confidence interval {CI}, 1.15–5.02]), and 95% (141/149) in children who were RDT+/PfPCR+ (OR, 10.52 [95% CI, 4.17–26.58]; Ptrend < .001). Furthermore, RDT+/PfPCR+ children followed by RDT–/PfPCR+ children had higher KSHV IgG and IgM antibody levels and reacted to more KSHV antigens compared to uninfected children.
Conclusions
Clinical malaria is associated with both increased KSHV seropositivity and antibody magnitude, suggesting that Pf is affecting KSHV immunity.
Keywords: clinical Plasmodium falciparum malaria, Ugandan children, Kaposi sarcoma–associated herpesvirus, Kaposi sarcoma–associated herpesvirus antibody levels, seropositivity
Plasmodium falciparum (Pf) malaria is associated with increased Kaposi sarcoma–associated herpesvirus (KSHV) seropositivity and higher KSHV antibody breadth and magnitude. Pf association with KSHV is highest in children with clinical malaria (rapid diagnostic test/polymerase chain reaction positive) compared to children without malaria.
Kaposi sarcoma (KS) is the commonest malignancy in people living with human immunodeficiency virus (HIV) in sub-Saharan Africa (SSA) [1]. In SSA, KS age-standardized incidence rates (ASIRs) are highest in Eastern Africa at 15.1 and 7.6 per 100 000 person-years in males and females, respectively. In Uganda, KS ASIRs are 24.0 and 14.1 in males and females, respectively [2]. Kaposi sarcoma–associated herpesvirus (KSHV) is the causative agent of KS [3]. KSHV infection is not ubiquitous worldwide; rather, there is very high KSHV seroprevalence in SSA where some populations have a seroprevalence >90% [1]. In SSA, KSHV seroprevalence also varies by region and even between geographically proximate areas, ranging from 60% to >90% in different urban or rural areas in Uganda [4].
Given the unique geographic distribution of KSHV, environmental risk factors have been suggested to play a role in either higher rates of KSHV transmission or increased susceptibility to infection. In SSA, pneumonia, diarrheal, and malarial diseases are the leading cause of death in children [5]. Infections with Plasmodium falciparum (Pf) malaria are very common throughout childhood starting as early as 6 months of age through 15 years of age [6]. Morbidity and mortality due to severe malaria disease are commonest in children aged <5 years [7]. However, immunity to severe malaria is obtained relatively quickly in endemic areas, but sterilizing immunity to infection is rarely achieved [6]. Therefore, school-going children (5–15 years of age) have the highest Pf infection rates despite being protected from severe disease by natural immunity [8].
There are very few studies that have determined primary infection with KSHV. Primary infection with KSHV occurs in childhood in SSA [9], and we have previously shown that infections with KSHV in SSA occur as early as 6 months of age [10], peaking around 15–24 years [11]. Furthermore, we have observed that children between 6 and 10 years of age have the highest rates of KSHV shedding [12]. The overlap between the age of malaria infection and KSHV acquisition, as well as KSHV viral reactivation among Pf-infected individuals, suggests a role for malaria in KSHV epidemiology. The relationship between Epstein-Barr virus (EBV) and childhood Pf malaria infection leading to endemic Burkitt lymphoma in African children is well-documented [13]. EBV is also a gammaherpesvirus, like KSHV, with a similar route of transmission and cell tropism. Several mechanisms through which Pf infection affects EBV infection, leading to an increased risk of Burkitt lymphoma, have been suggested. One mechanism is that impairment of EBV-specific T-cell immune surveillance by Pf infection leads to EBV viral reactivation [14]. The other is that the increased proliferation of B cells consequently increases the number of EBV-infected B cells [15]. We hypothesize that these mechanisms could similarly affect KSHV latent infections in CD19+ B cells. In support of this hypothesis, we have previously shown that children with asymptomatic Pf malaria are more likely to be KSHV seropositive and have higher antibody titers to KSHV ORF73 and K8.1 antigens as assessed by enzyme-linked immunosorbent assay (ELISA) [16–19]. Furthermore, we have shown that individuals with asymptomatic malaria have higher KSHV viral load in peripheral blood mononuclear cells (PBMCs) compared with uninfected individuals [20]. Increased antibody titers to KSHV proteins [21], and higher KSHV viral load in PBMCs are associated with KSHV-associated diseases [22]. Therefore, Pf infection could play a role in KSHV pathogenesis.
Microscopy is the gold standard for malaria diagnosis [23]. Due to the technical requirement of microscopy, rapid diagnostic tests (RDTs) were developed to allow point-of-care testing in resource-limited settings [24]. RDTs have highly comparable specificity to microscopy and are more sensitive [25]. However, both RDT and microscopy are not sensitive to diagnose low-parasite-density infections [25]. Molecular-based techniques such as quantitative real-time polymerase chain reaction (qPCR) are very sensitive because they detect low-parasite-density infections as well and are highly specific [26]. Therefore, our previous studies using RDT alone to evaluate malaria infection may have underestimated the burden of malaria. In addition, we did not evaluate KSHV serology in children with clinical malaria. In this study, we investigated KSHV antibody responses in symptomatic children with and without Pf infections to evaluate the association between Pf infection and KSHV seropositivity.
METHODS
Study Design
As part of the Environmental Determinants of KSHV Transmission in Rural Uganda (ENDKU) study [27], we conducted a case-control study within the General Population Cohort (GPC). The GPC is a community-based cohort of about 22 000 people in 25 adjacent villages in southwestern Uganda [28]. The seroprevalence of KSHV in the GPC is >90% in adults [29]. Between December 2020 and March 2022, sick children (aged 5–10 years, the age group previously shown to have a higher rate of KSHV shedding [12]) with a fever within 2 days of first contact were enrolled at the Kyamulibwa field station outpatient clinic. Clinical symptoms and an RDT (ONE STEP Malaria HRP-II [P.f] and pLDH [Pan] Antigen Rapid Test) were used to diagnose Pf infection. Hemoglobin (Hb) level was measured using the HemoCue HB analyzer. Children with a known chronic illness (eg, HIV, sickle cell disease, or tuberculosis), a severe neurological disease (eg, cerebral palsy or other known developmental delay), or Hb <7.0 g/dL requiring immediate medical attention at referral hospitals were excluded. HIV status was obtained from the GPC survey data. The child's age, sex, fever, history of recent fever, weight, and height were also recorded. Study data were recorded and maintained using the FileMaker Pro application [30]. Study participants were categorized into 3 groups: (1) clinical malaria: RDT positive, PCR positive (RDT+/PfPCR+); (2) malaria: RDT negative, PCR positive (RDT–/PfPCR+); and (3) malaria uninfected: RDT negative, PCR negative (RDT–/PfPCR–).
Ethical Approvals
This study was approved by the Uganda Virus Research Institute Research and Ethics Committee, the Uganda National Council for Science and Technology, the London School of Hygiene and Tropical Medicine Ethics Committee, and the Colorado Multiple Institutional Review Board. Written informed consent was obtained from parents or guardians of all study children, and written informed assent was obtained from study children aged 8–10 years.
Plasmodium falciparum qPCR
Plasmodium falciparum DNA was extracted from 200 μL of whole blood using the QIAamp blood kit (Qiagen, Valencia, California), following the manufacturer's instructions. Pf was quantified using the following primers and probe forward: 5′-TTA GAT TGC TTC CTT CAG TRC CTT ATG-3′, reverse: 5′-TGT TGA GTC AAA TTA AGC CGC AA-3′, probe 5′-FAM-TCA ATT CTT TTA ACT TTC TCG CTT GCG CGA-BHQ1-3′, using TaqMan Fast Advanced Master Mix (Applied Biosystems) on a ViiA7 machine (AB Applied Biosystems by Life Technologies). A Plasmodium plasmid of known concentration was used to quantify Pf [31]. Any DNA sample with a positive PfPCR amplification was considered positive for Pf; the highest cycle threshold value was 39.
KSHV qPCR
KSHV viral load in 2 million PBMCs was quantified using qPCR. KSHV DNA was detected using primers (forward primer: K6-10F 5′-CGCCTAATAGCTGCTGCTACGG-3′; reverse primer: K6-10R 5′-TGCATCAGCTGCCTAACCCAG-3′) and a probe (p-K6-10 5′-R-CACCCACCGCCCGTCCAAATTC-Q-3′). This procedure has been reported elsewhere [12].
KSHV Serology
Using the previously described multiplex bead assay [32], plasma samples were diluted 1:200 and used to measure anti-KSHV and anti–tetanus toxoid (TT) immunoglobulin G (IgG) and immunoglobulin M (IgM) antibody levels. Twenty-five KSHV recombinant proteins (ORF73, K10.5, K5, K14, ORF6, ORF11, ORF55, ORF50, ORF60, K3, ORF38, ORF52, ORF59, ORF65, ORF61, ORF18, K11, K8.1, ORF19, ORF25, ORF26, ORF72, ORF37, ORF44, and ORF63), plus 1 TT protein, were included in the assay panel. Each plate contained 3 negative control sera and 3 positive control sera replicates. Receiver operating characteristic (ROC) statistical analysis was used to compute the cutoff values for 11 antigens (K8.1, ORF73, ORF38, ORF65, ORF25, ORF61, ORF59, ORF6, ORF44, K10.5, K5) with an area under the curve (AUC) >70%. For the remaining antigens (K14, ORF11, ORF55, ORF50, ORF60, K3, ORF52, ORF18, K11, ORF19, ORF26, ORF72, ORF37, and ORF63) with a ROC AUC <70%, the cutoff was calculated as mean MFI (median fluorescent intensity) plus 2 times the standard deviation of 215 KSHV-uninfected US donor samples for each antigen [32].
Antibody isotype specificity was confirmed using plasma samples from Uganda by adsorbing either IgM or IgG before testing. In both cases, the isotype-specific signal was completely abated in treated samples (Supplementary Figure 1).
KSHV seropositivity was defined first as IgG reactivity to at least 1 KSHV recombinant protein of the 25 that were included in the assay. Since K8.1 and ORF73/LANA proteins have been widely used to define KSHV seropositivity previously, we also performed analyses using KSHV seropositivity defined as reactivity to either K8.1 or ORF73.
Statistical Analysis
Anti-KSHV antibody levels were log10 transformed for all analyses. First, associations between risk factors (including Pf infection, anemia [Hb <11.5 g/dL], fever [body temperature >37.5°C], age, and sex) and KSHV seropositivity (a binary outcome variable) were modeled using logistic regression. Risk factors (including Pf infection, anemia [Hb <11.5 g/dL], fever [body temperature >37.5°C], age, and sex) associated with anti-KSHV antibody levels (a continuous outcome variable) were determined using linear regression modeling. Associations between Pf and KSHV seropositivity or KSHV antibody levels were adjusted for anemia (Hb <11.5 g/dL), fever (body temperature >37.5°C), age, and sex. The χ2 test, Student t test, Kruskal–Wallis test, and 1-way analysis of variance were used for crude analyses, where appropriate. Correlation analyses were carried out using Spearman rank correlation. The false discovery rate was used to correct for multiple comparisons of antibody data. A P value <.05 was considered statistically significant. Statistical analyses were carried out using Stata version 13 (StataCorp, College Station, Texas) and R (R Foundation for Statistical Computing, Vienna, Austria) software.
RESULTS
Study Population Characteristics
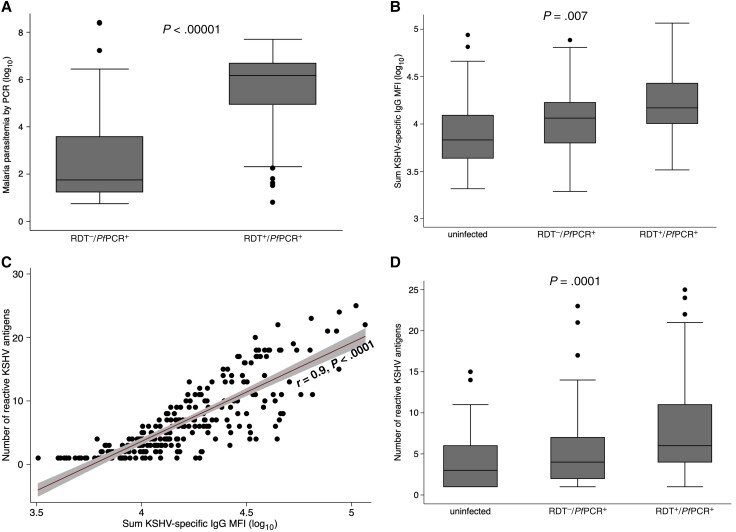
We enrolled 304 children aged 5–10 years with a mean age of 7 years. Forty-three percent of these children (132/304) were boys (Table 1). Of the 304, 149 had clinical Pf malaria as indicated by a recent fever of >37.5°C and RDT-positive results confirmed by qPCR to detect Pf DNA in whole blood. Of the 304, 77 study participants had RDT–/PfPCR+Pf infection as indicated by the absence of RDT reactivity but PfPCR positivity, and 78 were Pf uninfected as indicated by lack of RDT or qPCR positivity (Table 1). Children with clinical Pf malaria had 3-log higher mean Pf parasite load compared to children with RDT–/PfPCR+Pf infection, confirming that the RDT–/PfPCR+ parasitemic study participants had low parasitemia (Figure 1A). Age and sex distribution did not vary significantly between the 3 study groups. Children with clinical malaria were more likely to be anemic (34%) and to have a fever at the time of enrollment (36%); children with RDT–/PfPCR+ malaria were less likely to have anemia (19%) and fever (9%), and the malaria-uninfected children had the lowest proportions with anemia (10%) and fever (5%) (Table 1).
Table 1.
Characteristics of Study Participants
| Characteristic | Overall | Malaria Infected (RDT+/PfPCR+) | Malaria Infected (RDT–/PfPCR+) |
Malaria Uninfected (RDT−/PfPCR–) | P Value |
|---|---|---|---|---|---|
| No. of participants | 304 | 149 | 77 | 78 | |
| Age, y, mean (range) | 7 (5–10) | 7 (5–10) | 7 (5–10) | 7 (5–10) | .889a |
| Age group, y | |||||
| 5–7 | 56% (171/304) | 55% (82/149) | 57% (44/77) | 58% (45/78) | .933b |
| 8–10 | 44% (133/304) | 45% (67/149) | 43% (33/77) | 42% (33/78) | |
| Sex, male | 43% (132/304) | 46% (69/149) | 47% (36/77) | 35% (27/78) | .200b |
| Hb, g/dL, mean (range) | 12.3 (8–17.5) | 11.9 (8–17.5) | 12.5 (8.2–16) | 13.8 (8.1–15.2) | <.001c,* |
| Anemia | |||||
| Normal (Hb ≥11.5 g/dL) | 76% (230/304) | 66% (98/149) | 81% (62/77) | 90% (70/78) | <.001b,* |
| Anemic (Hb <11.5 g/dL) | 24% (74/304) | 34% (51/149) | 19% (15/77) | 10% (8/78) | |
| Body temperature (axillary) >37.5°C | 21% (64/300) | 36% (53/148) | 9% (7/75) | 5% (4/77) | <.001b,* |
RDTs and quantitative real-time PCR were used to determine malaria parasitemia.
Abbreviations: Hb, hemoglobin; PCR–, Plasmodium falciparum polymerase chain reaction negative; PCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
aKruskal–Wallis test.
bχ2 test.
cOne-way analysis of variance.
* P < .05 considered statistically significant.
Figure 1.
Malaria parasite load (A), sum total Kaposi sarcoma–associated herpesvirus (KSHV)–specific immunoglobulin G (IgG) median fluorescent intensity (MFI) levels (B), and the number of reactive KSHV proteins (D) by malaria status as well as the correlation between the number of reactive KSHV proteins and total KSHV-specific IgG level (C) in KSHV-seropositive children. The Student t test (A) (rapid diagnostic test negative [RDT–]/Plasmodium falciparum polymerase chain reaction positive [PfPCR+], n = 77; rapid diagnostic test positive [RDT+]/PfPCR+, n = 149), 1-way analysis of variance (B) (uninfected, n = 47; RDT–/PfPCR+, n = 61; RDT+/PfPCR+, n = 141), and Kruskal–Wallis test (D) (uninfected, n = 47; RDT–/PfPCR+, n = 61; RDT+/PfPCR+, n = 141) were used to analyze comparisons. KSHV-specific IgG to 25 KSHV recombinant proteins was measured using multiplex bead-based assay. RDTs and quantitative real-time PCR were used to determine malaria parasitemia.
Association Between Pf Infection Status and KSHV Seropositivity or KSHV Viral Load
When we defined KSHV seropositivity as IgG antibody reactivity to at least 1 of the 25 KSHV proteins, 95% of children with clinical malaria were KSHV seropositive compared to 79% of children with RDT–/PfPCR+ malaria and 60% of malaria-uninfected children (Table 2). Independent of age, sex, fever, and anemia, children with clinical malaria (adjusted odds ratio [aOR], 10.52 [95% confidence interval {CI}, 4.17–26.58]) and children with RDT–/PfPCR+ malaria (aOR, 2.41 [95% CI, 1.15–5.02]) were more likely to be KSHV seropositive compared to malaria-uninfected children (trend P < .001) (Table 2). Sex and fever were not associated with KSHV seropositivity, whereas every annual increase in age was associated with a higher risk of KSHV seropositivity (aOR, 1.21 [95% CI, 1.01–1.44]; P = .043) (Table 2).
Table 2.
Factors Associated With Kaposi Sarcoma–Associated Herpesvirus Seropositivity
| Factor | KSHV Seropositivity | Crude | Adjusteda | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Malaria | |||||
| RDT−/PfPCR− (uninfected) | 60% (47/78) | 1 | <. 001* (trend) | 1 | <.001* (trend) |
| RDT–/PfPCR+ | 79% (61/77) | 2.51 (1.23–5.13) | 2.41 (1.15–5.02) | ||
| RDT+/PfPCR+ | 95% (141/149) | 11.63 (5.00–27.05) | 10.52 (4.17–26.58) | ||
| Age, y | … | 1.20 (1.003–1.43) | .046* | 1.21 (1.01–1.44) | .043* |
| Sex | |||||
| Male | 84% (111/132) | 1 | .387 | 0.92 (.48–1.78) | .808 |
| Female | 80% (138/172) | 0.77 (.42–1.40) | … | ||
| Anemia | |||||
| Normal (Hb ≥11.5 g/dL) | 78% (180/230) | 1 | .006* | 1 | .065 |
| Anemic (Hb <11.5 g/dL) | 93% (69/74) | 3.83 (1.48–10.01) | 2.61 (.94–7.23) | ||
| Body temperature (axillary) | |||||
| ≤37.5°C | 79% (187/236) | 1 | .042* | 1 | .824 |
| >37.5°C | 91% (58/64) | 2.54 (1.03–6.21) | 0.89 (.31–2.52) | ||
KSHV-specific immunoglobulin G to 25 KSHV recombinant proteins was measured using multiplex bead-based assay. KSHV seropositivity was defined as seropositivity to at least 1 of 25 KSHV proteins.
Abbreviations: CI, confidence interval; Hb, hemoglobin; KSVH, Kaposi sarcoma–associated herpesvirus; OR, odds ratio; PCR–, Plasmodium falciparum polymerase chain reaction negative; PCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
aAdjusted for age, sex, anemia, body temperature, and malaria parasitemia. RDTs and quantitative real-time PCR were used to determine malaria parasitemia.
* P < .05 considered statistically significant.
Because our prior studies have focused on testing for antibodies to only K8.1 or ORF73, we performed an additional analysis where we limited our definition of KSHV seropositivity to the detection of IgG to either ORF73 or K8.1 antigens. KSHV seroprevalence was underestimated when defined by positivity to either K8.1 or ORF73 at 46% (Supplementary Table 1) versus 82% (Table 2), defined as positivity to at least 1 of 25 KSHV antigens. However, consistent with the findings where we analyzed antibodies to 25 KSHV antigens, children with clinical malaria and RDT–/PfPCR+ malaria were more likely to be KSHV seropositive compared with malaria-uninfected children (Supplementary Table 1).
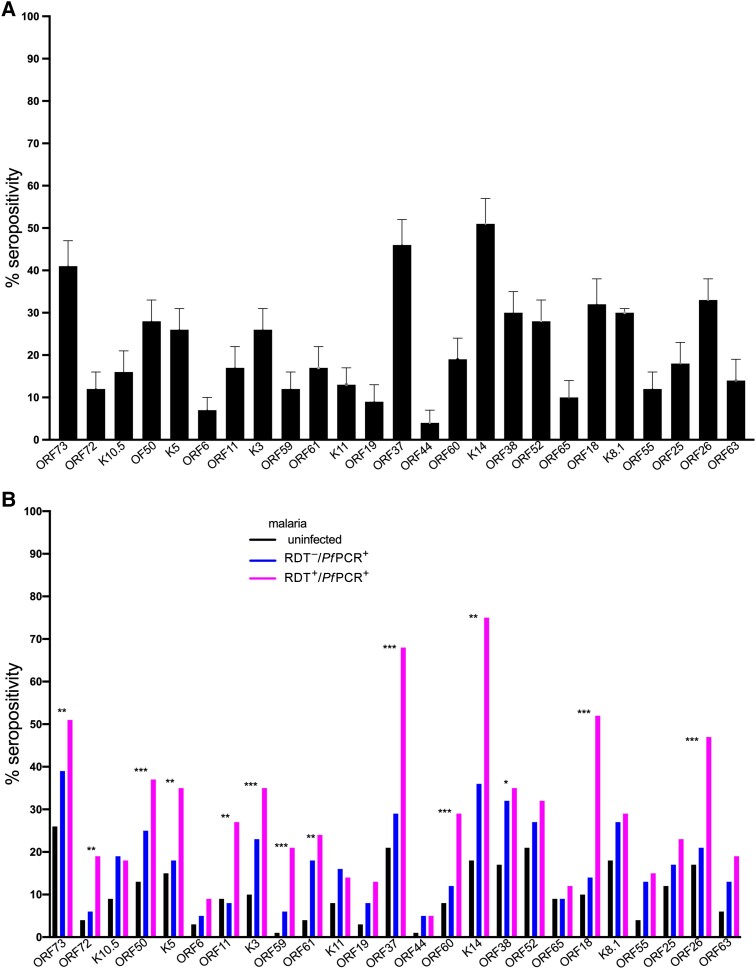
Overall, detection of antibodies to individual KSHV antigens was heterogenous with the largest proportion of children reacting to K14 (51%), followed by ORF37 (46%) and ORF73 (41%), whereas very few children reacted to ORF44 (4%) and 26% reacted to K8.1 (Supplementary Figure 2). Children with clinical malaria had the highest percentage positivity to anti-KSHV IgG anti-ORF73, anti-ORF72, anti-ORF50, anti-K5, anti-ORF11, anti-K3, anti-ORF59, anti-ORF61, anti-ORF37, anti-ORF60, anti-K14, anti-ORF38, anti-ORF18, and anti-ORF26, followed by children with RDT–/PfPCR+ malaria and lowest in malaria-uninfected children (Figure 2). Detection of other antibodies did not differ by malaria infection status (Figure 2).
Figure 2.
Percentage of participants with antibodies to individual Kaposi sarcoma–associated herpesvirus (KSHV) proteins (A) by malaria status (B). The χ2 test was used to analyze differences in antigen detection between groups, and the false discovery rate was used to adjust for multiple comparisons. KSHV-specific immunoglobulin G to 25 KSHV recombinant proteins measured using multiplex bead-based assay. Rapid diagnostic tests and quantitative real-time polymerase chain reaction were used to determine malaria parasitemia. Adjusted P values: *P < .05; **P < .01; ***P < .001. Abbreviations: PfPCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
KSHV viral load was detected in 20 of the 304 children tested. KSHV viral load was not different in the 3 study groups (Supplementary Figure 3).
Factors Associated With KSHV Antibody Breadth and Magnitude
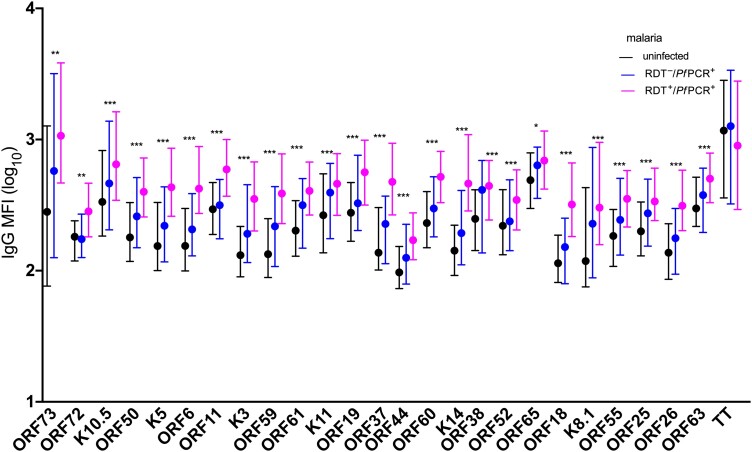
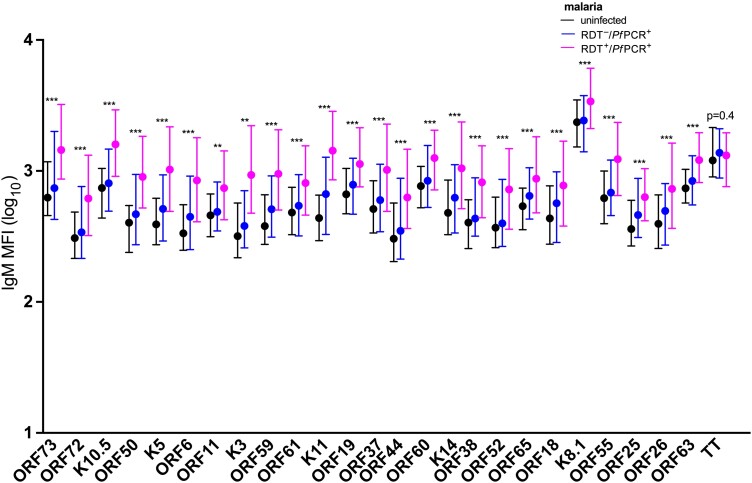
As another way to assess KSHV antibody reactivity, we summed the anti-IgG MFI from all KSHV proteins detected in an individual participant. Children with clinical malaria had higher sum KSHV antibody levels compared to malaria-uninfected children (adjusted regression coefficient, 0.25 [95% CI, .06–.45]; P = .014) (Table 3 and Figure 1B). The sum of KSHV antibody levels positively correlated with the number of reactive KSHV proteins per child (Figure 1C). Therefore, the number of reactive KSHV proteins was highest in children with clinical malaria, followed by those with RDT–/PfPCR+ malaria, and lowest in malaria-uninfected children (Figure 1D). Age, sex, fever, and anemia were not associated with sum KSHV antibody levels in KSHV-seropositive children (Table 3). Average levels of individual IgG antibodies to all of the 25 KSHV proteins were higher in children with clinical malaria, followed by children with RDT–/PfPCR+ malaria and lowest in malaria-uninfected children (Figure 3). IgG antibody levels to TT protein did not change by malaria infection status (Figure 3). Similarly, IgM levels of the individual KSHV proteins were higher in children with clinical malaria, followed by children with RDT–/PfPCR+ malaria and lowest in malaria-uninfected children (Figure 4). IgM antibody levels to TT did not change by malaria infection status (Figure 4). Furthermore, positive correlations of IgM and IgG antibody levels to specific KSHV antigens were observed (Supplementary Figure 4).
Table 3.
Factors Associated With Kaposi Sarcoma–Associated Herpesvirus (KSHV) Antibody Levels Among KSHV-Seropositive Children
| Factor | Crude | Adjusteda | ||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | P Value | Regression Coefficient (95% CI) | P Value | |
| Malaria | ||||
| RDT−/PfPCR− (uninfected) | Ref | .007* (trend) | Ref | .014* (trend) |
| RDT–/PfPCR+ | 0.12 (−.09 to .33) | 0.13 (−.09 to .35) | ||
| RDT+/PfPCR+ | 0.24 (.06–.43) | 0.25 (.06–.45) | ||
| Age, y | 0.03 (−.01 to .07) | .201 | 0.03 (−.01 to .07) | .190 |
| Sex | ||||
| Male | Ref | .935 | Ref | .867 |
| Female | −0.006 (−.15 to .13) | −0.01 (−.15 to .13) | ||
| Anemia | ||||
| Normal (Hb ≥11.5 g/dL) | Ref | .975 | Ref | .616 |
| Anemic (Hb <11.5 g/dL) | 0.002 (−.15 to .16) | −0.04 (−.20 to .12) | ||
| Body temperature (axillary) | ||||
| ≤37.5°C | Ref | .994 | Ref | .554 |
| >37.5°C | 0.001 (−.16 to .17) | −0.05 (−.22 to .12) | ||
Immunoglobulin G (IgG) to 25 Kaposi sarcoma–associated herpesvirus (KSHV) recombinant proteins was measured using multiplex bead assay. IgG median fluorescent intensity to the 25 KSHV proteins were summed and log10 transformed before linear regression modeling.
Abbreviations: CI, confidence interval; Hb, hemoglobin; PCR–, Plasmodium falciparum polymerase chain reaction negative; PCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
aAdjusted for age, sex, anemia, body temperature, and malaria parasitemia. RDTs and quantitative real-time PCR were used to determine malaria parasitemia.
* P < .05 considered statistically significant.
Figure 3.
Immunoglobulin G (IgG) antibody levels to Kaposi sarcoma–associated herpesvirus (KSHV) proteins and tetanus toxoid (TT) by malaria status. Kruskal–Wallis test was used to for analytic comparisons, and the false discovery rate was used to adjust for multiple comparisons. Medians and interquartile ranges are shown. KSHV-specific IgG to 25 KSHV recombinant proteins and TT antigen were measured using multiplex bead-based assay. Rapid diagnostic tests and quantitative real-time polymerase chain reaction were used to determine malaria parasitemia. Adjusted P values: *P < .05; **P < .01; ***P < .001. Abbreviations: IgG, immunoglobulin G; MFI, median fluorescent intensity; PfPCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
Figure 4.
Immunoglobulin M (IgM) antibody levels to Kaposi sarcoma–associated herpesvirus (KSHV) proteins and tetanus toxoid (TT) by malaria status. Kruskal–Wallis test was used to for analytic comparisons, and the false discovery rate was used to adjust for multiple comparisons. Medians and interquartile ranges are shown. KSHV-specific IgM to 25 KSHV recombinant proteins and TT antigen measured using multiplex bead-based assay. Rapid diagnostic tests and quantitative real-time polymerase chain reaction were used to determine malaria parasitemia. Adjusted P values: *P < .05; **P < .01; ***P < .001. Abbreviations: IgM, immunoglobulin M; MFI, median fluorescent intensity; PfPCR+, Plasmodium falciparum polymerase chain reaction positive; RDT–, rapid diagnostic test negative; RDT+, rapid diagnostic test positive.
DISCUSSION
In this study children with clinical malaria had a substantially higher prevalence of detectable antibodies against KSHV than did those without malaria (95% vs 60%). Furthermore, both higher KSHV IgG and IgM antibody levels (magnitude) and a larger number of reactive KSHV proteins (breadth) were characteristic of children infected with Pf malaria.
Two potential explanations could explain why children with Pf infection were more likely to be KSHV seropositive with a larger magnitude and breadth of KSHV-specific IgG antibodies. The first is the known effect of Pf on B cells. Pf encodes the erythrocyte membrane protein 1 (PfEMP1), a superantigen that polyclonally activates B cells and protects them from apoptosis [33]. Furthermore, hypergammaglobulinemia [33] and enhanced B-cell activation [34] are characteristics of Pf infection. These observations imply nonspecific effects of Pf infection on B cells. The expansion of B cells triggered by a malaria infection may lead to the higher magnitude and breadth of KSHV IgG antibody levels that we observed in this study. However, we did not observe a difference in antibody levels to TT in Pf-infected and -uninfected individuals, suggesting a more specific effect on KSHV.
A second potential explanation may be due to the impairment of immune surveillance to KSHV (leading to viral reactivation) by Pf infection. Pf has been implicated in the reactivation of other human herpesviruses including EBV [33], herpes simplex virus, and varicella zoster virus [35]. Therefore reactivation of KSHV by Pf infection is biologically plausible. We know that higher antibody levels of KSHV are observed in KSHV-associated diseases when immune systems are impaired [21]. KSHV viral reactivation could increase the breadth and magnitude of KSHV-specific IgG antibody levels due to the increased availability of KSHV antigens. A state of immunosuppression has been observed during acute Pf malaria infection [15]. Furthermore, higher frequencies of regulatory T cells (Tregs) are strongly associated with peripheral Pf parasitemia and parasite biomass in severe disease [36]. Tregs are known to suppress inflammatory responses to prevent immune pathology. This state of immunosuppression could affect immune surveillance in KSHV latently infected individuals, leading to viral reactivation [37]. Pf infection has also been shown to modulate dendritic cell function [38], stimulate regulatory T cells [39], reduced interferon gamma (IFN-γ) responses to unrelated antigens [40] and promote a T helper 2 (Th2) bias [41]. Impairment of dendritic cells could lead to loss of T-cell surveillance while upregulation of regulatory T cells could contribute to immunosuppression. IFN-γ responses to KSHV have been demonstrated in this and other populations [42, 43]; hence, if Pf infection reduces these IFN-γ responses, this could lead to KSHV reactivation. Finally, the Th2 bias seen in Pf infection could also lead to KSHV reactivation, because T helper 1 (Th1) cells promote IFN-γ and interleukin 2 antiviral responses, whereas Th2 responses that promote antibody production can impair antiviral immunity. Additionally, interleukin 4 (IL-4) production (a classical Th2 cytokine) was shown, in vitro, to reactivate KSHV from latency, and in a murine model, IL-4 was the principal mediator of gammaherpesvirus reactivation observed following helminthic infection [44].
KSHV seropositivity and the magnitude and breadth of KSHV-specific antibody levels were higher in children with clinical malaria compared to children with RDT–/PfPCR+ malaria. The parasite load was significantly different by several logs between these 2 groups, implying a dose-response effect seen between RDT–/PfPCR+ and clinical Pf malaria infection. This would suggest that the effect of Pf on KSHV or B cells increases with increasing malaria parasite burden.
KSHV encodes over 80 open reading frames, so the breadth of KSHV antibody responses may be missed by focusing on only 2 KSHV antigens. The use of the KSHV multiplex assay with 25 KSHV antigens in studies in Cameroon, Kenya, and Uganda has revealed a more dynamic KSHV antibody response, especially in children, and because of the larger dynamic range of the assay as compared to ELISA, a greater variation in antibody responses across different groups of individuals [45]. Using the 25-antigen multiplex bead assay, we were able to observe a different pattern of antibody reactivity to KSHV in children as compared to adults. In children, the pattern of antibody reactivity to KSHV proteins is heterogenous without the immune dominance of anti-ORF73 and anti-K8.1 IgG observed in adults [45]. One possible explanation is that following primary KSHV infection in children, no immunodominant KSHV antigen is present, unlike EBV where IgG to the viral capsid antigen is consistently found after primary EBV infection in children [46]. In adults who have years of persistent infection with KSHV, there is a selection for more immunodominant anti-K8.1 or anti-ORF73 IgG. It could also be that a more recent infection in children is not yet well immunologically controlled, compared with that in adults.
KSHV viral load was not associated with Pf infection status. This is attributed to the low detection rate of KSHV in peripheral circulation. Unlike EBV, KSHV DNA is rarely detected in plasma, even among individuals with KS [47]. On the other hand, KSHV can be detected in PBMCs and correlates with KS, suggesting that KSHV is majorly cell associated [22]. However, only about 50% of KS patients have detectable KSHV DNA in PBMCs and much less in plasma [48]. Therefore a larger sample size is required to detect differences in KSHV viral load.
There were several strengths of the current study. Our use of both RDT and qPCR to classify malaria parasitemia allowed us to identify a subgroup of children with RDT–/PfPCR+Pf infection that has not been included in previous studies. Our use of 25 KSHV proteins in a multiplex bead assay to quantify IgG to KSHV hence greatly increased the dynamic range, sensitivity, and specificity to detect KSHV antibody responses. Although the association between Pf and KSHV seropositivity could be detected even when using only 2 KSHV antigens, the seroprevalence in children is underestimated with 2 antigens as compared to using 25 KSHV antigens. The main weaknesses of the current study include the limitations of a case-control study design and unmeasured/unknown confounding factors. We did not enroll “healthy” children into our study and therefore were unable to compare KSHV serological measures in this group to our study participants. Finally, there was a lack of data about the timing of primary KSHV infection relative to the measurement of the KSHV antibodies at the time of enrollment in this study. However, infections with KSHV in SSA occur very early (as early as 6 months of age [10]), suggesting that KSHV acquisition preceded the current Pf infection for several years.
CONCLUSIONS
Clinical Pf malaria infections were associated with a higher risk of KSHV seropositivity and with the detection of an increased breadth and magnitude of IgG antibody levels to several KSHV latent and lytic proteins. These results suggest that Pf (possibly through Pf affecting immunity to KSHV) can affect KSHV latency and reactivation. The mechanisms through which Pf may impact KSHV latency and reactivation warrant further investigation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Angela Nalwoga, Department of Immunology and Microbiology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado; Cancer Epidemiology Programme, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda.
Katherine R Sabourin, Department of Immunology and Microbiology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado.
Wendell Miley, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Conner Jackson, Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado-Denver Anschutz Medical Campus, Aurora, Colorado.
Mahdi Maktabi, Department of Immunology and Microbiology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado.
Nazzarena Labo, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Joseph Mugisha, Cancer Epidemiology Programme, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda.
Denise Whitby, Viral Oncology Section, AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Rosemary Rochford, Department of Immunology and Microbiology, University of Colorado, Anschutz Medical Campus, Aurora, Colorado.
Robert Newton, Cancer Epidemiology Programme, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda; Department of Health Sciences, University of York, York, United Kingdom.
Notes
Acknowledgments . We thank the ENDKU team: Robert Newton, Rosemary Rochford, Angela Nalwoga, Katherine R. Sabourin, Vincent Arumadri, Joyce Aloyo, Joseph Tusubira, Brian Ssengendo, Aggrey Muzira, Naziifa Nakibuuka, Stellah Bilibagwa, Eva Ssejjemba, and Beatrice Kimono.
Financial support . This work was supported by the Department of Immunology and Microbiology, University of Colorado Anschutz Medical Campus (National Institutes of Health [NIH] grant number 1 RO1 CA239588-01 to R. N. and R. R.) and the National Cancer Institute (NCI)/NIH (contract number HHSN261201500003I and contract number 75N91019D00024 to D. W.). The UK Medical Research Council (MRC)/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit is jointly funded by the MRC and the UK Department for International Development (DFID) under the MRC/DFID Concordant agreement and is also part of the EDCTP2 Programme supported by the European Union.
References
- 1. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers 2019; 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motlhale M, Sitas F, Bradshaw D, et al. Epidemiology of Kaposi's sarcoma in sub-Saharan Africa. Cancer Epidemiol 2022; 78:102167. [DOI] [PubMed] [Google Scholar]
- 3. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 4. Nalwoga A, Webb EL, Muserere C, et al. Variation in KSHV prevalence between geographically proximate locations in Uganda. Infect Agent Cancer 2020; 15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batura N, Kasteng F, Condoane J, et al. Costs of treating childhood malaria, diarrhoea and pneumonia in rural Mozambique and Uganda. Malar J 2022; 21:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014; 383:723–35. [DOI] [PubMed] [Google Scholar]
- 7. Carneiro I, Roca-Feltrer A, Griffin JT, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One 2010; 5:e8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohee LM, Opondo C, Clarke SE, et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health 2020; 8:e1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dedicoat M, Newton R, Alkharsah KR, et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis 2004; 190:1068–75. [DOI] [PubMed] [Google Scholar]
- 10. Sabourin KR, Daud I, Ogolla S, et al. Malaria is associated with Kaposi sarcoma–associated herpesvirus seroconversion in a cohort of western Kenyan children. J Infect Dis 2021; 224:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newton R, Labo N, Wakeham K, et al. Kaposi sarcoma–associated herpesvirus in a rural Ugandan cohort, 1992–2008. J Infect Dis 2018; 217:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nalwoga A, Nakibuule M, Marshall V, et al. Risk factors for Kaposi's sarcoma–associated herpesvirus DNA in blood and in saliva in rural Uganda. Clin Infect Dis 2020; 71:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rochford R, Moormann AM. Burkitt's lymphoma. Curr Top Microbiol Immunol 2015; 390:267–85. [DOI] [PubMed] [Google Scholar]
- 14. Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A multifactorial role for P. falciparum malaria in endemic Burkitt's lymphoma pathogenesis. PLoS Pathog 2014; 10:e1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sánchez-Ponce Y, Fuentes-Pananá EM. The role of coinfections in the EBV-host broken equilibrium. Viruses 2021; 13:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nalwoga A, Cose S, Nash S, et al. Relationship between anemia, malaria coinfection, and Kaposi sarcoma–associated herpesvirus seropositivity in a population-based study in rural Uganda. J Infect Dis 2018; 218:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi's sarcoma–associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakeham K, Webb EL, Sebina I, et al. Parasite infection is associated with Kaposi's sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agent Cancer 2011; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakeham K, Webb EL, Sebina I, et al. Risk factors for seropositivity to Kaposi sarcoma–associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr 2013; 63:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nalwoga A, Nakibuule M, Marshall V, et al. Risk factors for Kaposi's sarcoma associated herpesvirus (KSHV) DNA in blood and in saliva in rural Uganda. Clin Infect Dis 2019; 71:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wakeham K, Johnston WT, Nalwoga A, et al. Trends in Kaposi's sarcoma–associated herpesvirus antibodies prior to the development of HIV-associated Kaposi's sarcoma: a nested case-control study. Int J Cancer 2015; 136:2822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinlivan EB, Zhang C, Stewart PW, Komoltri C, Davis MG, Wehbie RS. Elevated virus loads of Kaposi's sarcoma–associated human herpesvirus 8 predict Kaposi's sarcoma disease progression, but elevated levels of human immunodeficiency virus type 1 do not. J Infect Dis 2002; 185:1736–44. [DOI] [PubMed] [Google Scholar]
- 23. Hänscheid T. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin Lab Haematol 1999; 21:235–45. [DOI] [PubMed] [Google Scholar]
- 24. Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol 2006; 4:S7–20. [DOI] [PubMed] [Google Scholar]
- 25. Opoku Afriyie S, Addison TK, Gebre Y, et al. Accuracy of diagnosis among clinical malaria patients: comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar J 2023; 22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walk J, Schats R, Langenberg MC, et al. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J 2016; 15:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabourin KR, Nalwoga A, Whitby D, Newton R, Rochford R. Environmental determinants of Kaposi's sarcoma-associated herpesvirus (KSHV) transmission in rural Uganda (ENDKU study): contributions to research on KSHV infection and reactivation in African children; A longitudinal cohort study. Cancer Epidemiol 2022; 78:102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asiki G, Murphy G, Nakiyingi-Miiro J, et al. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int J Epidemiol 2013; 42:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newton R, Labo N, Wakeham K, et al. Kaposi's sarcoma associated herpesvirus in a rural Ugandan cohort: 1992–2008. J Infect Dis 2018; 217:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruth CJ, Huey SL, Krisher JT, et al. An electronic data capture framework (ConnEDCt) for global and public health research: design and implementation. J Med Internet Res 2020; 22:e18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wampfler R, Mwingira F, Javati S, et al. Strategies for detection of Plasmodium species gametocytes. PLoS One 2013; 8:e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Labo N, Miley W, Marshall V, et al. Heterogeneity and breadth of host antibody response to KSHV infection demonstrated by systematic analysis of the KSHV proteome. PLoS Pathog 2014; 10:e1004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chêne A, Donati D, Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog 2007; 3:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sebina I, Fogg LG, James KR, et al. IL-6 promotes CD4(+) T-cell and B-cell activation during Plasmodium infection. Parasite Immunol 2017; 39:e12455. [DOI] [PubMed] [Google Scholar]
- 35. Regunath H, Shivashankara KN, Sundeep KB, Bhaskar AP. Reactivation of herpes zoster in an adult with Plasmodium infection. J Vector Borne Dis 2008; 45:251–3. [PubMed] [Google Scholar]
- 36. Minigo G, Woodberry T, Piera KA, et al. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 2009; 5:e1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ueda K. KSHV genome replication and maintenance in latency. Adv Exp Med Biol 2018; 1045:299–320. [DOI] [PubMed] [Google Scholar]
- 38. Pinzon-Charry A, Woodberry T, Kienzle V, et al. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med 2013; 210:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frimpong A, Kusi KA, Tornyigah B, Ofori MF, Ndifon W. Characterization of T cell activation and regulation in children with asymptomatic Plasmodium falciparum infection. Malar J 2018; 17:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bejon P, Mwacharo J, Kai O, et al. The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol 2007; 179:4193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Studniberg SI, Ioannidis LJ, Utami RAS, et al. Molecular profiling reveals features of clinical immunity and immunosuppression in asymptomatic P. falciparum malaria. Mol Syst Biol 2022; 18:e10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nalwoga A, Roshan R, Moore K, et al. Kaposi's sarcoma–associated herpesvirus T cell responses in HIV seronegative individuals from rural Uganda. Nat Commun 2021; 12:7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roshan R, Labo N, Trivett M, et al. T-cell responses to KSHV infection: a systematic approach. Oncotarget 2017; 8:109402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reese TA, Wakeman BS, Choi HS, et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 2014; 345:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Labo N, Marshall V, Miley W, et al. Mutual detection of Kaposi's sarcoma–associated herpesvirus and Epstein-Barr virus in blood and saliva of Cameroonians with and without Kaposi's sarcoma. Int J Cancer 2019; 145:2468–77. [DOI] [PubMed] [Google Scholar]
- 46. Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 2012; 205:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haq IU, Dalla Pria A, Papanastasopoulos P, et al. The clinical application of plasma Kaposi sarcoma herpesvirus viral load as a tumour biomarker: results from 704 patients. HIV Med 2016; 17:56–61. [DOI] [PubMed] [Google Scholar]
- 48. Martró E, Cannon MJ, Dollard SC, et al. Evidence for both lytic replication and tightly regulated human herpesvirus 8 latency in circulating mononuclear cells, with virus loads frequently below common thresholds of detection. J Virol 2004; 78:11707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.