Abstract
We estimated the effectiveness of booster doses of monovalent and bivalent mRNA COVID-19 vaccines against Omicron-associated severe outcomes among adults aged ≥50 years in Ontario, Canada. Monovalent and bivalent mRNA COVID-19 booster doses provided similar strong initial protection against severe outcomes. Uncertainty remains around waning of protection from these vaccines.
Keywords: bivalent, COVID-19, mRNA, vaccine effectiveness, Omicron
Shortly after bivalent mRNA COVID-19 vaccines were introduced, monovalent and bivalent booster doses provided similar strong initial protection against severe outcomes among Ontario community-dwelling adults aged ≥50 years. Uncertainty remains around waning of protection.
According to clinical trial data, the Moderna BA.1 bivalent vaccine was found to be immunogenic against other Omicron sublineages [1], contributing to its authorization in Canada and other jurisdictions when the BA.4/BA.5 sublineage was predominant. On 12 September 2022, the vaccine was introduced to Ontario's COVID-19 vaccination program as a booster dose for adults at highest risk, including those aged ≥70 years, and was expanded to all adults on 26 September 2022 [2]. The Pfizer-BioNTech BA.4/BA.5 bivalent vaccine was introduced on 17 October 2022 [3]. Bivalent mRNA COVID-19 vaccines were recommended for booster doses, though monovalent vaccines were still available and accessible [4]. Canada's immunization advisory committee emphasized the importance of receiving booster doses, regardless of product, to maintain protection [4]. The effectiveness of bivalent COVID-19 vaccines has not yet been explored among older Ontario adults. Our objective was to compare the vaccine effectiveness (VE) of booster vaccinations using the Moderna BA.1 and Pfizer-BioNTech BA.4/BA.5 bivalent mRNA COVID-19 vaccines vs the original mRNA COVID-19 monovalent vaccines against Omicron-related severe outcomes.
METHODS
We conducted a test-negative design study among community-dwelling adults aged ≥50 years who had ≥1 real-time polymerase chain reaction test for SARS-CoV-2 between 19 June 2022 and 28 January 2023. We used provincial SARS-CoV-2 laboratory testing, COVID-19 surveillance (Public Health Case and Contact Management Solution), COVID-19 vaccination (COVaxON), and health administrative data sets. Our outcome of interest was severe COVID-19 (hospitalizations or death). Data entry guidelines from Public Health Case and Contact Management Solution specify that hospitalization data be entered only for those who received treatment for COVID-19 and/or if their length of stay was extended due to COVID-19 [5]. A COVID-19 death was defined as that resulting from a clinically compatible illness in a confirmed COVID-19 case, unless there was a clear alternative cause that could not be related to COVID-19 [5]. There were insufficient outcomes and, thus, statistical power to explore hospitalizations and deaths separately. Data were linked by unique encoded identifiers and analyzed at ICES. Projects that use data collected by ICES under section 45 of the Personal Health Information Protection Act and use no other data are exempt from research ethics board review.
We excluded adults who were immunocompromised (n = 10 715), received <4 doses (n = 40 422) or a vaccine not authorized by Health Canada (n = 13), tested positive within 60 days prior to the test (n = 36), or hospitalizations where specimen collection occurred >3 days after admission (n = 505) or the infection was flagged as nosocomial (n = 388). Third doses, or first booster doses, were excluded due to the time gap between third dose introduction (December 2021) and bivalent vaccine introduction (September 2022). Sublineage-predominant periods in the province were defined as ≥50% of sequenced samples being of a particular sublineage. From 19 June to 3 December 2022, Omicron BA.4/BA.5 was the predominant sublineage, and from 4 December 2022 to 28 January 2023, BQ was predominant (Supplementary Appendix, Supplementary Figure 1) [6].
We estimated the VE of the Moderna monovalent, Pfizer-BioNTech monovalent, Moderna BA.1 bivalent, and Pfizer-BioNTech BA.4/BA.5 bivalent booster doses against severe outcomes as compared with unvaccinated cases, stratified by time since vaccination (30-day periods up to 4 months). The Moderna BA.4/BA.5 bivalent vaccine was approved in Canada during the study period but was not available in Ontario. Controls had to be symptomatic (Supplementary Text) and test negative for SARS-CoV-2. Cases and controls were sampled by week of test. Once an individual became a case, that person could no longer reenter the study.
We used multivariable logistic regression to compare the odds of vaccination in cases vs test-negative controls. Few adults (<10%) appeared in models more than once, with minimal impact on confidence intervals; thus, clustering methods were not applied. Models were adjusted a priori for sex, age, prior influenza vaccination, geographic region, sociodemographic area–level variables (by quintile; household income, essential worker, persons per dwelling, self-identified visible), number of SARS-CoV-2 tests within 3 months prior to 14 December 2020, comorbidities, receipt of home care services, and week of test (Supplementary Appendix, Supplementary Table 1). VE of the Pfizer-BioNTech BA.4/BA.5 bivalent vaccine could be estimated only up to 89 days postvaccination due to more recent availability in Ontario. We calculated VE as 1 − adjusted odds ratio. As a secondary analysis, we stratified the study period by predominant sublineage (BA.4/BA.5 vs BQ).
RESULTS
We included 3755 Omicron cases and 14 338 test-negative controls (16 247 unique individuals). Cases were older than controls, and 57% and 69% had their fourth dose as their most recent dose, respectively (Supplementary Appendix, Supplementary Table 2). As compared with unvaccinated adults, fewer vaccinated adults were male or from areas with the lowest household incomes (Supplementary Table 3). From 19 June to 11 September 2022, when only monovalent COVID-19 vaccines were available, 33% and 66% of adults received the Moderna monovalent and Pfizer-BioNTech monovalent vaccine as their most recent dose. From 12 September 2022 to the end of the study period, 16%, 35%, 31%, and 18% received the Moderna monovalent, Pfizer-BioNTech monovalent, Moderna BA.1 bivalent, and Pfizer-BioNTech BA.4/BA.5 bivalent vaccine as their most recent dose.
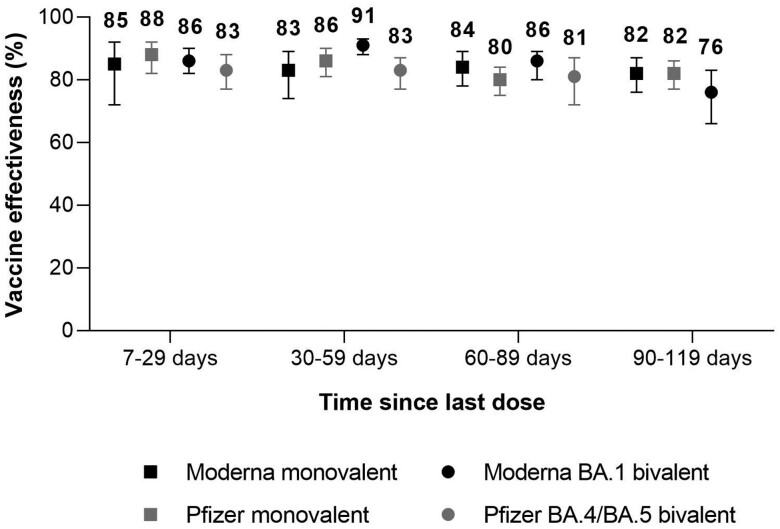
For the full study period, VE of the Moderna and Pfizer-BioNTech monovalent vaccines 7 to 29 days after vaccination was 85% (95% CI, 72%–92%) and 88% (95% CI, 82%–92%; Figure 1; Supplementary Appendix, Supplementary Table 4). At 90 to 119 days after vaccination, VE was 82% (95% CI, 76%–87%) for the Moderna monovalent vaccine and 82% (95% CI, 77%–86%) for the Pfizer-BioNTech monovalent vaccine. VE of the Moderna BA.1 bivalent vaccine was 86% (95% CI, 82%–90%) 7 to 29 days after vaccination and 76% (95% CI, 66%–83%) 90 to 119 days after vaccination. VE of the Pfizer-BioNTech BA.4/BA.5 bivalent vaccine was 83% (95% CI, 77%–88%) 7 to 29 days after vaccination and 81% (95% CI, 72%–87%) 60 to 89 days after vaccination.
Figure 1.
Vaccine effectiveness of monovalent and bivalent mRNA COVID-19 vaccines against Omicron-associated severe outcomes by time since vaccination among community-dwelling adults aged ≥50 years in Ontario, Canada, vs unvaccinated adults, 19 June 2022 to 28 January 2023 (BA.4/BA.5 and BQ periods combined). Error bars indicate 95% CI.
Comparisons of all vaccine products and intervals since vaccination were not possible by sublineage period due to different dates of vaccine introduction. For estimates that could be compared, VE was slightly lower during the BQ-predominant period than the BA.4/BA.5-predominant period (Supplementary Appendix, Supplementary Table 5, Supplementary Figure 2). For example, VE of the Moderna BA.1 bivalent vaccine and Pfizer-BioNTech BA.4/BA.5 bivalent vaccine was 93% (95% CI, 90%–95%) and 87% (95% CI, 71%–94%), respectively, 30 to 59 days after vaccination during the BA.4/BA.5-predominant period, whereas the corresponding estimates during the BQ-predominant period were 82% (95% CI, 71%–89%) and 82% (95% CI, 73%–88%).
DISCUSSION
Among community-dwelling adults aged ≥50 years in Ontario, monovalent and bivalent mRNA COVID-19 vaccines provided similar strong initial protection against hospitalization or death. VE of monovalent and bivalent vaccines ranged from 85% to 88% and 83% to 86%, respectively, 7 to 29 days after vaccination, with slight waning of protection across the 4-month period. After 90 to 119 days, monovalent VE decreased to 82% and Moderna BA.1 bivalent VE to 76%. VE appeared slightly lower in the BQ-predominant period vs the BA.4/BA.5 period, though uncertainty remains whether a true difference exists. Lower VE against BQ may contribute to lower VE in latter periods vs waning protection alone.
Few studies compared the VE of a COVID-19 monovalent vs bivalent booster across a similar period. Comparable to our findings, these studies suggested that the effectiveness of bivalent vaccines is either similar or moderately higher than the monovalent products [7–10]. Nonetheless, due to differences in circulating sublineages at the time of vaccine introduction, across the study period (eg, prevalence of a sublineage will differ even across 30-day intervals), and in rates of previous infection, direct comparisons of effectiveness between monovalent and bivalent vaccines within and across jurisdictions are difficult.
There is limited evidence on the VE of monovalent and bivalent COVID-19 vaccines against BQ-associated severe disease. Similar to our results, findings from England suggested that bivalent VE was lower against BQ- than BA.5-related hospitalizations ≥2 weeks after vaccination (∼10–percentage point difference) [11]. The study also expressed uncertainty around whether a difference really exists.
Evidence suggests that the VE of COVID-19 vaccines differs by past SARS-CoV-2 infection [12]. We were unable to account for prior infection as an effect modifier since there were few cases of previous infection in our data. Due to limited real-time polymerase chain reaction testing availability and no access to rapid antigen testing results, the majority of SARS-CoV-2 infections in Ontario are undocumented. If unvaccinated adults were more likely than vaccinated adults to have a previous infection, VE may be underestimated since unvaccinated adults would have some underlying infection-induced immunity. Nonetheless, increasing SARS-CoV-2 seroprevalence in Ontario (76% among adults as of 15 January 2023) [13] suggests that VE may be more generalizable to those previously infected. There is also potential for residual confounding since we were limited to the variables available in our data sets. A significant strength of our analysis is that, due to the continued availability and uptake of monovalent mRNA COVID-19 vaccines after bivalent vaccines were introduced, we were able to compare the VE of these products as booster doses across a similar study period.
Monovalent and bivalent mRNA COVID-19 vaccines provide comparable levels of initial protection against Omicron-related severe outcomes among community-dwelling adults aged ≥50 years in Ontario. Older adults continue to experience the highest rates of COVID-19–related severe outcomes and would benefit the most from additional vaccine doses. Longer follow-up is necessary to determine the long-term protection of bivalent vaccines and the effectiveness against newer Omicron sublineages such as XBB.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ramandip Grewal, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; Centre for Vaccine Preventable Diseases, University of Toronto.
Sarah A Buchan, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; Centre for Vaccine Preventable Diseases, University of Toronto; ICES, Toronto.
Lena Nguyen, ICES, Toronto.
Sharifa Nasreen, Dalla Lana School of Public Health, University of Toronto; ICES, Toronto.
Peter C Austin, ICES, Toronto; Institute of Health Policy, Management and Evaluation, University of Toronto.
Kevin A Brown, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; ICES, Toronto.
Jonathan Gubbay, Public Health Ontario, Toronto; Department of Pathology and Laboratory Medicine, British Columbia Children's and Women's Health Centre, Vancouver.
Nelson Lee, Dalla Lana School of Public Health, University of Toronto.
Kevin L Schwartz, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; ICES, Toronto.
Mina Tadrous, ICES, Toronto; Institute for Health System Solutions and Virtual Care, Women's College Hospital, Toronto; Leslie Dan Faculty of Pharmacy, University of Toronto.
Kumanan Wilson, Department of Medicine, University of Ottawa; Clinical Epidemiology Program, Ottawa Hospital Research Institute; Bruyere Research Institute, Ottawa.
Sarah E Wilson, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; Centre for Vaccine Preventable Diseases, University of Toronto; ICES, Toronto.
Jeffrey C Kwong, Public Health Ontario, Toronto; Dalla Lana School of Public Health, University of Toronto; Centre for Vaccine Preventable Diseases, University of Toronto; ICES, Toronto; Department of Family and Community Medicine, University of Toronto; Toronto Western Family Health Team, University Health Network, Toronto.
Notes
Acknowledgments . We acknowledge the Canadian Immunization Research Network’s Provincial Collaborative Network Investigators, Public Health Ontario, for access to vaccination data from COVaxON, case-level data from the Public Health Case and Contact Management Solution, and COVID-19 laboratory data, as well as assistance with data interpretation. We also thank the staff of Ontario's public health units who are responsible for COVID-19 case and contact management and data collection within Public Health Case and Contact Management Solution. We thank IQVIA Solutions Canada Inc for use of its Drug Information File. We are grateful to the Ontario residents without whom this research would be impossible. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File: this file is based on data licensed from Canada Post Corporation and/or adapted from the Ontario Ministry of Health Postal Code Conversion File, and it contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by the Ontario Ministry of Health, Ontario Health, the Canadian Institute for Health Information, Statistics Canada, and IQVIA Solutions Canada Inc.
Author contributions . R. G., S. A. B., L. N., and J. C. K. created the analysis plan. L. N. obtained the data and conducted the analyses (data set and variable creation and statistical modeling). R. G. drafted the manuscript. R. G., S. A. B., L. N., S. N., P. C. A., K. A. B., J. G., N. L., K. L. S., M. T., K. W., S. E. W., and J. C. K. contributed to the analysis plan, interpreted the results, critically reviewed and edited the manuscript, approved the final version, and agreed to be accountable for all aspects of the work. J. C. K. is the guarantor.
Data availability . The data set from this study is held securely in coded form at ICES. While legal data-sharing agreements between ICES and data providers (eg, health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca).
Disclaimer . The study sponsors did not participate in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Use of adapted data from Statistics Canada, Canadian Census 2016, does not constitute an endorsement by Statistics Canada of this product.
Financial support . This work was supported by funding from the Canadian Immunization Research Network through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944); the Public Health Agency of Canada through the Vaccine Surveillance Working Party and the COVID-19 Immunity Task Force. This study was supported by Public Health Ontario and ICES, the latter of which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care; the Ontario Health Data Platform, a Province of Ontario initiative to support Ontario's ongoing response to COVID-19 and its related impacts; and a Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine to J. C. K.
References
- 1. Chalkias S, Harper C, Vrbicky K, et al. A bivalent Omicron-containing booster vaccine against COVID-19. N Engl J Med 2022; 387:1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Government of Ontario . Ontarians aged 18+ eligible for bivalent COVID-19 booster dose. 2022. https://news.ontario.ca/en/release/1002277/ontarians-aged-18-eligible-for-bivalent-covid-19-booster-dose. Accessed 26 October 2022.
- 3. Government of Ontario . All Ontarians aged 12+ eligible for bivalent booster.2022. https://news.ontario.ca/en/release/1002384/all-ontarians-aged-12-eligible-for-bivalent-booster. Accessed 27 March 2023.
- 4. National Advisory Committee on Immunization . An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): recommendations on the use of bivalent Omicron-containing mRNA COVID-19 vaccines.Ottawa:National Advisory Committee on Immunization, 2022. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-bivalent-Omicron-containing-mrna-covid-19-vaccines.pdf. Accessed 28 October 2022. [Google Scholar]
- 5. Ontario Agency for Health Protection and Promotion, Public Health Ontario . COVID-19 CCM case investigation data entry guide, version 2.0. Toronto: Queen's Printer for Ontario, 2022. [Google Scholar]
- 6. Ontario Agency for Health Protection and Promotion, Public Health Ontario . SARS-CoV-2 whole genome sequencing in Ontario, February 17, 2023. Toronto: Queen’s Printer for Ontario, 2023. https://www.publichealthontario.ca/-/media/Documents/nCoV/Archives/Genome/2023/02/SARS-CoV-2-genomic-surveillance-report-2023-02-17.pdf?rev=0b6b17ef1f064e11add34c28fadd2443&sc_lang=en. Accessed 11 April 2023.
- 7. Lin D, Xu Y, Gu Y, et al. Effectiveness of bivalent boosters against severe Omicron infection. N Engl J Med 2023; 388:764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatzilena A, Hyams C, Challen R, et al. Relative vaccine effectiveness (rVE) of mRNA COVID-19 boosters in the UK vaccination programme, during the spring-summer (monovalent vaccine) and autumn-winter 2022 (bivalent vaccine) booster campaigns: a prospective test negative case-control study. medRxiv. Preprint posted online March 17, 2023. doi: 10.1101/2023.03.16.23287360 [DOI]
- 9. Andersson NW, Thiesson EM, Baum U, et al. Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: nationwide cohort study. BMJ 2023; 382:e075286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auvigne V, Tamandjou Tchuem CR, Schaeffer J, Vaux S, Parent Du Chatelet I. Protection against symptomatic SARS-CoV-2 infection conferred by the Pfizer-BioNTech Original/BA.4-5 bivalent vaccine compared to the mRNA Original monovalent vaccines—a matched cohort study in France. Vaccine 2023; 41:5490–3. [DOI] [PubMed] [Google Scholar]
- 11. UK Health and Security Agency . COVID-19 vaccine surveillance report: week 9. London: UK Health and Security Agency, 2023. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1139990/vaccine-surveillance-report-2023-week-9.pdf. Accessed 27 March 2023.
- 12. Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023; 23:556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. COVID-19 Immunity Task Force . Seroprevalence in Canada. https://www.covid19immunitytaskforce.ca/seroprevalence-in-canada/. Accessed 27 March 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.