Abstract
Background
In the previous (parent) study, 2 doses of different formulations of an investigational vaccine against respiratory syncytial virus (RSVPreF3 OA) were well tolerated and immunogenic in older adults. This multicenter phase 2b extension study assessed safety and immunogenicity of a revaccination (third) dose of the 120 μg RSVPreF3-AS01E formulation.
Methods
In total, 122 older adults (60–80 years), previously vaccinated with 2 doses of RSVPreF3-AS01E formulations (containing 30, 60, or 120 μg RSVPreF3 antigen), received an additional 120 μg RSVPreF3-AS01E dose 18 months after dose 2. Vaccine safety was evaluated in all participants up to 6 months and immunogenicity in participants who received 120 μg RSVPreF3-AS01E doses until 1 month after dose 3.
Results
Similar to the parent study, mostly mild-to-moderate solicited adverse events and no vaccine-related serious adverse events or potential immune-mediated disorders were reported. Neutralizing titers and cell-mediated immune responses persisted for 18 months after 2-dose vaccination. Dose 3 increased RSV-specific neutralizing titers against RSV-A and RSV-B and median CD4+ T-cell frequencies. After dose 3, RSV-specific neutralizing titers but not CD4+ T-cell frequencies were below levels detected 1 month after dose 1.
Conclusions
Revaccination with 120 μg RSVPreF3-AS01E 18 months after dose 2 is well tolerated and immunogenic in older adults.
Clinical Trials Registration
NCT04657198; EudraCT, 2020-000692-21.
Keywords: AS01E, RSV neutralizing titers, RSV vaccine, RSVPreF3, cell-mediated immunity, respiratory syncytial virus
This phase 2b extension study demonstrated an acceptable safety profile and robust immunogenicity of a third, revaccination, dose of an adjuvanted vaccine against respiratory syncytial virus administered 18 months after the second dose in older adults aged ≥60 years.
Respiratory syncytial virus (RSV) is a contagious seasonal virus causing respiratory tract infections in people of all ages [1, 2]. There are 2 main antigenic subtypes, RSV-A and RSV-B [1]. The subtypes are cocirculating with alternating predominance across seasons, with a varying pattern [1, 2].
RSV infections usually resolve without complications or sequelae in immune-competent persons [3]. However, in older adults (OAs) aged ≥60 years, RSV can cause more serious respiratory illnesses (including lower respiratory tract disease) [3], especially in people with underlying medical conditions or those who are immunocompromised [4, 5]. In OAs, RSV infections thus lead to a significant disease burden [6], which was underestimated for a long time [7–9]. According to a recent systematic review of data from high-income countries, the calculated pooled estimates of RSV acute respiratory infections in OAs aged ≥60 years were 1.62% (95% confidence interval [CI], .84%–3.08%) for attack rate, 0.15% (95% CI, .09%–.22%) for hospitalization rate, and 7.13% (95% CI, 5.40%–9.36%) for in-hospital case fatality rate [8]. Based on the described values and using the 2019 census data, the same review estimated that about 5 million cases of acute respiratory tract infection, half a million hospitalizations, and 33 000 in-hospital deaths of OAs could be attributed to RSV in 2019 [8].
The severity of RSV-associated disease in OAs has been ascribed to waning humoral and cellular immune responses (immunosenescence) that were induced by previous RSV infections [10–15]. A protective immune response against RSV is orchestrated by antibodies (eg, immunoglobulin A [IgA] and IgG, neutralizing antibodies [nAb]), and lymphocytes (both cluster-of-differentiation-4-expressing [CD4+] and CD8+ T cells) that produce a variety of cytokines such as interleukins (ILs) and interferons (IFNs), resulting in viral clearance and protection [14]. The RSV-specific immunity obtained after infection is not long lasting and, even though most people have some level of postinfection immunity, this does not prevent subsequent RSV infections. Due to a higher disease burden in the vulnerable OA population, the waning immune responses lead to an increased risk for more severe disease in OAs. Thus, approaches to overcome waning immunity (eg, vaccination) can help avoid serious RSV-associated disease in OAs [16].
Several vaccines based on the prefusion conformation of RSV fusion protein (PreF) and using different delivery systems were recently evaluated in clinical studies [17–26]. The RSV vaccine investigated in this study is based on PreF stabilized in its trimeric conformation (RSVPreF3) as the main antigen, and adjuvanted with AS01E [18, 27]. In a previous phase 1/2 study (hereafter referred to as the parent study), different formulations of the RSVPreF3-based vaccine were administered 2 months apart to OAs aged 60–80 years [18]. The vaccine formulation containing 120 µg of RSVPreF3 and adjuvanted with AS01E (hereafter referred to as 120 μg RSVPreF3-AS01E or RSVPreF3 OA) was selected for further clinical development, because it most potently induced humoral and cellular RSV-specific immune responses while retaining an acceptable safety profile in the parent study [18]. An ongoing vaccine efficacy study demonstrated a consistently high efficacy (point estimate of 82.6% with a 96.95% CI, 57.9%–94.1%) for the 120 μg RSVPreF3-AS01E formulation against RSV-related lower respiratory tract disease in OAs aged ≥60 years, thus meeting the primary study end point [27, 28]. The 120 μg RSVPreF3-AS01E formulation has then been licensed for use in OAs in the United States and European Union [29].
The overall objective of the present extension study was to evaluate the safety, reactogenicity, and immunogenicity of the selected 120 μg RSVPreF3-AS01E formulation, administered as the third vaccine dose (dose 3) 18 months after dose 2 (month 20 [M20]), in participants who had received 2 doses of the AS01E-adjuvanted formulations containing 30, 60, or 120 μg of RSVPreF3 (ie, 30 μg RSVPreF3-AS01E, 60 μg RSVPreF3-AS01E, or 120 μg RSVPreF3-AS01E) in the parent study.
METHODS
This phase 2b extension study (NCT04657198) was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization requirements. The study was approved by institutional ethics committees. The participating OAs were enrolled at 7 centers in the United States, and 3 centers in Belgium. The study was open label as all participants received the same 120 μg RSVPreF3-AS01E vaccine formulation.
Study Vaccine
The RSVPreF3 vaccine formulations have been described in detail [18]. In this extension study, only the 120 μg RSVPreF3-AS01E formulation was administered as the third dose.
Study Participants and Procedures
Eligible participants were healthy men and women who had received the 30-, 60-, or 120 μg RSVPreF3-AS01E formulation in the parent study [18]. Participants needed to be able and willing to comply with protocol requirements (as determined by investigator), and to have provided written informed consent prior to any study-specific procedures. Deviations from inclusion criteria were not allowed. Inclusion and exclusion criteria are listed in the Supplementary Material.
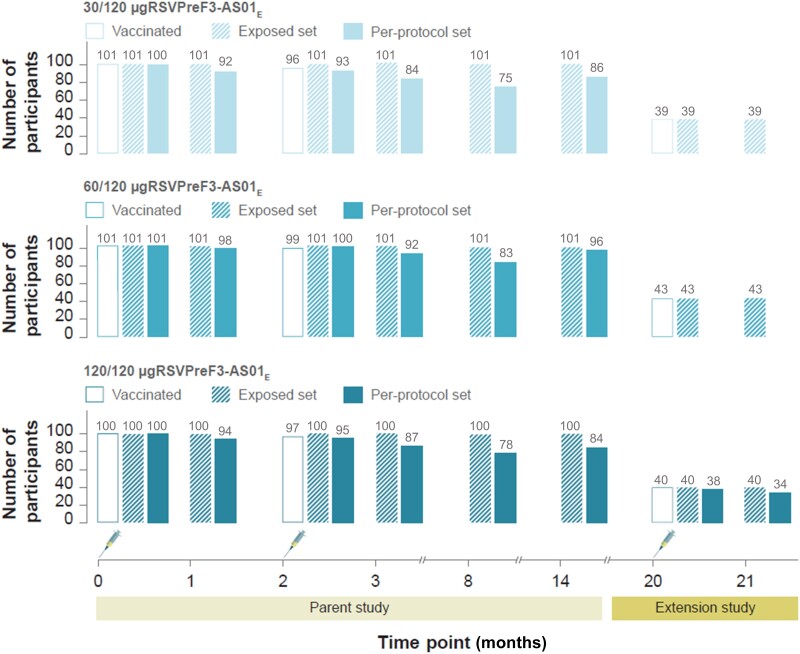
In the parent study [18], the OA participants were randomized to receive 2 doses of a given vaccine formulation or placebo on day 1 (also denoted as M0 time point) and day 61 (M2) (Figure 1). The follow-up period for OAs was up to 1 year after the second vaccination (M14). In this extension study, all OA recipients of the AS01E-adjuvanted formulations were invited to receive the third vaccine dose containing the 120 μg RSVPreF3-AS01E formulation at M20. Follow-up time was 6 months after dose 3 (M26). All vaccines were administered intramuscularly, into the deltoid region of the nondominant arm.
Figure 1.
Overview of parent and extension study designs. The parent study design and data have been published [18]. Syringe symbols represent vaccination. Time points 0, 1, 2, 3, 8, 14, 20, and 21 indicate study time points at M0 (day 1, dose 1 vaccination), M1 (day 31, 1 month after dose 1), M2 (day 61, dose 2 vaccination), M3 (day 91, 1 month after dose 2), M8, M14, M20 (dose 3 vaccination), and M21 (1 month after dose 3). Participants received 2 doses of the AS01E-adjuvanted vaccine formulation with 30, 60, or 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study, indicated by 30/120-, 60/120-, and 120/120 μg RSVPreF3-AS01E. Abbreviations: AS01E, adjuvant system; M, month; RSVPreF3, prefusion conformation of the respiratory syncytial virus fusion (F) protein.
Because the present manuscript refers to findings of both the parent and extension studies, the timeline details are provided here for ease of reference. Time points M0–M14 refer to the parent study and included M0 (day 1, baseline, dose 1 administration), M1 (day 31, 1 month after dose 1), M2 (day 61, dose 2 administration), M3 (day 91, 1 month after dose 2), M8, and M14. This extension study includes time points M20 (dose 3 administration), M21 (1 month after dose 3), and M26 (end of study, 6 months after dose 3) (Figure 1).
Participant groups were named 30/120 μg RSVPreF3-AS01E, 60/120 μg RSVPreF3-AS01E, and 120/120 μg RSVPreF3-AS01E according to the RSVPreF3-based vaccine formulations received in both studies (eg, the group 30/120 μg RSVPreF3-AS01E received 2 doses of the 30 μg RSVPreF3-AS01E formulation in the parent study and the 120 μg RSVPreF3-AS01E formulation in this extension study).
Occurrence of adverse events (AEs) was recorded for all participants in the following periods: 4 days after dose 3 for solicited AEs (administration site [pain, redness, swelling] and systemic [fever]) AEs, 30 days after dose 3 for unsolicited AEs, and up to 6 months after dose 3 for AEs leading to study withdrawal, serious AEs (SAEs) and potential immune-mediated disorders (pIMDs).
Blood samples for evaluation of humoral (approximately 20 mL) and cell-mediated immune (CMI) (approximately 25 mL) responses were collected at M20 and M21 time points only from participants in the 120/120 μg RSVPreF3-AS01E group. Neutralizing titers against RSV-A and RSV-B were measured by neutralization assays, and RSVPreF3-specific IgG concentrations were determined using an in-house enzyme-linked immunosorbent assay (ELISA) [18]. Frequencies of RSVPreF3-specific CD4+ and CD8+ T cells were evaluated using intracellular cytokine staining on peripheral blood mononuclear cells [18].
Study Objectives and End Points
Primary safety objectives were to evaluate the vaccine safety and reactogenicity in all participants up to 1 month after dose 3 in terms of occurrence of solicited AEs up to 4 days, and unsolicited AEs, SAEs, and pIMDs up to 30 days after dose 3. The primary immunogenicity objective was to evaluate humoral immune responses in the 120/120 μg RSVPreF3-AS01E group of participants in terms of neutralizing titers against RSV-A and RSV-B up to 1 month after dose 3 (M21).
The secondary safety objective was to determine the safety of dose 3 in all participants until study end (ie, 6 months after dose 3) in terms of occurrence of SAEs and pIMDs. The secondary immunogenicity objective was to evaluate the humoral response in terms of RSVPreF3-specific IgG concentration and CMI response in terms of frequency of RSVPreF3-specific CD4+ T cells expressing at least 2 markers (among IL-2, CD40 ligand [CD40L], tumor necrosis factor-α [TNF-α] and IFN-γ) in the 120/120 μg RSVPreF3-AS01E group of participants up to M21. Tertiary study objectives and end points are described in the Supplementary Material.
Statistical Analyses
Sample size for the parent study was previously presented in detail [18], and no additional estimations were done for this extension study. Analysis sets included enrolled set (participants who provided their informed consent to participate in the study), exposed set (participants who received dose 3), and per-protocol set (participants who received dose 3 with available immunogenicity data and without important protocol deviations including those leading to study exclusion [see Supplementary Material]). Safety was assessed on the exposed set, while immunogenicity was evaluated on the per-protocol set.
All data were analyzed using descriptive statistics. Categorical data were tabulated as the number and percentage of participants, while continuous data were described/plotted as mean with 95% CI or median with range (minimum and maximum).
The geometric mean titers/concentrations (GMTs/GMCs) were computed as the antilogarithm of the arithmetic mean of the log10 transformed titers/concentrations. Cutoff or lower limit of quantification (LLOQ) values for immunogenicity assays were: 18 estimated dilution 60 (ED60) (RSV-A nAb GMT), 30 ED60 (RSV-B nAb GMT), 25 ELISA units/mL (RSVPreF3-specific IgG GMC), and 590/106 cells (CD4+ T-cell frequencies). Titers/concentrations below the assay cutoff were given an arbitrary value of half the assay cutoff, while those above the assay's upper limit of quantification (ULOQ) were assigned the ULOQ value. For calculations of the fold change in frequencies of CD4+ T cells expressing at least 2 markers, frequencies below the LLOQ were imputed to the LLOQ value. Missing or nonevaluable measurements were not replaced.
RESULTS
Demographic and Baseline Characteristics of Study Participants
In the parent study, 1005 OA participants received at least 1 vaccine/placebo dose [18]. Of those, 302 received 1 and 291 received 2 doses of an RSVPreF3-AS01E vaccine formulation (Figure 1) [18].
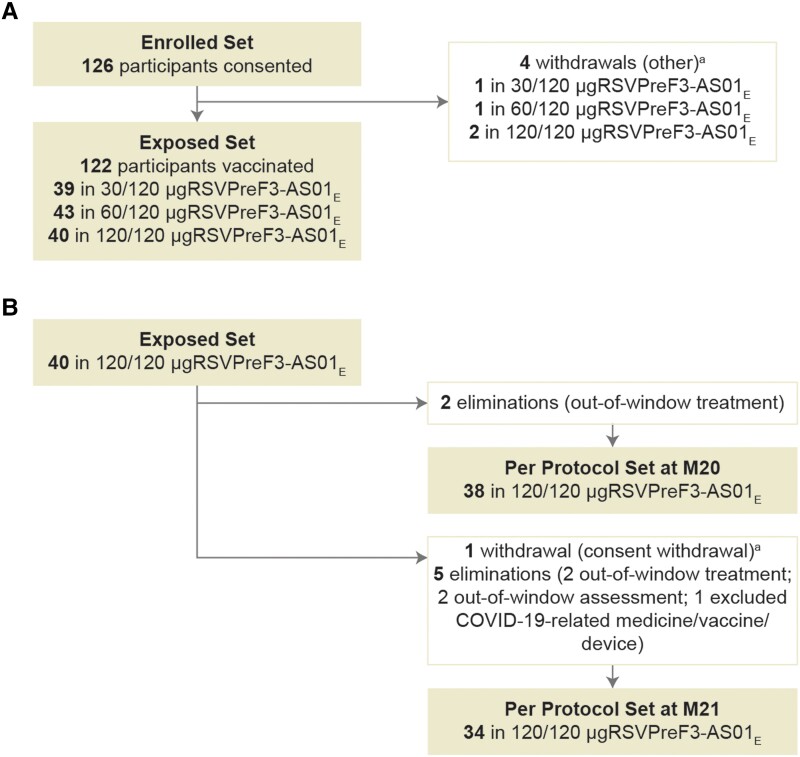
In this study, conducted between December 2020 and October 2021, 122 OA participants (39 in the 30/120 μg RSVPreF3-AS01E group, 43 in the 60/120 μg RSVPreF3-AS01E group, and 40 in the 120/120 μg RSVPreF3-AS01E group) were included in the exposed set (Figure 1 and Figure 2). The per-protocol set included 38 (95.0%) and 34 (85.0%) of 120/120 μg RSVPreF3-AS01E participants at M20 and M21 (Figure 2). The present study enrolled 72 (59.0%) female participants, and most participants were White (117, 95.9%) and of non-Hispanic or Latino ethnicity (121, 99.2%) (Table 1).
Figure 2.
Participant flow chart with (A) reasons for withdrawal and elimination from the exposed set and (B) per-protocol set. aParticipant withdrawal (including consent withdrawal) was due to a reason other than an adverse event and/or solicited adverse event, migration from study area, loss to follow-up, or sponsor study termination. Participants received 2 doses of the AS01E-adjuvanted vaccine formulation with 30, 60, or 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study, indicated by 30/120-, 60/120-, and 120/120 μg RSVPreF3-AS01E. M20 and M21 indicate study time points at month 20 (dose 3 vaccination) and month 21 (1 month after dose 3) in the extension study. Abbreviations: COVID-19, coronavirus disease 2019; M, month; RSVPreF3, prefusion conformation of the respiratory syncytial virus fusion (F) protein.
Table 1.
Demographic Characteristics of OA Participants in the Parenta and Extension Studies, Exposed Set
| OA Participants, 60─80 y, | Parent Study | Extension Study | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | 30 μg AS01E | 60 μg AS01E | 120 μg AS01E | Total | 30/120 μg RSVPreF3-AS01E | 60/120 μg RSVPreF3-AS01E | 120/120 μg RSVPreF3-AS01E | Total |
| No. of participants | 101 | 101 | 100 | 1005 | 39 | 43 | 40 | 122 |
| Age at first vaccination, y | ||||||||
| Mean (SD) | 67.8 (5.1) | 67.1 (5.6) | 67.6 (5.2) | 67.6 (5.2) | 69.1 (5.3) | 66.6 (5.6) | 68.3 (5.4) | 68.0 (5.5) |
| Median (min–max) | 67.0 (60.0–80.0) |
66.0 (60.0–79.0) |
67.0 (60.0–80.0) |
67.0 (60.0–80.0) |
69.0 (61.0–78.0) |
64.0 (60.0–79.0) |
68.5 (60.0–79.0) |
67.0 (60.0–79.0) |
| Age category, y, No. (%) | ||||||||
| 60–69 | 67 (66.3) | 66 (65.3) | 64 (64.0) | 660 (65.7) | 22 (56.4) | 28 (65.1) | 21 (52.5) | 71 (58.2) |
| 70–80 | 34 (33.7) | 35 (34.7) | 36 (36.0) | 345 (34.3) | 17 (43.6) | 15 (34.9) | 19 (47.5) | 51 (41.8) |
| Sex, No. (%) Female |
58 (57.4) | 57 (56.4) | 57 (57.0) | 573 (57.0) | 21 (53.8) | 24 (55.8) | 27 (67.5) | 72 (59.0) |
| Ethnicity, No. (%) not Hispanic or Latino |
98 (97.0) | 98 (97.0) | 97 (97.0) | 969 (96.4) | 39 (100.0) | 42 (97.7) | 40 (100.0) | 121 (99.2) |
| Race, No. (%) | ||||||||
| White | 88 (87.1) | 94 (93.1) | 93 (93.0) | 927 (92.2) | 37 (94.9) | 42 (97.7) | 38 (95.0) | 117 (95.9) |
| Black/African American | 12 (11.9) | 7 (6.9) | 5 (5.0) | 69 (6.9) | 2 (5.1) | 1 (2.3) | 1 (2.5) | 4 (3.3) |
| Otherb | 1 (1.0) | 0 (0.0) | 2 (2.0) | 9 (0.9) | 0 (0.0) | 0 (0.0) | 1 (2.5) | 1 (0.8) |
Abbreviations: min–max, minimum to maximum; No. (%), number and percentage of participants in a given category; OA, older adults; RSVPreF3, prefusion conformation of the respiratory syncytial virus fusion (F) protein.
aThe data have been published in Leroux-Roels et al [18].
bIncludes American Indian or Alaska Native and Asian participants; 30, 60, and 120 μg AS01E, participants who received at least 1 dose of the AS01E-adjuvanted vaccine formulation with 30, 60, or 120 μg of RSVPreF3 antigen in the parent study; 30/120, 60/120, and 120/120 μg RSVPreF3-AS01E, participants who received 2 doses of the AS01E-adjuvanted vaccine formulation with 30, 60, or 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study (see RSVPreF3 definition); AS01E, adjuvant system [18].
Safety Evaluation
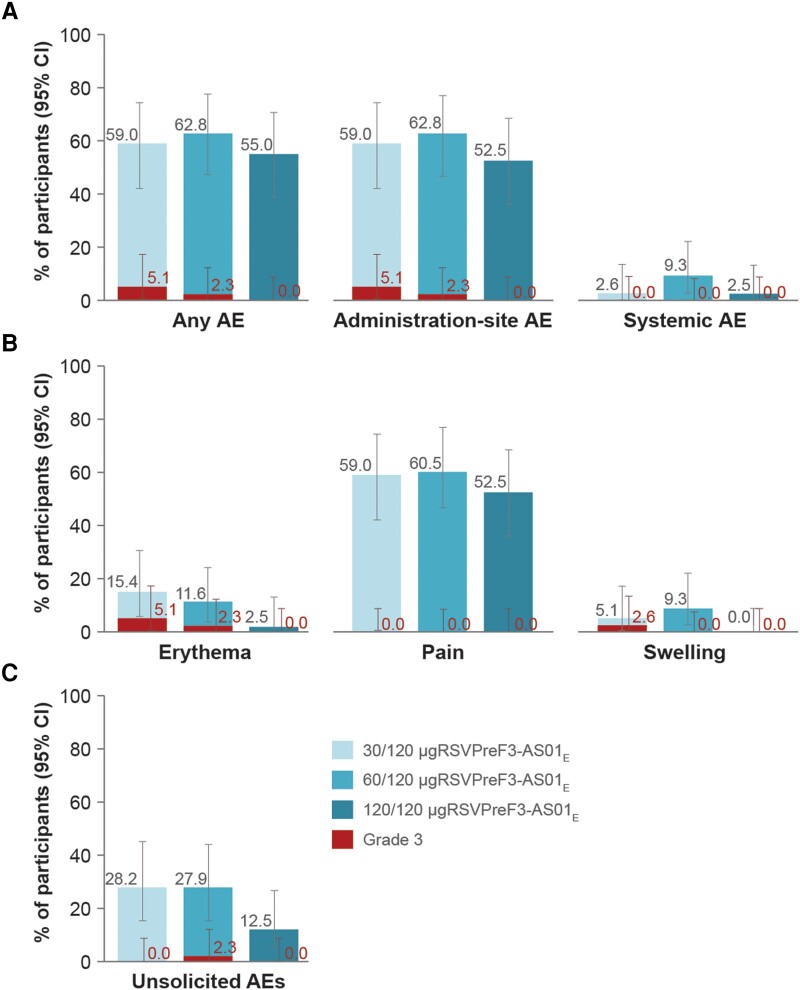
Within 4 days after dose 3, solicited administration-site AEs were reported in 23 (59.0%), 27 (62.8%), and 21 (52.5%) participants in the 30/120-, 60/120-, and 120/120 μg RSVPreF3-AS01E groups (Figure 3A). The most frequently reported solicited administration-site event was pain (in 23 [59.0%], 26 [60.5%], and 21 [52.5%] participants) (Figure 3B). Grade 3 administration-site erythema was reported in 2 (5.1%) and 1 (2.3%) participant in the 30/120- and 60/120 μg RSVPreF3-AS01E groups, respectively, and 1 (2.6%) participant in the 30/120 μg RSVPreF3-AS01E group reported grade 3 swelling. No participants in the 120/120 μg RSVPreF3-AS01E group reported grade 3 administration-site AEs (Figure 3).
Figure 3.
Percentage of participants reporting (A) at least 1 solicited AE (any, administration-site, and systemic adverse event) within 4 days, or (B) at least 1 solicited administration-site AE within 4 days, or (C) at least 1 unsolicited AE within 30 days after vaccination with the third dose of the 120 μg RSVPreF3-AS01E formulation (exposed set). The only collected systemic AE was fever, which was defined as body temperature ≥38°C (grade 3 fever was defined as temperature >39°C). Grade 3 erythema and swelling were defined as being >100 mm in diameter. No serious AEs, pIMDs, and deaths were reported within 30 days after dose 3. Participants received 2 doses of the AS01E-adjuvanted vaccine formulation with 30, 60, or 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study, indicated by 30/120-, 60/120-, and 120/120 μg RSVPreF3-AS01E. Abbreviations: AE, adverse event; AS01E, adjuvant system [18]; CI, confidence interval; RSVPreF3, prefusion conformation of the respiratory syncytial virus fusion (F) protein.
The only collected solicited systemic AE was fever, which was reported by 1 (2.6%) participant in the 30/120 μg RSVPreF3-AS01E group, 4 (9.3%) participants in the 60/120 μg RSVPreF3-AS01E group, and 1 (2.5%) participant in the 120/120 μg RSVPreF3-AS01E group. No grade 3 fever (>39.0°C) was reported (Figure 3A).
Within 30 days after dose 3, at least 1 unsolicited AE was reported by 11 (28.2%), 12 (27.9%), and 5 (12.5%) participants in the 30/120-, 60/120-, and 120/120 μgRSVPreF3-AS01E groups, respectively (Figure 3C). The most frequently reported unsolicited AE was headache, in 4 (10.3%; 30/120 μg RSVPreF3-AS01E), 1 (2.3%; 60/120 μg RSVPreF3-AS01E), and 1 (2.5%; 120/120 μg RSVPreF3-AS01E) participant. Only 1 grade 3 unsolicited AE was reported: headache in 1 (2.3%) participant in the 60/120 μg RSVPreF3-AS01E group. Seven (17.9% and 16.3%) participants in each of the 30/120 and 60/120 μg RSVPreF3-AS01E groups and 1 (2.5%) participant in the 120/120 μg RSVPreF3-AS01E group reported at least 1 unsolicited AE considered as related to vaccination by the investigators (Supplementary Table 1). No participant reported an SAE within 30 days after vaccination.
Until end of study (6 months after dose 3), 1 (2.6%) participant in the 30/120 μg RSVPreF3-AS01E group, 2 (4.7%) participants in the 60/120 μg RSVPreF3-AS01E group, and 1 (2.5%) participant in the 120/120 μg RSVPreF3-AS01E group reported at least 1 SAE (Supplementary Table 2). None of the SAEs were considered vaccine related by the investigators. No AEs led to withdrawal from the study, and no pIMDs or deaths were reported in this study.
Immunogenicity Evaluation
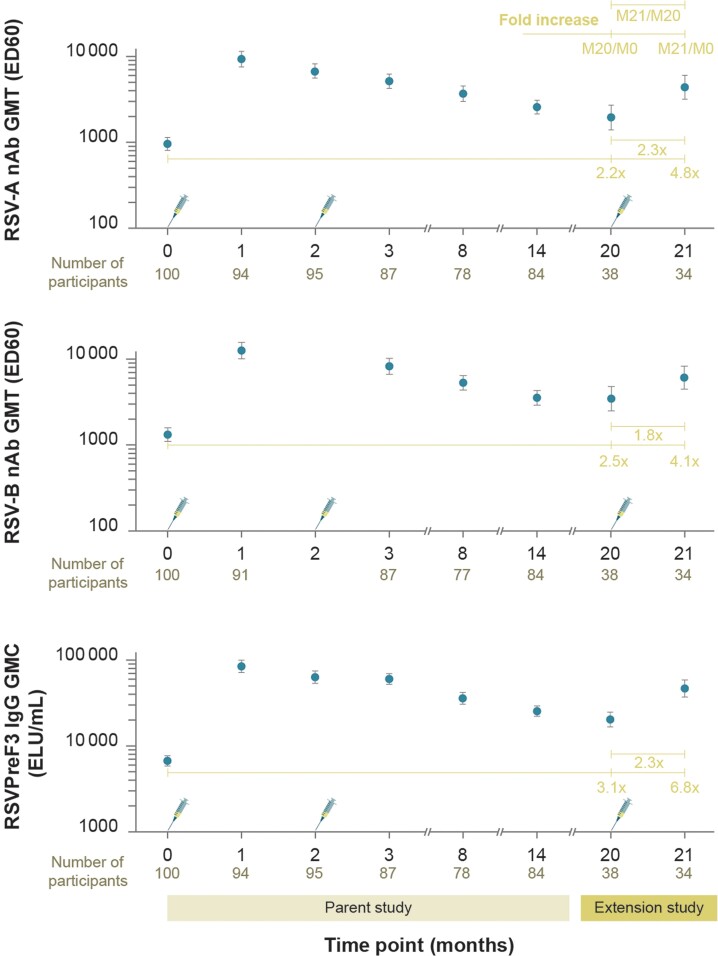
The RSV-A and RSV-B nAb GMTs (ED60) at M20 were 1957.4 (95% CI, 1404.4–2728.1) and 3459.6 (95% CI, 2492.5–4801.9) (Figure 4). These observed GMT values were lower than at M14 [18], but remained higher than prevaccination (baseline, M0) (Figure 4). At M21 (1 month after dose 3), the RSV-A and RSV-B nAb GMTs were 4394.9 (95% CI, 3191.3–6052.5) and 6094.3 (95% CI, 4476.8–8296.4). The geometric mean (GM) fold increases of neutralizing titers at M21 versus M20 were 2.3 (RSV-A) and 1.8 (RSV-B) (Figure 4 and Supplementary Figure 1). Compared to baseline (M0) values, the equivalent fold increases at M1, M14, and M21 were 9.5, 2.7, and 4.8 for RSV-A and 9.2, 2.8, and 4.1 for RSV-B nAb (Figure 4 and Supplementary Figure 1) [18].
Figure 4.
Humoral immune responses in terms of RSV-A and RSV-B nAb GMTs (ED60) and RSVPreF3-specific IgG GMCs (ELU/mL) in the 120/120 μg RSVPreF3-AS01E group (per-protocol set). Part of these data (until M14) have been published in the parent study [18]; only data for 120/120 μg RSVPreF3-AS01E formulation were obtained in the present (extension) study. Syringe symbols represent vaccination. Fold increase indicates fold increase in GMT and GMC values at M20 (before dose 3 in extension study) and M21 (1 month after dose 3 in the extension study) compared to M0 (before dose 1 in parent study) as well as GMT and GMC fold increase at M21 compared to M20. Time points 0, 1, 2, 3, 8, and 14 designate M0 (day 1), M1 (day 31), M2 (day 61), M3 (day 91), M8, and M14 in the parent study, respectively. Neutralizing titers against RSV-B were not measured at M2. Data are plotted as mean values with 95% confidence intervals. Participants received 2 doses of the AS01E-adjuvanted vaccine formulation with 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study, indicated by 120/120 μg RSVPreF3-AS01E. Abbreviations: AS01E, adjuvant system [18]; ED60, estimated dilution 60; ELU, enzyme-linked immunosorbent assay units; GMC/GMT, geometric mean concentration/titer; IgG, immunoglobulin G; M, month; nAb, neutralizing antibody; RSV-A and RSV-B, respiratory syncytial virus subtypes A and B; RSVPreF3, RSV fusion protein stabilized in its prefusion trimeric conformation.
The RSVPreF3-specific IgG GMCs (ELISA units/mL) were 20 202.5 (95% CI, 16 569.5–24 632.0) at M20 and 46 276.5 (95% CI, 36 821.3–58 159.6) at M21 (Figure 4). The observed GMC values at M20 were above baseline (M0) but lower than those at M14 [18]. At M21, the RSVPreF3-specific IgG GMC was 2.3-fold higher than that at M20 (Figure 4 and Supplementary Figure 1). Compared to baseline (M0) values, the equivalent increases of IgG GMCs at M1, M14, and M21 were 12.4, 3.6, and 6.8 (Figure 4, Supplementary Figure 1) [18].
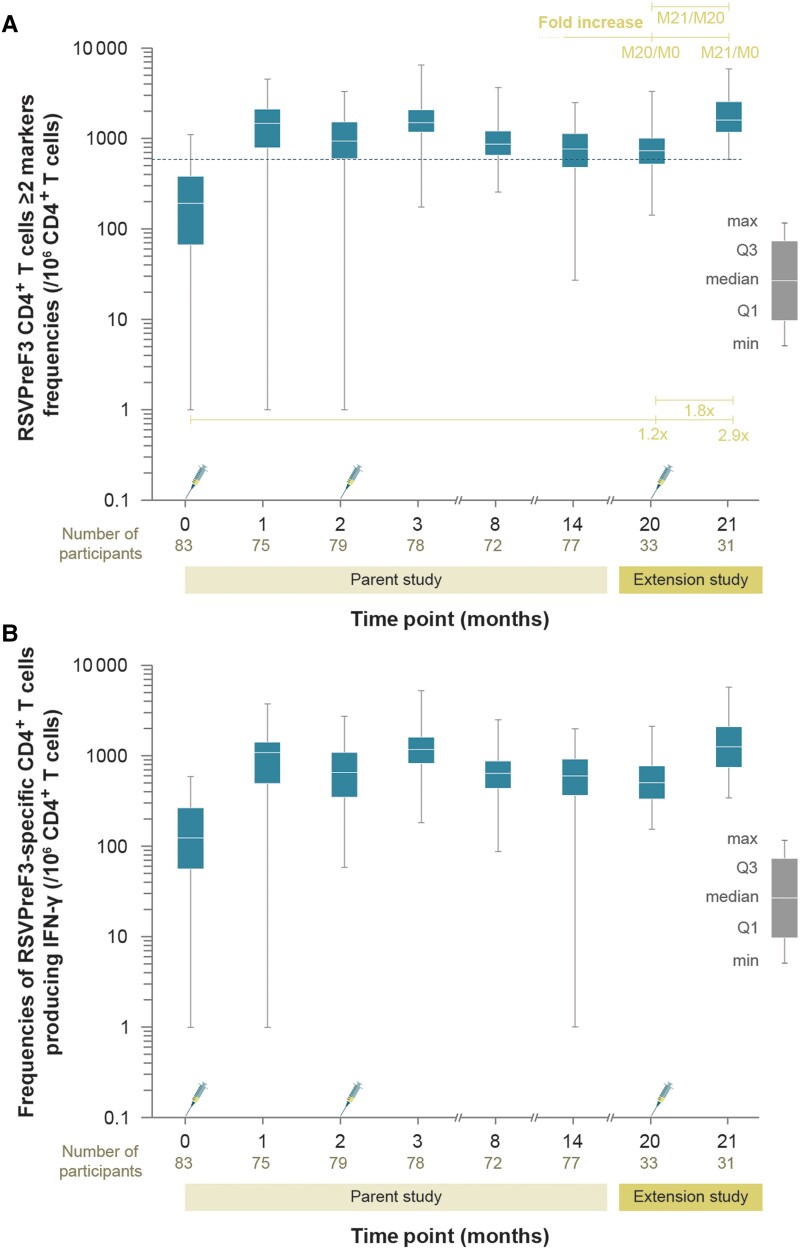
The median frequency (per 106 cells) of RSVPreF3-specific CD4+ T cells expressing at least 2 markers (among IL-2, CD40L, TNF-α, IFN-γ) was 731 (range, 142–3308) at M20, comparable to 764 (range, 27–2488) at M14 in the parent study (Figure 5A). At M21, the median frequency of these CD4+ T cells was 1601 (range, 589–5848), comparable to the M1 value (1466; range, 1–4593) [18]. At M21, frequencies of CD4+ T cells expressing at least 2 markers were 1.8- and 2.9-fold higher compared to M20 and baseline (M0), respectively (Figure 5A and Supplementary Figure 2). A similar profile was observed for CD4+ T cells producing at least IFN-γ (Figure 5B). Consistent with the previously reported results [18], no CD8+ T-cell responses were detected after vaccination with dose 3 of the 120 μg RSVPreF3-AS01E formulation (Supplementary Figure 3).
Figure 5.
Frequencies of RSVPreF3-specific CD4+ T cells expressing at least (A) 2 markers (among IL-2, CD40L, TNF-α, IFN-γ) or (B) IFN-γ in the 120/120 μg RSVPreF3-AS01E group (per-protocol set). Part of these data (time points to M14) have been published in the parent study [18]. Only data for 120/120 μg RSVPreF3-AS01E formulation were obtained in the present (extension) study. Syringe symbols represent vaccination. The dashed horizontal line represents the assay cutoff value of 590. Fold increase and the corresponding horizontal lines indicate fold increase in frequencies of CD4+ T cells at M20 (before dose 3 in extension study) and M21 (1 month after dose 3 in the extension study) compared to M0 (before dose 1 in parent study), as well as fold increase in frequencies at M21 compared to M20. Time points 0, 1, 2, 3, 8, and 14 designate M0 (day 1, dose 1 vaccination), M1 (day 31, 1 month after dose 1), M2 (day 61, dose 2 vaccination), M3 (day 91, 1 month after dose 2), M8, and M14 in the parent study. Data are plotted as box and whisker plots with a median, interquartile range (Q1 and Q3, first and third quartile), minimum and maximum. Participants received 2 doses of the AS01E-adjuvanted vaccine formulation with 120 μg of RSVPreF3 antigen in the parent study and a third dose of the AS01E-adjuvanted vaccine formulation containing 120 μg of RSVPreF3 antigen in the extension study, indicated by 120/120 μg RSVPreF3-AS01E. Abbreviations: AS01E, adjuvant system [18]; CD4+, cluster-of-differentiation-4-expressing; CD40L, cluster of differentiation 40 ligand; IFN-γ, interferon-γ; IL-2, interleukin 2; M, month; RSVPreF3, respiratory syncytial virus fusion protein stabilized in its prefusion trimeric conformation; TNF-α, tumor necrosis factor-α.
DISCUSSION
With the world population aging, disease prevention and reduced disease burden are important focus points of public health care. RSV is a common pathogen that can lead to severe respiratory disease in the OA population. OAs are susceptible to developing infection-associated morbidities and may be unable to mount an effective protective response against RSV [4, 16]. An RSV vaccine tailored toward the OA population will thus need to maximize the elicited immune responses, to overcome age-related immunosenescence, and to protect OAs against RSV-associated disease [4, 12]. Together with the ongoing phase 3 trials [30, 31], this extension study provides further insights into vaccine-induced immune responses in the OA population.
Prior to the first vaccination, the enrolled OA participants were seropositive for RSV-A and RSV-B nAb [18] due to previous exposure to RSV. Following the 2-dose vaccination in the parent study, both humoral (RSVPreF3-specific IgG GMCs, and RSV-A and RSV-B nAb GMTs) and CMI (frequencies of CD4+ T cells expressing at least 2 markers among IL-2, CD40L, TNF-α, and IFN-γ) responses were highest at 1 month after dose 1 (M1, day 31), without an added effect of RSVPreF3-based vaccine dose 2 (M3, day 91) [18]. These immune responses remained above baseline until 12 months after dose 2 (M14), although at lower levels than measured at M1 [18].
The described RSV-specific antibodies and CD4+ T cells persisted until revaccination in this study (M20), although with different kinetics. At the start of this extension study (M20), the IgG and nAb levels were lower than at M14 (parent study) but still higher than before dose 1 (M0). This is consistent with data reported for other RSV candidate vaccines [17, 24, 26, 32–36]. Importantly, however, the third 120 μg RSVPreF3-AS01E dose induced an increase in RSVPreF3-specific IgG and RSV-A and RSV-B nAb levels by approximately 2-fold at M21 compared to M20. These findings demonstrate that 120 μg RSVPreF3-AS01E–induced antibody levels remain above baseline for at least 18 months after the second vaccination and can be increased again by administering a third vaccine dose. The observed boosting of antibodies to levels below those measured after the first vaccination appears to be a common observation in the RSV vaccine field [23, 26, 35] and, thus, not specific to the RSVPreF3-based vaccine.
The observed humoral responses were coupled with the induction of CD4+ T-cell immunity. An important finding was that the frequencies of CD4+ T cells expressing at least 2 markers did not decrease further between M14 (parent study) and M20. Additionally, as measured at M21, the CD4+ T-cell compartment was stimulated to the level comparable to that observed 1 month after dose 1 (M1 in the parent study). Similar to the parent study [18], the predominant T-cell response profile was CD4+ T-helper cells 1 (Th1; cells expressing at least IFN-γ), without detectable CD8+ T-cell responses. It therefore appears that the 120 μg RSVPreF3-AS01E vaccine formulation induces stable CD4+ Th1-biased cellular immune responses, which persist for at least 18 months after the second vaccination and increase with revaccination. These findings suggest that T-cell memory induced by the primary schedule of 120 μg RSVPreF3-AS01E remained boostable 18 months after dose 2 (M20). The maintenance of a CD4+ Th1-biased cellular immune response through vaccinations with 120 μg RSVPreF3-AS01E is particularly important, as it is thought that Th1 CMI plays an important role in protecting against RSV disease [12, 14, 16].
An immunological correlate of protection for RSV is not yet established. However, the strong humoral and CMI responses elicited by the RSVPreF3 OA vaccine might be indicative of vaccine efficacy, as recently demonstrated in a phase 3 study [27, 28].
The safety findings of this extension study, in terms of solicited and unsolicited AE occurrences, are in line with previously published results [18]. Although the low number of enrolled participants was a limitation, the third dose of 120 μg RSVPreF3-AS01E was well tolerated when administered 18 months after dose 2 (AS01E-adjuvanted, containing either 30, 60, or 120 μg of RSVPreF3). No deaths, pIMDs, nor vaccine-related SAEs were reported during the extension study period.
The limitations of this study were the relatively low number of participants and the short follow-up time. Also, because the RSVPreF3 OA vaccine has been authorized as a single dose regimen in OAs, the generalizability of the present data is limited. However, even though these data as such will not be applicable to how the vaccine is authorized for the OA population, this study provides valuable information on the safety of a booster dose, as well as on the profile and persistence of immune responses after vaccination. The ongoing phase 3 studies are currently evaluating the long-term vaccine efficacy and immune responses after different revaccination schedules with 120 μg RSVPreF3-AS01E administered as a single dose in OAs [27, 28]. Study strengths include comprehensive immunogenicity evaluation and a close safety follow-up.
In conclusion, the third dose of the selected 120 μg RSVPreF3-AS01E formulation administered 18 months after the second dose was well tolerated and induced an increase in both humoral and CMI responses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Isabel Leroux-Roels, Centre for Vaccinology, Ghent University and Ghent University Hospital Ghent, Belgium.
Marc Van Ranst, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium.
Corinne Vandermeulen, Leuven University Vaccinology Centre, Katholieke Universiteit Leuven, Leuven, Belgium.
Carline Vanden Abeele, GSK, Wavre, Belgium.
Nathalie De Schrevel, GSK, Rixensart, Belgium.
Bruno Salaun, GSK, Rixensart, Belgium.
Céline Verheust, GSK, Wavre, Belgium.
Marie-Pierre David, GSK, Wavre, Belgium.
Shady Kotb, GSK, Wavre, Belgium.
Veronica Hulstrøm, GSK, Wavre, Belgium.
Notes
Author contributions . M. V. R., N. D. S., C. V., and M. P. D. contributed to the conceptualization of the study. I. L. R., M. V. R., N. D. S., B. S., C. Vm, S. K., and V. H. contributed to data collection or generation. M. V. R., N. D. S., and B. S. contributed to methodology. M. P. D., C. V. A., and S. K. contributed to formal analysis. I. L. R., M. V. R., C. V. A., N. D. S., B. S., C. V., M. P. D., S. K., and V. H. were involved in data analysis/interpretation. S. K. drafted the original draft of the manuscript. All authors contributed to manuscript review and editing and approved the final version of the manuscript.
Acknowledgments . We thank all study participants and their families, as well as the staff at the participating institutions. We thank staff at GSK Clinical Laboratory Sciences and GSK Clinical Department for technical assistance, in particular Anne-Marie Camier, Frederique Bossiroy, Ouafae Bouchikhi, Stephanie Howard, Geetha Kamath, Stacey Lewis, Anne Meulemans, Amoolya Modi, Maggie Schultz, Elisabeth Ngalula Kabanga, Melanie Steffens, and Jelena Tica. We also thank Yolanda Penders, Erik Jongert, and Cecile Felu for their critical review of the manuscript. Finally, we acknowledge the contribution of Frédéric Renaud, who was responsible for statistical analyses and review of the clinical study report. We also thank Akkodis Belgium for medical writing support (Irena Zurnic Bönisch), artwork design support, editorial assistance, and manuscript coordination, on behalf of GSK.
Data availability . Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com (study ID 213569). The study protocol, statistical analysis plan, and results summary are posted on ClinicalTrials.gov (NCT04657198).
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis and took responsibility for all costs associated with the development and the publishing of the article.
References
- 1. Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol 2013; 45:331–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Staadegaard L, Caini S, Wangchuk S, et al. . Defining the seasonality of respiratory syncytial virus around the world: national and subnational surveillance data from 12 countries. Influenza Other Respir Viruses 2021; 15:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev 2017; 30:277–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J of Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 5. Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ 2019; 366:l5021. [DOI] [PubMed] [Google Scholar]
- 6. Shi T, Denouel A, Tietjen AK, et al. . Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–S83. [DOI] [PubMed] [Google Scholar]
- 7. Kestler M, Munoz P, Mateos M, Adrados D, Bouza E. Respiratory syncytial virus burden among adults during flu season: an underestimated pathology. J Hosp Infect 2018; 100:463–8. [DOI] [PubMed] [Google Scholar]
- 8. Savic M, Penders Y, Shi T, Branche A, Pirçon J-Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses 2023; 17:e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schubert L, Steininger J, Lötsch F, et al. . Surveillance of respiratory syncytial virus infections in adults, Austria, 2017 to 2019. Sci Rep 2021; 11:8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherukuri A, Patton K, Gasser RA Jr, et al. . Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 2013; 20:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998; 177:463–6. [DOI] [PubMed] [Google Scholar]
- 12. Malloy AMW, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:211–31. [DOI] [PubMed] [Google Scholar]
- 13. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 14. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 16. Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines (Basel) 2021; 9:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langley JM, Aggarwal N, Toma A, et al. . A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. J Infect Dis 2017; 215:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leroux-Roels I, Davis MG, Steenackers K, et al. . Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J Infect Dis 2022; 227:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruckwardt TJ, Morabito KM, Phung E, et al. . Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir Med 2021; 9:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadoff J, De Paepe E, Haazen W, et al. . Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an influenza vaccine in older adults. J Infect Dis 2021; 223:699–708. [DOI] [PubMed] [Google Scholar]
- 21. Schwarz TF, Johnson C, Grigat C, et al. . Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J Infect Dis 2022; 225:2067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarz TF, McPhee RA, Launay O, et al. . Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in nonpregnant women: a phase 2, randomized trial. J Infect Dis 2019; 220:1816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh E, Falsey AR, Zareba AM, et al. . Respiratory syncytial virus prefusion F (RSVpreF) vaccination: antibody persistence and revaccination. In: 12th International RSV Symposium (29 September–2 October, ICC Belfast, Northern Ireland, UK). 2022. https://isirv.org/site/images/conferences/RSV/RSV2022/Oral%20Presentations%20at%20RSV2022_abstract%20reference%20order.pdf. Accessed 20 January 2023.
- 24. Walsh EE, Falsey AR, Scott DA, et al. . A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis 2022; 225:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walsh EE, Pérez Marc G, Zareba AM, et al. . Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388:1465–77 [DOI] [PubMed] [Google Scholar]
- 26. Williams K, Bastian AR, Feldman RA, et al. . Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J Infect Dis 2020; 222:979–88. [DOI] [PubMed] [Google Scholar]
- 27. Papi A, Ison M, Langley J, et al. . Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388:595–608. [DOI] [PubMed] [Google Scholar]
- 28. Ison MG, Papi A, Langley JM, et al. . A respiratory syncytial virus (RSV) prefusion F protein candidate vaccine (RSVPreF3 OA) is efficacious in adults ≥ 60 years of age (YOA). IDWeek 2022. Washington, DC. [Google Scholar]
- 29. Vidal Valero M. ‘A good day’: FDA approves world's first RSV vaccine. Nature 2023; 617:234–5. [DOI] [PubMed] [Google Scholar]
- 30. US National Library of Medicine . Efficacy study of GSK’s investigational respiratory syncytial virus (RSV) vaccine in adults aged 60 years and above.2022. https://clinicaltrials.gov/ct2/show/NCT04886596?term=NCT04886596&draw=2&rank=1. Accessed 13 February 2023.
- 31. US National Library of Medicine . Immunogenicity, safety, reactogenicity and persistence of an investigational respiratory syncytial virus (RSV) vaccine in adults aged 60 years and above.2022.https://clinicaltrials.gov/ct2/show/NCT04732871?term=NCT04732871&draw=2&rank=1. Accessed 13 February 2023.
- 32. Baber J, Arya M, Moodley Y, et al. . A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J Infect Dis 2022; 226:2054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falloon J, Talbot HK, Curtis C, et al. . Dose selection for an adjuvanted respiratory syncytial virus F protein vaccine for older adults based on humoral and cellular immune responses. Clin Vaccine Immunol 2017; 24:e00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falsey AR, Walsh EE, Scott DA, et al. . Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J Infect Dis 2022; 225:2056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jordan E, Lawrence SJ, Meyer TPH, et al. . Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J Infect Dis 2021; 223:1062–72. [DOI] [PubMed] [Google Scholar]
- 36. Stuart ASV, Virta M, Williams K, et al. . Phase 1/2a safety and immunogenicity of an adenovirus 26 vector respiratory syncytial virus (RSV) vaccine encoding prefusion F in adults 18–50 years and RSV-seropositive children 12–24 months. J Infect Dis 2022; 227:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.