Abstract
To test if biofilm formation in Staphylococcus epidermidis is dependent on the polysaccharide intercellular adhesin, whose biosynthesis is driven by the ica locus, a plasmid containing the ica locus was transferred to three ica-negative strains. Using in vitro biofilm assays and a rat central venous catheter infection model, we confirmed the importance of the ica locus for biofilm production and pathogenesis of S. epidermidis.
Staphylococcus epidermidis has become one of the most important pathogens of nosocomial infections associated with catheters and other indwelling medical devices (6, 7, 9). Previous studies have tried to determine factors that discriminate between commensal and invasive strains of S. epidermidis (5). Elucidation of the major differences between the two types of strains is believed to promote our understanding of S. epidermidis pathogenesis. Several studies have suggested that the ica locus, which encodes production of the N-acetylglucosamine polysaccharide intercellular adhesin (PIA), plays a critical role in distinguishing the two types of strains, indicating an important function of ica in invasiveness of S. epidermidis (4, 5, 17). Studies using insertional ica mutants, controllable expression of ica, and heterologous expression in Staphylococcus carnosus have further underscored the important role of ica in biofilm formation and pathogenesis of S. epidermidis (8, 10, 12-15). However, other studies have raised doubt about the critical function of the ica locus in causing S. epidermidis biofilm-associated infection (3). Therefore, to test the hypothesis that the ica locus is a major factor of S. epidermidis invasiveness, we attempted to convert commensal, ica-negative strains to invasive strains of S. epidermidis by ica locus expression.
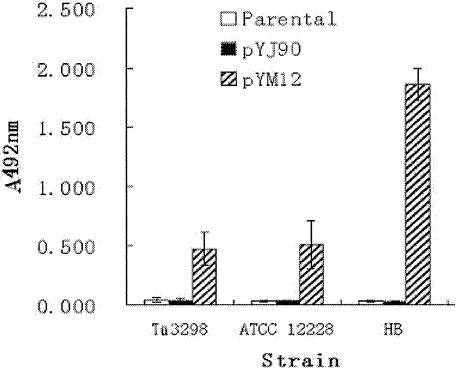
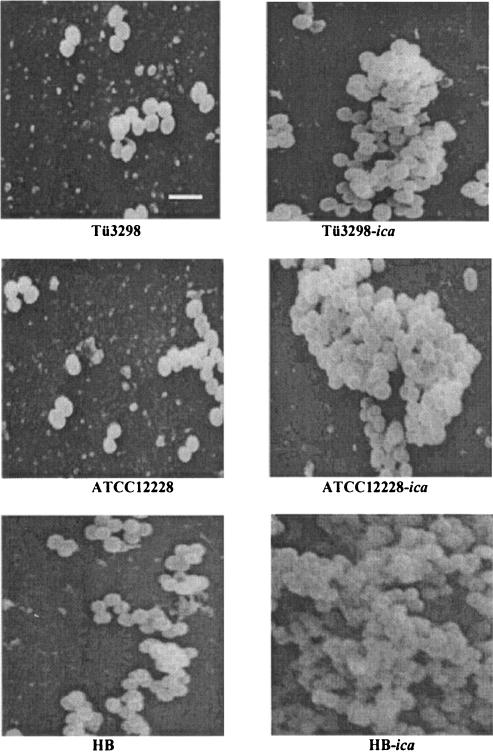
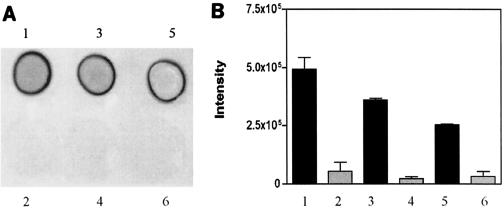
To investigate the impact of introducing the ica locus in ica-negative S. epidermidis strains, a plasmid containing the entire ica locus (icaRADBC) was constructed and transferred into ica-negative strains of S. epidermidis. A 4,215-bp fragment encompassing icaRADBC was PCR amplified using DNA from strain 97-337 as a template (16). The PCR product was cloned into vector pYJ90 and was confirmed by sequencing (GenBank accession number AY382582). The constructed plasmid was electroporated into three ica-negative strains, ATCC 12228, HB, and Tü3298 (1), as described previously to generate the respective isogenic strains ATCC 12228-ica, HB-ica and Tü3298-ica. The biofilm phenotypes of the strains were determined using semiquantitative biofilm assays and scanning electron microscopy (SEM) (2, 17). In the semiquantitative biofilm assay, the strains were allowed to form biofilm for 18 h at 37°C. The biofilm was stained with crystal violet and quantified by measuring the absorbance at 492 nm. ATCC 12228, HB, and Tü3298 were biofilm negative, whereas the strains with the ica locus expressed formed biofilm (Fig. 1 and 2). Biofilm formation of HB-ica was significantly more pronounced than that of ATCC 12228-ica (P = 0.0064) and Tü3298-ica (P = 0.0059). We also determined expression of PIA by immunodot blots using anti-PIA antiserum (Fig. 3). The three wild-type strains lacked PIA expression, whereas the ica locus-expressed strains were PIA positive by immunodot blot. In SEM, strains ATCC 12228, HB, and Tü3298 adhered as individual cells on the coverslips, while the ica locus-expressed strains formed biofilm, with that of strain HB appearing most dense. Thus, expression of ica resulted in conversion of biofilm-negative to biofilm-positive S. epidermidis in all three investigated cases, confirming the reported immense importance of ica in the accumulation phase of S. epidermidis biofilm development (8).
FIG. 1.
Semiquantitative biofilm assay of ica-negative S. epidermidis strains and corresponding ica locus-expressed isogenic strains. Plasmid pYM12 (containing icaRADBC) or the control plasmid pYJ90 was transformed into three ica-negative S. epidermidis strains, ATCC 12228, HB, and Tü3298 respectively. Each experiment was repeated eight times. When A492 exceeded 0.12, the strain was defined as biofilm positive. The mean of eight experiments ± standard error is shown.
FIG. 2.
Scanning electron microscopy of S. epidermidis biofilm. Bacteria adhered to the cover slides were fixed, treated, and observed by SEM. The bar represents a 1.5-μm scale.
FIG. 3.
PIA production in ica-negative S. epidermidis wild-type and corresponding ica locus-expressed strains. PIA was extracted from 24-h cultures by boiling with 0.5% EDTA for 5 min. Three-μl samples of the extract were spotted on nitrocellulose membrane, and PIA production was assayed by immunodot blot and evaluated by densitometry as described (15). The experiment was performed twice. (A) Representative immunodot blot. (B) Bars show the mean ± standard error: 1, Tü3298-ica; 2, Tü3298; 3, ATCC 12228-ica; 4, ATCC 12228; 5, HB-ica; 6, HB.
A rat central venous catheter (CVC)-associated infection model (11) was used to evaluate the relative virulence of the parental strains ATCC 12228, HB, and Tü3298 and their isogenic strains, respectively. Briefly, the neck of the rat was dissected and a Silastic catheter was inserted in the right external jugular vein and advanced into the superior vena cava. A definite quantity of bacteria (about 105 CFU) was injected into the catheters after 24 h following CVC placement. The catheters were flushed daily with a heparin solution, and the animals were sacrificed at day 8. The comparison of overall infection rates, defined as recovery of the bacteria from the blood, liver, kidney, and heart at sacrifice, showed that more rats developed CVC-associated infection when they were challenged with ATCC 12228-ica and HB-ica than when challenged with the parental strains ATCC 12228 and HB (chi-square test, both P < 0.0001). For all organ systems, there were more animals with metastasis disease in the group challenged with strains with ica. In addition, for almost all tested organ systems, the number of bacteria recovered per gram of tissue was greater in the animals challenged with ATCC 12228-ica and HB-ica than in those challenged with their parental strains. According to Wilcoxon's test, these differences were statistically significant. There were differences in the infection rate and the number of bacteria recovered per gram of tissue between Tü3298 and Tü3298-ica. However, the differences were not significant. Table 1 summarizes the results from defining the burden of metastasis disease in animals challenged with either parental strains or their isogenic ica locus-expressed strains. Notably, strains with higher production of PIA also caused more pronounced virulence in the infection model, underlining the importance of PIA in biofilm-associated S. epidermidis infection. In conclusion, our results demonstrate that presence of ica significantly increases the virulence of S. epidermidis, confirming previous work by Rupp et al., who compared wild-type to ica mutant strains (12-14). As in our work, ica was present in multiple copies on a plasmid: the differences seen might be more pronounced than in those studies. Further, the differences in the amount of biofilm formation that we observed in the ica locus-expressed strains suggest that factors other than ica contribute to biofilm formation in S. epidermidis strains.
TABLE 1.
Metastatic infections in different organs by S. epidermidis strains in the rat CVC-associated infection model
| Parameter and organ | Result for straina:
|
|||||
|---|---|---|---|---|---|---|
| ATCC 12228 | ATCC 12228-ica | HB | HB-ica | Tü3298 | Tü3298-ica | |
| Liver | ||||||
| No. of rats infected/ total | 3/10 | 7/10 | 2/6 | 8/8 | 6/9 | 7/9 |
| Median (range) CFU/g tissue | 0 (0 × 102-1.3 × 102) | 1.7 × 103 (0 × 105-2.7 × 105) | 1.7 (0 × 102-1 × 102) | 3.8 × 102 (3.3 × 101-6.2 × 103) | 3.3 × 102 (0 × 104-4 × 104) | 1 × 103 (0 × 104-8.3 × 104) |
| P value | 0.019 | 0.0078 | 0.1631 | |||
| Kidney | ||||||
| No. of rats infected/ total | 2/10 | 7/10 | 2/6 | 8/8 | 5/9 | 6/9 |
| Median (range) CFU/g tissue | 0 (0 × 102-2.3 × 102) | 2.7 × 102 (0 × 107-1.3 × 107) | 2.5 × 101 (0 × 102-8.5 × 102) | 2.6 × 103 (3.0 × 102-3.3 × 104) | 6.7 × 102 (0 × 105-3.2 × 105) | 4.3 × 103 (0 × 106-2.7 × 106) |
| P value | 0.015 | 0.0066 | 0.7502 | |||
| Heart | ||||||
| No. of rats infected/ total | 3/10 | 7/10 | 1/6 | 6/8 | 4/9 | 7/9 |
| Median (range) CFU/g tissue | 0 (0 × 102-3.6 × 102) | 3.8 × 102 (0 × 104-7.2 × 104) | 5 (0 × 101-8.3 × 101) | 2.0 × 102 (0 × 103-7.2 × 103) | 0 (0 × 103-2.7 × 103) | 2.3 × 103 (0 × 104-2.9 × 104) |
| P value | 0.023 | 0.0645 | 0.0792 | |||
| Blood | ||||||
| No. of rats infected/ total | 4/10 | 6/10 | 0/6 | 7/8 | 2/9 | 5/9 |
| Median (range) CFU/g tissue | 0 (0 × 102-1.3 × 102) | 1.5 × 102 (0 × 102-4.9 × 102) | 0 (0-0) | 8.6 × 102 (0 × 103-1.75 × 103) | 0 (0 × 102-6.0 × 102) | 10 (0 × 103-8.0 × 103) |
| P value | 0.123 | 0.0037 | 0.2649 | |||
| Infection | ||||||
| Infection rate (%) | 30.0 ± 8.2 | 67.5 ± 5.0 | 20.8 ± 16.0 | 90.6 ± 20.0 | 47.2 ± 19.0 | 69.4 ± 10.6 |
| P value | <0.0001 | <0.0001 | 0.056 | |||
For statistical significance (Wilcoxon test), results represent animals challenged with S. epidermidis ATCC 12228, HB, or Tü3298 versus those challenged with S. epidermidis ATCC 12228-ica, HB-ica, or Tü3298-ica.
Acknowledgments
We thank Yinduo Ji for providing plasmid pYJ90, Jan-Ingmar Flock for providing S. epidermidis strain HB, and Friedrich Götz for providing S. aureus RN4220.
This work was supported by Chinese National Natural Science Foundation grant 30170845 and 211 Project Grant—Functional Genomics of Important Pathogenic Microorganisms.
Editor: V. J. DiRita
REFERENCES
- 1.Augustin, J., and F. Götz. 1990. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol. Lett. 54:203-207. [DOI] [PubMed] [Google Scholar]
- 2.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francois, P., P. H. Tu Quoc, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, S. E. Cramton, F. Götz, and P. Vaudaux. 2003. Lack of biofilm contribution to bacterial colonisation in an experimental model of foreign body infection by Staphylococcus aureus and Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 35:135-140. [DOI] [PubMed] [Google Scholar]
- 4.Frebourg, N. B., S. Lefebvre, S. Baert, and J. F. Lemeland. 2000. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J. Clin. Microbiol. 38:877-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galdbart, J. O., J. Allignet, H. S. Tung, C. Ryden, and N. El Solh. 2000. Screening for Staphylococcus epidermidis markers discriminating between skin-flora strains and those responsible for infections of joint prostheses. J. Infect. Dis. 182:351-355. [DOI] [PubMed] [Google Scholar]
- 6.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 7.Heilmann, C., and G. Peters. 2000. Biology and pathogenicity of Staphylococcus epidermidis, p. 442-449. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 8.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis, W. R., and W. J. Martone. 1992. Predominant pathogens in hospital infections. J. Antimicrob. Chemother. 29(Suppl. A):19-24. [DOI] [PubMed] [Google Scholar]
- 10.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupp, M. E., and P. D. Fey. 2001. In vivo models to evaluate adhesion and biofilm formation by Staphylococcus epidermidis. Methods Enzymol. 336:206-215. [DOI] [PubMed] [Google Scholar]
- 12.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 13.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuong, C., C. Gerke, G. A. Somerville, E. R. Fischer, and M. Otto. 2003. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J. Infect. Dis. 188:706-718. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]
- 17.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]