Abstract
Salmonella strains are facultative intracellular pathogens that produce marked cytopathology during infection of host cells. Different forms of cytopathic effects have been associated with the virulence systems encoded by the two Salmonella pathogenicity islands (SPI-1 and SPI-2) and the spv locus. We used Salmonella enterica serovar Dublin to investigate the induction of cytopathology during infection of the human macrophage-like cell line THP-1. Analysis of host cells by flow cytometry using a fluorescent terminal deoxynucleotidyltransferase dUTP-biotin nick end labeling (TUNEL) assay revealed that 70% of THP-1 cells showed DNA fragmentation after 4 h of infection, increasing to greater than 90% by 5.5 h. Moreover, the results showed that gentamicin-killed or chloramphenicol-treated bacteria did not induce DNA fragmentation. Serovar Dublin strains with mutations in SPI-1, SPI-2, or spvB induced these cytopathic effects similar to wild-type bacteria. In contrast, a mutation in the phoP regulatory gene abolished DNA fragmentation in the TUNEL assay. Caspase-3 activation was detected during Salmonella infection of THP-1 cells, but caspase-8 and caspase-9 activities were not found. However, inhibition of caspase-3 did not block Salmonella-induced DNA fragmentation. These results identify a previously undetected apoptotic effect in Salmonella-infected cells that is dependent on phoP gene function.
Salmonella enterica comprises a group of bacteria that produce a variety of diseases, ranging from self-limited enteritis to fatal, disseminated infections (23, 25, 26). The hallmarks of Salmonella pathogenesis are bacterial invasion and intracellular infection, processes that require the expression of a number of bacterial virulence loci. Salmonella enterica strains encode two distinct type III protein secretion systems (TTSS) that function in each of these two phases of the pathogenic process (6, 11, 30). These two secretion systems deliver bacterial virulence proteins into the cytoplasm of the host cell to modulate key signal transduction processes. The system encoded by Salmonella pathogenicity island I (SPI-1) is required for invasion of the bacterium into intestinal epithelial cells, while systemic infections and intracellular accumulation of Salmonella are dependent on the function of SPI-2. Furthermore, a critical regulon controlled by the two-component PhoP/PhoQ system is essential for the intracellular survival and replication of Salmonella strains (5, 21). In addition, the spv locus, carried on virulence plasmids by certain serovars of the S. enterica subspecies I lineage, greatly enhances the systemic virulence of these strains (10). The spv virulence phenotype is primarily due to the SpvB protein, an ADP-ribosyl transferase that modifies actin monomers and prevents polymerization, leading to loss of the F-actin cytoskeleton in infected cells (17).
Salmonella possesses a variety of mechanisms to produce cytopathic effects in infected host cells. Salmonella induces apoptosis in intestinal epithelial cells by a process that involves invasion mediated by the SPI-1 effectors, but that also requires the functions of SPI-2 and the spv locus (24). Considerable work has focused on the cell death produced by Salmonella infection of macrophages. Experimental conditions, including the growth phase of the bacteria and treatment with opsonins such as normal serum, have a profound effect on the cytotoxic response of macrophages infected with Salmonella. Use of nonopsonized bacteria grown under conditions that maximize expression of SPI-1 leads to the rapid death and lysis of macrophages in a process that resembles necrosis as well as programmed death (2). This rapid cytotoxicity is mediated by the activation of caspase-1 by the SPI-1 effector protein SipB, but the detailed mechanism of cell death has not been established (13). A similar reaction has been described in dendritic cells (29). In caspase 1-deficient macrophages, SipB can activate caspase-2, with subsequent activation of caspase-3, -6, and -8, release of cytochrome c from mitochondria, and cytopathology with features of apoptosis (15). SipB also induces the formation of autophagic vesicles containing mitochondrial and endoplasmic reticulum components in caspase-1-deficient cells (12). A different cytotoxicity is seen when Salmonella is grown under conditions that repress expression of SPI-1, particularly if the bacteria are also opsonized with normal serum. Macrophages infected with these bacteria exhibit delayed cytopathology beginning about 12 h after infection (18, 19, 28). SipB and SPI-1 function are not required but SPI-2 is essential, and the process displays features of apoptosis, including nucleosome and DNA fragmentation. The Salmonella effectors mediating this delayed cytopathology have not been identified, although the SpvB protein appears to be required in human monocyte-derived macrophages (3, 17, 18).
In the present study, we used the human-derived macrophage-like cell line THP-1 to identify bacterial genes and host cell caspase activities involved in Salmonella-induced cell death. We found that Salmonella proliferated in THP-1 cells and induced extensive DNA fragmentation by 6 h following infection with S. enterica serovar Dublin. Live bacteria with active protein synthesis were required to induce this cytopathology. Mutations in SPI-1 (sipB), SPI-2 (ssaV and ssaJ), and spvB did not decrease the induction of DNA fragmentation. However, a phoP mutant caused much less cell death, despite similar numbers of intracellular bacteria. Caspase-3 activation was detected during infection with strains inducing cell death, but DNA fragmentation was not affected by blocking caspase-3 activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type S. enterica serovar Dublin and mutant derivatives ssaV::mTn5, ssaJ::mTn5, sipB::aphT, spvBmut1, and phoP::Tn10 (3, 5) were used in these studies. Bacteria were cultured aerobically at 37°C in Luria-Bertani (LB) broth or on LB agar with antibiotics as appropriate: kanamycin at 50 mg/liter or tetracycline at 10 mg/liter. To obtain stationary-phase bacteria for infection of THP-1 cells, LB broth was inoculated with a single colony and grown overnight with vigorous shaking. Bacteria were harvested by centrifugation, washed with Dulbecco's phosphate-buffered saline (PBS), and opsonized for 20 min at 37°C in 50% human fresh-frozen AB serum (SeraCare Life Sciences). Opsonized bacteria were diluted 10-fold in Hanks salt solution and used immediately for inoculation of THP-1 cells.
Cell culture and infection.
THP-1 cells (27) were maintained at a density of 2 × 105 to 1 × 106 cells/ml in RPMI 1640 with l-glutamine and 10% heat-inactivated fetal bovine serum supplemented with 2-mercaptoethanol (55 μM), penicillin (100 U/ml), and streptomycin (100 μg/ml). One day prior to infection, the cells were harvested, washed, and resuspended in fresh medium without 2-mercaptoethanol, penicillin, or streptomycin. To induce differentiation into adherent macrophage-like cells, 12-O-tetradecanoylphorbol-13-acetate (PMA) was added to 20 ng/ml and the cells were seeded into culture wells at a concentration of 7 × 105 cells/ml (27). The next day, medium and nonadherent cells were removed and replaced with fresh complete medium without antibiotics. After 2 hours, the cultures were infected with opsonized Salmonella strains at a multiplicity of infection of 10 bacteria/THP-1 cell. Culture plates were centrifuged at 200 × g for 5 min and incubated at 37°C for 30 min to allow phagocytosis to occur. Under these conditions, essentially all cells are infected with bacteria. The medium was then replaced with fresh medium containing gentamicin (20 μg/ml) and incubated for the indicated times. The total cell population in the well was harvested by gentle scraping with a cell scraper. To enumerate viable bacteria, an aliquot of the harvested cell population was centrifuged, the macrophages were lysed by 0.5% deoxycholate in Dulbecco's PBS, and the bacteria were diluted and plated on LB agar as described elsewhere (18).
Gentamicin and chloramphenicol treatment of the bacterial inocula.
To kill Salmonella strains with gentamicin, bacteria were cultured in LB broth with shaking as described above and grown to an optical density at 600 nm of 0.8. Gentamicin at 100 μg/ml was added, and the incubation continued for one more hour. Bacterial viability determined by colony counts decreased to less than 0.01% of the original number under these conditions (18). Gentamicin-treated bacteria were opsonized and used directly to inoculate THP-1 cells. For the chloramphenicol treatment, bacteria were grown and opsonized normally. Bacteria and chloramphenicol (100 μg/ml) were added simultaneously to THP-1 cells. After 30 min of phagocytosis, the medium was replaced with fresh medium without chloramphenicol but containing gentamicin (20 μg/ml), and the infected cells were harvested at the indicated times.
Labeling of DNA fragmentation with fluorescein and flow cytometric analysis.
THP-1 cytopathology was evaluated by flow cytometric analysis of cellular DNA fragmentation. At the indicated times after infection, cells were harvested as described and collected by centrifugation at 1,000 × g for 15 min, washed once with PBS, and fixed, and the DNA fragments were end labeled with fluorescein (terminal deoxynucleotidyltransferase dUTP-biotin nick end labeling [TUNEL] assay) using a commercial kit (Oncogene Research Products). Suspended cells were analyzed for fluorescence by flow cytometry; positive and negative controls were used to set the gating to determine the percent TUNEL-positive cells. The percent positive cells was determined from the fraction of cells with significantly increased fluorescence compared to simultaneous uninfected control cells.
Assay of caspase activity.
After infection with Salmonella, the THP-1 cells were collected by centrifugation at 1,000 × g for 15 min at room temperature and washed once with PBS. The cells were resuspended in lysis buffer at a density of 107 cells/ml and incubated on ice for 10 min. The cell debris was removed by centrifugation at 16,000 × g for 5 min at 4°C, and the supernatant was used for the colorimetric assay of caspase-3, -8, and -9 activities using commercial kits (caspase-3 kit from Sigma and caspase-8 and -9 kits from Calbiochem). Protein concentrations were determined using the Bio-Rad protein assay according to the manufacturer's instructions.
RESULTS
Growth of Salmonella strains in THP-1 cells.
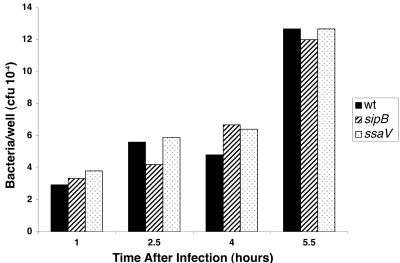
Previous studies have shown that wild-type S. enterica serovar Typhimurium is able to proliferate in THP-1 cells (27). We found similar growth of wild-type serovar Dublin inside THP-1 cells, as shown in Fig. 1. In addition, we found no difference in the phagocytosis or growth of strains with mutations in SPI-1 (sipB) or in SPI-2 (ssaV). Studies with independent mutants in SPI-1 or SPI-2 confirmed these results (data not shown). We have also found no effect of SPI-2 on the early intracellular growth of serovar Typhimurium in THP-1 cells (L. Sly and D. Guiney, unpublished data). Furthermore, mutations in spvB and phoP did not affect the early proliferation of Salmonella in THP-1 cells. Overall, THP-1 cells represent a permissive environment for Salmonella growth.
FIG. 1.
Growth of Salmonella strains in THP-1 cells. Opsonized bacteria were used to infect cells as described, the cultures were harvested, and aliquots from each culture well were processed for viable organisms at the indicated times.
Cytopathology induced by Salmonella infection of THP-1 cells.
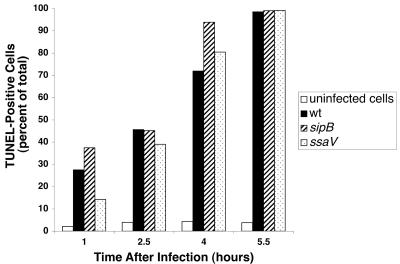
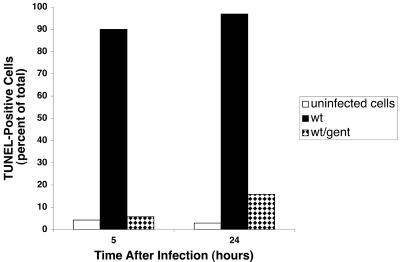
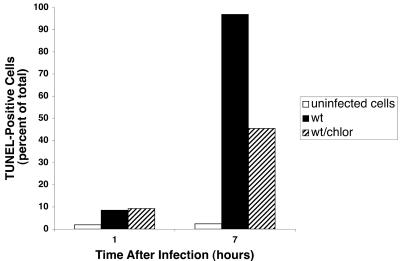
We examined the effects of Salmonella infection on DNA fragmentation in THP-1 cells as measured by flow cytometric analysis of TUNEL-stained cells. As shown in Fig. 2, the number of TUNEL-positive cells progressively increased with time after infection with wild-type serovar Dublin. Approximately 30% of cells were TUNEL positive after 1 hour of infection, increasing to nearly 100% by 5.5 h. The strains with mutations in sipB or ssaV also induced DNA fragmentation in THP-1 cells at the same level as wild type, and similar results were found with an ssaJ and an spvB mutant (data not shown). The induction of DNA fragmentation required live bacteria, since organisms killed by gentamicin treatment did not induce cell death, even after 24 h of infection (Fig. 3). Furthermore, transient inhibition of bacterial protein synthesis by simultaneous addition of bacteria and chloramphenicol to THP-1 cells, followed by removal of chloramphenicol after phagocytosis, reduced the cytopathic effect approximately 50% at 7 h postinfection (Fig. 4). This treatment did not affect phagocytosis or the viability of the infecting bacteria, although growth of the chloramphenicol-treated organisms was delayed as expected (data not shown).
FIG. 2.
Cytopathology caused by Salmonella strains in THP-1 cells. Cells from the same experiment as in Fig. 1 were processed for cytopathology in a fluorescent TUNEL assay as described in the text.
FIG. 3.
Effect of pretreatment of the bacterial inoculum with gentamicin. Equal numbers of untreated and gentamicin-treated wild-type Salmonella bacteria were used to infect THP-1 cells, and the cytopathology was determined at the indicated times.
FIG. 4.
Effect of chloramphenicol treatment at the time of bacterial infection of THP-1 cells. Cultures were treated with chloramphenicol during the time of phagocytosis as described in the text. The chloramphenicol was then removed and gentamicin was added to kill extracellular bacteria. Cytopathology was measured in a TUNEL assay at the indicated times.
Dependence of apoptosis on the Salmonella phoP gene.
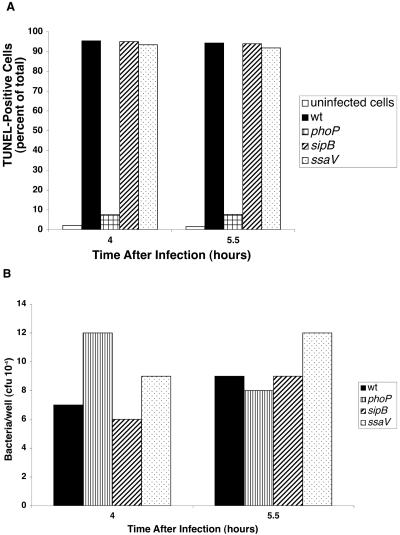
In contrast to the results with mutants in SPI-1, SPI-2, and spvB, we found that a phoP mutant was unable to induce DNA fragmentation in THP-1 cells. As shown in Fig. 5A, very low levels of TUNEL-positive cells were found after infection with the phoP mutant. The phoP mutant produced less than 10% positive cells even after 4 and 5.5 h of incubation, conditions that induced close to 100% positive cells with the other strains. As shown in Fig. 5B, comparable numbers of bacteria for all the strains were recovered from infected cells at both time points, excluding the possibility that the phoP result was due to lower numbers of intracellular organisms.
FIG. 5.
Effect of a phoP mutation on the cytopathology induced by Salmonella strains. A. Cells processed for TUNEL assay. B. Viable bacteria recovered from the same culture wells.
Caspase activation during Salmonella infection of THP-1 cells.
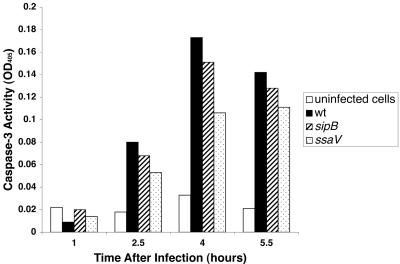
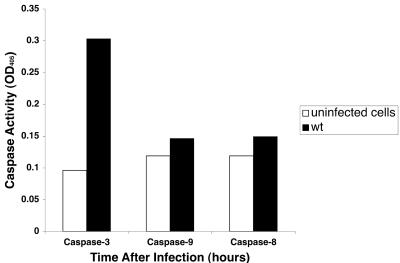
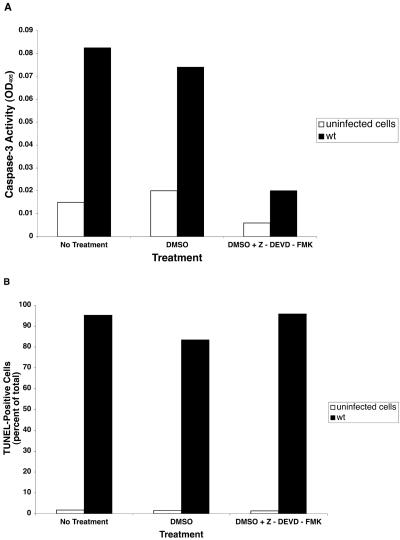
We determined whether caspase-3, -8, or -9 was activated by Salmonella infection by using biochemical assays of lysates from infected cells. As shown in Fig. 6, caspase-3 activity increased from 1 to 4 h following infection with wild-type Salmonella as well as with the sipB and ssaV mutant. At 4 h, caspase-3 activity was approximately fivefold higher in cells infected with the wild-type strain compared to uninfected controls. No major differences were found between caspase-3 activation by the wild type and the sipB or ssaV mutants. Infection with the phoP mutant gave results similar to uninfected cells (data not shown). However, we did not find significant activation of caspase-8 or caspase-9 in the same infected cells that displayed caspase-3 activation (Fig. 7). To investigate whether Salmonella-induced DNA fragmentation is dependent on caspase-3 activation, we used the caspase-3 inhibitor Z-DEVD-FMK. Although Z-DVED-FMK was effective in lowering caspase-3 activity in treated cells (Fig. 8A), the inhibition of caspase-3 did not affect the extent of DNA fragmentation (Fig. 8B). This result indicates that Salmonella-induced cell death in THP-1 cells resembles other systems in which caspase inhibitors are unable to prevent DNA fragmentation (1, 4, 12).
FIG. 6.
Caspase-3 activity in lysates from Salmonella-infected THP-1 cells. Cultures were infected as described in the text and harvested for caspase-3 assays at the indicated times.
FIG. 7.
Caspase-8 and -9 activities in lysates from Salmonella-infected THP-1 cells. Cultures were harvested 5 h after infection and processed for caspase-8 and -9 assays compared to caspase-3 activity in the same lysate.
FIG. 8.
Effect of the caspase-3 inhibitor Z-DEVD-FMK on Salmonella-induced DNA fragmentation. THP-1 cells were preincubated for 1 hour with 12 μM Z-DEVD-FMK in 0.6% dimethyl sulfoxide (DMSO), or the DMSO vehicle alone, and then infected with wild-type Salmonella serovar Dublin. After 5 h, the cells were harvested and processed for caspase-3 activity in cell lysates (A) and by FACS analysis for DNA fragmentation (B).
DISCUSSION
Salmonella enters macrophages and replicates within a vacuolar compartment (16). When entry is mediated primarily through the SPI-1-encoded invasion mechanism, the SipB protein triggers cell cytotoxicity through several mechanisms involving activation of caspase-1, caspase-2, and mitochondrial autophagy, leading to loss of macrophage viability within a few hours of infection (12, 13, 15). The significance of this cytotoxicity for the pathogenesis of Salmonella infection is unclear. This process may play a role during infection of intestinal macrophages, and caspase-1 is required for efficient dissemination of infection from the bowel in the murine model (14, 22). However, SipB and other components of the SPI-1 TTSS are not required for virulence in systemic disease in mice (7). A second form of cytopathology is seen in macrophages when bacteria enter by phagocytosis, the mechanism likely to account for intracellular infection during the systemic phase of disease (23). Bacteria taken up by phagocytosis proliferate over an extended period of time and reach high intracellular numbers. Cytopathology appears later in the infection and cell membrane function remains intact, as evidenced by the continued protection of bacteria from extracellular gentamicin (18). Cell death is manifested by DNA fragmentation before membrane integrity is compromised, one of the key features of apoptosis. In various macrophage systems, the SPI-2 TTSS and the spvB virulence gene have been implicated in this delayed cytopathology (18, 28).
We describe here a cytopathic effect induced by Salmonella in THP-1 cells that differs from both of the previously described processes. In THP-1 cells, opsonized Salmonella is phagocytized and rapidly proliferates. The uptake and early intracellular growth are not dependent on SPI-1, SPI-2, spvB, or phoP functions. This finding suggests that the Salmonella-containing vacuole in THP-1 cells is a permissive environment that does not require the function of specific Salmonella virulence factors for intracellular growth to be initiated. Previous work from our group has shown that induction of phagocyte NADPH oxidase activity by vitamin D3 is required to restrict the growth of Salmonella in THP-1 cells (27). In the present study, we have found that Salmonella infection induces DNA fragmentation in the vast majority of infected cells within 6 h. This cytopathology requires active bacterial protein synthesis, making it unlikely that the process is due only to the interaction of preexisting bacterial structural components with THP-1 cells. In fact, only a transient inhibition of bacterial protein synthesis with chloramphenicol at the time of phagocytosis is sufficient to reduce DNA fragmentation in infected cells, implying that proteins synthesized after phagocytosis by growing Salmonella are required to induce cytopathology.
Analysis of Salmonella mutants indicates that the virulence functions specified by SPI-1, SPI-2, or spvB are not involved in the induction of DNA fragmentation in THP-1 cells. This system allowed us to demonstrate a requirement for phoP that could not be detected using other macrophage systems. In murine macrophages, phoP is required for intracellular survival of Salmonella (5, 21). Therefore, it is difficult to assess an independent role for phoP in cytopathology. In primary human macrophages, phoP is required for the cytopathology mediated by SpvB, again preventing assessment of an independent role for phoP (D. Guiney, unpublished data). However, in THP-1 cells, the phoP mutant grew as well as the wild-type strain in the first few hours, although it did not produce cell death. Since PhoP is the response regulator of the two-component regulatory system using PhoQ as the sensor protein, these results suggest that one or more PhoP-regulated proteins are required for the induction of DNA fragmentation in THP-1 cells. PhoQ appears to sense low Mg2+ levels in the Salmonella-containing vacuole following phagocytosis (8). Our finding of the requirement for bacterial protein synthesis to induce DNA fragmentation is consistent with a model in which PhoP induces the synthesis of one or more proteins that promote cytopathology in THP-1 cells following entry of Salmonella strains. The PhoP-dependent effect appears to activate caspase-3 and DNA fragmentation in parallel without caspase-8 or -9 involvement (Fig. 2 and 6 to 8), in contrast to the classical extrinsic and intrinsic apoptosis pathways proceeding through caspase-8 or -9 to caspase-3 as the executioner initiating DNA fragmentation (20). It is significant that the phoP-mediated effect on cytopathology in THP-1 cells does not require the SPI-2 TTSS. A previous study defined a phoP-dependent phenotype involving inhibition of phagosome fusion that also did not require SPI-2 function (9). Taken together, these results suggest that phoP controls Salmonella virulence factors that affect phagocyte physiology and can be secreted by either the SPI-1 or SPI-2 TTSS or, alternatively, do not require these transport systems.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI-32178 and DK35108).
Editor: V. J. DiRita
REFERENCES
- 1.Andersson, M., A. Honarvar, J. Sjostrand, A. Peterson, and J. O. Karlsson. 2003. Decreased caspase-3 activity in human lens epithelium from posterior subcapsular cataracts. Exp. Eye Res. 76:175-182. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. [DOI] [PubMed] [Google Scholar]
- 3.Browne, S. H., M. L. Lesnick, and D. G. Guiney. 2002. Genetic requirements for Salmonella-induced cytopathology in human monocyte-derived macrophages. Infect. Immun. 70:7126-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings, B. S., G. R. Kinsey, L. J. Bolchoz, and R. G. Schnellmann. 2004. Identification of caspase-independent apoptosis in epithelial and cancer cells. J. Pharmacol. Exp. Ther. 310:126-134. [DOI] [PubMed] [Google Scholar]
- 5.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 7.Galan, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 86:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia Vescovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 9.Garvis, S. G., C. R. Beuzon, and D. W. Holden. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell. Microbiol. 3:731-744. [DOI] [PubMed] [Google Scholar]
- 10.Guiney, D. G., F. C. Fang, M. Krause, S. Libby, N. A. Buchmeier, and J. Fierer. 1995. Biology and clinical significance of virulence plasmids in Salmonella serovars. Clin. Infect. Dis. 21(Suppl. 2):S146-S151. [DOI] [PubMed] [Google Scholar]
- 11.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez, L. D., M. Pypaert, R. A. Flavell, and J. E. Galan. 2003. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163:1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvelainen, H. A., A. Galmiche, and A. Zychlinsky. 2003. Caspase-1 activation by Salmonella. Trends Cell. Biol. 13:204-209. [DOI] [PubMed] [Google Scholar]
- 15.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knodler, L. A., and O. Steele-Mortimer. 2003. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic 4:587-599. [DOI] [PubMed] [Google Scholar]
- 17.Lesnick, M. L., N. E. Reiner, J. Fierer, and D. G. Guiney. 2001. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 39:1464-1470. [DOI] [PubMed] [Google Scholar]
- 18.Libby, S. J., M. Lesnick, P. Hasegawa, E. Weidenhammer, and D. G. Guiney. 2000. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell. Microbiol. 2:49-58. [DOI] [PubMed] [Google Scholar]
- 19.Lindgren, S. W., I. Stojiljkovic, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsden, V. S., and A. Strasser. 2003. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21:71-105. [DOI] [PubMed] [Google Scholar]
- 21.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohl, M. E., and S. I. Miller. 2001. Salmonella: a model for bacterial pathogenesis. Annu. Rev. Med. 52:259-274. [DOI] [PubMed] [Google Scholar]
- 24.Paesold, G., D. G. Guiney, L. Eckmann, and M. F. Kagnoff. 2002. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell. Microbiol. 4:771-781. [DOI] [PubMed] [Google Scholar]
- 25.Pang, T., Z. A. Bhutta, B. B. Finlay, and M. Altwegg. 1995. Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 3:253-255. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez, M., I. de Diego, and M. C. Mendoza. 1998. Extraintestinal salmonellosis in a general hospital (1991 to 1996): relationships between Salmonella genomic groups and clinical presentations. J. Clin. Microbiol. 36:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sly, L. M., D. G. Guiney, and N. E. Reiner. 2002. Salmonella enterica serovar Typhimurium periplasmic superoxide dismutases SodCI and SodCII are required for protection against the phagocyte oxidative burst. Infect. Immun. 70:5312-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Velden, A. W., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Velden, A. W., M. Velasquez, and M. N. Starnbach. 2003. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J. Immunol. 171:6742-6749. [DOI] [PubMed] [Google Scholar]
- 30.Waterman, S. R., and D. W. Holden. 2003. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell. Microbiol. 5:501-511. [DOI] [PubMed] [Google Scholar]