Abstract
Host resistance to African trypanosomiasis is partially dependent on an early and strong T-independent B-cell response against the variant surface glycoprotein (VSG) coat expressed by trypanosomes. The repetitive array of surface epitopes displayed by a monotypic surface coat, in which identical VSG molecules are closely packed together in a uniform architectural display, cross-links cognate B-cell receptors and initiates T-independent B-cell activation events. However, this repetitive array of identical VSG epitopes is altered during the process of antigenic variation, when former and nascent VSG proteins are transiently expressed together in a mosaic surface coat. Thus, T-independent B-cell recognition of the trypanosome surface coat may be disrupted by the introduction of heterologous VSG molecules into the coat structure. To address this hypothesis, we transformed Trypanosoma brucei rhodesiense LouTat 1 with the 117 VSG gene from Trypanosoma brucei brucei MiTat 1.4 in order to produce VSG double expressers; coexpression of the exogenous 117 gene along with the endogenous LouTat 1 VSG gene resulted in the display of a mosaic VSG coat. Results presented here demonstrate that the host's ability to produce VSG-specific antibodies and activate B cells during early infection with VSG double expressers is compromised relative to that during infection with the parental strain, which displays a monotypic coat. These findings suggest a previously unrecognized mechanism of immune response evasion in which coat-switching trypanosomes fail to directly activate B cells until coat VSG homogeneity is achieved. This process affords an immunological advantage to trypanosomes during the process of antigenic variation.
The variant surface glycoprotein (VSG) coat covering the membrane of African trypanosomes consists of a densely packed array of 107 identical molecules that determine the antigenic phenotype of the parasite. VSG molecules are 55- to 65-kDa glycoproteins that contain internal antiparallel A and B α-helices that lend rigidity to the folded structure (29); the molecules are displayed as homodimers oriented with the hydrophilic N-terminal portion of the protein toward the extracellular space and with glycosylphosphatidylinositol anchors tethering the C terminus to the plasma membrane (9, 11, 29). Together, these characteristics permit a highly ordered packing of VSG molecules into the surface coat structure. Despite extensive primary sequence variation among different VSGs, secondary and tertiary structural features of these molecules are highly conserved (11, 12, 29, 31), perhaps ensuring that all VSG molecules are packaged similarly into a surface coat structure during the process of antigenic variation.
Stringent allelic exclusion ensures that only 1 of approximately 1,000 different VSG genes in the genome is transcribed at any given time from a chromosome telomere (reviewed in references 10, 13, and 17). Thus, normally only one species of VSG molecule is present within the trypanosome surface coat, resulting in the homogeneous display of identical surface epitopes in exposed N-terminal regions of the molecules; this exposed multiepitope array is capable of activating B cells in a T-independent manner, and early recognition by the immune system and clearance of trypanosomes from the bloodstream are largely mediated by a T-independent, VSG-specific immunoglobulin M (IgM) response (27, 32, 33, 36). In other microbial systems, a correlation has been established between T-independent activation of B cells and the degree of epitope repetitiveness, surface rigidity, spatial arrangement, and orientation of epitopes to achieve full B-cell activation (3, 40-42, 47). Thus, the VSG surface coat structure of trypanosomes both theoretically and functionally meets these criteria for T-independent B-cell activation.
However, during the process of antigenic variation, one homogeneous surface coat is replaced over a period of time by a new homogeneous coat. Transcription of a new VSG gene plus translation and trafficking of new mature VSG homodimers to the cell surface, coupled with residual mRNA and protein stability of the old VSG coat, result in transient expression of both the former and the nascent VSG species on the cell surface for up to 48 h during the process of antigenic variation (4, 18, 19, 38). This “mosaic” VSG surface coat has not previously been examined for its ability to prime the host immune system to the newly arising VSG species. The kinetics of antibody responses to VSG surface coats displayed by trypanosomes at the first peak of parasitemia and the kinetics of antibody responses to different VSG coats displayed by variant antigenic types (VATs) in subsequent waves of parasitemia are similar, suggesting that B cells are not primed to new VSG molecules expressed earlier in mosaic surface coats of preceding double expressers.
It is not known at present whether B cells are able to recognize and become activated by a mosaic display of old and new VSGs that are present on the cell surface during the process of antigenic variation. We hypothesized that a mosaic arrangement of surface epitopes may effectively prevent emerging VSG species from directly activating B cells until the trypanosome surface coat reaches homogeneity (e.g., when the previous surface coat has been completely replaced by the new one). In the present study, therefore, we tested this hypothesis by creating genetically modified trypanosomes that constitutively express both the endogenous LouTat 1 VSG gene from its native chromosome telomeric expression site and an exogenous 117 VSG gene transcribed from a ribosomal DNA locus. We have determined that VSG double-expresser parasites displaying a mosaic coat containing two different VSG species do not activate early T-independent B-cell responses to produce VSG-specific antibodies.
MATERIALS AND METHODS
Animals.
Female C57BL/6 wild-type (wt) and athymic nude (nu/nu) mice, 8 to 10 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME) and used for experimental infections. C57BL/6 mice are relatively resistant to trypanosome infection, surviving approximately 50 days postinfection. These animals have been extensively characterized for their immune response to variants of the Trypanosoma brucei rhodesiense LouTar serodeme (15, 16, 21, 22, 36, 45, 46). Outbred Swiss mice (Harlan Sprague-Dawley, Madison, WI) were used to expand frozen wt trypanosome stabilates. C57BL/6scid mice (scid mice) (Jackson Laboratory) were used to clone and expand stabilates of genetically transformed trypanosomes. All mice were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and were handled strictly according to National Institutes of Health and university guidelines.
Trypanosomes.
Frozen stabilates of Trypanosoma brucei rhodesiense clone LouTat 1 and Trypanosoma brucei brucei MiTat 1.4 were grown in Swiss mice prior to establishment of experimental infections. Swiss mice were immunosuppressed by cyclophosphamide treatment (300 mg/kg body weight; Sigma, St. Louis, MO) prior to intraperitoneal infection with LouTat 1 trypanosomes. This treatment suppresses B-cell responses to the VSG molecule and prevents immune selection for minor VATs (39); expression of the LouTat 1 VSG surface coat and expression of the LouTat 1 VSG gene from its telomeric expression site are relatively stable in the absence of immune selection (26). Parasites were isolated from the blood when parasitemia reached 109 trypanosomes/ml blood, as previously described (43). Briefly, animals were exsanguinated from the retrobulbar sinus into a heparinized tube. Blood was diluted with phosphate-buffered saline (PBS) supplemented with 1% glucose, pH 8 (PBSG), and passed over a Selectacel DEAE type 40 (Polysciences, Warrington, PA) column equilibrated with PBSG. By this technique, cellular blood components are bound to the column matrix while trypanosomes pass through (25). Trypanosomes were collected on ice, washed with ice-cold PBSG by centrifugation at 1,000 × g for 10 min at 4°C, and counted in a hemocytometer. As outlined below, LouTat 1 trypanosomes were genetically transformed with vector DNA containing the 117 VSG gene and a neomycin resistance gene (G418-selectable marker) and were subsequently propagated in G418-treated scid mice (50 mg G418/kg body weight given at 0, 24, and 48 h postinfection) prior to experimental infections of C57BL/6 wt and nu/nu mice in the absence of G418 selection.
Antibodies.
Antisera against LouTat 1 VSG and 117 VSG were generated in rabbits and mice. LouTat 1 VSG antiserum was affinity purified by positive selection on a LouTat 1 VSG-coupled AminoLink (Pierce Biotechnology, Rockford, IL) column. Bound proteins were eluted in glycine (pH 2.5) and the eluate negatively selected on a 117 VSG column. Likewise, 117 VSG antiserum was positively selected on a 117 VSG column and the eluate negatively selected on a LouTat 1 VSG column. VSG specificity was confirmed by Western blotting, immunoprecipitation, and immunofluorescence (data not shown). Species-specific Alexa 488- and Alexa 633-conjugated secondary antibodies, as well as 4′,6′-diamidino-2-phenylindole (DAPI) stain, were purchased from Molecular Probes (Eugene, OR).
Generation of VSG gene double expressers.
The pXS5neo117 constitutive bloodstream-stage expression vector was a generous gift from James Bangs (University of Wisconsin, Madison). pXS5neo has been previously described (2) and was provided to us with the 117 VSG gene cloned into the multiple cloning site (Fig. 1). Transformation of bloodstream-stage trypanosomes with linearized pXS5 has been previously described (2). Briefly, 107 LouTat 1 trypanosomes were mixed with 50 μg of linearized pXS5neo117 vector DNA in a final volume of 500 μl Cytomix in a 0.2-mm cuvette and were electroporated (R2, 1.5 kV, no capacitance) in a BTX600 cell manipulator (BTX Inc., San Diego, CA). The following adaptation was made from the published protocol for in vivo drug-selection of transformants, because T. b. rhodesiense LouTat 1 trypanosomes do not grow in vitro. Cells were rested in HMI-9 (23) for 24 h at 37°C, 5% CO2, prior to infection of G418-treated scid mice. A clonal population of successful transformants was derived by infection of G418-treated scid mice with a single parasite. Frozen stabilates were made from the resulting infection and were used for all future studies; expansion of stabilates for all experimental studies was carried out under drug selection in G418-treated scid mice.
FIG. 1.
pXS5 expression vector for ectopic VSG gene expression from the ribosomal locus of trypanosomes. From 5′ to 3′, the vector contains a nontranscribed targeting sequence from the 3′ end of a ribosomal repeat (Ribo 3′); the rRNA promoter (Ribo Prom); the 3′ end of the procyclic acidic repetitive protein (PARP) B2α splice acceptor site; the 117 VSG gene inserted into the multiple cloning site; the aldolase intergenic region (Aldo Int), containing a polyadenylation site and a 3′ splice acceptor site; the neomycin phosphotransferase gene (neor); and the tubulin αβ intergenic region (Tub αβ Int), also containing polyadenylation and slice acceptor sites. Linearization of the vector with XhoI targets the construct to replace a ribosomal repeat. Expression of neomycin phosphotransferase results in resistance to the neomycin analog G418 to allow for selection of transformants in vivo or in vitro. Asterisk, splice acceptor site; An, polyadenylation site.
Biology of VSG double expressers.
C57BL/6 mice, wt and scid, were infected intraperitoneally with 105 LouTat 1 trypanosomes or LT1:117 double-expresser trypanosomes in the absence of drug selection. Animals were monitored for parasitemia and survival. Blood was taken daily from mice via tail snips, and parasitemia was determined microscopically, based on a scale of 1 to 4 (1 = no visible parasites/10 400× fields; 4 = >100 parasites/single 400× field). Animals were monitored until they were in apparent physiological distress, at which time they were euthanized. Trypanosomes taken from blood at various points postinfection were fixed and assessed by immunofluorescent microscopy to determine the stability of VSGs expressed on the cell surface.
RT-PCR analysis of LT1:117 double expressers.
Drug-selected transformants were purified from whole blood as described above. Total RNA was isolated according to the manufacturer's instructions for the Ultraspec-II RNA isolation system (Biotecx Laboratories, Houston, TX). RNA was reverse transcribed and amplified using the Access reverse transcription-PCR (RT-PCR) system (Promega, Madison, WI) and gene-specific primers. The following synthetic deoxyoligonucleotides were designed using LaserGene primer analysis software (DNAStar, Inc., Madison, WI) and synthesized by Integrated DNA Technologies (Coralville, IA) (all sequences are 5′ to 3′): TLTF sense, ATTACAAAGGGGGAGGTGGAGACT; TLTF antisense, CCGCTTGTTCTGTTCATTCTGCT; LouTat 1 VSG sense (VSG 2383), GGACGCAAGTCTACCTAGCAGC; LouTat 1 VSG antisense (VSG 2382), CTGCAAGTGTCTACCGATGCC; 117 VSG sense (117p3U), TGGAGGCGATCAACGATGCT; 117 VSG antisense (117p3L), TCCGCTTGTTGTCAGTGTCCC.
Immunofluorescent microscopy.
Immunostaining and fluorescent microscopy were done by established methods (2). Briefly, fixed and permeabilized T. brucei LouTat 1, MiTat 1.4 (117 VSG), or VSG double-expresser trypanosomes were stained with affinity-purified anti-LouTat VSG or anti-117 VSG antibodies. Specific staining was visualized with appropriate species-specific Alexa 488- and Alexa 633-conjugated secondary reagents. Cells were counterstained with DAPI to visualize nuclear and kinetoplast components for cellular orientation. Stained cells were visualized at ×100 on a motorized Zeiss Axioplan IIi equipped with a rear-mounted excitation filter wheel, a triple-pass (DAPI-fluoroscein isothiocyanate-Texas Red) emission cube, and a Zeiss AxioCam black-and-white charge-coupled device camera. Fluorescence images were pseudocolored and merged using OpenLabs 3.0 software (Improvision, Inc., Lexington, MA).
Immunoprecipitation.
Radiolabeling of trypanosomes and immunoprecipitation with affinity-purified VSG-specific antibodies were performed as described previously (6, 7). Briefly, LouTat 1 and LT1:117 trypanosomes were radiolabeled with [35S]Met/Cys (Expre35S35S; DuPont, NEN) for 4 h at 37°C; VSG protein sequence information predicted that 23 Met/Cys (14 Met, 14 Cys) residues would be labeled for the 117 protein and that 18 Met/Cys (5 Met, 13 Cys) residues would be labeled for LouTat 1 protein. Cells were separated from medium and lysed in the presence of protease inhibitors. Lysates were incubated with an affinity-purified rabbit anti-LouTat 1 VSG or anti-117 VSG antibody preadsorbed to protein A-Sepharose CL-4B beads (Amersham Pharmacia); the two antibodies precipitated equivalent amounts of VSG from lysates of homotypic antigenic variants (data not shown). Beads were washed and bound proteins separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Bands were detected by autoradiography, and the resulting images were analyzed using GeneTools gel imaging software (Hitachi Genetic Systems; Hitachi Software Engineering Co., Ltd.). To confirm that VSGs in the LT1:117 double expressers were expressed only as homodimers and not as heterodimers, proteins immunoprecipitated with anti-LouTat VSG or anti-117 VSG were transferred to nitrocellulose filters and probed with anti-117 or anti-LouTat 1 VSG, respectively, followed by detection with the appropriate secondary reagents (data not shown).
VSG-specific ELISA and B-cell ELISPOT assays.
Enzyme-linked immunosorbent assays (ELISAs) to detect VSG-specific antibodies were performed as described previously (36). Briefly, 4 μg/ml soluble LouTat 1 VSG or 117 VSG in PBS was adsorbed to Immulon 4 ELISA plates (Thermo Lab Systems, Franklin, MA). Plates were incubated overnight at 4°C and washed. Serial dilutions of antisera from nu/nu mice infected with LouTat or LT1:117 trypanosomes for 0 through 5 days were then added to the plates. VSG-specific antibodies were detected with appropriate biotin-conjugated secondary reagents and p-nitrophenyl phosphate as a substrate. The B-cell enzyme-linked immunospot (ELISPOT) assays were done as we have described previously (36). Briefly, 10 μg/ml of the relevant VSG in PBS was bound to wells of Multiscreen-IP 96-well filtration plates (Millipore) at 4°C overnight. Plates were washed and blocked with PBS containing 5% fetal bovine serum; subsequently, 105 splenocytes in 100 μl RPMI with 10% fetal bovine serum were aliquoted into triplicate wells. Cells were cultured for 20 h, and bound cells secreting VSG-specific antibodies were detected with biotinylated anti-mouse IgM (Sigma) following extensive washing. Color was developed with alkaline phosphatase (Vectastain ABC-AP; Vector Laboratories) followed by 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (Sigma) substrate. Spots were counted using a dissecting microscope.
RESULTS
VSG gene double expressers.
Trypanosomes actively undergoing antigenic variation and expressing a VSG mosaic surface coat normally represent a very small percentage of the total parasite population at any given time (19). Additionally, expression of a dual VSG coat is transient and not amenable to long-term study. Therefore, in the present study we constitutively expressed an exogenous VSG gene from the ribosomal locus of T. b. rhodesiense LouTat 1 under control of the native ribosomal DNA promoter. We transformed LouTat 1 trypanosomes by electroporation with the expression vector pXS5neo117, which contains the gene encoding VSG 117 as well as the neomycin phosphotransferase gene, expression of which confers resistance to the neomycin analog G418. Following transformation and in vivo drug selection, both the 117 VSG gene and the neomycin resistance cassette were amplified from purified mRNA from the transformed VSG double expresser LT1:117 by gene-specific RT-PCR; these results confirmed that both genes had been successfully integrated and expressed (Fig. 2).
FIG. 2.
Amplification of LouTat 1 and 117 VSG mRNAs from LT1:117 VSG double expressers. LT1:117 total RNA was isolated using the Ultraspec-II RNA isolation system. The T-lymphocyte-triggering factor LouTat 1 VSG, and 117 VSG genes were amplified using gene-specific primers. A reaction control was provided with the Access RT-PCR core kit (Promega, Madison, WI). Appropriate negative controls were also run (not shown), and trypanosomes expressing monotypic coats expressed only the relevant VSG gene transcripts (not shown).
Infections.
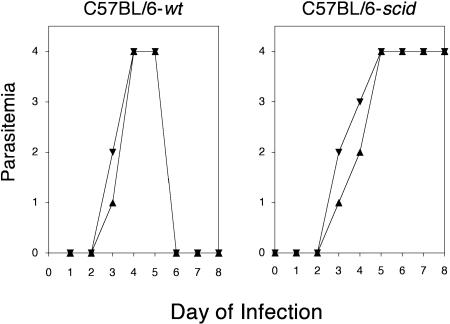
Previous studies of genetically modified VSG double expressers by Munoz-Jordan et al. demonstrated that expression of a second VSG in the surface coat did not adversely affect aspects of the normal biology of the trypanosome cell (30). We measured infectivity, survival, and growth properties of the LT1:117 double-expresser trypanosomes in comparison with those of the parental LouTat 1 strain. Infection of C57BL/6 wt and C57BL/6 scid mice with 105 LouTat 1 or LT1:117 trypanosomes resulted in higher levels of LT:117 parasites on days 3 to 4 postinfection but comparable peak parasitemias of approximately 109 trypanosomes/ml blood on days 4 to 5 postinfection (Fig. 3). Subsequent waves of parasitemia in infected immunocompetent mice were variable in terms of timing and peak numbers of parasites, both between individual animals and between trypanosome strains, as is common with infection of C57BL/6 mice; however, the second and subsequent waves of parasitemia in mice infected with the VSG double expresser appeared earlier and were much higher than those in mice infected with LouTat 1 (data not shown). Therefore, it is likely that mice infected with the VSG double expresser fail to control early growth of these trypanosomes and also fail to completely clear all the infecting variants from each peak compared to mice infected with the monotypic trypanosome. Survival times of immunocompetent mice infected with these two strains were comparable at 50 days or longer (data not shown). Trypanosome expression of LouTat 1 and 117 VSGs in the surface coat, measured by immunofluorescent microscopy, persisted for as long as 5 to 7 days in the absence of immune selection, demonstrating that surface expression was relatively stable in vivo during the period of immunological interest (Fig. 4). Overall, these data indicate that the transformed LT1:117 trypanosome strain expresses both LouTat VSG and 117 VSG in the surface coat and that coexpression of 117 VSG in the context of the LouTat 1 genetic background does not appear to alter the normal biology of the LouTat 1 parasite.
FIG. 3.
LouTat 1 and LT1:117 trypanosomes exhibit similar infectivity and growth patterns. C57BL/6 wt (left) and C57BL/6 scid (right) mice were infected with 105 viable trypanosomes and the early parasitemia profiles monitored. The data presented are from individual mouse infections but are representative of parasitemia profiles for 10 mice infected with each strain. Parasitemia scores: 4 = >100 trypanosomes per 400× field; 1 = 1 to 5 trypanosomes per 10 400× microscope fields. Symbols: ▴, LouTat 1; ▾, LT1:117.
FIG. 4.
LouTat 1 VSG and 117 VSG are stably coexpressed for up to 1 week by LT1:117 VSG gene double expressers in the absence of immune selection. C57BL/6 scid mice were infected with 105 LouTat 1 or LT1:117 trypanosomes in the presence or absence of G418 drug selection. VSG expression was monitored on trypanosomes by immunofluorescence staining with LouTat 1 VSG- and 117 VSG-specific antibodies at days 7 and 14 postinfection.
Colocalization of endogenous and exogenous VSGs.
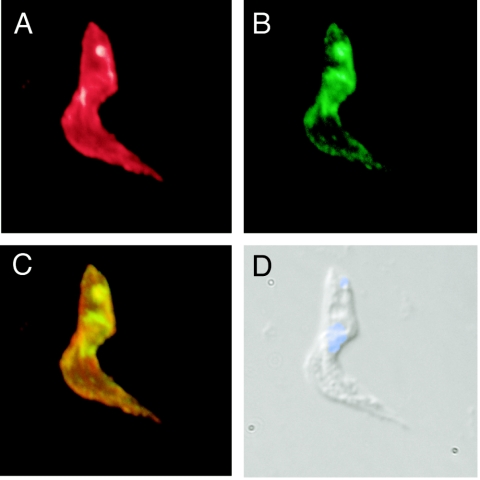
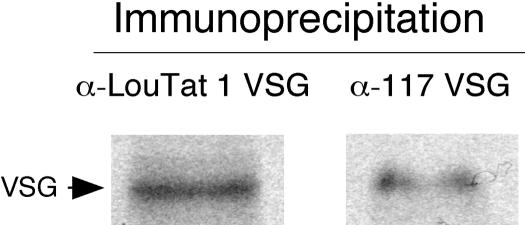
Naturally occurring VSG double-expresser trypanosomes detected during infection, as well as genetically manipulated stable transformants, demonstrate homogeneous distribution of two VSG species on the cell surface without apparent patching or capping (4, 19, 30). VSGs on these cells were observed only as homodimers, and there was no evidence of heterodimerization between VSG species. To determine the intracellular and extracellular distributions of VSG species in LT1:117 VSG double expressers, LouTat 1, MiTat 1.4 (VSG 117), and LT1:117 trypanosomes were fixed and permeabilized on glass slides. Cells were stained with mouse anti-LouTat 1 VSG and rabbit anti-117 VSG antibodies and were counterstained with DAPI to highlight nuclear and kinetoplast DNA. Following detection with appropriate fluorochrome-conjugated secondary antibodies, cells were viewed by immunofluorescence microscopy (Fig. 5). LouTat 1 VSG and 117 VSG were readily detected in LT1:117 cells, and the surface distribution appeared uniform, with colocalization of the two molecules apparent. It is notable that significant 117 VSG and LouTat 1 VSG were localized within a defined intracellular compartment (endoplasmic reticulum and Golgi apparatus) proximal to the nucleus; by fluorescence there appeared to be more 117 VSG than LouTat 1 VSG in this compartment. Additionally, LouTat 1 VSG and 117 VSG colocalized to the flagellar pocket, presumably as a result of protein trafficking and a high rate of VSG turnover in that location; again, there appeared to be more 117 VSG detectable in that location. Overall, both 117 and LouTat 1 VSGs trafficked to the cell surface and colocalized there without any apparent segregation. The relative amount of VSG protein being expressed by transformants was also measured by alternative means. We immunoprecipitated radiolabeled cell lysates from LT1:117 trypanosomes with anti-LouTat 1 VSG- or anti-117 VSG-adsorbed protein A-Sepharose (the two antibodies precipitated equivalent amounts of VSG from the lysates of homotypic antigenic variants [data not shown]). Quantitation of band density from the resulting SDS-PAGE gel showed that both LouTat 1 and 117 VSGs are detectable in extracts of the VSG double expressers, although total LouTat 1 VSG appeared to be about 30% more abundant than 117 VSG in these cells (Fig. 6).
FIG. 5.
LouTat 1 VSG and 117 VSG colocalize to the endoplasmic reticulum, flagellar pocket, and cell surface in LT1:117 VSG gene double expressers. G418-selected trypanosomes were fixed with 1% formaldehyde/0.05% glutaraldehyde and attached to slides by acetone/methanol fixation. Slides were stained with a mouse anti-LouTat 1 VSG monoclonal antibody and a rabbit anti-117 polyclonal serum. Positive cells were detected using Alexa 633-conjugated anti-mouse IgM (red) and Alexa 488-conjugated anti-rabbit IgM (green) and viewed by fluorescent microscopy. Immunostaining: (A) mouse anti-LouTat 1 VSG; (B) rabbit anti-117 VSG; (C) merged image; (D) differential interference contrast with DAPI counterstain. Control stains demonstrated that these antibodies do not cross-react with the heterologous VSG in this assay. Images were pseudocolored and merged using OpenLabs 3.0 software (Improvision, Inc., Lexington, MA).
FIG. 6.
Similar amounts of LouTat 1 VSG and 117 VSG are present in LT1:117 VSG double-expresser trypanosomes. Trypanosomes were metabolically labeled with [35S]methionine and [35S]cysteine in Met/Cys-depleted medium for 4 h. Cells were lysed, and the lysates were incubated with rabbit anti-LouTat 1 VSG or anti-117 VSG antibodies coupled to Sepharose A beads. Equivalent amounts of immunoprecipitated material were separated by SDS-PAGE and the bands detected by autoradiography. Band density was measured using GeneTools imaging software (Hitachi Genetic Systems; Hitachi Software Engineering Co., Ltd.). Scan pixel densities for precipitated LouTat 1 and 117 VSGs in autoradiograms were 1,422,050 and 1,036,078, respectively.
Overall, then, the combination of RT-PCR, immunoprecipitation, and cellular immunofluorescence results shows that both LouTat 1 and 117 VSG mRNA and protein were detectable in the VSG double-expresser trypanosomes. While both LouTat 1 and 117 VSGs were expressed at significant levels, with apparently uniform colocalization of the two VSGs in the surface coat, the intracellular levels may be somewhat different.
Immune response to the mosaic VSG coat.
We hypothesized that disruption of the homogeneous array of VSG epitopes present in the surface coat of trypanosomes by insertion of an additional VSG protein would negatively impact the ability of B cells to recognize and become activated by VSG epitopes within the surface coat. To test this hypothesis, we infected athymic C57BL/6 nu/nu mice with 105 LouTat 1 or LT1:117 trypanosomes. Both T-independent and T-dependent VSG-specific B-cell responses to VSG normally occur during infection of wt mice, but the T-dependent B-cell responses (including isotype switching) appear after the T-independent response has already cleared trypanosomes from the circulation (36). Thus, the use of athymic nude mice in the present study ensured that the B-cell responses detected were strictly T independent and represented the early B-cell response during infection. We examined B-cell activation during the first 5 days of infection by VSG-specific ELISA to measure antigen-specific antibody present in sera and by ELISPOT to enumerate splenic B cells secreting antibody. The focus on the first 5 days was reinforced by earlier data showing that the B-cell response during this period is normally sufficient to provide variant-specific immunity; the importance of T-independent VSG-specific IgM responses in controlling the first wave of parasitemia has been demonstrated (27, 28, 33). Also, we were concerned that a loss of 117 VSG gene and protein expression in even a subset of trypanosomes after 5 days would skew immunological results at later time points of infection in a manner that could not be controlled for. Therefore, we examined only animals infected with VSG double expressers during the 5-day period in which there was no demonstrable loss of ectopic VSG gene and protein expression in the organisms.
Infected C57BL/6 nu/nu mice were sacrificed on specific days postinfection, serum was isolated from whole blood and pooled, and relative antibody titers were measured by LouTat 1 VSG- and 117 VSG-specific ELISAs. Antibodies against both coat VSG constituents were depressed in animals infected with the VSG double expresser LT1:117; this was in contrast to the homogeneous single-VSG-expressing parental organism, which induced rapid and high levels of VSG-specific antibody (Fig. 7). Similarly, VSG-specific ELISPOT assays using splenocytes from trypanosome-infected C57BL/6 nu/nu mice clearly demonstrated that fewer B cells were stimulated to produce VSG-specific antibodies against coat determinants during LT1:117 infection than during LouTat 1 infection (Fig. 7). It is noteworthy that mice infected with the monotypic LouTat 1 variant generate cross-reactive antibodies against the 117 VSG molecule; this may be due to minor sub-sequence identity between the two VSGs, which are closely related with regard to type and class (see Materials and Methods). However, even this cross-reactive antibody response was depressed when animals were infected with the mosaic coat double expressers. This finding was also apparent in the B-cell ELISPOT data.
FIG. 7.
VSG-specific B-cell responses are depressed during infection with VSG double expressers relative to those for infection with the wt strain. C57BL/6 nu/nu mice were infected with 105 trypanosomes expressing either a homogeneous LouTat 1 VSG coat or the mosaic LT1:117 VSG coat. (Top) Serum was collected on the days indicated and analyzed by VSG-specific ELISAs using LouTat 1 VSG (left) or 117 VSG (right) as the capture molecule. Data are presented as relative change in optical density (OD) ± standard deviation from five separate experiments over an 8-month period. (Bottom) ELISPOT assays were performed with spleen cells from infected mice. Data are presented as mean number of antibody-forming cells (AFC)/105 spleen cells from a single experiment. Symbols: ▴, LouTat 1-infected mice; ▾, LT1:117-infected mice; ○, uninfected mice.
Thus, we determined from overall results that the ability of T-independent B cells to recognize and become activated by the VSG coat to produce antibodies is greatly reduced during infection with LT1:117 relative to the parental LouTat 1 strain during the first 5 days of infection. These data suggest that disruption of the homogeneous surface coat by insertion of a different VSG homodimer during the process of antigenic variation provides a window in which coat-switching trypanosomes are effectively not recognized by the host T-independent B-cell response.
DISCUSSION
The purpose of this study was to examine host T-independent B-cell responses to trypanosomes expressing two VSG molecules in the surface coat; such VSG double expressers arise naturally during the process of antigenic variation. It was previously unknown whether B cells are able to respond to such a mosaic antigen coat, or whether a transient period of mosaic coat expression by the trypanosome may result in an advantage for either host or parasite. Here we demonstrate for the first time that B cells exposed to a mosaic VSG coat during infection mount a significantly reduced T-independent antibody response to VSG epitopes. This result suggests that the process of antigenic variation not only provides the trypanosome with a new immunological identity upon completion of coat switching but also provides the parasite with an immunological “stealth coat” during the intermediate stages of coat switching, when two VSG species coexist in the glycoprotein surface coat.
Previous studies by other laboratories have shown that trypanosomes alter their surface coat during antigenic variation in vivo via establishment of a transient intermediate state in which two VSGs are expressed on the cell surface (1, 4, 19). Munoz-Jordan et al. demonstrated that there are no physiological or molecular barriers to constitutive, stable expression of two VSG species within the surface coat (30). Their study indicated that African trypanosomes were able to stably express two VSGs on the cell surface in a system in which an exogenous VSG gene was inserted into the active telomeric VSG gene expression site of the targeted parasite and was cotranscribed as part of site-specific polycistronic transcription, resulting in expression of both the endogenous and exogenous VSGs on the cell surface. Population doubling times and infectivity were similar for the parental single VSG expresser and the transformed VSG double-expresser strain, demonstrating for the first time that there were no intrinsic barriers to long-term expression of a mosaic VSG coat. However, no study detailing the effects of such a coat on the host immune response had been undertaken.
In the present study, we describe the construction of VSG double-expresser trypanosomes in which the VSG117 gene was expressed ectopically from the rRNA locus of T. b. rhodesiense LouTat 1. This model system and strategy were chosen for several reasons, the first and most prominent of which was that previous studies in this laboratory have extensively characterized the host immune response to the LouTat 1 variant in terms of both adaptive and innate immunity in several host genetic backgrounds (14-16, 21, 22, 32, 33, 35, 36, 45, 46). Relatively resistant mice (C57BL/6) infected with LouTat 1 trypanosomes live approximately 50 days postinfection and produce significant VSG-specific antibody responses during infection. Thus, we were extremely familiar with many aspects of the host-parasite interaction, and this disease model allowed us to study critically the host antibody response over time. Second, the VSG double expressers previously described by another lab were constructed in highly virulent strains of T. b. brucei that typically cause death of mice within 3 days, rendering such organisms unsuitable for most immunological studies (30). Third, ribosomal targeting of the 117 VSG gene was chosen because the pXS5neo117 vector was readily available and had been utilized in prior studies in T. b. brucei in which an exogenous VSG gene was expressed at high levels (5, 7). Fourth, VSG in the native telomeric expression site is transcribed by RNA polymerase I in vivo as part of a long polycistronic transcript; thus, one may predict that the same high level of transcription could be achieved by targeting the exogenous VSG gene to the ribosomal locus, where it would also be transcribed by RNA polymerase I behind the rRNA promoter (20, 24). We demonstrate here that the endogenous LouTat 1 VSG and the exogenous 117 VSG were produced in readily detectable quantities regardless of transcriptional loci and that the two VSG molecules trafficked to the cell surface in equivalent amounts to produce a mosaic coat. While we cannot prove that equimolar amounts of the two VSGs are present in the surface coat of the double expressers, it is clear that there is uniform distribution and colocalization of the two VSGs within the surface coat. Thus, the double-expresser surface coat is one in which the normally homogeneous LouTat 1 VSG coat has been disrupted by the presence of a different VSG molecule.
As in the earlier study of double expressers, introduction and expression of an exogenous VSG gene did not appear to alter the normal cell biology of the LouTat 1 parasite. Infectivity and the peak values observed in the first wave of parasitemia were similar for LT1:117 and the wt parental strain, although the double expressers initially grew to higher levels in infected mice on day 3, before the peak parasitemia occurred. Furthermore, subsequent waves of parasitemia in immunocompetent mice infected with the double expressers appeared to be less well-controlled than those with the wt trypanosomes. However, expression of an initial heterogeneous mosaic coat by the infecting parasite did not affect the overall survival time of the infected host. It is noteworthy that, given the reduced ability of host B cells to produce VSG-specific IgM antibodies against coat determinants of LT1:117, the double expresser initially grew to higher levels than LouTat 1. However, the LT:117 and LouTat 1 trypanosomes were eliminated with essentially the same kinetics following the first peak of parasitemia. One explanation for this observation may be that low levels of antibody generated by days 3 to 5 of infection against the 117 VSG component of the surface coat (Fig. 7) may have been sufficient to affect clearance. An alternative suggestion is that there were sufficient but small numbers of VSG double-expresser trypanosomes that displayed a mosaic coat with a greater degree of 117 VSG than LouTat 1 VSG expression (e.g., essentially resembling parasites completing the switch process); this may have provided sufficient cross-linking of the B-cell receptor to trigger a response. It is also possible that an attribute of the trypanosome, rather than a result of the host-parasite interaction, may be responsible. For example, recent studies have demonstrated that parasitemia is reduced significantly after the first peak during infection of IgM−/− mice (Sandor et al., unpublished data). This may reflect an innate ability of the organism to control its population size, perhaps by a mechanism similar to quorum sensing in prokaryotes, in the interest of sustaining a long-term infection to increase its chances of transmission (34, 44). It has also been suggested that parasitemia may be partially controlled by differentiation of dividing long-slender trypanosomes to senescent short-stumpy forms in preparation for transmission to the insect vector and that normal cell death of these nondividing parasites contributes to the apparent clearance of parasitemia (reviewed in reference 8). It is possible that LT1:117 double expressers differentiate to the short-stumpy morphology with different kinetics than the parental LouTat strain, although there is presently no evidence supporting this speculation. These ideas are clearly open for further investigation.
The technical approach taken in this study was not without drawbacks. As noted above, expression of the 117 VSG gene in double expressers was significantly down-regulated or lost during extended infection, despite drug selection of the organisms prior to each experimental infection (it is perhaps not unexpected that an ectopic gene could cease expression or be lost in the absence of continual drug selection). This phenomenon did not appear to affect the stability of surface expression of the ectopically expressed VSG during the first 5 to 7 days of an experimental infection (Fig. 4), but this observation was the basis for our decision to limit analyses of B-cell responses to the first 5 days of infection.
Originally, trypanosomes were cloned by limiting dilution and by visual confirmation to establish a clonal infection from a single double-expresser trypanosome, from which frozen stocks were prepared in order to establish future infections. Each experimental infection was preceded by expansion of a frozen stabilate in G418-treated C57BL/6 scid mice; thus, all experimental infectious populations were no more than a few passages from the original drug-selected transformants. However, frozen stocks that were stored at −80°C for extended periods also lost expression of the 117 VSG gene. Perhaps a very small percentage of LT1:117 trypanosomes down-regulate or lose expression of 117 VSG during infection, and these cells are better able to withstand storage for long periods, or possess an imperceptible but significant selective advantage over true double expressers under G418 selection when the stabilates are revived from frozen storage. It is unknown whether ribosomal targeting of the exogenous 117 VSG gene influences this phenomenon, but anecdotal evidence from several labs suggests this is true. As a result, work is ongoing in our lab to express the 117 VSG from the tubulin locus using pXS5neo117 in which the ribosomal targeting sequence has been removed and the plasmid linearized for insertion into the αβ-tubulin intergenic sequence. However, it is likely that the surest way to ensure stable insertion and transcription of the ectopic VSG gene is to directly insert the 117 VSG gene into the active LouTat 1 VSG expression site, in a manner similar to that employed by Munoz-Jordan et al. (30). Work is currently under way in our laboratory to establish such a system, although there is no VSG pseudogene upstream from the LouTat 1 VSG gene in the expression site to serve as a proper targeting site for gene insertion (Inverso et al., submitted for publication).
The data presented here indicate that the level of 117 VSG expressed on the surface of LT1:117 is sufficient to disrupt the overall surface architecture of the LouTat 1 surface coat, resulting in the comparative inability of T-independent B cells to produce an early, robust VSG-specific antibody response against surface determinants. It is apparent from immunoprecipitation analyses that relatively smaller quantities of total 117 VSG are being produced in this system (Fig. 6) but that both LouTat and 117 VSGs are being successfully expressed on the cell surface (Fig. 5). These data indicate that disruption of the homotypic LouTat surface coat with 117 VSG, even if these coat constituents are not present in equal proportions, is sufficient to modulate VAT-specific host B-cell responses to the parasite (Fig. 7). We believe we have maintained the gross surface architecture (i.e., changed only the VSG epitopes presented and not the VSG homodimer conformation within the coat architecture), since the 117 and LouTat 1 VSGs are predicted to have very similar three-dimensional structures, as both are class 1, type A, with respect to their C-terminal and N-terminal domain configurations, respectively (11, 37). We predicted that the 117 and LouTat 1 VSGs should integrate readily in the surface coat, and our data support that prediction, since the two VSGs were uniformly distributed and no patching or capping was evident. Therefore, we propose that disruption of the homogeneous array of identical epitopes that occurs following insertion of nascent VSG molecules into the coat during antigenic variation may provide the parasite a degree of antigenic stealth until the new coat reaches some prescribed degree of homogeneity that permits T-independent B cells to recognize and be activated by a surface array of identical epitopes. This constitutes a previously unrecognized mechanism of immune response evasion by the African trypanosomes.
Acknowledgments
This research was supported by funds from NIH grant AI-22441 to J.M.M. and by NIH training grants no. AI007414 (cell and molecular parasitology) and no. GM007215 (molecular biosciences) to M.E.D.
We thank G. A. M. Cross of the Rockefeller University for generous advice and helpful insights throughout this project. We are also grateful to James D. Bangs of the University of Wisconsin—Madison for assistance in this study.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Agur, Z., D. Abiri, and L. H. Van der Ploeg. 1989. Ordered appearance of antigenic variants of African trypanosomes explained in a mathematical model based on a stochastic switch process and immune-selection against putative switch intermediates. Proc. Natl. Acad. Sci. USA 86:9626-9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, D. L., K. J. Schwartz, A. E. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3253-3263. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., and R. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17:553-558. [DOI] [PubMed] [Google Scholar]
- 4.Baltz, T., C. Giroud, D. Baltz, C. Roth, A. Raibaud, and H. Eisen. 1986. Stable expression of two variable surface glycoproteins by cloned Trypanosoma equiperdum. Nature 319:602-604. [DOI] [PubMed] [Google Scholar]
- 5.Bangs, J. D. 1998. Surface coats and secretory trafficking in African trypanosomes. Curr. Opin. Microbiol. 1:448-454. [DOI] [PubMed] [Google Scholar]
- 6.Bangs, J. D., E. M. Brouch, D. M. Ransom, and J. L. Roggy. 1996. A soluble secretory reporter system in Trypanosoma brucei. Studies on endoplasmic reticulum targeting. J. Biol. Chem. 271:18387-18393. [DOI] [PubMed] [Google Scholar]
- 7.Bangs, J. D., D. M. Ransom, M. A. McDowell, and E. M. Brouch. 1997. Expression of bloodstream variant surface glycoproteins in procyclic stage Trypanosoma brucei: role of GPI anchors in secretion. EMBO J. 16:4285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, S. J., C. N. Sendashonga, C. O'Brien, N. K. Borowy, M. Naessens, P. Webster, and M. Murray. 1985. Regulation of parasitaemia in mice infected with Trypanosoma brucei. Curr. Top. Microbiol. Immunol. 117:93-118. [DOI] [PubMed] [Google Scholar]
- 9.Blum, J. L., J. A. Down, A. M. Gurnett, M. Carrington, M. J. Turner, and D. C. Wiley. 1993. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature 362:603-609. [DOI] [PubMed] [Google Scholar]
- 10.Borst, P., and S. Ulbert. 2001. Control of VSG gene expression sites. Mol. Biochem. Parasitol. 114:17-27. [DOI] [PubMed] [Google Scholar]
- 11.Carrington, M., N. Miller, M. Blum, I. Roditi, D. Wiley, and M. Turner. 1991. Variant specific glycoprotein of Trypanosoma brucei consists of two domains each having an independently conserved pattern of cysteine residues. J. Mol. Biol. 221:823-835. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, M. W., W. D. McCubbin, C. M. Kay, and T. W. Pearson. 1988. Physical studies of Trypanosoma brucei variant surface glycoproteins and their antigenic determinants. Biochemistry 27:405-413. [DOI] [PubMed] [Google Scholar]
- 13.Cross, G. A., L. E. Wirtz, and M. Navarro. 1998. Regulation of vsg expression site transcription and switching in Trypanosoma brucei. Mol. Biochem. Parasitol. 91:77-91. [DOI] [PubMed] [Google Scholar]
- 14.De Gee, A. L., G. Sonnenfeld, and J. M. Mansfield. 1985. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J. Immunol. 134:2723-2726. [PubMed] [Google Scholar]
- 15.Dempsey, W. L., and J. M. Mansfield. 1983. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J. Immunol. 130:405-411. [PubMed] [Google Scholar]
- 16.Dempsey, W. L., and J. M. Mansfield. 1983. Lymphocyte function in experimental African trypanosomiasis. VI. Parasite-specific immunosuppression. J. Immunol. 130:2896-2898. [PubMed] [Google Scholar]
- 17.Donelson, J. E. 2003. Antigenic variation and the African trypanosome genome. Acta Trop. 85:391-404. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers, B., J. Czichos, and P. Overath. 1987. RNA turnover in Trypanosoma brucei. Mol. Cell. Biol. 7:1242-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esser, K. M., and M. J. Schoenbechler. 1985. Expression of two variant surface glycoproteins on individual African trypanosomes during antigen switching. Science 229:190-193. [DOI] [PubMed] [Google Scholar]
- 20.Gunzl, A., T. Bruderer, G. Laufer, B. Schimanski, L. C. Tu, H. M. Chung, P. T. Lee, and M. G. Lee. 2003. RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot. Cell 2:542-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hertz, C. J., H. Filutowicz, and J. M. Mansfield. 1998. Resistance to the African trypanosomes is IFN-γ dependent. J. Immunol. 161:6775-6783. [PubMed] [Google Scholar]
- 22.Hertz, C. J., and J. M. Mansfield. 1999. IFN-γ-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell. Immunol. 192:24-32. [DOI] [PubMed] [Google Scholar]
- 23.Hirumi, H., and K. Hirumi. 1994. Axenic culture of African trypanosome bloodstream forms. Parasitol. Today 10:80-84. [DOI] [PubMed] [Google Scholar]
- 24.Kooter, J. M., and P. Borst. 1984. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 12:9457-9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanham, S. M., and D. G. Godfrey. 1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28:521-534. [DOI] [PubMed] [Google Scholar]
- 26.Levine, R. F., and J. M. Mansfield. 1984. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J. Immunol. 133:1564-1569. [PubMed] [Google Scholar]
- 27.Mansfield, J. M. 1994. T-cell responses to the trypanosome variant surface glycoprotein: a new paradigm? Parasitol. Today 10:267-270. [DOI] [PubMed] [Google Scholar]
- 28.Mansfield, J. M., R. F. Levine, W. L. Dempsey, S. R. Wellhausen, and C. T. Hansen. 1981. Lymphocyte function in experimental African trypanosomiasis. IV. Immunosuppression and suppressor cells in the athymic nu/nu mouse. Cell. Immunol. 63:210-215. [DOI] [PubMed] [Google Scholar]
- 29.Metcalf, P., M. Blum, D. Freymann, M. Turner, and D. C. Wiley. 1987. Two variant surface glycoproteins of Trypanosoma brucei of different sequence classes have similar 6 Å resolution X-ray structures. Nature 325:84-86. [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Jordan, J. L., K. P. Davies, and G. A. Cross. 1996. Stable expression of mosaic coats of variant surface glycoproteins in Trypanosoma brucei. Science 272:1795-1797. [DOI] [PubMed] [Google Scholar]
- 31.Reinitz, D. M., B. D. Aizenstein, and J. M. Mansfield. 1992. Variable and conserved structural elements of trypanosome variant surface glycoproteins. Mol. Biochem. Parasitol. 51:119-132. [DOI] [PubMed] [Google Scholar]
- 32.Reinitz, D. M., and J. M. Mansfield. 1988. Independent regulation of B cell responses to surface and subsurface epitopes of African trypanosome variable surface glycoproteins. J. Immunol. 141:620-626. [PubMed] [Google Scholar]
- 33.Reinitz, D. M., and J. M. Mansfield. 1990. T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect. Immun. 58:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuner, B., E. Vassella, B. Yutzy, and M. Boshart. 1997. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol. Biochem. Parasitol. 90:269-280. [DOI] [PubMed] [Google Scholar]
- 35.Schleifer, K. W., and J. M. Mansfield. 1993. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J. Immunol. 151:5492-5503. [PubMed] [Google Scholar]
- 36.Schopf, L. R., H. Filutowicz, X. J. Bi, and J. M. Mansfield. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect. Immun. 66:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schopf, L. R., and J. M. Mansfield. 1998. Characterization of a relatively rare class B, type 2 trypanosome variant surface glycoprotein gene. J. Parasitol. 84:284. [PubMed] [Google Scholar]
- 38.Seyfang, A., D. Mecke, and M. Duszenko. 1990. Degradation, recycling, and shedding of Trypanosoma brucei variant surface glycoprotein. J. Protozool. 37:546-552. [DOI] [PubMed] [Google Scholar]
- 39.Smith, C. J., R. F. Levine, and J. M. Mansfield. 1982. Cloning of African trypanosomes in mice immunosuppressed by cyclophosphamide treatment. Am. J. Trop. Med. Hyg. 31:1098-1102. [DOI] [PubMed] [Google Scholar]
- 40.Snapper, C. M., M. R. Kehry, B. E. Castle, and J. J. Mond. 1995. Multivalent, but not divalent, antigen receptor cross-linkers synergize with CD40 ligand for induction of Ig synthesis and class switching in normal murine B cells. A redefinition of the TI-2 vs T cell-dependent antigen dichotomy. J. Immunol. 154:1177-1187. [PubMed] [Google Scholar]
- 41.Snapper, C. M., and J. J. Mond. 1996. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J. Immunol. 157:2229-2233. [PubMed] [Google Scholar]
- 42.Snapper, C. M., F. R. Rosas, L. Jin, C. Wortham, M. R. Kehry, and J. J. Mond. 1995. Bacterial lipoproteins may substitute for cytokines in the humoral immune response to T cell-independent type II antigens. J. Immunol. 155:5582-5589. [PubMed] [Google Scholar]
- 43.Theodos, C. M., and J. M. Mansfield. 1990. Regulation of B cell responses to the variant surface glycoprotein molecule in trypanosomiasis. II. Down-regulation of idiotype expression is associated with the appearance of lymphocytes expressing antiidiotypic receptors. J. Immunol. 144:4022-4029. [PubMed] [Google Scholar]
- 44.Vassella, E., B. Reuner, B. Yutzy, and M. Boshart. 1997. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J. Cell Sci. 110:2661-2671. [DOI] [PubMed] [Google Scholar]
- 45.Wellhausen, S. R., and J. M. Mansfield. 1979. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J. Immunol. 122:818-824. [PubMed] [Google Scholar]
- 46.Wellhausen, S. R., and J. M. Mansfield. 1980. Lymphocyte function in experimental African trypanosomiasis. III. Loss of lymph node cell responsiveness. J. Immunol. 124:1183-1186. [PubMed] [Google Scholar]
- 47.Zinkernagel, R. M. 2000. What is missing in immunology to understand immunity? Nat. Immunol. 1:181-185. [DOI] [PubMed] [Google Scholar]