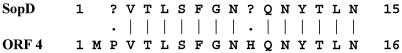Abstract
The ability of enteropathogenic salmonellae to recruit inflammatory cells and induce secretory responses in the infected ileum is considered to be a main feature in Salmonella-induced enteritis. Interactions between the pathogen and intestinal epithelial cells result in a variety of cellular responses mediating inflammation and fluid secretion. It is becoming apparent that proteins secreted by the Inv-Spa type III secretion system of Salmonella spp. play a key role in the induction of these responses. We have recently demonstrated that the SopB effector protein is translocated into eukaryotic cells via a Sip-dependent pathway and mediates inflammation and fluid secretion in infected ileal mucosa. However, SopB did not appear to be the only effector involved, as inactivation of the sopB gene only partially impaired enteropathogenicity. We suggested that at least some of such protein effectors are likely to be proteins of the same class as SopB, i.e., secreted effector proteins translocated into eukaroyotic cells via a Sip-dependent pathway. In this work, we identify SopD, another secreted protein belonging to the family of Sop effectors of Salmonella dublin. Using the cya reporter system we showed that SopD is translocated into eukaroyotic cells. We assessed the potential involvement of SopD in enteropathogenicity and found that inactivation of sopD has an additive effect in relation to the sopB mutation.
Infections with enteropathogenic salmonellae are a significant human and animal health problem worldwide. The pathology associated with enteric Salmonella infections is characterized by a large influx of polymorphonuclear leucocytes (PMN) into the intestinal mucosa and lumen (23, 24) and disruption of the normal movements of electrolytes and water, which results in a net fluid secretion into the lumen (19). Recent research has provided evidence that interactions between the pathogen and intestinal epithelial cells represent a crucial step in the induction of the host responses leading to the onset of enteritis (1, 4, 11–13).
The interaction between Salmonella spp. and epithelial cells is a complex process. This interaction involves many bacterial proteins and is largely dependent on the function of the Inv-Spa type III secretion system (for a review, see reference 3). We have recently shown that disruption of invH abolished Salmonella-induced secretory and inflammatory responses, indicating a key role for a functional Inv-Spa type III secretion system in enteropathogenicity (27). Type III secretion systems are highly conserved in a variety of gram-negative pathogenic bacteria (for a recent review, see reference 8). The main function of these systems is translocation of the effector proteins into the target eukaryotic cells. Four such effector proteins, SopB, SopE, AvrA, and SptP, have been identified in Salmonella to date (4, 6, 9, 28). The Sops are Salmonella secreted proteins, expression of which is significantly increased in Salmonella sip mutants (28). We have recently shown that the SopB protein has an important role in the induction of inflammatory response and fluid secretion in the infected ileum (4). Inactivation of the sopB gene resulted in a mutant strain which was substantially affected in its ability to induce the net fluid secretion and PMN influx into infected intestinal ligated loops. However, inactivation of sopB did not abolish enteropathogenesis entirely, suggesting that other effector proteins are also involved in the induction of enteritis (4). Here, we report the identification of the gene encoding another Sop effector protein of S. dublin, SopD. We present evidence that SopB and SopD act in a concerted manner to promote the inflammatory responses and fluid secretion in Salmonella-infected intestines.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Strains were grown in Luria-Bertani (LB) broth or on LB agar.
TABLE 1.
Bacterial strains and plamsids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | recA | BRL |
| S17.1 | λpir RP4-2 Tc::Mu-Km::Tn7 | 21 |
| S. dublin | ||
| 2229 | Wild-type, virulent field isolate of S. dublin | IAHa |
| B1 | sipB::pDM4, Cmlr | 29 |
| B2 | ΔsipB | This study |
| SB2 | ΔsopB | 4 |
| B2SD1 | ΔsipB sopD::pDM4, Cmlr | This study |
| SD1 | sopD::pDM4, Cmlr | This study |
| SB2SD1 | ΔsopB sopD::pDM4, Cmlr | This study |
| SpS | spaS::pDM4, Cmlr | This study |
| S. typhimurium 12/75 | Virulent field isolate | IAH |
| S. pullorum 449/87 | Virulent field isolate | IAH |
| S. choleraisuis A57 | Virulent field isolate | IAH |
| S. enteritidis 149 | Virulent field isolate | IAH |
| S. gallinarum 287/91 | Virulent field isolate | IAH |
| Shigella sonnei PB1 | Virulent field isolate | IAH |
| Plasmids | ||
| pDM4 | oriR6K, Cmlr | 15 |
| pBScya | Ampr, pBluescript-based cya gene fusion vector | K. Schesser |
| pDelsipB1 | pDM4 vector containing the 5′ and 3′ flanking regions of sipB | This study |
| pSS1 | pDM4 vector containing XbaI-EcoRV PCR-derived insert of spaS | This study |
| pMJ600 | sopD-cya fusion plasmid, Ampr | This study |
| pMW45 | sopD positive, isolated from a pBR322-based SauIIIA library of S. dublin | This study |
IAH, Institute for Animal Health.
DNA methods.
All recombinant DNA procedures were performed essentially as described by Sambrook et al. (20).
Protein purification.
Secreted proteins were isolated from insoluble protein filaments deposited by S. dublin FMB1 when grown at 37°C (28). The filaments were collected by pipette, washed in phosphate-buffered saline (PBS), and dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer as described previously (28). The proteins were separated by SDS-PAGE, blotted onto Immobilon membranes and stained with Coomasie blue. The SopD protein band was excised, and the protein was subjected to N-terminal sequence analysis.
Cloning of the sopD gene of S. dublin and construction of S. dublin mutants.
Based on the known nucleotide sequence of the ORF4 of S. typhimurium (16) we designed two oligonucleotide primers, SD1 (5′-ctc aaa gag gat cct cca atg cct tc-3′) and SD2 (5′-ttg gtt ata cag ctg act atc ttt atg t-3′), to generate a 1,225-bp DNA fragment containing an internal part of the gene. The corresponding DNA fragment was amplified by PCR with S. dublin 2229 chromosomal DNA as template. This DNA fragment was used as a probe to screen a plasmid library of S. dublin chromosomal DNA. Two positive clones were identified, one of which was denoted pMW45 and chosen for sequencing and transcomplementation experiments.
The sopD mutants of S. dublin were constructed as follows. An internal DNA fragment of sopD was amplified by PCR with the custom oligonucleotides SD3 (5′-tga tag taa aca gat ctt gat gag c-3′) and SD4 (5′-tta tgg gag tca ctt taa gct tcg gta a-3′) and was cloned into the suicide plasmid vector pDM4 (15). The resulting plasmid was conjugated from Escherichia coli S17.1 into S. dublin 2229, and Cmlr transconjugants were obtained. One of these clones was denoted S. dublin SD1 and was chosen for further experiments. The correct insertion of the suicide vector into the sopD gene was confirmed by PCR. The sopD mutation was then transfected by P22 transduction into the sopB mutant strain S. dublin SB2 to yield the mutant strain S. dublin SB2SD1.
The in-frame nonpolar deletion mutant of sipB, S. dublin B2, was constructed as follows: four oligonucleotides, SipB1 (5′-cag tcg tta aga tct aca aag tcg gt-3′), delB1 (5′-aat taa cat aat tat tcc ttt tct t-3′), delB2 (5′-att aat tat gtt aat tag taa tgt ggg-3′), and SipC1 (5′-cac tgg aag atc ttc cgc taa tat c-3′), designed from an S. dublin sequence (GenBank accession no. U66877), were used to generate a fusion DNA fragment covering the areas 5′ upstream and 3′ downstream of sipB with a deletion of the sipB gene. The two DNA fragments were amplified by PCR with S. dublin 2229 chromosomal DNA as a template with SipB1-delB1 and delB2-SipC1. These DNA fragments were then used as the template in a PCR with SipB1-SipC1. The resulting DNA fragment was cut with BamHI and BglII and cloned into the suicide plasmid pDM4 to yield pDelsipB1. pDelsipB1 was conjugated from E. coli S17.1 into S. dublin 2229, and Cmlr transconjugants were obtained. The suicide plasmid was then excised by a second recombination event as described by Milton et al. (15). The Cmls recombinants were obtained and screened for a mutated allele. Several clones carrying the deletion were identified. One of these was designated S. dublin B2 and was used in further experiments.
A double sipB-sopD mutant, S. dublin B2SD1, was constructed by P22 transduction of the chloramphenicol marker from S. dublin SD1 into S. dublin B2.
The spaS mutant of S. dublin was constructed as follows: an internal DNA fragment of spaS was amplified by PCR with SS1 (5′-tat gcg cac ggt tct aga tac ggt caa aac cc-3′) and SS2 (5′-cgc cag ttt gat atc gac gat ca-3′) primers, which were designed based on the known sequence of the S. typhimurium spaS gene (5) with chromosomal DNA of S. dublin 2229 as template. This fragment was digested with XbaI and EcoRV and cloned into the suicide vector pDM4. The resulting plasmid, pSS1, was conjugated into S. dublin 2229. The integration of the suicide plasmid into the S. dublin 2229 chromosome by a single recombination event resulted in an insertion mutant, denoted S. dublin SpS. S. dublin B1 (polar sipB mutant) and S. dublin SB2 (ΔsopB) have been described previously (4, 28).
Construction of sopD-cya gene fusion.
The plasmid pMJ600 containing sopD-cya was constructed by ligating the PCR fragment containing codons 1 to 202 and also 238 bp upstream of sopD in frame with the cya gene on plasmid pBScya (gift from Kurt Schesser, University of Umeå, Umeå, Sweden) so to form a C-terminal fusion of Cya onto SopD. The primers used for this were (forward) SD1 (5′-ctc aaa gag gat cct cca atg cct-3′) and (reverse) SD5 (5′-aat tga gtc ctg cag ttc gac aag cgg gtg-3′).
Measurement of translocation of SopD-Cya fusion protein in HeLa and J774 cells.
Bacterial cultures were grown overnight at 25°C with appropriate antibiotics. One hour before the experiment the cells were diluted 10-fold into fresh LB medium without antibiotics and incubated at 37°C to induce the expression of virulence factors. Bacteria were washed and resuspended in 1 ml of cell culture medium (Dulbecco’s minimal essential medium–10% fetal calf serum without antibiotics). A total of 100 μl of this bacterial suspension (multiplicity of infection [MOI], 20:1) was then added to monolayers of HeLa cells on 24-well plates. Cytochalasin D (final concentration, 1 μg/ml) was added 20 min prior to infection. After the appropriate incubation period the monolayer was washed with ice-cold PBS buffer, and then the cells were lysed with 100 μl of 0.1 M HCl with gentle agitation for 20 min. A total of 500 μl of 0.1 M NaOH–2% Na2CO3 was added to neutralize the pH (17). A total of 50 μl of the lysate was directly assayed with the Amersham Biotrak cyclic AMP (cAMP) assay kit (TRK 432) to determine the amount of cAMP present. Mixed infections were carried out with S. dublin strains in a 1:1 ratio, with a final MOI of 20:1. Control samples of uninfected cells were tested throughout the assay.
Protein determination.
Protein concentrations of the cAMP assay samples were determined with a bicinchoninic acid BCA protein assay kit according to the manufacturer’s instructions (Pierce).
Invasion of cultured eukaryotic cells.
The invasiveness of S. dublin strains for HeLa cells was determined by a gentamicin resistance assay as described by Wood et al. (28).
Bovine ligated ileal loop assays.
The experimental techniques used in the ligated loop assay have been described in detail elsewhere (25, 26). All experiments were performed within the mid-ilea of 28-day-old Friesian bull calves. The inocula were prepared by culturing bacteria overnight in LB broth at 25°C with shaking at 150 rpm. The cultures were diluted 1:100 in fresh LB broth and incubated at 37°C for 2 h. The bacterial cultures were adjusted to circa 109 CFU ml−1 by optical density, and 5 ml of culture was injected into each loop.
Approximately 50 ml of blood was removed from the calves approximately 1 h after injection of the loops. PMNs were isolated, labelled with 111In, and reinjected intravenously. The secretory response (volume of fluid within a loop/length of loop [ml cm−1]) and the influx of PMNs, as assessed by the magnitude of γ irradiation emitted from 111In-labelled PMNs within each loop, were recorded 12 h after injection of the loops. The PMN influx ratio was defined as the PMN influx in the test loops divided by the PMN influx in the negative-control loops. The PMN influx ratio in the negative-control loops was therefore equal to 1.00.
Nucleotide sequence accession number.
The sequence of sopD has been deposited into GenBank under accession no. AF030589.
RESULTS
Identification and mutagenesis of the gene encoding the SopD protein of S. dublin.
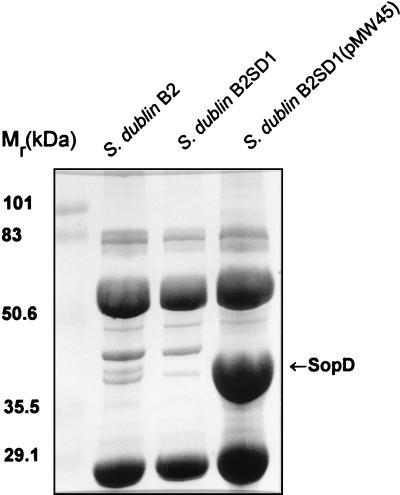
To further investigate Sops, we purified SopD by SDS-PAGE and subjected it to N-terminal amino acid sequencing. A sequence of 13 amino-terminal residues was obtained, and comparison of this with sequences in the available databases was carried out. This comparison revealed that the sequence was identical to that deduced from the DNA sequence beginning at codon 3 of ORF4 located downstream of the cysJIH operon in S. typhimurium (Fig. 1) (16). In addition, the molecular mass calculated for the deduced ORF4 peptide (36,068 Da) and the mass of the polypeptide, expressed in bacteria possessing plasmids carrying DNA fragments including ORF4 (∼38 kDa), were found to be in good agreement with the apparent molecular mass of S. dublin SopD (∼40 kDa) observed in our experiments (Fig. 2) (28). Taken together, these data suggest that orf4 described in reference 16 is sopD of Salmonella typhimurium. In order to further confirm this finding and identify sopD of S. dublin we designed two oligonucleotide primers, SD1 and SD2, based on the sequence of S. typhimurium orf4 and used them in a PCR to amplify a corresponding fragment of DNA with S. dublin chromosomal DNA as a template. The DNA fragment of expected size was obtained and used as a probe to screen a plasmid library of S. dublin chromosomal DNA. This screening yielded several positive clones, one of which was denoted pMW45 and was chosen for further experiments. The nucleotide sequence of the relevant part of the DNA fragment cloned into pMW45 was then obtained. This sequence included an open reading frame which was over 95% identical to ORF4 of S. typhimurium and the corresponding amino acid sequence included the N-terminal fragment identical to that obtained by the sequencing of SopD. This suggested that this open reading frame was the sopD gene of S. dublin. To investigate the expression of SopD we constructed a nonpolar in-frame sipB deletion mutant, S. dublin B2, and a double sipB-sopD mutant, S. dublin B2SD1. Comparative analysis of secreted proteins produced by S. dublin B2 and S. dublin B2SD1 (Fig. 2) revealed that the ∼40-kDa SopD protein was produced, secreted, and deposited in protein filaments by S. dublin B2 but was not expressed by the sopD mutant strain. No apparent effect of the mutation on the expression and secretion of other proteins was observed. The transcomplementation of the mutation by introduction of the pMW45 plasmid into S. dublin B2SD1 resulted in the production and secretion of SopD in elevated amounts (Fig. 2).
FIG. 1.
Sequence alignment of the N-terminal amino acid sequences of S. dublin SopD and S. typhimurium ORF4 (a hypothetical 36.1-kDa protein encoded by a gene located downstream of the cysJIH operon [16]). The SopD protein was isolated from filaments produced by S. dublin (28) and was purified by SDS-PAGE. The N-terminal amino acid sequence was determined by Edman degradation.
FIG. 2.
SDS-PAGE analysis of the proteins secreted by different S. dublin strains. The secreted proteins deposited into filaments were isolated as described earlier (28). The samples were separated by SDS–12% PAGE and stained with Coomassie blue.
To monitor the importance of SopD and its possible conjunction with SopB in Salmonella virulence, a sopD mutant of S. dublin, S. dublin SD1, and a sopB-sopD double mutant, S. dublin SB2SD1, were constructed. S. dublin SB2SD1 contains a deletion of sopB and an insertion mutation in sopD. These mutations did not cause any apparent effect on the growth characteristics of Salmonella and did not affect secretion of other proteins (data not shown).
Distribution of sopD in salmonellae.
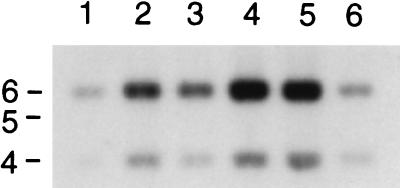
To investigate the distribution of sopD in different Salmonella serotypes, we performed a Southern hybridization experiment on the HpaI restriction digests of chromosomal DNA isolated from a variety of Salmonella serotypes by using a sopD-specific DNA probe. The result of this experiment clearly indicated that the sopD-specific sequence is present in all Salmonella tested, including ubiquitous, enteropathogenic, and host-specific serotypes (Fig. 3).
FIG. 3.
Southern blot analysis showing conservation of sopD in different Salmonella serotypes. Total cell DNA was isolated from S. choleraisuis A57 (lane 1), S. dublin 2229 (lane 2), S. enteritidis 149 (lane 3), S. gallinarum 287/91 (lane 4), S. pullorum 449/87 (lane 5), and S. typhimurium 12/75 (lane 6) and digested with HpaI. A DNA fragment including the full-length sopD was amplified by PCR, labelled, and used as a probe. The positions of size markers (in kilobases) are indicated on the left. The sopD gene of S. dublin is known to contain one HpaI site.
The S. dublin SopD protein does not appear to be required for entry into host cells.
To investigate the possible involvement of SopD in cell invasion, we assessed the sopD mutant strain in a HeLa cell invasion assay. The sopD mutant strain of S. dublin SD1 was as invasive as the wild-type S. dublin 2229 strain (data not shown). Thus, SopD does not appear to be involved in the invasiveness of Salmonella.
The SopD protein is translocated into the target cell via a Sip-dependent pathway.
To assess the potential site of action of SopD during the infection process, we constructed hybrid plasmid pMJ600 in which a DNA fragment encoding the sopD promoter area and the first 202 codons of SopD is followed by the cya gene. Similar constructs have been used to monitor the localization of virulence-associated proteins from Yersinia spp. and E. coli (10, 22). pMJ600 was introduced into the wild type, S. dublin 2229, and into sipB mutant S. dublin B1 to yield S. dublin 2229(pMJ600) and S. dublin B1(pMJ600), respectively. Both strains were found to produce and secrete a fusion protein of the expected size in vitro (data not shown).
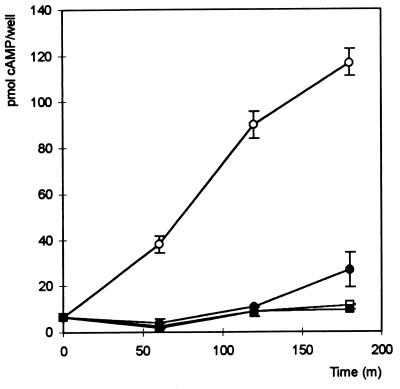
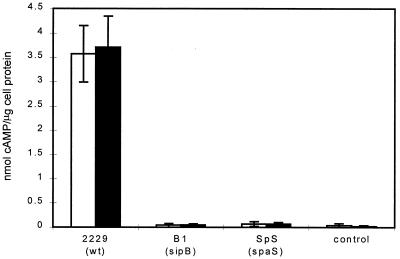
Overnight cultures of different S. dublin strains incubated at 25°C were diluted 10-fold in LB medium, grown for 1 h at 37°C, and used to infect cultured HeLa cells in the presence or absence of cytochalasin D. At appropriate time points after the onset of infection, HeLa cells were analyzed for the presence of adenylate cyclase activity by monitoring the intracellular concentration of cAMP. No significant increase in the level of intracellular cAMP over a period of 4 h was detected when the S. dublin 2229 wild type or the S. dublin B1 sipB mutant was used to infect HeLa cells (Table 2), indicating that infection with either of these strains did not affect intracellular cAMP levels due to endogenous adenylate cyclase activities. The infection of HeLa cells with S. dublin 2229(pMJ600) resulted in a steady increase of the intracellular levels of cAMP (Fig. 4). An increase in the intracellular cAMP level was also observed in the presence of cytochalasin D (Fig. 4), but it was with a reduced magnitude and was delayed compared to that seen in the absence of cytochalasin D. In contrast, no increase in the intracellular cAMP level was observed when the sipB mutant S. dublin B1(pMJ600), was used to infect HeLa cells. Together these data suggest that the SopD-Cya fusion protein was translocated into the HeLa cells via a Sip-dependent mechanism.
TABLE 2.
Accumulation of cAMP, after 2 and 4 h, as an indication of sopD translocation by different S. dublin strainsa
| Strain | cAMP produced (pmol) per well at:
|
|
|---|---|---|
| 2 h | 4 h | |
| S. dublin 2229 | 0.2 ± 0.08 | 0.50 ± 0.14 |
| S. dublin 2229(pMJ600) | 3.16 ± 1.16 | 3.5 ± 1.3 |
| S. dublin B1 | 0.26 ± 0.23 | 0.45 ± 0.18 |
| S. dublin B1(pMJ600) | 0.53 ± 0.09 | 0.5 ± 0.14 |
| No infection | 0.55 ± 0.32 | <0.1 |
HeLa cells were infected with S. dublin 2229, 2229(pMJ600), B1, and B1(pMJ600). A control of uninfected cells was used. The cAMP values (in picomoles ± standard deviations) are averages from three samples wells.
FIG. 4.
Accumulation of cAMP in HeLa cells over a period of time (in minutes) as a measure of the translocation of the SopD-Cya fusion protein mediated by S. dublin 2229 (squares) and S. dublin 2229(pMJ600) (circles). Filled symbols represent data from HeLa cells incubated in the presence of cytochalasin D (1 μg/ml). Each point is the mean result of samples from three wells. Error bars indicate standard deviations.
To ensure that the inability of S. dublin B1 to enter eukaryotic cells is not the major factor in stopping SopD-Cya translocation, a parallel study was carried out using the macrophage cell line J774.2. The strains S. dublin 2229, S. dublin B1, and S. dublin SpS, harboring, pMJ600, were used to infect the macrophage cell line J774.2. S. dublin 2229(pMJ600) and S. dublin B1(pMJ600) secreted the fusion protein in vitro, whereas S. dublin SpS(pMJ600) produced the protein but did not secrete it (data not shown). The three strains were all taken up in comparable levels by the macrophages, as determined by a gentamicin protection assay (data not shown). Only the wild-type strain, S. dublin 2229(pMJ600), caused an increase in intracellular cAMP production, indicating the importance of a fully functional translocation system (Fig. 5). To investigate this further, we carried out a mixed infection of HeLa cells, with both S. dublin 2229 (wild type) and S. dublin B1(pMJ600). In contrast to S. dublin 2229(pMJ600), a mixed infection with S. dublin 2229 and S. dublin B1(pMJ600) did not result in an increase of cAMP in the infected cells (data not shown), indicating that macropinocytosis induced by the wild-type Salmonella does not contribute to the translocation of SopD-Cya. Taken together our data suggest that functional Inv-Spa and Sip-dependent mechanisms are required for the secretion and translocation of the SopD protein fusion from bacteria into host cells. However, it appears that invasion or close prokaryotic-eukaryotic cell association might be required for efficient protein translocation.
FIG. 5.
Accumulation of cAMP within J774.2 cells in relation to total cell protein at 1 h (open bars) and 2 h (solid bars) after infection with different S. dublin strains harboring plasmid pMJ600. Each bar represents the mean result of samples from four wells. Error bars indicate standard deviations. cAMP values are given as nanomoles per microgram of protein.
Concerted effect of mutations in sopB and sopD on the ability of S. dublin to induce fluid secretion and inflammatory responses in the infected ileum.
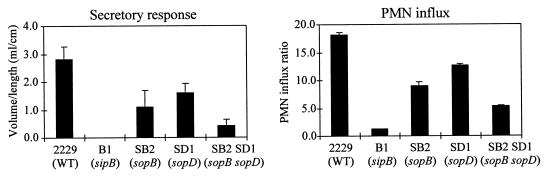
We have previously shown that one of the Sop effectors of Salmonella, the SopB protein, has an important role in the induction of enteritis (4). Our data have also suggested that some other secreted and/or translocated effector molecules could be involved in the enteropathogenesis of Salmonella, as a mutation in invH and sipB affected enteropathogenesis to a greater extent than did mutations in sopB (4, 27). To investigate this possibility further, we constructed a double sopB-sopD mutant, S. dublin SB2SD1. This mutant strain was assessed in a HeLa cell invasion assay, and these two mutations did not have a major effect on the invasiveness of S. dublin (data not shown). To study the possible role of SopD in Salmonella-induced enteritis, we assessed the ability of the different S. dublin strains to induce fluid secretion and PMN influx in ligated ileal loops in two calves. Compared with the wild-type strain, the sopD mutant S. dublin SD1 induced less fluid secretion and PMN influx (Fig. 6). In good agreement with our previously published data, sopB mutant strain S. dublin SB2 also induced lower secretory and inflammatory responses than the wild-type strain. However, introducing both sopB and sopD mutations into S. dublin 2229 resulted in additional attenuation as the double mutant S. dublin SB2SD1 induced even lower secretory and inflammatory responses than either of the single mutants (Fig. 6). This finding indicates that SopD has a role in the induction of enteritis and that SopB and SopD act in concert to promote fluid secretion and inflammatory responses in the infected ileum.
FIG. 6.
Effects of sopD, sopB, and double sopD-sopB mutations on the ability of S. dublin to induce fluid secretion and PMN influx. S. dublin 2229 (wild type), SB2 (sopB), SD1 (sopD), and SB2SD1 (sopB sopD) were used in ligated loop assays. The secretory response is defined as the volume of fluid within a loop per length of loop. The PMN influx is defined as radioactive counts of PMNs within test loops per radioactive count of PMNs in negative-control loops. Values are the means of three loops and are representative of results from three independent experiments. Error bars indicate standard deviations.
DISCUSSION
The influx of PMNs from the peripheral blood into infected intestines and lumen is a characteristic feature of infections by enteropathogenic salmonellae. This recruitment of PMNs is mediated by the responses of intestinal epithelial cells upon interaction with bacteria and is dependent on the production and release of chemoattractant mediators. Such epithelial responses appear to be triggered by specific contact-dependent bacterial factors reliant on the function of the Inv-Spa secretion system (1, 4, 11–13). We have recently characterized one such protein effector of S. dublin, the secreted protein SopB (4). SopB belongs to the group of five predominant secreted Sop proteins, the expression of which is enhanced in sip mutants (28). The results of studies of SopE and SopB indicated that Sops are important factors of Salmonella virulence (4, 7, 28).
As part of our ongoing effort to characterize the Sops further, we cloned and sequenced the sopD gene of S. dublin. No obvious homologies to the sopD gene product sequence were found among the GenBank-EMBL entries, which makes it difficult to suggest a possible function of SopD. A sequence of 13 N-terminal amino acids of purified SopD protein starting at residue 2 was found to be identical to that deduced from the DNA sequence beginning at codon 3 of sopD, indicating that only the initiator methionine is removed by processing to the mature secreted SopD, which is a rather common modification in Salmonella (14). Thus, SopD is secreted without cleavage of an N-terminal signal peptide, similar to other proteins secreted by type III secretion systems. This further suggested that SopD is one of the specific effectors of Salmonella virulence. Since the other Salmonella effector proteins, SopB, SopE, SptP, and AvrA, are found to be translocated into eukaryotic cells (2, 4, 6, 28), we assessed SopD translocation. The results of these experiments showed that SopD is translocated into eukaryotic cells via a Sip-dependent pathway and that the N terminus of the SopD protein is sufficient to direct its translocation into the eukaryotic cells. These results further support our suggestion that SopD is an effector protein.
Clearly cytochalasin D influenced the intracellular delivery of SopD. This may be a consequence of altered initial interactions between Salmonella and the target cell membrane. Alternatively, internalized bacteria may express and/or translocate Sops more efficiently than extracellular bacteria.
We assessed the potential involvement of SopD in enteropathogenicity and found that the inactivation of sopD is additive to the effects of the sopB mutation. Thus, SopB and SopD are secreted effectors which appear to act in concert to mediate Salmonella enteropathogenicity. The phenomenon of coordinated action of type III secreted bacterial effector proteins was first discovered for Yop effectors of Yersinia (18). The results of subsequent studies, including this one, clearly indicate that the pathogenic responses elicited by different bacterial pathogens in eukaryotic host cells result from the additive contribution of multiple effector proteins delivered inside host cells. It is not clear at present if other secreted effector proteins are also involved in Salmonella-induced induction of fluid secretion and intestinal inflammation. The biochemical functions of known effectors of enteropathogenicity, such as SopB, SopD, and the recently discovered Pip proteins (29), remain obscure. Our further research will concentrate on studies of these matters.
ACKNOWLEDGMENTS
We thank Kurt Schesser (University of Umeå, Umeå, Sweden) for supplying plasmid pBScya. We acknowledge Sam Hedges and Charlotte Rolph for technical support, Pat Barker for protein sequencing, and Sue Paulin for performing surgical procedures.
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Swindon, United Kingdom, including BBSRC ASD grant 201/A07439, and the Ministry of Agriculture, Fisheries and Food, London, United Kingdom.
REFERENCES
- 1.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu Y, Galán J E. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 4.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 5.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardt W-D, Galán J E. A secreted Salmonella protein with homology to a virulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardt W-D, Chen L-M, Schuebel K E, Bustelo X R, Galán J E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 8.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaniga K, Uralil J, Bliska J B, Galán J E. A secreted tyrosine phosphatase with modular effector domains encoded by the bacterial pathogen Salmonella typhimurium. Mol Microbiol. 1996;21:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 10.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolf C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithilial cells. EMBO J. 1998;17:2166–2167. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick B A, Colgan S P, Delp-Archer C, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick B A, Hofman P M, Kim J, Carnes D, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick B A, Miller S I, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995b;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C G, Strauch K L, Kukral A M, Miller J L, Wingfield P T, Mazzei G J, Werlen R C, Graber P, Movva N R. N-terminal methionine-specific peptidase in Salmonella typhimurium. Proc Natl Acad Sci USA. 1987;84:2718–2722. doi: 10.1073/pnas.84.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milton D L, O’Toole R, Hörstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski J, Wu J Y, Rueger D C, Miller B E, Siegel L M, Kredich N M. Characterization of the cysJIH regions of Salmonella typhimurium and Escherichia coli B. J Biol Chem. 1989;264:15726–15737. [PubMed] [Google Scholar]
- 17.Rozengurt E, Legg A, Strang G, Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci USA. 1981;78:4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 19.Rout W R, Formal S B, Dammin G J, Giannella R A. Pathophysiology of Salmonella diarrhea in the Rhesus monkey: intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974;67:59–70. [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Simon R, Preifer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 22.Sory M-P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull P C B, Richmond J E. A model of Salmonella enteritis: the behavior of Salmonella enteritidis in chick intestine studied by light and electron microscopy. Br J Exp Pathol. 1978;59:64–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Wallis T S, Hawker R J H, Candy D C A, Qi G-M, Clarke G J, Worton K J, Osborne M P, Stephen J. Quantification of the leukocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. J Med Microbiol. 1989;30:149–156. doi: 10.1099/00222615-30-2-149. [DOI] [PubMed] [Google Scholar]
- 25.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson P R, Paulin S M, Bland A P, Jones P W, Wallis T S. Characterization of intestinal invasion by Salmonella typhimurium and Salmonella dublin and effect of a mutation in the invH gene. Infect Immun. 1995;63:2743–2754. doi: 10.1128/iai.63.7.2743-2754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson P R, Galyov E E, Paulin S M, Jones P W, Wallis T S. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun. 1998;66:1432–1438. doi: 10.1128/iai.66.4.1432-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 29.Wood M W, Jones M A, Watson P R, Hedges S, Wallis T S, Galyov E E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29:883–892. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]