Abstract
Pneumocystis carinii pneumonia (PcP) is a clinically important infection of immunocompromised patients. Although the interaction of Pneumocystis with the alveolar epithelium has been well documented, very little information regarding the epithelial response to Pneumocystis is currently available. In order to study Pneumocystis-epithelium interactions, a murine cell line derived specifically from an alveolar epithelial cell (AEC) was utilized. The coculture of murine AECs with mouse Pneumocystis induced a dose- and time-dependent release of the CXC chemokine MIP-2. Importantly, the specific removal of Pneumocystis from the preparation, or the pretreatment of AECs with sulfasalazine, a potent and specific inhibitor of NF-κB, nearly completely abrogated the chemokine response to Pneumocystis. Since the murine MIP-2 promoter contains consensus κB binding sequences, the ability of Pneumocystis to stimulate NF-κB signaling in AECs was examined. Pneumocystis stimulation of an AEC line stably transfected with a κB-dependent reporter construct triggered the NF-κB signaling pathway and reporter production. These data were confirmed in gel shift assays, providing direct evidence that Pneumocystis induced the nuclear translocation of the p50/p65 heterodimeric form of NF-κB. Maximal NF-κB activation was dependent upon direct contact with viable Pneumocystis organisms. These data demonstrate that Pneumocystis activates NF-κB signaling in AECs and establish a reporter cell line for studying NF-κB activation in AECs. Given the global regulatory functions of the NF-κB family, these findings suggest that Pneumocystis directly alters AEC gene expression in a manner that promotes pulmonary immune and inflammatory responses.
Pneumocystis carinii is an opportunistic fungal pathogen that causes life-threatening pneumonia in patients suffering from various congenital or acquired immunodeficiencies. These include patients with certain genetic diseases, malignancies requiring chemo- or radiation therapy, and AIDS (10, 54, 56, 58). Despite improved treatments for human immunodeficiency virus infection and improved prophylaxis for P. carinii infection, PcP remains one of the most common AIDS-defining illnesses and is directly responsible for significant morbidity and mortality (12, 16, 17, 58, 62). Although significant progress has been made in our understanding of PcP, the exact mechanisms of lung injury are still largely undetermined. Because recent evidence has demonstrated that lung injury during PcP is not only a consequence of the direct effects of the organism but also a function of the host's response to infection (22, 23, 47, 48, 63, 67), the identification of signals that promote inflammation and contribute to injury is critical to understanding and treating this disease. Early studies demonstrated that the interaction of P. carinii with the alveolar epithelium is one of the initial events in the infectious process (28, 35, 38, 68). However, very little knowledge exists concerning how the pulmonary epithelium responds to P. carinii infection or what signaling pathways might be involved. An increasing body of evidence supports an immunomodulatory function for alveolar epithelial cells (AECs) (7, 15, 49, 52, 55, 61) and suggests that the interaction of P. carinii with alveolar epithelium could induce signals that help initiate and target the inflammatory response. Importantly, when P. carinii-infected severe combined immunodeficient (SCID) mice are immunologically reconstituted, inflammatory cells migrate specifically to infected regions of the alveolar epithelium while uninfected regions are not involved (64, 65). This finding suggests that the P. carinii-epithelium interactions occurring at sites of infection produce signals that direct the inflammatory response only to infected alveoli. In addition, it has been reported that a human pulmonary epithelial cell line (A549) secretes the proinflammatory cytokines interleukin-6 (IL-6), IL-8, and MCP-1 following stimulation with rat P. carinii (2, 44) and that P. carinii β-glucan stimulates MIP-2 secretion from primary cultures of rat AECs through a lactosylceramide-mediated mechanism (21). Together, these findings suggest that P. carinii stimulates changes in AEC gene expression that may function to initiate immunity in immunocompetent hosts and/or contribute to injury in susceptible hosts.
The NF-κB family of transcriptional regulators is involved in the rapid regulation of many immune- and inflammation-associated genes, including cytokines, chemokines, and adhesion molecules (5, 18, 31). NF-κB consists of at least five distinct subunits (p105/p50, RelA, p100/p52, RelB, and c-Rel), and various homo- and heterodimeric combinations of these subunits have DNA binding and transcriptional regulatory functions. NF-κB exists as a preformed complex with its inhibitor, IκB, in the cytoplasm of many cell types, and a variety of stimuli can induce the release of NF-κB from its inhibitor and the translocation of functional NF-κB to the nucleus for gene regulation. Although κB binding activity was originally identified in lymphocytes, NF-κB-like activity has been reported in many cell types. Stimulation of alveolar macrophages with whole P. carinii or purified cell wall β-glucan induces the nuclear translocation of NF-κB and may be an important signal transduction pathway regulating the macrophage response to P. carinii (29, 69). NF-κB is also an important mediator of signal transduction in epithelial cells, and cytokines, bacterial products, and toxicants have been reported to induce NF-κB activation in pulmonary epithelial cells (6, 20, 46, 51). Importantly, the promoters of all the AEC genes reportedly regulated by P. carinii stimulation contain consensus κB binding sites, suggesting that P. carinii could regulate AEC gene expression through this global transcription factor.
In this study, we hypothesized that the interaction of P. carinii with AECs induces the expression of proinflammatory genes that promote the generation and targeting of the immune response through a pathway that involves the activation of NF-κB signaling. Therefore, to test this hypothesis we developed an in vitro coculture system of mouse P. carinii and a murine AEC line. In addition, an AEC line that is stably transfected with a κB-dependent reporter construct was generated and used in conjunction with gel shift assays to directly test the ability of P. carinii to stimulate NF-κB activation in AECs.
MATERIALS AND METHODS
Cell culture.
The murine AEC line MLE-15 was derived from a pulmonary adenocarcinoma generated in a transgenic mouse expressing the simian virus 40 large T antigen under transcriptional control of the human surfactant protein C (SPC) promoter (24, 60). MLE-15 cells maintain morphological and gene expression characteristics of type II AECs, including a typical polygonal morphology, the appearance of multilamellar inclusion bodies, the expression of SPC mRNA, and production of SPC proprotein (24, 60). However, it has also been demonstrated that these cells express aquaporin-5, suggesting that they may also have some type I cell characteristics (3). MLE-15 cells were maintained at 37°C, 6% CO2 in Dulbecco's modified Eagle's medium nutrient mixture F-12 Ham (DMEM-F12; Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) and 1% penicillin and streptomycin. Cells were grown to confluency, washed with Hank's balanced salt solution, and then incubated for 8 h in DMEM-F12 lacking FBS prior to experimental treatments. Confluent monolayers of AECs were treated with freshly isolated murine P. carinii at cyst-to-AEC ratios of 0, 0.1, 0.2, 0.5, 1.0, 2.0, 3.0, and 4.0. In some experiments, cells were pretreated with 2 mM sulfasalazine (SSA) for 2 h prior to stimulation.
The MLE-pLUC/κB cell line was generated by transfecting the parent MLE-15 cell line with a reporter construct that consisted of the luciferase gene under the transcriptional control of the region −133 to +44 of the human IL-8 promoter (39, 40). A stable line was generated and cultured in DMEM-F12 medium supplemented with 10% FBS, 1% penicillin and streptomycin, and 1% geneticin (GIBCO, NY) to promote retention of the reporter construct.
Isolation and enumeration of mouse P. carinii organisms.
P. carinii-infected CB.17 scid/scid mice were treated with dexamethasone (4 mg/liter) and tetracycline (500 mg/liter) in the drinking water to increase the burden of organisms to approximately 108 P. carinii per mouse. All glassware and utensils used for the isolation procedure were thoroughly cleaned with detergent and baked at high temperature to prevent endotoxin contamination of the isolated P. carinii organisms. In addition, all test tubes, plasticware, buffers, and media used were certified pyrogen free. Briefly, the mouse was sacrificed and the pulmonary vasculature was perfused with 5 ml of sterile 0.9% NaCl (saline). The lungs were removed aseptically and homogenized with a sterile Tenbroeck tissue grinder in 10 ml of isolation buffer (1× Hank's balanced salt solution, 0.5% glutathione, 20 mM HEPES buffer, 1% penicillin and streptomycin; pH 7.2). The cell suspension was passed sequentially through 22- and 26-gauge needles and then centrifuged at 52 × g for 3 min. This force was sufficient to pellet larger tissue pieces while retaining P. carinii organisms in suspension. The supernatant was removed and centrifuged for 20 min at 1,600 × g. The pellet was resuspended in 2 ml of sterile, nonpyrogenic H2O for exactly 35 s to lyse erythrocytes, followed immediately by the addition of 2 ml of 2× phosphate-buffered saline (PBS) and 5.5 ml of isolation buffer. DNase was added to a final concentration of 10 μg/ml, and the mixture was incubated for 30 min at 37°C and then centrifuged for 5 min at 500 rpm. The supernatant was pushed through a 20-μm filter and centrifuged at 1,600 × g for 20 min, and the resulting P. carinii pellet was resuspended in serum-free medium. The volume of the P. carinii suspension was increased to 4 ml of serum-free medium per mouse and then plated on tissue culture dishes coated with anti-Ly-6G/6C (clone RB6-8C5), anti-CD16/CD32 (clone 2.4G2), and anti-CD45 (clone 30-F11) antibodies to remove any remaining hematopoietic cells, including macrophages, polymorphonuclear lymphocytes, NK cells, and dendritic cells. The plates were incubated at 37°C for 1.5 h, and the nonadherent P. carinii cells were collected and centrifuged at 1,600 × g for 20 min. The pellet was resuspended in ∼1 to 2 ml of serum-free medium per mouse and then aspirated through a 26-gauge needle three times. The final prep was then stained with ammoniacal silver to enumerate cysts and Diff-Quick to ensure no bacterial contamination was present. In addition, the preps were routinely plated on commercially available chocolate blood agar plates to test for the presence of other microorganisms and tested for endotoxin contamination using the limulus lysate assay. This procedure results in a mixed population of P. carinii forms consisting of approximately 10% cysts and 90% trophozoites.
Antibody-mediated depletion of P. carinii.
BioMag goat anti-mouse immunoglobulin G (IgG)-coated beads (1 mg/ml; QIAGEN) were prepared by washing three times in serum-free DMEM-F12 medium. The washed beads were resuspended in a final volume of 1 ml of serum-free medium and stored on ice until needed. Preliminary experiments indicated that 5 mg of beads was sufficient to deplete at least 5 × 107 P. carinii organisms. The P. carinii preparation was incubated with 1.4 ml of a pool of seven mouse anti-P. carinii IgG monoclonal antibodies (MAbs) in 10 ml of serum-free medium for 30 min at room temperature. Each of these MAbs has been demonstrated to bind to the surface of P. carinii by immunofluorescence assay (data not shown). The MAb-coated P. carinii organisms were then pelleted at 1,600 × g for 20 min at 4°C. The organisms were gently resuspended in 11 ml of serum-free medium, and 1 ml of the washed magnetic bead suspension was added. The P. carinii-bead mixture was incubated at 4°C for 30 min in a tumbler and then placed against a magnet for 20 min. The supernatant (depleted of P. carinii) was transferred to a fresh tube, pelleted at 1,600 × g for 20 min at 4°C, and then suspended in the original volume of the P. carinii suspension used. To assess the extent of P. carinii depletion achieved by this protocol, ammoniacal silver stain was used to enumerate cysts before and after depletion and real-time PCR was used to assess the number of copies of P. carinii DNA. For real-time PCR quantification of P. carinii, samples were boiled for 15 min, vigorously vortexed for 2 to 3 min, and then centrifuged for 5 min at 12,000 × g. The supernatant was carefully removed and stored at −80°C for real-time PCR analysis. Boiled samples were assayed by quantitative PCR using TaqMan primer/fluorogenic probe chemistry and an Applied Biosystems Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) as previously described (67). A primer/probe set specific for a 96-nucleotide region of the mouse P. carinii kexin gene was designed using the Primer Express software (Applied Biosystems) (30). The sequences of the primers and probe used were as follows: forward primer, 5′-GCACGCATTTATACTACGGATGTT-3′; reverse primer, 5′-GAGCTATAACGCCTGCTGCAA-3′; fluorogenic probe, 5′-CAGCACTGTACATTCTGGATCTTCTGCTTCC-3′. Quantitation was determined by extrapolation against standard curves constructed from serial dilutions of known copy numbers of plasmid DNA containing the target kexin sequence. Data were analyzed using the ABI Prism 7000 SDS version 1.0 software (Applied Biosystems) and are reported as total kexin DNA copies per sample.
Transient transfections.
MLE-15 cells were transfected using Lipofectamine (Invitrogen) according to the manufacturer's recommendations. The plasmid encoding the dominant negative mutated form of IκBα (pCMV4HA-IkBα) was a gift from S. Sun (19). Briefly, cells were plated in 12-well plates at a concentration of 3.0 × 105 cells per well in regular growth medium to ensure that cells were 90 to 95% confluent at the time of transfection. After 24 h, the medium was replaced with 1 ml of prewarmed Opti-MEMI (Invitrogen). The Lipofectamine complex was prepared by diluting 3.2 μl of Lipofectamine in 100 μl of Opti-MEMI and incubating for 5 min at room temperature. At the same time, 1.6 μg of DNA was diluted in 100 μl of OPTI-MEMI and also incubated for 5 min at room temperature. Following incubation, the diluted DNA was mixed with the diluted Lipofectamine (DNA/Lipofectamine ratio, 1:2), mixed gently, and incubated for 20 min at room temperature to allow the DNA-Lipofectamine complexes to form. After incubation, the DNA-Lipofectamine complexes were added to the cells and mixed gently by rocking the plate back and forth. The cells were incubated at 37°C, 6% CO2 for 6 h, and then the medium was replaced with regular growth medium. Eighteen hours later, the cells were stimulated as described. In separate experiments, transfection of MLE-15 cells with a green fluorescent protein (GFP)-expressing construct demonstrated a transfection efficiency of >75% for these cells.
MIP-2 ELISA.
Culture supernatants from stimulated MLE-15 cells were collected, centrifuged at 12,000 × g for 5 min to remove debris, and then stored at −80°C. MIP-2 concentrations were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Luciferase assays.
Cells were assayed for luciferase activity using the commercially available luciferase assay system from Promega (Madison, WI). Briefly, MLE-pLUC/κB cells were grown to confluency in 24-well tissue culture dishes and then stimulated for 6 h with the indicated amounts of freshly isolated P. carinii or with P. carinii-depleted preparations. The cells were then washed twice with 1 ml of PBS and dissolved in 60 μl of passive lysis buffer. The lysed cells were centrifuged at 12,000 × g for 15 seconds at room temperature, and 20 μl of the cell lysate supernatant was mixed with 100 μl of the luciferase assay substrate in a luminometer tube. The light intensity was determined with a luminometer. Data were expressed as fold increase over nonstimulated cells transfected with the same construct.
EMSAs.
Nuclear extracts were prepared from stimulated and unstimulated MLE-15 cells as described previously (50). Briefly, cells were washed in ice-cold PBS, resuspended in 50 μl of ice-cold lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.4% NP-40, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 1× protease/phosphatase inhibitor cocktail [Sigma]), and then placed on ice for 2 min. Lysates were centrifuged at 20,000 × g for 1 min at 4°C, and the supernatant with cytoplasmic proteins was removed. The pellets were resuspended in 40 μl of cold hypertonic buffer (20 mM HEPES [pH 7.9], 0.4 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1× protease/phosphatase inhibitor cocktail [Sigma]), incubated for 10 min at 4°C shaking, and then centrifuged for 1 min. Nuclear protein-containing supernatants were removed, quantified with the Bio-Rad protein assay kit (Bio-Rad, CA), and then stored at −80°C. For electrophoretic mobility shift assays (EMSAs), 5 μg of nuclear extract was mixed with a radiolabeled, double-stranded oligonucleotide probe encoding a consensus κB binding site (5′-CAACGGCAGGGGAATTCCCCTCTCCTT-3′). The reaction was carried out in EMSA reaction buffer (12 mM HEPES [pH 7.9], 100 mM NaCl, 0.25 mM EDTA, 1 mM DTT, and 1 mM PMSF) at room temperature for 10 min, followed by resolution of the protein-DNA complexes on nondenaturing 6% polyacrylamide gels. The gels were dried and placed against X-AR film (Kodak, Rochester, NY) to visualize bound proteins. For quantitation, the dried gels were used to expose PhosphorImager screens, and the intensity of each band was measured using a computer-linked PhosphorImager and the ImageQuant software. For antibody supershift assays, 1 μg of antiserum recognizing each of the NF-κB subunits (Santa Cruz Biotechnology Inc., Santa Cruz, CA) was added to the EMSA reaction mixture 10 min prior to electrophoresis. Specificities and reactivities of the antibodies were confirmed as described previously (36, 37).
RNA isolation and RPAs.
MLE-15 cells were grown to confluency in 12-well plates and then stimulated for the indicated times. Total RNA was isolated from the cells using TRIzol reagent according to the manufacturer's instructions (Life Technologies Inc., Grand Island, NY). Each final RNA pellet was resuspended in 20 μl of diethylpyrocarbonate-treated water. The RNA concentration and purity were quantified with the GeneQuant RNA/DNA calculator (Pharmacia Biotech, Piscataway, N.J.). Quantitation of steady-state cytokine mRNA levels was performed by a previously described multicytokine RNase protection assay (RPA) (64). The mCK-5 template set (PharMingen) was used to transcribe radiolabeled, antisense riboprobes for murine lymphotactin, RANTES, eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, MCP-1, TCA-3, the murine ribosomal protein L32, and glyceraldehyde-3-phosphate dehydrogenase as previously described (65). In addition, a custom template set was used to transcribe radiolabeled, antisense riboprobes for IκBα and murine L32. The protected RNA duplexes were purified by phenol-chloroform extraction and ethanol precipitation, and the pellets were resuspended in 5-μl portions of RPA loading buffer (80% formamide, 0.5× Tris-borate-EDTA, 0.05% bromphenol blue [Sigma]). The protected, radiolabeled RNA fragments were electrophoresed on a 5% acrylamide-8 M urea sequencing gel, and the dried gel was used to expose X-AR film (Kodak, Rochester, NY). For quantitation, the dried gels were placed against PhosphorImager screens (Molecular Dynamics, Sunnyvale, Calif.). The intensity of each specific chemokine band was measured with a computer-linked PhosphorImager and ImageQuant software (Molecular Dynamics). To correct for RNA loading, each intensity score was normalized to the intensity of hybridization for the L32 gene.
Isolation of primary type II cells.
Primary type II cells were isolated from female C57BL/6 mice by modification of a previously published method (9). Briefly, the lungs were exposed and the vasculature was perfused with sterile heparinized saline. Two milliliters of dispase solution (Collaborative Biomedical) was instilled into the lungs through a tracheal catheter, followed immediately by slow insertion of 0.45 ml of low-melting-point agarose at 45°C. The lungs were immediately covered with ice for 2 min and then removed from the mouse and incubated for 45 min at room temperature (25°C) in 2 ml of dispase solution. The lungs were placed in room temperature DMEM with 0.01% DNase I, and the lung tissue was microdissected away from the airways. The cell suspension was incubated briefly at room temperature and then filtered through nylon monofilament screens (100, 40, and 25 μm; Tetko Inc.). The cells were collected by centrifugation for 8 min at 130 × g at 4°C and resuspended in DMEM supplemented with 25 mM HEPES and 10% FBS. The recovered cells were then incubated with biotinylated anti-CD32/CD16 and anti-CD45 antibodies for 30 min at 37°C. The cells were collected by centrifugation and resuspended in DMEM with 25 mM HEPES. Streptavidin-coated magnetic beads were added to the cells and incubated with gentle tumbling for 30 min at room temperature. The cell suspension was placed against a magnet for 15 min, and the unbound cells were removed to a fresh tube. The cells were recovered by centrifugation and resuspended in DMEM supplemented with 25 mM HEPES and 10% FBS. The cells were placed in 60-mm tissue culture dishes for 4 to 6 h to remove any mesenchymal cells. Nonadherent cells were recovered and enumerated, and viability was assessed. The purity of the type II cell preparation was assessed by modified Papanicolaou staining and intracellular staining for pro-SPC. Using this procedure, we typically recover a type II preparation with >95% viability and approximately 95% purity based on SPC expression. For P. carinii stimulation of primary AECs, 106 freshly isolated AECs were incubated with or without P. carinii (P. carinii cyst/AEC ratio of 3:1) for 2 h in a 12-well tissue culture dish. The cells were recovered by centrifugation at 130 × g for 8 min at 4°C, and nuclear extracts were isolated as described above.
Statistical analysis.
One-way and two-way analyses of variance (ANOVAs) were performed with the SigmaStat 2.0 software (Jandel, San Rafael, Calif.) to determine the confidence intervals of observed variations in chemokine protein and mRNA levels in AEC cultures. The Student-Newman-Keuls method was used for all pairwise multiple comparisons of experimental groups.
RESULTS
P. carinii induces MIP-2 secretion from murine AECs.
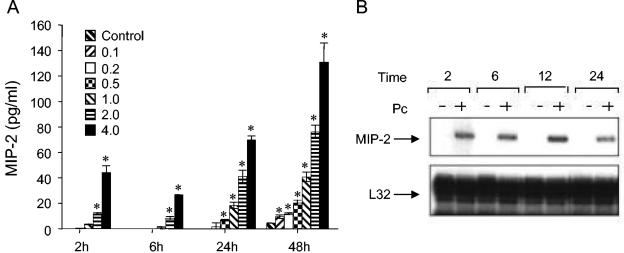
Confluent monolayers of the murine lung epithelial cell line MLE-15 were inoculated with freshly isolated murine P. carinii at cyst to AEC ratios of 0, 0.1, 0.2, 0.5, 1.0, 2.0, and 4.0. At 2, 6, 24, and 48 h postinoculation, the culture supernatants were removed and assayed for MIP-2 levels by ELISA. Stimulation of AECs with P. carinii induced a dose- and time-dependent increase in MIP-2 secretion (Fig. 1A). MIP-2 concentrations were significantly elevated in AECs treated with 2.0 and 4.0 ratios of P. carinii by 2 h postinoculation compared to unstimulated cells (P ≤ 0.002). At 24 h, MIP-2 secretion from AECs cocultured with 0.5, 1.0, 2.0, and 4.0 ratios of P. carinii were significantly elevated compared to unstimulated controls (P ≤ 0.003), and by 48 h the MIP-2 concentrations in all P. carinii-stimulated cultures were significantly elevated (P ≤ 0.018). To further support the significance of these data, the mean MIP-2 values for the 0.0, 0.5, 1.0, 2.0, and 4.0 ratios from eight independent 24-h experiments were pooled (n ≥ 18 for each ratio), and two-way ANOVA was performed using the Student-Newman-Keuls method for pairwise multiple comparisons. The mean MIP-2 value ± 1 standard error measurement for AECs stimulated with a 4:1 ratio of P. carinii to AEC was 223 ± 14 pg/ml. This value was statistically different from the mean MIP-2 values from AECs treated with 2:1 (109 ± 15 pg/ml; P < 0.001), 1:1 (40 ± 16 pg/ml; P < 0.001), and 0.5:1 (12 ± 16 pg/ml; P < 0.001) ratios of P. carinii, as well as untreated AECs (2 ± 14 pg/ml; P < 0.001). The mean MIP-2 value for the pooled 2:1 ratio was also statistically different from all other groups (P ≤ 0.003). RPA analysis demonstrated that the P. carinii-stimulated increase in MIP-2 protein secretion was accompanied by a concomitant increase in the steady-state level of MIP-2 mRNA (Fig. 1B). MIP-2 mRNA was elevated by 2 h postinoculation and remained elevated at 6, 12, and 24 h in AECs stimulated with a 3:1 ratio of P. carinii. In contrast, unstimulated cells exhibited no detectable MIP-2 mRNA expression.
FIG. 1.
MIP-2 protein secretion and mRNA expression by P. carinii-stimulated AECs. Confluent monolayers of MLE-15 cells were inoculated with freshly isolated mouse Pneumocystis at P. carinii-to-AEC ratios of 0.1, 0.2, 0.5, 1.0, 2.0, and 4.0. At the indicated time points, culture supernatants were removed and assayed for MIP-2 protein by ELISA (A). Control samples were from untreated cells. The values are expressed as the mean ± 1 standard error measurement (n = 3). *, P < 0.05 compared to unstimulated cells. In a separate experiment cells were stimulated with a 3:1 ratio of P. carinii to AEC, and RNA was isolated 2, 6, 12, and 24 h later. The steady-state MIP-2 and L32 mRNA levels were then determined by RPA (B). The experiment shown is a representative of at least three separate experiments.
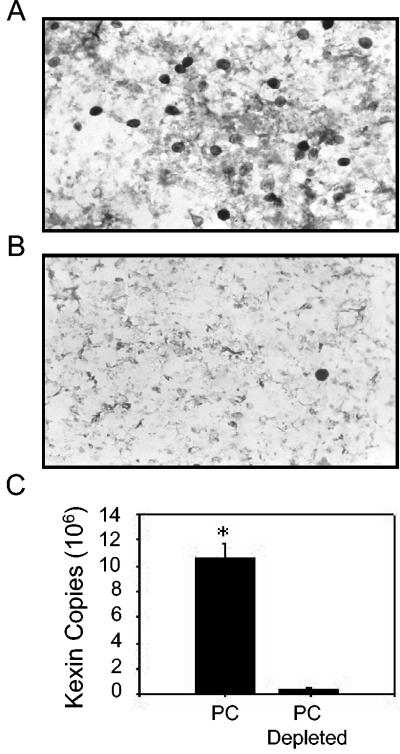
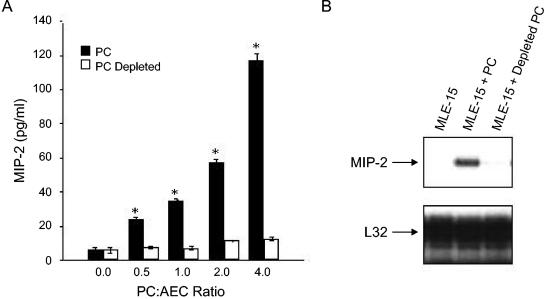
Since there is no reliable in vitro culture system for mouse P. carinii, the organisms used in these experiments were purified from the lungs of infected SCID mice. Therefore, to ensure that MIP-2 secretion by AECs was a response to P. carinii and not to potentially copurified contaminants, such as lipopolysaccharide (LPS) or mouse lung proteins, an antibody and magnetic bead-based technique for the specific removal of P. carinii from the preparation was employed. The purified preparation was depleted of P. carinii by using magnetic beads coated with a pool of anti-P. carinii antibodies. Enumeration of cysts before and after P. carinii depletion, as well as real-time PCR, demonstrated that this method removed >96% of the P. carinii organisms (Fig. 2A, B, and C). Equal amounts of P. carinii and of depleted preparations were used to stimulate AECs. As expected, stimulation of AECs with a 3:1 ratio of P. carinii to epithelial cells caused a significant increase in MIP-2 mRNA expression and a significant release of MIP-2 protein (Fig. 3A and B). In contrast, antibody-mediated removal of the P. carinii from the preparation abolished the MIP-2 response, demonstrating that the AECs are responding to the P. carinii (Fig. 3A and B) and not a copurified contaminant. These data demonstrate that the MLE-15 cell line responds to P. carinii stimulation with MIP-2 production, similar to primary cultures of rat AECs, and suggest that these cells are useful for in vitro studies of the P. carinii-epithelium interaction.
FIG. 2.
Specific antibody-mediated depletion of P. carinii from purified preparations. To provide a control for in vitro experiments using P. carinii purified from mouse lungs, a technique to deplete P. carinii was developed and assessed. Freshly isolated P. carinii organisms were incubated with a pool of seven P. carinii-specific MAbs and then incubated with magnetic beads coated with rat anti-mouse IgG. The P. carinii was removed from the preparation using magnetic separation, and the remaining material was collected. A silver stain of purified P. carinii demonstrates abundant cysts (A), while a silver stain of an identical volume of depleted P. carinii prep shows a nearly complete removal of cysts (B). Real-time PCR of P. carinii and P. carinii-depleted preps using a primer-probe set specific for the P. carinii kexin gene confirmed the level of depletion (C). Data are the mean ± 1 standard error measurement of four replicates (P < 0.05).
FIG. 3.
MIP-2 response of AECs is P. carinii specific. MLE-15 cells were stimulated with identical amounts of P. carinii or P. carinii-depleted preps for 24 h, and MIP-2 secretion was assessed by ELISA (A). Depletion of P. carinii resulted in nearly complete abrogation of MIP-2 secretion. The values are expressed as the mean ± 1 standard error measurement (n = 3). *, P < 0.05 compared to P. carinii-stimulated cells at the same P. carinii/AEC ratio. To assess MIP-2 mRNA expression, MLE-15 cells were stimulated with a P. carinii/AEC ratio of 3.0 or an equivalent amount of P. carinii-depleted prep for 6 h (B). Total RNA was isolated, and an RPA was used to determine steady-state levels of MIP-2 and L32 mRNAs.
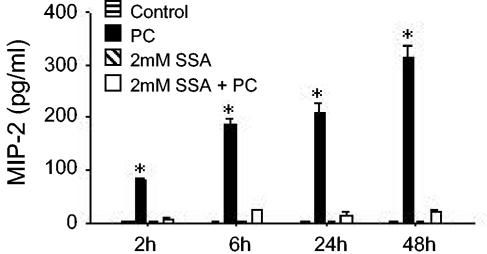
SSA blocks P. carinii-stimulated MIP-2 secretion.
The murine MIP-2 promoter contains consensus κB binding sites that are important for the inducible expression of this gene (26). Therefore, to determine whether P. carinii-stimulated activation of NF-κB signaling was involved in AEC chemokine production, the NF-κB inhibitor sulfasalazine (SSA) was utilized. Recent studies have demonstrated that SSA is a potent and specific inhibitor of NF-κB that directly inhibits IKK-α and -β (57, 59). Preliminary studies found that a concentration of 2 mM SSA had no effect on the viability of MLE-15 cells compared to untreated cells but nearly completely abrogated tumor necrosis factor (TNF)-stimulated NF-κB activation (data not shown). When MLE-15 cells were pretreated with SSA for 2 h prior to P. carinii stimulation, inducible MIP-2 synthesis was almost completely blocked (Fig. 4) (P < 0.05). In contrast, 2 mM SSA treatment in the absence of P. carinii stimulation had no demonstrable effect on the AEC line. Together, these data indicate that P. carinii-stimulated MIP-2 production by AECs could be inhibited by SSA and suggested that the interaction of P. carinii with AECs induces activation of the important transcription factor NF-κB.
FIG. 4.
Effect of SSA on MIP-2 production by P. carinii-stimulated AECs. MLE-15 cells were left unstimulated, stimulated with a 4:1 ratio of P. carinii, treated with 2 mM SSA, or treated with 2 mM SSA and stimulated with P. carinii. Culture supernatants were collected at 2, 4, 6, 24, and 48 h and assayed for MIP-2 by ELISA. The values are expressed as the mean ± 1 standard error measurement (n = 3). *, P < 0.05 compared with unstimulated cells, cells treated with SSA alone, and cells treated with SSA and stimulated with P. carinii. The experiment shown is a representative of at least three separate experiments.
P. carinii induces κB-dependent reporter gene expression in AECs.
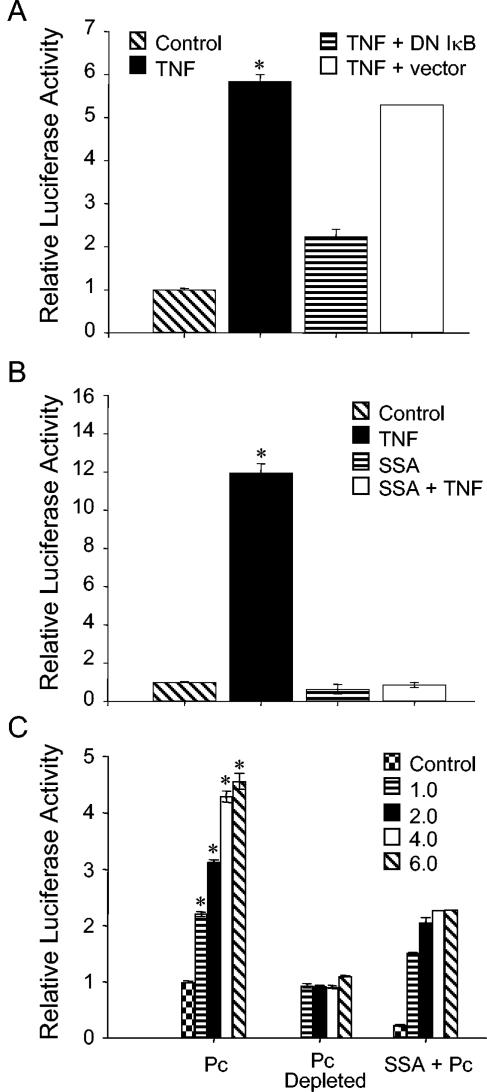
To study NF-κB-dependent gene expression in P. carinii-stimulated murine AECs, MLE-15 cells were stably transfected with a κB-dependent luciferase reporter construct derived from the human IL-8 promoter (39, 40). In order to characterize the NF-κB response in these cells and to determine if they were suitable for use as reporter cells, they were stimulated with recombinant murine TNF, a known inducer of NF-κB activation and signaling. As expected, TNF induced the NF-κB-dependent production of luciferase in the MLE-pLUC/κB cell line (Fig. 5A). Importantly, luciferase production by TNF-stimulated cells was blocked by transient transfection of cells with a constitutively expressed dominant negative form of IκB but unaffected by transient transfection with parent vector (Fig. 5A). Additionally, the NF-κB inhibitor SSA also blocked luciferase production by TNF-stimulated MLE-pLUC/κB cells (Fig. 5B). These data demonstrated that MLE-pLUC/κB cells could serve as a reporter cell line to study NF-κB activation and signaling in response to P. carinii.
FIG. 5.
P. carinii stimulation induces κB-dependent reporter expression in AECs. MLE-pLUC/κB cells, MLE-pLUC/κB cells transiently transfected with dominant-negative IκB, and MLE-pLUC/κB cells transiently transfected with control vector were stimulated with 5 ng/ml TNF (A). In a separate experiment, untreated cells and cells pretreated with 2 mM SSA for 2 h were stimulated with 10 ng/ml TNF (B). To assess the effect of P. carinii stimulation on reporter expression, MLE-pLUC/κB cells were stimulated with P. carinii or equivalent amounts of P. carinii-depleted preps. In addition, cells pretreated with 2 mM SSA were stimulated with P. carinii (C). For all experiments the cells were recovered and assayed for luciferase activity after 6 h of stimulation. Untreated cells were used as controls in all experiments, and the values are expressed as the mean fold change in luciferase activity ± 1 standard error measurement (n = 3, except forthe “TNF + vector” condition in panel A [n = 2]). *, P < 0.05 compared to unstimulated cells, cells stimulated with equivalent amounts of P. carinii-depleted preps, and cells pretreated with 2 mM SSA and then stimulated with equivalent amounts of P. carinii.
To determine whether P. carinii is capable of inducing NF-κB-dependent gene expression in murine AECs, the MLE-pLUC/κB cell line was utilized. P. carinii stimulation resulted in a dose-dependent increase in NF-κB-dependent luciferase activity. MLE-pLUC/κB cells stimulated with P. carinii to AEC ratios of 0.5, 1.0, 2.0, and 3.0 demonstrated respective 2-fold, 3-fold, 4.25-fold, and 4.5-fold increases in luciferase activity compared to unstimulated cells (Fig. 5C) (P < 0.05 for all groups). Similar to the data obtained for inducible MIP-2 production, luciferase induction was dependent upon the presence of P. carinii, since luciferase activity in MLE-pLUC/κB cells treated with preparations depleted of P. carinii was not statistically different from that of untreated cells (Fig. 5C). Finally, the NF-κB inhibitor SSA also blocked luciferase reporter expression by P. carinii-stimulated cells (Fig. 5C). Together, these data suggested that P. carinii induces NF-κB activation and κB-dependent gene expression in AECs.
P. carinii stimulates κB binding activity in the nucleus of AECs.
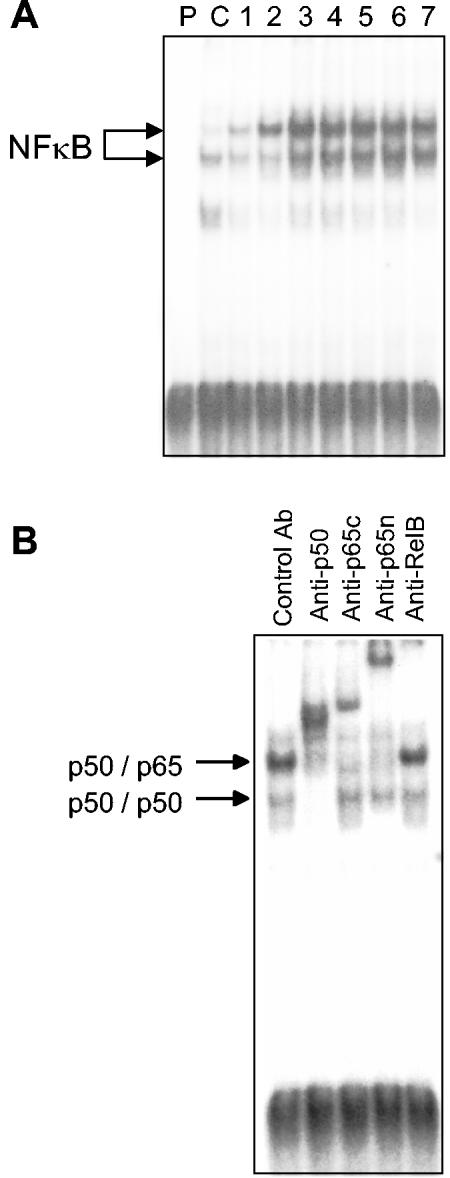
To directly demonstrate that P. carinii induces κB binding activity in the nucleus of AECs, gel shift assays were performed on nuclear extracts isolated from MLE-15 cell cultures at 0, 1, 1.5, 2, 2.5, 3, 4, and 6 h postinoculation. There was low-level baseline κB binding activity in the nucleus of untreated AEC cultures. In contrast, P. carinii treatment induced a significant increase in a slower-migrating κB binding activity in the nucleus. Elevated κB binding activity was detected by 1 h postinoculation, peaked at 1 to 2 h, and was declining by 6 h (Fig. 6). To identify the subunit composition of the induced κB binding activity, supershift assays were performed with control antibodies and antibodies specific for the p50, p65, and RelB subunits of NF-κB. These assays demonstrated that the main P. carinii-stimulated κB binding activity was composed of p50/p65 heterodimers, while the faster-migrating activity present at low levels in the untreated AECs consisted of p50/p50 homodimers (Fig. 6B).
FIG. 6.
P. carinii stimulation induces nuclear translocation of the p50/p65 heterodimeric form of NF-κB. MLE-15 cells were stimulated with a 3:1 ratio of P. carinii to AEC, and EMSAs were performed on nuclear extracts taken from the cells at 1, 1.5, 2, 2.5, 3, 4, and 6 h after inoculation (lanes 1 to 7, respectively) (A). Unbound probe (lane P) and unstimulated cells (lane C) were used as controls. The gel is representative of at least three independent experiments. (B) Representative supershift assay on the nuclear extract from MLE-15 cells stimulated with a 3:1 ratio of P. carinii for 2 h. Antibodies specific for p50, the C-terminal portion of p65 (anti-p65c), the N-terminal portion of p65 (anti-p65n), and RelB were used to determine the composition of the P. carinii-inducible form of NF-κB.
Direct contact of viable P. carinii with AECs is critical for maximal activation of NF-κB.
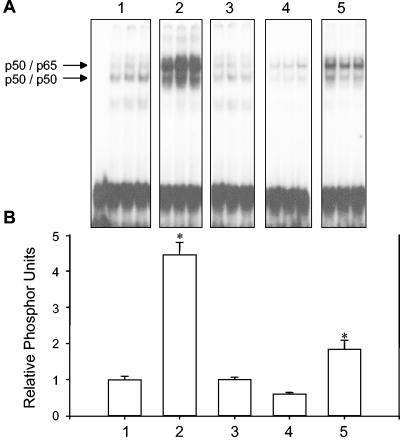
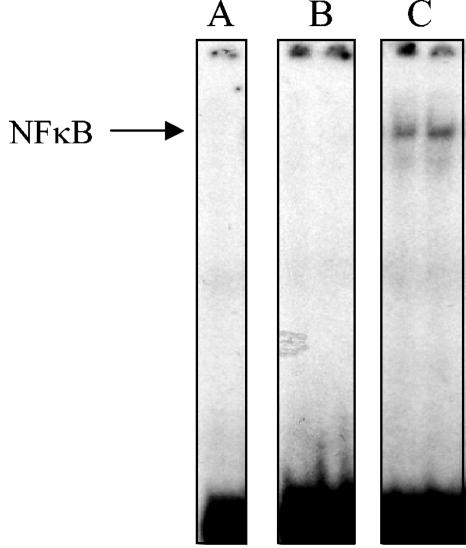
In order to further characterize the nature of NF-κB activation in treated AECs, the requirement of direct P. carinii-AEC contact, and of P. carinii viability, was examined. EMSAs were performed in triplicate on stimulated and unstimulated cells (Fig. 7A), and the intensity of each band corresponding to the p50/p65 heterodimer was quantified using a PhosphorImager (Fig. 7B). As expected, unstimulated cells exhibited little κB binding activity (Fig. 7, lanes 1), while treatment of AECs with P. carinii induced significant NF-κB nuclear translocation (Fig. 7, lanes 2) (P < 0.05). Specific antibody-magnetic bead removal of P. carinii from the preparation demonstrated that inducible p50/p65 nuclear translocation is dependent upon the presence of P. carinii organisms (Fig. 7, lanes 3). In addition, to determine whether direct P. carinii-AEC contact was required for NF-κB activation, experiments were performed in which the P. carinii organisms were separated from the AECs by Transwell inserts. Although soluble mediators were freely diffusible between the AEC and P. carinii compartment, the lack of direct contact prevented NF-κB activation (Fig. 7, lanes 4). Finally, the requirement of P. carinii viability for the NF-κB response in AECs was assessed. Five cycles of freezing and thawing P. carinii in serum-free medium reduced the viability of the isolated P. carinii by approximately 90% based on incorporation of the vital dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (data not shown). When the killed P. carinii was used to stimulate AECs, NF-κB activation was significantly reduced compared to freshly isolated live P. carinii (Fig. 7, lanes 5). These data demonstrated that NF-κB activation in P. carinii-stimulated AECs requires the direct contact of viable P. carinii with the AEC monolayer.
FIG. 7.
Specificity of NF-κB activation in P. carinii-stimulated AECs. Nuclear extracts were isolated from MLE-15 cells stimulated for 2 h with equal amounts (a 3:1 ratio) of either P. carinii (lanes 2), P. carinii-depleted preps (lanes 3), P. carinii separated from the AECs by Transwell inserts (lanes 4), or killed P. carinii (lanes 5). Unstimulated cells were used as controls. EMSAs were performed on nuclear extracts with a labeled κB probe (A). The dried gel was then used to expose PhosphorImager screens, and the relative intensity of each p50/p65 band was quantified using ImageQuant software (B). Values are means ± 1 standard error measurements. *, P < 0.05 compared to untreated cells.
NF-κB activation is associated with increased IκBα expression in P. carinii-stimulated AECs.
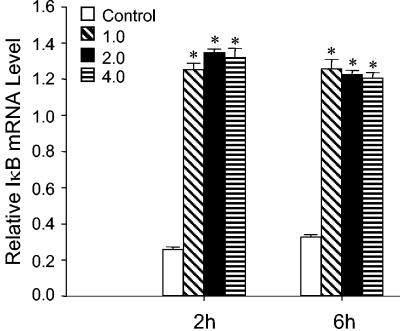
The activation and nuclear translocation of NF-κB involves the degradation of its cytoplasmic inhibitor, IκBα. In order to control stimulus-mediated alterations in cellular gene expression, the activation of NF-κB induces the transcription and resynthesis of IκB. As additional evidence confirming the induction of κB-dependent gene expression in P. carinii-stimulated AECs, steady-state levels of IκBα mRNA were examined. When AECs were treated with P. carinii, a greater-than-10-fold increase in the steady-state level of IκBα compared to unstimulated cells was observed at 2 and 6 h postinfection (Fig. 8) (P < 0.05). These data supported our conclusion that many NF-κB-responsive genes are altered in P. carinii-stimulated AECs and suggested that resynthesis of IκBα is likely an attempt to control the NF-κB response of AECs to P. carinii.
FIG. 8.
Expression of IκBα mRNA in P. carinii-stimulated AECs. Total RNA was extracted from MLE-15 cells stimulated with 1.0, 2.0, and 4.0 ratios of P. carinii to AEC for the indicated times, and IκBα mRNA levels were assessed by RPA. Values are means ± 1 standard error measurements (n = 3). *, P < 0.05 compared to untreated cells.
P. carinii stimulates κB binding activity in the nucleus of primary murine AECs.
The data presented thus far demonstrated that P. carinii activates NF-κB signaling in a murine AEC line. Therefore, to validate that primary AECs respond to P. carinii in a similar fashion to the AEC line, EMSA was performed on nuclear extracts from primary murine AECs following P. carinii stimulation. Nuclear extracts from unstimulated primary type II cells contained no detectable κB binding activity as assessed by EMSA. In contrast, κB binding activity was detected in type II cells following 2 h of coculture with P. carinii at a P. carinii/AEC ratio of 3:1 (Fig. 9). Furthermore, the major form of NF-κB induced in primary cells is consistent with the migration pattern of p50/p65 heterodimers. These data support the validity of the results obtained using the MLE-15 cell line and strengthen our data suggesting that P. carinii induces NF-κB signaling in AECs.
FIG. 9.
P. carinii stimulation induces nuclear translocation of NF-κB in primary murine AECs. Primary type II cells were stimulated with a 3:1 ratio of P. carinii to AECs, and EMSAs were performed on nuclear extracts taken from the cells at 2 h after inoculation (C). Unbound probe (A) and unstimulated primary cells (B) were used as controls. The gel is representative of at least three independent experiments, and the samples shown were performed in duplicate.
DISCUSSION
Given that P. carinii is acquired by aerosol spread, the interaction of P. carinii with the alveolar epithelium is likely the initial step in the infectious process leading to PcP. Our data and reports from other groups suggest that this interaction induces changes in AEC gene expression that may contribute to the generation and targeting of the host's immune response. In a normal host these changes may lead to the generation of effective immunity, while in a compromised host they may contribute to immune-mediated lung injury. The studies presented herein confirm recent reports that P. carinii stimulates the release of MIP-2 from cultured AECs and support our contention that the MLE-15 cell line responds to P. carinii similarly to primary cells. In addition, we have expanded upon these data by providing direct evidence that P. carinii stimulation of AECs induces the nuclear translocation of the classic p50/RelA heterodimeric form of NF-κB and, subsequently, κB-dependent gene expression. The finding that P. carinii induces NF-κB signaling suggests that in addition to the known genes that are altered by the interaction of P. carinii with AECs, many other NF-κB-responsive genes, including adhesion molecules, cytokines, and chemokines, and acute phase proteins that are involved in inflammatory reactions may also be regulated. Therefore, this study adds to the growing amount of evidence supporting an immunomodulatory role for AECs in defense against infectious agents.
Prior studies have demonstrated that stimulation of primary rat AECs and rat and human AEC lines with P. carinii or purified P. carinii β-glucan induces expression of proinflammatory genes, including MIP-2, IL-6, MCP-1, and IL-8 (2, 21, 44). Importantly, the promoter regions of all of these genes contain consensus κB DNA binding sequences, and we have demonstrated that P. carinii induces NF-κB activation in AECs. Therefore, it is likely that NF-κB-dependent signal transduction pathways play a critical role in P. carinii-induced alteration of AEC gene expression. Although we have not identified the specific molecular associations required for activation of the NF-κB pathway, we have demonstrated that direct contact with viable P. carinii is necessary for activation to occur. Several modes of interaction between P. carinii and AECs have been proposed. Both direct binding of P. carinii to AECs as well as indirect binding using extracellular matrix (ECM) proteins as bridging molecules have been reported (1, 32-34, 41-43, 45). In addition, the interaction of P. carinii with pattern recognition molecules expressed on AECs, such as β-glucan receptors, has been reported. Hahn et al. recently reported that purified P. carinii β-glucan induced MIP-2 secretion from AECs through a lactosylceramide-mediated mechanism (21). However, they did not assess the contribution of NF-κB to this process. While our study did not address the role of lactosylceramide in AEC NF-κB activation because of a lack of murine-specific blocking reagents, we did find that viable P. carinii organisms were required for maximal NF-κB activation. This finding suggests that β-glucan is not solely responsible for AEC response to intact P. carinii and indicates that other mechanisms of the P. carinii-AEC interaction exist. However, it does not exclude the possibility that purified β-glucan alone can stimulate AECs. The obvious difference between the present study and that of Hahn et al. is that cells were stimulated with whole organisms in the present study, in contrast to the particulate antigen preparation used by Hahn et al. which was 96% carbohydrate and highly enriched for β-glucan (21). This major technical difference makes it impossible to directly compare the results of these two studies. Furthermore, although the studies by Hahn et al. used defined quantities of β-glucan, it is difficult to normalize this for a specific number of P. carinii organisms. Therefore, it is highly probable that Hahn et al. used an amount of free β-glucan that greatly exceeds the amount contained in the P. carinii organisms we used to stimulate the AECs. The establishment of this in vitro model will allow further studies to examine the critical interactions that lead to NF-κB activation.
The proinflammatory responses of alveolar macrophages (AMs) to P. carinii stimulation has also been well documented, and P. carinii β-glucan plays an important role in the AM response (29, 53). However, our data suggest that AECs and AMs may recognize P. carinii via distinct receptor-ligand interactions. Although P. carinii β-glucan is quite efficient in activating NF-κB in macrophages, the data presented herein suggest that the AEC response to P. carinii may not be exclusively to β-glucan. There are many documented differences in the response of AECs and macrophages to a variety of stimuli. For example, macrophages respond much more vigorously to LPS than do cells of epithelial origin, probably owing to differential receptor expression. We believe that this may also be the situation with macrophage and AEC responses to P. carinii β-glucan. A recent report has demonstrated that a newly identified β-glucan receptor, Dectin-1, is critical to the macrophage response to P. carinii (53). However, Dectin-1 expression is specific to phagocytic cells, and there is no evidence that Dectin-1 is expressed on AECs. Furthermore, it has been reported that the AEC response to β-glucan is mediated by lactosylceramide (21). Therefore, our data are consistent with the existence of distinct pathogen recognition mechanisms utilized by AECs and AMs.
While the main focus of the current study was the direct activation of NF-κB by P. carinii, it is also well documented that TNF induces NF-κB activation in pulmonary epithelial cells (6, 20). Prior studies have demonstrated that little detectable TNF is present in the lungs of P. carinii-infected SCID mice (8, 64). However, following immune reconstitution a large spike in TNF mRNA levels in the lung and protein levels in bronchoalveolar lavage fluid is observed (64). Importantly, increased TNF production coincides with immune-mediated pulmonary dysfunction (63). Therefore, in addition to direct stimulation of NF-κB by P. carinii, local TNF production may also contribute to NF-κB activation in AECs proximal to P. carinii infection and contribute to inflammatory cell targeting and lung injury. We have also demonstrated that TNF-induced NF-κB activation in AECs is blocked by the potent and specific inhibitor SSA (57, 59) (Fig. 5). While further in vivo studies are required to determine the contribution of NF-κB signaling in AECs to PcP-related immune-mediated lung injury, these data do suggest that NF-κB inhibitors could have some beneficial effects for individuals suffering from PcP.
The consequences of NF-κB activation in the AECs of P. carinii-infected animals remain unclear. However, it is plausible that altered AEC gene expression could promote the immune-mediated lung injury associated with PcP (63, 66, 67). We have demonstrated that following the immunological reconstitution of P. carinii-infected SCID mice, immune and inflammatory cells are recruited specifically to alveolar regions of infection and not to uninfected alveoli (64, 65). This finding suggests that the interaction of P. carinii with AECs in vivo produces signals that direct the inflammatory response to infected alveoli. Therefore, if the recruitment of inflammatory cells to the lung were a direct consequence of NF-κB-mediated signal transduction in AECs, then blockade of NF-κB might alleviate the lung injury resulting from immune-mediated mechanisms. While the present study has focused on the P. carinii-stimulated activation of NF-κB in AECs, it has also been demonstrated that P. carinii and P. carinii β-glucan stimulate NF-κB in AMs (29, 69). Furthermore, it has been well documented that NF-κB is important for lymphocyte activation and proliferation, and a direct role for lymphocytes in PcP-related lung injury has been demonstrated (63, 66). Therefore, the inhibition of NF-κB signaling may interfere with the generation of an injurious host response to P. carinii on several fronts and could provide a promising therapeutic intervention to lessen immune-mediated respiratory impairment during PcP. Several classes of NF-κB inhibitors have been developed, and this signaling pathway is becoming an important target for antiinflammatory therapeutics to alleviate many different diseases (11). For example, SSA is used therapeutically in humans to alleviate the symptoms of inflammatory bowel disease and rheumatoid arthritis (4, 13, 14, 25, 27). Therefore, further studies should be performed to determine whether appropriate anti-inflammatory treatments, including NF-κB inhibitors, could provide a valuable therapeutic adjunct for PcP treatment.
In vitro studies of P. carinii-AEC interactions are made significantly more difficult because of the inability to grow and culture significant quantities of P. carinii in vitro. Instead, organisms purified from the lungs of SCID mice must be used for these studies. While significant improvement has been made to the purification protocol, there always exists the possibility that a non-P. carinii contaminant will be copurified and contribute to the observed AEC response. Therefore, in addition to routinely testing P. carinii preparations for endotoxin contamination and the presence of other microorganisms, we have developed a MAb-linked magnetic bead method by which greater than 96% of the P. carinii is removed from the preparations in a specific manner (Fig. 2). The remaining non-P. carinii contaminants (including LPS, bioactive inflammatory particles, or ECM breakdown products) are then used to stimulate AEC cultures. Using this depletion technique it was demonstrated that the removal of P. carinii from the preparation nearly completely abolished both P. carinii-induced NF-κB activation and MIP-2 secretion. We have also validated this technique in vivo by demonstrating that cytokine responses to intratracheally inoculated P. carinii are blocked when P. carinii is first depleted in this manner (data not shown). Thus, we are confident in concluding that P. carinii is directly responsible for the observed alterations in AEC gene expression.
Although the dose-dependent MIP-2 response of P. carinii-stimulated AECs exhibited a very small degree of variability in each individual experiment, it must be noted that we often observed greater variability among replicate samples from independent experiments (Fig. 1, 3, and 4). The most likely explanation for this observation is variation in the enumeration and/or viability of individual P. carinii preparations. To quickly enumerate the P. carinii organisms isolated from SCID mouse lungs, we performed cyst counts on silver-stained slides. While this provides an easy, rapid method for determining a relative P. carinii count, it does not give an absolute number of all P. carinii organisms, as our isolation procedure recovers both cyst and trophozoite forms. Importantly, we have found that the cyst/trophozoite ratio can vary from 1:10 to 1:15 in the lungs of SCID mice, depending upon the level of infection. Therefore, it is possible that one experiment may actually use more total P. carinii (or more trophozoites) than another experiment because we have normalized the inocula to cyst counts only. These differences would likely produce the observed variation, especially if there were a difference in the capacity of a cyst or trophozoite to interact with an AEC. However, in our experience this variation has not been so great that it creates an obstacle to our data interpretation, since we typically use a range of P. carinii inocula. Furthermore, to ensure that the MIP-2 results were statistically relevant, the 24-h data from eight independent experiments, utilizing eight different P. carinii preparations and four different P. carinii doses, were pooled and analyzed by two-way ANOVA using the Student-Newman-Keuls method for pairwise multiple comparisons. This analysis demonstrated that the difference in mean MIP-2 values of AECs treated with different P. carinii ratios was statistically significant when allowing for variation in the individual P. carinii preparations (P < 0.001) and also confirmed the dose dependence of the MIP-2 response at P. carinii-to-AEC ratios of 4.0 and 2.0.
The temporal pattern of NF-κB activation, IκB resynthesis, and gene regulation has been extensively studied in several model systems. However, in the current study we did not specifically examine transcriptional regulation in P. carinii-stimulated AECs. While the elevated levels of MIP-2 mRNA and protein at 24 h (Fig. 1) may seem counterintuitive to our data showing that κB binding activity is decreasing and IκB expression is increasing at 6 h, there are several possible explanations for this. First, the amount of κB binding activity in the nucleus at 6 h is decreasing but still elevated above controls. Thus, NF-κB-dependent mRNA and protein production and accumulation could continue to later time points. A second possibility is that following stimulation, MIP-2 mRNA exhibits increased stability and remains present even at 24 h. Since we are measuring steady-state levels of MIP-2 mRNA, and not specific transcriptional activity of the MIP-2 promoter (which is directly controlled by NF-κB), the persistence of MIP-2 mRNA could continue in the absence of further NF-κB-dependent transcription. Finally, this phenomenon could be explained by the continued presence of P. carinii in the culture. Because there is no method to easily remove adherent P. carinii from the cultured AECs, the stimulus remains throughout the experimental time course. Thus, we may be observing a second wave of NF-κB activation after the first wave has been blunted by NF-κB-stimulated IκB resynthesis.
Although we report data obtained using a murine AEC line, it is important to note that similar results were obtained using another pulmonary epithelial cell line (2), and also primary rat AECs (21). In addition, PcP-related MIP-2 production in rodents and IL-8 production in humans have been demonstrated in vivo. Thus, since our data are in agreement with both primary culture and in vivo experiments, we are optimistic that this system will provide a valuable in vitro model for studying the interaction of P. carinii with AECs. In addition, this cell line can be genetically modified to perform signal transduction studies that may not be possible in vivo, or with primary epithelial cells. For example, we were able to construct an NF-κB reporter cell line by stable transfection of MLE-15 cells with a κB-dependent reporter construct. This cell line should prove valuable for studies evaluating the mechanism of P. carinii-induced NF-κB signaling.
The data presented herein demonstrate that direct interaction with P. carinii induces NF-κB activation in type II-like AECs. In contrast, the majority of in vivo studies have described the physical association between P. carinii and type I cells (28, 35, 38, 68), with less common reports of binding to type II cells (35). However, we believe that the in vivo interaction of P. carinii with the type II cell may be underrepresented. Since the vast majority of the lung surface area is covered by type I cells, the structure of the lung makes it much more likely that in vivo studies would identify P. carinii in direct contact with these cells. However, since the structure of the alveoli is such that type I cells are in close proximity to type II cells, it is likely that ample opportunity exists for P. carinii to contact the apical surface of the type II cell. Furthermore, activation of signal transduction pathways may not necessarily require firm attachment to the type II cell, and direct contact with P. carinii may induce NF-κB activation without the firm adherence characteristic of the P. carinii-type I cell interaction. In support of our contention that P. carinii-type II interactions may be important during in vivo infection, several other groups have reported that cells with type II-like characteristics bind and respond to P. carinii (2, 21, 33, 34, 42, 44). Alternatively, it is possible that, because type II cells are precursors to type I cells, the MLE-15 and other cell lines can take on some characteristics of type I cells in culture, making them more responsive to direct interaction with P. carinii. More definitive studies will be required to determine the differential responses of type I and type II cells to P. carinii.
In summary, we have demonstrated that stimulation of AECs with viable P. carinii activates the NF-κB signaling pathway and alters chemokine gene expression. The P. carinii-stimulated activation and nuclear translocation of the classic p50/p65 form of NF-κB suggests that in addition to MIP-2, the expression of other immune- and inflammatory- related genes may also be altered in AECs following P. carinii stimulation. More in-depth studies of the role of NF-κB in AECs may provide insight into how AECs affect immunity to P. carinii and also the role of AECs in promoting and maintaining the immune-mediated lung injury observed during PcP.
Acknowledgments
This work was supported by Public Health Service grants HL-64559 (T.W.W.) and PO1 HL-71659 from the National Heart, Lung, and Blood Institute.
Editor: T. R. Kozel
REFERENCES
- 1.Beck, J. M., A. M. Preston, J. G. Wagner, S. E. Wilcoxen, P. Hossler, S. R. Meshnick, and R. Paine III. 1998. Interaction of rat Pneumocystis carinii and rat alveolar epithelial cells in vitro. Am. J. Physiol. 275:L118-L125. [DOI] [PubMed] [Google Scholar]
- 2.Benfield, T. L., B. Lundgren, J. H. Shelhamer, and J. D. Lundgren. 1999. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur. J. Clin. Investig. 29:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Borok, Z., X. Li, V. F. Fernandes, B. Zhou, D. K. Ann, and E. D. Crandall. 2000. Differential regulation of rat aquaporin-5 promoter/enhancer activities in lung and salivary epithelial cells. J. Biol. Chem. 275:26507-26514. [DOI] [PubMed] [Google Scholar]
- 4.Brookes, M. J., and J. R. Green. 2004. Maintenance of remission in Crohn's disease: current and emerging therapeutic options. Drugs 64:1069-1089. [DOI] [PubMed] [Google Scholar]
- 5.Caamano, J., and C. A. Hunter. 2002. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15:414-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casola, A., A. Henderson, T. Liu, R. P. Garofalo, and A. R. Brasier. 2002. Regulation of RANTES promoter activation in alveolar epithelial cells after cytokine stimulation. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L1280-L1290. [DOI] [PubMed] [Google Scholar]
- 7.Center, D. M. 2000. A role for the alveolar epithelium in recruitment of mononuclear cells into the lung. J. Clin. Investig. 106:741-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corti, M., A. R. Brody, and J. H. Harrison. 1996. Isolation and primary culture of murine alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 14:309-315. [DOI] [PubMed] [Google Scholar]
- 10.Cushion, M. T. 2004. Pneumocystis: unraveling the cloak of obscurity. Trends Microbiol. 12:243-249. [DOI] [PubMed] [Google Scholar]
- 11.D'Acquisto, F., M. J. May, and S. Ghosh. 2002. Inhibition of nuclear factor kappa B (NF-κB): an emerging theme in anti-inflammatory therapies. Mol. Intervent. 2:22-35. [DOI] [PubMed] [Google Scholar]
- 12.Dore, G. J., Y. Li, A. McDonald, H. Ree, J. M. Kaldor, and J. M. Kaldo. 2002. Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J. Acquir. Immune Defic. Syndr. 29:388-395. [DOI] [PubMed] [Google Scholar]
- 13.Dougados, M., P. Emery, E. Lemmel, C. Zerbini, S. Brin, and P. van Riel. 2005. When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide? Ann. Rheum. Dis. 64:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan, L. J., and W. J. Sandborn. 1998. Inhibition of nuclear factor κB by sulfasalazine: a new target for inflammatory bowel disease therapy? Gastroenterology 115:1295-1296. [DOI] [PubMed] [Google Scholar]
- 15.Fehrenbach, H. 2001. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest, D. M., E. Seminari, R. S. Hogg, B. Yip, J. Raboud, L. Lawson, P. Phillips, M. T. Schechter, M. V. O'Shaughnessy, and J. S. Montaner. 1998. The incidence and spectrum of AIDS-defining illnesses in persons treated with antiretroviral drugs. Clin. Infect. Dis. 27:1379-1385. [DOI] [PubMed] [Google Scholar]
- 17.Forrest, D. M., C. Zala, O. Djurdjev, J. Singer, K. J. Craib, L. Lawson, J. A. Russell, and J. S. Montaner. 1999. Determinants of short- and long-term outcome in patients with respiratory failure caused by AIDS-related Pneumocystis carinii pneumonia. Arch. Intern. Med. 159:741-747. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 19.Good, L. F., S. B. Maggirwar, A. Kealiher, M. Uhlik, and S. C. Sun. 1996. Multiple structural domains within I kappa B alpha are required for its inducible degradation by both cytokines and phosphatase inhibitors. Biochem. Biophys. Res. Commun. 223:123-128. [DOI] [PubMed] [Google Scholar]
- 20.Haddad, J. J. 2002. Recombinant TNF-alpha mediated regulation of the I kappa B-alpha/NF-kappa B signaling pathway: evidence for the enhancement of pro- and anti-inflammatory cytokines in alveolar epithelial cells. Cytokine 17:301-310. [DOI] [PubMed] [Google Scholar]
- 21.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 278:2043-2050. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, H. H., G. Kaufmann, P. Sendi, and M. Battegay. 2004. Immune reconstitution in HIV-infected patients. Clin. Infect. Dis. 38:1159-1166. [DOI] [PubMed] [Google Scholar]
- 23.Hori, S., T. L. Carvalho, and J. Demengeot. 2002. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda, K., J. C. Clark, C. J. Bachurski, K. A. Wikenheiser, J. Cuppoletti, S. Mohanti, R. E. Morris, and J. A. Whitsett. 1994. Immortalization of subpopulations of respiratory epithelial cells from transgenic mice bearing SV40 large T antigen. Am. J. Physiol. 267:L309-L317. [DOI] [PubMed] [Google Scholar]
- 25.Jansen, G., D. H. van, R. Oerlemans, W. F. Lems, I. Ifergan, R. J. Scheper, Y. G. Assaraf, and B. A. Dijkmans. 2004. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum. 50:2130-2139. [DOI] [PubMed] [Google Scholar]
- 26.Kim, D. S., J. H. Han, and H. J. Kwon. 2003. NF-κB and c-Jun-dependent regulation of macrophage inflammatory protein-2 gene expression in response to lipopolysaccharide in RAW 264.7 cells. Mol. Immunol. 40:633-643. [DOI] [PubMed] [Google Scholar]
- 27.Korpela, M., L. Laasonen, P. Hannonen, H. Kautiainen, M. Leirisalo-Repo, M. Hakala, L. Paimela, H. Blafield, K. Puolakka, and T. Mottonen. 2004. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum. 50:2072-2081. [DOI] [PubMed] [Google Scholar]
- 28.Lanken, P. N., M. Minda, G. G. Pietra, and A. P. Fishman. 1980. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am. J. Pathol. 99:561-588. [PMC free article] [PubMed] [Google Scholar]
- 29.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-κB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 30.Lee, L. H., F. Gigliotti, T. W. Wright, P. J. Simpson-Haidaris, G. A. Weinberg, and C. G. Haidaris. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141-150. [DOI] [PubMed] [Google Scholar]
- 31.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 32.Limper, A. H., and W. J. Martin II. 1990. Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J. Clin. Investig. 85:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limper, A. H., S. T. Pottratz, and W. J. Martin. 1991. Modulation of Pneumocystis carinii adherence to cultured lung cells by a mannose-dependent mechanism. J. Lab. Clin. Med. 118:492-499. [PubMed] [Google Scholar]
- 34.Limper, A. H., J. E. Standing, O. A. Hoffman, M. Castro, and L. W. Neese. 1993. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect. Immun. 61:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long, E. G., J. S. Smith, and J. L. Meier. 1986. Attachment of Pneumocystis carinii to rat pneumocytes. Lab. Investig. 54:609-615. [PubMed] [Google Scholar]
- 36.Maggirwar, S. B., S. Ramirez, N. Tong, H. A. Gelbard, and S. Dewhurst. 2000. Functional interplay between nuclear factor-κB and c-Jun integrated by coactivator p300 determines the survival of nerve growth factor-dependent PC12 cells. J. Neurochem. 74:527-539. [DOI] [PubMed] [Google Scholar]
- 37.Maggirwar, S. B., P. D. Sarmiere, S. Dewhurst, and R. S. Freeman. 1998. Nerve growth factor-dependent activation of NF-κB contributes to survival of sympathetic neurons. J. Neurosci. 18:10356-10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millard, P. R., A. E. Wakefield, and J. M. Hopkin. 1990. A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. Int. J. Exp. Pathol. 71:895-904. [PMC free article] [PubMed] [Google Scholar]
- 39.Mukaida, N., M. Morita, Y. Ishikawa, N. Rice, S. Okamoto, T. Kasahara, and K. Matsushima. 1994. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J. Biol. Chem. 269:13289-13295. [PubMed] [Google Scholar]
- 40.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 41.Narasimhan, S., M. Y. Armstrong, K. Rhee, J. C. Edman, F. F. Richards, and E. Spicer. 1994. Gene for an extracellular matrix receptor protein from Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 91:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pottratz, S. T., and W. J. Martin II. 1990. Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J. Clin. Investig. 85:351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pottratz, S. T., J. Paulsrud, J. S. Smith, and W. J. Martin II. 1991. Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J. Clin. Investig. 88:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pottratz, S. T., S. Reese, and J. L. Sheldon. 1998. Pneumocystis carinii induces interleukin 6 production by an alveolar epithelial cell line. Eur. J. Clin. Investig. 28:424-429. [DOI] [PubMed] [Google Scholar]
- 45.Pottratz, S. T., and A. L. Weir. 1995. Attachment of Pneumocystis carinii to primary cultures of rat alveolar epithelial cells. Exp. Cell Res. 221:357-362. [DOI] [PubMed] [Google Scholar]
- 46.Poynter, M. E., C. G. Irvin, and Y. M. Janssen-Heininger. 2003. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 170:6257-6265. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi, M. H., A. G. Harmsen, and B. A. Garvy. 2003. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J. Immunol. 170:1002-1009. [DOI] [PubMed] [Google Scholar]
- 48.Ruan, S., C. Tate, J. J. Lee, T. Ritter, J. K. Kolls, and J. E. Shellito. 2002. Local delivery of the viral interleukin-10 gene suppresses tissue inflammation in murine Pneumocystis carinii infection. Infect. Immun. 70:6107-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saffiotti, U. 1996. Alveolar type II cells at the crossroad of inflammation, fibrogenesis, and neoplasia. Am. J. Pathol. 149:1423-1426. [PMC free article] [PubMed] [Google Scholar]
- 50.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shishodia, S., P. Potdar, C. G. Gairola, and B. B. Aggarwal. 2003. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-κB activation through inhibition of IκBα kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 24:1269-1279. [DOI] [PubMed] [Google Scholar]
- 52.Stadnyk, A. W. 1994. Cytokine production by epithelial cells. FASEB J. 8:1041-1047. [DOI] [PubMed] [Google Scholar]
- 53.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas, C. F. J., and A. H. Limper. 2004. Pneumocystis pneumonia. N. Engl. J. Med. 350:2487-2498. [DOI] [PubMed] [Google Scholar]
- 55.Vanderbilt, J. N., E. M. Mager, L. Allen, T. Sawa, J. Wiener-Kronish, R. Gonzalez, and L. G. Dobbs. 2003. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am. J. Respir. Cell Mol. Biol. 29:661-668. [DOI] [PubMed] [Google Scholar]
- 56.Varthalitis, I., and F. Meunier. 1993. Pneumocystis carinii pneumonia in cancer patients. Cancer Treat. Rev. 19:387-413. [DOI] [PubMed] [Google Scholar]
- 57.Wahl, C., S. Liptay, G. Adler, and R. M. Schmid. 1998. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 101:1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson, J. 2002. Pneumocystis carinii: where are we now? J. HIV Ther. 7:8-12. [PubMed] [Google Scholar]
- 59.Weber, C. K., S. Liptay, T. Wirth, G. Adler, and R. M. Schmid. 2000. Suppression of NF-κB activity by sulfasalazine is mediated by direct inhibition of IκB kinases alpha and beta. Gastroenterology 119:1209-1218. [DOI] [PubMed] [Google Scholar]
- 60.Wikenheiser, K. A., D. K. Vorbroker, W. A. Rice, J. C. Clark, C. J. Bachurski, H. K. Oie, and J. A. Whitsett. 1993. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc. Natl. Acad. Sci. USA 90:11029-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, M. C. 2003. Alveolar type I cells: molecular phenotype and development. Annu. Rev. Physiol. 65:669-695. [DOI] [PubMed] [Google Scholar]
- 62.Wolff, A. J., and A. E. O'Donnell. 2003. HIV-related pulmonary infections: a review of the recent literature. Curr. Opin. Pulm. Med. 9:210-214. [DOI] [PubMed] [Google Scholar]
- 63.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function contributing to the pathogenesis of Pneumocystis pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1997. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am. J. Respir. Cell Mol. Biol. 17:491-500. [DOI] [PubMed] [Google Scholar]
- 65.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1999. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect. Immun. 67:3452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright, T. W., R. H. Notter, Z. Wang, A. G. Harmsen, and F. Gigliotti. 2001. Pulmonary inflammation disrupts surfactant function during Pneumocystis carinii pneumonia. Infect. Immun. 69:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright, T. W., G. S. Pryhuber, P. R. Chess, Z. Wang, R. H. Notter, and F. Gigliotti. 2004. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J. Immumol. 172:2511-2521. [DOI] [PubMed] [Google Scholar]
- 68.Yoneda, K., and P. D. Walzer. 1980. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect. Immun. 29:692-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang, J., J. Zhu, A. Imrich, M. Cushion, T. B. Kinane, and H. Koziel. 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect. Immun. 72:3147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]