Abstract
Genome sequencing of the relapsing fever spirochetes Borrelia hermsii and Borrelia turicatae identified three open reading frames (ORFs) on the chromosomes that contained internal, tandemly repeated amino acid sequences that were absent in the Lyme disease spirochete Borrelia burgdorferi. The predicted amino acid sequences of these genes (BH0209, BH0512, and BH0553) have hydrophobic N termini, indicating that these proteins may be secreted. B. hermsii transcribed the three ORFs in vitro, and the BH0512- and BH0553-encoded proteins (PBH-512 and PBH-553) were produced in vitro and in experimentally infected mice. PBH-512 and PBH-553 were on the spirochete's outer surface, and antiserum to these proteins reduced the adherence of B. hermsii to red blood cells. PCR analyses of 28 isolates of B. hermsii and 8 isolates of B. turicatae demonstrated polymorphism in each gene correlated with the number of repeats. Serum samples from relapsing fever patients reacted with recombinant PBH-512 and PBH-553, suggesting that these proteins are produced during human infection. These polymorphic proteins may be involved in the pathogenicity of these relapsing fever spirochetes and provide a mechanism for antigenic heterogeneity within their populations.
Relapsing fevers are a group of closely allied acute infectious disorders characterized by recurrent febrile symptoms that are caused by blood spirochetes of the genus Borrelia (40). The human body louse and soft ticks are the arthropod vectors of relapsing fever spirochetes. While louse-borne relapsing fever is often epidemic, tick-borne relapsing fever is an endemic zoonotic infection, with cases regularly reported in Africa, Asia, and the Americas (10). The case fatality rate of tick-borne relapsing fever is approximately 5% in adult patients and can reach more than 20% in patients below 1 year of age (40). In the United States, Borrelia hermsii and Borrelia turicatae are the primary agents of tick-borne relapsing fever. These two Borrelia species are transmitted to humans through the bites of the soft ticks Ornithodoros hermsi and Ornithodoros turicata, respectively. These fast-feeding ticks transmit the bacteria to their host during the 15 to 90 min required for their blood meal. In mammals, the bacteremia associated with relapsing fever can achieve a cell density as high as 108 spirochetes/ml, and each relapse is associated with a new bacteremic peak. In B. hermsii, antigenic variation associated with these relapses involves the sequential production of variable large proteins and variable small proteins (Vsp) to evade the mammalian immune response (4) and prolong the mammal's infectivity to fast-feeding ticks (34).
A more prevalent tick-borne disorder is Lyme disease, caused by Borrelia burgdorferi in North America and by Borrelia afzelii and Borrelia garinii as well as B. burgdorferi in Eurasia. Although B. burgdorferi and the tick-borne relapsing fever spirochetes are closely related (14) and transmitted by ticks, there are major differences in the pathogenicities of these two types of spirochetes. First, B. hermsii causes recurrent, acute, and temporally restricted symptoms, whereas B. burgdorferi-related symptoms may be chronic and persistent. Relapsing fever spirochetes achieve high cell densities in the blood, while B. burgdorferi concentrations are much lower (not over 104cells/ml) (45). In unfed Ixodes scapularis ticks, B. burgdorferi is usually restricted to the midgut. However, during the several days of tick feeding, B. burgdorferi replicates and disseminates from the midgut to the salivary glands for transmission in the saliva (30, 47). In contrast, relapsing fever spirochetes in unfed Ornithodoros ticks are present in many tissues and persistently infect the salivary glands (34). As a consequence, relapsing fever spirochetes are positioned for efficient transmission to mammalian hosts during the short time these ticks feed. The differences between the two microorganisms indicate that they have evolved with their hosts and with very different vectors to maximize their horizontal transmissions in nature. However, most of the mechanisms controlling vector specificities and differences in pathogenicities remain unknown.
To study the specific behaviors of relapsing fever spirochetes by comparative genomics with the B. burgdorferi DNA sequence (7, 11), genomic sequencing of two relapsing fever spirochetes is in progress in our laboratory. In the present study, we identified three open reading frames (ORFs) containing repeated sequences unique to relapsing fever spirochetes. We studied the polymorphism of these genes among numerous isolates of relapsing fever spirochetes, and we quantified their mRNA in various conditions by quantitative reverse transcription-PCR (qRT-PCR). We also produced specific polyclonal antibodies to assess the expression of these genes in vitro and in vivo and to evaluate the surface exposure of the proteins by protease accessibility experiments. To investigate the antigenic variability of the corresponding proteins, we analyzed B. hermsii isolates by Western blotting. The immunoreactivity of these recombinant proteins was examined with serum samples from human relapsing fever patients.
MATERIALS AND METHODS
Borrelia strains and cultivation.
A total of 37 Borrelia isolates were studied: 28 isolates of B. hermsii, 1 isolate of B. burgdorferi, and 8 isolates of B. turicatae (Table 1). These isolates originated primarily from ticks or humans from North America. All spirochetes were cultured in modified Kelly's medium (complete BSK-H medium) (Sigma-Aldrich, St. Louis, Mo.) supplemented to 12% rabbit serum.
TABLE 1.
Borrelia species and isolates used in this study
| Strain code | Source | Reference, origin, or designation |
|---|---|---|
| B. hermsii | ||
| CAR | Human | 26 |
| BAK | Human | 26 |
| SWA | Human | 26 |
| CON | Human | 17 |
| BYM | Human | 26 |
| FRE | Human | 36 |
| BRO | Human | 26 |
| HAL | Human | 26 |
| DAH | Human | 2 |
| MIL | Human | 26 |
| RAL | Human | 12 |
| SIS | Ornithodoros hermsi | 12 |
| FRO | Human | 37 |
| GAR | Human | 26 |
| EST | Chipmunk | 43 |
| MAN | Human | 17 |
| WAD | Human | 12 |
| CMC | Human | 26 |
| GMC | Human | 26 |
| RUM | Human | 26 |
| HAN | Human | 36 |
| OKA-1 | Human | 2 |
| OKA-2 | Human | 2 |
| OKA-3 | Human | 2 |
| REN | Human | 36 |
| YOR | Human | 17 |
| SIL | Human | 26 |
| LAK | Human | 35 |
| B. turicatae | ||
| TCB-1 | Dog | Texas |
| TCB-2 | Dog | Texas |
| FCB | Dog | 5 |
| 95PE570 | Ornithodoros turicata | Texas |
| RML | O. turicata | Texas |
| 99PEI-326 | O. turicata | Texas |
| 99PE1807 | O. turicata | Texas |
| 91E-135 | O. turicata | Texas |
| B. burgdorferi B31 | Ixodes scapularis | ATCC 35210 |
PCR.
Total genomic DNAs were purified from 500-ml cultures as described previously (39) and quantified by UV spectrophotometry, and 50 ng of genomic DNA was used as a template for each PCR. Taq DNA polymerase, deoxynucleoside triphosphates, and buffer were used according to the recommendations of the manufacturer (Perkin-Elmer Life and Analytical Sciences, Boston, Mass.). PCR amplification of fragments shorter than 2 kb included an initial denaturation for 3 min at 94°C, followed with 30 cycles (94°C for 15 s, 55°C for 30 s, 72°C for 45 s) and 1 extension cycle at 72°C for 10 min. PCR amplification of fragments used for cloning in an expression vector were performed using the Expand high fidelity PCR system (Roche Diagnostics Corporation, Indianapolis, Ind.) with 10 cycles of 94°C for 10 s, 55°C for 30 s, and 68°C for 10 min, followed by 20 cycles with the time of each successive extension step increasing by 20 s. After PCR amplification, the products were electrophoresed in a 1% agarose gel and visualized with ethidium bromide staining and UV transillumination. The lengths of the amplification products were estimated with Quantity One software from Bio-Rad Laboratories (Hercules, Calif.).
Production and purification of His-tagged fusion proteins.
The BH0209, BH0512, and BH0553 genes of B. hermsii DAH were amplified from genomic DNA by PCR using the respective primer pairs Up-BH209 and Low-BH209, Up exp-BH512 and Low exp-BH512, and Up exp-BH553 and Low exp-BH553 (Table 2). The PCR products were cloned into the pTRC-His TOPO TA vector according to the instructions of the manufacturer (Invitrogen, Carlsbad, Calif.). The resulting plasmids were transformed into Escherichia coli BL21(DE3). Following selection on LB agar medium supplemented with ampicillin (100 μg/ml), recombinant clones were tested for the expression and synthesis of recombinant proteins after induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma-Aldrich, St. Louis, Mo.). Purifications of His-tagged fusion proteins were achieved under denaturing conditions by use of a nickel Sepharose high performance chromatography column according to the instructions of the manufacturer (Amersham Biosciences, Uppsala, Sweden).
TABLE 2.
Oligonucleotides used for PCR
| Gene | Primer | Sequence (5′ to 3′) | Purposea |
|---|---|---|---|
| BH0209 | Up-BH209 | ATTGATCTTTTAAGAAATAAA | P, E |
| Low-BH209 | CCAAATGAAGCAATAAGTA | P, E | |
| BH0512 | Up-BH512 | CTTGCTTTGAAATATGC | P |
| Low-BH512 | TATCGCTTCTTTCTCTT | P | |
| Up exp-BH512 | TTAAAGGATAAAATTAGTGG | E | |
| Low exp-BH512 | AAAACTATCCCTTAATCATC | E | |
| BH0553 | Up-BH553 | ATATGGATCTCAACGCTGAA | P |
| Low-BH553 | TAAAAGTGCAAAAAGCCAATA | P | |
| Up exp-BH553 | AGTGACTATATAATAAATCAC | E | |
| Low exp-BH553 | GGTATTAAGACCTTCAG | E | |
| BT0209 | Up-BT209 | AACGAGGCGAGGGTTAT | P |
| Low-BT209 | GTTATTTGCAAGTTCGGAAAG | P | |
| BT0512 | Up-BT512 | AAGATATCTGCTCTTAAGTCA | P |
| Low-BT512 | GATTCAATGCATGTATTTCT | P | |
| BT0553 | Up-BT553 | TTTGTCTTTTAGTGCATTGAT | P |
| Low-BT553 | ACACATATGATATGCATGCT | P |
P, polymorphism study; E, expression of recombinant protein.
Polyclonal antibody production.
Fusion proteins were excised from sodium dodecyl sulfate (SDS)-polyacrylamide gels, minced, suspended in 10 ml of phosphate-buffered saline (PBS), and used to immunize rabbits by subcutaneous and intramuscular inoculation. The rabbits received one primary immunization and three boosts at 3-week intervals. Three weeks after the last boost, the rabbits were bled, and sera were collected from the rabbits to obtain specific antibodies raised against the C termini of PBH-209 (amino acids [aa] 188 to 593) and PBH-512 (aa 1301 to 2394). This work was approved by the Rocky Mountain Laboratories Animal Care and Use Committee.
Antibodies specific to PBH-553 were also produced in rabbits; however, the immunization protocol included 5 mg of two synthetic peptides, NKINNNKETLKDALHKNTHKLC and KINNNKETLNNNIQRLSDLDDC, representing aa 307 to 327 and 383 to 403, respectively, of PBH-553 coupled to bovine serum albumin with cysteine residues added to their C termini (Eurogentec, San Diego, Calif.). Freund's complete adjuvant (Sigma-Aldrich) was used with the primary injection, and Freund's incomplete adjuvant (Sigma-Aldrich) was used with the boosts.
Prediction of secondary structure.
Amino acid sequences of PBH-209, PBH-512, and PBH-553 were analyzed for secondary structure using a combination of three methods provided by Pôle BioInformatique Lyonnais (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_seccons.html): SOPM, DSC, and PHD (13, 16, 31).
Adhesion assay of B. hermsii to red blood cells.
To measure the effect of polyclonal antibodies to PBH-512 and PBH-553 on B. hermsii adhesion, each heat-inactivated serum sample was diluted to a ratio of 1/50 in 50 μl of BSK-H medium supplemented with 6% of rabbit serum and incubated with 106 live B. hermsii cells at 34°C for 2 h. An equivalent volume of BSK-H medium mixed with citrated human whole blood containing 105 human red blood cells was then added, and the mixtures were incubated in 96-well plates at 34°C and with 5% CO2 for 1 h. The percentages of live B. hermsii cells bound to red blood cells were recorded from a total of 10 microscopic fields using a phase-contrast microscope and a magnification of ×400. A mouse monoclonal antibody to flagellin (H9724) and a normal rabbit serum were used as negative controls.
SDS-PAGE and immunoblotting.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed according to the method of Laemmli (20) with a 4% stacking gel and a 4-to-15% separating gel. After electrophoresis, gels were stained with Coomassie brilliant blue R-250 (Invitrogen). Proteins were transferred onto nitrocellulose membranes (Bio-Rad) for immunoblotting as described by Towbin et al. (42). Histidine-tagged proteins were detected using the alkaline phosphatase-conjugated anti-His mouse monoclonal antibody (Invitrogen) according to the instructions of the manufacturer. To detect specific B. hermsii proteins, cell lysates representing 108 cells were used. Human relapsing fever sera used in this study were diluted at a ratio of 1/100 and were as previously described (36). Polyclonal rabbit antibodies produced in this study were used at a dilution ratio of 1/500. Bound antibodies were detected with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Zymed Laboratories, South San Francisco, Calif.) or 125I-labeled protein A (Amersham Biosciences, Piscataway, N.J.).
PK treatment.
B. hermsii DAH cultures (108cells/ml) were treated with proteinase K (PK) as described previously (19). Briefly, B. hermsii cells from BSK-H medium with 12% rabbit serum were centrifuged at 4,300 × g for 15 min at 4°C. The resulting pellet was then washed three times in PBS with 5 mM MgCl250 mM sucrose and suspended to a final cell concentration of approximately 108cells/ml. The cell suspension was then split into 1-ml aliquots and mixed with a 1/10 volume of a proteinase K solution to give final concentrations ranging from 50 to 400 mg/ml. An equivalent volume of water was added to an untreated sample as a negative control. After 40 min of incubation at 37°C, phenylmethylsulfonyl fluoride was added to a final concentration of 1 mM to inhibit proteinase K activity, and the cells were washed three times in PBS with 5 mM MgCl2-50 mM sucrose-1 mM phenylmethylsulfonyl fluoride. Lysates were analyzed by SDS-PAGE and immunoblotting with rabbit anti-PBH-512, anti-PBH-553, or anti-GlpQ sera (36) or mouse anti-Vsp33 monoclonal antibody, H4825 (3).
In vivo studies.
Two adult female RML mice (Rocky Mountain Laboratories Animal Facility) were inoculated intraperitoneally with approximately 2.5 × 108 B. hermsii DAH cells. Bacteremias were monitored in the mice during the next 3 days by examining the peripheral blood from the tail vein by nonphase, bright-field microscopy. The spirochetemic mice were bled while anesthetized with isoflurane. B. hermsii cells were separated from the red blood cells with a low-speed centrifugation (100 × g, 15 min), followed by a high-speed centrifugation of 16,000 × g for 15 min.
RNA isolation.
Isolations of total RNA from 500-ml B. hermsii DAH cultures (5 × 107 cells/ml) were performed with the RNeasy kit (QIAGEN, Inc., Valencia, Calif.). Contaminating DNA in the RNA preparations was removed using the DNA-free kit (Ambion, Inc., Austin, TX). RNA quality and integrity were assessed with the RNA 6000 Nano LabChip kit with a bioanalyzer (model 2100; Agilent Technologies, Inc., Wilmington, Del.). Concentrations of total RNA were determined with an Ultrospec 3000 UV spectrophotometer (Amersham Biosciences Corp., Piscataway, N.J.).
Quantitative RT-PCR.
Oligonucleotides and probes (Table 3) were designed with Primer Express software (version 2.0; Applied Biosystems, Foster City, Calif.) and purchased from Applied Biosystems. The probes consisted of an oligonucleotide labeled at the 5′ end with 6-carboxyfluorescein as the reporter and at the 3′ end with carboxytetramethylrhodamine as the quencher. qRT-PCR was performed with the TaqMan one-step RT-PCR master mix reagents kit according to the manufacturer's instructions (Applied Biosystems). The RT-PCR mixture (25 μl) contained 6.25 U of multiscribe reverse transcriptase, 10 U of RNase inhibitor, 500 nM each gene-specific primer, 100 nM each probe, and 10 ng of RNA template. Amplification and detection of specific products were performed with the ABI Prism 7700 detection system (Applied Biosystems) with the following conditions: 1 cycle at 48°C for 30 min, 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s and at 60°C for 1 min. The amount of RNA for each gene was normalized to the quantity of flaB RNA in each sample and was calculated with the annealing efficiency of each probe.
TABLE 3.
Oligonucleotides and fluorescent probes used for real-time RT-PCR
| Gene | Primers and probe | Sequence (5′ to 3′) |
|---|---|---|
| BH0209 | Up RT-209 | CTCTTGAAAAACTTGACAAAAGTCTCA |
| Low RT-209 | CCATGATCTTGTTGCTGTCCTATG | |
| Probe BH0209 | AAACAGAAGTGAACACATTG | |
| BH0512 | Up RT-512 | TGGCAAAGGGAAGTAGGTT |
| Low RT-512 | ATGTCCTGAGAGTTCATTGAAAAT | |
| Probe BH0512 | TCACTAAGTTCTTTTCCCTTAGTCCTTTGAGTTT | |
| BH0553 | Up RT-553 | GGCAAAAAGATACTTCCAATAC |
| Low RT-553 | TTTGGGCTCTCCAAAAAG | |
| Probe BH0553 | AATGTGCACGAAAATAGCATAAGAATTCT | |
| flaB | Up-FlaB | CAGCTAGTGATGCTGGTGTGTTAAT |
| Low-FlaB | TTTGCGGGTTGCATTCCAAGCTCTT | |
| Probe FlaB | AAGTCAGCTGCTCAAAATGTAAAAAC | |
| vsp33 | Up-Vsp33 | AGAAGGGAAGTGATGATTTCTTAACC |
| Low-Vsp33 | GCATTACCTTTCTTTATAGCCTTTGG | |
| Probe Vsp33 | GCACTTTGACCAAGATTATTATGCTGCGCTTT |
Nucleotide sequence accession numbers.
GenBank accession numbers of genes or coding DNA sequences (CDS) described in this study are the following: for B. hermsii DAH BH0209, AY692275; for BH0512, AY692276; for BH0553, AY692277; for B. turicatae 91E135 BT0209, AY692272; for BT0512, AY692273; for BT0553, AY692274; for B. hermsii CON CDS encoding the repeated domains of BH0512, AY823089; for B. hermsii CMC CDS encoding the repeated domains of BH0512, AY823090; for B. hermsii SIL CDS encoding the repeated domains of BH0512, AY823091; for B. hermsii CMC CDS encoding the repeated domains of BH0553, AY823087; for B. hermsii FRO CDS encoding the repeated domains of BH0553, AY823088; and for B. hermsii RUM CDS encoding the repeated domains of BH0553, AY823086.
RESULTS
In silico analysis of BH0209, BH0512, and BH0553.
DNA sequencing of the B. hermsii DAH chromosome identified three ORFs that were homologous to ORFs on the chromosome of B. burgdorferi (designated BB0209, BB0512, and BB0553) (11) but contained numerous, direct tandemly repeated sequences absent in the B. burgdorferi homologs (Fig. 1). We have designated these three ORFs in B. hermsii DAH as BH0209, BH0512, and BH0553 (Table 4).
FIG. 1.
Illustration of the three chromosomal genes, BH0209 (A), BH0512 (B), and BH0553 (C), and respective hydropathy profiles of their deduced amino acid sequences using the Kyte and Doolittle algorithm (18). In the large arrows at the top, the locations of DNA coding the unique repeat domains and putative signal sequences are represented by gray and black boxes, respectively. Putative signal sequences, deduced amino acid sequences, and the respective number of repeats are shown below the large arrows. Broken lines represent regions omitted from the diagram of BH0512 (7,185 bp) to shorten its schematic length. Hydropathy profiles were determined using a window size of nine amino acids. The vertical axis displays relative hydrophobicity with negative scores indicating relative hydrophilicity. The horizontal axis indicates amino acid numbers. Thin single-headed arrows indicate hydrophobic N termini (N-ter); thin double-headed arrows indicate the repeat regions of the proteins.
TABLE 4.
Chromosomal ORFs in B. hermsii and B. turicatae that contain direct, internal repeated sequences
| Gene | bp | no. of aa | kDa | pI |
|---|---|---|---|---|
| BH0209 | 1,782 | 593 | 67 | 9.27 |
| BH0512 | 7,185 | 2,394 | 280 | 4.49 |
| BH0553 | 2,283 | 760 | 90 | 6.87 |
| BT0209 | 1,689 | 563 | 67.2 | 9.12 |
| BT0512 | 6,888 | 2,296 | 269 | 4.77 |
| BT0553 | 2,127 | 709 | 83.5 | 6.31 |
From aa 223 to 564, the predicted amino acid sequence of the BH0209 protein, PBH-209, contained a repetitive domain that contained 19 identical copies of the 18-aa repeat KEPQINKKEPTGKDKPAD (Fig. 1A). Hydropathy analysis of PBH-209 (Fig. 1A) (18) showed that the repeat region was highly hydrophilic, while the N terminus was hydrophobic, which is consistent with the presence of a potential signal peptide. The PBH-209 amino acid sequence was submitted to the signalP program (23) to predict its subcellular localization. This analysis suggested that PBH-209 may be secreted, since it possessed a predicted N-terminal signal sequence (Fig. 1A). Alignment of B. hermsii PBH-209 with the predicted protein of B. burgdorferi BB0209 revealed a 36.4% identity (69% identity after exclusion of the repeated domain) and showed that the repeated motifs were unique to B. hermsii. BLAST searches also revealed 28% and 29% identities between PBH-209 and multiple banded antigens (MBAs) of Ureaplasma parvum and Ureaplasma urealyticum, respectively (46). The MBAs consist of small repeating units associated with antigen size variation in invasive Ureaplasma isolates. The MBAs also contain serovar-specific epitopes that are dominant antigens recognized during Ureaplasma infections of humans. PBH-209 also shared 16.3% identity with VraA of B. burgdorferi. VraA is a surface-exposed protein encoded on the linear plasmid lp28-4 (19). Like PBH-209, VraA contains repeated domains and confers partial protection against experimental infections with Lyme disease spirochetes (19). There is an adjacent ORF (BB0210) on the B. burgdorferi chromosome (7, 11) that contains hydrophilic repeats, although there is no sequence similarity with BH0209. Computer analysis for secondary structure predicted that PBH-209 is composed primarily of random coil structures (72.5%). The percentage of predicted random coil structures increased to 100% when the analysis was performed only on the repeated domain (Table 5).
TABLE 5.
Secondary structure prediction analysis of PBH-209, PBH-512, and PBH-553 and their respective repeated domainsa
| Analyzed protein or repeated domain | % α-Helix | % β-Sheet | % Random coil | % Ambiguous |
|---|---|---|---|---|
| PBH-209 | 21.9 | 4.8 | 72.5 | 0 |
| PBH-209 repeated domain (aa 223-564) | 0 | 0 | 100 | 0 |
| PBH-512 | 78.1 | 6.8 | 10.6 | 0 |
| PBH-512 repeated domain (aa 1319-1582) | 55.7 | 14.8 | 19.3 | 0 |
| PBH-P553 | 62.1 | 10.1 | 20.9 | 0 |
| PBH-553 repeated domain (aa 260-529) | 71.5 | 1.7 | 16.5 | 10.2 |
Values represent percentiles of each secondary structure component and are averages of DSC, PHD, and SOPM predictions.
From aa 1319 to 1582, the predicted amino acid sequence of the BH0512 protein, PBH-512, contained eight copies of the amino acid motif KYAXLEDKYDEKXLXVEKKIDDKTXXXDXLIAS, where X's represent polymorphic sites with variable amino acids (Fig. 1B). Hydropathy analysis of PBH-512 (Fig. 1B) identified an N-terminal hydrophobic core consistent with the presence of a potential signal peptide (Fig. 1B). Alignment of B. hermsii PBH-512 with the amino acid sequence of the B. burgdorferi BB0512 protein revealed a 51.1% identity (55.9% identity after exclusion of the repeated domain). PBH-512 also had 12.8% identity with the 235-kDa rhoptry protein of Plasmodium yoelii yoelii, a protein involved with merozoite attachment and the invasion of red blood cells in rodents (15). Although the identity shared between PBH-512 and the 235-kDa rhoptry protein is low, both proteins are very large, and identical amino acids were found throughout the two sequences. Additionally, there is only a 16.8-to-18.8% identity between the P. yoelii yoelii 235-kDa rhoptry protein and heterologous proteins of other Plasmodium species. Despite this low level of identity, these plasmodium proteins all share the same binding properties (9, 44). Furthermore, like PBH-512, the 235-kDa rhoptry protein contains repeated domains. The predicted secondary structure of PBH-512 and its repeated domain (aa 1319 to 1582) suggested that both are primarily composed of α-helix structures, which accounted for 78.1 and 55.7%, respectively, of their compositions (Table 5).
From aa 260 to 301, the predicted amino acid sequence of the BH0553 protein, PBH-553, contained two identical copies of the peptide LNELNDKINNNKDALNNNIHR. From aa 308 to 382, there was a second motif, KINNNKETLKDALHKNTHKLSELDX, repeated three times. This 25-aa motif was conserved among the three repeats, except that the C-terminal asparagine residue in the first two copies was replaced by an aspartic acid in the third repeat. From aa 383 to 529, an identical 21-aa sequence, KINNNKETLNNNIQRLSDLDD, was repeated seven times (Fig. 1C). Hydropathy analysis showed that the repeat region was very hydrophilic (Fig. 1C) while the N terminus was hydrophobic, and there was a putative signal sequence (Fig. 1C). Alignment of PBH-553 revealed only 22.5% identity with the B. burgdorferi BB0553 protein, while the identity increased to 55.9% after exclusion of the repeated domain. BLAST searches did not reveal any other proteins in the databases with significant sequence similarities. Submission of the complete PBH-553 and its repeated domain (aa 260 to 529) to DSC, PHD, and SOPM programs suggested that the entire protein and the repeated domain were composed mainly of α-helix structures (Table 5).
PCR analysis of BH0209, BH0512, and BH0553.
To determine if BH0209, BH0512, and BH0553 were polymorphic genes, we designed specific sets of primers that flanked their respective repeated domains (Table 2) and performed PCR analysis with 28 isolates of B. hermsii (Table 1). The amplicons were electrophoresed and their sizes were estimated with Quantity One analysis software. The numbers of repeats were then calculated based on the sizes of the amplicons minus the numbers of nucleotides outside the repeated domains. PCR amplification of the repeated domain of BH0209 gave single amplicons ranging in size from 990 to 2,430 bp (Fig. 2A). The estimated number of repeats for each isolate was between 9 and 35.7, based also on the assumption that the repeated motif sequences were fully conserved. To further assess the conservation of BH0209 repeats, a PstI restriction analysis was performed on the PCR fragments (data not shown). These results showed a common restriction pattern composed of three fragments of 156, 140, and 54 bp in all the isolates, which also supports the notion that the sequence of repeated motifs was identical for each isolate.
FIG. 2.
Polymorphism analyses of BH0209 (A), BH0512 (B), and BH0553 (C) in 28 isolates of B. hermsii (as labeled with their codes above each gel). After PCR amplification, the products were electrophoresed in a 1% agarose gel and visualized after ethidium bromide staining. The lengths of the amplification products were estimated using Quantity One software. The positions of the molecular size markers (M) are indicated on both sides of the gels in bp.
PCR analysis of the BH0512 repeated domain in B. hermsii produced single amplicons ranging in size from 594 to 1,118 bp (Fig. 2B), with the estimated number of repeats varying from 3 to 8.3. To confirm BH0512 polymorphism, DNA sequences encoding the repeat domains of three other B. hermsii isolates (CON, CMC, SIL) were determined. Alignment of the repeated domains deduced from these DNA sequences corroborated that BH0512 polymorphism was due to variation in the number of repeated motifs as well as in the amino acid sequences (Fig. 3).
FIG. 3.
Alignments of repeated domains encoded by BH0512 from the B. hermsii isolates DAH, SIL, COM, and CMC. Black boxes with white type represent amino acid residues conserved in the four isolates, and white boxes with boldface type represent amino acid residues present in at least 50% of the sequences.
PCR analysis of the BH0553 repeated domain in the 28 isolates of B. hermsii yielded single amplicons ranging in size from 2,330 to 2,920 bp, with the number of repeats for each isolate estimated to be between 10 and 19.4. Polymorphism in this locus was analyzed further by DNA sequencing the repeated domain from additional isolates (FRO, CMC, RUM). These results confirmed that the polymorphism of BH0553 was directly related to the number of repeated motifs (Table 6) but also that there was heterogeneity in the sequences among these isolates.
TABLE 6.
Repeated motifs encoded by BH0553 from four B. hermsii isolatesa
| Isolate | Repeated motif I | Repeated motif II | Repeated motif III |
|---|---|---|---|
| DAH | 2× LNELNDKINNNKDALNNNIHR | 3× KINNNKETLKDALHKNTHKLSELDX | 7× KINNNKETLNNNIQRLSDLDD |
| FRO | 2× LNELNDKINNNKDALNNNIHR | 3× KINNNKETLKDALHKNTHKLSELDX | 5× KINNNKETLNNNIQRLSDLDD |
| CMC | 3× SELDDKIINNKEALKDALHKNTHKL | 2× SELDNKIINNKDTINNNIHRL | 2× SELDNKIINNKDTINNNIHRLL |
| RUM | 4× SELDDKIINNKEALKDALHKNTHKL | 3× SELDNKIINNKDTINNNIHRL | 7× SELDNKIINNKDTXNNNIQRL |
X represents a variable amino acid.
In silico and PCR analysis of BT0209, BT0512, and BT0553.
The B. turicatae 91E135 chromosome also contained three ORFs homologous to BH0209, BH0512, and BH0553 (designated BT0209, BT0512, and BT0553, respectively) with repeated motifs absent in B. burgdorferi (Table 4).
Analysis of the BT0209-encoded protein, PBT-209, with the signalP program predicted a signal sequence with a cleavage site after aa 26. From aa 220 to 507, there were eight repeats, which were XXNTQEHMXKKDXXNTQENMSKKDNXXTQEHMXKKD. PBT-209 and PBH-209 were 49.3% identical, and the identity increased to 84.7% when the repeats were excluded from the alignment. PCR analysis of the BT0209 repeat region in eight isolates of B. turicatae produced single amplicons ranging in size from 1,623 to 1,849 bp, corresponding to 8.5 to 10.6 copies of the 36-aa repeated motif.
PBT-512, the protein encoded by BT0512, contained a predicted signal sequence with a cleavage site after aa 30. The repeated domain contained five copies of the 33-aa motif KYDEKHLLVEKKINXKTNSVEELIAXKYAEXXX present from aa 1,326 to 1,490. PBT-512 and PBH-512 were 83.1% identical, and the identity increased to 87.8% when the repeats were excluded from the alignment. PCR analysis of BT0512 produced a single amplicon of 678 bp, which suggested that all of the isolates contained 5.7 copies of the 33-aa repeat (Fig. 4B).
FIG. 4.
Polymorphism analyses of BT0209 (A), BT0512 (B), and BT0553 (C) in eight isolates of B. turicatae (as labeled above each gel). After PCR amplification, the products were electrophoresed in a 1% agarose gel and visualized after ethidium bromide staining. The lengths of the amplification products were estimated using Quantity One software. The positions of the molecular size markers (M) are indicated on both sides of the gels in bp.
The protein encoded by BT0553, PBT-553, contained a predicted signal peptide sequence with a cleavage site after aa 22. PBT-553 contained a repeated domain from aa 274 to 477 with three tandem repeats of the peptide KINNNKDTLNNNTHKLSELDN, two repeats of KINNNKDTINNNTHKLSELDX,and six repeats of KINNNKDTLNNNTHKLNELGD, in that order. PBH-553 and PBT-553 were 77.8% identical, but the identity decreased slightly to 75.7% when the repeats were excluded from the alignment. PCR amplification of the BT0553 repeat region produced single amplicons ranging in size from 824 to 1,102 bp (Fig. 4C), indicating that the number of repeated motifs ranged from 10.4 to 14.8 in the eight B. turicatae isolates.
Quantitative RT-PCR analysis of BH0209, BH0512, and BH0553 transcripts.
Transcription levels of BH0209, BH0512, and BH0553 in B. hermsii DAH grown in vitro were determined by qRT-PCR. Experiments were performed in triplicate with RNA samples isolated from three independent exponential-phase cultures (5 × 107 cells/ml) grown at 34°C and 24°C (Table 7). Standard curves were generated for each gene with genomic DNA isolated from B. hermsii DAH to determine the relative quantities of specific transcripts. The quantity of RNA for each gene was normalized to the quantity of flaB RNA present in each preparation. vsp33 was used as a positive control because this gene is up-regulated at the lower temperature (33). Specific RNAs were detected for all three genes, although no significant differences in the amounts of transcripts at the two temperatures were observed. None of the transcripts were as abundant as the transcript for flaB, and vsp33 transcripts were 3.60-fold less abundant at 34°C than at 24°C.
TABLE 7.
Relative amounts of mRNA of flaB, vsp33, BH0209, BH0512, and BH0553 genes in B. hermsii DAH grown at 34°C or 24°C as determined by qRT-PCR analysis
| Gene | Transcript amount (ng) from growth ata:
|
Fold variation in mRNA amountsb | |
|---|---|---|---|
| 34°C | 24°C | ||
| flaB | 16.58 ± 0 | 16.58 ± 0 | 1.00 |
| vsp33 | 18.84 ± 5.66 | 68.54 ± 21.74 | −3.64c |
| BH0209 | 0.86 ± 0.32 | 0.92 ± 0.24 | −1.06 |
| BH0512 | 0.15 ± 0.05 | 0.09 ± 0.01 | 1.66 |
| BH0553 | 2.97 ± 1.35 | 2.02 ± 0.46 | 1.47 |
Measurements of transcript amounts are based on each probe efficiency and are normalized to flaB.
Relative amounts of mRNA in B. hermsii DAH at 34°C and at 24°C.
P < 0.001.
In vitro and in vivo synthesis of specific proteins.
The synthesis of PBH-209, PBH-512, and PBH-553 by B. hermsii was examined with polyclonal antibodies produced in rabbits immunized with either purified recombinant proteins or synthetic peptides. Immunoblot analysis with polyclonal antibodies produced with recombinant PBH-209 were nonreactive with B. hermsii DAH lysates, although the antibodies reacted strongly with the recombinant protein (data not shown). PBH-512 antiserum reacted strongly with a 245-kDa protein (Fig. 5A, lane 1) and weakly with a 235-kDa protein in B. turicatae 91E135 (Fig. 5A, lane 2), suggesting that both species of relapsing fever spirochetes produced this protein. In contrast, PBH-512 antiserum failed to detect any protein in B. burgdorferi (Fig. 5A, lane 3). PBH-553 antiserum reacted with proteins of 86, 60, and 57 kDa in B. hermsii (Fig. 5B, lane 1), with an 82-kDa protein in B. turicatae, and with an 86-kDa protein in B. burgdorferi (Fig. 5B, lanes 2 and 3), suggesting that all three species produced PBH-553 homologues in vitro.
FIG. 5.
Immunoblot analyses of B. hermsii DAH, B. turicatae 91E135, and B. burgdorferi B31 probed with PBH-512 antibodies (A) and PBH-553 antibodies (B). Comparative immunoblot analyses of B. hermsii DAH lysates produced in vitro and in vivo using PBH-512 antibodies (C) and PBH-553 antibodies (D). Primary bound antibodies were detected with horseradish peroxidase-linked goat anti-rabbit IgG. The positions of the molecular mass markers are indicated on the left of each immunoblot in kDa.
The in vivo production in mice of PBH-209, PBH-512, and PBH-553 by B. hermsii was examined. Spirochetes were collected from the blood of infected mice and compared to in vitro-grown spirochetes by SDS-PAGE and immunoblot analysis (Fig. 5C and D). PBH-512 antiserum reacted with a 245-kDa protein in B. hermsii produced in vitro and in vivo (Fig. 5C), and PBH-553 antiserum reacted strongly with an 86-kDa protein in spirochetes grown under either condition (Fig. 5D). Therefore, B. hermsii produced PBH-512 and PBH-553 in mammals, while PBH-209 was not detected.
Localization of PBH-512 and PBH-553.
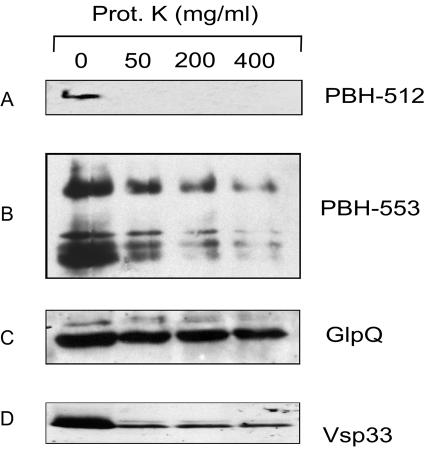
Computer analysis predicted that PBH-512 and PBH-553 contained signal sequences; therefore we sought evidence for their possible locations on the outer surfaces of the spirochetes by performing protease accessibility experiments. Freshly harvested, intact B. hermsii was treated with PK, fractionated by SDS-PAGE, blotted, and probed with PBH-512- or PBH-553-specific antisera (Fig. 6). We included samples for detecting Vsp33 and GlpQ, because previous work demonstrated that the former protein is on the spirochete's outer surface (32) while the latter is periplasmic (36). The amounts of PBH-512 and PBH-553 detected were reduced after PK digestion, as was that of Vsp33, while that of GlpQ was not (Fig. 6). These results demonstrated that PBH-512 and PBH-553 were on the cell's outer surface.
FIG. 6.
Localization of B. hermsii PBH-512 and PBH-553. Immunoblot analyses of B. hermsii cells treated or not treated with proteinase K (Prot. K) by use of specific antibodies to detect removal of PBH-512 (A) and PBH-553 (B). Control proteins GlpQ (periplasmic) (C) and Vsp33 (surface exposed) (D) were respectively used for accessibility to proteinase K degradation. After proteinase K treatment, the amounts of PBH-512 and PBH-553 were significantly reduced. Bound antibodies were detected with horseradish peroxidase-linked goat anti-rabbit IgG.
Antigenic polymorphism of PBH-512 and PBH-553.
The presence and size variation of PBH-512 and PBH-553 were examined in B. hermsii. Twenty-six of 28 (93%) isolates grown in vitro contained proteins that reacted with anti-PBH-512 antiserum (Fig. 7A). Additionally, a marked size polymorphism, with sizes ranging from 245 to 189 kDa, was evident for this protein. Antiserum raised against the repeated motifs of PBH-553 reacted with only 18 of 28 isolates tested (64%) (Fig. 7B). The 18 positive isolates exhibited an MBA pattern comprised of proteins with molecular masses ranging from 57 to 115 kDa. Interestingly, the reactive isolates all belonged to the recently identified Genomic Group I of B. hermsii based on 16S rRNA, flaB, gyrB, and glpQ sequence analysis (26). In contrast, 10 isolates of B. hermsii in Genomic Group II were no more reactive with the immune sera than with the preimmune sera. These results suggested that each genomic group produced its own specific, repeated motif. Therefore, we determined the DNA sequence of the repeated region of PBH-553 in B. hermsii CMC (a Genomic Group II isolate) and showed that it had only 75% identity with B. hermsii DAH (a Genomic Group I isolate).
FIG. 7.
Immunoblot analyses of 28 isolates of B. hermsii using antisera specific for PBH-512 (A) and PBH-553 (B). The codes for the isolates are indicated at the tops of the immunoblots. Brackets indicate the isolates from Genomic Groups I and II based on 16S rRNA, flaB, gyrB, and glpQ sequence analysis (26). The positions of the molecular mass markers are indicated on the left in kDa. Bound antibodies were detected with 125I-labeled protein A.
Immunoreactivity of human relapsing fever sera.
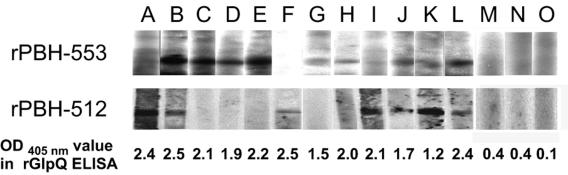
Recombinant PBH-209, PBH-512, and PBH-553 were examined with immunoblot analysis and serum samples from 12 human relapsing fever patients from Washington and Idaho and from British Columbia, Canada (36). Three nonimmune human sera were used as negative controls. The reactivity of the samples was examined with recombinant His-GlpQ in an enzyme-linked immunosorbent assay as described previously (27). Immune sera had optical densities at 405 nm greater than 1.0 (Fig. 8, lanes A through L), whereas control sera had optical densities at 405 nm below 0.5 (Fig. 8, lanes M through O). None of the sera reacted with recombinant PBH-209, suggesting that this protein is not produced or is not antigenic in humans (data not shown). In contrast, 9 of the 12 sera (75%) reacted with recombinant PBH-553, and 7 (58%) reacted with recombinant PBH-512 (Fig. 8, lanes A through L). None of the control sera reacted with the recombinant proteins (Fig. 8, lanes M through O). These results suggested that both PBH-512 and PBH-553 were produced and antigenic in infected humans.
FIG. 8.
Immunoblot analyses of purified recombinant PBH-512 and PBH-553 using 12 human relapsing fever sera and 3 naïve human sera (labeled A through O above immunoblots). Seven of the human relapsing fever sera (58%) reacted with recombinant PBH-512, and eight (67%) reacted with recombinant PBH-553. All human relapsing fever sera were positive, with anti-glpQ antibodies determined by enzyme-linked immunosorbent assay (ELISA) and with rGlpQ indicated at the bottom. As negative controls, experiments were also performed with three normal human sera, none of which immunoreacted significantly with recombinant GlpQ, PBH-512, or PBH-553. Bound antibodies were detected with 125I-labeled protein A. OD, optical density.
Adherence of B. hermsii to red blood cells.
Having shown that PBH-512 and PBH-553 were synthesized in vivo, were on the spirochete's outer surface, and contained polymorphic tandemly repeated motifs, we hypothesized that these proteins may be involved in the adherence of B. hermsii to red blood cells (Fig. 9A). To test this hypothesis, we determined whether antibodies specific for these antigens inhibited spirochete binding to red blood cells. B. hermsii DAH incubated with rabbit antisera raised against PBH-512 and PBH-553 showed a significantly lower percentage of spirochetes attached to red blood cells compared to spirochetes incubated with anti-flagellin or with normal rabbit serum (Fig. 9B).
FIG. 9.
Antibody-mediated inhibition of B. hermsii DAH adhesion to red blood cells. (A) Microphotograph of B. hermsii DAH bound to red blood cells. (B) Percentages of B. hermsii DAH bound to red blood cells after incubation with antibodies against PBH-553 or PBH-0512, control antibody (anti-flagellin), or normal serum. The percentages of bound B. hermsii cells were 46.3 and 39.1% for anti-PBH-512 serum and anti-PBH-553 serum, respectively. Error bars indicate standard deviations of the results from three separate experiments.*, Student's t test P value = 0.01 versus experiment with normal rabbit serum; **, Student's t test P value = 0.004 versus experiment with normal rabbit serum.
DISCUSSION
In the present study, we identified three chromosomal ORFs of B. hermsii and B. turicatae (BH/T0209, BH/T0512, and BH/T0553) that encode proteins with unique, tandemly repeated sequences that are absent in the B. burgdorferi genome (11). These ORFs are highly polymorphic, due to variations in the number of tandem repeats and, in a few cases examined, also in the primary sequences. These repeats may play a role in crucial functions that are specific to relapsing fever spirochetes and their interactions with their mammalian hosts and tick vectors.
B. hermsii BH0209 contained repeated sequences that comprised more than 50% of the entire gene and encoded a very hydrophilic region of the protein. These repeated sequences were identical at the nucleotide level, suggesting that this region is either of recent origin or essential for the function of the encoded protein. Sequence similarities were found between PBH-209 and the MBA of U. parvum and U. urealyticum, which contain a variable number of repeated motifs that exhibit phase variation (22). PCR analyses of the repeat region of BH0209 and BT0209 revealed extensive genetic polymorphism in 36 isolates of two species of relapsing fever spirochete. Although BH0209 was transcribed in vitro, antiserum to recombinant PBH-209 failed to detect any proteins in B. hermsii lysates. Also, human relapsing fever sera were nonreactive with recombinant PBH-209, despite the protein's predicted antigenicity. Consequently, PBH-209 appears to be produced neither in infected humans nor when grown in vitro. Therefore, this protein may be regulated at the translational level and produced in ticks only; however, further experiments are needed to address this possibility.
The second relapsing fever gene encoding unique, tandem repeats was BH0512. This ORF is homologous to BB0512, which encodes the largest protein in B. burgdorferi but which lacks the internal repeats (11). PCR analyses of the BH0512 and BT0512 repeat regions demonstrated significant polymorphism among 28 isolates of B. hermsii but none among 8 isolates of B. turicatae, which, for the latter species, may be due to the smaller number of isolates examined from a more restricted geographic area. PBH-512 demonstrated significant antigenic polymorphism among 28 B. hermsii isolates and was localized to the spirochete's outer surface. PBH-512 was compared with the 235-kDa protein of P. yoelii yoelii, a protein that binds to red blood cells and contains tandemly repeated sequences involved with clonal phenotypic variation (28, 29). Based on several properties similar to those of the plasmodial 235-kDa protein (predicted signal sequence, low but dispersed amino acid identity, presence of repeated domains, very large size), we speculate that PBH-512 may play a role in the adherence of B. hermsii to red blood cells. Cook first described this interaction in 1904 when he confirmed the presence of spirochetes in the blood of tick fever patients in Uganda (8).
BLAST searches with BH0553 did not reveal significant identity with any reported sequence except BB0553 of B. burgdorferi, which lacks the repeated domains. PCR analyses demonstrated that the repeat domains of BH0553 and BT0553 were present in all relapsing fever isolates examined. These tandemly repeated sequences were polymorphic, and antiserum reacted with three proteins of different apparent molecular masses (86, 60, and 57 kDa). This result suggests that the 60- and 57-kDa proteins could be either isoforms or degradation products of the 86-kDa protein. PBH-553 antiserum revealed polymorphism in this protein but a lack of immunoreactivity with Genomic Group II isolates (26). This result agrees with the finding that the sequences of repeated domains from the two genomic groups were different. This result also demonstrates that in addition to having a variation due to the number of repeated sequences, PBH-553 is also polymorphic at the amino acid level.
The most often-reported function of tandem repeats is binding to proteins during processes such as protein transport, complex assembly, and regulation (1). When surface exposed, these proteins can play a role in host cell adhesion. For example, proteins referred to as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) in gram-positive bacteria contain internal, tandemly repeated domains that promote bacterial binding to the extracellular matrices of host tissues (24, 25). An increase in the number of repeats via intragenic duplication can enlarge the protein's surface and thereby enhance its binding properties. Repeated sequences are also generally thought to confer advantages to microorganisms and to be a part of adaptive evolution (1). Recently, a comparative genomics study of eukaryotes and prokaryotes suggested that repeated sequences are recent evolutionary events (21). Some prokaryotes are also believed to vary their number of repeated sequences to generate novel surface antigens and thereby adapt to changing environments (41).
One host cell that relapsing fever spirochetes adhere to is the circulating red blood cells. Cook noted the following: “The organisms have a curious adhesiveness to the corpuscles, and one can often see them break off by apparently a great effort and locomote through the plasma, till sooner or later they bump against another corpuscle and adhere to it.” (8). The adherence to red blood cells is especially dramatic with Borrelia crocidurae, which causes a striking rosette formation with these cells in infected mice (6, 38). Our work with B. hermsii in mice over many years has routinely demonstrated that this spirochete also adheres to red blood cells as observed with microscopy with freshly collected blood from spirochetemic animals. Therefore, the binding properties of PBH-512 and PBH-553 were partially investigated by measuring the adherence of B. hermsii to red blood cells in the presence of PBH-512 and PBH-553 antibodies. Preliminary experiments suggested that PBH-512 and PBH-553 could have binding properties. Nevertheless, to fully address such questions, alternative strategies using gene inactivation are currently in progress in our laboratory. Also, PBH-512 and PBH-553 may contribute to antigenic diversity in B. hermsii since they are antigenic, immunogenic, surface exposed, and polymorphic. Many but not all human relapsing fever sera reacted with recombinant PBH-512 and PBH-553. Thus, these two proteins appear to elicit varying immune responses in infected humans, which is in accordance with the hypothesis that their antigenic polymorphism may be a mechanism for spirochetes to evade preexisting immunity from prior or persistent infections.
Acknowledgments
We thank Gary Hettrick for graphic arts work, Ralph Larson for animal help and antibody production, and Patricia Rosa for reviewing the manuscript. Julie Rawlings, Glena Teltow, Donald Anderson, and Marlyn Whitney provided some of the spirochete isolates included in this study.
Editor: J. T. Barbieri
REFERENCES
- 1.Andrade, M. A., C. Perez-Iratxeta, and C. P. Ponting. 2001. Protein repeats: structures, functions, and evolution. J. Struct. Biol. 134:117-131. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, S. N., M. Banerjee, K. Fernando, W. Burgdorfer, and T. G. Schwan. 1998. Tick-borne relapsing fever in British Columbia, Canada: first isolation of Borrelia hermsii. J. Clin. Microbiol. 36:3505-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitschwerdt, E. B., W. L. Nicholson, A. R. Kiehl, C. Steers, D. J. Meuten, and J. F. Levine. 1994. Natural infections with Borrelia spirochetes in two dogs from Florida. J. Clin. Microbiol. 32:352-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burman, N., A. Shamaei-Tousi, and S. Bergstrom. 1998. The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever. Infect. Immun. 66:815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 8.Cook, A. R. 1904. Relapsing fever in Uganda. J. Trop. Med. 7:24-26. [Google Scholar]
- 9.Duraisingh, M. T., T. Triglia, S. A. Ralph, J. C. Rayner, J. W. Barnwell, G. I. McFadden, and A. F. Cowman. 2003. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 22:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenfeld, O. 1971. Borrelia: strains, vectors, human and animal borreliosis. W. H. Green, St. Louis, Mo.
- 11.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 12.Fritz, C. L., L. R. Bronson, C. R. Smith, M. E. Schriefer, J. R. Tucker, and T. G. Schwan. 2004. Isolation and characterization of Borrelia hermsii associated with two foci of tick-borne relapsing fever in California. J. Clin. Microbiol. 42:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geourjon, C., and G. Deleage. 1994. SOPM: a self-optimized method for protein secondary structure prediction. Protein Eng. 7:157-164. [DOI] [PubMed] [Google Scholar]
- 14.Hyde, F. W., and R. C. Johnson. 1986. Genetic analysis of Borrelia. Zentbl. Bakteriol. Mikrobiol. Hyg. 1 Abt. Orig. A 263:119-122. [DOI] [PubMed] [Google Scholar]
- 15.Khan, S. M., W. Jarra, H. Bayele, and P. R. Preiser. 2001. Distribution and characterisation of the 235 kDa rhoptry multigene family within the genomes of virulent and avirulent lines of Plasmodium yoelii. Mol. Biochem. Parasitol. 114:197-208. [DOI] [PubMed] [Google Scholar]
- 16.King, R. D., and M. Sterberg. 1996. Identification and application of the concepts important for accurate and reliable protein secondary structure prediction. Protein Sci. 5:2298-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurashige, S., M. Bissett, and L. Oshiro. 1990. Characterization of a tick isolate of Borrelia burgdorferi that possesses a major low-molecular-weight surface protein. J. Clin. Microbiol. 28:1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 19.Labandeira-Rey, M., E. A. Baker, and J. T. Skare. 2001. VraA (BBI16) protein of Borrelia burgdorferi is a surface-exposed antigen with a repetitive motif that confers partial protection against experimental Lyme borreliosis. Infect. Immun. 69:1409-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Marcotte, E. M., M. Pellegrini, T. O. Yeates, and D. Eisenberg. 1999. A census of protein repeats. J. Mol. Biol. 293:151-160. [DOI] [PubMed] [Google Scholar]
- 22.Monecke, S., J. H. Helbig, and E. Jacobs. 2003. Phase variation of the multiple banded protein in Ureaplasma urealyticum and Ureaplasma parvum. Int. J. Med. Microbiol. 293:203-211. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 24.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 25.Perkins, S., E. J. Walsh, C. C. Deivanayagam, S. V. Narayana, T. J. Foster, and M. Hook. 2001. Structural organization of the fibrinogen-binding region of the clumping factor B MSCRAMM of Staphylococcus aureus. J. Biol. Chem. 276:44721-44728. [DOI] [PubMed] [Google Scholar]
- 26.Porcella, S. F., S. J. Raffel, J. D. E. Anderson, S. Gilk, J. L. Bono, M. E. Schrumpf, and T. G. Schwan. The variable tick protein (Vtp) in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America, submitted for publication. [DOI] [PMC free article] [PubMed]
- 27.Porcella, S. F., S. J. Raffel, M. E. Schrumpf, M. E. Schriefer, D. T. Dennis, and T. G. Schwan. 2000. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 38:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preiser, P. R., W. Jarra, T. Capiod, and G. Snounou. 1999. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature 398:618-622. [DOI] [PubMed] [Google Scholar]
- 29.Preiser, P. R., S. Khan, F. T. Costa, W. Jarra, E. Belnoue, S. Ogun, A. A. Holder, T. Voza, I. Landau, G. Snounou, and L. Renia. 2002. Stage-specific transcription of distinct repertoires of a multigene family during Plasmodium life cycle. Science 295:342-345. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro, J. M., T. N. Mather, J. Piesman, and A. Spielman. 1987. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 24:201-205. [DOI] [PubMed] [Google Scholar]
- 31.Rost, B., and C. Sander. 1993. Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol. 232:584-599. [DOI] [PubMed] [Google Scholar]
- 32.Sadziene, A., M. Jonsson, S. Bergstrom, R. K. Bright, R. C. Kennedy, and A. G. Barbour. 1994. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect. Immun. 62:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280:1938-1940. [DOI] [PubMed] [Google Scholar]
- 34.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwan, T. G., P. F. Policastro, Z. Miller, R. L. Thompson, T. Damrow, and J. E. Keirans. 2003. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 9:1151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan, T. G., M. E. Schrumpf, B. J. Hinnebusch, D. E. Anderson, Jr., and M. E. Konkel. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 34:2483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan, T. G., W. J. Simpson, M. E. Schrumpf, and R. H. Karstens. 1989. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J. Clin. Microbiol. 27:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamaei-Tousi, A., P. Martin, A. Bergh, N. Burman, T. Brannstrom, and S. Bergstrom. 1999. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J. Infect. Dis. 180:1929-1938. [DOI] [PubMed] [Google Scholar]
- 39.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Borrelia burgdorferi contains repeated DNA sequences that are species specific and plasmid associated. Infect. Immun. 58:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Southern, P. M., and J. P. Sanford. 1969. Relapsing fever: a clinical and microbiological review. Medicine 48:129-149. [Google Scholar]
- 41.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 42.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trevejo, R. T., M. E. Schriefer, K. L. Gage, T. J. Safranek, K. A. Orloski, W. J. Pape, J. A. Montenieri, and G. L. Campbell. 1998. An interstate outbreak of tick-borne relapsing fever among vacationers at a Rocky Mountain cabin. Am. J. Trop. Med. Hyg. 58:743-747. [DOI] [PubMed] [Google Scholar]
- 44.Urquiza, M., M. A. Patarroyo, V. Mari, M. Ocampo, J. Suarez, R. Lopez, A. Puentes, H. Curtidor, J. Garcia, L. E. Rodriuez, R. Vera, A. Torres, M. Laverde, A. P. Robles, and M. E. Patarroyo. 2002. Identification and polymorphism of Plasmodium vivax RBP-1 peptides which bind specifically to reticulocytes. Peptides 23:2265-2277. [DOI] [PubMed] [Google Scholar]
- 45.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng, X., L. J. Teng, H. L. Watson, J. I. Glass, A. Blanchard, and G. H. Cassell. 1995. Small repeating units within the Ureaplasma urealyticum MB antigen gene encode serovar specificity and are associated with antigen size variation. Infect. Immun. 63:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zung, J. L., S. Lewengrub, M. A. Rudzinska, A. Spielman, S. R. Telford, and J. Piesman. 1989. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can. J. Zool. 67:1737-1748. [Google Scholar]