Abstract
Two-dimensional gel electrophoretic analysis of cell lysates from Brucella abortus 2308 and the isogenic hfq mutant Hfq3 revealed that the RNA binding protein Hfq (also known as host factor I or HF-I) is required for the optimal stationary phase production of the periplasmic Cu,Zn superoxide dismutase SodC. An isogenic sodC mutant, designated MEK2, was constructed from B. abortus 2308 by gene replacement, and the sodC mutant exhibited much greater susceptibility to killing by O2− generated by pyrogallol and the xanthine oxidase reaction than the parental 2308 strain supporting a role for SodC in protecting this bacterium from O2− of exogenous origin. The B. abortus sodC mutant was also found to be much more sensitive to killing by cultured resident peritoneal macrophages from C57BL6J mice than 2308, and the attenuation displayed by MEK2 in cultured murine macrophages was enhanced when these phagocytes were treated with gamma interferon (IFN-γ). The attenuation displayed by the B. abortus sodC mutant in both resting and IFN-γ-activated macrophages was alleviated, however, when these host cells were treated with the NADPH oxidase inhibitor apocynin. Consistent with its increased susceptibility to killing by cultured murine macrophages, the B. abortus sodC mutant also displayed significant attenuation in experimentally infected C57BL6J mice compared to the parental strain. These experimental findings indicate that SodC protects B. abortus 2308 from the respiratory burst of host macrophages. They also suggest that reduced SodC levels may contribute to the attenuation displayed by the B. abortus hfq mutant Hfq3 in the mouse model.
The Brucella spp. are gram-negative facultative intracellular pathogens capable of infecting a wide variety of mammalian hosts. Brucella abortus is the etiological agent of bovine brucellosis, an infection that leads to spontaneous abortion and infertility (10). Human brucellosis presents as a debilitating febrile illness commonly referred to as Malta Fever or undulant fever (59), and an active infection is characterized by fever, malaise, chills, night sweats, and anorexia. Human brucellosis is a zoonotic disease; therefore, the incidence of disease in humans is directly related to its occurrence in animals (43). Although considered mainly an occupational hazard in many countries, human brucellosis remains a significant problem where the disease is endemic in food animals and raw milk or other unpasteurized dairy products are still consumed.
Brucella spp. are not found free living, nor are they commensal organisms (23). The preferred ecological niche for the brucellae is within the phagosomal compartment of host macrophages, and the capacity of this organism to establish and maintain chronic infections is dependent upon its ability to survive and replicate within these phagocytic cells (48). Experimental evidence indicates that the production of reactive oxygen intermediates (ROIs) represents one of the primary mechanisms utilized by host macrophages for limiting the intracellular replication of the brucellae. For instance, detoxifiers of the superoxide anion (e.g., superoxide dismutase) and hydrogen peroxide (e.g., catalase) dampen the brucellacidal effects of cultured macrophages (30). Conversely, treatment of cultured macrophages with the electron-accepting compound methylene blue, which increases macrophage ROI production, decreases the number of surviving intracellular brucellae in these phagocytes. Remarkably, although opsonization of the brucellae with immunoglobulin G (IgG) or activation of macrophages with gamma interferon (IFN-γ), factors that are known to increase ROI production, enhances the brucellacidal activity of these phagocytes, virulent strains of Brucella still resist killing by these host cells and demonstrate net intracellular replication (16, 29). Accordingly, the brucellae appear to be well equipped to deal with the exposure to ROIs they encounter during their intracellular residence in host macrophages (32, 48).
Genetic and biochemical studies have identified an antioxidant that would appear to be ideally suited to protect the brucellae from the respiratory burst of host macrophages, the periplasmic Cu,Zn cofactored superoxide dismutase (SOD) encoded by sodC (4, 8). The SODs are a family of metalloenzymes containing either iron, manganese, or copper and zinc at their active sites (5, 31, 58). These enzymes catalyze the dismutation of superoxide (O2−) to hydrogen peroxide (H2O2) (37, 38), which can then be further detoxified through the action of catalases and peroxidases. Most aerobic bacteria contain either a Mn or Fe cofactored SOD in their cytoplasm that serves to detoxify endogenous superoxide arising as a by-product of aerobic metabolism (19). Once thought to be strictly a eukaryotic enzyme, the function of Cu,Zn SOD in bacteria is not as clearly understood as its cytosolic counterparts (34). Superoxide is a weakly anionic species, (39), and is not thought to cross the cytoplasmic membrane (26). In fact, experimental evidence indicates that bacterial SODs only detoxify O2− in the intracellular compartment within which they reside (51). The inability of O2− to cross the cytoplasmic membrane, coupled with the periplasmic location of SodC, has led to the proposal that SodC most likely protects bacteria from superoxide of exogenous origin (54). For bacterial pathogens such as Brucella that survive and replicate in host macrophages, one obvious source of exogenous O2− would be exposure to the respiratory burst of host phagocytes. Indeed, genetic evidence suggests that SodC homologs play important roles in protecting Salmonella enterica serovar Typhimurium, Neisseria meningitidis, and Mycobacterium tuberculosis from oxidative killing by host macrophages (11, 12, 44). Previous studies, however, have left the role of SodC in protecting Brucella from the oxidative killing of host macrophages unresolved (35, 55).
In this study we report conclusive data demonstrating the role of the B. abortus Cu,Zn SOD (SodC) in protecting these cells from oxidative killing in vitro and in conferring resistance to the actions of the respiratory burst of host-derived macrophages ex vivo. We also present evidence supporting a regulatory link between the RNA binding protein Hfq and optimal stationary-phase sodC expression in B. abortus 2308 and discuss the possible implications that inefficient SodC production might have on the phenotype displayed by the B. abortus hfq mutant Hfq3 (46).
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Routine cultivation of Escherichia coli strains was carried out in Luria-Bertani (LB) broth or on tryptic soy agar (TSA) plates with appropriate antibiotic supplementation as necessary. Brucella abortus strains were routinely grown in either brucella broth at 37°C with aeration, on Schaedler agar supplemented with 5% bovine blood (SBA) at 37°C under 5% CO2, or on Schaedler agar supplemented with 1,000 U/ml of bovine liver catalase (Sigma) as indicated in the text. Gerhardt's minimal medium (GMM) was prepared as previously described (21). Culture media were supplemented with 100 μg/ml ampicillin, 5 μg/ml chloramphenicol, or 45 μg/ml kanamycin when appropriate. Frozen stocks of the bacteria were maintained at −80°C in 25% glycerol-75% brucella broth.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli DH5-α | F− φ80dlacZΔM15Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 phoA supE441-thi-1 gyrA96 relA1 | Gibco-BRL |
| Brucella abortus | ||
| 2308 | Virulent challenge strain | Laboratory stock |
| Hfq3 | 2308 hfq::aphA | 46 |
| MEK2 | 2308 sodC::cat | This study |
| MEK2Cm | MEK2 carrying pJG22 | This study |
| MEK2Cm* | MEK2 carrying pMEK15 | This study |
| Plasmids | ||
| pUC9 | Cloning vector; ColE-1 origin of replication; ampicillin resistance | Gibco-BRL |
| pUC18 | Cloning vector; ColE-1 origin of replication; ampicillin resistance | Gibco-BRL |
| pBluescriptII KS+ | Cloning vector; ColE-1 origin of replication; ampicillin resistance | Stratagene |
| pBBR1MCS-4 | Cloning vector; broad-host range; moderate copy number (∼10 copies per cell); ampicillin resistance | 33 |
| pMR10 | Cloning vector; broad-host range; low copy number (2 to 4 copies per cell); kanamycin resistance | NCBI accession no. AJ606312 |
| pBlue-Cm2 | 945-bp PCR amplified cat gene from pBC-SK+ cloned into pBluescriptII KS+ | 47 |
| pBA23 | 3.2-kb Sau3A fragment containing sodC cloned into the BamHI site of pUC9 | 35 |
| pBA23.10 | 2-kb HindIII/EcoRV fragment containing sodC cloned into pBluescriptII KS+ | This study |
| pBA23.10::cat | Derivative of pBA23.10 in which a 226-bp SmaI/NaeI fragment internal to the sodC coding region has been replaced with the cat gene from pBlue Cm2 | This study |
| pMEK15 | 2-kb EcoRV/HindIII fragment containing sodC cloned into pBBR1MCS-4 | This study |
| pJG22 | 2-kb EcoRI/XhoI fragment containing sodC cloned into pMR10 | This study |
Preparation of bacterial cell lysates for two-dimensional gel electrophoresis.
B. abortus 2308 and Hfq3 were grown for 96 h in GMM. Bacterial cells were harvested by centrifugation and washed three times with 10 mM Tris HCl, pH 8.0, and then suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.3% SDS, 0.2 M dithiothreitol, 0.05 M Tris HCl, pH 8.0). After boiling for 10 min to ensure loss of viability, the cells were lysed by sonication. The sonicated suspension was boiled for an additional 10 min and then placed on ice. The suspension was treated with DNase I and RNase A at final concentrations of 0.1 mg/ml and 0.025 mg/ml, respectively, for 20 min on ice. Protein concentrations were determined using the Coomassie Plus Protein Assay Reagent (Pierce).
Two-dimensional gel electrophoresis.
Fifty to 200 μg of total protein was diluted to 400 μl with thiourea buffer, which contained 5 M urea, 2 M thiourea, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 2% SB 3-10 (Bio-Rad), 40 mM Tris, 0.2% Bio-lyte 3/10, 2 mM tributyl phosphine, and was allowed to rehydrate with an Immobiline Drystrip, pH 4 to 7 (Amersham Pharmacia), overnight under oil. Isoelectric focusing was carried out in an IPG pHaser (Genomic Solutions) according to the manufacturer's instructions. After focusing, the immobilized pH gradient strip was equilibrated in equilibration buffer (6 M urea, 2% dithiothreitol, 30% glycerol, 5% SDS, 0.112 M Tris-acetate, and 0.01% bromophenol blue) for 15 min. A second equilibration was carried out in the same solution, except dithiothreitol was replaced with 2.5% iodoacetamide for another 15 min. After equilibration, the strip was sealed on top of a precast 10% Duracryl-Tris/Tricine/SDS gel (Genomic Solutions) using 0.5% low-melting agarose dissolved in cathode running buffer (0.2 M Tris, 0.2 M Tricine, and 0.4% SDS). Second-dimension separation of proteins was performed in the Investigator 2D Electrophoresis System (Genomic Solutions) following the manufacturer's instructions.
After electrophoresis, the resolved protein spots were either stained using the Protein Silver Staining kit (Amersham Pharmacia) with glutaraldehyde removed from the sensitization step or transferred to polyvinylidene difluoride (PVDF) membranes (Hyperbond; Porton Instruments) without prior staining. Images of the gels were captured with the Investigator Proteomic Analyzer Camera System (Genomic Solutions) and analyzed by Investigator HT Proteomic Analysis Software (Genomic Solutions). Identities of protein spots were determined by matrix-assisted laser desorption ionization-time-of-flight peptide mass fingerprinting or N-terminal sequence analysis.
N-terminal protein sequence analysis.
To obtain N-terminal amino acid sequence data on selected proteins, proteins resolved in two dimensions were transferred to PVDF membranes by the semiwet transfer method (Investigator Graphite Electroblotter System; Genomic Solutions). The membranes were then stained with Coomassie brilliant blue according to previously described procedures (56, 57). The desired protein spots were cut out based on comparisons to similarly run silver stained gels and subjected to microsequence analysis using an automated sequencer. Searches for sequence identity or homology were performed using the FASTA program. The blastp algorithm was used to compare the N-terminal amino acid sequence data obtained with the predicted products of the open reading frames annotated in the B. melitensis and B. suis genome sequences (http://www.ncbi.nlm.gov/genomes/MICROBES/Complete.html).
Construction of a Brucella abortus sodC mutant.
Cloning, subcloning, and isolation of plasmid DNA from recombinant E. coli strains were performed using standard procedures (52). A previously described gene replacement strategy (24) was used to construct a B. abortus sodC mutant from virulent strain 2308 in the following manner. A 226-bp SmaI/NaeI fragment internal to the sodC coding region in pBA23 (Table 1) was replaced with a 945-bp PCR-generated fragment containing the cat gene from pBlue-CM2 (47). The resulting plasmid, designated pBA23.10sodC::cat, was introduced into B. abortus 2308 by electroporation as described previously (14), and one transformant was selected based upon its resistance to chloramphenicol and sensitivity to ampicillin. This transformant was assigned the designation MEK2 (Table 1). Genomic DNA isolation was isolated from B. abortus MEK2 using the methods of Pitcher et al. (45), and the predicted genotype of this mutant was verified by Southern blot analysis with sodC-, cat-, and vector-specific probes (data not shown).
To facilitate genetic complementation of the B. abortus sodC mutant, low- and medium-copy plasmids bearing intact copies of sodC were constructed. A 2-kb EcoRV/HindIII fragment containing the sodC gene from pBA23 was first cloned into the medium-copy plasmid pBBR1MCS-4 (33) to yield plasmid pMEK15 (Table 1). A 2.0-kb EcoRI/XhoI-digested fragment containing the sodC gene from pMEK15 was also subsequently cloned into the low-copy RK2-based plasmid pMR10 (GenBank accession no. AJ606312), producing pJG22. These sodC-containing plasmids were introduced into B. abortus MEK2 by electroporation yielding strains designated MEK2Cm [MEK2 (pJG22)] and MEK2Cm* [MEK2 (pMEK15)].
Resistance of Brucella abortus strains to H2O2 and the O2− generating compound pyrogallol in a disk sensitivity assay.
Brucella abortus 2308, MEK2, and MEK2Cm* were grown to mid-log phase in brucella broth, and the cultures were adjusted to an optical density at 600 nm of 0.15 (approximately 109 CFU/ml). One-hundred μl of each cell suspension was spread onto Schaedler agar (SA) for the H2O2 sensitivity assays or SA supplemented with 1,000 U/ml of bovine liver catalase (Sigma) for the pyrogallol sensitivity assay. A 7-mm-diameter Whatman filter paper disk was placed in the center of each plate and impregnated with 10 μl of one of the following solutions: 970 mM H2O2, 9.7 M H2O2, or 1 M pyrogallol. After approximately 3 days of incubation at 37°C with 5% CO2, the diameter of the zone of inhibition surrounding each disk on the plates was measured to the nearest millimeter. The diameters of the zones of inhibition from five separate plates were measured for each strain examined.
Resistance of B. abortus strains to exogenous O2− generated by the xanthine oxidase reaction.
Brucella abortus 2308, MEK2, and MEK2Cm were grown to mid-log phase in brucella broth, harvested by centrifugation, washed twice in phosphate-buffered saline (PBS), and adjusted to a density of approximately 5 × 107 cells per ml in PBS. Xanthine at a final concentration of 1,000 μM and 0.5 U/ml of xanthine oxidase were added to these cell suspensions along with 1,000 U/ml of bovine liver catalase to detoxify any H202 generated by spontaneous dismutation of the O2− generated by the xanthine oxidase reaction. At selected time points after initiating the xanthine oxidase reaction, the number of surviving bacteria in the cell suspensions was determined by 10-fold serial dilution and plating on SA supplemented with 1,000 U/ml of bovine liver catalase. Plates were incubated for approximately 3 days at 37°C with 5% CO2, and the results from three independent platings of each bacterial cell suspension were calculated and averaged, and the data obtained were expressed as log10 CFU/ml at each sampling time.
Survival and replication of the Brucella abortus strains in cultured murine macrophages.
Previously described procedures (15) were used to harvest and infect cultured murine resident peritoneal macrophages with B. abortus 2308, MEK2, and MEK2Cm. Briefly, 6- to 8-week old female C57BL6J mice were euthanized by isoflurane overdose. Immediately following euthanasia, cells from the peritoneal cavity were harvested via lavage using 8 ml of Dulbecco's minimal essential medium (DMEM) supplemented with 5% fetal calf serum (FCS) and 5 U/ml heparin. Total cell numbers and viability were determined using a hemacytometer and the trypan blue exclusion technique. Explanted cells were then seeded at a density of 1.5 × 105 cells per well in sterile 96-well microtiter plates in DMEM supplemented with 5% FCS and 5 μg/ml gentamicin.
After overnight incubation at 37°C in 5% CO2, cell cultures were enriched for macrophages by washing away nonadherent cells. Cultured macrophages were then infected with either 2308, MEK2, or MEK2Cm opsonized for 30 min with a subagglutinating dilution (1:1,500) of hyperimmune C57BL6J mouse serum at a multiplicity of infection (MOI) of 50 brucellae per macrophage (MOI of 50:1) and incubated at 37°C with 5% CO2 for 2 h to allow for phagocytosis of the brucellae. After this 2-h period, cell culture medium was removed and replaced with DMEM supplemented with 5% FCS and 50 μg/ml of gentamicin, and incubation at 37°C with 5% CO2 continued for 1 h to kill any remaining extracellular brucellae. The macrophages were then washed with PBS supplemented with 0.5% FCS and maintained thereafter in DMEM with 5% FCS and 12.5 μg/ml of gentamicin. Cell culture medium was replaced with fresh medium every 24 h. At 3, 27, and 51 h after infection, macrophages were washed with PBS supplemented with 0.5% FCS and lysed with 0.1% deoxycholic acid in PBS. After 5 min of incubation at room temperature, serial 10-fold dilutions of the lysates were prepared in sterile PBS and plated on SBA. Platings were performed in triplicate from 3 wells per strain at each time point, and the data obtained were expressed as log10 intracellular brucellae. Host cell lysates were also plated on SBA supplemented with 5 μg/ml of chloramphenicol and/or SBA containing a combination of 5 μg/ml chloramphenicol and 45 μg/ml kanamycin to confirm the identity of reisolates and monitor the stability of the plasmid in MEK2Cm.
Parallel experiments were also performed to examine the effects of treatment of the cultured macrophages with (i) IFN-γ; (ii) the NADPH oxidase inhibitor apocynin (25); or (iii) a combination of IFN-γ and apocynin on the capacity of these phagocytes to kill the intracellular brucellae. Twenty-five U/ml of IFN-γ (Roche) and/or 500 μM (final concentration) of apocynin were added to each treated well of phagocytes immediately after plating. Apocynin was also added to treated wells again 6 h prior to infection with the brucellae. Microscopic analysis of nitroblue tetrazolium reduction was used to monitor the oxidative burst capacity of the cultured macrophages (7), and control experiments were performed to ensure that apocynin at this concentration had no brucellacidal effect in DMEM (data not shown).
Experimental infection of C57BL6J mice.
Brucella strains were grown on SBA with or without antibiotics as appropriate, and infection doses were prepared as described previously (14). Briefly, 6- to 8-week old female C57BL6J mice were infected via the intraperitoneal route with approximately 5 × 104 brucellae. At 1, 4, and 8 weeks postinfection, 5 mice per experimental group were sacrificed by isoflurane overdose. Immediately following euthanasia, spleens were harvested aseptically and homogenized in sterile PBS. Spleen homogenates were then serially diluted 10-fold in sterile PBS and plated onto SBA to determine the number of brucellae present at each time point. Spleen homogenates were also plated in parallel on SBA supplemented with the appropriate antibiotics to confirm the identity of the isolates and monitor the stability of the plasmid in MEK2Cm. Mean averages of counts from each test group were determined, and the data were expressed as log10 brucellae/spleen.
Statistical analysis.
All statistical analyses were performed using the Student's two-tailed t test. P values ≤ 0.05 were considered significant (50).
RESULTS
Hfq is required for the optimal production of SodC during stationary phase in B. abortus 2308.
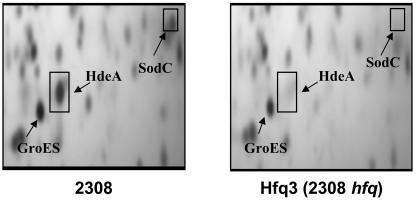
Previous studies in our laboratory have shown that the B. abortus hfq mutant Hfq3 exhibits increased susceptibility to a variety of environmental stresses (including exposure to ROIs) during stationary phase, and this phenotype is most pronounced when this mutant is cultivated in a defined minimal medium (46). Interestingly, two-dimensional gel electrophoretic analysis of cell lysates from B. abortus 2308 and the isogenic hfq mutant Hfq3 cultures grown to stationary phase in GMM shows that wild-type production of SodC by B. abortus 2308 is dependent upon the presence of Hfq (49) (Fig. 1). This observation suggests that inefficient sodC expression may make a significant contribution to the ROI sensitivity displayed by the B. abortus hfq mutant Hfq3 and possibly the attenuation displayed by this mutant in experimentally infected mice (46).
FIG. 1.
Hfq is required for the wild-type expression of sodC in B. abortus 2308. Panels represent corresponding segments from silver-stained polyacrylamide gels following two-dimensional gel electrophoretic analysis of cell lysates from cultures of B. abortus 2308 and the isogenic hfq mutant Hfq3 grown for 96 h in Gerhardt's minimal medium. Protein spots corresponding to GroES (which is produced at equivalent levels in B. abortus 2308 and Hfq3 under these cultural conditions) and HdeA (the product of another gene requiring Hfq for its efficient stationary-phase expression in B. abortus 2308 [49]) are shown as a basis for comparison. SodC, HdeA, and GroES were identified by matrix-assisted laser desorption ionization-time-of-flight peptide mass fingerprinting and/or N-terminal amino acid sequence analysis as described in Materials and Methods.
The B. abortus sodC mutant is sensitive to exogenous superoxide.
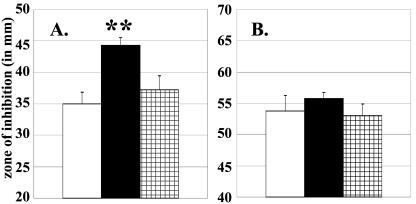
Based upon the proposed function and periplasmic location of SodC, we predicted that a B. abortus sodC mutant would be defective in its ability to resist exposure to exogenous superoxide. To evaluate the sensitivity of MEK2 (sodC::cat; see Table 1) to superoxide, we performed disk diffusion assays with the compound pyrogallol (1,2,3-trihydroxybenzene), whose spontaneous auto oxidation results in the generation of the superoxide anion (36). Because O2− production by pyrogallol does not require a reaction between this compound and the metabolic intermediates NADH or NADPH, O2− is generated in the extracellular environment of cells exposed to pyrogallol. The B. abortus sodC mutant MEK2 was significantly more sensitive to pyrogallol than the parental strain 2308 in the disk sensitivity assay (Fig. 2A), and resistance to exogenous superoxide could be restored to a level equivalent to that exhibited by 2308 by introducing a plasmid-borne copy of sodC into MEK2. In contrast, 2308 and MEK2 were found to be equally resistant to H2O2 exposure in the disk diffusion assays (Fig. 2B).
FIG. 2.
Resistance of B. abortus 2308 (white bars), MEK2 (2308 sodC) (black bars), and MEK2Cm* [MEK2 (pMEK15)] (hatched bars) to killing by (A) pyrogallol or (B) H2O2 in a disk sensitivity assay. Error bars represent the standard deviations taken from the averages of five zones per strain. Significance values: the double asterisk indicates P ≤ 0.01 for comparisons of MEK2 versus 2308 and MEK2Cm*.
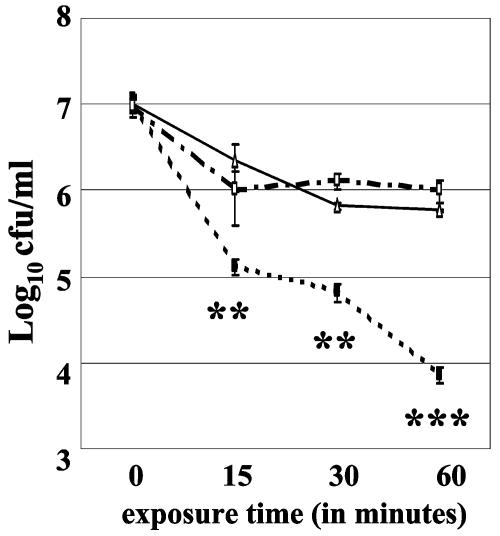
Although increased sensitivity to pyrogallol is a characteristic phenotype exhibited by bacterial sodC mutants (53), disk assays represent a chronic exposure to O2− stress that is dependent upon the diffusion of the compound into the plating medium over time. Considering the short half-life of O2− as well as its propensity to spontaneously dismute to H2O2, we wanted a more definitive assessment of the sensitivity of the B. abortus sodC mutant to exogenous O2−. Therefore, we performed a second assay using the xanthine oxidase reaction to generate exogenous superoxide (11). Xanthine oxidase converts xanthine to urate, and O2− is generated during the course of this reaction (18). Xanthine oxidase is too large to cross cell membranes, and consequently the xanthine oxidase reaction has been widely used as a method for generating O2− in the extracellular environment of both prokaryotic and eukaryotic cells. Following a 1-h exposure to the xanthine oxidase reaction, survival of the B. abortus sodC mutant MEK2 was reduced approximately 100-fold compared to that displayed by the parental 2308 strain (Fig. 3), and introduction of a plasmid-borne copy of sodC into MEK2 restored the resistance of this mutant to the xanthine oxidase reaction to wild-type levels (Fig. 3).
FIG. 3.
Resistance of B. abortus 2308 (▵), MEK2 (2308 sodC) (▪), and MEK2Cm [MEK2 (pJG22)] (□) to killing by O2− generated by the xanthine oxidase reaction. The error bars represent standard deviations in average log10 CFU/ml values obtained from triplicate platings taken at each time point. Significance values: **, P ≤ 0.01; ***, P ≤ 0.005 for comparisons of MEK2 versus 2308 and MEK2Cm.
SodC is required for wild-type survival and replication of Brucella abortus 2308 in cultured murine macrophages.
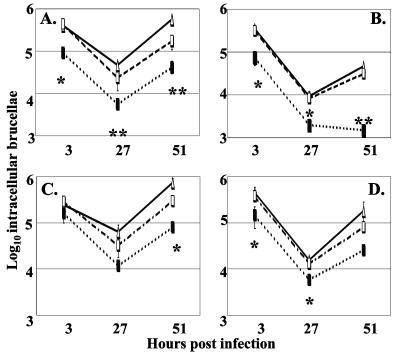
In keeping with the demonstrated sensitivity of MEK2 to exposure to exogenous O2− in vitro, we predicted that this mutant might be defective in its capacity to replicate within cultured murine macrophages due to an inability to detoxify the O2− generated by the respiratory burst of these phagocytes. Consistent with this prediction, the B. abortus sodC mutant MEK2 displayed a significant reduction in its capacity to resist killing by cultured murine macrophages compared to 2308, as evidenced by the reduced number of intracellular MEK2 versus 2308 being recovered from the cultured murine macrophages at all three sampling times postinfection (Fig. 4A), and the attenuation exhibited by MEK2 compared to 2308 in the cultured murine macrophages was enhanced when these phagocytes were treated with IFN-γ prior to infection (Fig. 4B). The attenuation displayed by MEK2 compared to 2308 in both nonactivated and IFN-γ activated macrophages, however, was reduced considerably when the host cells were treated with the NADPH oxidase inhibitor apocynin (Fig. 4C and D). These experimental findings indicate that the respiratory burst of the cultured murine macrophages contributes significantly to the attenuation displayed by the B. abortus sodC mutant in these phagocytes. MEK2Cm, a derivative of MEK2 carrying sodC on a pMR10-based plasmid, displayed a level of resistance to killing by the cultured murine macrophages that was not statistically different from that exhibited by B. abortus 2308 under all of the experimental conditions employed (Fig. 4A to D). These results verify the link between the sodC mutation in MEK2 and the increased sensitivity of this mutant to the oxidative killing pathways of the cultured murine macrophages. More importantly, these findings coupled with those obtained with the NADPH oxidase inhibitor apocynin are consistent with SodC functioning as an antioxidant that protects B. abortus 2308 from exposure to O2− generated by the respiratory burst of host phagocytes.
FIG. 4.
Intracellular survival and replication of B. abortus 2308 (▵), MEK2 (2308 sodC) (▪), and MEK2Cm [MEK2 (pJG22)] (□) in primary cultures of resident peritoneal macrophages from C57BL6J mice. A) untreated macrophages; B) macrophages treated with 25 U/ml per well of IFN-γ; C) macrophages treated with 500 μM apocynin; D) macrophages treated with 25 U/ml of IFN-γ and 500 μM apocynin. The error bars represent standard deviation in average log10 intracellular brucellae from triplicate platings taken at each time point. Significance values: *, P ≤ 0.05; and **, P ≤ 0.01 for comparisons of MEK2 versus 2308 and MEK2Cm.
SodC is essential for the establishment and maintenance of chronic infection in experimentally infected mice.
To test whether the reduced intracellular survival observed for the sodC mutant in explanted nonactivated or IFN-γ-activated macrophages would translate into reduced virulence in an experimental host, the spleen colonization profiles of B. abortus 2308, MEK2, and MEK2Cm in C57BL6J mice were determined. The B. abortus sodC mutant MEK2 displayed significant attenuation compared to 2308 at 1, 4, and 8 weeks postinfection in C57BL6J mice (Fig. 5). MEK2Cm, the MEK2 derivative carrying sodC on the pMR10-based plasmid pJG22, displayed a spleen colonization profile in mice that was intermediate between that displayed by B. abortus 2308 and MEK2 at 1 week postinfection. However, the numbers of B. abortus 2308 and MEK2 recovered from the spleens of mice infected with these strains were not statistically different at 4 and 8 weeks postinfection. These experimental findings not only establish a correlation between the increased susceptibility of the B. abortus sodC mutant to killing by macrophages and attenuation in mice, but they also support the link between the sodC mutation in MEK2 and the phenotype displayed by this mutant in the mouse model from a genetic standpoint.
FIG. 5.
Spleen colonization profiles of B. abortus 2308 (▵), MEK2 (2308 sodC) (▪), and MEK2Cm (MEK2pJG22) (□) in C57BL6J mice. The error bars represent the standard deviation in the average of the number of brucellae recovered from the spleens of five mice per strain at each time point. Significance values: *, P ≤ 0.05; and **, P ≤ 0.01 for comparisons between 2308 and MEK2; †, P ≤ 0.05; and ††, P ≤ 0.01 for comparisons between MEK2 and MEK2Cm.
DISCUSSION
The experimental findings described in this report establish SodC as an antioxidant that protects B. abortus 2308 from O2− of exogenous origin. More importantly, from the perspective of the intracellular lifestyle of the brucellae, the phenotype displayed by the B. abortus sodC mutant MEK2 during its interactions with cultured murine macrophages suggests that SodC protects the parent strain from O2− generated by the respiratory burst of host macrophages. IFN-γ activation of host macrophages leads to the increased production of ROIs by these phagocytes (6, 42). This relationship is consistent with our findings that differential killing of 2308 and MEK2 was enhanced in cultured murine macrophages activated with IFN-γ compared to the differences in killing observed for these strains in nonactivated macrophages. A positive correlation between ROI production and the enhanced killing activity of the IFN-γ activated macrophages for the B. abortus sodC mutant was further supported by the finding that apocynin treatment reduced the attenuation displayed by MEK2 in IFN-γ-activated phagocytes, allowing this strain to display net intracellular replication in these cells between 27 and 51 h postinfection. Interestingly, apocynin treatment failed to totally alleviate the attenuation displayed by the B. abortus sodC mutant in the cultured murine macrophages. One possibility for this experimental finding is that the concentration of apocynin used in these experiments did not completely inhibit the respiratory burst of these cultured phagocytes. Another possibility is that the B. abortus sodC mutant possesses a defect in its ability to resist the microbicidal activities of host macrophages that is unrelated or indirectly related to the reduced capacity of this strain to defend itself against the oxidative burst of these phagocytes. Regardless, the results of these studies demonstrate that the B. abortus SodC performs a similar function to that ascribed to its counterparts in Salmonella (11) and Mycobacterium tuberculosis (44) and protects this bacterium from O2− generated by the oxidative burst of host macrophages.
Mutants of B. abortus that exhibit defects in their ability to survive and replicate within macrophages are generally attenuated in the mouse model of chronic infection (2, 3), and this relationship held true when the virulence of the B. abortus sodC mutant MEK2 in C57BL6J mice was compared to that displayed by the virulent parent strain. Notably, the attenuation displayed by MEK2 in mice appears to be biphasic. At 1 week postinfection, the numbers of MEK2 recovered from the spleens of experimentally infected mice was considerably lower than the inoculum, while in contrast the numbers of the parent strain recovered from the spleens of infected mice increased fivefold compared to the inoculum during this same period. These contrasting phenotypes are consistent with the B. abortus sodC mutant being much more sensitive to the ROI burst during internalization into resting or nonactivated macrophages during the early stages of infection than the virulent parental strain. Between 1 and 4 weeks postinfection, however, the number of MEK2 recovered from the spleens of infected mice appears to stabilize. This phenotype is also consistent with our finding that once the B. abortus sodC mutant is internalized into nonactivated macrophages, it can survive and replicate within these phagocytes. The second phase of attenuation displayed by MEK2 in mice begins at some point beyond 4 weeks postinfection. Specifically, between 4 and 8 weeks postinfection, the B. abortus sodC mutant displays accelerated clearance from the spleens of C57BL6J mice compared to the parent strain 2308. This is the period during which previous studies have shown that cellular immunity is induced in mice experimentally infected with B. abortus (1), which would lead to an increased number of IFN-γ-activated macrophages being present. Consequently, accelerated clearance of MEK2 from the spleens of experimentally infected mice beginning at 4 weeks postinfection or shortly thereafter would be consistent with the increased susceptibility of the B. abortus sodC mutant to killing by IFN-γ activated macrophages compared to the parental strain observed in the host cell culture assays.
Previous studies evaluating the virulence of B. abortus sodC mutants in experimentally infected mice generated conflicting results. Latimer et al. (35), for instance, reported no differences between the spleen colonization profiles of B. abortus 2308 and an isogenic sodC mutant at 1 and 4 weeks postinfection when these strains were evaluated in BALB/c mice. Tatum et al. (55), on the other hand, found significant differences in the spleen colonization profiles of B. abortus 2308 and an isogenic sodC mutant throughout the course of a 16-week infection, with the sodC mutant displaying significant attenuation compared to 2308 at most of the experimental time points examined. These latter authors also reported observing no significant differences between the intracellular survival and replication patterns of B. abortus 2308 and the sodC mutant in HeLa cells or in the murine macrophage-like cell line J774 (55). The reasons underlying the discrepancies between these earlier reports and the studies presented here are unknown. It is quite possible, however, that the macrophages isolated from the peritoneal cavities of the C57BL6J mice produce a more robust oxidative burst in primary culture than the J774 and HeLa cell lines, and this could explain the differential behavior of the B. abortus sodC mutants in these different host cell types. It has also been reported that BALB/c mice experience an interruption in IFN-γ production during infection with Brucella strains that is not observed in C57BL6J mice (41). Considering the well-documented link between IFN-γ activation, ROI production, and the brucellacidal activity of murine macrophages (29, 30), it is also quite possible that the B. abortus sodC mutant experiences a less intense exposure to O2− of phagocyte origin during its residence in BALB/c mice than it does during its residence in C57BL6J mice.
The nature of the regulatory link between Hfq and stationary phase sodC expression in B. abortus 2308 is presently under investigation. Bacterial sodC genes are typically regulated in a growth-phase-dependent manner, and their expression is usually maximal during stationary phase (22, 53). In enteric bacteria such as Escherichia coli and Salmonella enterica serovar Typhimurium, Hfq is an RNA binding protein that facilitates efficient translation of the rpoS transcript coincident with entry into stationary phase (9, 40). RpoS is an alternate stationary-phase-specific sigma factor (σS), and increased levels of RpoS lead to the increased expression of a number of different gene products involved in resistance to a variety of environmental stresses upon entry into stationary phase (13, 27, 28). In E. coli and S. enterica serovar Typhimurium, RpoS, and consequently Hfq, are responsible for increased expression of sodC during stationary phase (17, 22). Based on recent experimental findings with the sodB gene of E. coli (20), the possibility also exists that Hfq plays a direct role in regulating sodC expression in B. abortus 2308 at the posttranscriptional level. The B. abortus hfq mutant Hfq3 displays increased sensitivity to O2− in in vitro assays compared to the parental 2308 strain (J. Gee, unpublished observations), and this mutant is also highly attenuated in both cultured murine macrophages and experimentally infected mice (44). The phenotype displayed by the B. abortus sodC mutant MEK2 certainly suggests that inefficient sodC expression might play a role in the generalized ROI sensitivity displayed by the B. abortus hfq mutant Hfq3 in vitro and potentially contributes to the extreme attenuation displayed by this mutant in the mouse model. It is important to note, however, that experiments to date suggest that greater than 40 B. abortus 2308 genes require Hfq for their efficient expression during stationary phase (49), and experimental analysis of other Hfq regulated genes has determined that inefficient sodC expression is not the sole basis for the attenuation displayed by the B. abortus hfq mutant in experimentally infected mice (M. W. Valderas, R. B. Alcantara, J. B. Baumgartner, J. M. Gee, and R. M. Roop II, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. E-065, 2003) (48).
Acknowledgments
This work was supported by a grant from the National Institute of Allergy and Infectious Disease (AI 48499) to R.M.R. II.
Editor: V. J. DiRita
REFERENCES
- 1.Araya, L. N., P. H. Elzer, G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 2.Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella, p. 363-380. In B. S. Zwilling and T. K. Eisenstein (ed.) Macrophage-pathogen interactions. Marcell Dekker, New York, N.Y.
- 3.Baldwin, C. L., and R. M. Roop II. 1999. Brucella infections and immunity, p. 255-279. In L. J. Paradise, H. Friedman, and M. Bendinelli (ed.), Opportunistic intracellular pathogens and immunity. Plenum Press, New York, N.Y.
- 4.Beck, B. L., L. B. Tabatabai, and J. E. Mayfield. 1990. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry 29:372-376. [DOI] [PubMed] [Google Scholar]
- 5.Benov, L. T., and I. Fridovich. 1994. Escherichia coli expresses a copper and zinc containing superoxide dismutase. J. Biol. Chem. 269:25310-25314. [PubMed] [Google Scholar]
- 6.Bogdan, C. M., M. Rollinghoff, and A. Drefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 7.Bowe, F., and F. Heffron. 1994. Isolation of Salmonella mutants defective for intracellular survival. Methods Enzymol. 236:509-526. [DOI] [PubMed] [Google Scholar]
- 8.Bricker, B. J., L. B. Tabatabai, B. A. Judge, B. L. Deyoe, and J. E. Mayfield. 1990. Cloning, expression and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect. Immun. 58:2935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, L., and T. Elliot. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA binding protein encoded by the hfq gene. J. Bacteriol. 178:3763-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbel, M. J. 1989. Brucellosis: epidemiology and prevalence worldwide, p. 25-40. In E. J. Young and M. J. Corbel (ed.), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, Fla.
- 11.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vasquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn, K. L., J. L. Farrant, P. R. Langford, and J. S. Kroll. 2003. Bacterial [Cu,Zn]-cofactored superoxide dismutase protects opsonized, encapsulated Neisseria meningitidis from phagocytosis by human monocytes/macrophages. Infect. Immun. 71:1604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenstark, A., M. J. Calcutt, M. Becker-Hapak, and A. Ivanova. 1996. Role of Escherichia coli rpoS and associated genes in defense versus oxidative damage. Free Rad. Biol. Med. 21:975-993. [DOI] [PubMed] [Google Scholar]
- 14.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high temperature requirement A (htrA) deletion mutant. Infect. Immun. 62:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elzer, P. H., R. W. Phillips, G. T. Robertson, and R. M. Roop II. 1996. The HtrA stress response protease contributes to the resistance of Brucella abortus to killing by murine phagocytes. Infect. Immun. 64:4838-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Bäumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu,Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96:7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridovich, I. 1970. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J. Biol. Chem. 245:4053-4057. [PubMed] [Google Scholar]
- 19.Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64:97-112. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann, T. A., and D. Touati. 2004. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 23:396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhardt, P. 1958. The nutrition of brucellae. Microbiol. Rev. 22:81-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gort, A. S., D. M. Ferber, and J. A. Imlay. 1999. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol. Microbiol. 32:179-191. [DOI] [PubMed] [Google Scholar]
- 23.Gorvel, J. P., and E. Moreno. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281-297. [DOI] [PubMed] [Google Scholar]
- 24.Halling, S. M., P. G. Detilleux, F. M. Tatum, B. A. Judge, and J. E. Mayfield. 1991. Deletion of the BCSP31 gene of Brucella abortus by replacement. Infect. Immun. 59:3863-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart, B. A., and J. M. Simmons. 1992. Metabolic activation of phenols by stimulated neutrophils: a concept for a selective type of anti-inflammatory drug. Biotechnol. Ther. 3:119-135. [PubMed] [Google Scholar]
- 26.Hassan, H. M., and I. Fridovich. 1979. Intracellular production of superoxide radical and of hydrogen peroxide by redox-active compounds. Arch. Biochem. Biophys. 196:385-395. [DOI] [PubMed] [Google Scholar]
- 27.Hengge-Aronis, R. 1993. Survival of hunger and stress: the role of rpoS in stationary phase gene regulation in Escherichia coli. Cell 72:165-168. [DOI] [PubMed] [Google Scholar]
- 28.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1572. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology Press, Washington, D.C.
- 29.Jiang, X., and C. L. Baldwin. 1993. Effects of cytokines on the intracellular growth of Brucella abortus. Infect. Immun. 61:124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, X., B. Leonard, R. Benson, and C. L. Baldwin. 1993. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 151:309-319. [DOI] [PubMed] [Google Scholar]
- 31.Keele, B. B. J., J. M. McCord, and I. Fridovich. 1970. Superoxide dismutase from Escherichia coli B. A new manganese containing enzyme. J. Biol. Chem. 245:6176-6181. [PubMed] [Google Scholar]
- 32.Köhler, S., S. Michaux-Charachon, F. Porte, M. Ramuz, and J. P. Liautard. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 11:215-219. [DOI] [PubMed] [Google Scholar]
- 33.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 34.Kroll, J. S., P. R. Langford, K. E. Wilks, and A. D. Keil. 1995. Bacterial [Cu-Zn]-superoxide dismutase: phylogenetically distinct from the eucaryotic enzyme, and not so rare after all! Microbiology 141:2271-2279. [DOI] [PubMed] [Google Scholar]
- 35.Latimer, E., J. Simmers, N. Sriranganathan, R. M. Roop II, G. G. Schurig, and S. M. Boyle. 1992. Brucella abortus deficient in copper/zinc superoxide dismutase is virulent in BALB/c mice: Microb. Pathog. 12:105-113. [DOI] [PubMed] [Google Scholar]
- 36.Marklund, S., and G. Marklund. 1974. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47:469-474. [DOI] [PubMed] [Google Scholar]
- 37.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase: an enzymatic function for erthrocuprein (hemocuprein). J. Biol. Chem. 244:6049-6055. [PubMed] [Google Scholar]
- 38.McCord, J. M., B. B. Keele, Jr., and I. Fridovich. 1971. An enzyme based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 68:1024-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, R. A., and B. E. Brittigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muffler, A., D. D. Traulsen, D. Fischer, R. Lange, and R. Hengge-Aronis. 1997. The RNA binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 179:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, E. A., M. Parent, J. Sathiyaseelan, X. Jiang, and C. L. Baldwin. 2001. Immune control of Brucella abortus 2308 infections in BALB/c mice. FEMS Immunol. Med. Microbiol. 32:85-88. [DOI] [PubMed] [Google Scholar]
- 42.Nathan, C. F., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicoletti, P. L. 1989. Relationship between animal and human disease, p. 41-52. In E. J. Young and M. J. Corbel (ed.), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, Fla.
- 44.Piddington, D. L., F. C. Fang, T. Laessig, A. M. Cooper, I. M. Orme, and N. A. Buchmeier. 2001. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 69:4980-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitcher, D. G., N. A. Saunders, and R. J. Owens. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 46.Robertson, G. T., and R. M. Roop II. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:790-800. [DOI] [PubMed] [Google Scholar]
- 47.Robertson, G. T., A. Reisenauer, R. Wright, R. B. Jensen, A. Jensen, L. Shapiro, and R. M. Roop II. 2000. The Brucella abortus CcrM DNA methyltransferase is essential for viability, and its overexpression attenuates intracellular replication in murine macrophages. J. Bacteriol. 182:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roop, R. M., II, B. H. Bellaire, M. W. Valderas, and J. M. Cardelli. 2004. Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 52:621-630. [DOI] [PubMed] [Google Scholar]
- 49.Roop, R. M., II, J. M. Gee, G. T. Robertson, J. M. Richardson, W.-L. Ng, and M. E. Winkler. 2003. Brucella stationary phase gene expression and virulence. Annu. Rev. Microbiol. 57:57-76. [DOI] [PubMed] [Google Scholar]
- 50.Rosner, B. 2000. Fundamentals of biostatistics, 5th ed. Duxbury, Pacific Grove, Calif.
- 51.Sadosky, A. B., J. W. Wilson, H. M. Steinman, and H. A. Shuman. 1994. The iron superoxide dismutase of Legionella pneumophila is essential for viability. Infect. Immun. 176:3790-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schnell, S., and H. M. Steinman. 1995. Function and stationary phase induction of periplasmic copper-zinc superoxide dismutase and catalase peroxidases in Caulobacter crescentus. J. Bacteriol. 177:5924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinman, H. M. 1993. Function of periplasmic copper-zinc superoxide dismutase in Caulobacter crescentus. J. Bacteriol. 175:1198-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatum, F. M., P. G. Detilleux, J. M. Sacks, and S. M. Halling. 1992. Construction of a Cu/Zn superoxide dismutase deletion mutant of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect. Immun. 60:2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira-Gomes, A. P., A. Cloeckaert, and M. S. Zygmunt. 2000. Characterization of heat, oxidative, and acid stress responses in Brucella melitensis. Infect. Immun. 68:2954-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teixeira-Gomes, A. P., A. Cloeckaert, G. Bezard, G. Dubray, and M. S. Zygmunt. 1997. Mapping and identification of Brucella melitensis proteins by two dimensional electrophoresis and microsequencing. Electrophoresis 1:156-162. [DOI] [PubMed] [Google Scholar]
- 58.Yost, F. J. J., and I. Fridovich. 1973. An iron containing superoxide dismutase from Escherichia coli. J. Biol. Chem. 248:4905-4908. [PubMed] [Google Scholar]
- 59.Young, E. J. 2000. Brucella species, p. 2386-2393. In G. L. Mandel, J. E. Bennett, and R. Dolin, (ed.), Principle and practice of infectious disease, 5th ed. Churchill-Livingstone, Philadelphia, Pa.