Abstract
We examined the roles of Th1-Th2 cytokine cross talk in Legionella pneumophila-infected bone marrow-derived (BM) macrophages in the presence of costimulation with interleukin-12 (IL-12) and IL-18. Treatment with gamma interferon (IFN-γ) alone or treatment with IL-12 in combination with IL-18 resulted in a 3- or 2-log reduction in bacterial numbers, respectively, in BM macrophages, whereas treatment with IL-12 or IL-18 alone had no effect. Significant amounts of IFN-γ were detected in the culture supernatants of infected macrophages stimulated with IL-12 and IL-18 in combination but not independently. Neutralization of IFN-γ by antibody completely abolished the growth inhibitory effects of IL-12 and IL-18. Interestingly, higher infectivity ratios of L. pneumophila or the addition of increasing concentrations of heat-killed bacteria (HKB) suppressed the production of IFN-γ, which resulted in the increased intracellular growth of bacteria. Significant amounts of IL-10 were detected in culture supernatants when Legionella-infected macrophages were cocultured with HKB. Furthermore, neutralization of IL-10 by antibody resulted in an increase in IFN-γ production by infected BM macrophages when cocultured with HKB. Treatment of HKB with trypsin but not polymyxin B attenuated the growth-promoting effects of HKB, suggesting the involvement of a protein component(s) in regulation of the growth of L. pneumophila. These findings demonstrate a crucial role of Th1-Th2 cross talk in L. pneumophila-infected BM macrophages. Our results also suggest that L. pneumophila modulates the cytokine balance from IFN-γ-driven Th1 to more Th2 responses, likely through the induction of IL-10 by a bacterial protein component(s). These data provide new insights not only into the cellular mechanisms of Th1-Th2 cross talk in Legionella-infected macrophages but also into the pathogenesis of L. pneumophila pneumonia in humans.
Legionella pneumophila is a gram-negative intracellular pathogen that often causes serious and life-threatening pneumonia in humans (21, 36). A recent epidemiological survey estimated that 17,000 to 50,000 patients with Legionella disease have been hospitalized annually in the United States (21, 36). Unfortunately, high mortality rates reaching 50% have been observed, illustrating the fact that Legionella pneumonia is still a challenging infectious disease, especially in immunocompromised individuals (10, 35, 47).
Legionella organisms usually infect humans via inhalation of contaminated aerosols from waterborne environmental sources. In the lungs, bacteria infect cells through binding to complement receptors on the surface and multiply predominantly in monocytes/macrophages (16, 24, 28, 34). The development of the A/J mouse model of L. pneumophila pneumonia has provided a valuable tool for analyzing the pathogenesis of this disease (1, 51). Macrophages of A/J mice are believed to be specifically permissive for L. pneumophila, and pneumonia induced in these animals resembles human disease in both pathological findings and cytokine responses (1). Previous studies demonstrated protective roles of Th1 cytokines, such as gamma interferon (IFN-γ), interleukin-12 (IL-12), and IL-18, in an L. pneumophila infection model (2, 3, 13, 38, 41). For example, we have reported a crucial role of neutrophil-derived IL-12 in driving Th1-type host responses in murine L. pneumophila pneumonia (46). In contrast, the Th2 cytokine IL-10 facilitates the growth of this organism in macrophages, due in part to IL-10-mediated suppression of Th1 cytokines (33). Th1-polarized cytokine production may be a critical event for resistance to intracellular pathogens, including Legionella, although how the Th1-Th2 balance is organized in L. pneumophila infection is still poorly understood.
IL-12 (6, 20) and IL-18 (31, 48) were separately recognized as IFN-γ-inducing factors. Furthermore, recent studies demonstrated that IL-12 and IL-18 synergistically induce the production of IFN-γ in a variety of cell types (25, 31, 32, 52, 53). An essential role of costimulation with IL-12 and IL-18 in the induction of IFN-γ was demonstrated in several infection models, such as Cryptococcus (53) and Listeria (30). Using a murine L. pneumophila pneumonia model, Brieland and collaborators demonstrated that blocking of endogenous IL-12 and IL-18 suppressed the production of IFN-γ in the lungs, a result which suggested the synergistic induction of IFN-γ by IL-12 and IL-18 in vivo (2). Recently, Munder and collaborators demonstrated that murine bone marrow-derived (BM) macrophages secrete IFN-γ upon stimulation with IL-12 and IL-18 in combination (27). These findings suggest that macrophages not only are key cells responding to IFN-γ but also are potent IFN-γ-producing cells. However, the roles and significance of IFN-γ-mediated autocrine macrophage activation in host inflammatory and immunological processes remain to be investigated.
In the present study, we examined the cross talk of Th1 and Th2 cytokines, especially IFN-γ induction and counterregulation by IL-10, in L. pneumophila-infected BM macrophages treated with IL-12 and IL-18 in combination. Our data suggest that L. pneumophila modulates the cytokine balance from IFN-γ-driven Th1 to more Th2 responses, probably through the induction of IL-10 by a bacterial protein component(s). The cellular mechanisms of Th1-Th2 cross talk in Legionella-infected macrophages and their contributions to the pathogenesis of L. pneumophila pneumonia are discussed.
MATERIALS AND METHODS
Medium and reagents.
Culturing of cells for infection was performed with RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 1% nonessential amino acids, 1% sodium pyruvate, 1% 2-mercaptoethanol, 1% l-glutamine, and penicillin/streptomycin (GIBCO, Grand Island, NY).
Mice.
Female A/J mice, 6 to 8 weeks old (Sankyo Laboratory, Tokyo, Japan), were used in these studies. They were housed and cared for at the Toho University School of Medicine animal facility.
Bacteria.
Clinical isolates of L. pneumophila Suzuki (44), a serogroup 1 strain originally isolated at Toho University Hospital, were grown over 3 to 4 days at 37°C on buffered charcoal-yeast extract (BCYE) agar supplemented with l-cysteine and ferric nitrates. A single colony was transferred to 3 ml of buffered yeast extract broth and incubated overnight at 37°C with constant shaking. The bacterial suspension was transferred to fresh buffered yeast extract broth as serial fivefold dilutions and incubated overnight under the same conditions as those described above. After confirmation of bacterial motility, the concentration of bacteria in the broth was determined by measuring the absorbance at 600 nm. Post-exponential-phase bacteria were used as challenge organisms (4). Heat-killed L. pneumophila was prepared by incubating the bacterial suspension in normal saline at 60°C for 10 min. After the heat treatment, the sterility of the bacterial suspension was confirmed by plating it on BCYE agar. The numbers of viable bacteria in the challenge suspension and the pre-heat treatment suspension were determined by plating and incubating organisms on BCYE agar for 4 days.
Preparation of BM macrophages.
Mouse macrophages were prepared from bone marrow exudates of female A/J mouse femurs as described previously (5, 43). The cells were seeded in 96-well tissue culture plates (104 cells/well) overnight and used as BM macrophages. After the medium was changed, the BM macrophages were infected with L. pneumophila Suzuki at several infectivity ratios (macrophage/bacteria, 1:0.2 to 1:1). At 2 h after infection, nonphagocytized and nonadherent bacteria were removed by two washes with medium. The infected macrophages were cultured for an additional 7 days at 37°C in a humidified atmosphere containing 5% CO2. In some experiments, various concentrations of heat-killed L. pneumophila were added to the infected macrophages 2 h after infection.
Addition of cytokines and neutralizing antibodies.
Recombinant mouse cytokines (IL-12, IL-18, and IFN-γ) and antibodies (anti-IFN-γ or anti-IL-10) were added to the infected macrophages 1 day after infection with L. pneumophila. All cytokines and antibodies used were obtained from R&D Systems (Minneapolis, MN).
Determination of bacterial numbers.
At various times after infection, culture supernatants were collected and infected macrophages were lysed by mechanical disruption in phosphate-buffered saline (pH 7.4). Harvested bacterial suspensions were plated on BCYE agar as serial 10-fold dilutions and incubated at 37°C for 4 days.
ELISAs for cytokines.
The suspensions used for the determination of bacterial numbers were centrifuged (10,000 rpm, 5 min). The supernatants were collected for cytokine assays and stored at −80°C until used. The concentrations of IL-10, tumor necrosis factor alpha (TNF-α), and IFN-γ were determined by sandwich enzyme-linked immunosorbent assays (ELISAs) with antibody pairs from R&D Systems in accordance with the manufacturer's instructions. The sensitivities of the ELISAs for the cytokines were 31 pg/ml for IL-10, 30 pg/ml for IFN-γ, and 16 pg/ml for TNF-α.
Trypsin and polymyxin B treatments of heat-killed L. pneumophila and effects of Legionella lipopolysaccharide (LPS) on bacterial numbers.
Heat-killed L. pneumophila (approximately 1010 CFU/ml) was diluted to the desired concentrations in RPMI 1640 medium. Trypsin treatment was performed as described previously (9, 12). Briefly, heat-killed L. pneumophila Suzuki was treated with trypsin (Sigma) for 2 h, and then soybean trypsin inhibitor (Sigma) was added for 1 h. This suspension was added to wells 2 h after infection. Final concentrations of trypsin were 80, 2,000, and 50,000 μg/ml, and that of trypsin inhibitor was 2.5 mg/ml. According to the manufacturer's instructions, 1 mg of trypsin inhibitor will inhibit 1.3 to 2.5 mg of trypsin. We did not observe significant effects of these concentrations of trypsin and/or trypsin inhibitor on bacterial numbers in the presence or absence of IL-12 plus IL-18.
Polymyxin B was obtained from Pfizer Japan Inc., and the suspension form of heat-killed L. pneumophila was treated with polymyxin B as described previously (49). Polymyxin B was incubated with the suspension of heat-killed bacteria for 3 h at room temperature. This suspension was added to wells 2 h after infection. Final concentrations of polymyxin B were 2 and 50 μg/ml.
LPS of L. pneumophila Suzuki was prepared as described previously (14). Purified LPS demonstrated 429,500 endotoxin units/ml, which is equivalent to approximately 2 × 109 to 4 × 109 CFU/ml. L. pneumophila-infected macrophages were incubated with various concentrations of Legionella LPS, and bacterial numbers in wells were determined on day 7.
RESULTS
Effects of Th1 cytokines on the growth of L. pneumophila in BM macrophages.
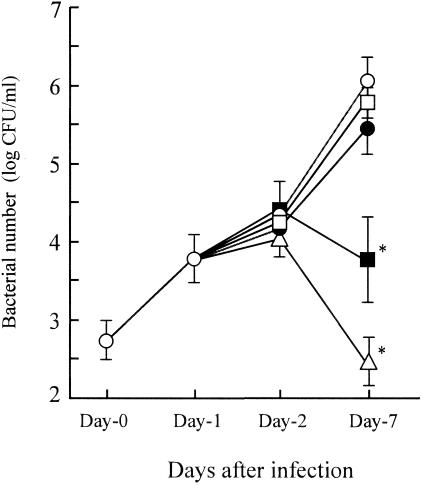
BM macrophages from A/J mice were infected with L. pneumophila at an infectivity ratio of 1 (macrophages:bacteria, 1:1) on day 0, and then macrophages were treated with IL-12, IL-18, IL-12 plus IL-18, or IFN-γ (20 ng/ml, each) on day 1. Bacterial numbers at day 0, 1, 2 and 7 after the infection were then determined (n = 5). As shown in Fig. 1, L. pneumophila displayed continuous growth in untreated control BM macrophages, reaching a concentration of approximately 106 CFU/ml on day 7. We did not observe growth inhibitory effects when macrophages were treated with IL-12 or IL-18 separately. In contrast, IL-12 plus IL-18, or IFN-γ alone resulted in a significant reduction in bacterial numbers on day 7. Specifically, incubation with IL-12 and IL-18 in combination, or IFN-γ induced 2- and 3-log reductions in bacterial numbers on day 7, respectively. These data suggest that the combination of IL-12 and IL-18 induces growth inhibition of L. pneumophila in BM macrophages, even at the concentrations in which each cytokine is ineffective individually. Furthermore, our data indicates that IFN-γ serves as the most potent stimulus for bacterial growth inhibition by macrophages.
FIG. 1.
Effects of Th1 cytokines on the growth of L. pneumophila in BM macrophages. BM macrophages (105 cells) were infected with L. pneumophila (105 CFU; infectivity ratio, 1:1) on day 0. Cytokines (20 ng/ml) were added to the wells of macrophage cultures at 24 h after infection (day 1). At the indicated times, supernatants and macrophages were collected and viable bacterial numbers were compared in the control group (open circle) and in groups treated with IL-12 (closed circle), IL-18 (open square), IL-12 plus IL-18 (closed square), and IFN-γ (open triangle). The results are expressed as means ± standard deviations for five wells per group. Similar results were obtained in three independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the control group.
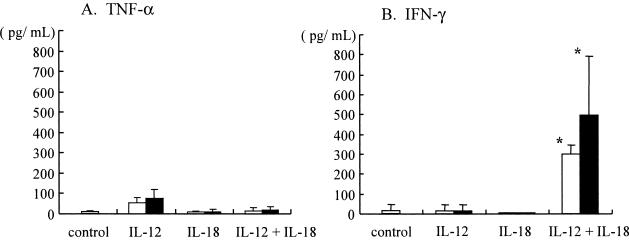
TNF-α and IFN-γ expression in culture supernatants of infected BM macrophages treated with IL-12 plus IL-18.
To define the nature of growth inhibitory effects induced by treatment of BM macrophages with IL-12 and IL-18 in combination, we examined levels of TNF-α and IFN-γ in culture supernatants of the infected BM macrophages. In these experiments, infected BM macrophages were treated with IL-12 or IL-18 (10 and 20 ng/ml) alone or in combination on day 1, incubated for additional 6 days, then culture supernatants were collected on day 7 and examined for TNF-α and IFN-γ by ELISAs (n = 5). As shown in Fig. 2, we were unable to detect significant amounts of TNF-α in any of these treatment groups. In contrast, the combination of IL-12 and IL-18 (at doses of 10 and 20 ng/ml) induced significant IFN-γ production in Legionella-infected BM macrophages, whereas each cytokine alone failed to induce detectable levels of IFN-γ. These data suggest that the treatment of infected BM macrophages with IL-12 and IL-18 in combination stimulates induction of IFN-γ, which may be associated with growth inhibitory effects on L. pneumophila.
FIG. 2.
TNF-α and IFN-γ levels in culture supernatants of infected BM macrophages treated with IL-12 plus IL-18. Cytokines at concentrations of 10 ng/ml (open bars) and 20 ng/ml (closed bars) were added to the wells of L. pneumophila-infected BM macrophages (105 cells) at 24 h after infection (n = 5). Culture supernatants were collected 7 days after infection, and TNF-α and IFN-γ levels were determined. The results represent means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the control group.
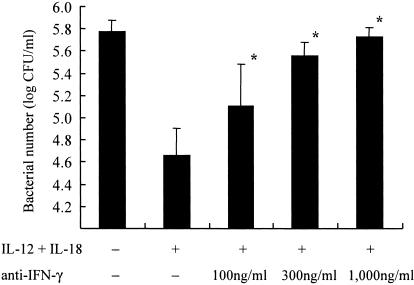
Effects of IFN-γ neutralization on suppression of the growth of L. pneumophila mediated by IL-12 and IL-18 in combination.
To further analyze the growth inhibitory effects observed by treatment of macrophages with IL-12 plus IL-18, we examined the effects of anti-IFN-γ antibodies on the growth of L. pneumophila in BM macrophages. Anti-IFN-γ antibodies (100, 300, and 1,000 ng/ml), in combination with IL-12 plus IL-18 (20 ng/ml), were added to the infected BM macrophages on day 1. Bacterial numbers in wells were determined on day 7 (n = 5). As previously noted, a significant reduction in bacterial numbers in BM macrophages was observed when these cells were treated with IL-12 plus IL-18. Under these experimental conditions, anti-IFN-γ antibodies abolished the growth-suppressive effects of therapy with IL-12 plus IL-18 (Fig. 3). Anti-IFN-γ antibodies effects were observed in a concentration-dependent fashion, and 1,000 ng/ml of antibody completely restored the growth of L. pneumophila to the control level. These results demonstrate that the induction of IFN-γ induction by IL-12 plus IL-18 was the major mechanism accounting for the growth inhibitory effects observed.
FIG. 3.
Effects of anti-IFN-γ antibodies on suppression of the growth of L. pneumophila in BM macrophages treated with IL-12 plus IL-18. Cytokines (20 ng/ml) were added to the wells of L. pneumophila-infected BM macrophages (105 cells) at 24 h after infection (n = 5). Several concentrations of anti-IFN-γ antibodies were added simultaneously with IL-12 and IL-18. Bacterial numbers in the wells were determined on day 7 after infection. The results represent means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the group receiving IL-12 plus IL-18 but not antibodies.
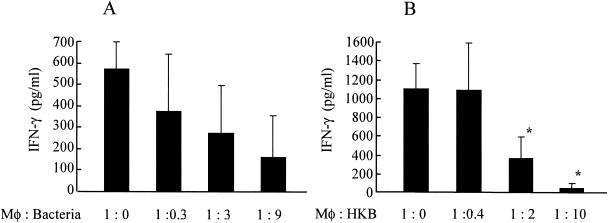
Suppression of IFN-γ production by live and heat-killed L. pneumophila.
To further characterize roles of Th1-Th2 cytokines in the pathogenesis of L. pneumophila infection, we examined IFN-γ induction in the setting of several magnitudes of infectivity ratio. As previously shown (Fig. 2), IFN-γ production was observed in the supernatants of L. pneumophila-infected BM macrophages when these cells were treated with IL-12 and IL-18. Interestingly, we observed less IFN-γ production in higher infectivity ratio although it was not statistically significant, as shown in Fig. 4A. These data suggest that L. pneumophila organism may suppress IFN-γ production from BM macrophages. However, we could not exclude the possibility of cytotoxic effects of bacteria at higher infectivity ratio as a reason for a reduction of IFN-γ, because previous data demonstrated that Legionella organisms induce cell death of macrophages, probably through induction of apoptosis (29, 54). To simplify the experimental conditions, we next examined effects of heat-killed L. pneumophila on IFN-γ production in BM macrophages. In these experimental conditions, we did not observe decrease of macrophage viability in the presence of heat-killed organisms. As shown in Fig. 4B, heat-killed L. pneumophila reduced production of IFN-γ in a dose-dependent fashion, similar to that observed with live organisms (Fig. 4B). These data demonstrate that L. pneumophila suppress IFN-γ production from macrophages stimulated with IL-12 plus IL-18. Moreover, our data suggest that heat-stable components of the organisms may be responsible for this IFN-γ-suppressing activity in BM macrophages.
FIG. 4.
Suppression of IFN-γ production by live and heat-killed L. pneumophila. (A) BM macrophages (105 cells) were infected with several concentrations of bacteria on day 0, and IL-12 plus IL-18 (10 ng/ml) was added to the wells on day 1. Culture supernatants were collected on day 7, and the concentrations of IFN-γ were determined (n = 5). (B) BM macrophages (105 cells) were infected with 105 CFU of bacteria on day 0. Heat-killed bacteria (HKB) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 2 h and 24 h after infection, respectively. Culture supernatants were collected on day 7, and the concentrations of IFN-γ were determined (n = 5). The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the groups not receiving heat-killed bacteria.
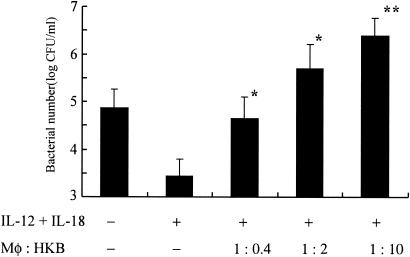
Heat-killed L. pneumophila enhances the replication of live organisms in BM macrophages.
Next, we examined effects of heat-killed L. pneumophila on growth of the organisms in BM macrophages. Various concentrations of heat-killed organisms were added to the culture of infected BM macrophages 2 h after the infection with live L. pneumophila, and then bacterial numbers were compared on day 7 (n = 5). Interestingly, the addition of heat-killed organisms promoted the growth of L. pneumophila (Fig. 5). This growth promoting effect of heat-killed organisms was concentration dependent, and more than a 1-log increase on bacterial numbers was observed when 1:10 heat-killed organisms (macrophages:heat-killed bacteria) were added to the culture. These data are consistent with the observed reduction of IFN-γ in the presence of heat-killed organisms. Taken together, the present results suggest that IFN-γ is a critical factor for growth suppression of L. pneumophila, and the presence of heat-killed organisms suppresses IFN-γ induction, which may be associated with the growth promotion of this organism in BM macrophages.
FIG. 5.
Heat-killed L. pneumophila augments replication of the organisms in BM macrophages. BM macrophages (105 cells) were infected with L. pneumophila on day 0. Heat-killed bacteria (HKB) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 2 h and 24 h after infection, respectively. Seven days after infection, bacterial numbers in the wells were determined. The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. A single asterisk indicates a P value of <0.05 and a double asterisk indicates a P value of <0.01 in a comparison with the group receiving IL-12 plus IL-18 but not heat-killed bacteria.
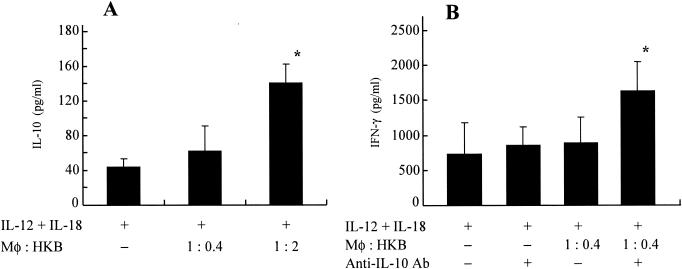
Heat-killed L. pneumophila induces IL-10 production in infected BM macrophages.
To examine the potential mechanism(s) of suppression of IFN-γ by heat-killed organisms, we determined IL-10 levels in the infected BM macrophages cocultured with heat-killed organisms (n = 5). As shown in Fig. 6A, significant amounts of IL-10 were detected in the culture supernatants of infected BM macrophages when these cells were cocultured with 1:2 heat-killed organisms (macrophages:heat-killed organisms). These results prompted us to examine effect of anti-IL-10 antibodies on IFN-γ production in these experimental conditions. As shown in Fig. 6B, the neutralization of IL-10 by specific antibody resulted in significant increase of IFN-γ in the infected BM macrophages when cocultured with heat-killed organisms. These findings suggest an important role of IL-10 induction by heat-killed L. pneumophila, which may explain, at least in part, suppression of IFN-γ and the resultant growth promotion of the organisms observed in the presence of heat-killed bacteria.
FIG. 6.
Heat-killed L. pneumophila induces IL-10 production in Legionella-infected BM macrophages. (A) BM macrophages (105 cells) were infected with L. pneumophila on day 0. Heat-killed bacteria (HKB) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 2 h and 24 h after infection, respectively. Seven days after infection, IL-10 production in supernatants was determined. The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the group receiving IL-12 plus IL-18 but not heat-killed bacteria. (B) BM macrophages (105 cells) were infected with L. pneumophila on day 0. Heat-killed bacteria (HKB) were added to the wells at 2 h after infection. Anti-IL-10 antibodies (Ab) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 24 h after infection. Seven days after infection, IFN-γ production in the wells was determined. The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the group receiving IL-12 plus IL-18 and heat-killed bacteria but not antibodies.
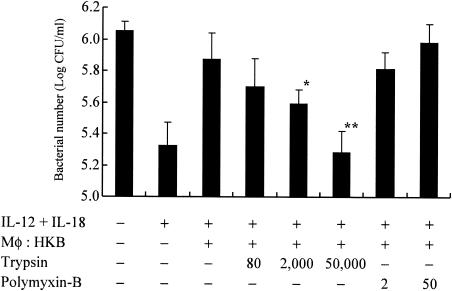
Characterization of a bacterial component(s) responsible for growth promotion in heat-killed L. pneumophila.
We next characterized the bacterial component(s) responsible for growth inhibitory effects mediated by heat-killed organisms. Heat-killed bacteria were treated with the proteinase trypsin for protein digestion (12) or polymyxin B for lipopolysaccharide inactivation (49), then added to the culture of infected BM macrophages, and the bacterial numbers on day 7 were examined. As shown in Fig. 7, the treatment of heat-killed L. pneumophila with trypsin significantly abolished the growth enhancing effects of heat-killed organisms, whereas inactivation of lipopolysaccharide by polymyxin B failed to alter growth of bacteria. These data suggests that a heat-stable protein component(s) of L. pneumophila may be responsible for growth promoting effects observed in the infected BM macrophages cocultured with heat-killed organisms.
FIG. 7.
Characterization of a bacterial component(s) responsible for bacterial growth promotion by heat-killed L. pneumophila. BM macrophages (105 cells) were infected with L. pneumophila on day 0. Heat-killed bacteria (HKB) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 2 h and 24 h after infection, respectively. Trypsin-treated (80, 2,000, or 50,000 μg/ml) or polymyxin B-treated (2 or 50 μg/ml) heat-killed bacteria were added to the wells of infected BM macrophages as described in Materials and Methods. Seven days after infection, bacterial numbers in the wells were determined. The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, in a comparison with the groups not receiving trypsin treatment.
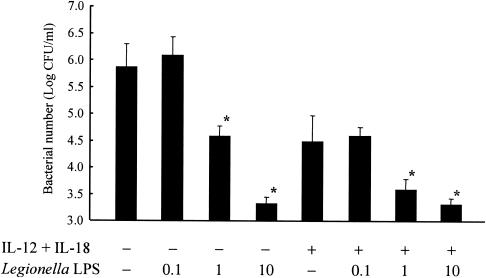
Effects of Legionella LPS on bacterial numbers.
Finally, we examined effects of purified L. pneumophila LPS on bacterial numbers in macrophages. As shown in Fig. 8, we observed reductions in bacterial numbers in the presence of Legionella LPS, in a concentration-dependent manner. The Legionella LPS effects were demonstrated in despite of the presence or absence of IL-12 plus IL-18. These data are consistent with data of Fig. 7, and further suggested that Legionella LPS is not an effector of growth promotion observed in the presence of heat-killed bacteria.
FIG. 8.
Effects of Legionella LPS on the growth of bacteria. BM macrophages (105 cells) were infected with L. pneumophila on day 0. Legionella LPS (in multiplicities of infection) and IL-12 plus IL-18 (10 ng/ml) were added to the wells at 2 h and 24 h after infection, respectively. Seven days after infection, bacterial numbers in the wells were determined. The results are expressed as means ± standard deviations for five wells. Similar results were obtained in at least two independent experiments. An asterisk indicates a P value of <0.05 in a comparison with the control group.
DISCUSSION
In the present study, cross talk between Th1 and Th2 cytokines was examined in L. pneumophila-infected BM macrophages. Our data are consistent with previous reports indicating a critical role of IFN-γ in anti-Legionella activity, and further demonstrate that L. pneumophila modulates cytokine balance, probably through IL-10-mediated suppression of IFN-γ production. The present results highlight the critical involvement of bacterial protein component(s), not only in Th1-Th2 cytokine cross talk, but also in regulation of growth of L. pneumophila.
Synergistic effects of IL-12 and IL-18 on IFN-γ induction has been demonstrated in several types of cells, such as T cells (25, 37), B cells (52) and NK cells (53). Furthermore, a recent report demonstrated that murine macrophages also produce efficient amount of IFN-γ in response to these cytokines, which suggests a novel pathway of autocrine macrophage activation (27). Salins and associates have demonstrated that expression of IFN-γ mRNA was detected in the Legionella-infected macrophages of BALB/c mice (growth restrictive), but not in the macrophages of A/J mice (growth-permissive), further supporting the importance of macrophage-derived IFN-γ in in vitro L. pneumophila infection (38). Brieland and collaborators have demonstrated that blocking of endogenous IL-12 and IL-18 by administration of specific neutralization antibody suppressed production of IFN-γ in lungs of mice infected with L. pneumophila, confirming a contribution of IL-12 and IL-18 to induction of IFN-γ in vivo (2). In this regard, we did not find a protective role of exogenous IL-12 in neutropenic A/J mouse model of L. pneumophila pneumonia, although combination treatment of IL-12 and IL-18 was not examined (46). In the present study, we observed IFN-γ production in L. pneumophila-infected BM macrophage from A/J mice, although costimulation with IL-12 and IL-18 was required. Since neutralization of IFN-γ by specific antibody completely abolished the growth inhibitory effects of treatment with IL-12 plus IL-18, it was concluded that IFN-γ-mediated macrophage activation is the principle reason for exaggerated bactericidal activity. These data suggest that production of macrophage-associated IFN-γ under costimulation with IL-12 and IL-18 may be important for regulation of growth of intracellular pathogens, including L. pneumophila. Moreover, our results suggest a potential role of therapy with IL-12 plus IL-18 against L. pneumophila infection, which is an ongoing study in our laboratory.
During these experiments, we noted suppression of IFN-γ when BM macrophages were infected with higher numbers of bacteria, which prompted us to examine effect of heat-killed organisms. Interestingly, we observed that heat-killed L. pneumophila also inhibited the production of IFN-γ in a dose-dependent manner (Fig. 4). The presence of heat-killed organisms in L. pneumophila-infected BM macrophages promoted the bacterial growth, an effect which correlated with suppression of IFN-γ. These results suggest that cellular component(s) of L. pneumophila may produce extracellular signals that modulate IFN-γ production and intracellular multiplication of bacteria in BM macrophages. Matsunaga and colleagues demonstrated that LPS-induced production of IL-12 was down-regulated in macrophages by infection with virulent L. pneumophila, but other cytokines, such as IL-6 and IL-10, were not affected (22). They also suggested an involvement of the mitogen-activated protein kinase cascade in the inhibition of IL-12 production by L. pneumophila (23) Since virulent L. pneumophila, but not avirulent or UV-killed bacteria, suppressed IL-12 production in macrophages, it was speculated that IL-12 suppression may be dependent on L. pneumophila virulence and its viability in the macrophages. Although a correlation between IL-12 suppression by virulent organisms and the present data of IFN-γ inhibition by heat-killed L. pneumophila is unknown, altering the production of the Th1-driving cytokines may be a critical means by which bacteria evade host immune systems.
Significant amounts of IL-10 were detected in L. pneumophila-infected BM macrophages when these cells were cocultured with heat-killed bacteria. Furthermore, neutralization of IL-10 by specific antibody resulted in a reciprocal increase of IFN-γ in these culture conditions. These data suggest that the production of IL-10 in response to heat-killed organisms contributes to IFN-γ suppression, which may be associated with growth promotion effects by heat-killed L. pneumophila. The present results are in agreement with the notion that bacteria can alter host cytokine networks as a means to enhance pathogenicity (50). IL-10 is a strong suppressor for IFN-γ and a key factor for driving Th2-type host responses. The role of IL-10 in microbial infection has been investigated for several bacterial and parasitic pathogens (26). In particular, a pathogen-mediated IL-10 production as microbial strategy to evade host immune system was demonstrated in Leishmania major (17) and Yersinia spp (39, 40). Sing and associates have demonstrated that Y. enterocolitica releases a virulence factor LcrV, which induces IL-10 through Toll-like receptor-2 and CD14 (40). Although LcrV homolog, PcrV, has been discovered in Pseudomonas aeruginosa as a part of type III protein secretion/translocation apparatus (39), the presence of LcrV homolog in L. pneumophila has not yet been demonstrated.
L. pneumophila possesses a variety of virulence factors, including LPS, flagella, heat-shock protein (Hsp60) and toxins (7, 42). More recently, lvgA, a surface-expressed novel virulence factor, has been identified (8, 9). Since treatment with a protease trypsin, but not the LPS inhibitor polymyxin B, decreased the growth promoting effect by heat-killed organisms, it appears likely that protein component(s) of bacteria may be responsible for these mechanisms. In fact, we did not observe the growth promotion effect by adding purified Legionella LPS. On the other hand, Legionella flagella (15) and a toxin (11) have been reported thus far as protein components with heat-stable characteristics. Hawn and colleagues have reported that flagella proteins of L. pneumophila are a principal stimulant of proinflammatory cytokine production in lung epithelial cells (15). They demonstrated that heat-killed L. pneumophila (65°C, 15 min), but not flagellum-deficient mutant, stimulated IL-8 production in A549 and Calu-3 cells, although the effects of Legionella flagella proteins on macrophages were not examined. Another important characteristic of L. pneumophila is the surface expression of Hsp60, a member of the GroEL family of chaperonins, as a virulence factor (12). In a study addressing bacterial Hsp60-host cell interactions, it was shown that the pretreatment of HeLa cells with Hsp60 of L. pneumophila reduced the adherence and invasiveness of bacteria at 3 h after infection (12), although the effect of Hsp60 pretreatment on long-term growth, or the stability of this protein after heat treatment has not been investigated. Which protein component(s) of L. pneumophila is responsible for the growth promotion effects, as well as potential receptor-ligand interaction and down-stream signaling mechanisms governing growth permissiveness in macrophages, are of great interest.
While our study has identified a protein component(s) of L. pneumophila as an autocrine and/or paracrine enhancer of intracellular growth within macrophages, the roles and significance of these phenomenon in vivo remain to be investigated. L. pneumophila pneumonia is characterized by severe hypoxemia and a high frequency of complications, such as acute respiratory distress syndrome and multiple organ failure (10, 35, 47). It is well known that patients with L. pneumophila pneumonia excrete bacterial antigens into urine, which is now widely accepted as a diagnostic tool for the disease. Interestingly, urinary antigens are generally detectable within a few days of illness onset and can remain so for several months to >300 days after initiation of appropriate antimicrobial therapy (18). These findings suggest that bacteria or at least bacterial components/products may exist at sites of infection during those periods. Previously, we found less extensive IFN-γ and IL-12 responses in the lungs when larger numbers of L. pneumophila organisms were inoculated into the lungs of A/J mice, suggesting the suppression of Th1-type cytokine responses by this organism in vivo (unpublished results). In contrast, in the serum of L. pneumophila pneumonia patients, we did not observe a correlation between the severity of disease and levels of IFN-γ, although most patients consistently demonstrated strong Th1-type cytokine responses (45). Recently, Lettinga and associates reported reduced IFN-γ responses in patients who had recovered from L. pneumophila pneumonia 1 year earlier, in comparison with control individuals who were also exposed but had not developed the disease (19). Since smaller amounts of IFN-γ were released after whole-blood stimulation with LPS or IL-12 in the former patients, they concluded that impaired IFN-γ production may contribute to susceptibility to L. pneumophila infection. Based on our current observations, an alternative interpretation of these results is that suppression of IFN-γ production may be mediated by ongoing exposure to L. pneumophila or its antigens, although the duration and persistence of IFN-γ-suppressing effects by heat-killed bacteria have not yet been examined. Further investigations are warranted in order to better define the mechanisms of pathogenesis in L. pneumophila infection, the molecular and cellular aspects of Th1-Th2 cytokine cross talk, and the clinical significance of these responses in disease.
Acknowledgments
We greatly appreciate suggestions made by Paul H. Edelstein, University of Pennsylvania. We also thank Tsuneharu Maeda and Chiharu N. Kimura for technical assistance and helpful discussions.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan.
Editor: J. L. Flynn
REFERENCES
- 1.Brieland, J., P. Freeman, R. Kunkel, C. Chrisp, M. Hurley, J. Fantone, and C. Engleberg. 1994. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am. J. Pathol. 145:1537-1546. [PMC free article] [PubMed] [Google Scholar]
- 2.Brieland, J. K., C. Jackson, S. Hurst, D. Loebenberg, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2000. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect. Immun. 68:6567-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brieland, J. K., D. G. Remick, M. L. LeGendre, N. C. Engleberg, and J. C. Fantone. 1998. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect. Immun. 66:65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, S. H., B. Perussia, J. W. Gupta, M. Kobayashi, M. Pospisil, H. A. Young, S. F. Wolf, D. Young, S. C. Clark, and G. Trinchieri. 1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianciotto, N. P. 2001. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 291:331-343. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelstein, P. H., B. Hu, F. Higa, and M. A. Edelstein. 2003. LvgA, a novel Legionella pneumophila virulence factor. Infect. Immun. 71:2394-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.el-Ebiary, M., X. Sarmiento, A. Torres, S. Nogue, E. Mesalles, M. Bodi, and J. Almirall. 1997. Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am. J. Respir. Crit. Care Med. 156:1467-1472. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, R. L., B. H. Iglewski, and R. D. Miller. 1980. Identification of a cytotoxin produced by Legionella pneumophila. Infect. Immun. 29:271-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garduno, R. A., E. Garduno, and P. S. Hoffman. 1998. Surface-associated hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect. Immun. 66:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebran, S. J., Y. Yamamoto, C. Newton, T. W. Klein, and H. Friedman. 1994. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III). Infect. Immun. 62:3197-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard, R., T. Pedron, S. Uematsu, V. Balloy, M. Chignard, S. Akira, and R. Chaby. 2003. Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J. Cell Sci. 116:293-302. [DOI] [PubMed] [Google Scholar]
- 15.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires' disease. J. Exp. Med. 198:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J. Clin. Investig. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 18.Kohler, R. B., W. C. Winn, Jr., and L. J. Wheat. 1984. Onset and duration of urinary antigen excretion in Legionnaires' disease. J. Clin. Microbiol. 20:605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lettinga, K. D., S. Weijer, P. Speelman, J. M. Prins, T. Van Der Poll, and A. Verbon. 2003. Reduced interferon-gamma release in patients recovered from Legionnaires' disease. Thorax 58:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locksley, R. M. 1993. Interleukin 12 in host defense against microbial pathogens. Proc. Natl. Acad. Sci. USA 90:5879-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marston, B. J., H. B. Lipman, and R. F. Breiman. 1994. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch. Intern. Med. 154:2417-2422. [PubMed] [Google Scholar]
- 22.Matsunaga, K., T. W. Klein, C. Newton, H. Friedman, and Y. Yamamoto. 2001. Legionella pneumophila suppresses interleukin-12 production by macrophages. Infect. Immun. 69:1929-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga, K., H. Yamaguchi, T. W. Klein, H. Friedman, and Y. Yamamoto. 2003. Legionella pneumophila suppresses macrophage interleukin-12 production by activating the p42/44 mitogen-activated protein kinase cascade. Infect. Immun. 71:6672-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDade, J. E., C. C. Shepard, D. W. Fraser, T. R. Tsai, M. A. Redus, and W. R. Dowdle. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297:1197-1203. [DOI] [PubMed] [Google Scholar]
- 25.Micallef, M. J., T. Ohtsuki, K. Kohno, F. Tanabe, S. Ushio, M. Namba, T. Tanimoto, K. Torigoe, M. Fujii, M. Ikeda, S. Fukuda, and M. Kurimoto. 1996. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26:1647-1651. [DOI] [PubMed] [Google Scholar]
- 26.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 27.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1984. Interaction between the Legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J. Clin. Investig. 74:771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumeister, B., M. Faigle, K. Lauber, H. Northoff, and S. Wesselborg. 2002. Legionella pneumophila induces apoptosis via the mitochondrial death pathway. Microbiology 148:3639-3650. [DOI] [PubMed] [Google Scholar]
- 30.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 32.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70:281-312. [DOI] [PubMed] [Google Scholar]
- 33.Park, D. R., and S. J. Skerrett. 1996. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J. Immunol. 157:2528-2538. [PubMed] [Google Scholar]
- 34.Payne, N. R., and M. A. Horwitz. 1987. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J. Exp. Med. 166:1377-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedro-Botet, M. L., M. Sabria-Leal, N. Sopena, J. M. Manterola, J. Morera, R. Blavia, E. Padilla, L. Matas, and J. M. Gimeno. 1998. Role of immunosuppression in the evolution of Legionnaires' disease. Clin. Infect. Dis. 26:14-19. [DOI] [PubMed] [Google Scholar]
- 36.Reingold, A. L. 1988. Role of legionellae in acute infections of the lower respiratory tract. Rev. Infect. Dis. 10:1018-1028. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, D., K. Shibuya, A. Mui, F. Zonin, E. Murphy, T. Sana, S. B. Hartley, S. Menon, R. Kastelein, F. Bazan, and A. O'Garra. 1997. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 7:571-581. [DOI] [PubMed] [Google Scholar]
- 38.Salins, S., C. Newton, R. Widen, T. W. Klein, and H. Friedman. 2001. Differential induction of gamma interferon in Legionella pneumophila-infected macrophages from BALB/c and A/J mice. Infect. Immun. 69:3605-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 40.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skerrett, S. J., and T. R. Martin. 1994. Intratracheal interferon-gamma augments pulmonary defenses in experimental legionellosis. Am. J. Respir. Crit. Care Med. 149:50-58. [DOI] [PubMed] [Google Scholar]
- 42.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 43.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tateda, K., J. C. Deng, T. A. Moore, M. W. Newstead, R. Paine III, N. Kobayashi, K. Yamaguchi, and T. J. Standiford. 2003. Hyperoxia mediates acute lung injury and increased lethality in murine Legionella pneumonia: the role of apoptosis. J. Immunol. 170:4209-4216. [DOI] [PubMed] [Google Scholar]
- 45.Tateda, K., T. Matsumoto, Y. Ishii, N. Furuya, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. Serum cytokines in patients with Legionella pneumonia: relative predominance of Th1-type cytokines. Clin. Diagn. Lab. Immunol. 5:401-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 47.Tkatch, L. S., S. Kusne, W. D. Irish, S. Krystofiak, and E. Wing. 1998. Epidemiology of Legionella pneumonia and factors associated with legionella-related mortality at a tertiary care center. Clin. Infect. Dis. 27:1479-1486. [DOI] [PubMed] [Google Scholar]
- 48.Ushio, S., M. Namba, T. Okura, K. Hattori, Y. Nukada, K. Akita, F. Tanabe, K. Konishi, M. Micallef, M. Fujii, K. Torigoe, T. Tanimoto, S. Fukuda, M. Ikeda, H. Okamura, and M. Kurimoto. 1996. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 156:4274-4279. [PubMed] [Google Scholar]
- 49.Wiese, A., M. Munstermann, T. Gutsmann, B. Lindner, K. Kawahara, U. Zahringer, and U. Seydel. 1998. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J. Membr. Biol. 162:127-138. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, M., R. Seymour, and B. Henderson. 1998. Bacterial perturbation of cytokine networks. Infect. Immun. 66:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto, Y., T. W. Klein, C. A. Newton, R. Widen, and H. Friedman. 1988. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect. Immun. 56:370-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimoto, T., H. Okamura, Y. I. Tagawa, Y. Iwakura, and K. Nakanishi. 1997. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc. Natl. Acad. Sci. USA 94:3948-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, T., K. Kawakami, M. H. Qureshi, H. Okamura, M. Kurimoto, and A. Saito. 1997. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect. Immun. 65:3594-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu-Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]