Abstract
In Borrelia burgdorferi-infected C3H-scid mice, antiserum to a differentially expressed, 37-kDa spirochetal outer-surface protein, termed arthritis-related protein (Arp), has been shown to prevent or reduce the severity of arthritis. In this study, we determined the immunoglobulin G (IgG) antibody responses to this spirochetal protein in single serum samples from 124 antibiotic-treated human patients with early or late manifestations of Lyme disease and in serial serum samples from 20 historic, untreated patients who were followed longitudinally from early infection through the period of arthritis. These 20 patients were representative of the spectrum of the severity and duration of Lyme arthritis. Among the 124 antibiotic-treated patients, 53% with culture-proven erythema migrans (EM) had IgG responses to recombinant glutathione S-transferase (GST)-Arp, as did 59% of the patients with facial palsy and 68% of those with Lyme arthritis. In addition, 75 to 80% of the 20 past, untreated patients had reactivity with this protein when EM was present, during initial episodes of joint pain, or during the maximal period of arthritis. There was no association at any of these three time points between GST-Arp antibody levels and the severity of the maximal attack of arthritis or the total duration of arthritis. Thus, after the first several weeks of infection, 60 to 80% of patients had IgG antibody responses to GST-Arp, but this response did not correlate with the severity or duration of Lyme arthritis.
Lyme disease in the United States is caused by the tick-borne spirochete Borrelia burgdorferi (18). The infection usually begins in summer with an expanding skin lesion, erythema migrans (EM), which occurs at the site of the tick bite (17). Days to weeks later, during or soon after the phase of spirochetal dissemination, patients may develop brief episodes of joint pain or heart or nervous system involvement. The most common neurologic abnormality is facial nerve paralysis (16). Months later, untreated patients often have intermittent or continuous arthritis which can last for several years with marked joint swelling and pain, primarily in large joints, especially the knee (20). It is now known that all stages of the disease can usually be treated successfully with antibiotic therapy (22).
A model of Lyme arthritis in C3H mice duplicates many of the features of the acute phase of human Lyme arthritis (3). In C3H mice, B. burgdorferi infection induces marked edema and inflammation 10 to 14 days after inoculation of the spirochete. In mice, the arthritis occurs mainly in the tibiotarsal joints, peaks 3 to 5 weeks after infection, and usually resolves within weeks, despite persistent infection. In B. burgdorferi-infected, C3H-scid (severe combined immunodeficient) mice that lack T- and B-cell responses, passive transfer of immune sera proved that the resolution of arthritis was an antibody-mediated event (4).
To identify the borrelial antigens targeted by arthritis-resolving antibodies, Feng et al. screened a B. burgdorferi strain N40 genomic expression library with sera from actively infected mice (9). Of 46 immunoreactive clones, antibody to one clone had arthritis-resolving activity. When the clone was sequenced, it was shown to be a 37-kDa protein, which the researchers called arthritis-related protein (Arp). The gene sequence of B. burgdorferi N40 arp had 99% identity with the sequence located on linear plasmid lp28-1 of B. burgdorferi strain B31 (bbf01) (12). In B. burgdorferi-infected, C3H-scid mice, antiserum to this single recombinant, glutathione S-transferase (GST)-cleaved, Arp protein, passively transferred prior to or during the period of arthritis, prevented or reduced the severity of arthritis, whereas antiserum to GST alone had no effect (9). However, despite the resolution of arthritis, the infection continued. In contrast, antibodies to outer-surface protein A (OspA), OspB, and decorin-binding protein A (DbpA) prevent infection but do not resolve established arthritis (4, 11, 13).
Antibody responses to Arp have not yet been studied in human Lyme disease. In the present study, we determined the frequency of immunoglobulin G (IgG) antibody responses to recombinant GST-Arp in patients with early or late manifestations of the infection. In addition, using a unique set of archival serum samples from untreated patients followed longitudinally throughout their illness, we compared the strength of GST-Arp antibody responses prior to and during the period of arthritis with the severity and duration of joint swelling.
MATERIALS AND METHODS
Patients.
Serum samples were selected from 124 patients with early or late manifestations of Lyme disease (erythema migrans, facial palsy, or arthritis) who were treated with appropriate antibiotic therapy for their manifestation of the illness. Acute- and convalescent-phase serum samples were tested from 30 patients with culture-confirmed EM. For this group, every third name was selected from an alphabetical list of 91 patients who participated in a recent field study of early Lyme disease in Wakefield, Rhode Island, or East Lyme, Connecticut (19, 21). Single samples were also tested from all 32 patients in our files who had facial paralysis for whom samples were available at the time of paralysis. In addition, a single sample was tested from 62 patients with active arthritis. For this group, every second name was selected from an alphabetical list of 121 patients who participated in the study of immunity in Lyme arthritis between 1987 and 2004 (1, 7). Samples from 50 healthy subjects from the same geographic area served as a control group. Nitin Damle in Wakefield, Rhode Island, and Vijay Sikand in East Lyme, Connecticut, collected the samples from EM patients and control subjects. Finally, the enzyme-linked immunosorbent assays (ELISAs) were calibrated using the same serum samples from seven healthy individuals that we have used in previous studies (8). The ranges of absorbance values of these samples to sonicated Borrelia antigens were previously shown to be representative of those observed in healthy control subjects.
Serial serum samples were also tested from all 20 historic, untreated patients with Lyme disease for whom at least three serum samples were available: one when erythema migrans was present, a second during initial episodes of joint pain, and a third during the maximal period of arthritis. These 20 patients, who were representative of the spectrum of the severity and duration of Lyme arthritis, were followed throughout the illness by one of us (A.C.S) at the Lyme Disease Clinic at Yale University School of Medicine during the late 1970s, prior to the use of antibiotic therapy for Lyme disease. At the mild end of the spectrum, a few of these patients had only one episode of arthritis lasting several weeks; in the middle, some patients had brief, intermittent attacks of arthritis over a 1- to 2-year period, and at the far end of the spectrum, other patients had severe, continuous arthritis for several years. Serial samples were not available from untreated patients in whom EM was the only manifestation of the illness. Clinical data were recorded in the patients' charts, and serum samples from each visit were stored at −70°C. The severity of arthritis was based on the volume of knee effusions, which had been determined by joint aspiration or estimated by physical examination at each visit according to the following scale: 1 to 10 ml, score of 1; 10 to 30 ml, score of 2; 30 to 50 ml, score of 3; and >50 ml, score of 4. Maximal arthritis was defined as the most prolonged episode of continuous joint swelling, and the total duration of arthritis was the sum of all periods of active arthritis.
All 144 patients met the criteria of the Centers for Disease Control for the diagnosis of Lyme disease (5). They had EM or a later manifestation of the disease and a positive antibody response to B. burgdorferi by ELISA and Western blotting, interpreted according to the criteria of the Centers for Disease Control and Prevention (6). The study was approved by the Human Investigations Committee at Tufts-New England Medical Center, where the study was begun.
Recombinant fusion protein.
The Escherichia coli DH5α cells containing the GST-Arp plasmid were a kind gift of Stephen Barthold, University of California at Davis. This construct, which contained the Arp sequence from the N40 strain of B. burgdorferi (GenBank accession no. AF050212), was used previously to express the recombinant protein for murine studies of Feng et al. (9). Isolation of the recombinant GST-Arp fusion protein was performed according to a published protocol (2), with minor modifications. Briefly, the cells were incubated for 5 h, and then recombinant GST-Arp was induced for 2 h with 1 M isopropyl-1-thio-β-d-galactopyranoside (IPTG). The cells were centrifuged at 4,000 rpm for 10 min, and the pellets were resuspended in 10 ml phosphate-buffered saline (PBS) (1:10) with 1% Triton X-100 and 100 mM phenylmethylsulfonyl fluoride (PMSF). After sonication, Triton X-100 was added to a 3% final concentration. The sonicates were centrifuged to remove insoluble material. Polyacrylamide gels stained with Gelcode Blue Stain reagent (Pierce Biotechnology, Rockford, IL) showed that GST-Arp was in the soluble fraction. The supernatants containing GST-Arp were mixed with glutathione-Sepharose 4B beads (Pharmacia, Amersham Biosciences, Piscataway, NJ) in a 15-ml tube and incubated for 1 h on a rocker at room temperature. After the beads were washed with cold PBS and centrifuged, they were resuspended in 250 μl of reduced glutathione elution buffer (50 mM Tris-HCl-10 mM reduced glutathione, pH 8.0) and incubated for 10 min on a rocker at room temperature. After centrifugation, the supernatant containing GST-Arp was removed and saved, and the elution step was repeated twice. Afterwards, the preparations of GST-Arp were combined, aliquoted, and frozen at −80°C.
ELISA.
Easy Wash plates (Costar; Corning, Inc., Corning, NY) were coated with 50 μl of GST-Arp (0.05 μM) or GST alone (0.4 μM) (Sigma) in 50 mM carbonate buffer (pH 9.6) and incubated overnight at 4°C. After the wells were washed three times with PBS containing 0.05% Tween 20 (PBS-T) (pH 7.6), each well was blocked with 200 μl of PBS-T containing 5% nonfat dry milk and incubated for 1 h at 37°C. After the wells were washed three times with PBS-T, serum samples (50 μl), diluted 1:100 in milk buffer, were added in duplicate and incubated for 1 h at 37°C. The plates were then washed three times with PBS-T, and 50 μl of the conjugate, goat anti-human IgG linked with alkaline phosphatase (Biosource, Int., Camarillo, CA) diluted 1:1,000 in milk buffer, were added to each well and incubated for 1 h at 37°C. Serial serum samples from the same patient were analyzed at the same time on the same plate. Finally, the plates were washed three times with PBS-T and three times with PBS alone, followed by the addition of 200 μl of the substrate, p-nitrophenyl phosphate (5 mg/ml), to each well. After incubation at room temperature for 10 min, the plates were read in a microplate reader (Bio-Rad) at an optical density at 405 nm, and the values in duplicate wells were averaged. The cutoff optical density for a positive response was defined as 3 standard deviations above the mean absorbance value of the samples from seven seronegative, healthy control subjects included on each plate. For four patients with minimally positive responses to GST, repeating the assay with equimolar GST-Arp to GST alone (0.05 μM) did not significantly alter the results. For comparison, samples were tested for IgG responses to a sonicate preparation of B. burgdorferi (strain G39/40) by ELISA, as previously described (8).
Statistics.
The identity of groups was compared in 2 × 2 tables by Fisher's exact test, and the distributions of values among groups were compared by Mann-Whitney U test. In past, untreated patients, the GST-Arp antibody levels at each of three time points were correlated with the severity and duration of arthritis, using Spearman correlation test. All P values are two tailed.
RESULTS
Evaluation of antibiotic-treated patients.
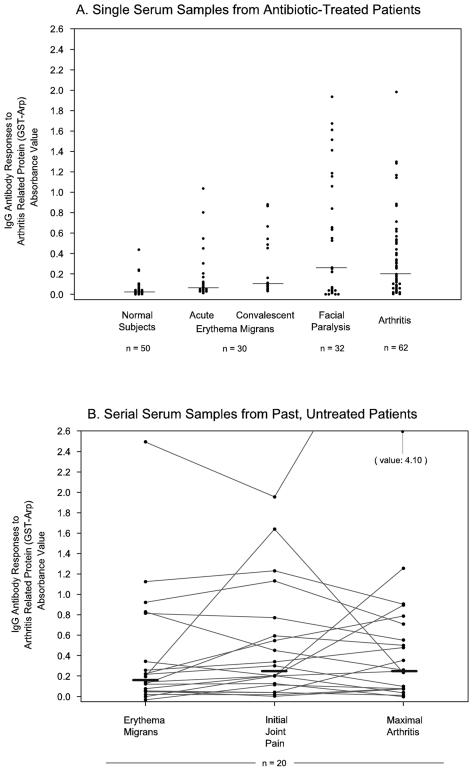
To determine the frequency of IgG responses to the GST-Arp fusion protein in patients with early or late manifestations of Lyme disease, serum samples from 124 antibiotic-treated patients with erythema migrans, facial palsy, or Lyme arthritis were tested. Of the 30 patients with culture-proven EM, 13 (43%) had IgG antibody responses to GST-Arp in acute-phase samples a median of 4 days (range, 1 to 21 days) after the onset of signs and symptoms of the infection (Table 1). During convalescence 3 to 4 weeks later, at the conclusion of antibiotic therapy, 16 of the 30 patients (53%) had such responses. Among the 32 patients with facial palsy, which occurred weeks after the onset of symptoms, 19 (59%) had IgG responses to GST-Arp. Finally, 42 of the 62 patients (68%) with arthritis, which occurred months after the onset of symptoms, had such reactivity. In contrast, only 2 of the 50 samples (4%) from healthy control subjects responded to GST-Arp. Three of the 174 samples had minimal reactivity with GST alone (data not shown), but these patients had a significantly greater response to GST-Arp so were still included in the analysis. Compared with the responses to GST-Arp, fewer patients with EM had positive IgG responses to the whole-cell sonicate preparation of B. burgdorferi, but a greater number of those with facial palsy or arthritis had reactivity with this preparation, including all 59 (100%) with Lyme arthritis in whom sufficient sample volume remained for testing.
TABLE 1.
Frequency of IgG antibody responses to GST-Arp or B. burgdorferi sonicate according to the manifestation of Lyme disease
| Infection stage | Single serum samples from treated patientsa
|
Serial serum samples from untreated patientsb
|
||||
|---|---|---|---|---|---|---|
| Time after onset of infection | No. positive/total patientsc (%)
|
Time after onset of infection | No. positive/total patients (%)
|
|||
| GST-Arp | Sonicated | GST-Arp | Sonicate | |||
| Erythema migranse | ||||||
| Acute | 1-21 days | 13/30 (43) | 7/30 (23) | 2-12 wks | 15/20 (75) | 7/19 (37) |
| Convalescent | 16-39 days | 16/30 (53) | 20/30 (67) | |||
| Facial palsy/arthralgia | 2-6 wks | 19/32 (59) | 21/32 (66) | 2-12 mo | 16/20 (80) | 12/19 (63) |
| Arthritis | 6-28 mo | 42/62 (68) | 59/59 (100) | 7-52 mo | 15/20 (75) | 17/17 (100) |
Thirty patients with culture-confirmed erythema migrans, 32 patients with facial palsy, and 62 patients with arthritis.
Twenty patients with untreated Lyme arthritis; serial serum samples were obtained during erythema migrans, during the initial episodes of joint pain (arthralgia), and during the maximal period of arthritis.
Number of patients with positive IgG antibody response/total no. of patients.
Sonicate results were not obtained in single serum samples from three treated patients and in five samples from three untreated patients because insufficient serum remained.
Samples were obtained at the time of initial presentation (acute) and at the conclusion of antibiotic therapy 2 to 4 weeks later (convalescent) in treated patients.
When the magnitude of the responses to GST-Arp was compared among the Lyme disease groups, the patients with facial palsy or arthritis tended to have higher values than those with EM (Fig. 1A). These differences reached statistical significance for the comparison of the facial palsy group with the EM group during the acute phase of the illness (P < 0.01). Each of the Lyme disease groups had significantly greater reactivity with GST-Arp than healthy control subjects (in each instance, P < 0.001).
FIG. 1.
IgG antibody responses to GST-Arp are shown (A) in single serum samples from 124 antibiotic-treated patients with erythema migrans, facial palsy, or Lyme arthritis and (B) in serial serum samples from 20 past, untreated patients who were followed longitudinally from early infection when EM was present, through initial episodes of joint pain, and during the maximal period of arthritis. The bars show the median values in the patients' responses (in panel A, healthy [normal] control subjects, 0.02; acute erythema migrans, 0.06; convalescent erythema migrans, 0.1; facial paralysis, 0.25; arthritis, 0.19; in panel B, erythema migrans, 0.17; initial joint pain, 0.25; maximal arthritis, 0.26).
Longitudinal evaluation of past, untreated patients.
To correlate the IgG antibody responses to GST-Arp prior to and during the period of arthritis with the severity and duration of joint swelling, reactivity with this protein was evaluated in serum samples from 20 past, untreated patients with Lyme disease who were followed longitudinally from early infection through the period of arthritis, prior to the use of antibiotic therapy for this illness. These patients were representative of the spectrum of Lyme arthritis, ranging from mild and brief to severe and prolonged arthritis. Fifteen of the 20 patients (75%) had IgG responses to GST-Arp early in the illness when EM was present; 16 (80%) had such responses during initial episodes of joint pain (arthralgias), and 15 (75%) had reactivity with this protein during the maximal period of arthritis (Table 1). One of the samples had minimal reactivity with GST alone (data not shown), but this patient's samples had no reactivity with GST-Arp at any time so the data were included in the analysis.
Compared with the responses to GST-Arp, fewer patients had positive IgG responses to the whole-cell Borrelia sonicate when EM was present or during initial episodes of joint pain, but more patients had such responses during the period of maximal arthritis (Table 1). Although the percentage of patients with positive responses to GST-Arp was somewhat greater at the first two time points in past, untreated patients than in antibiotic-treated patients, the first time point in past patients was often several weeks later and the second time point was months later in the course of the infection (Table 1). During the period of maximal arthritis, a similar percentage of both past, untreated and antibiotic-treated patients had responses to GST-Arp.
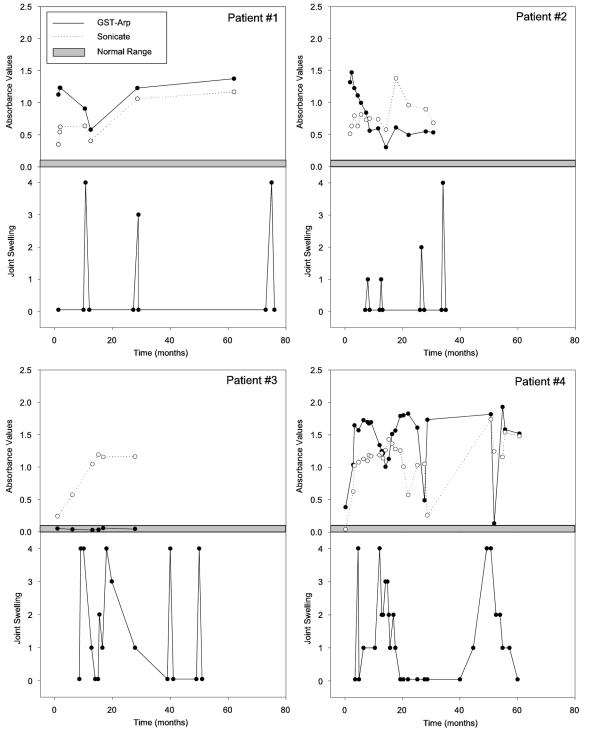
When the IgG antibody responses to GST-Arp were examined longitudinally, no dominant pattern was observed (Fig. 1B). In some patients, the response was greatest when EM was present and declined thereafter. Conversely, in other patients, reactivity increased throughout the infection and was greatest during the maximal period of arthritis. In still others, the response declined after EM was present but increased again during the maximal period of arthritis. Finally, some patients had no reactivity with this spirochetal protein at any time in the illness. The courses of four representative patients for whom all available samples were tested demonstrate this variability in GST-Arp responses (Fig. 2).
FIG. 2.
The clinical courses and IgG antibody responses to GST-Arp and whole-cell Borrelia sonicate are shown in four representative patients. Patient 1, who had three brief episodes of arthritis, had a strong response to GST-Arp early in the illness, which declined after the first attack, but the response increased again during the subsequent episodes of arthritis. Patient 2 had strong reactivity with GST-Arp prior to the onset of arthritis, and this response declined throughout the subsequent four attacks of arthritis. Patient 3, who had more prolonged arthritis, had no reactivity with GST-Arp at any time in the illness. In contrast, patient 4, who also had prolonged attacks of arthritis, had marked reactivity with GST-Arp throughout the illness. In patients 1 and 4, the responses to GST-Arp sometimes decreased along with attacks of arthritis. However, reactivity with Borrelia sonicate also declined at these times, and thus, this pattern was not specific for GST-Arp. The gray area shows the range in seven healthy (normal) control subjects used to calibrate the ELISA.
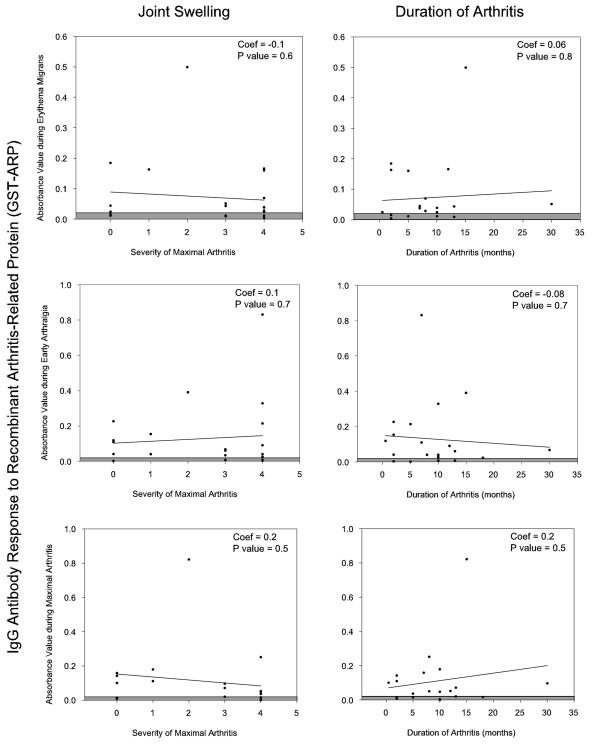
In an effort to determine whether IgG reactivity with GST-Arp prior to or during the period of arthritis was associated with less severe arthritis of shorter duration, GST-Arp antibody levels during erythema migrans, early joint pain (arthralgias), or maximal arthritis (Fig. 1B) were each correlated with the severity of the maximal attack of arthritis and the total duration of arthritis. However, no association was found at any of the three time points between the antibody levels to GST-Arp and the severity or duration of arthritis (Fig. 3).
FIG. 3.
Correlation of the IgG antibody responses to GST-Arp during erythema migrans, early arthralgia, or maximal arthritis with the severity and duration of arthritis. At each of these three time points, there was no association between the antibody levels to GST-Arp and the severity of duration of arthritis. Coef, coefficient.
DISCUSSION
After the first several weeks of infection, approximately 60 to 80% of the study patients with Lyme disease had IgG antibody responses to the 37-kDa arthritis-related protein (Arp) of B. burgdorferi. Among the patients followed longitudinally, multiple patterns were observed, including early responses that declined or increased during the period of arthritis, high responses that were sustained throughout the infection, or no responses at any time in the illness. This variability is consistent with the observations that Arp is a differentially expressed, outer-surface protein of the spirochete (10, 14). In contrast, the percentage of patients with positive responses to whole-cell Borrelia sonicate increased at each stage of the illness and was highest among those with arthritis late in the infection.
This study was carried out because of findings in B. burgdorferi-infected, C3H-scid mice in which Arp antibody, passively transferred prior to or during the period of arthritis, prevented or reduced the severity of arthritis (9). The mechanism of this effect is not clear. C3H-scid mice have no adaptive immune responses, and therefore, they have a higher spirochetal burden in affected joints than immunocompetent C3H mice (13). Moreover, in infected C3H-scid mice, transcription of Arp and several other lipoprotein genes is higher than that in C3H mice (14). For example, in these mice, B. burgdorferi persistently expressed OspC, but the administration of anti-OspC antibody selected for spirochetes that did not express this protein (15). By analogy, the lack of Arp antibody in these immunodeficient mice may lead to sustained high expression of Arp. This may be an important factor in explaining why passively transferred Arp antibody modulated joint inflammation in this model system.
In contrast with the findings in passively immunized C3H-scid mice, the IgG antibody response to Arp in human patients did not correlate with the severity or duration of arthritis. How does one explain these differences? In an effort to reduce methodologic differences, we attempted to translate the conditions of the experimental Lyme arthritis model as closely as possible. Most important, we employed serial serum samples from past, untreated patients followed longitudinally, removing the issue of antibiotic-induced resolution of arthritis. Second, the same recombinant fusion protein, GST-Arp, was used in both the murine and human studies. Although mice and humans may respond to different epitopes, the same epitopes of this highly conserved protein were available for antibody binding.
Despite these efforts to translate the mouse experiments closely, critical differences remained in the immune competence of the host. Humans develop adaptive immune responses that are thought to kill spirochetes, and reduction of the spirochetal burden may reduce joint inflammation. In addition, the spirochete itself may have different expression among patients in response to variable host immune pressure (14). For this reason, it is possible that Arp antibody dampened joint inflammation in some patients but not in others, thereby masking the significance of this single response. However, using the same set of serum samples that were employed here, we previously showed an association between several other antibody responses to B. burgdorferi and the severity and duration of arthritis (1). Thus, the likely explanation for the differences between mice and humans in protective Arp antibody responses is that the more uniform and artificial conditions in inbred, immunodeficient mice do not duplicate the more variable pathogenetic process in outbred, immunocompetent human patients with Lyme arthritis.
In summary, most of the study patients with Lyme disease had IgG antibody responses to Arp. Reactivity with this protein usually developed relatively early in the infection and sometimes persisted throughout the illness. The lack of association between this response and the severity or duration of arthritis suggests that it is not a singular or dominant arthritis-resolving event in human patients with Lyme arthritis.
Acknowledgments
We thank Stephen Barthold for the construct used to express the recombinant GST-Arp fusion protein, Nitin Damle and Vijay Sikand for collecting serum samples from patients with EM and healthy control subjects, Gail McHugh for laboratory aid, and Colleen Squires for help with preparation of the manuscript.
This study was supported in part by cooperative agreement CCU110291 from the Centers for Disease Control and Prevention; NIH grant AR-20358; the English, Bonter, Mitchell Foundation; the Lyme/Arthritis Research Fund; and the Eshe Fund. E. Drouin received support from the Lincoln Financial Group Foundation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2000. Expression and purification of glutathione-S-transferase fusion proteins, p. 16.71-16.75. In Current protocols in molecular biology. Wiley, New York, N.Y. [DOI] [PubMed]
- 3.Barthold, S. W., M. S. DeSouza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25:S9-S17. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1990. Case definitions for public health surveillance. Morb. Mortal. Wkly. Rep. 39:1-43. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second international conference on serologic diagnosis of Lyme disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 7.Chen, J., J. A. Field, L. Glickstein, P. J. Molloy, B. T. Huber, and A. C. Steere. 1999. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer-surface protein A (OspA) of Borrelia burgdorferi. Arthritis Rheum. 42:1813-1822. [DOI] [PubMed] [Google Scholar]
- 8.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 9.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 11.Fikrig, E., H. Tao, M. Chen, S. W. Barthold, and R. A. Flavell. 1995. Lyme borreliosis in transgenic mice tolerant to Borrelia burgdorferi OspA or B. J. Clin. Investig. 96:1706-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 13.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodzic, E., S. Feng, K. J. Freet, and S. W. Barthold. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 71:5042-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pachner, A. R., and A. C. Steere. 1985. The triad of neurologic manifestations of Lyme disease: meningitis, cranial neuritis, and radiculoneuritis. Neurology 35:47-53. [DOI] [PubMed] [Google Scholar]
- 17.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 18.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steere, A. C., G. McHugh, C. Suarez, J. Hoitt, N. Damle, and V. J. Sikand. 2003. Prospective study of coinfection in patients with erythema migrans. Clin. Infect. Dis. 36:1078-1081. [DOI] [PubMed] [Google Scholar]
- 20.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 21.Vaz, A., L. Glickstein, J. A. Field, G. McHugh, V. K. Sikand, N. Damle, and A. C. Steere. 2001. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect. Immun. 69:7437-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, and B. J. Luft. 2000. Practice guidelines for the treatment of Lyme disease. Clin. Infect. Dis. 31:S1-S14. [DOI] [PubMed] [Google Scholar]