Abstract
Bacterial hydroxy fatty acids and alpha-hydroxy fatty acids have been demonstrated in complex lipid extracts of subgingival plaque and gingival tissue. However, little is known about the relationship between these hydroxy fatty acids in plaque and gingival tissues or the significance of these complex lipids in promoting inflammatory periodontal disease. The present study determined the percentages of ester-linked and amide-linked hydroxy fatty acids in complex lipids recovered from plaque and gingival tissue samples and the relationship between bacterial hydroxy fatty acids and alpha-hydroxy fatty acids in the lipid extracts. To evaluate a potential role for these hydroxy fatty acids in inflammatory periodontal disease, gingival tissue samples were examined for a relationship between prostaglandin E2 (PGE2) and hydroxy fatty acids recovered in gingival lipid. This investigation demonstrated that alpha-hydroxy fatty acids are only ester linked in plaque lipids but are largely amide linked in gingival tissue lipids. Furthermore, the level of alpha-hydroxy fatty acid in gingival lipid is directly related to the level of the bacterial hydroxy fatty acid 3-OH iso-branched C17:0 (3-OH iC17:0) in the same lipid extract. However, the relationship between hydroxy fatty acids in gingival lipids does not parallel the fatty acid relationship observed in plaque lipids. Finally, alpha-hydroxy fatty acid levels in gingival tissue lipids correlate directly with the recovery of PGE2 in the same tissue samples. These results demonstrate that alpha-hydroxy fatty acid levels in gingival lipids are directly related to both 3-OH iC17:0 bacterial lipid levels and PGE2 levels. These results indicate that in periodontal tissues there are unusual host-parasite interactions involving penetration of bacterial lipid in association with an altered gingival lipid metabolism and prostaglandin synthesis.
A prominent hydroxy fatty acid recovered in gingival tissues is 2-OH C16:0, an alpha-hydroxy fatty acid which is held virtually exclusively within complex lipids (24). Another alpha-hydroxy fatty acid, 2-OH C14:0, is recovered in smaller amounts within lipid extracts of gingival tissue than is 2-OH C16:0 (24). Among the beta-hydroxy fatty acids, 3-OH C14:0 and 3-OH iso-branched C17:0 (3-OH iC17:0) are recovered in the gingival lipids, but a significant percentage of 3-OH C14:0 is recovered in aqueous soluble components as well (23). Of these fatty acids, 3-OH iC17:0 is synthesized by members of only four genera of oral microorganisms: Bacteroides, Capnocytophaga, Prevotella, and Porphyromonas (12, 13, 22, 25, 34); the remaining hydroxy fatty acids may be synthesized to a variable extent either by microorganisms or by the host. Therefore, recovery of 3-OH iC17:0 in gingival lipids is presumed to represent penetration of specific bacterial lipids into the tissue. It is unclear whether other hydroxy fatty acids in gingival complex lipids are synthesized by the host or represent plaque lipids which penetrate into gingival tissues. To explore this question, the present investigation examined the linkage of hydroxy fatty acids to complex lipids recovered in plaque and gingival tissue and the relationship between hydroxy fatty acids recovered in lipid extracts from subgingival plaque and gingival tissue samples.
The levels of prostaglandins, particularly prostaglandin E2 (PGE2), are elevated in diseased gingival tissues. Prostaglandins are considered to be important proinflammatory and tissue-destructive mediators. Although many bacterial and host-derived factors are known to stimulate prostaglandin synthesis and secretion from host cells in culture, little evidence has demonstrated a direct correlation between bacteria or bacterial factors and prostaglandin levels in tissue samples. Because hydroxy fatty acids are prevalent in complex lipid extracts from diseased gingival tissue, the second goal of this investigation was to determine whether there exists a direct relationship between levels of hydroxy fatty acids recovered in gingival lipids and prostaglandins in the same tissue samples. Complicating this evaluation is the capacity of lipopolysaccharide to stimulate prostaglandin synthesis in a variety of cell types and the fact that lipopolysaccharide contains either 3-OH C14:0 or 3-OH iC17:0. Therefore, lipopolysaccharide must be excluded from gingival lipids before attempting to correlate prostaglandin levels with complex lipids containing hydroxy fatty acids. According to a recent report, a common phospholipid extraction procedure partitions lipopolysaccharide into the aqueous phase, whereas complex lipids reside in the organic-solvent phase (24). Therefore, the present investigation compared the recovery of prostaglandins with that of hydroxy fatty acids in either aqueous or organic-solvent extracts of gingival tissue samples.
MATERIALS AND METHODS
Collection of plaque samples.
Subgingival plaque samples were obtained from sites of either gingivitis or adult periodontitis. The site characteristics are listed below. Each periodontal site was dried, the supragingival plaque was removed with gauze, and then coarse endodontic paper points (3) were placed into the sulcus. The points were removed after approximately 10 s and placed into individual glass vials. The vials were stored at −20°C. The paper points for each site were processed by the Bligh and Dyer procedure (2) described below, and the organic-solvent extracts were dried under nitrogen.
Collection of gingival tissue samples.
Gingival tissue samples were obtained from patients who had not provided plaque samples. Gingival tissue samples were taken from patients in the Periodontics and Oral and Maxillofacial Surgery clinics at the University of Connecticut School of Dental Medicine. Tissue samples were collected for three independent studies. The first study examined the levels of ester- and amide-linked hydroxy fatty acids in lipid extracts from subgingival plaque and gingival tissue samples. The second study examined the relationships between hydroxy fatty acids in complex lipids extracted from gingival tissue samples and compared this distribution with the hydroxy fatty acid distribution observed in subgingival plaque lipids. The third study evaluated the relationships between prostaglandins and hydroxy fatty acids recovered from the same gingival tissue samples. Gingival tissue samples which would normally be discarded at the time of periodontal surgery were retained if they met the following selection criteria.
The gingival sites demonstrated a spectrum of clinical findings ranging from gingival health to severe periodontal breakdown. The most severe disease manifested at a surgical site determined the disease category to which the site was assigned. For the prostaglandin study, the site data also included maximal pocket depth, gingival index score (17), and presence or absence of bleeding on probing. Healthy and gingivitis tissue samples were obtained during crown lengthening procedures. Gingivitis sites exhibited inflammatory disease but no attachment loss. Adult periodontitis sites demonstrated periodontal attachment loss of varying severity. Juvenile periodontitis sites were identified in subjects from 13 to 20 years of age with periodontal destruction limited to molars and/or incisors. All periodontitis sites were treated with scaling and root planing prior to the surgical excision of the gingival tissue (generally this occurred months before the surgical excision). Upon excision, each tissue sample was immediately frozen and stored at −20°C. Tissue samples were used for the prostaglandin study only if the patient refrained from using nonsteroidal anti-inflammatory drugs for at least 7 days prior to the periodontal surgery.
For the prostaglandin study, D4-PGF2α, D4-PGE2, D4-PGE1, D4-PGA2, D4-6-keto-PGF1α, and 4D4-thromboxane B2 (200 ng/sample of gingiva; Cayman Chemical Co., Ann Arbor, Mich.) were added to each glass homogenizer tube. The tissue samples (approximately 60 mg per sample) were weighed, thawed, and quickly cut into small pieces by using a no. 15 scalpel blade. Each minced tissue specimen was then placed into a homogenizer tube, and 1.33 ml of ice-cold methanol containing 10−5 M indomethacin was added. The samples were then homogenized (15-ml glass tube and pestle homogenizer; Belco Glass, Inc. Vineland, N.J.), with the tubes being kept ice cold at all times. After homogenization, 0.67 ml of CHCl3 and 0.5 ml of H2O were added to each tube. The contents were transferred to a glass tube, and 2 ml of methanol (MeOH)-CHCl3 (2:1, vol/vol) and 0.5 ml of H2O were added to each homogenization tube; after being vortexed, the sample was combined with the original extract. After a 2-h room temperature incubation, 1.5 ml of CHCl3 and 1.5 ml of 2 N KCl–0.5 N K2PO4 were added to each tube and the contents were vortexed. The test tubes were centrifuged (1,000 × g, 10 min) to achieve phase separation and minimize aspiration of the tissue homogenate pellet. The organic phase (lower phase) was aspirated, and a second aliquot of 1.5 ml of CHCl3 was added to the homogenate; this was followed by vortexing and centrifugation as before. The second organic-solvent extract was aspirated, the organic extracts were combined, and the combined extracts were evaporated under N2.
The tissue homogenate and associated aqueous phase were supplemented with concentrated formic acid to achieve a pH of 3.5 while maintaining the tubes on ice. The acidic aqueous phase was rapidly extracted twice with 2 ml of CHCl3, and the combined organic extracts, containing the prostaglandins, were evaporated under N2. Preliminary experiments demonstrated that this rapid acidic extraction does not promote hydrolysis of Salmonella typhimurium lipopolysaccharide (data not shown). The prostaglandin samples (acidic organic extracts) were derivatized directly for gas chromatography-mass spectrometry (GC-MS) analysis.
For studies examining hydroxy fatty acids in gingival lipids, tissue samples were homogenized as described above and lipids were extracted by the procedure of Bligh and Dyer (2). Each tissue sample was weighed, thawed, minced, and placed in a homogenizer tube containing 1.33 ml of MeOH. The samples were homogenized (15-ml glass tube and pestle homogenizer; Belco Glass, Inc.). After homogenization, 0.67 ml of CHCl3 and 0.5 ml of H2O were added to each tube. The contents were transferred to glass tubes, and 2 ml of MeOH-CHCl3 (2:1, vol/vol) and 0.5 ml of H2O were added to each homogenization tube; after being vortexed, the contents were combined with the original extract. After a 2-h incubation at room temperature, 1.5 ml of CHCl3 and 1.5 ml of 2 N KCl–0.5 N K2HPO4 were added to each tube and the contents were vortexed. The test tubes were centrifuged (1,000 × g, 10 min) to achieve phase separation and minimize aspiration of the tissue homogenate pellet.
Hydrolysis of aqueous and organic-solvent extracts and preparation of derivatives.
For the evaluation of ester-linked and total hydroxy fatty acid contents in gingival tissue and subgingival plaque lipids, the organic-solvent extract of each sample was divided in half, each half was supplemented with 120 ng of nonadecanoic acid (CH3-C19:0; Matreya, Inc., Pleasant Gap, Pa.) and the samples were evaporated under N2. For determination of ester-linked hydroxy fatty acid, one lipid sample from each tissue specimen was supplemented with 0.03 ml of tetrahydrofuran plus 0.1 ml of 0.25 N NaOCH3 and incubated for 15 min at 50°C. Glacial acetic acid (0.02 ml) and water (0.5 ml) were added, and each sample was extracted four times with (1 ml per extraction) hexane (6). The hexane extracts were evaporated under N2. The samples were then hydrolyzed in 4 N KOH (0.5 ml; 90 min at 100°C). After the samples were cooled, concentrated HCl was added to each (pH to <1.0); this was followed by two hexane extractions (2 ml each). The hexane was evaporated under N2.
For determination of total hydroxy fatty acids, the remaining half of each tissue lipid extract was supplemented with 2 N HCl (0.5 ml) and heated overnight at 100°C (20). The sample was cooled, and 16 N KOH (0.37 ml) was added; this was followed by incubation at 100°C for 90 min. Concentrated HCl (0.40 ml) was added, and the sample was extracted twice with hexane (2 ml each). The hexane was evaporated under N2. The level of amide-linked hydroxy fatty acids was determined by subtracting the ester-linked hydroxy fatty acid content from the total hydroxy fatty acid recovered in each subgingival plaque and gingival tissue sample.
For the evaluation of the relationships between hydroxy fatty acids in subgingival plaque and gingival tissue samples, each lipid extract from gingiva or plaque was supplemented with 960 ng of nonadecanoic acid and hydrolyzed in 0.5 ml of 4 N KOH (90 min, 100°C). After the samples were cooled, concentrated HCl (0.2 ml) was added to each, and the samples were extracted twice with hexane (2 ml each). The hexane extracts were dried under nitrogen.
For experiments examining the relationship between prostaglandin and hydroxy fatty acid contents in gingiva, the prostaglandins were first extracted from the homogenized tissue samples and the remaining aqueous phase was supplemented with 960 ng of nonadecanoic acid. The aqueous phase was adjusted to a final concentration of 4 N KOH by addition of 16 N KOH. The samples were then hydrolyzed (90 min, 100°C). After the samples were cooled, concentrated HCl was added to each tube to adjust the pH to <1.0; this was followed by two hexane extractions (2 ml each). Each hexane extract containing aqueous phase-associated fatty acids was then evaporated under N2.
All derivatizing agents were obtained from Pierce Chemical Corp., Rockford, Ill. Prostaglandin samples were derivatized by the method of Waddell et al. (36). Prostaglandin samples were first treated with 2% methoxylamine hydrochloride in pyridine (50 μl). After being allowed to stand overnight, the samples were dried under N2, dissolved in acetonitrile (30 μl), and treated with pentafluorobenzyl bromide (10 μl, 35% [vol/vol] in acetonitrile) and diisopropylethylamine (10 μl). The samples were vortexed, incubated for 20 min at 40°C, and dried under N2. The residue was then treated with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA; 50 μl) and allowed to stand for 4 to 5 days. Prostaglandin derivatives in BSTFA were diluted 1:1 (vol/vol) in hexane and allowed to stand for 30 min. The prostaglandin samples were then centrifuged (1,000 × g, 10 min), and the supernatants were transferred to automatic sampling vials.
Hydroxy fatty acids were derivatized for GC-MS as follows. Samples were dissolved in acetonitrile (30 μl) and treated with pentafluorobenzyl bromide (10 μl, 35% [vol/vol] in acetonitrile) and diisopropylethylamine (10 μl) (24). The samples were then dried under N2, treated with BSTFA (50 μl), and allowed to stand overnight. Hydroxy fatty acid derivatives in BSTFA were transferred to automatic sampling vials without being diluted in hexane.
GC-MS was carried out on a model 5890 gas chromatograph interfaced with a model 9988A mass spectrometer (both from Hewlett-Packard). Samples were applied to an HP-1 (Ultra-1; 12 m in length by 0.2 mm [internal diameter]; Hewlett-Packard, Avondale, Pa.) column held at 100°C. Hydroxy fatty acid samples and prostaglandins were analyzed separately. Hydroxy fatty acid samples were injected in the splitless mode (30 s), and the column was heated to 170°C at a rate of 10°C/min and then to 270°C at 5°C/min. Prostanoid samples were analyzed by using a temperature program of 2°C/min from 100 to 240°C. The injector block was held at 260°C. The mass spectrometer was used in the negative-ion chemical ionization mode with the source temperature at 100°C, the electron energy at 240 eV, and the emission current of 300 mA. Methane, used as the analyte gas, was maintained at 0.5 torr in the ion source. Product levels were quantified by selected ion monitoring of the characteristic base peak ions for prostanoids, hydroxy fatty acids, and internal standards. The recovered prostanoid and hydroxy fatty acid levels were then normalized based on the tissue sample mass.
Data analysis involved two steps: (i) exploratory data analysis and (ii) linear regression analysis. Exploratory data analysis included the calculation of sample distribution characteristics and outcome measures (means and standard errors of the means). Multiple linear regression analysis was performed to estimate the relationship (correlation coefficient and coefficient of determination) between hydroxy fatty acid levels or between prostaglandins and hydroxy fatty acids in gingival tissue samples.
RESULTS
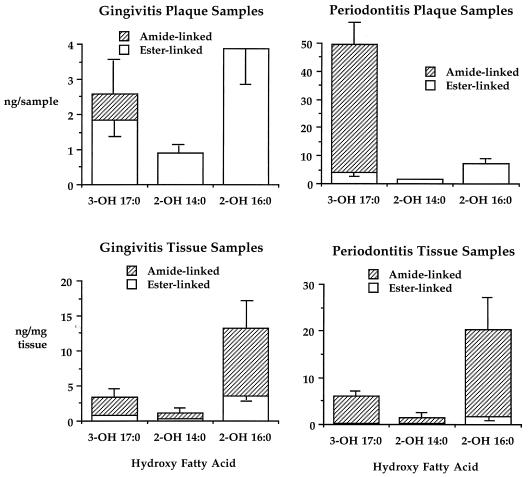
Figure 1 shows the recovery of ester-linked and total hydroxy fatty acids in lipid extracts from gingival tissue and subgingival plaque samples. The difference between ester-linked and total hydroxy fatty acid recoveries represents the amide-linked hydroxy fatty acid content (20). Results for 14 gingivitis plaque samples and 40 adult periodontitis plaque samples and for 5 gingivitis tissue samples and 27 adult periodontitis tissue samples are shown. The alpha-hydroxy fatty acids (2-OH C16:0 and 2-OH C14:0) in lipid extracts of plaque samples are virtually completely ester linked, but in lipid extracts of gingival tissue samples, particularly periodontitis tissue samples, they are mostly amide linked. Analysis of lipid extracts of plaque and gingival tissue samples from periodontitis sites revealed that most 3-OH iC17:0 is amide linked in complex lipids.
FIG. 1.
Ester-linked and amide-linked hydroxy fatty acids in lipid extracts of subgingival plaque and gingival tissue samples. The characteristics and number of sites sampled are listed in Results. Lipids were extracted as described in Materials and Methods. The histogram bars depict the recovery of ester-linked and total hydroxy fatty acids in lipid extracts of subgingival plaque or gingival tissue samples. Subtracting the ester-linked fatty acid content from the total fatty acid content determines the recovery of amide-linked fatty acid. Error bars represent the standard errors of the means.
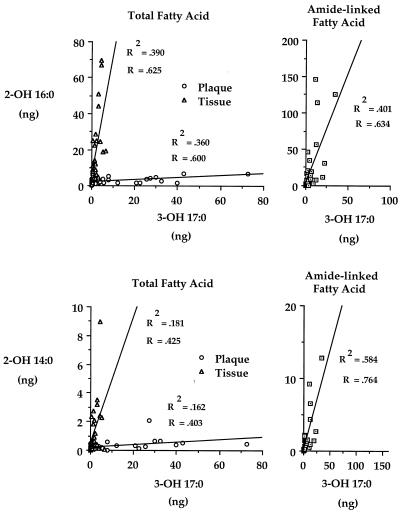
The left panels of Fig. 2 show that the relationships between total 3-OH iC17:0 and total 2-OH C16:0 or 2-OH C14:0 are markedly different for plaque and gingival tissue lipid extracts. Results for 16 healthy or gingivitis tissue samples, 16 adult periodontitis tissue samples, 14 gingivitis plaque samples, and 15 adult periodontitis plaque samples are shown. When the linear regression slopes for tissue and plaque lipid extracts are compared, the slopes do not overlap at confidence interval levels of 99.95% for 3-OH iC17:0 versus 2-OH C16:0 and of 95% for 3-OH iC17:0 versus 2-OH C14:0. This analysis demonstrates that the regression slopes comparing 3-OH iC17:0 with 2-OH C16:0 in plaque and gingival tissue lipids (0.054 and 6.82, respectively) differ by more than 2 orders of magnitude. The regression slopes comparing 3-OH iC17:0 with 2-OH C14:0 differ by almost 2 orders of magnitude (0.009 for plaque and 0.442 for gingival tissue).
FIG. 2.
Linear regression analysis comparing total and amide-linked hydroxy fatty acids in subgingival plaque and gingival tissue lipids. The characteristics and number of sites sampled are listed in Results. Samples were processed for GC-MS analysis as described in Materials and Methods. The hydroxy fatty acid levels for plaque samples were divided by 10 to facilitate data presentation. Regression analysis of amide-linked hydroxy fatty acids in plaque lipids was not performed because negligible levels of alpha-hydroxy fatty acid are amide linked in plaque lipids. The correlation coefficients and coefficients of determination are listed for each linear regression.
Additional regression analyses were performed to evaluate the relationship between amide-linked hydroxy fatty acids (shown in the right panels of Fig. 2). The linear regressions of amide-linked hydroxy fatty acids for lipid extracts from 39 tissue samples are depicted; 12 samples were healthy or gingivitis samples, and the remainder were adult periodontitis samples. These regressions demonstrated slope characteristics and regression coefficients virtually identical to those shown for total hydroxy fatty acids detected in gingival tissue lipid (shown in the left panels of Fig. 2).
For the investigation of prostaglandin levels in gingival tissue samples, the distribution of gingival tissue samples by disease category was as follows: healthy, n = 1; mild gingivitis, n = 2; moderate gingivitis, n = 2; mild periodontitis, n = 4; moderate periodontitis, n = 10; severe periodontitis, n = 9; and juvenile periodontitis, n = 3. For the results presented in Fig. 3 and 4, tissue samples were grouped into the following categories: healthy/gingivitis samples, adult periodontitis samples, and juvenile periodontitis samples.
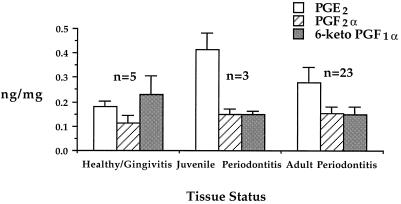
FIG. 3.
Recovery of PGE2, PGF2α, and 6-keto-PGF1α recovery from gingival tissue excised from healthy sites as well as sites of gingivitis, adult periodontitis, and juvenile periodontitis. Gingival tissue samples were assigned to disease categories as described in Results. Samples were processed for GC-MS analysis as described in Materials and Methods. Histogram bars show the recovery of prostaglandin in nanograms per milligram of gingival tissue. Each histogram bar represents the mean recovery of prostaglandin (n = the number of samples), with the error bar indicating the standard error of the mean.
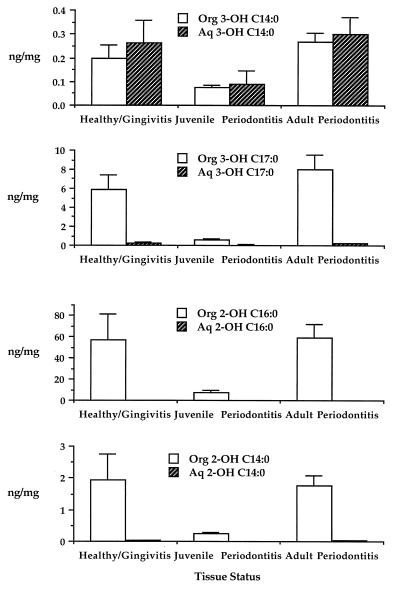
FIG. 4.
The distribution of 3-OH C14:0, 3-OH iC17:0, 2-OH C16:0, and 2-OH C14:0 in organic-solvent and aqueous extracts of the same gingival tissue samples shown in Fig. 2. Samples were extracted and processed for GC-MS analysis as described in Materials and Methods. Histogram bars show the recovery of hydroxy fatty acid (in nanograms per milligram of tissue) in organic-solvent extracts (Org) (open bars) and aqueous extracts (Aq) (cross-hatched bars). Each histogram bar represents the mean recovery of hydroxy fatty acid in the respective extract, with the error bar indicating the standard error of the mean.
The deuterated PGE2 internal standard was shown to contain a small amount of PGE2 (PGE2 represented 1.05% of the deuterated internal standard recovery). This level of contamination of the deuterated internal standard is consistent with the reported deuterium substitution efficiency of the 4D-PGE2 internal standard (≥98%; Cayman Chemical Co.) and represents a contribution of approximately 2 ng of PGE2 to each gingival tissue sample. Recovery of PGE2 from gingival tissue samples ranged from 0 to 72 ng and in most cases far exceeded the background levels of PGE2 contained in the deuterated internal standard. Nevertheless, the background contribution of PGE2 from the internal standard was subtracted before calculation of the recovery of PGE2 for each tissue sample. The deuteration substitution efficiency for other prostanoid internal standards was comparable to that reported for PGE2 (≥98%), and background contamination was determined and corrected for with other prostaglandin determinations. Only the most frequently recovered prostaglandins are depicted in Fig. 3.
Figure 3 shows prostaglandin recovery from healthy or gingivitis tissue samples compared with that from juvenile periodontitis and adult periodontitis samples. PGE2 levels of healthy/gingivitis samples and adult periodontitis samples were not significantly different. However, there was a significant difference between the PGE2 levels of juvenile periodontitis samples and healthy/gingivitis samples (P < 0.044; t test). By contrast, the mean levels of PGF2α and 6-keto-PGF1α were not significantly different between tissue samples classified by disease category.
Hydroxy fatty acid contents recovered in aqueous and organic-solvent extracts of gingival tissue samples were averaged by tissue category as shown in Fig. 4. The recovery of hydroxy fatty acids was higher in organic-solvent extracts than in aqueous extracts, and it was approximately equal for healthy/gingivitis tissue samples and adult periodontitis samples but considerably lower in juvenile periodontitis tissue samples. These differences were not statistically significant.
Because juvenile periodontitis tissue samples contained high PGE2 levels and low hydroxy fatty acid levels, these samples were excluded from the prostaglandin regression analyses. Table 1 shows the results of a linear regression analysis of PGE2 and hydroxy fatty acids recovered from aqueous and organic extracts of the same gingival tissue samples. The linear regression slopes with high significance levels (P < 0.01) include PGE2 versus 3-OH iC17:0 recovered in aqueous solvent extracts, PGE2 versus 2-OH C16:0 recovered in aqueous extracts or organic-solvent extracts, and PGE2 versus 2-OH C14:0 recovered in organic-solvent extracts. Importantly, the linear regression comparing PGE2 with 3-OH C14:0 recovered in aqueous-solvent extracts demonstrated no significant relationship.
TABLE 1.
Linear regression analysis of PGE2 versus hydroxy fatty acids in gingival tissue extractsa
| Tissue extract | Linear regression formula | r | r2 | P |
|---|---|---|---|---|
| Aq 3-OH C14:0 | y = −0.010x + 0.305 | 0.009 | 0.0001 | |
| Org 3-OH C14:0 | y = 0.257x + 0.184 | 0.391 | 0.120 | 0.0397 |
| Aq 3-OH iC17:0 | y = 0.467x + 0.103 | 0.533 | 0.285 | 0.0035 |
| Org 3-OH iC17:0 | y = 6.932x + 5.644 | 0.272 | 0.074 | |
| Aq 2-OH C16:0 | y = 0.785x + 0.063 | 0.621 | 0.386 | 0.0004 |
| Org 2-OH C16:0 | y = 105.316x + 31.653 | 0.486 | 0.237 | 0.0087 |
| Aq 2-OH C14:0 | y = 0.047x + 0.020 | 0.376 | 0.142 | 0.0483 |
| Org 2-OH C14:0 | y = 3.660x + 0.841 | 0.667 | 0.445 | 0.0001 |
Tissue samples were processed as described in Materials and Methods. Prostaglandin levels were compared with hydroxy fatty acid recovered in either the aqueous extracts (Aq) or organic solvent extracts (Org) of the same gingival tissue samples. The correlation coefficients (r), coefficients of determination (r2), and the slope significance levels (P) are listed for each comparison.
DISCUSSION
As shown in Fig. 1, the distributions of hydroxy fatty acids in subgingival plaque lipids differ between gingivitis and periodontitis sites. Lipids of subgingival plaque from gingivitis sites contain mostly ester-linked 3-OH iC17:0, whereas lipids of subgingival plaque from periodontitis sites contain mostly amide-linked 3-OH iC17:0. More importantly, the recovery of 2-OH C16:0 in plaque lipids from periodontitis sites is relatively low compared with that of 3-OH iC17:0, but 2-OH C16:0 is considerably more abundant than 3-OH iC17:0 in gingival tissue lipid, regardless of the disease status of the tissue. This evidence demonstrates that first-order uptake of subgingival plaque lipids into gingiva does not account for the recovery of alpha-hydroxy fatty acids in gingival tissue lipids. Furthermore, if gingiva accumulates alpha-hydroxy fatty acids from plaque lipids, ester-linked 2-OH C16:0 must be released from plaque lipids and then metabolically incorporated primarily into gingival lipids which hold fatty acid in an amide linkage. Transfer of 2-OH C16:0 into gingival lipids also must occur without a comparable transfer of 2-OH C14:0 into gingival lipids. Finally, the selective transfer of 2-OH C16:0 into gingival complex lipids must occur preferentially, by a factor of approximately 25-fold over the penetration of plaque lipid containing 3-OH iC17:0, as seen at periodontitis sites. From a biological standpoint, such a process appears to be unlikely.
Because a relatively constant relationship exists between 3-OH iC17:0 and 2-OH C16:0 in gingivitis and periodontitis tissue lipid extracts (Fig. 1), bacterial lipid containing 3-OH iC17:0 may be influencing the recovery of gingival lipid containing 2-OH C16:0. A possible explanation is that gingival tissue synthesizes lipid containing amide-linked 2-OH C16:0 in proportion to the amount of lipid containing 3-OH iC17:0 in gingival tissue. To further examine this possibility, linear regressions of total 3-OH iC17:0 versus total 2-OH C16:0 or 2-OH C14:0 in gingival lipids were compared with those of subgingival plaque lipids (Fig. 2). Linear regression analysis revealed that the recovery of 2-OH C16:0 or 2-OH C14:0 in gingival lipids is directly related to the recovery of lipid containing 3-OH iC17:0. The lipid containing 3-OH iC17:0 is presumed to be of bacterial origin since mammalian tissues have not been reported to synthesize this fatty acid. Additional regression analyses evaluated the relationships between amide-linked hydroxy fatty acids in gingival lipids (Fig. 2) and showed slope characteristics virtually identical to those observed with total hydroxy fatty acid regressions. Minimal recoveries of 3-OH iC17:0, 2-OH C16:0, and 2-OH C14:0 in gingival tissue lipids from juvenile periodontitis sites (shown in Fig. 4) also support a direct relationship between penetration of bacterial lipid containing 3-OH iC17:0 and recovery of gingival lipid containing 2-OH C16:0 and 2-OH C14:0.
Sphingolipids comprise a group of complex lipids with a common lipid structure consisting of either a saturated, unsaturated, or hydroxylated fatty acid in an amide linkage to a long-chain base. Virtually all other saponifiable complex lipids hold fatty acids in an ester linkage. Mayberry reported that all 3-OH iC17:0 recovered in Bacteroides fragilis and Bacteroides asaccharolyticus lipids was amide linked (20); however, the specific class or classes of complex lipids holding 3-OH iC17:0 were not identified in this report. Preliminary work has also shown that 20 to 70% of the 3-OH iC17:0 in lipid extracts from isolates of Porphyromonas gingivalis, a suspected periodontal pathogen, is in an amide linkage (data not shown). In contrast to the data on 3-OH iC17:0, a previous report demonstrated that the highest levels of alpha-hydroxy fatty acids are recovered in sphingolipids of brain and neural tissues and that considerably smaller amounts of alpha-hydroxy fatty acids are recovered in most other tissue sources, including skin (14). That 3-OH iC17:0 and alpha-hydroxy fatty acids are held primarily in amide linkages in diseased gingival tissue suggests the presence of sphingolipid. Furthermore, the direct relationship between amide-linked 3-OH iC17:0 and either 2-OH C16:0 or 2-OH C14:0 in gingival lipid extracts also suggests that bacterial sphingolipid may be influencing the recovery of gingival sphingolipid containing 2-OH C16:0 and 2-OH C14:0. Experiments to determine the types of sphingolipids present in diseased gingival tissue and the biological relationships between these lipids are currently in progress.
Prior studies comparing prostaglandin recovery from healthy and diseased gingival tissues have generally found higher PGE2 levels in gingivitis or periodontitis sites than in healthy sites (7–9, 21, 28, 31). Dewhirst et al. reported a study of 33 gingival tissue samples, ranging from healthy to severe periodontitis, with PGE2 being detected primarily in deep granulation tissue (7) at a mean value of 122 pg/mg. Ohm et al. demonstrated PGE2 levels of 1.7 pg/mg in healthy gingival tissue samples, 5.7 pg/mg in gingivitis tissue samples, and 23.2 pg/mg in periodontitis tissue samples (28). Offenbacher et al. reported PGE2 levels of 32 and 179.5 pg/μl in crevicular fluid from gingivitis and periodontitis sites, respectively (26). In a later study, Offenbacher and coworkers reported PGE levels of 144 and 57.5 ng/ml in juvenile periodontitis and adult periodontitis crevicular fluid samples, respectively, and observed a direct correlation between the tissue levels and crevicular fluid levels of PGE (27). The reason for elevated levels of PGE in juvenile periodontitis gingival samples was not established. The previously cited studies quantified prostaglandin levels by radioimmunoassay techniques, and independent verification of the immunoassay determinations has not been reported.
In the present study, in which only superficial gingival tissue was evaluated, PGE2 was detected in 26 of 29 gingival tissue samples, with average concentrations of 180 pg/mg for healthy/gingivitis samples, 280 pg/mg for adult periodontitis tissue samples, and 413 pg/mg for juvenile periodontitis tissue samples. While the results presented here are generally consistent with the trends reported in earlier studies, GC-MS determinations yielded higher mean levels of PGE2 in gingival tissue samples than has been reported previously, suggesting significant losses of PGE2 during tissue extraction procedures by other investigators. In the present study, addition of deuterated prostaglandin standards to gingival tissue samples prior to sample processing allowed for correction of extraction losses and resulted in better recovery of prostaglandins, particularly from healthy or minimally diseased tissue samples. When corrected for tissue water content (approximately 70% of the wet weight being water), the mean level of PGE2 recovered in healthy/gingivitis tissue samples approached 1 μM, and in the other two disease categories this value was exceeded. According to Klein and Raisz (15), 0.01 and 1 μM concentrations of PGE2 will promote virtually identical levels of osteoclast-mediated bone resorption in vitro. Assuming that gingival tissue contains 70% water by weight, the level of PGE2 in the single healthy gingival tissue sample was 0.16 μM, well above the threshold for stimulation of osteoclast-mediated bone resorption. Most other tissue samples from adult periodontitis sites contained higher levels of PGE2.
Before examining the relationship between prostaglandins and hydroxy fatty acids in gingiva, the significance of bacterial hydroxy fatty acid recovery in gingival tissue will be reviewed. Although most hydroxy fatty acids in gingiva are recovered predominantly in complex lipids (Fig. 4), some hydroxy fatty acids may also be constituents of lipopolysaccharide. Previous investigations have shown that the oral genera Bacteroides, Prevotella, and Porphyromonas incorporate 3-OH iC17:0 into lipopolysaccharide (12, 13, 23, 25, 32), and lipopolysaccharide isolated from Bacteroides species can stimulate release of PGE2 from monocytes (10, 25). Lipopolysaccharide can facilitate attachment of organisms to epithelial surfaces (1, 16, 29) and can penetrate into the gingiva (30, 33). Under these circumstances, hydroxy fatty acid associated with lipopolysaccharide should be recovered with the gingival tissue sample. A previous report demonstrated that the phospholipid extraction procedure of Bligh and Dyer partitions lipopolysaccharide into the aqueous phase and complex lipids into the organic-solvent phase (24). This approach revealed that organisms containing 3-OH iC17:0, or their lipopolysaccharides, are not prevalent in diseased gingival tissue relative to the bacterial lipids containing 3-OH iC17:0 (24). The results shown in Fig. 4 confirm that 3-OH iC17:0 is recovered in negligible amounts in aqueous extracts of diseased gingival tissue, therefore indicating that very little 3-OH iC17:0-containing lipopolysaccharide is bound to or present in gingival tissue relative to lipids containing 3-OH iC17:0.
Of the remaining hydroxy fatty acids examined, 3-OH C14:0 is a major constituent of lipopolysaccharides of most other gram-negative periodontal organisms evaluated to date (19). However, recovery of 3-OH C14:0 in aqueous extracts of gingival tissue may also reflect the presence of substances derived from the host, because 3-OH C14:0 is synthesized in mammalian tissue during β-oxidation. The extent to which thiol coenzyme A esters of 3-OH C14:0 are soluble in aqueous solvent may affect the recovery of 3-OH C14:0 in aqueous extracts of gingival tissues. Uncertainty regarding the source of 3-OH C14:0 in aqueous extracts of gingival tissue will confound the interpretation of a relationship between 3-OH C14:0 and inflammatory mediators in gingival tissue, should a direct relationship exist. However, regression analysis of all healthy and diseased gingival tissue samples, except for juvenile periodontitis samples, did not reveal a correlation between PGE2 and aqueous soluble 3-OH C14:0 in these samples (Table 1). Therefore, these results do not indicate a relationship between 3-OH C14:0-containing lipopolysaccharide and PGE2 in gingival tissue.
In the case of juvenile periodontitis tissue samples, recovery of 3-OH C14:0 in aqueous extracts may reflect the presence of lipopolysaccharide from Actinobacillus actinomycetemcomitans (3). Lipopolysaccharide from this organism, like that of most enterobacteria and subgingival periodontal organisms, contains 3-OH C14:0 as the predominant hydroxy fatty acid (19). Although A. actinomycetemcomitans is usually the dominant organism at juvenile periodontitis sites (18, 35, 37) and may penetrate into gingival tissue (4, 5, 11), the results shown in Fig. 3 indicate that the recovery of 3-OH C14:0 in aqueous extracts of juvenile periodontitis tissue samples is relatively low compared with that from healthy/gingivitis or adult periodontitis gingival tissue samples. This evidence does not support the concept of substantial A. actinomycetemcomitans penetration into the gingiva at juvenile periodontitis sites. When all tissue samples are compared, the highest levels of PGE2 and the lowest levels of all hydroxy fatty acids are recovered in gingival tissue samples from juvenile periodontitis sites. Because of these unusual characteristics, the juvenile periodontitis tissue samples were not included in the regression analyses of healthy/gingivitis and adult periodontitis tissue samples.
The relationship between PGE2 and hydroxy fatty acids recovered in gingival tissue extracts was evaluated by linear regression analysis. The regression analysis data shown in Table 1 demonstrate that PGE2 directly correlates with 3-OH iC17:0 recovered in aqueous extracts of gingival tissue samples (r2 = 0.533). Although a recent report indicated that lipopolysaccharide containing 3-OH iC17:0 is recovered from gingival tissue at low levels relative to lipids containing 3-OH iC17:0 (24), linear regression analysis demonstrated a relationship between 3-OH iC17:0 in aqueous extracts and PGE2 in gingival tissue, suggesting a lipopolysaccharide effect. However, the majority of 3-OH iC17:0 in gingival tissue is recovered in complex lipids (3-OH iC17:0 is at least 50 times more plentiful in organic-solvent extracts than in aqueous extracts of gingival tissue). The identities of these complex lipids containing 3-OH iC17:0 remain to be established. Regression analysis revealed a very weak association between the recovery of PGE2 and lipid-associated 3-OH iC17:0 in all gingival tissue samples (Table 1), again excluding juvenile periodontitis samples. This evidence shows that bacterial lipids containing 3-OH iC17:0 have little relationship to the synthesis of PGE2 in gingival tissue.
It is of interest that 2-OH C16:0 is synthesized to a negligible extent in Porphyromonas gingivalis or Prevotella intermedia and is recovered at low levels in subgingival plaque lipids relative to 3-hydroxy fatty acids (23). However, 2-OH C16:0 is considerably more abundant than 3-hydroxy fatty acids in gingival lipid extracts of adults. Regression analysis revealed that PGE2 levels directly correlate with the recovery of 2-OH C16:0 in gingival complex lipids and aqueous soluble products (Table 1). Since the Bligh and Dyer procedure will not quantitatively extract all lipid, the trace amount of 2-OH C16:0 recovered in aqueous extracts (<1%) probably represents unextracted lipid containing 2-OH C16:0. Therefore, the correlation between PGE2 and aqueous-solvent-extracted 2-OH C16:0 probably reflects traces of 2-OH C16:0-containing lipid remaining in the aqueous phase. Recovery of 2-OH C16:0 in aqueous and organic-solvent extracts correlates with the recovery of PGE2 to approximately the same extent as the relationship between PGE2 and 3-OH iC17:0 recovered in aqueous extracts of gingival tissue samples. More importantly, linear regression analysis demonstrates a stronger correlation between PGE2 and 2-OH C14:0 in gingival tissue lipids than that observed between PGE2 and aqueous extractable 3-OH iC17:0.
Additional regression analyses failed to identify significant correlations between PGF2α or 6-keto-PGF1α and the above-mentioned hydroxy fatty acids in aqueous and organic-solvent extracts of gingival tissues (data not shown). Furthermore, regression analysis did not reveal significant relationships between PGE2 and PGF2α or 6-keto-PGF1α in gingival tissue samples (data not shown). Therefore, the direct correlations observed between PGE2 and hydroxy fatty acids in gingival tissue samples are specific for these products.
In summary, complex lipids extracted from gingival tissue samples of adults contain predominantly amide-linked 2-OH C16:0, suggesting that the majority of this fatty acid is held in the form of sphingolipid. In contrast to gingival lipid, subgingival plaque lipids hold 2-OH C16:0 exclusively in an ester linkage. Either 2-OH C16:0 is derived from plaque and is transferred into gingival lipids predominantly of an amide type, or gingival lipids containing 2-OH C16:0 are primarily derived from the host. More importantly, the recovery of alpha-hydroxy fatty acid in gingival tissue lipid of adults, measured as either total or amide-linked hydroxy fatty acid, is directly related to the level of gingival lipid containing 3-OH iC17:0, a fatty acid of bacterial origin. This evidence suggests that gingival complex lipid containing 3-OH iC17:0, presumably of bacterial origin, may be influencing the synthesis of sphingolipid containing alpha-hydroxy fatty acid. Finally, a direct relationship exists between PGE2 and gingival lipids containing alpha-hydroxy fatty acids in adults. These observations indicate new and potentially important relationships between disparate bacterium- and host-derived biochemical markers in gingival tissues of adults. Future work will examine the cellular mechanisms responsible for these observed relationships.
ACKNOWLEDGMENTS
We are grateful to the residents, staff, and faculty members of the Departments of Oral and Maxillofacial Surgery and Periodontology of the University of Connecticut School of Dental Medicine for their assistance in obtaining plaque and gingival tissue samples.
This work was supported by grants from the American Society of Orthodontics and the Northeast Society of Orthodontics.
REFERENCES
- 1.Bélanger M, Dubreuil D, Harel J, Girard C, Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990;58:3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Brondz I, Olsen I. Chemical differences in lipopolysaccharides from Actinobacillus (Haemophilus) actinomycetemcomitans and Haemophilus aphrophilus: clues to differences in periodontopathogenic potential and taxonomic distinction. Infect Immun. 1989;57:3106–3109. doi: 10.1128/iai.57.10.3106-3109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carranza F A, Saglia R, Newman M G, Valentin P L. Scanning and transmission electron microscopy of tissue invading microorganisms in localized juvenile periodontitis. J Periodontol. 1983;54:598–623. doi: 10.1902/jop.1983.54.10.598. [DOI] [PubMed] [Google Scholar]
- 5.Christersson L A, Wikesjö U M E, Albini B, Zambon J J, Genco R E. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluoresence and culture techniques. J Periodontol. 1987;58:540–545. doi: 10.1902/jop.1987.58.8.540. [DOI] [PubMed] [Google Scholar]
- 6.Christie W M. Lipid analysis: isolation, separation, identification and structural analysis of lipids. Oxford, United Kingdom: Pergamon Press; 1982. pp. 53–54. [Google Scholar]
- 7.Dewhirst F E, Moss D E, Offenbacher S, Goodson J M. Levels of prostaglandin E2, thromboxane, and prostacyclin in periodontal tissues. J Periodontal Res. 1983;18:156–163. doi: 10.1111/j.1600-0765.1983.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 8.ElAttar T M A, Lin H S. Relative conversion of arachidonic acid through lipoxygenase and cyclooxygenase pathways by homogenates of diseased periodontal tissues. J Oral Pathol. 1983;12:7–10. doi: 10.1111/j.1600-0714.1983.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 9.ElAttar T M A, Lin H S, Killoy W J, Vanderhoek J Y, Goodson J M. Hydroxy fatty acids and prostaglandin formation in diseased human periodontal tissue. J Periodontal Res. 1986;21:169–176. doi: 10.1111/j.1600-0765.1986.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrison S W, Holt S C, Nichols F C. Lipopolysaccharide stimulated PGE2 release from human monocytes: comparison of lipopolysaccharides prepared from suspected periodontal pathogens. J Periodontol. 1988;59:684–687. doi: 10.1902/jop.1988.59.10.684. [DOI] [PubMed] [Google Scholar]
- 11.Gillett R, Johnson N W. Bacterial invasion of the periodontium in a case of localized juvenile periodontitis. J Clin Periodontol. 1982;9:93–100. doi: 10.1111/j.1600-051x.1982.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Johne B, Bryn K. Chemical composition and biological properties of a lipopolysaccharide from Bacteroides intermedius. Acta Pathol Microbiol Immunol Scand Sect B. 1986;94:265–271. doi: 10.1111/j.1699-0463.1986.tb03051.x. [DOI] [PubMed] [Google Scholar]
- 13.Johne B, Olsen I, Bryn K. Fatty acids and sugars in lipopolysaccharides from Bacteroides intermedius, Bacteroides gingivalis and Bacteroides loescheii. Oral Microbiol Immunol. 1988;3:22–27. doi: 10.1111/j.1399-302x.1988.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto Y, Radin N S. Occurrance of 2-hydroxy fatty acids in animal tissues. J Lipid Res. 1963;4:139–143. [PubMed] [Google Scholar]
- 15.Klein D C, Raisz L G. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 16.Licht T R, Krogfelt K A, Cohen P S, Poulsen L K, Urbance J, Modlin S. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect Immun. 1996;64:3811–3817. doi: 10.1128/iai.64.9.3811-3817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 18.Mandell R, Socranski S S. A selective medium for Actinobacillus actinomycetemcomitans and incidence of the organism in juvenile periodontitis. J Periodontol. 1981;52:593–598. doi: 10.1902/jop.1981.52.10.593. [DOI] [PubMed] [Google Scholar]
- 19.Mashimo J, Yoshida M, Ikeuchi K, Hata S, Arata S, Kasai N, Okuda K, Takazoe I. Fatty acid composition and Shwartzmann activity of lipopolysaccharides from oral bacteria. Microbiol Immunol. 1985;29:395–403. doi: 10.1111/j.1348-0421.1985.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 20.Mayberry W R. Cellular distribution and linkage of d-(−)-3-hydroxy fatty acids in Bacteroides species. J Bacteriol. 1980;144:200–204. doi: 10.1128/jb.144.1.200-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendieta C F. Biosynthesis of prostaglandins in gingiva of patients with chronic periodontitis. J Periodontol. 1985;56:44–47. doi: 10.1902/jop.1985.56.1.44. [DOI] [PubMed] [Google Scholar]
- 22.Moore, W. E. C. Personal communication.
- 23.Nair B C, Mayberry W R, Dziak R, Chen P B, Levine M J, Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983;18:40–49. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 24.Nichols F C. Distribution of 3-hydroxy iC17:0 in subgingival plaque and gingival tissue samples: relationship to adult periodontitis. Infect Immun. 1994;62:3753–3760. doi: 10.1128/iai.62.9.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols F C, Peluso J F, Tempro P J, Garrison S W, Payne J B. Prostaglandin E release from human monocytes treated with lipopolysaccharides isolated from Bacteroides intermedius and Salmonella typhimurium: potentiation by gamma interferon. Infect Immun. 1991;59:398–406. doi: 10.1128/iai.59.1.398-406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offenbacher S, Farr D H, Goodson J M. Measurement of prostaglandin E in crevicular fluid. J Clin Periodontol. 1981;8:359–367. doi: 10.1111/j.1600-051x.1981.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 27.Offenbacher S, Odle B M, Gray R C, Van Dyke T E. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J Periodontal Res. 1984;19:1–13. doi: 10.1111/j.1600-0765.1984.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohm K, Albers H-K, Lisboa B P. Measurement of eight prostaglandins in human gingival and periodontal disease using high pressure liquid chromatography and radioimmunoassay. J Periodontal Res. 1984;19:501–511. doi: 10.1111/j.1600-0765.1984.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 29.Paradis S-E, Dubreuil D, Rioux S, Gottschalk M, Jacques M. High-molecular-mass lipopolysaccharides are involved in Actinobacillus pleuropneumoniae adherence to porcine respiratory tract cells. Infect Immun. 1994;62:3311–3319. doi: 10.1128/iai.62.8.3311-3319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranney R R, Montgomery E H. Vascular leakage resulting from topical application of endotoxin to the gingiva of the beagle dog. Arch Oral Biol. 1973;18:963–970. doi: 10.1016/0003-9969(73)90177-5. [DOI] [PubMed] [Google Scholar]
- 31.Rifkin B R, Tai H H. Elevated thromboxane B2 levels in periodontal disease. J Periodontal Res. 1981;16:194–198. doi: 10.1111/j.1600-0765.1981.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 32.Schifferle R E, Reddy M S, Zambon J J, Genco R J, Levine M J. Characterization of a polysaccharide antigen from Bacteroides gingivalis. J Immunol. 1990;143:3035–3042. [PubMed] [Google Scholar]
- 33.Schwartz J, Stinson F, Parker R. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972;43:270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- 34.Shah H N, Collins M D. Genus Bacteroides: a chemotaxonomical perspective. J Appl Bacteriol. 1983;55:403–416. doi: 10.1111/j.1365-2672.1983.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 35.Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982;15:606–609. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waddell K A, Blair I A, Wellby J. Combined capillary column gas chromatography-negative ion chemical ionization mass spectrometry of prostanoids. Biomed Mass Spectrom. 1983;10:83–88. doi: 10.1002/bms.1200110205. [DOI] [PubMed] [Google Scholar]
- 37.Zambon J J, Christersson L A, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease: prevalence in patient groups and distribution of the biotypes and serotypes within families. J Periodontol. 1983;54:707–711. doi: 10.1902/jop.1983.54.12.707. [DOI] [PubMed] [Google Scholar]