Opinion statement
Metformin is a first-line drug in the clinical treatment of type 2 diabetes. Its main molecular mechanism involves the activation of adenosine 5′-monophosphate-activated protein kinase (AMPK), which regulates cell energy metabolism. Many clinical studies have shown that metformin can reduce the incidence and mortality of cancer in patients with or without diabetes. In vitro studies also confirmed that metformin can inhibit proliferation, promote apoptosis, and enhance the response of cells to chemical drugs and other anticancer effects on a variety of leukemia cells. In recent years, leukemia has become one of the most common malignant diseases. Although great progress has been made in therapeutic approaches for leukemia, novel drugs and better treatments are still needed to improve the therapeutic efficacy of these treatments. This article reviews the application status and possible mechanism of metformin in the treatment of leukemia to further understand the anticancer mechanism of metformin and expand its clinical application.
Keywords: Leukemia, Metformin, AMPK
Introduction
Leukemia is a hematopoietic system disease caused by the abnormal proliferation, enhanced self-renewal ability, blocked differentiation, and reduced apoptosis of leukemic cells caused by hematopoietic cell mutations [1]. Leukemia accounts for 2.8% of all new cancer cases and 3.4% of new cancer deaths [2]. At present, leukemia is still treated mainly by combined chemotherapy, but these traditional treatments have serious side effects and can lead to drug resistance. Inter- and intra-tumor heterogeneity (ITH) of leukemia has been indicated as a major obstacle to effective treatment. Although a variety of targeted therapies and immunotherapies can improve the treatment efficacy for leukemia, the prognosis of patients with recurrent leukemia is still poor, revealing the urgent need for further leukemia study and novel treatment opportunities.
Metformin is a lipophilic biguanide compound that can inhibit liver glycosylation and improve peripheral glucose utilization, mainly through the regulation of mitochondrial function. Metformin is widely used to treat diabetes because of its excellent safety and efficacy. Recent studies have shown that metformin has potential antitumor activity, especially in regulating inflammation, metabolism, and cell cycle arrest, and can be used to enhance the antitumor effect of chemotherapy through these effects [3]. Compared with those in normal tissue cells, mitochondrial defects in cancer cells lead to the utilization of glucose mainly through anaerobic glycolysis rather than adenosine triphosphate (ATP) production by glucose through oxidative phosphorylation (OXPHOS) in mitochondria, as in normal cells [4]. Although ATP production in cancer cells is much lower than normal cells, substance utilization can be optimized due to metabolic reprogramming to adapt to this metabolic change [5, 6]. Leukemic cells, like cancer cells, need active mitochondria to survive [7•]. Therefore, it is possible to kill leukemic cells by inhibiting the electron transport chain or mitochondrial translation or by targeting mitochondrial respiratory function. Furthermore, metformin can also affect tumor immunity to achieve antitumor effects [8••]. A recent meta-analysis showed that the long-term use of metformin was associated with a reduction in the incidence of multiple solid tumors [9]. However, studies have shown that diabetic patients who continue to take metformin may have an increased risk of leukemia [10]. This potential cancer-promoting mechanism suggests that the use of metformin may be a double-edged sword for the occurrence of leukemia and is not limited by the known hypoglycemic and antitumor effects, which various from diseases to disease. Considering that the role of metformin in leukemia has not been elucidated, this article further explored the specific mechanism of metformin in the treatment of leukemia to provide a new treatment strategy for this disease.
Mechanisms by which metformin exerts anticancer effects
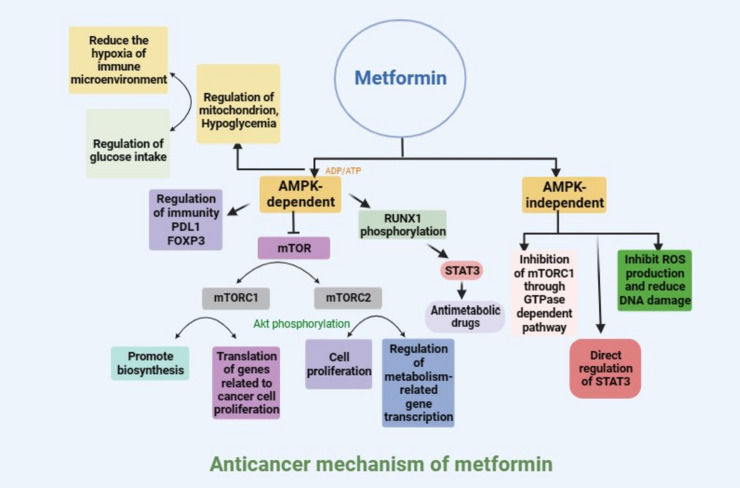
AMPK is a widely reported downstream effector of metformin [11]. Metformin inhibits oxidative respiration by acting on complex I of the mitochondrial respiratory chain, thus inhibiting the synthesis of ATP, increasing the adenosine diphosphate (ADP)/ATP ratio and adenosine monophosphate (AMP)/ATP ratio, and promoting the activation of AMPK [12]. In addition, studies have shown that metformin-induced glucose starvation can also lead to the activation of AMPK through the lysosomal v-ATPase-Ragulator complex [13]. AMPK is a key signal integration factor crucial for the control of mitochondrial health and metabolism and is also closely related to cell senescence and cell fate. Studies have shown that metformin-induced AMPK activation can further participate in the regulation of tumor cell growth and apoptosis through a variety of pathways [14, 15]. The activation of AMPK can inhibit the activity of mammalian target of rapamycin (mTOR). mTOR is the active center component of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which can co-stimulate cell growth [16, 17]. mTORC1 controls the synthesis of proteins, lipids, and nucleic acids, and mTORC2 can phosphorylate protein kinase B (AKT) to promote cell proliferation [18]. AMPK can also promote the phosphorylation of mTORC2 to block its nuclear translocation and binding to phosphate CRE-binding proteins, thus interfering with the transcription of key gluconeogenesis molecules, such as peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), glucose-6-phosphatase (G6P), and phosphoenolpyruvate carboxykinase (PEPCK) [19]. Metformin blocks the activation of mTORC1 and blocks the initiation of translation, especially the translation of c-myc, cyclin D1, and B-cell lymphoma-extra large (Bcl-xl), which are essential for cancer proliferation [20]. Evidence showed that the RUNX1/signal transducer and activator of transcription 3 (STAT3) also involved in the function of metformin via AMPK pathway [21]. Metformin-induced AMPK activation is closely involved in the regulation of tumor cell fate. For example, a study by Zheng et al. showed that metformin can lead to the overexpression of BAX and tumor cell death through the phosphorylation of AMPK/SIRT1 and the downstream nuclear factor-kappa B (NF-kB) p65 subunit [22]. Metformin can also promote ferroptosis in breast cancer cells by upregulating miR-324-3p [23].
AMPK is a key regulator of cell metabolism and a regulatory molecule of a series of important metabolic enzymes, such as acetyl-CoA carboxylase and HMG-CoA reductase. It can directly regulate the activity of enzymes through phosphorylation. Moreover, the expression of metabolic enzymes at the transcription level can be affected by the phosphorylation of transcription factors and their coactivators, such as acetyltransferase p300. AMPK can participate in the regulation of cell cycle progression through p53 activation and cell metabolism checkpoints and thus participate in the regulation of tumor growth [24, 25]. In addition, AMPK activation can also inhibit ATP formation through fatty acid oxidation (FAO) and stimulate glycolysis through phosphorylation-induced phosphofructokinase-2 (PFK2) [26]. Therefore, interference with glycolysis shed a light on influencing tumor growth.
The anticancer effect of metformin may also be mediated through an AMPK-independent mechanism. It has been reported that metformin can inhibit cell DNA damage by preventing the production of reactive oxygen species (ROS) [27]. In the absence of AMPK, metformin can also affect AKT/mTOR signaling by directly inhibiting mTORC1 signaling [28]. In addition, metformin has been shown to inhibit cyclin D1, an important regulator of the cell cycle [29]. It has been reported that this inhibition is related to the p53-dependent upregulation of Redd1, an effect of the DNA damage response. Redd1 is a negative regulator of mTOR and a new molecular target of metformin [30]. Next, metformin was found to upregulate apoptosis and autophagy through the bcl-2 pathway, thereby inhibiting tumor growth. This effect is mediated by the inactivation of the STAT3/bcl-2 pathway [31]. After the knockout of AMPK, this pathway changed only slightly, indicating that the contribution of AMPK is quite small [31].
The anticancer effect of metformin can also inhibit tumor growth through dietary control, such as reducing blood glucose and insulin resistance and further reducing the levels of insulin and insulin-like growth factor-1 (IGF-1), thus inhibiting the growth of cancer cells [32]. Metformin can weaken the function of hexokinase, which catalyzes the production of G6P, thus inhibiting the utilization of glucose by tumor cells [33]. It has also been reported that metformin reduces glucose uptake in cancer patients. This changes the energy source and energy metabolism of tissues and cells, resulting in improved mitochondrial function [34].
Metformin can also exert its anticancer effect by affecting the immune microenvironment and stimulating the immune response to cancer cells. Metformin potentially ameliorates hypoxia in the tumor microenvironment through its potential effect on mitochondrial function and downstream oxygen production, and it has been reported that metformin can cause the degradation of oxygen-dependent hypoxia inducible factor 1α (HIF-1α) in hepatocellular carcinoma cells, thus improving the phenotype of the inhibitory tumor microenvironment caused by hypoxia [35]. A decrease in HIF-1α levels can also lead to a decrease in CD39 + and CD73 + immunosuppressive myeloid cells in the tumor microenvironment [36]. Metformin can improve immunotherapy resistance by changing the oxygen consumption of small cell lung cancer cells [37]. In addition, metformin may affect the immune response of tumor cells by directly reducing PD1/PD-L1 expression in tumor cells. For example, Cha et al. showed that metformin-induced AMPK activation can increase the degree of phosphorylation of PD-L1 S195 and increase the degradation of PD-L1 in the endoplasmic reticulum, thus reducing the expression level of PD-L1 on the tumor cell surface [38]. AMPK activation can also promote the phosphorylation of the mTOR and S6 proteins and reduce the expression of FOXP3, thus reducing the immunosuppressive function of Treg cells (Fig. 1) [39].
Fig. 1.
Anticancer mechanism of metformin.
Research progress on metformin in different types of leukemia
Acute lymphocytic leukemia (ALL)
ALL is a neoplastic disease characterized by the abnormal malignant proliferation of B or T cells in the bone marrow (BM). Some studies have shown that PTEN deficiency in tumor suppressor genes leads to the constitutive activation of the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia (T-ALL) [40] and is related to the poor prognosis of patients with T-ALL [41]. Metformin significantly reduces the levels of c-myc and Bcl-xl by stimulating AMPK to inhibit mTOR, thus inhibiting the metabolism and proliferation of cancer cells [42]. In addition, metformin induces the compensatory antiapoptotic activation of Akt and PIM-2, which is reversed by the corresponding inhibitors, which induce cell death when combined with metformin. However, metformin does not affect the survival of normal T lymphocytes, suggesting that metformin can be used to treat ALL [43, 44].
Metformin can also enhance the sensitivity to chemotherapeutic drugs by promoting apoptosis and inhibiting the cell cycle. Clinical data show that the recurrence rate of ALL in a metformin combined with chemotherapy group was lower than that in a chemotherapy group and that this treatment improved the survival rate of patients [45]. Metformin has been shown to enhance the antitumor effect of anthracyclines and reduce growth and survival of ALL cells [46]. In addition to the antitumor effects of synergistic chemotherapeutic drugs, the combination of metformin with chemotherapeutic drugs can help reduce the clinical dose of chemotherapeutic drugs, thus reducing cardiotoxicity and other adverse reactions to anthracyclines [47]. In addition, a clinical study of metformin combined with the chemotherapy drugs vincristine, dexamethasone, doxorubicin and l-asparaginase in the treatment of recurrent childhood ALL (NCT01324180) revealed good clinical effects. The synergistic mechanism may involve metformin inhibiting the unfolded protein response (UPR) and upregulating endoplasmic reticulum stress by activating AMPK, thus mediating the apoptosis of ALL cells [48]. In addition, metformin can enhance the apoptosis of leukemic cells induced by the Bcl-2 inhibitor ABT-737 by inducing mitochondrial membrane depolarization [49].
In vitro cell experiments showed that metformin not only inhibited the malignant proliferation of ALL cells but also reversed drug resistance. Metformin can induce cell cycle arrest and apoptosis in drug-resistant ALL cells [50]. Further studies revealed that high ATP-binding cassette subfamily B member 1 (ABCB1) expression in cancer cells was positively correlated with drug resistance in ALL. The survival rate of patients with high ABCB1 expression is lower than that of patients with low ABCB1 expression, and this difference in survival rate affects patient prognosis. Metformin can increase the sensitivity of ALL patients with high ABCB1 expression to chemotherapeutic drugs, reduce the recurrence rate, and increase the survival rate [51, 52]. Leukemia stem cells (LSCs) are rare cells of leukemic origin and are the cause of leukemia recurrence because they are inherently resistant to chemotherapy [53]. Studies have shown that metformin and chemotherapeutic drugs can achieve antitumor effects by inhibiting the proliferation of cancer stem cells (CSCs), which were also demonstrated in and that ALL model as well [54, 55].
Chronic lymphocytic leukemia (CLL)
CLL is caused by the clonal accumulation of resting and antiapoptotic malignant B lymphocytes. The tumor cells are monoclonal B lymphocytes that are similar to normal mature small lymphocytes and accumulate in the blood, BM, and lymphoid tissue. In fact, most CLL tumor cells are arrested in the G0/G1 cell cycle stage [56]. Bruno et al. [57] found that metformin induced the death of quiescent CLL cells and inhibited the entry of CLL cells into the cell cycle but had no effect on normal lymphocytes. Metformin induces AMPK phosphorylation, reduces glucose metabolism, and inhibits cell cycle progression in CLL cells [58].
Analyses of glucose dependence in CLL patients showed that different patients had different sensitivities to glucose deprivation. Further evaluation of the metabolic dependence of CLL cells resistant to glucose deprivation showed that glucose transporter type 4 (GLUT4) was upregulated [59]. CLL cells were treated with ritonavir, a human immunodeficiency virus (HIV) protease inhibitor that can inhibit GLUT4, and the toxicity was similar to that caused by glucose deprivation. CLL cells resistant to ritonavir exhibited enhanced drug sensitivity when combined with metformin, potentially by targeting compensatory mitochondrial complex 1 activity [59]. Similarly, metformin has a synergistic effect with the glycolysis inhibitor sodium dichloroacetate (DCA), which can inhibit the expression of the antiapoptotic protein MCI-1, thus promoting CLL cell death [60]. Metformin combined with thymoquinone (TQ) could increase the level of cleaved poly (ADP-ribose) polymerase (PARP) in primary CLL cells, decrease the level of proliferation regulatory proteins, and inhibit the Akt and NF-kB signaling pathways, suggesting that metformin can be used as a therapeutic strategy for CLL [61]. However, the effect and mechanism of action of metformin in CLL are not perfect, and many basic and clinical studies are still needed to determine its therapeutic effect on CLL.
Acute myeloid leukemia (AML)
AML is a group of heterogeneous malignant diseases characterized by the unrestricted proliferation of BM primitive hematopoietic stem cells (HSCs) that are blocked in the early stage of differentiation. The accumulation of immature undifferentiated cells in the BM and peripheral blood replaces normal terminally differentiated blood cells, leading to progressive anemia, thrombocytopenia, and neutropenia. AML is the most common type of acute leukemia in adults; among those with AML, the majority are males, and the median age is approximately 70 years. The French-American-British (FAB) classification is still in use. The FAB classification is based mainly on the morphological characteristics of leukemic cells [62]. Moreover, there has been no significant change in the clinical treatment of AML in the past 40 years and includes 7–10 days of treatment with cytarabine combined with 3 days of anthracycline treatment as remission induction therapy. In patients younger than 60 years, this treatment yields a complete remission (CR) rate of approximately 60–90%. In addition, several courses of high-dose cytarabine or allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be used as consolidation therapy. Among elderly patients for whom intensive therapy is not suitable, this treatment, including low-dose cytarabine or demethylation therapy and supportive therapy, does not yield good clinical results [63]. At present, new therapeutic drugs for AML, including monoclonal antibodies, small inhibitors, and epigenetic regulators, are in early clinical trials. Currently, the most successful drug therapy for acute lymphoblastic leukemia (APL) is still induced differentiation therapy. APL is a special subtype of AML that is characterized by the translocation of chromosomes 15 and 17. APL is a fatal disease with a high incidence of early hemorrhagic death. It has been successfully treated with all-trans retinoic acid (ATRA) and chemotherapy. The induced CR rate is 90%, and the cure rate is approximately 80%. In the later stage, arsenic trioxide can be introduced for the treatment of refractory or recurrent APL, and better clinical results have been obtained [64]. Huai et al. reported that metformin inhibits the proliferation and differentiation of the APL cell line NB4 by inhibiting extracellular regulated protein kinase (ERK), live kinase B1 (LKB1)/AMPK, and other signaling pathways to activate the apoptosis-related protease caspase-3, subsequently promoting apoptosis and reducing tumor cell adhesion [65].
In a recent retrospective study, the use of metformin did not significantly improve the overall disease-free survival of patients with AML [66]. Compared with normal cells, leukemic cells exhibit high levels of AKT phosphorylation, glucose consumption, and glycolysis, which may reduce the metformin-induced Pasteur effect and induce resistance to metformin-induced apoptosis. Therefore, treatment with deoxyglucose or AKT inhibitors can increase the sensitivity of leukemic cells to metformin. Therefore, when cultured in low-glucose medium or when glycolysis is downregulated by 2-DG or AKT inhibitors, AML cells may be more sensitive to metformin [67]. Liu et al. showed that metformin can inhibit OXPHOS. AML cells with the MLL/AF9 genotype are highly dependent on oxidative phosphorylation and can be targeted by metformin. Metformin significantly inhibits the proliferation of MLL/AF9 AML cells by inhibiting mitochondrial respiration [68]. In AML, metformin can block the expression of proto-oncogenes by inhibiting mTOR, inhibiting the proliferation of G0/G1 or S-G2/M cells, interfering with cancer cell proliferation and colony formation activity, and inducing apoptosis but not affecting the proliferation and differentiation of normal HSCs [20]. Numerous in vitro experiments have confirmed that metformin, an AMPK agonist, can inhibit the growth of the AML cell lines OCI/AML2, OCI/AML3, and THP-1 in vitro; is a potential drug for adjuvant therapy for leukemia; and inhibits the growth and survival of chronic myeloid leukemia (CML) cells expressing BCR-ABL mutations in vitro [69, 70]. The inhibitory effect of metformin has also been observed in the phorbol-12-myristate-13-acetate (PMA)-mediated differentiation of the acute monocytic leukemia cell line THP-1 [71]. The role of metformin in AML may depend on the basic activity of mTOR and ERK, which are downstream targets of AMPK [72]. In addition, metformin-mediated apoptosis and inhibition of proliferation in AML cells were preserved when AMPK expression was downregulated by small interfering RNA (si-RNA), indicating that metformin may have an AMPK-independent regulatory effect on AML [67, 73]. Increasing evidence shows that the different effects of metformin on hematopoietic and immune cell differentiation and its subsequent immunomodulatory effect may be another mechanism by which metformin exerts antitumor activity [74].
In addition to the direct inhibitory effect of metformin on AML, metformin can also be used as an adjuvant to enhance the therapeutic efficacy of treatment for AML. First, some studies have shown that metformin can inhibit the transformation of stromal cells into AML cells by blocking mitochondria, a process that can significantly enhance the sensitivity of AML cells to small molecule chemical drugs [75–77]. Second, metformin can also inhibit mTORC1 and increase the sensitivity of AML to cytarabine through its classical regulatory pathway [78]. The antileukemic effect of the Flt3 inhibitor sorafenib was investigated in FLT3-ITD-positive AML cells, which are associated with poor prognosis. Metformin enhances the antileukemia activity of sorafenib by downregulating the expression of related genes in the mTOR pathway [79]. In addition, in the study of AML, it has been found that metformin combined with the nonsteroidal anti-inflammatory drugs diclofenac and diflunisal can induce the apoptosis of AML cells [80].
Recently, as the only Bcl-2 selective inhibitor approved for sale worldwide, venetoclax was approved by the Food and Drug Administration (FDA) for use in newly diagnosed AML patients, especially those who are not suitable for routine chemotherapy. Although venetoclax has been shown to have ideal therapeutic efficacy in clinical practice, the acquisition of drug resistance induced by the activation of the apoptosis pathway by ABT-199 is still an important clinical problem. For this reason, Zhou et al. confirmed in vitro and in vivo that compared with metformin or ABT-199 alone, the combination of these two drugs has synergistic effects on promoting apoptosis, resulting in greater antileukemia effects [58]. In addition, the combined use of metformin and ABT-199 significantly decreased the expression of the apoptosis-related protein myeloid cell leukemia-1 (Mcl-1) induced by ABT-199 alone and inhibited the expression of another antiapoptotic protein, BCL-xl, to some extent [58].
Chronic myeloid leukemia (CML)
CML is characterized by the production of many immature white blood cells, which accumulate in the BM and inhibit normal hematopoiesis in the BM. It often occurs in adults between the ages of 40 and 60 years. The cause is usually the rearrangement of two designated chromosomes (chromosomes 9 and 22) to form the so-called Philadelphia chromosome. The Philadelphia chromosome encodes an abnormal enzyme, tyrosine kinase, which leads to an increase in the number of white blood cells produced in abnormal growth patterns in patients with CML [81]. Tyrosine kinase inhibitors (TKIs) are first-line treatments for CML. A complete cytogenetic response (CCyR) has been shown to be associated with increased overall survival, with only 66% of patients reaching CCyR after 1 year of TKI treatment [81]. In preclinical studies, metformin has been shown to inhibit the viability of imatinib-resistant CML cells (K562R) and BCR-ABL-mutant CML cells, induce apoptosis, and downregulate the mTORC1 signaling pathway [82]. In a clinical study, metformin and TKIs can increase the proportion of patients with CML to achieve CCyR and shorten the time to reach a major molecular response (MMR) or complete molecular response (CMR) [83]. Nilotinib is used to treat patients with imatinib-sensitive or drug-resistant CML. However, in recent years, there have been cases of nilotinib resistance. Researchers have found that the nilotinib-resistant CML cell lines K562 and KU812 are produced by exposing cells to gradually increasing doses of nilotinib. Several studies have shown the driving force of resistance to nilotinib and the effect of metformin on the driving force. Metformin can enhance the apoptosis of CML cells induced by nilotinib and restore the sensitivity of drug-resistant cells to nilotinib by reducing Jun N-terminal kinase (JNK) phosphorylation and inhibiting Bcl-xl expression. The results of the above studies suggest that the combination of metformin and nilotinib may have enhanced the efficacy of leukemia treatments and overcome resistance to nilotinib [84]. Metformin can also inhibit the growth and promote the apoptosis of the human CML cell line K562 by inhibiting glycolysis and the PI3K/Akt/mTOR pathway. Metformin may be a promising adjuvant for the treatment of CML (Table 1) [85].
Table 1.
Research progress on the use of metformin in different types of leukemia
| Type of leukemia | Key findings | Combination drug | Reference |
|---|---|---|---|
| ALL | Induces apoptosis through AMPK-dependent inhibition of UPR signaling | - | [44] |
| Inhibits mTOR signaling to enhance the antitumor effect | Anthracycline | [46] | |
| Inhibits the UPR and upregulates endoplasmic reticulum stress by activating AMPK | Vincristine, dexamethasone, PEG-asparaginase, doxorubicin | [48] | |
| Induces mitochondrial membrane depolarization to enhance apoptosis | ABT-737 | [49] | |
| Inhibits cell proliferation by arresting cells in the G2/M and S phases of the cell cycle | - | [50] | |
| CLL | Induces AMPK phosphorylation and reduces glucose metabolism | Fludarabine, ABT-737 | [57] |
| Downregulates the expression of Mcl-1 and Bcl-xl by inhibiting protein synthesis | ABT-199 (venetoclax) | [58] | |
| Potentially targets compensatory mitochondrial complex 1 activity to sensitize CLL cells resistant to ritonavir | Ritonavir | [59] | |
| Induces synergistic apoptotic cell death coupled substantial Mcl-1 protein downregulation | DCA | [60] | |
| Increases the level of cleaved PARP and inhibits the AKT and NF-kB signaling pathways | TQ | [61] | |
| AML | Inhibits ERK, LKB1/AMPK and other signaling pathways to activate caspase-3 | ATRA | [65] |
| Decreases electron transport chain complex I activity, oxygen consumption, and mitochondrial ATP synthesis while stimulating glycolysis for ATP and metabolic effects | Deoxyglucose, AKT inhibitor | [67] | |
| Represses cell proliferation by inhibiting mitochondrial respiration | - | [68] | |
| Blocks the expression of proto-oncogenes by inhibiting mTOR and then inhibits the proliferation of cells in the G0/G1 or S-G2/M phase | - | [20] | |
| Downregulates the expression of TYRO3 and phosphorylation of MERTK, partly due to the inhibition of TAM kinases | TP-0903 (a small molecule AXL inhibitor) | [70] | |
| Inhibits THP-1 macrophage-derived foam cell formation induced by LPS, reduces intracellular lipid accumulation, and downregulates the expression of ADRP | PMA | [71] | |
| Inhibits the mitochondrial transfer and OXPHOS activity | Ara-C | [75] | |
| Inhibits the mTORC1/P70S6K pathway and increases the sensitivity of AML to Ara-C | Ara-C | [78] | |
| Increases LC3 levels and reduces the expression of proteins in the mTOR/p70S6K/4EBP1 pathway, without appreciably altering the cell cycle | Sorafenib | [79] | |
| CML | Suppresses survival, induces apoptosis, and downregulates the activation of the mTORC1 pathway | Imatinib | [82] |
| Reduces Bcl-xl expression, decreases phosphorylated JNK levels, and restores nilotinib sensitivity | Nilotinib | [84] | |
| Inhibits the growth and proliferation of K562 cells and promotes the apoptosis of K562 cells by inhibiting glycolysis energy metabolism | - | [85] |
ALL acute lymphocytic leukemia, AMPK adenosine 5′-monophosphate (AMP)-activated protein kinase, UPR unfolded protein response, mTOR mammalian target of rapamycin, CLL chronic lymphocytic leukemia, Mcl-1 myeloid cell leukemia-1, Bcl-xl B-cell lymphoma-extra large, DCA dichloroacetate, PARP poly(ADP-ribose) polymerase, AKT protein kinase B, NF-kB nuclear factor-kappa B, TQ thymoquinone, ERK extracellular regulated protein kinases, LKB1 live kinase B1, AML acute myeloid leukemia, ATRA all-trans retinoic acid, ATP adenosine triphosphate, TYRO3 protein tyrosine kinase gene, MERTK tyrosine-protein kinase mer, TAM tumor-associated macrophage, LPS lipopolysaccharide, PMA phorbol-12-myristate-13-acetate, ADRP adipose differentiation-related protein, OXPHOS oxidative phosphorylation, mTORC1 mTOR complex 1, P70S6K phosphoprotein 70 ribosomal protein S6 kinase, Ara-C cytarabine, LC3 microtubule-associated protein 1 light chain 3, 4EBP1 eukaryotic initiation factor 4E-binding protein 1, CML chronic myeloid leukemia, JNK Jun N-terminal kinase
Clinical trials
Although metformin may theoretically be used as a potential adjuvant for a variety of leukemias and many preclinical in vitro or in vivo trials provide supporting data, there is still a lack of high-quality evidence from clinical trials. Most of the existing clinical studies have focused on the application of metformin in ALL patients. In a randomized controlled study (NCT00500240) reported in 2012, Khanh et al. studied the hypoglycemic effect of metformin in patients with diabetic ALL and reported that metformin not only had a hypoglycemic effect but was also associated with improved PFS; moreover, multivariate analysis revealed that blood glucose level was an independent risk factor for OS in patients with ALL [86]. In 2014, a randomized controlled trial in Spain showed that metformin can lead to a 56% reduction in the risk of relapse according to Cox regression analysis [87]. In 2018, another randomized controlled study (NCT03118128) by Christian et al. showed that for ALL patients with high ABCB1 expression, metformin combined with the LALHGM07 chemotherapy regimen improved survival and reduced the risk of treatment failure (OR = 0.07, 95% CI = 0.0037–1.53) and early recurrence (OR = 0.05, 95% CI = 0.0028–1.153). It is suggested that metformin can be used as an adjuvant therapy for specific patient subgroups [52]. In addition, two related clinical studies are underway, one focusing on the potential therapeutic value of metformin for adolescent ALL (NCT05326984) and the expression of biomarkers and the other on the effect of metformin on the risk of leukemia in patients with clonal cytopenia of undetermined significance (CCUS) or lower-risk myelodysplastic neoplasms (LR-MDS). We also look forward to obtaining additional evidence-based medicine data to support the clinical application of metformin in patients with leukemia in the future. We also hope that additional studies will focus on patients with types of leukemia other than ALL to provide insight into the clinical application of metformin in leukemia treatment (Table 2).
Table 2.
Clinical trials
| Clinical trial ID | Official title | Status |
|---|---|---|
| NCT05326984 | Effect of metformin on ABCB1 and AMPK expression in adolescents with newly diagnosed ALL | Recruiting |
| NCT04741945 | Repurposing metformin as a leukemia-preventive drug in CCUS and LR-MDS | Recruiting |
ABCB1 ATP-binding cassette subfamily B member 1, AMPK adenosine 5′-monophosphate (AMP)-activated protein kinase, ALL acute lymphocytic leukemia, CCUS clonal cytopenia of undetermined significance, LR-MDS lower-risk myelodysplastic neoplasms
Conclusions and perspectives
Metformin is a safe and inexpensive small molecule antidiabetic drug that has been used for the treatment of diabetes for several decades. In recent years, metformin has aroused widespread interest for its antitumor, immunoregulatory, antiaging, and other effects. To date, numerous medical studies have suggested that metformin is beneficial for patients with leukemia, but most of these studies are limited to preclinical studies, and the results of a small number of clinical trials are also different. In our previous in vitro study, we found that venetoclax and metformin alone inhibited the proliferation of AML cells, promoted apoptosis, and reduced the mitochondrial membrane potential. The effects of inhibiting proliferation and promoting apoptosis were time and concentration dependent. In animal experiments in the same study, metformin combined with venetoclax reduced the leukemia load and prolonged the survival of AML xenograft mice. Therefore, studying the pharmacological effects of metformin and the efficacy of metformin in treating hematopoietic diseases may also be helpful for understanding the pathophysiological process of the occurrence and development of hematological diseases. Therefore, determining whether metformin can be used as a therapeutic drug for leukemia and how to correctly evaluate its clinical efficacy still require a large amount of new data. In the future, scholars should conduct more in-depth research on the role of metformin in combination with other anticancer drugs.
Author contributions
Qian Wang drafted the manuscript; Xu-Dong Wei congtributed to revising the manuscript for important content
Data availability
No datasets were generated or analyzed during the current study.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ci T, Zhang W, Qiao Y, Li H, Zang J, Li H, et al. Delivery strategies in treatments of leukemia. Chem Soc Rev. 2022;51:2121–2144. doi: 10.1039/D1CS00755F. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Cunha Junior AD, Bragagnoli AC, Costa FO, Carvalheira JBC. Repurposing metformin for the treatment of gastrointestinal cancer. World J Gastroenterol. 2021;27:1883–1904. doi: 10.3748/wjg.v27.i17.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartman CR, Weilandt DR, Shen Y, Lee WD, Han Y, TeSlaa T, et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature. 2023;614:349–357. doi: 10.1038/s41586-022-05661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johar D, Elmehrath AO, Khalil RM, Elberry MH, Zaky S, Shalabi SA, et al. Protein networks linking Warburg and reverse Warburg effects to cancer cell metabolism. BioFactors. 2021;47:713–728. doi: 10.1002/biof.1768. [DOI] [PubMed] [Google Scholar]
- 6.Alden RS, Kamran MZ, Bashjawish BA, Simone BA. Glutamine metabolism and radiosensitivity: beyond the Warburg effect. Front Oncol. 2022;12:1070514. doi: 10.3389/fonc.2022.1070514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141–162. doi: 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafi F, Rajati F, Sarokhani D, Bavandpour M, Moradinazar M. The Relationship between metformin consumption and cancer risk: an updated umbrella review of systematic reviews and meta-analyses. Int J Prev Med. 2023;14:90. doi: 10.4103/ijpvm.ijpvm_62_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng CH. Metformin use and leukemia risk in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 2020;11:541090. doi: 10.3389/fendo.2020.541090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603:159–165. doi: 10.1038/s41586-022-04431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL, et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526–540. doi: 10.1016/j.cmet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Xu D, Trefts E, Lv M, Inuzuka H, Song G, et al. Metabolic orchestration of cell death by AMPK-mediated phosphorylation of RIPK1. Science. 2023;380:1372–1380. doi: 10.1126/science.abn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugan M, Dufay Wojcicki A, d’Hayer B, Boudy V. Metformin: an anti-diabetic drug to fight cancer. Pharmacol Res. 2016;113:675–685. doi: 10.1016/j.phrs.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101:1371–1426. doi: 10.1152/physrev.00026.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Reis F. mTOR signaling: new insights into cancer, cardiovascular diseases, diabetes and aging. Int J Mol Sci. 2023;24:13628. [DOI] [PMC free article] [PubMed]
- 18.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 20.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116:4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 21.Gayatri MB, Kancha RK, Behera A, Patchva D, Velugonda N, Gundeti S, et al. AMPK-induced novel phosphorylation of RUNX1 inhibits STAT3 activation and overcome imatinib resistance in chronic myelogenous leukemia (CML) subjects. Cell Death Discov. 2023;9:401. doi: 10.1038/s41420-023-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-kappaB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19:1089–1104. doi: 10.1080/15384101.2020.1743911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Y, Cai S, Yu S, Lin H. Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim Biophys Sin (Shanghai) 2021;53:333–341. doi: 10.1093/abbs/gmaa180. [DOI] [PubMed] [Google Scholar]
- 24.Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Liu L, Chen HY, Yan X, Li RL, Lan J, et al. Mogrol suppresses lung cancer cell growth by activating AMPK-dependent autophagic death and inducing p53-dependent cell cycle arrest and apoptosis. Toxicol Appl Pharmacol. 2022;444:116037. doi: 10.1016/j.taap.2022.116037. [DOI] [PubMed] [Google Scholar]
- 26.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/S0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D, Yu H. Metformin attenuates UVA-induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int J Mol Sci. 2022;23(13):6960. [DOI] [PMC free article] [PubMed]
- 28.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raafat SN, El Wahed SA, Badawi NM, Saber MM, Abdollah MRA. Enhancing the anticancer potential of metformin: fabrication of efficient nanospanlastics, in vitro cytotoxic studies on HEP-2 cells and reactome enhanced pathway analysis. Int J Pharm X. 2023;6:100215. doi: 10.1016/j.ijpx.2023.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang SK, Hong SE, Lee DH, Kim JY, Kim JY, Ye SK, et al. Inhibition of mTORC1 through ATF4-induced REDD1 and Sestrin2 expression by metformin. BMC Cancer. 2021;21:803. doi: 10.1186/s12885-021-08346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini C, Salani B, Massollo M, Amaro A, Esposito AI, Orengo AM, et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12:3490–3499. doi: 10.4161/cc.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YC, Li H, Wang J. Mechanisms of metformin inhibiting cancer invasion and migration. Am J Transl Res. 2020;12:4885–4901. [PMC free article] [PubMed] [Google Scholar]
- 33.Salani B, Marini C, Rio AD, Ravera S, Massollo M, Orengo AM, et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559:637–641. doi: 10.1038/s41586-018-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Chen J, Yi G, Deng M, Liu H, Liang M, et al. Metformin suppresses hypoxia-induced stabilization of HIF-1α through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7:873–884. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78:1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y, Vagia E, Viveiros P, Kang CY, Lee JY, Gim G, et al. Overcoming acquired resistance to PD-1 inhibitor with the addition of metformin in small cell lung cancer (SCLC) Cancer Immunol Immunother. 2021;70:961–965. doi: 10.1007/s00262-020-02703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71:606–620.e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, Guo P, Zuo Y, Wang Y, Zhao H, Lan T, et al. Folic acid intervention changes liver Foxp3 methylation and ameliorates the damage caused by Th17/Treg imbalance after long-term alcohol exposure. Food Funct. 2022;13:5262–5274. doi: 10.1039/D1FO04267J. [DOI] [PubMed] [Google Scholar]
- 40.Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jotta PY, Ganazza MA, Silva A, Viana MB, da Silva MJ, Zambaldi LJ, et al. Negative prognostic impact of PTEN mutation in pediatric T-cell acute lymphoblastic leukemia. Leukemia. 2010;24:239–242. doi: 10.1038/leu.2009.209. [DOI] [PubMed] [Google Scholar]
- 42.Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25:463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimaldi C, Chiarini F, Tabellini G, Ricci F, Tazzari PL, Battistelli M, et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2012;26:91–100. doi: 10.1038/leu.2011.269. [DOI] [PubMed] [Google Scholar]
- 44.Leclerc GM, Leclerc GJ, Kuznetsov JN, DeSalvo J, Barredo JC. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS ONE. 2013;8:e74420. doi: 10.1371/journal.pone.0074420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos Penafiel C, Olarte Carrillo I, Ceron Maldonado R, Miranda Peralta E, Rozen Fuller E, KassackIpina JJ, et al. Effect of metformin added to chemotherapy on the survival of patients with acute lymphoblastic leukemia. Rev Med Chil. 2018;146:846–853. doi: 10.4067/s0034-98872018000700846. [DOI] [PubMed] [Google Scholar]
- 46.Pan J, Chen C, Jin Y, Fuentes-Mattei E, Velazquez-Tores G, Benito JM, et al. Differential impact of structurally different anti-diabetic drugs on proliferation and chemosensitivity of acute lymphoblastic leukemia cells. Cell Cycle. 2012;11:2314–2326. doi: 10.4161/cc.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trucco M, Barredo JC, Goldberg J, Leclerc GM, Hale GA, Gill J, et al. A phase I window, dose escalating and safety trial of metformin in combination with induction chemotherapy in relapsed refractory acute lymphoblastic leukemia: metformin with induction chemotherapy of vincristine, dexamethasone, PEG-asparaginase, and doxorubicin. Pediatr Blood Cancer. 2018;65:e27224. doi: 10.1002/pbc.27224. [DOI] [PubMed] [Google Scholar]
- 49.Velez J, Pan R, Lee JT, Enciso L, Suarez M, Duque JE, et al. Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget. 2016;7:51435–51449. doi: 10.18632/oncotarget.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Lirio A, Perez-Yarza G, Fernandez-Suarez MR, Alonso-Tejerina E, Boyano MD, Asumendi A. Metformin induces cell cycle arrest and apoptosis in drug-resistant leukemia cells. Leuk Res Treatment. 2015;2015:516460. doi: 10.1155/2015/516460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olarte Carrillo I, Ramos Penafiel C, Miranda Peralta E, Rozen Fuller E, Kassack Ipina JJ, Centeno Cruz F, et al. Clinical significance of the ABCB1 and ABCG2 gene expression levels in acute lymphoblastic leukemia. Hematology. 2017;22:286–291. doi: 10.1080/10245332.2016.1265780. [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Penafiel C, Olarte-Carrillo I, Ceron-Maldonado R, Rozen-Fuller E, Kassack-Ipina JJ, Melendez-Mier G, et al. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J Transl Med. 2018;16:245. doi: 10.1186/s12967-018-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grey W, Rio-Machin A, Casado P, Gronroos E, Ali S, Miettinen JJ, et al. CKS1 inhibition depletes leukemic stem cells and protects healthy hematopoietic stem cells in acute myeloid leukemia. Sci Transl Med. 2022;14:eabn3248. doi: 10.1126/scitranslmed.abn3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan C, Liu S, Song Q, Hu Y. Metformin inhibits self-renewal of colorectal cancer stem cells by inhibiting mitochondrial oxidative phosphorylation. Nan Fang Yi Ke Da Xue Xue Bao. 2023;43:1279–1286. doi: 10.12122/j.issn.1673-4254.2023.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biondani G, Peyron JF. Metformin, an anti-diabetic drug to target leukemia. Front Endocrinol (Lausanne) 2018;9:446. doi: 10.3389/fendo.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. JAMA. 2023;329:918–932. doi: 10.1001/jama.2023.1946. [DOI] [PubMed] [Google Scholar]
- 57.Bruno S, Ledda B, Tenca C, Ravera S, Orengo AM, Mazzarello AN, et al. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. Oncotarget. 2015;6:22624–22640. doi: 10.18632/oncotarget.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou FJ, Zeng CX, Kuang W, Cheng C, Liu HC, Yan XY, et al. Metformin exerts a synergistic effect with venetoclax by downregulating Mcl-1 protein in acute myeloid leukemia. J Cancer. 2021;12:6727–6739. doi: 10.7150/jca.60208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adekola KU, Dalva Aydemir S, Ma S, Zhou Z, Rosen ST, Shanmugam M. Investigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk Lymphoma. 2015;56:450–459. doi: 10.3109/10428194.2014.922180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voltan R, Rimondi E, Melloni E, Gilli P, Bertolasi V, Casciano F, et al. Metformin combined with sodium dichloroacetate promotes B leukemic cell death by suppressing anti-apoptotic protein Mcl-1. Oncotarget. 2016;7:18965–18977. doi: 10.18632/oncotarget.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glamoclija U, Mahmutovic L, Bilajac E, Soljic V, Vukojevic K, Suljagic M. Metformin and thymoquinone synergistically inhibit proliferation of imatinib-resistant human leukemic cells. Front Pharmacol. 2022;13:867133. doi: 10.3389/fphar.2022.867133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiNardo CD, Erba HP, Freeman SD, Wei AH. Acute myeloid leukaemia. Lancet. 2023;401:2073–2086. doi: 10.1016/S0140-6736(23)00108-3. [DOI] [PubMed] [Google Scholar]
- 63.Bazinet A, Kantarjian HM. Moving toward individualized target-based therapies in acute myeloid leukemia. Ann Oncol. 2023;34:141–151. doi: 10.1016/j.annonc.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630–1643. doi: 10.1182/blood-2019-01-894980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huai L, Wang C, Zhang C, Li Q, Chen Y, Jia Y, et al. Metformin induces differentiation in acute promyelocytic leukemia by activating the MEK/ERK signaling pathway. Biochem Biophys Res Commun. 2012;422:398–404. doi: 10.1016/j.bbrc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Ceacareanu AC, Nimako GK, Wintrob ZAP. Missing the benefit of metformin in acute myeloid leukemia: a problem of contrast? J Res Pharm Pract. 2017;6:145–150. doi: 10.4103/jrpp.JRPP_17_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scotland S, Saland E, Skuli N, de Toni F, Boutzen H, Micklow E, et al. Mitochondrial energetic and AKT status mediate metabolic effects and apoptosis of metformin in human leukemic cells. Leukemia. 2013;27:2129–2138. doi: 10.1038/leu.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L, Patnana PK, Xie X, Frank D, Nimmagadda SC, Rosemann A, Liebmann M, Klotz L, Opalka B, Khandanpour C. High Metabolic dependence on oxidative phosphorylation drives sensitivity to metformin treatment in MLL/AF9 acute myeloid leukemia. Cancers (Basel). 2022;14(3):486. [DOI] [PMC free article] [PubMed]
- 69.Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–6402. doi: 10.1182/blood-2011-01-332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito T, Itoh M, Tohda S. Metformin suppresses the growth of leukemia cells partly through downregulation of AXL receptor tyrosine kinase. Leuk Res. 2020;94:106383. doi: 10.1016/j.leukres.2020.106383. [DOI] [PubMed] [Google Scholar]
- 71.Xie Z, Yuan Y, Shi J, Shi X, Gao X, Zhao Y, et al. Metformin inhibits THP-1 macrophage-derived foam cell formation induced by lipopolysaccharide. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:168–72. [PubMed] [Google Scholar]
- 72.Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci U S A. 2014;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han Y, Bagchi P, Yun CC. Regulation of the intestinal Na(+)/H(+) exchanger NHE3 by AMP-activated kinase is dependent on phosphorylation of NHE3 at S555 and S563. Am J Physiol Cell Physiol. 2023 doi: 10.1152/ajpcell.00540.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Limagne E, Thibaudin M, Euvrard R, Berger H, Chalons P, Végan F, et al. Sirtuin-1 activation controls tumor growth by impeding Th17 differentiation via STAT3 deacetylation. Cell Rep. 2017;19:746–759. doi: 10.1016/j.celrep.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 75.You R, Wang B, Chen P, Zheng X, Hou D, Wang X, et al. Metformin sensitizes AML cells to chemotherapy through blocking mitochondrial transfer from stromal cells to AML cells. Cancer Lett. 2022;532:215582. doi: 10.1016/j.canlet.2022.215582. [DOI] [PubMed] [Google Scholar]
- 76.Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128:253–264. doi: 10.1182/blood-2015-07-655860. [DOI] [PubMed] [Google Scholar]
- 77.Griessinger E, Moschoi R, Biondani G, Peyron JF. Mitochondrial transfer in the leukemia microenvironment. Trends Cancer. 2017;3:828–839. doi: 10.1016/j.trecan.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Yuan F, Cheng C, Xiao F, Liu H, Cao S, Zhou G. Inhibition of mTORC1/P70S6K pathway by metformin synergistically sensitizes acute myeloid leukemia to Ara-C. Life Sci. 2020;243:117276. doi: 10.1016/j.lfs.2020.117276. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Liu Z, Zeng J, Zhu H, Li J, Cheng X, et al. Metformin synergistically sensitizes FLT3-ITD-positive acute myeloid leukemia to sorafenib by promoting mTOR-mediated apoptosis and autophagy. Leuk Res. 2015;39:1421–1427. doi: 10.1016/j.leukres.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Renner K, Seilbeck A, Kauer N, Ugele I, Siska PJ, Brummer C, et al. Combined metabolic targeting with metformin and the NSAIDs diflunisal and diclofenac induces apoptosis in acute myeloid leukemia cells. Front Pharmacol. 2018;9:1258. doi: 10.3389/fphar.2018.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortes J, Pavlovsky C, Saußele S. Chronic myeloid leukaemia. Lancet. 2021;398:1914–1926. doi: 10.1016/S0140-6736(21)01204-6. [DOI] [PubMed] [Google Scholar]
- 82.Shi R, Lin J, Gong Y, Yan T, Shi F, Yang X, et al. The antileukemia effect of metformin in the Philadelphia chromosome-positive leukemia cell line and patient primary leukemia cell. Anticancer Drugs. 2015;26:913–922. doi: 10.1097/CAD.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 83.Pokorny R, Stenehjem DD, Gilreath JA. Impact of metformin on tyrosine kinase inhibitor response in chronic myeloid leukemia. J Oncol Pharm Pract. 2022;28:916–923. doi: 10.1177/10781552221077254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Na YJ, Yu ES, Kim DS, Lee DH, Oh SC, Choi CW. Metformin enhances the cytotoxic effect of nilotinib and overcomes nilotinib resistance in chronic myeloid leukemia cells. Korean J Intern Med. 2021;36:S196–s206. doi: 10.3904/kjim.2019.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen HL, Ma P, Chen YL, Sun L, Xing Y, Wang F, et al. Effect of metformin on proliferation capacity, apoptosis and glycolysis in K562 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27:1387–1394. doi: 10.19746/j.cnki.issn.1009-2137.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Vu K, Busaidy N, Cabanillas ME, Konopleva M, Faderl S, Thomas DA, et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:355–362. doi: 10.1016/j.clml.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos-Penafiel CO, Martinez-Murillo C, Santoyo-Sanchez A, Jimenez-Ponce F, Rozen-Fuller E, Collazo-Jaloma J, et al. Effect of metformin addition to an acute lymphoblastic leukemia chemotherapy treatment. Rev Med Inst Mex Seguro Soc. 2014;52:270–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.