Abstract
Objectives
Computed tomography (CT)–derived fractional flow reserve (FFRCT) decreases continuously from proximal to distal segments of the vessel due to the influence of various factors even in non-obstructive coronary artery disease (NOCAD). It is known that FFRCT is dependent on vessel-length, but the relationship with other vessel morphologies remains to be explained.
Purpose
To investigate morphological aspects of the vessels that influence FFRCT in NOCAD in the right coronary artery (RCA).
Methods
A total of 443 patients who underwent both FFRCT and invasive coronary angiography, with < 50% RCA stenosis, were evaluated. Enrolled RCA vessels were classified into two groups according to distal FFRCT: FFRCT ≤ 0.80 (n = 60) and FFRCT > 0.80 (n = 383). Vessel morphology (vessel length, lumen diameter, lumen volume, and plaque volume) and left-ventricular mass were assessed. The ratio of lumen volume and vessel length was defined as V/L ratio.
Results
Whereas vessel-length was almost the same between FFRCT ≤ 0.80 and > 0.80, lumen volume and V/L ratio were significantly lower in FFRCT ≤ 0.80. Distal FFRCT correlated with plaque-related parameters (low-attenuation plaque, intermediate-attenuation plaque, and calcified plaque) and vessel-related parameters (proximal and distal vessel diameter, vessel length, lumen volume, and V/L ratio). Among all vessel-related parameters, V/L ratio showed the highest correlation with distal FFRCT (r = 0.61, p < 0.0001). Multivariable analysis showed that calcified plaque volume was the strongest predictor of distal FFRCT, followed by V/L ratio (β-coefficient = 0.48, p = 0.03). V/L ratio was the strongest predictor of a distal FFRCT ≤ 0.80 (cut-off 8.1 mm3/mm, AUC 0.88, sensitivity 90.0%, specificity 76.7%, 95% CI 0.84–0.93, p < 0.0001).
Conclusions
Our study suggests that V/L ratio can be a measure to predict subclinical coronary perfusion disturbance.
Clinical relevance statement
A novel marker of the ratio of lumen volume to vessel length (V/L ratio) is the strongest predictor of a distal CT-derived fractional flow reserve (FFRCT) and may have the potential to improve the diagnostic accuracy of FFRCT.
Key Points
• Physiological FFR CT decline depends not only on vessel length but also on the lumen volume in non-obstructive coronary artery disease in the right coronary artery.
• FFR CT correlates with plaque-related parameters (low-attenuation plaque, intermediate-attenuation plaque, and calcified plaque) and vessel-related parameters (proximal and distal vessel diameter, vessel length, lumen volume, and V/L ratio).
• Of vessel-related parameters, V/L ratio is the strongest predictor of a distal FFR CT and an optimal cut-off value of 8.1 mm 3 /mm.
Supplementary information
The online version contains supplementary material available at 10.1007/s00330-023-09972-8.
Keywords: Coronary artery disease, Computed tomography angiography, Ischemia
Introduction
Computed tomography (CT)–derived fractional flow reserve (FFRCT) is a feasible tool for assessing the hemodynamic significance of coronary stenosis non-invasively with a high diagnostic accuracy of 81.9% [1]. The implementation of FFRCT can change treatment strategies and improve diagnostic efficiency and effectiveness in patients with coronary artery disease (CAD) [2]. Even in non-obstructive CAD (NOCAD), FFRCT gradually decreases from the proximal to the distal segments of the vessel (physiological FFRCT decline) [3, 4]. FFRCT is influenced by various factors such as vessel length [3, 4], bifurcation angle [5, 6], plaque burden [3], left ventricular mass [7], and collateral circulation [8]. FFRCT is modified by multiple variables, resulting in 37% of vessels presenting FFRCT of ≤ 0.80 even with normal coronary arteries [9]. Vessel length plays an important role in physiological FFRCT decline [3, 4]. It is empirically proven that differing FFRCT changes occur depending on the lumen volume even with the same vessel length. The lumen cross-sectional area decreases towards the distal segments [10], resulting in vessel tapering. The lumen cross-sectional area correlates with FFRCT [11]. Furthermore, with a higher dose of nitroglycerin as a vasodilator agent, lumen volume increases, resulting in FFRCT elevation at the distal vessel segments [12]. Hence, physiological FFRCT decline may be related to lumen volume [9]. The relationship between vessel length and lumen volume and FFRCT remains to be explained. The present study aimed to investigate morphological aspects of the vessels and to identify predictive factors forphysiological FFRCT decline in NOCAD in the right coronary artery (RCA).
Methods
Patient population
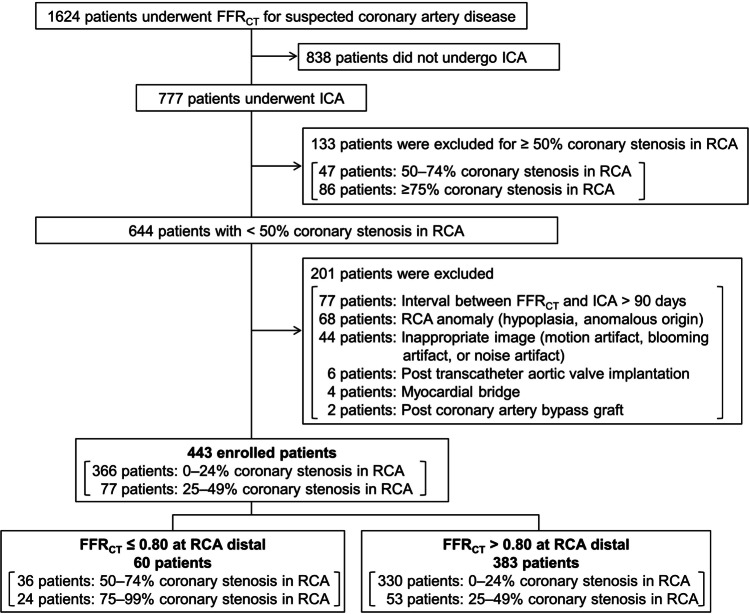
A total of 1624 outpatients with suspected CAD and who had a CT angiography (CTA) with FFRCT analysis examined at the Universitair Ziekenhuis Brussel between January 2017 and January 2023 were included in the study. A retrospective cohort study was performed. Approval from the ethical board at the Universitair Ziekenhuis Brussel was obtained with protocol number B.U.N. 143202000302. Inclusion criteria were NOCAD on ICA at the RCA. The presence of coronary stenosis in the left coronary artery was irrelevant. NOCAD was defined as vessels with < 50% coronary stenosis or luminal irregularities. The severity of coronary stenosis on CTA was determined as 0–2 according to CAD reporting and data system (CAD-RADS) classification [13] by experts in cardiac radiology imaging (T.T., and K.T.). The severity of coronary stenosis was assessed visually by experienced interventional cardiologists (JF.A. and C.B.). A total of 838 patients who had not undergone ICA and 133 patients with ≥ 50% coronary stenosis were excluded from the study. Although not included in the current study, patients with heart failure and old myocardial infarction were excluded. The following categories of patients were also excluded from the study: time interval between FFRCT and ICA > 90 days (n = 77), RCA anomaly (hypoplasia, anomalous origin) (n = 68), inappropriate image quality (motion artifact, blooming artifact, or noise artifact) (n = 44), post transcatheter aortic valve implantation (n = 6), myocardial bridge (n = 4), post coronary artery bypass graft (n = 2). As a result, 443 patients were enrolled and divided into two groups according to RCA distal vessel FFRCT: FFRCT ≤ 0.80 (n = 60) and FFRCT > 0.80 (n = 383). The patient selection flowchart is presented in Fig. 1.
Fig. 1.
Patient selection flow diagram showing patient flow in the study. CTA, computed tomography angiography; ICA, invasive coronary angiography; RCA, right coronary artery
Coronary CT angiography
All coronary CTA scans were acquired with a 320 slice GE Revolution scanner (GE Healthcar) enabling to image the heart in one heartbeat. This protocol used a single-rotation axial scan with prospective gating, 16-cm detector coverage, noise index 30 (noise level fixed over all kVp levels and body sizes), and gantry rotation time of 0.28 s. Tube potential ranged between 70 and 120 kV depending on body size. Iodinated contrast agent was administered with Iopromide 370 (Bayer Schering Pharma) using the bolus tracking method at a rate of 5.0 ml/s depending on body size. The volume of iodinated contrast injected was individualised according to body size (50–80 mL). Beta-blockers were administered when necessary to obtain a target heart rate of < 60 beats/min. According to guidelines [14], sublingual nitrates (two sprays of 0.8 mg) were administered 5 min prior to CT scanning in all patients. The images were reconstructed at 75 ± 10% of the R-R interval.
FFRCT
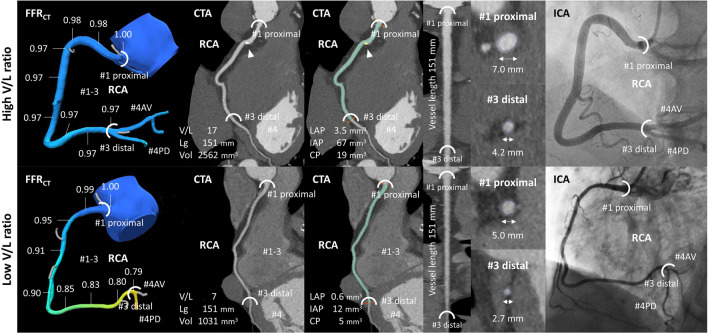
FFRCT was analysed by HeartFlow Inc. Computational fluid dynamics and blood flow simulations were performed to calculate the FFRCT at any arbitrary point in the coronary artery. The RCA was classified into 4 segments (#1–#4) based on the American Heart Association classification (AHA) [15]. To avoid the effects of turbulence due to branching, FFRCT values were measured from RCA ostium (#1) to the bifurcation (#3 distal end) of the atrioventricular nodal branch (#4AV) and posterior descending branch (#4PD) (Fig. 2). Moreover, each segment was divided into three categories (proximal, middle, and distal). The magnitude of change in FFRCT (ΔFFRCT) was measured from the proximal to the distal at the RCA. A positive FFRCT was defined as a value ≤ 0.80 in accordance with previously published literature [16–19].
Fig. 2.
Representative cases of high V/L ratio (top panel) and low V/L ratio (bottom panel). Analysis range is from #1 proximal to #3 distal. Arrowhead (CP). AV, atrioventricular nodal; CP, calcified plaque; IAP, intermediate-attenuation plaque; LAP, low-attenuation plaque; Lg, vessel length; PD, posterior descending; Vol, lumen volume
Coronary artery morphology and composition
Vessel diameter, vessel length, lumen volume, and composition of each vessel were measured using GE AW server 3.2 software (GE Healthcare). The ratio of lumen volume and vessel length was defined as V/L ratio (mm3/mm). Plaque characterisation and vessel morphology measurements were performed semi-automatically with Color Code Plaque (GE Healthcar). Vessel constituents were characterised based on Hounsfield units (HU) into low-attenuation plaque (LAP) (< 30 HU), intermediate-attenuation plaque (IAP) (30–150 HU), and calcified plaque (CP) (> 150 HU) [20, 21].
Invasive coronary angiography
All ICA images were evaluated by Philips Azurion with ClarityIQ (Philips Healthcare). Iodixanol 320 (GE Healthcare) was administered as an iodinated contrast agent.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). The 95% confidence interval was calculated as ± 1.96 SDs from the mean. Two groups (FFRCT > 0.80 and ≤ 0.80) comparisons were performed with paired t test or 1-way analysis of variance for means if the data were normally distributed or with Mann–Whitney U test or Kruskal–Wallis test if the data were not normally distributed. Fisher’s or χ2 test was used to analyse the categorical data. Associations between distal FFRCT, ΔFFRCT, and cardiac parameters were assessed by Pearson correlation analysis. Multivariable linear regression analyses were performed to examine the independent correlates between distal FFRCT, ΔFFRCT, and cardiac parameters. Receiver operating characteristic curves and their area under the curve were estimated based on Mann–Whitney U test, and the null hypothesis on the area under the curve C (H0: area under the curve = 0.5) was tested. Receiver operating characteristics curves were generated to determine the cut-off value of the highest diagnostic performance of an FFRCT ≤ 0.80 at the distal vessel. Intra-observer (T.T.) and inter-observer (T.T. and K.T.) agreement was assessed in 15 randomly selected subjects using Bland–Altman analyses. p < 0.05 was considered statistically significant. All statistical analyses were performed with JMP 11.0 statistical software (SAS Institute) or R version 4.4.2 (R Foundation for Statistical Computing).
Results
Physical, CT acquisition condition, FFRCT, and vessel characteristics
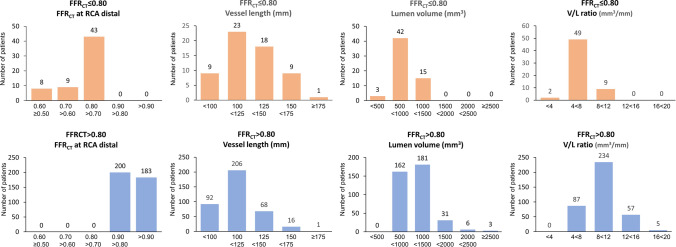
Among 443 patients, 383 patients (86%) had FFRCT > 0.80, whereas 60 patients (14%) showed a positive FFRCT even in NOCAD in the RCA (Fig. 3). Table 1 summarises the physical, CT acquisition condition, FFRCT, vessel, and myocardial characteristics in the RCA in our study population. Physical characteristics did not differ between FFRCT ≤ 0.80 and > 0.80. The time phase in the cardiac cycle during CT images could be acquired at the mid-diastolic phase in all patients. Proximal FFRCT was identical between FFRCT ≤ 0.80 and > 0.80, but distal FFRCT (0.72 ± 0.09 vs. 0.90 ± 0.04, p < 0.0001) and ΔFFRCT (0.28 ± 0.09 vs. 0.10 ± 0.04, p < 0.0001) were significantly lower in FFRCT ≤ 0.80 than in > 0.80 (Fig. 4, Table 1, and Supplementary Table 1). Vessel length did not differ between FFRCT ≤ 0.80 and > 0.80 (118.3 ± 22.3 vs. 113.4 ± 17.4 mm, p = 0.11). Proximal vessel diameter, distal vessel diameter, lumen volume (825.7 ± 255.0 vs. 1113.0 ± 337.9 mm3, p < 0.0001), and V/L ratio (6.5 ± 1.6 vs. 9.9 ± 2.4 mm3/mm, p < 0.0001) were significantly lower in FFRCT ≤ 0.80 than in > 0.80. Vessel length was almost the same, but vessel morphology (proximal vessel diameter, distal vessel diameter, lumen volume, and V/L ratio) was significantly lower in FFRCT ≤ 0.80. Distal FFRCT was lower in ≤ FFRCT > 0.80. LAP volume was almost the same comparing FFRCT ≤ 0.80 and > 0.80, but IAP and CP volume were higher in FFRCT ≤ 0.80 (Table 1).
Fig. 3.
Distribution of FFRCT and vessel morphology
Table 1.
Physical, CT acquisition condition, FFRCT, vessel, and myocardial characteristics in the right coronary artery
| All n = 443 |
FFRCT ≤ 0.80 n = 60 |
FFRCT > 0.80 n = 383 |
|
|---|---|---|---|
| Physical characteristics | |||
| Age (years) | 67.2 ± 9.9 | 69.3 ± 7.8 | 66.9 ± 10.2 |
| Men, n (%) | 304 (69%) | 36 (60%) | 310 (81%) |
| Height (cm) | 172.6 ± 9.5 | 171.4 ± 11.2 | 172.8 ± 9.2 |
| Body weight (kg) | 81.0 ± 15.2 | 77.7 ± 17.2 | 81.5 ± 14.8 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.9 ± 0.3 | 1.9 ± 0.2 |
| Body mass index (kg/m2) | 27.1 ± 4.3 | 26.2 ± 4.3 | 27.2 ± 4.3 |
| Hypertension, n (%) | 92 (51%) | 11 (58%) | 81 (51%) |
| Dislipidemia, n (%) | 73 (41%) | 8 (42%) | 65 (41%) |
| Diabetes, n (%) | 33 (19%) | 5 (26%) | 28 (18%) |
| Current smoking, n (%) | 57 (32%) | 7 (35%) | 50 (31%) |
| CT images acquisition condition | |||
| Time phase in the cardiac cycle during CT imaging | 75.0 ± 5.4 | 74.7 ± 4.6 | 75.1 ± 5.5 |
| Heart rate (beats/min) | 60.3 ± 8.6 | 62.5 ± 9.2 | 59.9 ± 8.5 |
| Atrial fibrillation, n (%) | 46 (10%) | 7 (12%) | 39 (10%) |
| Systolic blood pressure (mmHg) | 143.8 ± 19.8 | 147.2 ± 10.9 | 142.3 ± 19.3 |
| Diastolic blood pressure (mmHg) | 83.1 ± 12.1 | 87.8 ± 12.8 | 82.4 ± 11.9 |
| Interval between FFRCT and invasive coronary angiography (days) | 22.0 ± 18.4 | 21.0 ± 15.0 | 22.1 ± 18.8 |
| FFRCT characteristics | |||
| Proximal FFRCT | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Distal FFRCT | 0.88 ± 0.08 | 0.72 ± 0.09† | 0.90 ± 0.04 |
| ΔFFRCT | 0.12 ± 0.08 | 0.28 ± 0.09† | 0.10 ± 0.04 |
| Vessel morphology | |||
| Vessel length (mm) | 114.0 ± 18.1 | 118.3 ± 22.3 | 113.4 ± 17.4 |
| Lumen volume (mm3) | 1074.0 ± 342.3 | 825.7 ± 255.0† | 1113.0 ± 337.9 |
| Proximal vessel diameter (mm) | 4.5 ± 1.0 | 3.9 ± 0.8† | 4.6 ± 1.0 |
| Distal vessel diameter (mm) | 2.8 ± 0.6 | 2.4 ± 0.5† | 2.9 ± 0.6 |
| V/L ratio (mm3/mm) | 9.4 ± 2.6 | 6.5 ± 1.6† | 9.9 ± 2.4 |
| LAP volume (mm3) | 15.6 ± 18.5 | 18.6 ± 18.0 | 15.1 ± 18.6 |
| IAP volume (mm3) | 156.6 ± 174.9 | 224.7 ± 194.5† | 145.9 ± 169.4 |
| CP volume (mm3) | 29.6 ± 68.2 | 67.7 ± 89.9† | 23.6 ± 62.2 |
| Myocardial morphology | |||
| LV mass (g) | 109.6 ± 31.9 | 107.6 ± 31.2 | 109.8 ± 32.0 |
| LV mass index (g/m2) | 56.0 ± 14.6 | 57.4 ± 15.8 | 55.8 ± 14.4 |
CP, calcified plaque; IAP, intermediate-attenuation plaque; LAP, low-attenuation plaque; LV, left ventricular
*p < 0.05 vs. FFRCT > 0.80. †p < 0.01 vs. FFRCT > 0.80
Fig. 4.
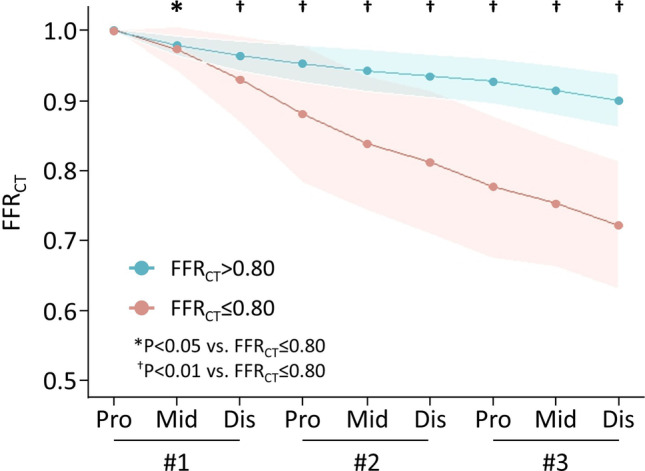
Changes in FFRCT between FFRCT ≤ 0.80 and > 0.80
Effects of RCA branches on FFRCT
To investigate the effects of RCA branches on FFRCT, changes in FFRCT were compared in the following groups: (1) presence of both right ventricular branch and acute marginal branch confirmed by FFRCT (n = 305); (2) presence of right ventricular branch confirmed by FFRCT (n = 99); (3) presence of acute marginal branch confirmed by FFRCT (n = 28); (4) absence of both right ventricular branch and acute marginal branch confirmed by FFRCT (n = 11). Distal FFRCT (group 1, 0.87 ± 0.08; group 2, 0.88 ± 0.08; group 3, 0.88 ± 0.06, and group 4, 0.89 ± 0.03) and ΔFFRCT (group 1, 0.13 ± 0.08; group 2, 0.12 ± 0.08; group 3, 0.12 ± 0.06, and group 4, 0.11 ± 0.03) did not significantly differ between the four groups (Supplementary Table 2).
Univariate and multivariate analysis of the relationship between FFRCT and vessel morphology
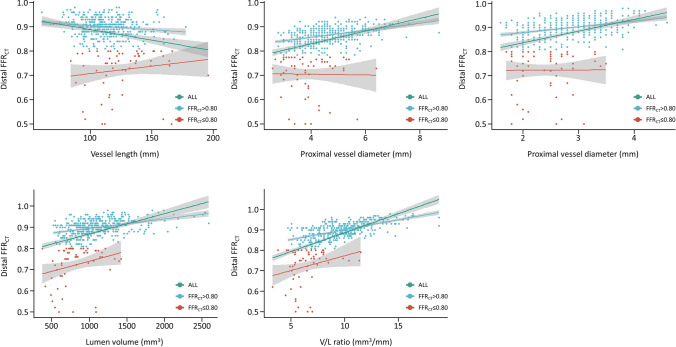
In all patients (FFRCT ≤ 0.80 and > 0.80), distal FFRCT and ΔFFRCT were correlated with vessel-related parameters (proximal and distal vessel diameters, vessel length, lumen diameter, and V/L ratio) and plaque-related parameters (LAP, IAP, and CP volume). Among all vessel-related parameters, V/L ratio showed the highest correlation with distal FFRCT (r = 0.61, p < 0.0001) (Fig. 5 and Supplementary Table 3) and ΔFFRCT (r = − 0.61, p < 0.0001) (Supplementary Fig. 1 and Supplementary Table 4). Multivariable analysis showed that CP volume was the strongest predictor of distal FFRCT (β-coefficient = − 0.12, p = 0.01), followed by V/L ratio (β-coefficient = 0.48, p = 0.03) (Table 2). Furthermore, as for ΔFFRCT, CP plaque volume (β-coefficient = 0.12, p = 0.01) was the strongest predictor, and was followed by V/L ratio (β-coefficient = − 0.48. p = 0.03) (Supplementary Table 5). Among vessel-related parameters, V/L ratio of 8.1 mm3/mm had the highest diagnostic performance for an FFRCT at distal RCA ≤ 0.80 (AUC = 0.88, 95% CI 0.84–0.93, p < 0.0001, sensitivity 90.0%, specificity 76.7%) (Fig. 6 and Supplementary Table 6).
Fig. 5.
Relationship between distal FFRCT and vessel morphology
Table 2.
Univariable and multivariable analysis for distal FFRCT
| Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | t-value | p-value | β | 95% CI | t-value | p-value | |
| Vessel length | − 0.22 | − 0.001 to − 0.0005 | − 4.70 | < 0.0001 | ||||
| Lumen volume | 0.42 | 0.00008 to 0.001 | 9.82 | < 0.0001 | ||||
| Proximal vessel diameter | 0.36 | 0.02 to 0.04 | 8.18 | < 0.0001 | 0.09 | 0.0002 to 0.01 | 2.02 | 0.04 |
| Distal vessel diameter | 0.37 | 0.04 to 0.06 | 8.37 | < 0.0001 | ||||
| V/L ratio | 0.61 | 0.016 to 0.02 | 16.30 | < 0.0001 | 0.48 | 0.001 to 0.03 | 2.14 | 0.03 |
| LAP volume | − 0.16 | − 0.0003 to − 0.04 | − 3.30 | 0.001 | ||||
| IAP volume | − 0.17 | − 0.0001 to 0.00003 | − 3.51 | 0.0005 | ||||
| CP volume | − 0.19 | − 0.0003 to − 0.0001 | − 4.09 | < 0.0001 | − 0.12 | − 0.0002 to − 0.00003 | − 2.54 | 0.01 |
| LV mass index | − 0.07 | − 0.0009 to 0.0002 | − 1.32 | 0.19 | ||||
CI, confidence interval; CP, calcified plaque; IAP, intermediate-attenuation plaque; LAP, low-attenuation plaque; LV, left ventricular
Fig. 6.
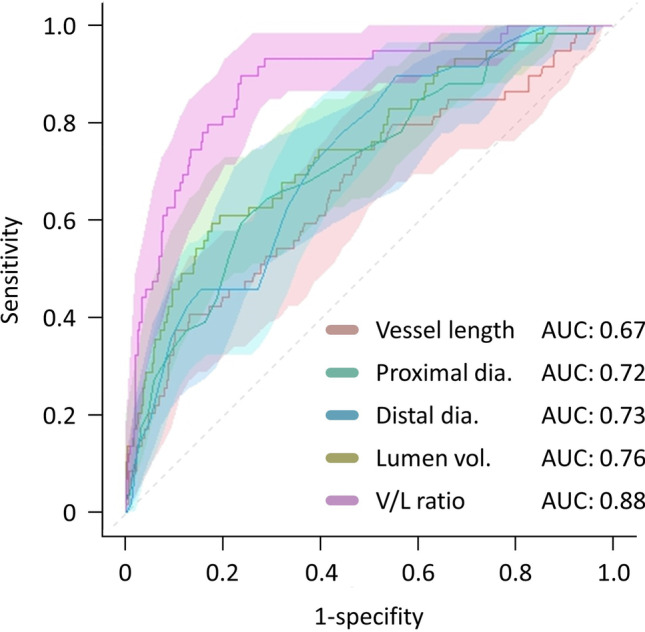
Receiver operating curve of vessel morphology for predicting distal FFRCT. dia., diameter; vol., volume
Repeatability and reproducibility
Intra-observer and inter-observer variability for vessel-related parameters and myocardium-related parameters is summarised in Supplementary Figs. 2 and 3. Intra-observer and inter-observer analyses showed good repeatability and reproducibility.
Discussion
In patients with an ICA showing no or minimal CAD in the RCA, our study highlighted the following: (1) vessel length was almost the same in FFRCT ≤ 0.80 and > 0.80, but lumen volume and V/L ratio were significantly lower in the FFRCT ≤ 0.80 group; (2) FFRCT correlated with plaque-related parameters (LAP, IAP, and CP) and vessel-related parameters (proximal and distal vessel diameter, vessel length, lumen volume, and V/L ratio); (3) of all parameters (plaque-related parameters and vessel related-parameters), the strongest predictor of a distal FFRCT value was CP volume followed by V/L ratio and an optimal cut-off value of 8.1 mm3/mm. Our study reports for the first time that FFRCT decline depends on V/L ratio in patients with NOCAD in the RCA.
The progressive and continuous FFRCT [3, 4] and invasive FFR [22] decline from the proximal to the distal segments of the vessel even in NOCAD is a commonly observed radiological finding. In our study, 60 patients (14%) with NOCAD had FFRCT < 0.80. Physiological FFRCT decline was dependent on iodine contrast attenuation and the full algorithm remains unclear for commercial reasons. However, FFRCT hemodynamics have been reported to be influenced by various factors The degree of FFRCT decline was not hemodynamically uniform in major three vessels and that RCA was smaller than LAD and LCX. This finding may be related to energy loss due to turbulence by the bifurcation from the left main trunk to LAD and LCX, or splitting into large branches such as diagonal branch, obtuse marginal branch, or posterior descending branch. Our previous study on the ramus artery demonstrated that the presence of a large ramus artery yields energy loss due to turbulence around the bifurcation angle, resulting in distal FFRCT decline in both LAD and LCX [23, 24]. Turbulent eddies generated by the presence of large branches could contribute to energy loss, resulting in impact on FFRCT hemodynamics. RCA may also have side branches such as the right ventricular branch or acute marginal branch (Supplementary Table 2). Turbulence flow generated in the RCA side branches was small and unlikely to affect FFRCT. Therefore, RCA with no major branches was included in the present study.
The finding that the degree of FFRCT decline depended on vessel length was consistent with previous reports [3, 4]. Interestingly, even though vessel length was almost the same between FFRCT ≤ 0.80 and > 0.80, proximal/distal vessel diameters, lumen volume, and V/L ratio were significantly lower in FFRCT ≤ 0.80. Vessel morphology including proximal/distal vessel diameters, lumen volume, and V/L ratio may have the potential to play an important role in physiological FFRCT hemodynamics. Several studies have reported the effects of vessel morphology on FFRCT from two perspectives: luminal cross-sectional area (2D) and vessel volume (3D). At the 2D level (luminal cross-sectional area), Collet et al [11] reported that FFRCT was significantly correlated with the luminal cross-sectional area. At the 3D level (lumen volume), Holmes et al [12] reported that increase in homogeneous lumen volume caused by the vasodilator agent of nitroglycerin resulted in FFRCT elevation. According to Hagen-Poiseuille flow, the change in pressure is proportional to the length, and the volumetric flow rate, and inversely proportional to the fourth power of the lumen diameter radius [25]. Baumgartner et al [26] reported that positive remodelling which was heterogeneous vasodilatation was associated with an increase in FFRCT. The streamlined vessel morphology caused by positive remodelling leads to pressure recovery phenomena, which may be responsible for the FFRCT elevation. Tapering as a morphological change of the vessel might contribute to FFRCT decline. A small distal vessel diameter was related to FFRCT ≤ 0.80 at the distal segment [9]. Our results of multivariable analysis were consistent with the results of previous studies. Of the vessel morphology-related parameters, V/L ratio was the most predictive factor for distal FFRCT. Hence, a thinner and longer vessel contributes to a larger FFRCT decline. Conversely, a shorter and thicker vessel causes a smaller FFRCT decline.
Differences between FFRCT ≤ 0.80 and > 0.80 could be attributed to hemodynamic stress. FFRCT depends on coronary artery flow. Coronary artery flow is determined by hemodynamic stress following three factors: (1) wall shear stress; (2) perfusion pressure; (3) vascular resistance. Of these factors, wall shear stress is affected by vessel diameter (inversely related to vessel diameter). Wall shear stress generates nitric oxide, which increases coronary artery flow, resulting in greater pressure drop and FFRCT decline [27]. In our study, FFRCT ≤ 0.80 group tended to have smaller vessel diameters than > 0.80, which might contribute to high shear stress and small FFRCT values. To our knowledge, this is the first report to investigate the effect of the ratio of lumen volume to vascular length on FFRCT. The addition of a novel parameter, V/L ratio assessment in addition to the conventional FFRCT interpretation may have the potential to overcome some drawbacks of FFRCT. Decision making based on FFRCT values at distal coronary artery segments should be performed with the greatest caution and by a thorough consideration of lumen volume.
Limitations
Our study has some limitations. First, invasive FFR was not performed because included patients were NOCAD. Since FFRCT is highly concordant with invasive FFR, it can be used as an alternative testing method [28–30]. Our study excluded a history of old myocardial infarction, but may not have completely ruled out the effects of left coronary artery disease. Old myocardial infarction causes left ventricular (LV) dysfunction, resulting in reduced left ventricular myocardial–related parameters such as LV wall thickness or LV mass. Fairbairn et al reported that the ratio of lumen volume to LV mass (V/M ratio) and FFRCT were inversely related. In our previous study, LV mass index was the most influential factor on FFRCT among LV myocardial-related parameters, with a cut-off value of 66.5 g/m2. Furthermore, LV dysfunction due to old myocardial infarction or heart failure causes an increase in LV end-diastolic pressure or low cardiac output, resulting in a decrease in right ventricular perfusion. In the setting of low right ventricular perfusion, the pressure drop is lower than normally expected coronary blood flow, resulting in higher FFRCT values. Collateral circulation generates unusual coronary hemodynamics from donor artery perfusion. FFRCT values depend on the donor and recipient arteries balance (Supplementary Fig. 4). In our study, patients with old myocardial infarction or heart failure were excluded, and it is unlikely that right ventricular perfusion would be compromised due to low cardiac output. LV mass index was below the threshold to affect FFRCT hemodynamics and was not a strong predictor of distal FFRCT and Δ FFRCT in multivariate analysis. Furthermore, there was no evidence of collateral circulation. Therefore, left coronary artery disease was unlikely to affect FFRCT in the RCA. Second, this study focused on only RCA vessels to avoid the effects of energy loss due to the bifurcation branches. Our results should be confirmed with all vessels including left coronary arteries. Third, despite the fact that FFRCT is affected by plaque volume [3, 31–33], this study did not completely eliminate plaque volume even though only NOCAD were selected. However, it is controversial to investigate FFRCT in simulated vessel models that completely eliminates the effects of plaque characteristics. Simulated vessel models are assumed to have a rigid wall rather than an elastic wall; therefore, the simulation does not reflect the physiological situation [34].
Conclusions
A novel marker for the predictor of distal FFRCT, V/L ratio is useful in assessing the physiological changes in subclinical perfusion disturbance in coronary arteries.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CAD
Coronary artery disease
- CAD-RADS
CAD reporting and data system
- CP
Calcified plaque
- CTA
CT angiography
- FFR
Fractional flow reserve
- FFRCT
Computed tomography–derived fractional flow reserve
- HU
Hounsfield units
- IAP
Intermediate-attenuation plaque
- ICA
Invasive coronary angiography
- LAD
Left anterior descending artery
- LAP
Low-attenuation plaque
- LCX
Left circumflex artery
- LV
Left ventricular
- NOCAD
Non-obstructive CAD
- RCA
Right coronary artery
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Johan De Mey.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Universitair Ziekenhuis Brussel Institutional Review Board (B.U.N. 143202000302) approval was obtained.
Study subjects or cohorts overlap
None.
Methodology
retrospective
observational
performed at one institution
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cook CM, Petraco R, Shun-Shin MJ, et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol. 2017;2:803–810. doi: 10.1001/jamacardio.2017.1314. [DOI] [PubMed] [Google Scholar]
- 2.Nous FMA, Budde RPJ, Lubbers MM, et al. Impact of machine-learning CT-derived fractional flow reserve for the diagnosis and management of coronary artery disease in the randomized CRESCENT trials. Eur Radiol. 2020;30:3692–3701. doi: 10.1007/s00330-020-06778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsugu T, Tanaka K, Belsack D, et al. Impact of vascular morphology and plaque characteristics on computed tomography derived fractional flow reserve in early stage coronary artery disease. Int J Cardiol. 2021;343:187–193. doi: 10.1016/j.ijcard.2021.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Cami E, Tagami T, Raff G, et al. Assessment of lesion-specific ischemia using fractional flow reserve (FFR) profiles derived from coronary computed tomography angiography (FFRCT) and invasive pressure measurements (FFRINV): importance of the site of measurement and implications for patient referral for invasive coronary angiography and percutaneous coronary intervention. J Cardiovasc Comput Tomogr. 2018;12:480–492. doi: 10.1016/j.jcct.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Tsugu T, Tanaka K, Nagatomo Y et al (2023) Impact of coronary bifurcation angle on computed tomography derived fractional flow reserve in coronary vessels with no apparent coronary artery disease. Eur Radiol 33:1277–1285 [DOI] [PMC free article] [PubMed]
- 6.Tsugu T, Tanaka K (2022) Differences in fractional flow reserve derived from coronary computed tomography angiography according to coronary artery bifurcation angle. Turk Kardiyol Dern Ars 50:83–84 [DOI] [PubMed]
- 7.Tsugu T, Tanaka K, Belsack D, et al. Effects of left ventricular mass on computed tomography derived fractional flow reserve in significant obstructive coronary artery disease. Int J Cardiol. 2022;355:59–64. doi: 10.1016/j.ijcard.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Tsugu T, Tanaka K, Belsack D, Jean-Francois A, Mey J. Impact of collateral circulation with fractional flow reserve derived from coronary computed tomography angiography. Turk Kardiyol Dern Ars. 2021;49:694–695. doi: 10.5543/tkda.2021.04567. [DOI] [PubMed] [Google Scholar]
- 9.Gassenmaier S, Tsiflikas I, Greulich S, et al. Prevalence of pathological FFRCT values without coronary artery stenosis in an asymptomatic marathon runner cohort. Eur Radiol. 2021;31:8975–8982. doi: 10.1007/s00330-021-08027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodge JT, Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. doi: 10.1161/01.CIR.86.1.232. [DOI] [PubMed] [Google Scholar]
- 11.Collet C, Katagiri Y, Miyazaki Y, et al. Impact of coronary remodeling on fractional flow reserve. Circulation. 2018;137:747–749. doi: 10.1161/CIRCULATIONAHA.117.031478. [DOI] [PubMed] [Google Scholar]
- 12.Holmes KR, Fonte TA, Weir-McCall J, et al. Impact of sublingual nitroglycerin dosage on FFRCT assessment and coronary luminal volume-to-myocardial mass ratio. Eur Radiol. 2019;29:6829–6836. doi: 10.1007/s00330-019-06293-7. [DOI] [PubMed] [Google Scholar]
- 13.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) coronary artery disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach S, Rudolph T, Rieber J, et al. Performing and interpreting fractional flow reserve measurements in clinical practice: an expert consensus document. Interv Cardiol. 2017;12:97–109. doi: 10.15420/icr.2017:13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairbairn TA, Dobson R, Hurwitz-Koweek L, et al. Sex differences in coronary computed tomography angiography-derived fractional flow reserve: lessons from ADVANCE. JACC Cardiovasc Imaging. 2020;13:2576–2587. doi: 10.1016/j.jcmg.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Gaur S, Taylor CA, Jensen JM, et al. FFR derived from coronary CT angiography in nonculprit lesions of patients with recent STEMI. JACC Cardiovasc Imaging. 2017;10:424–433. doi: 10.1016/j.jcmg.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Nadjiri J, Hausleiter J, Jahnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 2016;10:97–104. doi: 10.1016/j.jcct.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 22.De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "normal" coronary angiography. Circulation. 2001;104:2401–2406. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 23.Tsugu T, Tanaka K, Nagatomo Y, Belsack D, De Maeseneer M, De Mey J. Paradoxical changes of coronary computed tomography derived fractional flow reserve. Echocardiography. 2022;39:398–403. doi: 10.1111/echo.15307. [DOI] [PubMed] [Google Scholar]
- 24.Tsugu T, Tanaka K, Nagatomo Y, et al. Impact of ramus coronary artery on computed tomography derived fractional flow reserve (FFR(CT) ) in no apparent coronary artery disease. Echocardiography 2023 Feb;40(2):103-112 . 2023;40:103–112. doi: 10.1111/echo.15526. [DOI] [PubMed] [Google Scholar]
- 25.Pfitzner J. Poiseuille and his law. Anaesthesia. 1976;31:273–275. doi: 10.1111/j.1365-2044.1976.tb11804.x. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner H, Stefenelli T, Niederberger J et al (1999) “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol 33:1665–1661 [DOI] [PubMed]
- 27.Urschel K, Tauchi M, Achenbach S, Dietel B (2021) Investigation of wall shear stress in cardiovascular research and in clinical practice-from bench to bedside. Int J Mol Sci 26;22:5635. [DOI] [PMC free article] [PubMed]
- 28.Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 29.Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015;36:3359–3367. doi: 10.1093/eurheartj/ehv444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min JK, Taylor CA, Achenbach S, et al. Noninvasive fractional flow reserve derived from coronary CT angiography: clinical data and scientific principles. JACC Cardiovasc Imaging. 2015;8:1209–1222. doi: 10.1016/j.jcmg.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Gaur S, Ovrehus KA, Dey D, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J. 2016;37:1220–1227. doi: 10.1093/eurheartj/ehv690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otaki Y, Han D, Klein E, et al. Value of semiquantitative assessment of high-risk plaque features on coronary CT angiography over stenosis in selection of studies for FFRct. J Cardiovasc Comput Tomogr. 2022;16:27–33. doi: 10.1016/j.jcct.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Driessen RS, Stuijfzand WJ, Raijmakers PG, et al. Effect of plaque burden and morphology on myocardial blood flow and fractional flow reserve. J Am Coll Cardiol. 2018;71:499–509. doi: 10.1016/j.jacc.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 34.Chaichana T, Sun Z, Jewkes J (2010) Computation of hemodynamics in the left coronary artery with variable angulations. J Biomech 44:1869–1878 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.