Abstract
Upon infection with Salmonella, a host develops an immune response to limit bacterial growth and kill and eliminate the pathogen. Salmonella has evolved mechanisms to remain dormant within the body, only to reappear (reactivate) at a later time when the immune system is abated. We have developed an in vivo model for studying reactivation of Salmonella enterica serovar Typhimurium infection in mice. Upon subcutaneous infection, C3H/HeN (Ityr) mice showed an increase in bacterial numbers in livers and spleens, which reached a peak on day 19. After full recovery from the infection, these mice were irradiated or depleted of CD4+ T cells. The mice displayed a secondary infection peak in livers and spleens with a course similar to that of the primary infection. We concluded that CD4+ T cells are involved in active suppression of S. enterica serovar Typhimurium during latency. The role of CD4+ T cells during primary infection with S. enterica serovar Typhimurium is well established. This is the first study to describe a role of CD4+ T cells during the latent phase of S. enterica serovar Typhimurium infection.
Salmonellae are gram-negative, facultative intracellular pathogens that can cause a range of diseases in both animals and humans that vary from mild diarrhea to severe infections such as typhoid fever. It predominantly invades mononuclear phagocytes, and despite antimicrobial mechanisms present in phagocytic cells, Salmonella is able not only to enter but to survive and even replicate within these cells. The bacterium can cause chronic or persistent infection by evasion of the host defense (13). This ability of Salmonella to replicate within phagocytic cells is essential for its survival, as mutants unable to do so are avirulent (5). Although the exact mechanisms for intracellular survival of Salmonella after phagocytosis are still uncertain, it is clear that Salmonella responds to the specific host environment by expressing factors crucial for intracellular survival (3, 6, 7, 13, 20).
Upon infection, the host mounts an immune response to limit bacterial growth and to eventually kill and eliminate the pathogen. B cells, T cells, and macrophages are important for host resistance and their protective effects are mediated by cytokines such as gamma interferon (IFN-γ), interleukin-12, and tumor necrosis factor alpha (4, 14, 16-18, 23). This integrated response results in activation of macrophages, which in turn kill the Salmonella. Although the macrophages are the main host cells, are necessary for survival and replication of Salmonella within the host, and mediate the Salmonella-induced pathology, macrophages also play a crucial role in host defense against Salmonella (27). They are necessary for the early local control of infection and, subsequently, for the induction of acquired immunity (10, 15), as well as for restriction of bacterial growth in immune mice (27).
Even in the presence of an acquired immune response, Salmonella has evolved mechanisms to persist within the body and reappear (reactivate) at a later time. Several studies and case reports have shown that patients who underwent total-body irradiation or received an organ transplant and were treated with glucocorticosteroids or other immunosuppressive drugs, as well as patients suffering from human immunodeficiency virus infection (11) or interleukin-12 receptor β1 deficiency (24), can suffer from recurrent infections with a Salmonella strain that persists within the host.
By investigating the possibility that S. enterica serovar Typhimurium persists and reactivates after immune intervention in a mouse model of latent S. enterica serovar Typhimurium infection, we aimed to gain insight into the mechanisms by which the host continually suppresses Salmonella from reactivating at a later time.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female Salmonella-resistant (Ityr) C3H/HeN mice were obtained from Harlan (Horst, The Netherlands). Mice were maintained according to institutional guidelines with water and food ad libitum in filter top cages which were opened only inside a laminar flow cabinet. Studies were carried out in accordance with and after approval of the animal research ethics committee of the Leiden University Medical Center.
Bacteria.
For in vivo infection experiments S. enterica serovar Typhimurium strain 14028s (50% lethal doses after intraperitoneal injection, 5 × 103 bacteria for Ityr mice and <102 for Itys mice) was grown to the end of the log phase and then washed and diluted in sterile phosphate-buffered saline (PBS). The number of CFU in the inoculum was determined microbiologically.
Antibodies.
Monoclonal antibodies (mAbs) directed to mouse T-cell surface antigen CD4 were obtained from supernatant of cultured hybridoma GK1.5 (rat anti-mouse CD4; American Type Culture Collection). The hybridoma was cultured in protein-free medium (Gibco), and supernatant was concentrated with a capillary dialyzer, filter sterilized, and stored at −20°C. Fluorescein isothiocyanate-conjugated rat anti-mouse CD4 (L3T4) and phycoerythrin-conjugated rat anti-mouse CD8 (Ly-2) monoclonal antibodies were obtained from BD Biosciences.
S. enterica serovar Typhimurium infection.
Mice were inoculated subcutaneously in the flanks with 0.1 ml of a bacterial suspension containing 3 × 104 CFU of S. enterica serovar Typhimurium 14028s. For each group at each time three or four mice were used. Mice were sacrificed by carbon dioxide inhalation, and blood was taken by cardiac puncture. Spleens, livers, and inguinal lymph nodes were removed, single-cell suspensions were prepared by using sterile 70-μm-mesh-size cell strainers (Falcon), and lysates were made. The number of bacteria per organ was determined microbiologically by plating serial dilutions of the lysates. The lowest numbers of bacteria that could be detected in this way were 30 CFU for the spleens and lymph nodes and 50 CFU for the livers.
Leukocyte count and blood cell differentiation.
Leukocyte numbers were determined by counting the number of nucleated cells in heparinized blood and by making blood smears for the differentiation of the blood cells. Blood smears were fixed in methanol and stained with Giemsa stain, and relative percentages of the different types of blood cells were determined. By combining the data from leukocyte counts and blood smears, we calculated the numbers of lymphocytes, monocytes, and polymorphonuclear leukocytes present in the blood at the different times. The number of lymphocytes was used together with fluorescence-activated cell sorter (FACS) analysis data to calculate the number of CD4+ and CD8+ T cells.
Gamma irradiation and in vivo T-cell depletion.
For total -body gamma irradiation, mice were put in a small perspex box and were irradiated until a dose of 6 Gy was reached. For depletion of CD4+ T cells, mice were injected intraperitoneally (i.p.) with 200 μg of rat anti-CD4 GK1.5 antibody. Mice received second and third injections of 100 μg of this antibody on days 2 and 4 after the first injection. Infection controls were injected i.p. with an equal volume of PBS.
Flow cytometry.
To determine CD4+- and CD8+-T-cell counts in blood, 100 μl of heparinized blood was used. After the erythrocytes were lysed and washed with PBS, the cells were labeled for 30 min with fluorescein isothiocyanate-conjugated rat anti-mouse CD4 mAb and phycoerythrin-conjugated rat anti-mouse CD8 mAb. Flow cytometry was performed with a FACSCaliber system (Becton Dickinson).
Detection of S. enterica serovar Typhimurium-specific antibodies.
Induction of S. enterica serovar Typhimurium-specific antibodies was determined by a whole-cell enzyme-linked immunosorbent assay (ELISA) (25). Maxisorp plates (Nunc) were coated with S. enterica serovar Typhimurium 14028s, and serial dilutions of the sera were added after nonspecific binding was blocked. Sera from naïve mice were included as a control. Wavelength absorbance was measured at 490 nm using an ELISA plate reader (VICTOR2 1420 multilabel counter; Perkin-Elmer Life and Analytical Sciences). The titer was defined as the dilution for which the optical density at 450 (OD450) of the sample was more than the OD450 of the naïve serum plus 2 standard deviations.
Statistical analysis.
For comparison between treatments we used Student's t tests for CD4+- and CD8+-T-cell counts and Mann-Whitney rank order tests for CFU counts. For all analyses, a P value of <0.05 was considered significant.
RESULTS
Replication of S. enterica serovar Typhimurium 14028s during a primary infection in C3H/HeN mice.
Mice were inoculated subcutaneously in the flanks with 3 × 104 CFU of S. enterica serovar Typhimurium 14028s and showed an infection comparable to that described previously (26). S. enterica serovar Typhimurium 14028s was detectable in the lymph nodes on day 1 after infection; from there the infection spread to the spleens and livers, and within these organs bacterial loads of up to 3 × 107 CFU per organ were reached. The bacterial loads were highest on day 19. The bacterial loads eventually declined, which coincided with reductions in the spleen and liver weights (data not shown).
Reactivation of the S. enterica serovar Typhimurium infection by gamma irradiation.
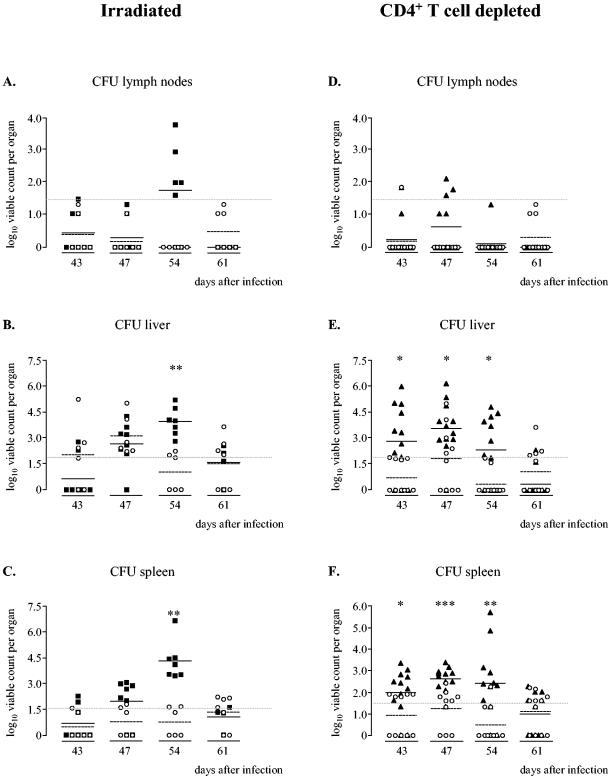
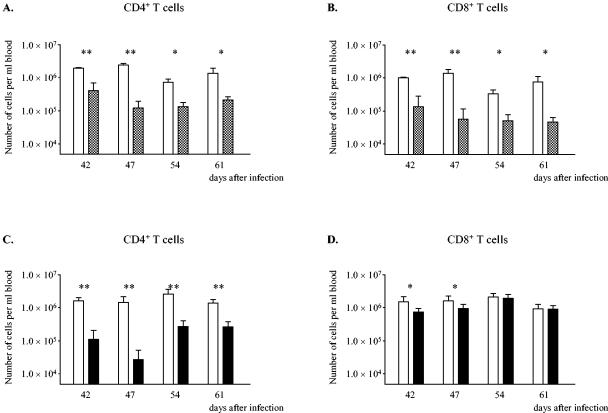
On day 41, when the bacterial loads in the organs were below the detection limit, the mice received sublethal total-body irradiation (6 Gy). Infection controls were not treated. The irradiated mice showed signs of illness, like ruffled fur and malaise, between days 54 and 61. The effects of the irradiation on the bacterial numbers in the organs are shown in Fig. 1. In the infection control group, 60% of the mice showed detectable amounts of bacteria in the livers (Fig. 1B), but the averages were around or below the detection limit and no increase in bacterial numbers could be observed. The bacterial numbers in the lymph nodes and spleens stayed below or around the detection limits (Fig. 1A and C). In the irradiated mouse population, on the other hand, we observed increases in the numbers of S. enterica serovar Typhimurium cells in the livers and spleens (Fig. 1B and C) upon immune intervention, and in all the mice the infection peaked on day 54. This secondary infection (i.e., reactivation) was milder than the primary infection, as shown by the lower maximal bacterial numbers in the organs reached, but otherwise it followed a course that was similar to that of the primary infection peak (Fig. 1). The irradiated mice showed reduced leukocyte counts in the blood as soon as day 1 after the irradiation (day 42) compared to the untreated infection controls, and the numbers remained lower up to day 61 after infection (Table 1). Table 1 and Fig. 2A and B also show that the numbers of granulocytes and CD4+ and CD8+ T cells declined in the irradiated mice.
FIG. 1.
Bacterial loads within the lymph nodes (A), livers (B), and spleens (C) of mice infected with S. enterica serovar Typhimurium 14028s that were irradiated on day 41 (▪) and untreated infection controls (○) and bacterial loads within the lymph nodes (D), livers (E), and spleens (F) of mice depleted of CD4+ T cells (▴) and infection controls (○). On days 39, 41, and 43 after infection, the mice were injected i.p. with 200 μg, 100 μg, and 100 μg of rat anti-CD4 GK1.5 antibody, respectively. The infection controls were injected i.p. with an equal volume of PBS. At different times, livers, spleens, and lymph nodes were aseptically removed, and cell lysates were made. The viable counts in the organs were determined by plating serial dilutions of the cell lysates and are expressed as log10 viable counts (means ± standard errors of the means). Data from two independently performed experiments are shown. Asterisks indicate statistically significant differences compared to the infection controls (one asterisk, P < 0.05; two asterisks, P < 0.005; Mann-Whitney rank order test), and the gray dashed lines indicate the detection limits of the microbiological method (50 CFU for the livers and 30 CFU for the spleens and lymph nodes).
TABLE 1.
Numbers of leukocytes, lymphocytes, monocytes, and granulocytes in the blooda
| Day | Treatment | No. (105) of cells/ml
|
|||
|---|---|---|---|---|---|
| Leukocytes | Lymphocytes | Monocytes | Granulocytes | ||
| 1 | None | 54.7 ± 8.3 | 38.7 ± 12.9 | 1.1 ± 0.5 | 14.3 ± 8.9 |
| 5 | None | 85.1 ± 13.5 | 48.3 ± 9.4 | 1.8 ± 1.4 | 29.9 ± 7.3 |
| 12 | None | 77.3 ± 24.4 | 30.4 ± 12.7 | 0.8 ± 0.7 | 42.3 ± 13.6 |
| 19 | None | 100.7 ± 28.5 | 33.8 ± 13.8 | 2.8 ± 2.2 | 52.2 ± 13.1 |
| 26 | None | 78.0 ± 13.4 | 39.3 ± 5.6 | 1.2 ± 1.0 | 33.8 ± 11.7 |
| 33 | None | 110.8 ± 37.6 | 52.2 ± 10.3 | 1.8 ± 1.9 | 53.3 ± 29.2 |
| 42 | None | 86.2 ± 29.7 | 53.7 ± 18.0 | 0.7 ± 0.7 | 27.9 ± 14.2 |
| irrb | 23.5 ± 9.4 | 7.4 ± 3.8 | 0.0 ± 0.0 | 15.5 ± 6.3 | |
| 43 | deplc | 50.2 ± 11.3 | 26.3 ± 6.9 | 1.1 ± 0.9 | 21.6 ± 13.0 |
| 47 | None | 87.8 ± 23.9 | 58.5 ± 20.6 | 1.0 ± 0.5 | 24.7 ± 7.6 |
| irr | 9.1 ± 4.4 | 5.1 ± 2.6 | 0.2 ± 0.2 | 3.1 ± 2.3 | |
| depl | 61.1 ± 21.2 | 31.4 ± 11.8 | 1.5 ± 1.6 | 26.2 ± 17.1 | |
| 54 | None | 50.8 ± 29.0 | 29.0 ± 22.4 | 0.6 ± 0.7 | 19.3 ± 10.2 |
| irr | 10.6 ± 5.1 | 5.8 ± 3.0 | 0.1 ± 0.1 | 2.8 ± 3.4 | |
| depl | 89.6 ± 21.1 | 39.0 ± 16.2 | 2.1 ± 1.4 | 39.0 ± 16.2 | |
| 61 | None | 94.6 ± 24.1 | 45.6 ± 14.3 | 1.0 ± 1.1 | 41.9 ± 27.4 |
| irr | 29.5 ± 7.6 | 8.7 ± 2.2 | 0.2 ± 0.2 | 15.8 ± 4.5 | |
| depl | 55.0 ± 14.2 | 29.2 ± 8.5 | 0.7 ± 0.4 | 23.2 ± 11.9 | |
Values are means ± standard deviations.
irr, mice received total-body irradiation (6 Gy).
depl, mice were depleted of CD4+ T cells by injection of anti-CD4 antibodies.
FIG. 2.
Numbers of CD4+ T cells (A and C) and CD8+ T cells (B and D) per ml of blood of C3H/HeN control mice infected with S. enterica serovar Typhimurium (open bars), mice that received total-body irradiation (cross-hatched bars), and mice that were depleted of CD4+ T cells (solid bars). The numbers of CD4+ and CD8+ T cells were calculated by combining data from leukocyte counts, cell differentiation analysis, and FACS analysis. Two asterisks indicate that the P value is <0.005, and one asterisk indicates that the P value is <0.05.
These results suggest that the observed reactivation of the S. enterica serovar Typhimurium infection upon irradiation could have been due to the reduction in the numbers of either granulocytes, CD4+ T lymphocytes, CD8+ T lymphocytes, or a combination of cells.
Reactivation following T-cell depletion.
Reactivation of a latent S. enterica serovar Typhimurium infection in people has been described for patients suffering from AIDS. This strongly suggests a role for the CD4+ T cells in the suppression of S. enterica serovar Typhimurium during the persistence phase. Since the irradiated mice also showed a reduction in granulocytes and CD8+ T cells, we wondered whether reducing the number of CD4+ T cells alone by in vivo depletion could also result in the reactivation of a latent S. enterica serovar Typhimurium infection in C3H/HeN mice. In the infection control group we observed no reactivation of the infection, as the bacterial numbers stayed around or below the detection limits in all the organs up to day 61. In the lymph nodes of the mice that were depleted of CD4+ T cells, we observed no detectable outgrowth of S. enterica serovar Typhimurium (Fig. 1D). In the livers and spleens, on the other hand, we observed increases in bacterial numbers that were significantly different from those in the infection controls, and the reactivation reached a peak on day 47 (Fig. 1E and F). As observed for the irradiated mice, this reactivation peak was lower than the peak observed for the primary infection, but it followed a course that was similar to that of the primary infection. FACS analysis of the lymphocyte population revealed a strong decrease in the number of CD4+ T cells in the depleted mice, indicating that the injection of the rat-anti CD4 antibody resulted in successful depletion of the CD4+-T-cell population (Fig. 2C) and, as expected, had little effect on the number of CD8+ T cells (Fig. 2D).
Anti-Salmonella immunoglobulin G (IgG) antibodies in the serum of S. enterica serovar Typhimurium-infected mice.
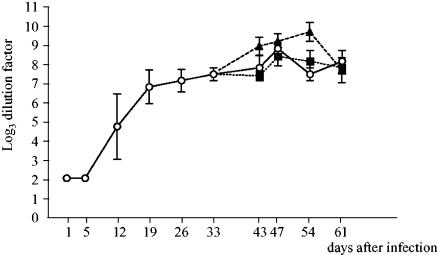
To determine whether the mice had antibodies to the pathogen, serum was collected from the mice that were sacrificed at each time, and anti-Salmonella IgG antibodies were detected using a whole-cell ELISA. The infected mice started to produce antibodies to S. enterica serovar Typhimurium 14028s between days 5 and 12, when the infection reached its peak in the lymph nodes and spleens. The titers increased further until day 43 after infection and then remained around a log3 dilution factor of 8.5 (Fig. 3). The mice that were irradiated or depleted of CD4+ T cells and that showed reactivation of the S. enterica serovar Typhimurium infection had serum antibody levels that were similar to those of the infection controls. Thus, despite the fact that these mice had serum antibodies to S. enterica serovar Typhimurium, they still showed reactivation of the S. enterica serovar Typhimurium infection.
FIG. 3.
Anti-Salmonella IgG antibodies in the serum of S. enterica serovar Typhimurium-infected mice that received no further treatment (○), received total-body irradiation (▪), or were depleted of CD4+ T cells (▴). The titer was defined as the dilution for which the optical density at 450 (OD450) of the sample was more than the OD450 of the naïve serum plus 2 standard deviations.
DISCUSSION
The main finding of the present study of reactivation of Salmonella after clearance of a primary systemic infection in C3H/HeN (Ityr) mice is that after total-body irradiation or selective CD4+-T-cell depletion, the numbers of bacteria in livers and spleens increased at a rate identical to that in the primary infection. The only difference between the outgrowth curves was that upon reactivation, S. enterica serovar Typhimurium infection was controlled more rapidly than it was in the primary infection (i.e., by about 2 instead of 3 weeks). The presence of Salmonella-specific antibodies did not prevent reactivation but may help explain the difference in outgrowth rates between irradiated and CD4+-T-cell-depleted mice, since antibodies act as opsonins for uptake by granulocytes which are not affected by CD4+-T-cell depletion.
We used subcutaneous S. enterica serovar Typhimurium infection of C3H/HeN (Ityr) mice in the inguinal region to set up a model for reactivation of S. enterica serovar Typhimurium infection. By infecting subcutaneously, a reservoir is established near draining lymph nodes, from which Salmonella spreads via the lymph and becomes systemic, reaching the liver and spleen (2). This model gives rise to a more subtle infection than the intraperitoneal or intravenous models that result in peracute and overwhelming infections. An advantage over oral infection is that subcutaneously injected bacteria can be dosed precisely, while in an oral infection the actual dose depends on the number of bacteria that pass through the stomach and cross the intestinal mucosa. Using subcutaneous infection, we set up a new in vivo model for reactivation of latent S. enterica serovar Typhimurium infection, in which total-body irradiation or in vivo depletion of CD4+ T cells in C3H/HeN (Ityr) mice that had fully recovered from a primary infection with S. enterica serovar Typhimurium resulted in outgrowth of bacteria that persisted within the body. In our reactivation model we accepted that some of the control mice still showed some low number of bacteria in the organs, just above the limit of detection. Otherwise, we would have needed many more animals to find only a few in which S. enterica serovar Typhimurium persisted and reactivated upon irradiation or T-cell depletion.
Reactivation of a latent S. enterica serovar Typhimurium infection in humans has been described in human immunodeficiency virus/AIDS patients (9), which (1, 8, 12) suggests a role for CD4+ T cells in the suppression of S. enterica serovar Typhimurium during persistence. This is supported by a study of Hung et al. in Taiwan showing that the risk of recurrent nontyphoidal Salmonella bacteremia decreased dramatically after the introduction of highly active antiretroviral therapy and coincides with recovery of CD4+-T-cell counts and reconstitution of immunity (11).
We investigated whether reducing the number of CD4+ T cells could result in reactivation of a (latent) S. enterica serovar Typhimurium infection. Like irradiated mice, CD4+-T-cell-depleted mice showed reactivation of S. enterica serovar Typhimurium infection in both livers and spleens. This reactivation occurred despite the presence of high titers of anti-S. enterica serovar Typhimurium antibodies (Fig. 3). This is consistent with the observation that protection against Salmonella requires both immune serum and T cells (19).
Recently, Monack et al. described a model for chronic carriage of S. enterica serovar Typhimurium in Ityr mice (21). In contrast to our latent infection model in which bacteria could no longer be detected in the lymph nodes after 43 days, these mice showed high numbers of bacteria in the mesenteric lymph nodes up until 268 days after oral infection and periodic fecal shedding, as observed for chronic carriers of S. enterica serovar Typhi and S. enterica serovar Paratyphi in humans. Monack et al. showed that IFN-γ plays an essential role in the control of chronically persistent S. enterica serovar Typhimurium infection, since neutralization resulted in reactivation (21). Neutralization of IFN-γ precludes activation of infected macrophages by all types of IFN-γ-producing cells and results in reactivation of S. enterica serovar Typhimurium infection. In our reactivation model of latent infection, however, we depleted mice of CD4+ T cells, precluding the production of IFN-γ by this type of cell, but the IFN-γ-producing NK and CD8+ T cells were still present (Fig. 2D). Apparently, the amounts of IFN-γ produced by these cells are not sufficient to appropriately activate macrophages and prevent reactivation. Our data, together with those described by Monack et al., indicate that IFN-γ produced by CD4+ T cells is necessary to suppress bacterial growth during the persistence phase and suggest that IFN-γ produced by NK cells and CD8+ T cells does not play a pivotal role in this respect.
It is generally accepted that CD4+ T cells play an important role in the clearance of bacteria during a primary infection with S. enterica serovar Typhimurium. Mice depleted of CD4+ T cells on the day of infection are highly susceptible to S. enterica serovar Typhimurium and rapidly die due to the lack of CD4+-T-cell-mediated defense against Salmonella (22). Our study is the first study that describes a role of CD4+ T cells in preventing reactivation of S. enterica serovar Typhimurium infection in Ityr mice during the persistence phase. The in vivo reactivation mouse model is suitable for further studies on reactivating S. enterica serovar Typhimurium infections and might provide insight into bacterial strategies that S. enterica serovar Typhimurium uses to persist in its host. Detailed knowledge of these mechanisms is necessary to develop new approaches for preventing relapsing infections with salmonellae in immunocompromised hosts.
Editor: A. D. O'Brien
REFERENCES
- 1.Burckhardt, B., P. Sendi, D. Pfluger, W. Zimmerli, R. Nuesch, H. C. Bucher, J. Drewe, N. Gyr, and M. Battegay. 1999. Rare AIDS-defining diseases in the Swiss HIV Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 18:399-402. [DOI] [PubMed] [Google Scholar]
- 2.Chen, Z. M., and M. K. Jenkins. 1999. Clonal expansion of antigen-specific CD4 T cells following infection with Salmonella typhimurium is similar in susceptible (Itys) and resistant (Ityr) BALB/c mice. Infect. Immun. 67:2025-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demple, B. 1991. Regulation of bacterial oxidative stress genes. Annu. Rev. Genet. 25:315-337. [DOI] [PubMed] [Google Scholar]
- 4.Everest, P., M. Roberts, and G. Dougan. 1998. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect. Immun. 66:3355-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay, B. B., and S. Falkow. 1989. Common themes in microbial pathogenicity. Microbiol. Rev. 53:210-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 8.Glaser, J. B., L. Morton-Kute, S. R. Berger, J. Weber, F. P. Siegal, C. Lopez, W. Robbins, and S. H. Landesman. 1985. Recurrent Salmonella typhimurium bacteremia associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 102:189-193. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, M. A., H. T. Banda, M. Gondwe, S. B. Gordon, M. J. Boeree, A. L. Walsh, J. E. Corkill, C. A. Hart, C. F. Gilks, and M. E. Molyneux. 2002. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633-1641. [DOI] [PubMed] [Google Scholar]
- 10.Hormaeche, C. E. 1979. The natural resistance of radiation chimeras to S. typhimurium C5. Immunology 37:329-332. [PMC free article] [PubMed] [Google Scholar]
- 11.Hung, C. C., S. M. Hsieh, C. F. Hsiao, M. Y. Chen, and W. H. Sheng. 2001. Risk of recurrent non-typhoid Salmonella bacteraemia after early discontinuation of ciprofloxacin as secondary prophylaxis in AIDS patients in the era of highly active antiretroviral therapy. AIDS 15:645-647. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson, M. A., S. M. Hahn, J. L. Gerberding, B. Lee, and M. A. Sande. 1989. Ciprofloxacin for Salmonella bacteremia in the acquired immunodeficiency syndrome (AIDS). Ann. Intern. Med. 110:1027-1029. [DOI] [PubMed] [Google Scholar]
- 13.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 14.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 15.Maskell, D. J., C. E. Hormaeche, K. A. Harrington, H. S. Joysey, and F. Y. Liew. 1987. The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microb. Pathog. 2:295-305. [DOI] [PubMed] [Google Scholar]
- 16.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastroeni, P., J. A. Harrison, J. A. Chabalgoity, and C. E. Hormaeche. 1996. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 64:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastroeni, P., J. N. Skepper, and C. E. Hormaeche. 1995. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 63:3674-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastroeni, P., B. Villarreal-Ramos, and C. E. Hormaeche. 1993. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect. Immun. 61:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monack, D. M., D. M. Bouley, and S. Falkow. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J. Exp. Med. 199:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nauciel, C. 1990. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 145:1265-1269. [PubMed] [Google Scholar]
- 23.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staretz-Haham, O., R. Melamed, M. Lifshitz, N. Porat, C. Fieschi, J. L. Casanova, and J. Levy. 2003. Interleukin-12 receptor beta1 deficiency presenting as recurrent Salmonella infection. Clin. Infect. Dis. 37:137-140. [DOI] [PubMed] [Google Scholar]
- 25.Van der Ley, P., H. Amesz, J. Tommassen, and B. Lugtenberg. 1985. Monoclonal antibodies directed against the cell-surface-exposed part of PhoE pore protein of the Escherichia coli K-12 outer membrane. Eur. J. Biochem. 147:401-407. [DOI] [PubMed] [Google Scholar]
- 26.Van Diepen, A., T. van Der Straaten, S. M. Holland, R. Janssen, and J. T. van Dissel. 2002. A superoxide-hypersusceptible Salmonella enterica serovar Typhimurium mutant is attenuated but regains virulence in p47(phox−/−) mice. Infect. Immun. 70:2614-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijburg, O. L., C. P. Simmons, N. van Rooijen, and R. A. Strugnell. 2000. Dual role for macrophages in vivo in pathogenesis and control of murine Salmonella enterica var. Typhimurium infections. Eur. J. Immunol. 30:944-953. [DOI] [PubMed] [Google Scholar]