Abstract
Staphylococcus aureus and coagulase-negative staphylococci, primarily Staphylococcus epidermidis, are recognized as a major cause of nosocomial infections associated with the use of implanted medical devices. It has been established that clinical isolates often produce a biofilm, which is involved in adherence to biomaterials and provides enhanced resistance of bacteria against host defenses and antibiotic treatments. It has been thought that the staphylococcal biofilm contains two polysaccharides, one responsible for primary cell adherence to biomaterials (polysaccharide/adhesin [PS/A]) and an antigen that mediates bacterial aggregation (polysaccharide intercellular adhesin [PIA]). In the present paper we present an improved procedure for preparation of PIA that conserves its labile substituents and avoids contamination with by-products. Based on structural analysis of the polysaccharide antigens and a thorough overview of the previously published data, we concluded that PIA from S. epidermidis is structurally identical to the recently described poly-β-(1→6)-N-acetylglucosamine from PS/A-overproducing strain S. aureus MN8m. We also show that another carbohydrate-containing polymer, extracellular teichoic acid (EC TA), is an essential component of S. epidermidis RP62A biofilms. We demonstrate that the relative amounts of extracellular PIA and EC TA produced depend on the growth conditions. Moderate shaking or static culture in tryptic soy broth favors PIA production, while more EC TA is produced in brain heart infusion medium.
Staphylococcus aureus and coagulase-negative staphylococci, including Staphylococcus epidermidis as a leading species, have emerged as the most frequently isolated pathogens in nosocomial sepsis and cause more infections associated with use of medical devices than any other group of microorganisms (28).
S. epidermidis, a predominant inhabitant of the skin and mucous membranes, is presently known to be an opportunistic pathogen, causing infections in immunocompromised hosts or patients with implanted medical devices, such as intravascular and peritoneal dialysis catheters, prosthetic heart valves, or orthopedic prostheses. These infections can be described as “chronic polymer-associated infections” (15). A characteristic feature of this kind of infection is the ability of the causative microorganisms to colonize surfaces of biomaterials in multilayered biofilms, which are structured communities of cells enclosed in a self-produced polymeric matrix, an amorphous slimy material, which is loosely bound to staphylococcal cells. This ability to form biofilms is believed to make the microorganisms more resistant to administered antibiotics and to host defense mechanisms (46). Due to a certain existing nonuniformity in terminology, in this paper we use one adopted by Hussain et al. (22), and use the term “biofilm” to refer to an accumulated biomass of bacteria and extracellular material on a solid surface. The more ambiguous term “slime” is defined as a mucoid extracellular substance holding a community of bacterial cells as cell aggregates (22).
Extracellular antigenic markers of staphylococci related to slime or biofilm production have been intensively investigated in the past decade, and S. epidermidis RP62A (ATCC 35984), which is considered a reference biofilm-positive strain, was used as a preferential model strain for such studies by a number of authors. The extracellular antigens of this organism were isolated and studied independently by three different research groups (Table 1). An extracellular capsular polysaccharide adhesin (PS/A) was first isolated by Tojo et al., as members of the group of G. Pier (Boston, Mass.), from the culture supernatant of S. epidermidis strain RP62A in 1988 (45). PS/A has been reported to be the component of the bacterial cell surface and biofilm layer that mediates cell adherence to biomaterials and protects the bacterial cells from host defenses (38). Two years later, Christensen et al. (6) described a slime-associated antigen (SAA) isolated from the same strain that had a similar function. SAA was claimed to be different from PS/A due to a relatively high glucose content. However, after reinvestigation of the chemical composition of SAA, Baldassari et al. (1) suggested that SAA and a hexosamine-containing polysaccharide intercellular adhesin (PIA) of S. epidermidis strains RP62A and 1457, described at that time by Mack et al. (34), could be the same antigenic molecule.
TABLE 1.
N-Acetylglucosamine-containing polysaccharides of biofilm-positive staphylococcal strains
| Polysaccharide | Strain | Analytical artifact | Reference(s) |
|---|---|---|---|
| PS/A | S. epidermidis RP62A | 45 | |
| PS/A, PNSG | S. carnosus(pCN27) | N-Succinyl substitution | 38 |
| S. aureus MN8m | 39, 40 | ||
| PS/A, PNAG | S. aureus MN8m | 36 | |
| PIA | S. epidermidis RP62A | 33 | |
| S. epidermidis 1457 | |||
| SAA | S. epidermidis RP62A | Presence of glucose | 6 |
| S. epidermidis clinical isolates | 1 | ||
| SAE | S. aureus MN8m | 25 |
Later, Mack et al. (33) were the researchers to elucidate a chemical structure of PIA. Using a combination of analytical methods and two-dimensional nuclear magnetic resonance (NMR) spectroscopy, PIA was identified as a linear β-(1→6)-linked N-acetylglucosaminoglycan partially substituted with O-succinyl groups. Part of the N-acetylglucosamine units was found to be deacetylated and therefore positively charged in aqueous media. The genes encoding PIA biosynthesis are organized in the ica (intercellular adhesion) operon (icaADBC), which consists of four open reading frames with a transcriptional repressor gene, icaR, located upstream and transcribed in the opposite orientation (7). The ica locus was later found in a number of S. aureus strains, and its presence was related to the ability of the bacteria to form biofilms in vitro (8). To define the activities of the polymer synthesized by the ica-encoded proteins, a fragment of chromosomal DNA of S. epidermidis RP62A containing the icaABC locus was cloned and transferred to a biofilm-negative strain of Staphylococcus carnosus, strain TM300. The recombinant strain S. carnosus TM300(pCN27), unlike the parent strain, was adherent to glass and was able to form intercellular aggregates as well as to produce PIA (18). McKenney et al. (38) subsequently demonstrated that the recombinant S. carnosus TM300(pCN27) antigen was identical to PS/A and “chemically related” to but distinct from PIA in molecular size, solubility, and substitution of the majority of the amino groups of the glucosamine residues with succinate. This polymer, named poly-N-succinyl-β-(1→6)-glucosamine (PNSG), was later found to be produced as a major surface polysaccharide antigen of clinical strains of S. aureus isolates from patient lung and sputum samples. Cultivation of these isolates in vitro resulted in decreased production of PNSG. It was suggested that as a prominent target for protective antibodies, PNSG had potential as a vaccine candidate for use against staphylococcal infections (39, 40). However, subsequent studies carried out by the same group showed that the presence of the N-succinyl groups in PNSG was an analytical artifact.
An interest in further investigations of a relationship between PIA and PS/A was indicated recently (15, 32); however, no direct comparative structural study of PS/A [or PNSG, poly-β-(1→6)-N-acetylglucosamine (PNAG), or S. aureus exopolysaccharide (SAE)] and PIA has been carried out yet. Such a study seems even more necessary in view of functional differences still attributed to these two polysaccharides (see the recent review by von Eiff et al. [47]).
The relationship between PIA and staphylococcal slime also requires clarification. In a recent review on staphylococcal biofilms it was suggested that, “in retrospect, in most cases ‘slime’ was very likely PIA,” and in particular, in the well-studied strains S. epidermidis RP62A and O-47 “slime” and PIA are the same (15). This postulate, however, contradicts previous data from studies in which S. epidermidis “slime” was reported to consist of teichoic acid (TA) (about 80% [wt/wt]) and 20% protein (22).
TA is another extracellular carbohydrate-containing polymer produced by S. epidermidis RP62A (19, 20, 45). While cell wall (CW) TA is a common component of all gram-positive bacteria, extracellular (EC) TA has been discovered only in a limited number of species (10, 23). Hussain et al. (19) found that the chemical compositions of CW and EC TAs of S. epidermidis RP62A were very similar. Both TAs contained glycerol, phosphate, glucose, glucosamine, and d-alanine. In our recent study, we established the chemical structure of the CW and EC TAs of S. epidermidis RP62A (43) and showed that they are (1→3)-linked poly(glycerol phosphate), substituted at the 2 position of glycerol residues with α-Glc, α-GlcNAc, d-Ala, and, most interestingly, α-Glc6Ala. The importance of teichoic acid and particularly the importance of the presence of d-alanine substitution in the cell wall teichoic acid in biofilm formation by S. aureus were recently demonstrated (16). In S. epidermidis, the CW TA significantly enhances adhesion of the bacterial cells to fibronectin-coated surfaces, which suggests a possible role as a bridging molecule between microorganisms and immobilized fibronectin in early stages of S. epidermidis pathogenesis (21).
However, there has been no clear indication of the role that extracellular teichoic acid may play as a constituent of biofilm or slime of S. epidermidis. Interestingly, Karamanos et al. (26) suggested that the main component of the extracellular slime of S. epidermidis, including strain RP62A, was neither PIA nor teichoic acid but rather was a 20-kDa polysaccharide, the chemical structure of which has not been established.
In the present study, we characterized the extracellular poly-β-(1→6)-N-acetylglucosamine produced by S. epidermidis model biofilm-positive strain RP62A and confirmed its structural identity to the PNAG (PS/A, SAE) produced by the “PS/A-hyperproducing strain” S. aureus MN8m (36) under the same growth conditions and isolated using similar purification procedures. We also assayed the production of the two major cell-associated extracellular carbohydrate-containing polymers produced by S. epidermidis RP62A, PIA and extracellular teichoic acid, depending on the growth conditions, and we demonstrated that they both are constituents of a biofilm produced by this strain.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The S. epidermidis RP62A (ATCC 35984) and S. aureus MN8m strains were kindly provided by Gerald Pier (Harvard Medical School, Boston, MA). Cells were grown at 37°C for 24 h under different conditions of shaking and aeration, as follows: (i) in 1 liter of tryptic soy broth (TSB) (Becton Dickinson, Le Pont de Claix, France) or brain heart infusion broth (BHI) (Becton, Dickinson, Le Pont de Claix, France) supplemented or not supplemented with glucose (1%, wt/vol) in a 3-liter Erlenmeyer flask with vigorous shaking (aerobic onditions, 80 strokes/min; Gerhard Laboshake shaker; Fisher Scientific, Elancourt, France); (ii) in 3 liters of TSB or BHI supplemented or not supplemented with glucose (1%, wt/vol), in a 5-liter Erlenmeyer flask with moderate shaking (aero-anaerobic conditions, 60 strokes/min); and (iii) in Erlenmeyer flasks (approximately 1 ml of culture per cm2 of bottom surface) in TSB or BHI for 24 h at 37°C without shaking. All cultures were inoculated with 3% (vol/vol) of a 20-h aerobic preculture grown in the same medium.
Preparation of cell-associated polysaccharides.
Crude extracellular matrix was prepared and fractionated essentially as described by Mack et al. (33). Briefly, bacterial cells were collected by centrifugation (4,500 × g, 4°C, 20 min), suspended in phosphate-buffered saline (PBS) (pH 7.4) or 0.9% NaCl (pH 5.9), and sonicated on ice in a plastic container (IKASONIC sonicator; 50% amplitude, cycle 0.5, four times for 30 s; IKA Labortechnik, Staufen, Germany) to extract the cell-associated extracellular material. Cells and insoluble material were removed by centrifugation (4,500 × g, 30 min at 4°C). The supernatant was further clarified by a short high-speed centrifugation (12,000 × g, 15 min at 4°C) and filter sterilized to give a crude extract, which was further concentrated using an Amicon ultrafiltration cell with a 10-kDa-cutoff membrane (Millipore, Bedford, MA). The concentrated crude extract was applied to a Sephacryl S-300 column directly or after precipitation of proteins by addition of trichloroacetic acid (TCA) to a concentration of 5%. Fractions corresponding to PIA (PNAG) were pooled and then freeze-dried or concentrated using an Amicon cell. Fractions corresponding to EC TA were freeze-dried and, if necessary, purified as described previously (43).
For preparation of carbohydrate-containing polymers from biofilm, cells were grown in glass flasks without shaking, as described above. Medium and nonadherent cells were removed, and the biofilm, which formed on the bottom, was washed twice with 0.9% NaCl. The biofilm was removed from the glass surface by gentle scraping and suspended in 0.9% NaCl. The crude extract was prepared and fractionated on the Sephacryl S-300 column as described above.
For estimation of the relative amount of extracellular TA, an aliquot of the crude extract (corresponding to 1 liter of culture) after protein precipitation was dialyzed and freeze-dried. In order to remove possible trace amounts of low-molecular-weight impurities, the residue was redissolved in 1 ml of water, desalted on a P10 cartridge (Amersham Biosciences, Succursale, France), and freeze-dried. To an aliquot (1/4) of the high-molecular-weight material 200 μg of an internal standard (meso-erythritol; Acros Organic, Noisy-le-Grand, France) was added. The sample was freeze-dried, depolymerized with 48% hydrofluoric acid (HF) (Acros Organic, Noisy-le-Grand, France) for 48 h at 4°C. The HF was evaporated under a stream of nitrogen, and the residue was hydrolyzed with 4 M trifluoroacetic acid (Acros Organic, Noisy-le-Grand, France) at 120°C for 2 h and peracetylated using a 1:1 (vol/vol) mixture of acetic anhydride and pyridine for 50 min at 100°C. The amount of teichoic acid was assayed by gas-liquid chromatography (GLC) (with program b [see below]), with judging by using the relative ratio of glycerol to erythritol, and was expressed in μg of glycerol per liter of culture.
Isolation of the polysaccharide fractions in TSB and BHI.
Five grams of Bacto Tryptic Soy Broth was dissolved in 3 ml of water. Proteins were precipitated by addition of TCA to a concentration of 5% and were removed by centrifugation, and the clear supernatant was fractionated on a Sephadex G-50 column. Fractions corresponding to the void volume (TSBPS) were pooled, freeze-dried, and analyzed by GLC as alditol acetates and by 1H-NMR spectroscopy. The polysaccharide fraction of the BHI medium (BHIPS) was prepared and characterized in a similar way.
General and analytical methods.
Glycerol and monosaccharides were identified by GLC with a Shimadzu GC-14 B gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a Zebron ZB-5 capillary column (30 m by 0.25 mm; Phenomenex, Torrance, CA) by using hydrogen as the carrier gas and the following temperature programs: 170°C for 3 min and then an increase to 260°C at a rate of 5°C/min (program a) and 140°C for 3 min and then an increase to 260°C at a rate of 10°C/min (program b). Polysaccharide samples were converted to alditol acetates by conventional methods following hydrolysis with 4 M trifluoroacetic acid at 120°C for 2 h. Samples containing TA were depolymerized with 48% HF prior to hydrolysis.
Gel permeation chromatography was carried out on a Sephacryl S-300 column (1 by 90 cm; Pharmacia Biotech, Uppsala, Sweden) and a Sephadex G-50 column (1.6 by 95 cm; Pharmacia Biotech, Uppsala, Sweden) irrigated with water. Fractions (5 ml) were assayed colorimetrically for aldose (13) and amino sugars (14). In order to study the charge distribution in PIA, anion- and cation-exchange chromatographic analyses were performed. Anion-exchange chromatography was carried out on a column (0.7 by 7 cm) of Q-Sepharose fast flow (Pharmacia Biotech, Uppsala, Sweden) equilibrated with 25 mM phosphate buffer (pH 7.4). Charged fractions were eluted from the column with 1 M NaCl in the same buffer. Fractions (2 ml) were collected and assayed for neutral sugars and amino sugars. Cation-exchange chromatography was performed on a column (0.7 by 5 cm) of carboxymethyl cellulose (Sigma, Saint Quentin Falavier, France) equilibrated with 25 mM sodium acetate buffer (pH 4.5). Charged material was eluted with 1 M NaCl in the same buffer. Fractions (2 ml) were assayed for neutral sugars (13), total amino sugars (14), and nonacetylated amino groups of hexosamine as described by Smith and Gilkerson (44) without the initial treatment with 0.5 M HCl. Glucosamine and N-acetylglucosamine (0.1 mg/ml) were used as standards. Dialysis was performed with Spectra/Por membranes (Spectrum Laboratories, Inc., Rancho Dominguez, CA) with molecular weight cutoffs of 6 to 8 kDa against deionized water.
NMR spectra were recorded at 25°C in D2O with a Varian UNITY INOVA 500 instrument using acetone as the reference (1H, δ 2.225 ppm; 13C, δ 31.5 ppm). Freeze-dried PIA samples were dissolved in 5 M DCl (30 μl), and the total volume was adjusted to 1 ml with D2O. Alternatively, PIA samples were prepared essentially as described by Maira-Litran et al. (36) (i.e., dissolved in 5 M DCl/D2O and neutralized with an equal volume of 5 M NaOD, and the resulting solutions were exchanged into D2O by five cycles of concentration and dilution by using a Microcon-YM cartridge [Amicon, Bedford, MA] with molecular weight cutoff of 30 kDa).
RESULTS
Optimization of PIA production and purification.
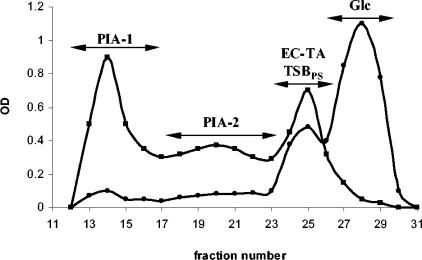
To establish the optimum growth conditions for reproducible and sufficient production of PIA by S. epidermidis RP62A, cells were grown in TSB under different conditions as described above. The production of “slime” was observed visually as formation of cell aggregates, indicating the adherent aspect of these bacterial cells in culture. For all growth conditions tested, cells expressing an adherent extracellular material were collected by centrifugation, and the extracellular matrix (crude slime) was extracted from the pellet by sonication on ice under conditions excluding cell lysis. To ensure minimal cell lysis, cell viability was checked in preliminary experiments by viable counting before and after sonication at different intensities (data not shown). Such mild extraction conditions allowed us to avoid contamination with polymers of cellular origin (CW TA, intracellular proteins, and nucleic acids). The extract was concentrated and directly applied to a Sephacryl S-300 column. Fractions were assayed for neutral and amino sugars (Fig. 1) and analyzed by 1H-NMR spectroscopy, and, upon complete acid hydrolysis, the sugar composition was determined by GLC as the corresponding alditol acetates. Since the phenol-sulfuric acid assay for neutral hesoxes (13) has very low sensitivity for glucosamine, simultaneous screening of fractions using colorimetric assays for neutral and amino sugars was required to establish the elution profile for PIA. The profiles obtained were corroborated by a Smith-Gilkerson assay (44) with and without preliminary acid hydrolysis for total amino sugars and amino sugars with a free amino group, respectively. We have found that the Elson-Morgan assay is more suitable for monitoring the PIA purification and fractionation procedure than the generally used Smith-Gilkerson assay (32, 33, 38), since it is less laborious and less sensitive to the presence of salts and other impurities. Preliminary experiments allowed us to obtain relatively high yields of PIA when cells were grown in TSB supplemented with glucose under the aero-anaerobic conditions described in Materials and Methods with moderate shaking (40 to 60 rpm) and aeration (the volume of the bacterial culture was 60 to 70% of the volume of the Erlenmeyer flask). Production of “slime” under these conditions was quite pronounced. A typical gel filtration profile on the Sephacryl S-300 column of the crude PBS extract of S. epidermidis RP62A grown in TSB supplemented with 1% glucose under aero-anaerobic conditions is presented in Fig. 1. PIA was eluted at the beginning in the void volume of the column, followed by a relatively long, sometimes slowly declining, profile. We referred to these two fractions of PIA as PIA-1 and PIA-2 to distinguish them from “polysaccharide I” and “polysaccharide II” of S. epidermidis 1457 (33), representing neutral and negatively charged fractions of PIA, respectively, as separated by anion-exchange chromatography. GLC analysis revealed that glucosamine was the only monosaccharide component of both PIA-1 and PIA-2. Both fractions were poorly soluble in water but well soluble in 5 M HCl, as described by Maira-Litran et al. (36).
FIG. 1.
Typical elution profile of the crude extracellular slime of S. epidermidis RP62A on a Sephacryl S-300 column. Cells were grown in aero-anaerobic conditions in 6 liters of TSB supplemented with glucose (1%, wt/vol). Aliquots (100 μl) of each 5-ml fraction were assessed for neutral sugars (•) (at an optical density at 485 nm) and amino sugars (▪) (at an optical density at 530 nm). OD, optical density.
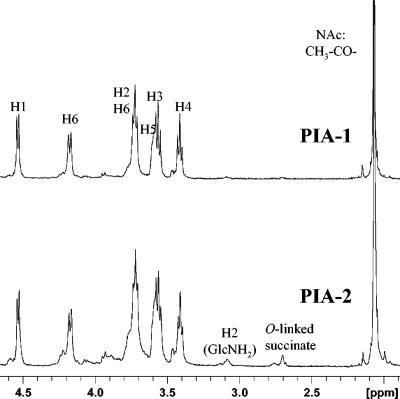
For NMR analysis, samples were dissolved in 30 μl of 5 M HCl/D2O, diluted with D2O, and exchanged several times with D2O using an ultrafiltration cartridge as described by Maira-Litran et al. (36). Their 1H-NMR spectra were similar to those reported for the poly-β-(1→6)-N-acetylglucosamine backbone of polysaccharide I (33) or PNAG (36) (Fig. 2). According to GLC analysis, only trace amounts of polysaccharide components from the TSBPS contaminated PIA-2. Screening of the fractions eluted from the Sephacryl S-300 column for protein showed that these contaminants usually had lower molecular weights and therefore were easily separated from PIA by column chromatography. Moreover, the amount of proteins in the crude extract could be significantly reduced by adding TCA to a concentration of 5% prior to gel filtration.
FIG. 2.
1H-NMR spectra of PIA-1 and PIA-2 prepared in 5 M HCl, followed by several cycles of concentration and dilution. The signals of ring protons and of the N-acetyl methyl group of β-(1→6)-GlcNAc are shown.
Charge distribution in PIA and its solubility characteristics.
Total PIA (PIA-1 and PIA-2) could be separated into two practically equal fractions by cation-exchange chromatography on carboxymethyl cellulose at pH 4.5. In the neutral fraction, only ∼4% of glucosamine residues had free amino groups, while up to 30% of glucosamine residues in the charged fraction were nonacetylated and carried a positive charge. The average content of nonacetylated glucosamine residues in total PIA (17%) was in good agreement with the data reported by Mack et al. (33).
Using a Q-Sepharose (pH 7.4) anion-exchange column, PIA was separated into neutral and negatively charged fractions with a relative ratio of about 5:1, a value close to that obtained by Mack et al. (33) for polysaccharides I and II isolated from S. epidermidis 1457 (about 7:1).
In most preparations, PIA-1 and PIA-2, once lyophilized, were very poorly soluble in water and usually precipitated from aqueous solutions at concentrations higher than 1 mg/ml. In some cases, however, the solubility of lyophilized PIA in water was good. All PIA preparations were soluble in 5 M HCl (36), forming viscous solutions.
Sample preparation for NMR analysis and evidence of O succinylation.
Maira-Litran et al. (36) used the following procedure for preparation of extracellular polysaccharide samples from S. aureus MN8m prior to NMR analysis. Samples were dissolved in 5 M DCl, neutralized with 5 M NaOD, and exchanged with D2O by several cycles of concentration and dilution using an ultrafiltration cartridge. The 1H-NMR spectrum of the polysaccharide (referred to as PS/A and PNAG) prepared in such a way was practically identical to the spectrum of polysaccharide I (33) and corresponded to the homogeneous β-(1→6)-N-acetylglucosamine backbone with traces of free amino groups (indicated by a very small and relatively broad peak at ca. 2.7 ppm). While polysaccharide I represented the fraction of PIA lacking the negative charge bearing substituents, in case of PS/A (PNAG) from S. aureus the O-succinyl substituents were, most probably, cleaved off the polymer during sample preparation for NMR analysis and thus could not be detected even though they were possibly present in the original sample.
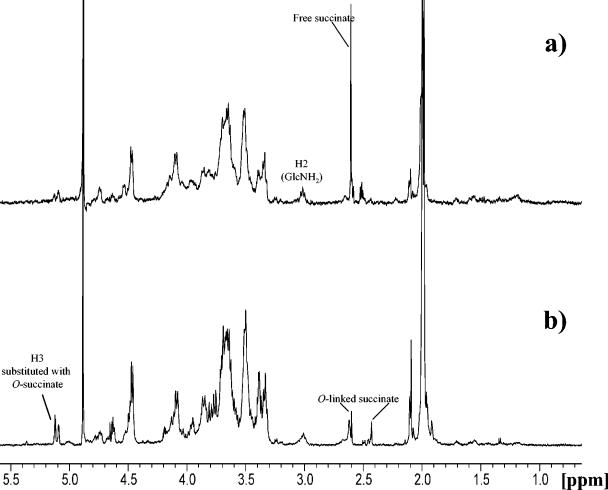
To address the problem of the presence of O-succinyl groups in PIA from S. epidermidis RP62A, we first obtained a negatively charged (and, therefore, O-succinyl-enriched) fraction of the PIA by anion-exchange chromatography. The negatively charged fraction was dialyzed, concentrated to a small volume, and de-O-acylated with 10% ammonium hydroxide. The residue was lyophilized, solubilized in DCl, and analyzed by 1H-NMR spectroscopy. As expected, an intensive sharp peak of the free succinate released due to alkaline hydrolysis was present at 2.6 ppm; this peak was similar to the one observed for the free succinate in SAE samples prepared in alkaline buffer (25). The resonance at 3.05 ppm, corresponding to H-2 of the nonacetylated glucosamine residue, was also present in the spectrum (Fig. 3a). The 1H-NMR spectrum of the positively charged fraction of PIA (see above), prepared without concentration/dilution steps, also showed resonances characteristic for the O-succinyl substitution: two small signals of O-succinyl group methylene protons centered at 2.4 and 2.6 ppm, as well as a signal of H-3 at 5.1 ppm assigned to a β-GlcNAc residue carrying the O-succinyl substituent (25) (Fig. 3b). Interestingly, in some preparations native PIA had a 1H-NMR spectrum corresponding to the homogeneous β-(1→6)-N-acetylglucosamine backbone, thus showing that the charged groups were not always present in the macromolecule. Moreover, the positively charged fraction of PIA (retained on the carboxymethyl cellulose column) appeared to be also substituted with O-succinyl groups, while no O-succinyl substituents were found in the neutral fraction. In a separate preparation, PIA was fractionated on an anion-exchange column as described above, and the fractions were assayed for total amino sugars (14) and amino sugars with free amino groups (Smith-Gilkerson assay without preliminary hydrolysis). In the negatively charged fraction retained on the column, ca. 25% of glucosamine residues were deacetylated, while the neutral fraction contained only 5% deacetylated glucosamine residues. This fact might lead to speculation that two populations of PIA are synthesized by S. epidermidis RP62A, a mostly neutral β-(1→6)-N-acetylglucosaminoglycan, “polysaccharide I” (33), and “polysaccharide II,” in which both positive (free amino groups) and negative (O-succinyl groups) are present. The relative quantities of the two populations vary from one preparation to another.
FIG. 3.
(a) 1H-NMR spectrum of the negatively charged fraction of PIA treated with 10% NH4OH; (b) 1H-NMR spectrum of the positively charged fraction of PIA. Samples were dissolved in 30 μl of 5 M DCl and diluted with D2O.
Determination of the ester-linked phosphate.
In order to avoid possible contamination of the PIA with phosphate-containing compounds, we used 0.9% NaCl instead of PBS for crude slime extraction, and, for the subsequent chromatographic step, water was used as an eluent. PIA prepared in such a way contained no detectable inorganic or organic phosphate (5). The absence of phosphate was confirmed by NMR analysis (data not shown).
Structural relationship between PIA and PNAG (SAE) from S. aureus MN8m.
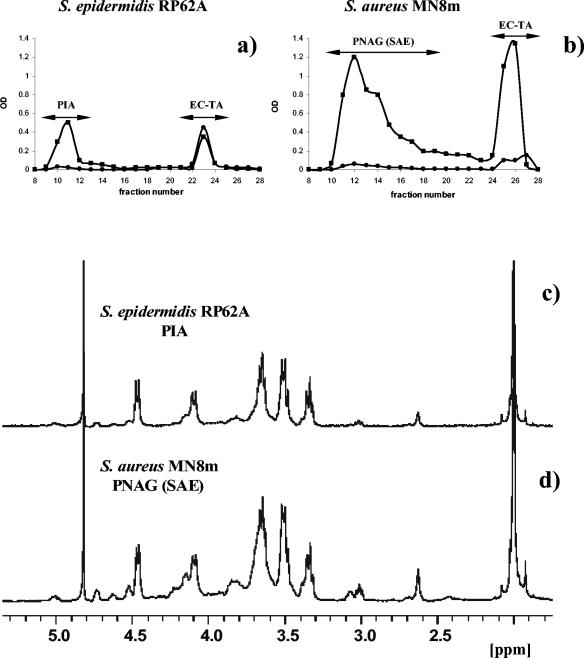
To address the question of structural relationship between PIA and PNAG (SAE), we isolated extracellular cell-associated polysaccharides from S. epidermidis RP62A and S. aureus MN8m grown under the same aero-anaerobic conditions favorable for slime production with moderate shaking or directly as a biofilm on a glass surface without shaking. Cell-associated polysaccharides were isolated using a simple procedure described in Materials and Methods. The elution profiles of crude extracts obtained from the two strains on a Sephacryl S-300 column were very similar (Fig. 4). The 1H-NMR spectra of the polysaccharides were practically identical (Fig. 4), indicating the close similarity of the two polysaccharides. The only detectable difference between the two polymers seems to be a higher proportion of both negatively (O-succinates) and positively (free amino groups) charged groups in PNAG from S. aureus MN8m (25), as judged by the relative intensities of the peaks at 5.1, 2.7, and 3.0 ppm, respectively (Fig. 4a and b).
FIG. 4.
Elution profiles of crude extracellular extracts of S. epidermidis RP62A (a) and S. aureus MN8m (b) grown as biofilms on a glass surface and 1H-NMR spectra of the corresponding high-molecular-weight fractions (c and d). Aliquots (200 μl) of each 5-ml fraction were assessed for neutral sugars (•) (at an optical density at 485 nm) and amino sugars (▪) (at an optical density at 530 nm). The data were obtained for 1 g (wet weight) of adherent biofilm for both strains. For NMR analysis, samples were dissolved in 30 μl of 5 M DCl and diluted with D2O. The peak assignments are the same as those in Fig. 2. OD, optical density.
Production of cell-associated PIA and EC TA under different growth conditions.
In our previous study, we characterized the extracellular teichoic acid of S. epidermidis RP62A produced under aerobic conditions in TSB (43). However, the structural role of EC TA in biofilm formation and slime production by biofilm-positive staphylococci still remains unclear. To address this question, we first studied the relative amounts of PIA and EC TA in cell-associated polymeric material of S. epidermidis RP62A produced under different growth conditions in TSB and BHI. The relative amount of cell-associated carbohydrate-containing polymers could be estimated by examining the Sephacryl S-300 elution profiles, as shown in Fig. 5. All the profiles were produced by analyzing material recovered from 2 liters of bacterial culture and crude extracts prepared by identical extraction methods. The amount of PIA could be estimated by the size of the high-molecular-mass amino sugar-positive fractions. EC TA was eluted later in the fractions positive for both neutral and amino sugars, in agreement with the fact that it contained both glucose and glucosamine (43).
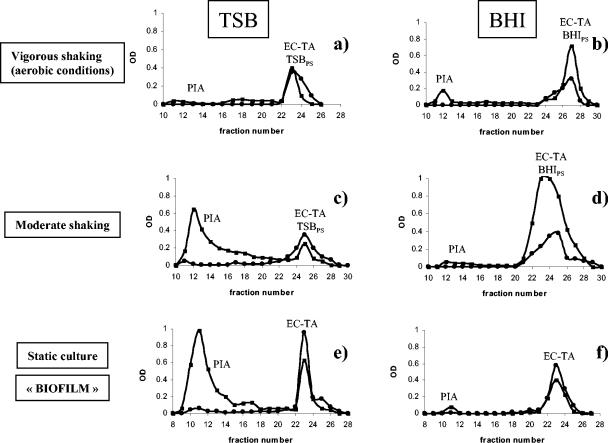
FIG. 5.
Typical chromatographic profiles of crude extracellular matrix of S. epidermidis RP62A grown under various conditions at 37°C on a Sephacryl S-300 column. Aliquots (200 μl) of each 5-ml fraction were assessed for neutral sugars (•) (at an optical density at 485 nm) and amino sugars (▪) (at an optical density at 530 nm). The data are data for 2 liters of each bacterial culture. Cells were grown with vigorous shaking in TSB (a) and BHI (b), under aero-anaerobic conditions with moderate shaking in TSB (c) and BHI (d), and as biofilms on glass surfaces without shaking in TSB (e) and BHI (f). OD, optical density; TSBPS, polysaccharide fraction of TSB medium; BHIPS, polysaccharide fraction of BHI medium.
Moderate shaking.
As mentioned above, we found that moderate shaking under aero-anaerobic conditions in TSB favored slime and PIA production (Fig. 5c). Only small amounts of cell-associated EC TA (less than 50 μg of glycerol per liter of culture) were produced under these conditions. In BHI, with moderate shaking, formation of cell clusters and adherence of cells to the glass surface were almost as notable as they were in TSB. The PIA production, however, was much lower than that in TBS under the same conditions (Fig. 5d), while the production of EC TA was higher (around 300 μg of glycerol per liter of culture). The amount of protein in the crude cell-associated matrix was also higher in BHI than in TSB (data not shown). We observed that the crude cell-associated matrix formed in BHI had good solubility in water, which was not always the case for the matrix formed in TSB. We assumed that poorly soluble PIA in this case could have formed a more soluble complex with EC TA and proteins. This complex sometimes eluted as a single broad peak from the S-300 column (data not shown). Interestingly, TAs are known to form stable soluble macromolecular complexes with glycogen and bovine plasma albumin in alcohol solutions (11).
Vigorous shaking.
Under aerobic conditions with vigorous shaking in both TSB and BHI, slime production was much less apparent, and the production of PIA in TSB was five- to sevenfold lower than that under aero-anaerobic conditions with moderate shaking in the same medium (Fig. 5a and b). Surprisingly, under both conditions, addition of glucose (1%, wt/vol) to TSB did not seem to affect PIA production (data not shown), although the ability of glucose to increase biofilm formation by staphylococci has been described by several authors (35, 42).
Without shaking.
In TSB, S. epidermidis RP62A forms a thick adherent biofilm on a glass surface. We found that PIA is the main constituent polysaccharide of this biofilm (Fig. 5e), which is in a good agreement with previous data indicating that PIA is involved in cell adherence to glass surfaces (17) and also plays a role as an “extracellular glue” at the later stages of biofilm formation (15). In BHI, formation of a biofilm on glass was less pronounced, which correlated with reduced production of PIA in this medium (Fig. 5f).
It is important to emphasize that in both media the EC TA was indeed clearly shown to be an integral part of the extracellular matrix and of biofilms in particular. GLC analysis of the lower-molecular-weight fractions (Fig. 5) indicated the presence of glycerol, glucose, and glucosamine, which is a characteristic composition of EC TA of the strain used (43).
DISCUSSION
It has been established that coagulase-negative staphylococci and S. aureus produce under the appropriate conditions biofilms that mediate adherence of bacterial cells to biomaterials and help the organisms avoid the host immune defense (15). The role of polysaccharides in constitution of these biofilms was also unambiguously demonstrated previously, and the icaADBC locus containing the genes responsible for biosynthesis of the linear β-(1→6)-linked N-acetylglucosamine polymer was identified both in S. aureus and S. epidermidis, which are major nosocomial staphylococcal pathogens (8). In the present study, we developed a simple cultivation protocol favorable for PIA production by a model biofilm-positive strain, S. epidermidis RP62A. We established that aero-anaerobic conditions in TSB with moderate shaking or without shaking ensured the best yields of PIA. These results are in agreement with the previously published data. TSB is known to stimulate biofilm formation by S. epidermidis (32), and anaerobic growth conditions were recently shown to induce PIA expression and ica gene transcription in S. aureus and S. epidermidis (9). High yields of PIA directly correlated with a “slimy” appearance of bacterial cultures (formation of cell clusters and adherence to glass surfaces of Erlenmeyer flasks).
A simple PIA purification protocol is also suggested here. Much attention was paid to exclusion of the possibility of misinterpretation of the monosaccharide composition of the PIA due to contaminating polysaccharides arising from the cultivation media, since several erroneous reports that arose due to such mistakes have been presented in the past (12). In contrast to the previously used methods (32), in this protocol we avoided using alkaline eluents and included only one chromatographic step, which allowed separation of PIA and EC TA and a colorimetric assay of these compounds in the eluted fractions. Extracellular teichoic acid fractions and proteins eluted separately from PIA on a Sephacryl S-300 column. Thus, the PIA purification scheme suggested here is simpler than the one described by Mack et al. (32) and results in PIA of sufficient purity. Moreover, PIA obtained using this mild procedure is close to its native form; all the charged groups in the molecule have been conserved because of the absence of any harsh treatment or ion-exchange chromatography purification steps. Besides, the lack of phosphate in both extraction and elution media allowed us to avoid erroneous phosphate identification, as has occurred previously (33). PIA was characterized by chemical methods and using high-field NMR spectroscopy. In agreement with previously published data (33), our results showed the structure of a β-(1→6)-linked N-acetylglucosaminoglycan, with part of the N-acetylglucosamine residues deacetylated. Approximately 20% of the PIA had a net negative charge due to the partial O-succinyl substitution.
To date, very little NMR evidence for the presence of the charged groups in PIA from S. epidermidis has been presented. Using hetero-correlated 1H-13C NMR techniques, Mack et al. (33) showed that the small proton peak at 2.87 ppm correlated with the carbon resonance at 57.92 ppm and assigned it to H-2 of the free glucosamine residue. This was the only NMR evidence for the presence of nonacetylated glucosaminyl residues, and additional proof was obtained by chemical analysis. Even though enzymatic assays indicated that ∼6% of glucosamine residues in polysaccharide II were O succinylated, there was no NMR evidence for the presence of the O-succinyl groups in PIA from S. epidermidis. This is the first time that the presence of O-succinyl groups in S. epidermidis RP62A PIA was unambiguously confirmed by an NMR investigation.
There are currently several terms that are used to describe extracellular staphylococcal polysaccharides that are involved in biofilm composition and to which the role of mediating adhesion to biomaterials is ascribed. A list of the terms used for description of N-acetylglucosamine-containing antigens from staphylococci together with the corresponding references is shown in Table 1. It seems especially important to clarify the structural relationship between “PS/A” and “PIA,” since traditionally different functions are attributed to these two polysaccharides (primary attachment of bacterial cells to an artificial surface and “consolidation” of the accumulated extracellular matrix in the biofilm created, respectively) (47). It is noteworthy that in the early studies, the term PS/A was assigned by researchers from the group of G. Pier (45) to the glucosamine-containing extracellular antigen of S. epidermidis RP62A, the same strain for which the term “PIA” was later introduced by a different research group (33). In subsequent studies, Pier and coworkers applied the term “PS/A” to the extracellular polysaccharide from S. aureus strain MN8m, which was afterwards called PNSG (40), PNAG (36), and SAE (25). Recently, Joyce et al. (25) demonstrated the quasi-identity of SAE (PNAG) isolated from “PS/A-overproducing strain” S. aureus MN8m (36) and PIA of S. epidermidis, which are both described as the “products of the same locus with minor structural variations arising from growth- or strain-dependent factors.” The two main differences between SAE and “the published description of PIA” (25) were the O phosphorylation and the molecular mass distribution. The absence of O phosphorylation in PIA was clearly demonstrated in the present study. Concerning the molecular size of PIA, it was initially estimated to be ca. 130 β-(1→6)-linked N-acetylglucosamine residues (33) on the basis of methylation analysis. We, however, believe that this degree of polymerization leading to a calculated average molecular mass of 28 kDa (36) is a mistake due to the very probable depolymerization of PIA under the highly alkaline conditions of the methylation reaction that resulted in an exaggerated high ratio for the terminal glucosamine residue. Polysaccharides are known to undergo depolymerization under alkaline conditions of methylation (30). In order to avoid this, borohydride reduction of the reducing unit is recommended to be performed prior to methylation analysis, particularly for amino sugar-containing polysaccharides (24). Interestingly, the data obtained by the same group contradict such a low estimate for the molecular mass of PIA, since according to Mack et al., the polysaccharide was detected near the void volume of the columns when it was eluted on Sephacryl S-200 or S-300 (32). This result clearly implies that the true molecular mass of PIA is in the range of hundreds of thousands, which is in accordance with our observations (Fig. 1 and 5). Furthermore, the direct comparison of the elution profiles for crude biofilms and the 1H-NMR spectra of the corresponding polysaccharides in the present study clearly demonstrated the structural identity of PIA from S. epidermidis RP62A and PNAG (PS/A, SAE) from S. aureus MN8m (Fig. 4). Interestingly, recent studies have shown that the initial distinction between PIA and PS/A was due to some trivial differences in polystyrene microtiter plates manufactured in Europe and the United States (17, 37).
Therefore, it seems clear that SAE is PS/A and chemically PNAG. Analysis of the previously published data and results presented here clearly show that under identical growth conditions, a biofilm on a glass surface, the two strains produce identical polysaccharide antigens. The functions of the polysaccharide in these strains are, most probably, mediation of primary bacterial adherence to solid surfaces during the first phase of biofilm formation (a feature assigned to PS/A) and accumulation of bacterial cells into a biofilm during the second phase of biofilm formation, which is considered to be the property of PIA (31). Therefore, our data confirm that, as stated by Maira-Litran et al. (37), “PIA and PS/A are the same chemical entity—PNAG.”
Since the ica operon encoding the biosynthesis of PIA (PS/A, PNAG, SAE) is present in a large number of staphylococcal strains (49), it seems legitimate to propose that this linear β-(1→6)-linked N-acetylglucosaminoglycan is a common extracellular antigen of staphylococci involved in biofilm formation. To date, this antigen has been structurally characterized for S. epidermidis RP62A (33; this study), S. epidermidis 1457 (33), and S. aureus MN8m (25, 36). It has also been shown that in some S. epidermidis clinical isolates the ica locus seems to encode a polysaccharide antigen different from PIA, an “immunochemical variant of PIA,” which was called PIAv (29). The chemical structure of PIAv remains unknown. Determination of the chemical structures of the products of the ica operon in different biofilm-positive staphylococci requires further investigation.
Recently, an unbranched poly-β-(1→6)-N-acetylglucosamineinvolved in biofilm formation by Escherichia coli was isolated and characterized. The pgaABCD operon, related to biosynthesis of this compound, was found in a variety of eubacteria (48). It is interesting that PNAG is an antigen which may play an important role in biofilm formation in a number of bacterial species, including both gram-positive and gram-negative species.
Another question addressed in the current study was analysis of the totality of the cell-associated carbohydrate-containing polymers in S. epidermidis RP62A. The structural role of PIA and the EC TA in the extracellular matrix under different growth conditions was therefore investigated. Hussain et al. (20) isolated an “extracellular high-molecular-weight carbohydrate polymer (EC)” from S. epidermidis RP62A grown in chemically defined medium and found that its composition was identical to that of the cell wall teichoic acid of this strain (19). In a recent study, we established the exact chemical structure of both EC and CW TAs of this strain (43). It was still not clear, however, under which conditions the EC TA was produced and, more precisely, whether it can be considered a constituent of the biofilm layer of S. epidermidis RP62A. In this study this question was addressed by using the very mild method for extraction of the extracellular matrix described here, where cells were not disrupted and therefore only EC TA, and not CW TA, was present in the crude extract of S. epidermidis RP62A. We established that the EC TA, along with a moderate amount of PIA, was a constituent of the extracellular matrix under aerobic growth conditions in TSB and BHI (Fig. 5a and b). When cells were grown with moderate shaking, which is favorable for formation of cell clusters, the composition of the extracellular matrix (slime) differed for the two growth media used. In TSB, the matrix contained mostly PIA and only trace amounts of EC TA, while in BHI the amount of PIA was smaller and the quantity of EC TA was significantly larger (Fig. 5c and d).
It is known that the rich complex medium BHI modifies the physiological state of another gram-positive bacterium, Enterococcus faecalis, rendering it more resistant to osmotic stress (41). On the other hand, production of exopolysaccharides by bacteria is thought to be related to their protective effect under environmental stress conditions (27). The osmoprotective property of BHI could be one of the possible explanations for the low production of PIA by S. epidermidis RP62A in this medium.
We should point out that EC TA was clearly identified in this study as a part of a biofilm formed on a glass surface in both TSB and BHI. Under these conditions, the contamination of the EC TA with medium components was minimized because the biofilm was washed with 0.9% NaCl prior to scraping and extraction, and thus the elution profiles adequately illustrated the composition of biofilms (Fig. 5e and f). It is therefore clear that, along with proteins, the biofilm formed by strain S. epidermidis RP62A contains two carbohydrate components, PIA and EC TA. The poly(glycerol phosphate) EC TA is a highly polar and hydrophilic molecule, while PIA is rich in relatively hydrophobic N-acetyl groups. The amounts of these two polymers vary depending on the growth conditions. Both macromolecules possess positive and negative charges due to substitution with charged groups (free amino groups and O-succinyl substituents in PIA, d-alanyl esterification in EC TA), the amount of which may vary and also can be influenced by the growth conditions. It can be concluded that the capacity to regulate positive and negative charges, as well as hydrophilic properties of its biofilm constituents, should increase the ability of S. epidermidis RP62A to form biofilms on surfaces with different physicochemical properties and to survive and proliferate under various environmental conditions.
In summary, the present study demonstrates that PIA and the EC TA are the two main carbohydrate-containing polymers of the extracellular matrix and of the biofilm of S. epidermidis RP62A. Common extracellular antigens have been described previously for other biofilm-forming bacterial species, such as alginate in Pseudomonas aeruginosa (2). An acidic exopolysaccharide containing a branched heptasaccharide repeating unit was characterized for a mucoid strain of Burkholderia cepacia (4) and was later found in a number of clinical and environmental Burkholderia cepacia strains (3). Whether the product of the ica locus and the extracellular TA are common constituents of biofilms of S. epidermidis and other biofilm-forming staphylococci has not been established yet. In this context, a more general comprehensive investigation of the role of PIA (or PIA-like polysaccharides) and EC TA as constituents of biofilms in other staphylococcal strains is a very important subject for future research.
Acknowledgments
This work was supported by the Regional Council of Nord-Pas-de-Calais and INSERM. G.K. is a recipient of the Nord-Pas-de-Calais Fellowship in 2003-2004.
We thank G. Pier, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, for providing the bacterial strains S. epidermidis RP62A and S. aureus MN8m (Material Transfer Agreement A4969) and for invaluable scientific discussions. We thank J. Jeanfils and P. Hardouin (Université du Littoral-Côte d'Opale) for their support.
Editor: V. J. DiRita
REFERENCES
- 1.Baldassarri, L., G. Donnelli, A. Gelosia, M. C. Voglino, A. W. Simpson, and G. D. Christensen. 1996. Purification and characterization of the staphylococcal slime-associated antigen and its occurrence among Staphylococcus epidermis clinical isolates. Infect. Immun. 64:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, A., and A. M. Chakrabarty. 1995. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J. Ind. Microbiol. 15:162-168. [DOI] [PubMed] [Google Scholar]
- 3.Cérantola, S., J.-D. Bounéry, C. Segonds, N. Marty, and H. Montrozier. 2000. Exopolysaccharide production by mucoid and non-mucoid strains of Burkholderia cepacia. FEMS Microbiol. Lett. 185:243-246. [DOI] [PubMed] [Google Scholar]
- 4.Cerantola, S., A. Lemassu-Jacquier, and H. Montrozier. 1999. Structural elucidation of a novel exopolysaccharide produced by a mucoid clinical isolate of Burkholderia cepacia. Characterization of a trisubstituted glucuronic acid residue in a heptasaccharide repeating unit. Eur. J. Biochem. 260:373-383. [DOI] [PubMed] [Google Scholar]
- 5.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 6.Christensen, G. D., L. P. Barker, T. P. Mawhinney, L. M. Baddour, and W. A. Simpson. 1990. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect. Immun. 58:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer, W., F. J. Kruyssen, and J. T. Wouters. 1981. Cell wall metabolism in Bacillus subtilis subsp. niger: accumulation of wall polymers in the supernatant of chemostat cultures. J. Bacteriol. 146:877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle, R. J., A. N. Chatterjee, U. N. Streips, and F. E. Young. 1975. Soluble macromolecular complexes involving bacterial teichoic acids. J. Bacteriol. 124:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drewry, D. T., L. Galbraith, B. J. Wilkinson, and S. G. Wilkinson. 1990. Staphylococcal slime: a cautionary tale. J. Clin. Microbiol. 28:1292-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois, M., K. A. Gilles, J. F. Hamilton, P. A. Rebers, and F. Smyth. 1956. Colorimetric methods for determination of sugars and related substances. Anal. Biochem. 28:350-356. [Google Scholar]
- 14.Enghofer, E., and H. Kress. 1979. An evaluation of the Morgan-Elson assay for 2-amino-2-deoxy sugars. Carbohydr. Res. 76:233-238. [DOI] [PubMed] [Google Scholar]
- 15.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 16.Gross, M., S. E. Cramton, F. Götz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 19.Hussain, M., J. G. Hastings, and P. J. White. 1992. Comparison of cell-wall teichoic acid with high-molecular-weight extracellular slime material from Staphylococcus epidermidis. J. Med. Microbiol. 37:368-375. [DOI] [PubMed] [Google Scholar]
- 20.Hussain, M., J. G. M. Hastings, and P. J. White. 1991. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in chemically defined medium. J. Infect. Dis. 163:534-541. [DOI] [PubMed] [Google Scholar]
- 21.Hussain, M., C. Heilmann, G. Peters, and M. Herrmann. 2001. Teichoic acid enhances adhesion of Staphylococcus epidermidis to immobilized fibronectin. Microb. Pathog. 31:261-270. [DOI] [PubMed] [Google Scholar]
- 22.Hussain, M., M. H. Wilcox, and P. J. White. 1993. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol. Rev. 10:191-207. [DOI] [PubMed] [Google Scholar]
- 23.Jacques, N. A., L. Hardy, K. W. Knox, and A. J. Wicken. 1979. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect. Immun. 25:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jay, A. 1996. The methylation reaction in carbohydrate analysis. J. Carbohydr. Chem. 15:897-923. [Google Scholar]
- 25.Joyce, J. G., C. Abeygunawardana, Q. Xu, J. C. Cook, R. Hepler, C. T. Przysiecki, K. M. Grimm, K. Roper, C. C. Yu Ip, L. Cope, D. Montgomery, M. Chang, S. Campie, M. Brown, T. B. McNeely, J. Zorman, T. Maira-Litran, G. B. Pier, P. M. Keller, K. U. Jansen, and G. E. Mark III. 2003. Isolation, structural characterization, and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr. Res. 338:903-922. [DOI] [PubMed] [Google Scholar]
- 26.Karamanos, N. K., A. Syrokou, H. S. Panagiotopoulou, E. D. Anastassiou, and G. Dimitracopoulos. 1997. The major 20-kDa polysaccharide of Staphylococcus epidermidis extracellular slime and its antibodies as powerful agents for detecting antibodies in blood serum and differentiating among slime-positive and -negative S. epidermidis and other staphylococci species. Arch. Biochem. Biophys. 342:389-395. [DOI] [PubMed] [Google Scholar]
- 27.Keith, L. M., and C. L. Bender. 1999. AlgT (σ22) controls alginate production and tolerance to environmental stress in Pseudomonas syringae. J. Bacteriol. 181:7176-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloos, W. E., and T. E. Bannerman. 1994. Update on clinical significance of coagulase-negative staphylococci. Clin. Microbiol. Rev. 7:117-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knobloch, J. K., M. A. Horstkotte, H. Rohde, P. M. Kaulfers, and D. Mack. 2002. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J. Antimicrob. Chemother. 49:683-687. [DOI] [PubMed] [Google Scholar]
- 30.Kochetkov, N. K., A. F. Bochkov, B. A. Dmitriev, A. I. Usov, O. S. Chizhov, and V. N. Shibaev. 1967. Chemistry of carbohydrates. Khimija, Moscow, USSR.
- 31.Mack, D. 1999. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect. 43:S113-125. [DOI] [PubMed] [Google Scholar]
- 32.Mack, D., K. Bartscht, C. Fischer, H. Rohde, C. de Grahl, S. Dobinsky, M. A. Horstkotte, K. Kiel, and J. K. Knobloch. 2001. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. Methods Enzymol. 336:215-239. [DOI] [PubMed] [Google Scholar]
- 33.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maira-Litran, T., A. Kropec, D. Goldmann, and G. B. Pier. 2004. Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine 22:872-879. [DOI] [PubMed] [Google Scholar]
- 38.McKenney, D., J. Hubner, E. Muller, Y. Wang, D. A. Goldmann, and G. B. Pier. 1998. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect. Immun. 66:4711-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenney, D., K. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Döring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 2000. Vaccine potential of poly-1-6 β-d-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. J. Biotechnol. 83:37-44. [DOI] [PubMed] [Google Scholar]
- 40.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 41.Pichereau, V., S. Bourot, S. Flahaut, C. Blanco, Y. Auffray, and T. Bernard. 1999. The osmoprotectant glycine betaine inhibits salt-induced cross-tolerance towards lethal treatment in Enterococcus faecalis. Microbiology 145:427-435. [DOI] [PubMed] [Google Scholar]
- 42.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadovskaya, I., E. Vinogradov, J. Li, and S. Jabbouri. 2004. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 339:1467-1473. [DOI] [PubMed] [Google Scholar]
- 44.Smith, R. L., and E. Gilkerson. 1979. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal. Biochem. 98:478-480. [DOI] [PubMed] [Google Scholar]
- 45.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 46.von Eiff, C., C. Heilmann, and G. Peters. 1999. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:843-846. [DOI] [PubMed] [Google Scholar]
- 47.von Eiff, C., G. Peters, and C. Heilmann. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 48.Wang, X., J. F. Preston III, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziebuhr, W., C. Heilmann, F. Gotz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]