Abstract
This study was conducted to define adhesive characteristics of the acid-stable moiety of the Candida albicans phosphomannoprotein complex (PMPC) on adherence of this fungus to marginal zone macrophages of the mouse spleen. Complete digestion of the acid-stable moiety (Fr.IIS) of the C. albicans PMPC with an α-mannosidase or hydrolysis with 0.6 N sulfuric acid destroyed adhesin activity, as determined by the inability of the soluble digests to inhibit yeast cell adherence to the splenic marginal zone. Fr.IIS adhesin activity was decreased following digestion with an α-1,2-specific mannosidase. Oligomannosyls consisting of one to six mannose units, which were isolated from the acid-stable part of the PMPC, did not inhibit yeast cell binding and thus do not function alone as adhesin sites in the PMPC. To gain more insight into the minimum requirements for adhesin activity, PMPCs were isolated from a Saccharomyces cerevisiae wild-type strain and from mutant strains mnn1, mnn2, and mnn4; the PMPCs were designated scwt/Fr.II, scmn1/Fr.II, scmn2/Fr.II, and scmn4/Fr.II, respectively. S. cerevisiae scmn2/Fr.II lacks oligomannosyl side chain branches from the outer core mannan, and scmn2/Fr.II was the only PMPC without adhesin activity. S. cerevisiae scwt/Fr.II, scmn1/Fr.II, and scmn4/Fr.II showed adhesin activities less than that of C. albicans Fr.II. These three S. cerevisiae PMPCs are generally similar to Fr.IIS, except that the S. cerevisiae structure has fewer and shorter side chains. Immunofluorescence microscopy show that the acid-stable part of the PMPC is displayed homogeneously on the C. albicans yeast cell surface, which would be expected for a surface adhesin. Our results indicate that both the mannan core and the oligomannosyl side chains are responsible for the adhesin activity of the acid-stable part of the PMPC.
Candida albicans adherence to host receptors is important for establishment of colonization and for initiation of invasion into host tissue (6, 8, 9, 15, 20). Many candidate adhesin molecules on yeast cells have been reported (3, 5, 12, 16, 21, 26), and the cell wall phosphomannoprotein complex (PMPC) has received the most attention (4, 28, 29, 31, 35).
We have focused our studies on interactions between C. albicans and macrophages found in the marginal zone of mouse splenic tissue and certain macrophages in peripheral lymph nodes (9, 13, 15, 17–19, 27). This site for interaction studies was chosen because of its clear importance in trapping of yeast cells during candidemia. Use of an ex vivo assay (30) has led to preliminary data indicating that a mannose receptor as characterized by Stahl (34) is significantly different from the receptor for C. albicans (reference 16 and our unpublished data). Candida adhesins involved in the attachment to the macrophages appear to be entirely associated with the phosphomannan complex on the fungal cell surface.
The phosphomannan part of the C. albicans PMPC can be divided into acid-stable and acid-labile moieties (24, 32, 33). Both parts are involved in adherence of this fungus to host tissues such as spleen and lymph node (19, 27). Chaffin et al. (7) isolated a C. albicans cell surface mutant lacking the acid-labile moieties. In wild-type cells, these moieties consist of β-1,2-linked oligomannosyl residues phosphodiester linked to the acid-stable part. The mutant strain was reported to be reduced in adherence ability compared to the wild type. Li and Cutler (27) defined an adhesin site on the acid-labile part of C. albicans PMPC as a β-1,2-linked mannotetraose; Fradin et al. showed that this oligomannoside functions as an adhesin in attachment of yeast cells to a macrophage cell line (11). Furthermore, antigenic factor 6, which consists of a β-1,2-linked mannoside located on the acid-stable part of the mannoprotein of serotype A C. albicans (23), acts as an adhesin in the binding of C. albicans to epithelial cells (29). These various data strongly support an adhesin function for β-1,2-linked oligomannosyls in the PMPC of C. albicans. In other studies, we showed strong mannan adhesin activity that was not associated with β-1,2-linked oligomannosyls (19). A serotype B strain, which lacks β-1,2-glycosides bonds in the acid-stable part, had strong adhesin activity associated with the acid-stable part (19, 24). The PMPC acid-stable parts from Saccharomyces cerevisiae and C. albicans are similar (2), but the S. cerevisiae acid-stable part of the PMPC and the phospholinked glycosides do not have β-1,2-linked oligomannosyl residues. These features and the availability of well-defined cell wall mannan mutants (2) make S. cerevisiae a useful tool for understanding the nature of the adhesin site(s) in the acid-stable part of the C. albicans PMPC.
In this study, we isolated PMPCs from S. cerevisiae wild-type and three mnn mutant strains and compared their adhesin activities with that of the C. albicans acid-stable PMPC mannan fraction, Fr.IIS, which was previously isolated (19). We show that the adhesin activity of the acid-stable moiety depends on both number and length of mannosyl side chains bound to the outer core mannan, and not on the kind of side-chain linkages.
For our work, Fr.IIS was obtained from a serotype B strain of C. albicans, because the PMPC structure from this strain contains β-1,2-oligomannosyl residues only in the acid-labile part of the structure, whereas serotype A strains have these β-1,2-glycosidic linkages in the acid-labile and acid-stable parts (23). The use of serotype B strains in these studies does not bias the results because both strains appear to bind to splenic marginal zone macrophages by the same mechanism(s) (18).
MATERIALS AND METHODS
Organisms and culture conditions.
C. albicans A9 (serotype B), Saccharomyces cerevisiae wild-type parent strain X2180, and three derived mnn mutant strains, S. cerevisiae mnn1, mnn2 and mnn4, were used. The C. albicans strain was described previously (13, 17–19, 30). S. cerevisiae mnn1 mannan lacks α-1,3-linked mannoses at the ends of the side chains, S. cerevisiae mnn2 mannan lacks all side chains, and mannan of S. cerevisiae mnn4 lacks phosphate-bound mannose chains (2). The mnn mutants were kindly provided by Clinton Ballou, University of California, Berkeley. Stock cultures of the strains maintained in 50% glycerol at −80°C were spread onto a GYEP (2% glucose, 0.3% yeast extract, 1% peptone) agar plate and cultured for 48 h at 30°C, and a single colony of each strain was inoculated into GYEP broth. For ex vivo adherence assays, hydrophilic C. albicans yeast cells were obtained by culturing for 24 h at 37°C in GYEP broth under aeration by reciprocal shaking at 100 strokes per min. The stationary-phase hydrophilic Candida cells were washed in cold distilled water; their hydrophobicity was determined by attachment of latex microspheres to the cell wall surface as described previously (15), and the cell number was adjusted to 1.5 × 108 per ml in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Chemical Co., St. Louis, Mo.), (pH 7.4) (18, 19, 30). For isolation of PMPC, C. albicans was cultured in GYEP broth for 24 h at 37°C, and S. cerevisiae was cultured in GYEP broth for 36 h at 30°C as described above.
Acid-stable part of the C. albicans PMPC.
The acid-stable part of the C. albicans PMPC was obtained by acid treatment of the complex followed by size exclusion column chromatography as described previously (19). In brief, hydrophilic yeast cells of C. albicans were treated with 300 mM β-mercaptoethanol (2-ME) in 0.1 M EDTA (pH 9.0) for 30 min at 20 to 25°C; the resultant supernatant was dialyzed against distilled water and lyophilized, and the material was called 2-ME extract. 2-ME extract was dissolved in 25 mM phosphate buffer (PB; pH 7.2) containing 0.15 N NaCl (PBS), applied to a concanavalin A (ConA)-agarose (Honen Co., Tokyo, Japan) column, and washed with PBS, and materials were eluted with 0.5 M α-methyl-d-mannopyranoside. The eluted fractions were pooled, forming the PMPC, and designated Fr.II (18). Fr.II was dissolved in 10 mM HCl, hydrolyzed for 60 min at 100°C, and applied to a size exclusion column (Toyopearl HW 40S; Toyo Soda Manufacturing Company Ltd., Tokyo, Japan), and fraction eluting in the void volume was lyophilized. This fraction, the acid-stable part of the PMPC, was designated Fr.IIS (19). Mannose, α-1,2-linked mannobiose, and α-1,2-linked mannotriose, which were kindly supplied by Nobuyuki Shibata, Tohoku College of Pharmacy, Sendai, Japan, were used to determine the peaks corresponding to mono-, di-, and trisaccharides eluted from the size exclusion columns.
Acetolysis products of Fr.IIS.
Alpha-linked oligomannosyls were isolated from Fr.IIS of C. albicans NIH B-792 by acetolysis and size exclusion column chromatography (32). The resulting oligomannosyls, Man, Man α1-2Man, Man α1-2Man α1-2Man, Man α1-2Man α1-2Man α1-2Man, Man α1-3Man α1-2Man α1-2Man α1-2Man, and Man α1-2Man α1-3Man α1-2Man α1-2Man α1-2Man, were kindly supplied by Nobuyuki Shibata; these residues were designated M1 to M6, respectively.
PMPCs of S. cerevisiae.
PMPCs were obtained from S. cerevisiae strains by methods similar to those used for C. albicans (18). In brief, stationary-phase yeast cells of the various strains were grown in GYEP broth for 48 h at 30°C and treated with 300 mM 2-ME. The resulting 2-ME extracts were applied to a ConA-agarose column, and 0.5 M α-methyl-d-mannopyranoside-eluted fractions were pooled. These pools, the PMPCs, were designated scwt/Fr.II for the S. cerevisiae parent strain X2180, scmn1/Fr.II for mutant mnn1, scmn2/Fr.II for mutant mnn2, and scmn4/Fr.II for mutant mnn4.
PMPCs of S. cerevisiae and of C. albicans that were used in this study are listed in Table 1.
TABLE 1.
PMPCs used in this study
| Source | PMPC | Mannose linkage(s) present |
|---|---|---|
| C. albicans A9 (serotype B) | Fr.IIa | α-1,2, α-1,3, α-1,6, β-1,2 |
| Fr.IISb | α-1,2, α-1,3, α-1,6 | |
| S. cerevisiae | ||
| X2180 | Fr.II/scwtc | α 1,2, α-1,3, α-1,6 |
| mnn1 | Fr.II/scmn1d | α-1,2, α-1,6 |
| mnn2 | Fr.II/scmn2e | α-1,6 |
| mnn4 | Fr.II/scmn4f | α-1,2, α-1,3, α-1,6 |
Composed of acid-stable and acid-labile mannan parts.
Does not contain phosphate-bound β-1,2-linked mannose.
Contains α-1,6-linked mannose core, α-1,2-, and α-1,3-linked mannose side chains bound with the core, and phosphate-bound side chains consisting of α-1,2-and α-1,3-linked mannose.
Contains α-1,6-linked mannose core, α-1,2-linked mannose side chains bound with the core, and phosphate-bound side chains but does not contain α-1,3-linked mannose.
Contains only α-1,6-linked mannose core.
Contains side chains consisting of α-1,2-and α-1,3-linked mannose chains and α-1,6-linked mannose core but does not contain phosphate-bound side chains.
Enzymatic digestion and chemical treatment.
Fr.IIS was dissolved in 50 mM citrate buffer (pH 5.0) at 200 μg/ml, with each milliliter containing 0 to 20 μU of α-1,2-mannosidase (Glyko Inc., Novato, Calif.). This enzyme was isolated from Aspergillus saitoi and is highly specific for α-1,2-linked mannose at the nonreducing end. Alternatively, 0 to 1.13 U of α-mannosidase (Glyko) was used. This enzyme was isolated from Canavalia ensiformis and breaks α-1,2-, α-1,3-, and α-1,6-linked mannose bonds. Samples were incubated for 18 h at 37°C and then treated at 100°C for 3 min to inactivate enzymatic activity. For proteinase K treatment, Fr.IIS was dissolved in 25 mM Tris-HCl buffer (pH 7) containing proteinase K (100 μg/ml; Wako Pure Chemicals, Osaka, Japan), incubated for 60 min at 37°C, and then incubated for 3 min at 100°C to inactivate the proteinase K. All samples were diluted 1:10 in DMEM and tested for the ability to inhibit yeast adherence to mouse splenic tissue in the ex vivo assay as described below.
To break the glycoside linkage of the nonreducing end on the oligomannosyl side chains, Fr.IIS was dissolved in 0.6 N H2SO4 at 10 mg/ml and heated at 100°C for 0, 0.75, 1.5, 3.0, 4.5, and 6 h (22). At each treatment period, each aliquot of sample solution was collected, cooled to room temperature, neutralized with 4 N NaOH, and concentrated threefold by incomplete drying in vacuo. Each sample was applied to a size exclusion column Toyopearl HW 40S (Toyo Soda) and eluted at 21 to 25°C with distilled water at a flow rate of 12.5 ml/h (2.5 ml/tube). Each fraction was monitored for carbohydrate content by the phenol-sulfuric acid method (10), and fractions corresponding to each peak were pooled and lyophilized for activity by the inhibition test in the ex vivo assay as described below.
Lectin and antibodies.
Horseradish peroxidase (HRP)-conjugated ConA (Honen Co.) was used to detect mannans in the fractions isolated from C. albicans and S. cerevisiae. Antisera specific for antigenic factors 5 and 9 of C. albicans were obtained from a commercial source (Candida Check, Iatron Laboratories Inc., Tokyo, Japan). Factor 5 corresponds to β-1,2-linked oligomannosyl residues of C. albicans mannan (32, 33), and factor 9 corresponds to the α-1,6-mannan core without mannose side chains (1). Mouse immunoglobulin M (IgM) monoclonal antibody (MAb) B6.1, specific for a β-1,2-linked mannotriose (14), was also used to monitor for β-linked mannose chains.
Antibodies were raised against scmn1/Fr.II or scmn4/Fr.II, neither of which contains β-1,2-mannosides. Both antisera were treated twice with S. cerevisiae mnn2 yeast cells for 2 h at 4 to 6°C to adsorb antibodies against proteins or other mannan epitopes. The adsorbed antisera were used for detection of various moieties within the acid-stable part of the PMPC on the surface of C. albicans. The slide agglutination titer against C. albicans yeast cells was 40 for both antisera.
Dot blots.
Antibodies reacting with PMPCs from C. albicans and S. cerevisiae were tested in a dot blot format. One microliter of each PMPC extract (10 mg/ml) from C. albicans and S. cerevisiae was placed on nitrocellulose sheets (Cellulosenitrat, EBA 85; Schleicher & Schuell, Dassel, Germany) and air dried for 30 min. The sheets were soaked in 25 mM Tris-HCl buffer (pH 7.5) containing 0.5 N NaCl (Tris-buffered saline [TBS]) and 3% skim milk for 30 min at 20 to 25°C. Each sheet was reacted overnight at 4 to 6°C with one of the following antisera: anti-factor 5 (1:100) rabbit serum, anti-factor 9 (1:100) rabbit serum, anti-Fr.II/scmn1 (1:200) rabbit serum, anti-Fr.II/scmn4 (1:200) rabbit serum, and mouse MAb B6.1 (4 μg/ml). All antibody preparations were diluted in TBS containing 1% skim milk. After being washed in TBS–1% skim milk, the sheets were incubated with HRP-conjugated anti-rabbit IgG goat serum (1:2,000; Cappel Research Products, Durham, N.C.) or HRP-conjugated anti-mouse IgM goat serum (1:1,000; Jackson ImmunoResearch Laboratories, Inc.) for 3 h at 20 to 25°C. For HRP-ConA, the sheet blotted with the mannoprotein fractions was soaked in TBS containing 3% bovine serum albumin (BSA) and reacted with HRP-conjugated ConA (2 μg/ml) for 60 min at 20 to 25°C. Finally, all sheets were incubated in a mixture of 4-chloro-1-naphthol and H2O2 in TBS (18).
IFM and flow cytometry.
Hydrophilic yeast cells of C. albicans grown in GYEP for 24 h at 37°C were fixed with 3% formalin in 25 mM PB (pH 7.4) for 2 h at 20 to 25°C. After several washes with PB, the fixed samples were treated with PBS–3% BSA for 30 min at 20 to 25°C and then incubated with rabbit anti-scmn1/Fr.II (1:100 in PBS containing 1% BSA) rabbit serum or rabbit anti-scmn4/Fr.II (1:100 in 1% BSA–PBS) for 2 h at 37°C. After washing with 1% BSA–PBS, the samples were reacted with fluorescein isothiocyanate-conjugated anti-rabbit IgG goat serum (diluted 1:50 in PBS containing 1% BSA) (Cappel Research Products) for 2 h at 20 to 25°C. For indirect immunofluorescence microscopy (IFM) of yeast cells adhered to the splenic tissue, cryosections of the tissue with adherent yeast cells were fixed with 3% formalin for 2 h at 20 to 25°C. The sections were further processed by the same procedures as described for yeast cells. As controls, yeast cells were incubated with the antisera in the presence of Fr.IIS at 250 μg/ml, or antibodies against PMPC were omitted in the first incubation. Samples were observed with a BH2-RFK epifluorescence microscope (Olympus Optical Co., Ltd., Tokyo, Japan) and photographed on Kodak Tmax 400 film. This method was used to determine the distribution patterns of the adhesin complexes on the yeast cell surface.
For flow cytometry, hydrophilic C. albicans yeast cells were prepared as for IFM and analyzed in a flow cytometer (EPICS XL; Coulter Corporation, Miami, Fla.) equipped with an argon laser (488 nm) (14). This method was used to define the proportion of C. albicans cells that expressed mannan adhesins on the cell wall surface.
Determination of adhesion activity.
To test adhesin activity of the yeast PMPC, both an ex vivo adherence inhibition assay and an ex vivo binding assay were used as described previously (18, 19). In brief, for the inhibition assay, each PMPC sample at a concentration of 10, 20, 50, or 100 μg/ml (diluted in DMEM) was added to splenic cryosections mounted on glass slides and incubated for 15 min at 4 to 6°C. After being washed in DMEM, C. albicans yeast cells were added to the sections, and subsequent yeast binding to the splenic tissue was determined under a light microscope. For ex vivo binding assays, latex beads were coated with each PMPC of C. albicans or S. cerevisiae as before (19, 27). Latex beads (PolybeadR Microparticles; Polysciences Inc., Warrington, Pa.) were incubated with one of the PMPC fractions (1 mg/ml) in 0.067 M carbonate buffer (pH 9.6) for 2 h at 21 to 25°C. After several washes, the beads were blocked with 1% BSA (Sigma) for 2 to 3 h at 21 to 25°C. BSA-coated latex beads were used as a negative control. The beads were washed in DMEM three times and stored at 4°C. PMPC-coated latex beads (1.5 × 108 beads/ml) were added to the cryosections mounted on glass slides and incubated for 20 min at 4 to 6°C. After being fixed with 2.5% glutaraldehyde and washed in DMEM, the coated beads that attached to the splenic marginal zone were viewed in an Olympus BH-2 microscope equipped with phase-contrast optics. In both adherence experiments, the mean number of yeast cells or the adhesin-coated latex beads was calculated from 20 different fields of the splenic marginal zone (five fields per section; we examined four sections). This experiments was repeated twice. To evaluate the results of adhesin activity among the PMPCs, Student’s t test was performed.
Nuclear magnetic resonance (NMR) analysis.
The presence of α-1,2-, α-1,3-, and β-1,2-linked mannose chains in each PMPC of C. albicans and S. cerevisiae was analyzed in a JEOL-GSX 400 spectrometer as described previously (32).
Measurement of carbohydrate.
Total amount of carbohydrates in each PMPC was determined by the phenol-sulfuric acid method (10) with d-mannose as a standard. The contents of carbohydrate in Fr.IIS, Fr.II/scwt, Fr.II/scmn1, Fr.II/scmn2, and Fr.II/scmn4 used were 95.6, 93.5, 91.4, 93.8, and 92.8%, respectively.
RESULTS
Characterization of various PMPCs.
In dot blots, ConA reacted with all PMPC samples isolated from C. albicans and S. cerevisiae wild-type and mnn mutant strains. Anti-factor 5 and MAb B6.1, which react with β-1,2-linked mannosyls of C. albicans, did not react with Fr.IIS or with any PMPC derived from S. cerevisiae. Anti-factor 9 rabbit serum, which detects core α-mannan, reacted strongly with scmn2/Fr.II (data not shown). Both anti-scmn1/Fr.II and anti-scmn4/Fr.II reacted with PMPCs from C. albicans and S. cerevisiae wild-type and mutant strains but not with scmn2/Fr.II). NMR analysis of Fr.IIS from C. albicans and PMPCs from S. cerevisiae showed no evidence of β-1,2-linked oligomannosyl residues, scmn1/Fr.II did not contain α-1,3-linked mannosyl residues, and scmn2/Fr.II did not contain α-1,2-or α-1,3-linked mannose chains (data not shown). These were the results expected, based on previous reports (2, 32).
Effects of enzymatic digestion on the adhesin activity of Fr.IIS.
The acid-stable part (Fr.IIS) of the C. albicans PMPC has adhesin activity, as determined by its ability to inhibit binding of C. albicans yeast cells to the splenic marginal zone (19). The ability of Fr.IIS to inhibit the binding was strongly reduced after treatment with an α-mannosidase that generally degrades α-mannan (18). Also, α-1,2-specific mannosidase reduced the adhesin activity of Fr.IIS, but to a lesser extent than α-mannosidase treatment (Table 2). In contrast, proteinase K treatment had no effect on the adhesin activity of Fr.IIS (Table 2).
TABLE 2.
Effects of α-mannosidases and proteinase K on adhesin activity of Fr.IIS
| Enzymea | % Adhesin activity of Fr.IIS (SD)b |
|---|---|
| α-Mannosidase (U)c | |
| 0 (buffer only) | 90.7 (4.5) |
| 0.44 | 71.0 (8.3) |
| 0.55 | 43.2 (10.3) |
| 0.66 | 23.1 (6.5) |
| 0.89 | 6.3 (9.8) |
| 1.13 | 1.8 (5.7) |
| α-1,2-Mannosidase (μU)d | |
| 0 (buffer only) | 94.5 (2.3) |
| 0.5 | 83.2 (7.8) |
| 1 | 72.2 (11.5) |
| 5 | 46.9 (13.4) |
| 10 | 41.3 (8.9) |
| 20 | 30.2 (17.8) |
| Proteinase K (μg) | |
| 0 (buffer only) | 87.4 (2.9) |
| 100 | 83.3 (2.1) |
The indicated amount of each enzyme was added to 1 ml containing 200 μg of Fr.IIS. Samples were incubated with α-mannosidase or α-1,2-mannosidase for 18 h at 37°C or with proteinase K for 60 min at 37°C.
Assessed by the ability of the fractions to block binding of C. albicans to the splenic marginal zone as described previously (17, 18).
Isolated from C. ensiformis; releases α-1-2-, α-1-3-, and α-1-6-linked mannoses.
Isolated from A. saitoi; releases α-1-2-linked mannoses at the nonreducing end.
Adhesin activity of acid-stable oligomannosyls obtained from acetolysis of the C. albicans PMPC.
We tested the oligomannosyls (M1 to M6) isolated as acetolysis products from the acid-stable part of C. albicans PMPC for the ability to inhibit yeast cell adherence by adding each to the splenic cryosections prior to addition of yeast cells. The binding of C. albicans to the marginal zone was not inhibited by any of the oligomannosyls tested at 20 μg/ml (inhibition of 2 to 3%), and only slight (3 to 10%) inhibition occurred when each was tested at 100 μg/ml.
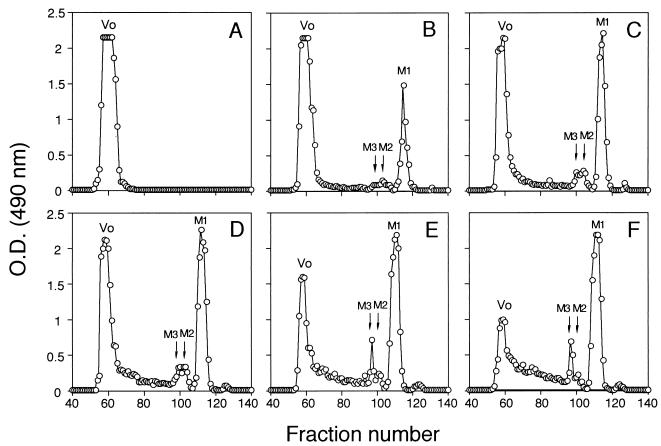
Sulfuric acid hydrolysis inactivates adhesin activity of Fr.IIS.
To release mannose units from the nonreducing ends of the oligomannosyl side chains, Fr.IIS was treated with 0.6 N sulfuric acid for 0 to 6 h at 100°C. Samples from each time period were separated on a size exclusion column (Fig. 1). A peak corresponding to a single released mannose (M1) increased during the treatment, and peaks of di- and trisaccharides (M2 and M3) also slightly increased during the treatment; in contrast, the amount of void-volume material decreased by 6 h of chemical treatment (Fig. 1). During the acid hydrolysis, oligosaccharides greater than trisaccharides were not detected. The fractions corresponding to M1, M2 plus M3, and the void-volume material were pooled and lyophilized. All samples were added to the splenic cryosections at 20 μg/ml to test for adhesin activity by the ability to inhibit adherence of yeast cells to the splenic marginal zone in the ex vivo assay. In all cases, M1 and a mixture of M2 and M3 did not show adhesin activity (Table 3). The adhesin activity of the void-volume material decreased as a function of time upon treatment with sulfuric acid (Table 3). By 6 h of treatment, essentially all Fr.IIS adhesin activity was destroyed and none of the released degradation products had activity. These data suggested that oligomannosyl side chains and side chain length are important in the adhesin activity of the acid-stable part of the PMPC.
FIG. 1.
Size exclusion column elution patterns of Fr.IIS sulfuric acid hydrolysis products. Fr.IIS was treated with 0.6 N H2SO4 for 0 (A), 0.75 (B), 1.5 (C), 3 (D), 4.5 (E) and 6 (F) h at 100°C. At each time period, the treated samples were applied to a size exclusion column and eluted with distilled water, and carbohydrate content was monitored by the phenol-sulfuric acid method. M1, M2, and M3 indicate mono-, di-, and trisaccharides, respectively, removed from Fr.IIS by the sulfuric acid treatment. The amount of M1, M2, and M3 increased during the treatment, whereas the fractions eluted in the void volume (Vo) decreased with the same treatment. The elution pattern of scmn1/Fr.II treated with the sulfuric acid was essentially the same as that from hydrolysis of Fr.IIS (data not shown). O.D., optical density.
TABLE 3.
Adhesin inactivation by sulfuric acid hydrolysis
| Treatment time (h)a | % inhibition (SD)b
|
||
|---|---|---|---|
| Void-volume material | M3 + M2 | M1 | |
| 0 | 96.2 (3.2) | ||
| 0.75 | 83.5 (6.7) | 3.0 (2.1) | 2.1 (4.6) |
| 1.5 | 45.3 (10.9) | 2.5 (1.9) | 2.3 (1.8) |
| 3.0 | 38.5 (21.3) | 2.2 (3.3) | 2.0 (6.7) |
| 4.5 | 18.5 (11.4) | 3.8 (2.9) | 2.1 (2.4) |
| 6.0 | 6.5 (17.3) | 3.0 (1.3) | 1.9 (4.2) |
Fr.IIS was treated with 0.6 N sulfuric acid at 100°C for the indicated times.
Fractions corresponding to each peak were pooled and lyophilized for determination of adhesin activity. Each fraction was added to cryosections at 20 μg/ml and incubated for 20 min at 4 to 6°C; subsequent binding of C. albicans to the splenic marginal zone was scored by microscopic evaluation as described previously (18, 19).
Adhesin activity of PMPC from S. cerevisiae wild-type and mnn mutants.
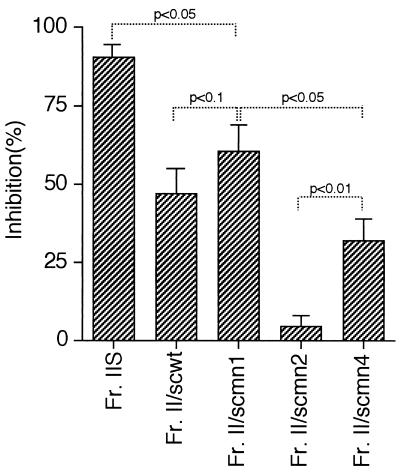
PMPC samples of S. cerevisiae strains were tested for adhesin activity at 10 μg/ml in the ex vivo assay as described above for PMPC from C. albicans. The greatest adhesin activity was associated with PMPC from S. cerevisiae mutant mnn1 (Fig. 2). scmn1/Fr.II caused a 60% inhibition of C. albicans yeast cell adherence to the splenic marginal zone. This inhibition was slightly stronger than that of scwt/Fr.II (P < 0.1) but weaker than that of C. albicans Fr.IIS (P < 0.05), which gave about 90% inhibition when tested at 10 μg/ml. scmn4/Fr.II inhibited yeast binding to a lesser extent than scmn1/Fr.II (P < 0.05), but scmn2/Fr.II showed essentially no inhibitory activity at 10 μg/ml (Fig. 2). When tested at 50 μg/ml, adhesin activity was also negligible (inhibition of C. albicans yeast cells binding was only 7.2% ± 6.3%).
FIG. 2.
Inhibitory activities of PMPC from S. cerevisiae wild-type and mnn mutant strains. scwt/Fr.II, scmn1/Fr.II, scmn2/Fr.II, and scmn4/Fr.II were added to the cryosections of mouse spleen mounted on glass slides and compared with Fr.IIS for ability to inhibit binding of C. albicans to the splenic marginal zone. scwt/Fr.II, scmn1/Fr.II, and scmn4/Fr.II inhibited strongly the binding of C. albicans, whereas scmn2/Fr.II did not inhibit yeast binding; scmn1/Fr.II showed the strongest inhibition among the S. cerevisiae PMPCs, but its adhesin activity was slightly lower than that of Fr.IIS. These results suggested that α-1,2-linked side chains are critical for adhesin activity of the PMPC; this inference is supported by the results from Table 1. Percent inhibition was calculated as 100 − (100 × number of yeasts/files adhered in the presence of PMPC)/(number of yeasts/field adhered in the DMEM without PMPC). P values obtained by Student’s t test are shown.
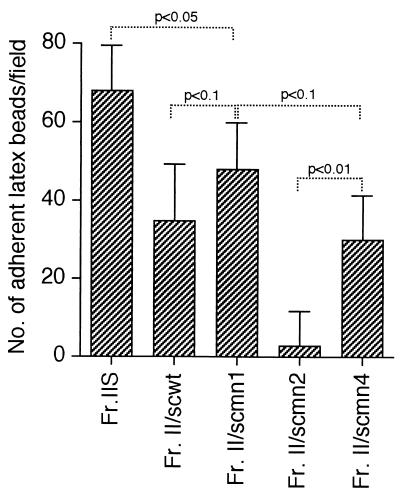
In direct tests for the adhesin activity of S. cerevisiae PMPC, latex beads were coated with each extract and used in the ex vivo splenic binding assay. All latex beads except those coated with scmn2/Fr.II adhered to the splenic marginal zone (Fig. 3). The numbers of latex beads coated with Fr.IIS, scwt/Fr.II, scmn1/Fr.II, scmn2/Fr.II, and scmn4/Fr.II that adhered to the marginal zone were 68.7 ± 12.2, 35.6 ± 15.1, 48.5 ± 12.1, 3.3 ± 8.8, and 30.4 ± 10.1 per field, respectively. These results correlated well with the adhesin activity of the S. cerevisiae PMPCs as demonstrated above by the ex vivo inhibition assay (Fig. 2).
FIG. 3.
Adherence of S. cerevisiae PMPC-coated latex beads to the splenic marginal zone. Latex beads coated with Fr.IIS, scwt/Fr.II, scmn1/Fr.II, scmn2/Fr.II, or scmn4/Fr.II were added to splenic cryosections mounted on glass slides. The glass slides were incubated for 20 min at 4 to 6°C, and the number of the latex beads that adhered to the marginal zone was determined. Specific binding of the latex beads to the marginal zone was found with the latex beads coated with Fr.IIS, scwt/Fr.II, scmn1/Fr.II, and scmn4/Fr.II. Few latex beads coated with Fr.II/scmn2 bound to the marginal zone. These results are consistent with those obtained in the ex vivo inhibition test shown in Fig. 2. P values obtained by Student’s t test are shown.
Expression of the C. albicans acid-stable moiety of the PMPC on the yeast cell surface.
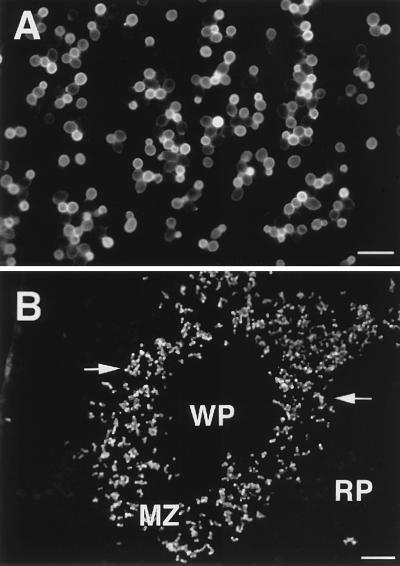
If the acid-stable moiety is an adhesin, it should be expressed on the cell wall surface. We used rabbit polyclonal antibodies, anti-scmn1/Fr.II and anti-scmn4/Fr.II rabbit polyclonal antibodies, and IFM to analyze expression. Stationary-phase hydrophilic C. albicans yeast cells incubated with anti-scmn1/Fr.II showed bright fluorescence over the entire surface of all yeast cells (Fig. 4A). This was confirmed by flow cytometric analysis, which showed that 99.8% of the stationary-phase C. albicans yeast cells expressed the PMPC acid-stable moiety on the cell wall surface (data not shown); Fig. 4B shows the same expression pattern for yeast cells that adhered to the splenic marginal zone. Results were similar when anti-scmn4/Fr.II was used (data not shown). Negative controls, which consisted of yeast cells reacted with normal rabbit serum, showed no detectable fluorescence (data not shown). In addition, reaction of C. albicans cell walls with the two antibodies was inhibited by adding of C. albicans Fr.IIS at 250 μg/ml at the time of addition of the antibodies (data not shown).
FIG. 4.
Expression of the acid-stable part of the PMPC on the surface of C. albicans cell wall. Yeast cells of C. albicans that were grown in GYEP for 24 h at 37°C (A) or allowed to adhere to splenic sections in an ex vivo assay (B) were incubated with anti-scmn1/Fr.II polyclonal rabbit serum. In both, a bright fluorescence showing the expression of the acid-stable PMPC on the surface of C. albicans cell wall was observed. Staining patterns similar to that for the anti-scmn1/Fr.II antibody were observed when anti-scmn4/Fr.II was used in IFM (data not shown). Marginal zone (MZ), white pulp (WP), and red pulp (RP) are labeled; arrows indicate adhered yeast cells to the marginal zone. Bars represent 10 μm (A) and 100 μm (B).
DISCUSSION
The C. albicans PMPC is composed of acid-stable mannan and phosphate-bound acid-labile mannan parts, the entire complex of which is N linked to protein (24, 32). We have studied adhesin activity of the C. albicans PMPC in relation to adherence of yeast cells to macrophages of the mouse splenic marginal zone (13, 17, 18) and found that the adhesin activity of the complex is due to acid-labile and acid-stable parts (19, 27). The adhesin associated with the acid-labile part of the PMPC is a β-1,2-mannotetraose (27). Since adhesin activity associated with the acid-stable part of the molecule appears to be stronger than that of the mannotetraose (19), in this study we sought to define the minimum chemical requirement for adhesin activity of this adhesin moiety.
The acid-stable part consists of an α-1,6-linked mannan outer core unit and α-1,2-and α-1,3-linked oligomannosyl chains side chains bound to the core (23). More recently, an α-1,6-linked mannose side chain was found in the acid-stable part of the C. albicans PMPC (33). The findings reported here demonstrate that digestion of Fr.IIS with a general α-mannosidase completely destroyed the adhesin activity of Fr.IIS, as evidenced by loss of the ability of the digest to inhibit binding of C. albicans yeast cells to the splenic marginal zone in an ex vivo assay. This result is similar to our earlier findings (18) and indicates that mannose chains are essential for adhesin activity of the acid-stable part of the PMPC. Our finding that α-1,2-mannosidase treatment incompletely decreased the adhesin activity of Fr.IIS suggests that α-1,2-linked oligomannosyl side chains are partially responsible for the adhesin activity. These side chains are not sufficient, however, for adhesin activity because yeast binding was not inhibited by any acetolysis-released oligomannosyl side chains (M1 to M6) at 20 μg/ml and only slightly affected at 100 μg/ml; the latter value is more than 10 times the concentration required for inhibition of adherence due to Fr.IIS.
One interpretation of the results from the α-1,2-mannosidase digestion and subsequent lack of adhesin activity associated with the isolated oligomannosyl side chains is that the α-1,6-mannan core is responsible for the acid-stable adhesin activity. However, the results from experiments using scmn2/Fr.II, which contains the α-1,6-mannan core but lacks all α-1,2-and α-1,3-linked oligomannosyl side chains (2), showed that the core alone is not sufficient for adhesin activity. These results imply that both the mannan outer core and the oligomannosyl side chains are essential for adhesin activity of the acid-stable part of the PMPC.
To investigate the relationship between side chain length and adhesin activity, we used partial sulfuric acid hydrolysis. Others have shown that sulfuric acid cleaves glycosidic linkages at the terminal mannoses of the oligomannosyl side chains (22). When Fr.IIS was treated with sulfuric acid for various lengths of time and elution profiles of the hydrolysates were monitored on a size exclusion column, the relative amount of monomeric mannose (M1) increased during the treatment, and peaks corresponding to M2 and M3 also increased slightly with time. These results are consistent with hydrolysis preference for terminal mannose units rather than random breakage of glycoside bonds. Fractions eluted in the void volume, therefore, would be composed of the α-mannan core with truncated versions of the oligomannosyl side chains, the lengths of which were proportional to the time of hydrolysis. The adhesin activity of the fraction eluted in the void volume was clearly reduced as a function of time of hydrolysis (Table 3). The above results strongly suggest that adhesin activity is directly related to the mannosyl chain length. This suggestion is supported by the experiments using the S. cerevisiae PMPCs. Wild-type S. cerevisiae PMPCs have shorter oligomannosyl side chains than C. albicans (2). Accordingly, the PMPC from the wild-type strain, S. cerevisiae X2180, had less adhesin activity than the C. albicans Fr.IIS. We used the simpler PMPC structure from S. cerevisiae wild-type and mnn mutant strains to explore the minimum chemical requirements for adhesin activity of the acid-stable part of the PMPC. All PMPC extracts except that from S. cerevisiae mnn2 showed adhesin activity, but lower than that of PMPC from C. albicans. These results confirm that mannosyl side chains are essential for adhesin activity and indicate that chain length is also important. The nature the side chain linkages appears to be of less significance; in fact, our results for the mnn1 mutant suggests that terminal α-1,3-linked mannose units may even interfere with adhesin activity. Our conclusion that side chain length is important was also supported by preliminary experiments in which the more elaborate PMPC from a serotype A strain of C. albicans had more adhesin activity than PMPC from a serotype B strain which has shorter side chains (32).
Molecules involved in adherence of yeast cells to host cells or tissues should be expressed on the surface of the fungal cell wall. In accordance with this supposition, several morphological observations by electron microscopy have shown that C. albicans mannans are located on the surface of cell wall and along the plasmalemma (27, 36). In this study, polyclonal antibodies against S. cerevisiae mannan used to detect the acid-stable part of the C. albicans PMPC reacted with the entire surface of yeast cells, as observed by IFM. Flow cytometric analysis revealed that about 99.8% yeast cells expressed this moiety on the cell wall surface. These findings were consistent with the acid-stable adhesin activity of the cell wall PMPC of C. albicans. In addition, the acid-labile adhesin moiety of the PMPC was expressed over the entire surface of yeast cells (14).
Antibodies against the Candida phosphomannan complex might be expected to block adherence, but they do not. Possible explanations are that although the antibodies react with the phosphomannan complex on the cell wall surface, their specificity is for nonadhesin sites within this large complex. Thus, the adhesin sites may still be free to attach to Candida receptors in the splenic marginal zone. This is a logical explanation because the antibody was generated against S. cerevisiae mannan, which would preclude having antibodies with specificities against certain adhesin sites in the acid-stable and acid-labile parts of the phosphomannan complex of C. albicans. Adherence of the Candida yeast cells may occur in vivo even in the presence of antibodies specific for adhesin sites in the phosphomannan complex, provided that the antibodies on the yeast surface results in rapid complement activation and adherence via C3 receptors on marginal zone macrophages. In the presence of Candida-specific antibodies, complement activation on the yeast cell surface has been shown to occur within 1 to 2 min (25), which would be rapid enough to promote adherence to the splenic marginal zone macrophages.
ACKNOWLEDGMENTS
We thank Leo Dewald for excellent assistance and N. Shibata, Tohoku College of Pharmacy, for his special assistance with NMR analysis and for providing oligomannosyls of C. albicans.
This work was supported by grants for Scientific Researcher from the Ministry of Education, Science and Culture (grant 09670278) and from the Ministry of Health and Welfare (to T.K.) and grant 5RO1 AI 24912 to J.E.C. from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ataoglu H, Zueco J, Sentandreu R. Characterization of epitopes recognized by Candida factors 1 and 9 antisera by use of Saccharomyces cerevisiae mnn mutants. Infect Immun. 1993;61:3313–3317. doi: 10.1128/iai.61.8.3313-3317.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballou C E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with non-conditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. doi: 10.1016/0076-6879(90)85038-p. [DOI] [PubMed] [Google Scholar]
- 3.Brassart D, Woltz A, Golliard M, Neeser J-R. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fucα1-2Galβ-bearing complex carbohydrates. Infect Immun. 1991;51:337–343. doi: 10.1128/iai.59.5.1605-1613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casanova M, Lopez-Ribot J L, Monteagudo C, Llombart-Bosch A, Sentandreu R, Martinez J P. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect Immun. 1992;60:4221–4229. doi: 10.1128/iai.60.10.4221-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- 6.Calderone R A, Braun P C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaffin W L, Collins B, Marx J N, Cole G T, Morrow K J., Jr Characterization of mutant strains of Candida albicans deficient in expression of a surface determinant. Infect Immun. 1993;61:3499–3458. doi: 10.1128/iai.61.8.3449-3458.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;133:629–636. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 9.Cutler J E, Brawner D L, Hazen K C, Jutila M A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990;58:1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 11.Fradin, C., T. Jouault, A. Mallet, J. M. Mallet, D. Camus, P. Sinay, and D. Poulain. β-1,2-Linked oligomannosides inhibit Candida albicans binding to murine macrophage. J. Leukoc. Biol. 60:81–87. [DOI] [PubMed]
- 12.Fukayama M, Calderone R A. Adherence of cell surface mutants of Candida albicans to buccal epithelial cells and analyses of the cell surface proteins of the mutants. Infect Immun. 1991;59:1341–1345. doi: 10.1128/iai.59.4.1341-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Rooijen H V, Cutler J E. Binding of Candida albicans yeast cells to mouse popliteal lymph node tissue is mediated by macrophages. Infect Immun. 1993;61:3244–3247. doi: 10.1128/iai.61.8.3244-3249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazen K C, Brawner D L, Riesselman M H, Jutila M A, Cutler J E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Lucho V, Ginsburg V, Krivan H C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Galβ1-4Glcβ1-1Cer), a possible adhesion receptor for yeasts. Infect Immun. 1990;58:2085–2090. doi: 10.1128/iai.58.7.2085-2090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanbe T, Jutila M A, Cutler J E. Evidence that Candida albicans binds via a unique adhesion system on phagocytic cells in the marginal zone of the mouse spleen. Infect Immun. 1992;60:1972–1978. doi: 10.1128/iai.60.5.1972-1978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanbe T, Cutler J E. Evidence for adhesin activity in the acid-stable moiety of the phosphomannoprotein cell wall complex of Candida albicans. Infect Immun. 1994;62:1662–1668. doi: 10.1128/iai.62.5.1662-1668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klotz S A. Fungal adherence to the vascular compartment: a critical step in the pathogenesis of disseminated candidiasis. Clin Infect Dis. 1992;14:340–347. doi: 10.1093/clinids/14.1.340. [DOI] [PubMed] [Google Scholar]
- 21.Klotz S A, Smith R L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991;163:604–609. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Shibata N, Yonezu T, Suzuki S. Application of mild acetolysis to confirm the comb-like structure of cell wall mannan from Pichia pastoris IFO 0948 strain. Chem Pharm Bull. 1988;36:3168–3172. [Google Scholar]
- 23.Kobayashi H, Shibata N, Suzuki S. Evidence for oligomannosyl residues as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun. 1992;60:2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi H, Shibata N, Nakada M, Chaki S, Mizugami K, Ohkubo Y, Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990;278:195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- 25.Kozel T R, Weinhold L C, Lupan D M. Distinct characteristics of initiation of the classical and alternative complement pathway by Candida albicans. Infect Immun. 1996;64:3360–3368. doi: 10.1128/iai.64.8.3360-3368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehrer N, Segal E, Lis H, Gov Y. Effect of Candida albicans cell wall components on the adhesion of the fungus to human and murine vaginal mucosa. Mycopathologia. 1988;102:115–121. doi: 10.1007/BF00437448. [DOI] [PubMed] [Google Scholar]
- 27.Li R K, Cutler J E. Chemical definition of an epitope/adhesin molecule on Candida albicans. J Biol Chem. 1993;268:18293–18299. [PubMed] [Google Scholar]
- 28.McCourtie J, Douglas L J. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol. 1985;131:495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- 29.Miyakawa Y, Kuribayashi T, Kagaya K, Suzuki M. Role of specific determinants in mannan of Candida albicans serotype A in adherence to human buccal epithelial cells. Infect Immun. 1992;60:2493–2499. doi: 10.1128/iai.60.6.2493-2499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riesselman H M, Kanbe T, Cutler J E. Improvements and important considerations of an ex vivo assay to study interactions of Candida albicans with splenic tissue. J Immunol Methods. 1991;145:153–160. doi: 10.1016/0022-1759(91)90321-6. [DOI] [PubMed] [Google Scholar]
- 31.Sandin R L, Rogers A L, Paterson R J, Beneke E S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982;35:79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as β-1,2-linked oligomannosyl residues. Infect Immun. 1992;60:4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata N, Ikuta K, Imai T, Satoh Y, Satoh R, Suzuki A, Kojima C, Kobayashi H, Hisamichi K, Suzuki S. Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem. 1995;270:1113–1122. doi: 10.1074/jbc.270.3.1113. [DOI] [PubMed] [Google Scholar]
- 34.Stahl D. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992;4:49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- 35.Tosh F D, Douglas J J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun. 1992;60:4734–4739. doi: 10.1128/iai.60.11.4734-4739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tronchin G, Poulain D, Biguet J. Cytochemical and ultrastructural studies of the cell wall of Candida albicans. Arch Microbiol. 1979;1234:245–249. doi: 10.1007/BF00406657. [DOI] [PubMed] [Google Scholar]