Abstract
A Brucella suis mgtC mutant is defective for growth within macrophages and in low-Mg2+ medium. These phenotypes are strikingly similar to those observed with mgtC mutants from Salmonella enterica and Mycobacterium tuberculosis, two other pathogens that proliferate within phagosomes. MgtC appears as a remarkable virulence factor that would have been acquired by distantly related intracellular pathogens to contribute to the adaptation to a low-Mg2+ environment in the phagosome.
Intracellular bacterial pathogens such as Salmonella enterica, Mycobacterium tuberculosis, and Brucella species have evolved virulence strategies to modulate host cell biology and survive within the harsh environment of the phagosome (1, 8, 17). S. enterica serovar Typhimurium and M. tuberculosis, two unrelated intracellular pathogens producing different diseases, both require the MgtC protein for survival inside macrophages and growth in a low-Mg2+ medium (2, 5). The macrophage survival defect of an S. enterica serovar Typhimurium mgtC mutant can be partially suppressed by adding Mg2+ to the cell culture medium, suggesting that the growth defect is related to a rate-limiting Mg2+ concentration inside the phagosome and that MgtC somehow has a role in Mg2+ uptake (2). Other studies based on mgtC transcriptional induction within macrophages have suggested that the Salmonella-containing phagosome is a Mg2+-deprived environment (6, 18), and we have proposed that M. tuberculosis also faces a low-Mg2+ environment inside phagosomes (5). MgtC is a predicted inner membrane protein of unknown function that contains an N-terminal transmembrane domain and a C-terminal soluble domain (19). In S. enterica serovar Typhimurium, mgtC is cotranscribed with mgtB, encoding a Mg2+ transporter (19). The mgtC gene is not essential for membrane insertion or transport function of MgtB (20), and MgtC does not appear itself as a cation transporter (14).
Genes encoding MgtC-like proteins are found in a limited number of eubacterial genomes, including those of Brucella spp., and analysis of the phylogeny of MgtC-like proteins and mgtC chromosomal regions suggests that mgtC has been acquired by horizontal gene transfer repeatedly throughout bacterial evolution (3). Phylogenetic analysis shows that the S. enterica serovar Typhimurium and M. tuberculosis MgtC are grouped with a subclass of MgtC-like proteins, which includes that of Brucella melitensis. In B. melitensis, MgtC is encoded on an 8-kb region of the chromosome II that is absent in the genome of the closely related bacterium Mesorhizobium loti (3). This region is adjacent to a tRNA gene, arguing in favor of the hypothesis of a region acquired by horizontal gene transfer (7). The origin of mgtC remains unknown, and genes linked to mgtC are not conserved with the exception of mgtB that is found both in S. enterica serovar Typhimurium and Brucella spp. (3). We have proposed that MgtC-like proteins phylogenetically close to the S. enterica serovar Typhimurium and M. tuberculosis MgtC proteins might exhibit a similar function and play a role in the virulence of pathogens such as Brucella spp., which also replicate within a phagosome (4). In the present study, we investigate the role of the Brucella suis MgtC protein for growth in macrophages and low-Mg2+ medium.
Construction of a mgtC mutant in B. suis.
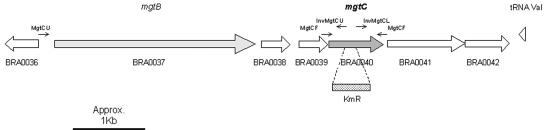
The chromosome II region of B. suis harboring mgtC is schematized in Fig. 1. The MgtC protein of B. suis, which is 100% identical to that of B. melitensis, exhibits 40% overall sequence identity with the MgtC proteins of S. enterica serovar Typhimurium and M. tuberculosis, with the highest level of homology (65%) found in the N-terminal part of the protein. Some differences in genetic organization are evident, for example in B. suis mgtC is found downstream of mgtB (Fig. 1) whereas a mgtCB operon is found in serovar Typhimurium (no clear MgtB homologue is found in M. tuberculosis). The intergenic region (770 bp) contains two putative open reading frames, and it is not clear whether the mgtB and mgtC genes are grouped in the same operon in Brucella spp. A transposon insertion mutation in mgtB has been recovered in a genetic screening of B. melitensis mutants deficient for growth in macrophages (12). If mgtB and mgtC are organized as an operon, the attenuation might be due to a polar effect on mgtC. To investigate the role of mgtC in B. suis, we have constructed a mutant harboring a deletion and a kanamycin resistance cassette in the mgtC gene (Fig. 1).
FIG. 1.
Schematic representation of the B. suis 1330 chromosome II region around mgtC (16). Orientation of the different genes is indicated by large arrows. The orientation and the location of the different primers used in this study are indicated by small arrows. The position of the resistance cassette (KmR) inserted during the construction of the mgtC::Kan mutant by allelic replacement is indicated.
The mgtC gene was amplified by PCR from B. suis 1330 (ATCC 23444T) genomic DNA with MgtCF (5′-TAATCCAGAAAGGCCACCGG-3′) and MgtCR (5′-CGGCGATACGCAGTGGCAGG-3′) primers. A similar fragment was amplified when genomic DNA from B. melitensis, B. ovis, B. canis, and B. abortus was used as template, showing that the gene is conserved throughout the Brucella genus (not shown). The PCR product from B. suis 1330 was cloned into the pGEMT vector (Promega, Madison, WI), which confers resistance to ampicillin and is unable to replicate in Brucella spp. A fragment of 152 bp within the mgtC gene was deleted by an inverse PCR using InvMgtCU (5′-TGCAGAGGCTCAGGTGCGCG-3′) and InvMgtCL (5′-CGCCGCAAAGCACGCCAACA-3′) primers. The 152-bp fragment was replaced by a 1.2-kb HincII fragment carrying the kanamycin resistance gene from plasmid pUC4K (Pharmacia, Peapack, NJ), giving pGEMT-mgtC::kan. B. suis was transformed with this suicide plasmid by electroporation (10). Double recombination events between the chromosomal mgtC gene and the inactivated mgtC gene on the suicide plasmid led to a mgtC::Kanr ampicillin-sensitive strain. The chromosomal insertion within mgtC was verified by Southern blot analysis (data not shown). To complement the mgtC::Kan mutant, the mgtC gene was cloned into pBBR1MCS (11), which confers resistance to chloramphenicol and replicates in Brucella. Because the location of the mgtC promoter is unknown, we cloned a region upstream of mgtC that includes the mgtBC intergenic region and the mgtB gene and its putative promoter region (Fig. 1). The mgtBC region was amplified by PCR from B. suis genomic DNA with MgtCU (5′-GGTCAGGCGGCTCAGGAAAGCC-3′) and MgtCR primers and was first cloned into pGEMT easy (Promega). An EcoRI digestion fragment harboring the mgtBC region was then subcloned into pBBR1MCS, giving pBBR1-mgtBC.
A B. suis mgtC mutant is attenuated for growth within J774 macrophages.
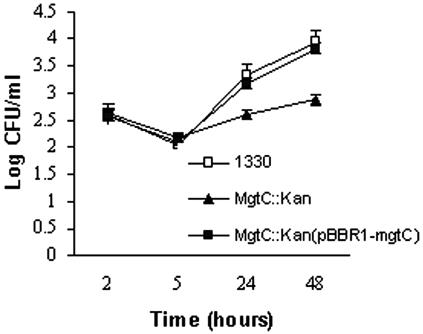
The role of B. suis mgtC gene in intracellular survival was evaluated in murine J774 macrophage-like cells as described previously (15). Briefly, 24-well plates were seeded with 5 × 105 cells in RPMI 1640 medium supplemented with 10% fetal calf serum and infected 24 h later with B. suis at a multiplicity of infection of 20. After 30 min, cells were washed three times with phosphate-buffered saline and reincubated in cell medium supplemented with 30 μg ml−1 gentamicin. The number of intracellular bacteria was measured at 2, 5, 24 and 48 h postinfection. As shown in Fig. 2, no significant difference was found at 2 h or 5 h postinfection between the wild type and the mgtC::Kan mutant, indicating that mgtC is not involved in the early stages of intramacrophage growth. However, the mgtC::Kan strain showed significantly lower levels of intracellular multiplication at 24 h and 48 h postinfection (Fig. 2). The multiplication defect of the mgtC::kan mutant can be completely suppressed by complementation with the pBBR1-mgtBC plasmid, since the complemented strain multiplies at a rate similar to that of the wild-type strain (Fig. 2). These results indicate that MgtC is required for the intramacrophage growth of B. suis.
FIG. 2.
The mgtC gene of B. suis is required for intramacrophagic survival. Numbers of CFU were determined at the indicated times. Values are the geometric means ± standard errors of the results from one representative experiment done in triplicate out of four.
MgtC is required for B. suis growth in low-Mg2+ medium.
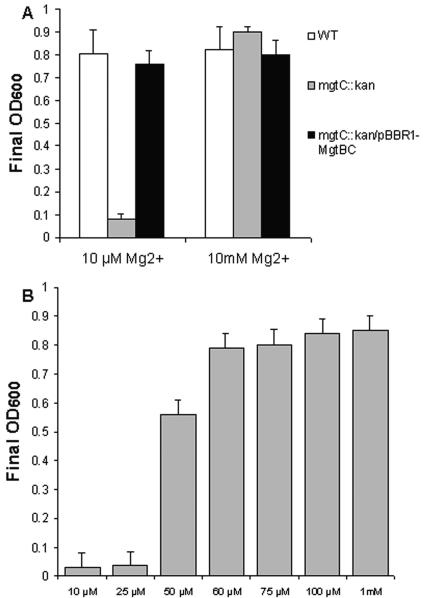
To further compare the similarities between the role of MgtC in B. suis and that described in serovar Typhimurium and M. tuberculosis, we investigated the growth of the mgtC::Kan mutant in low-Mg2+ liquid medium. Wild-type and mgtC::Kan bacteria were grown overnight at 37°C in tryptic soy broth and diluted 1:100 in NCE-minimal medium (13) supplemented with 0.1% Casamino Acids, 1% arabinose, 1% yeast extract, and either 10 μM or 10 mM MgCl2. Optical density at 600 nm (OD600) was measured after 24 h growth. The mgtC::Kan mutant is severely impaired for growth in NCE supplemented with 10 μM Mg2+ compared to the wild-type strain. The growth defect is due to the low-Mg2+ concentration because both strains reached similar OD in the presence of 10 mM Mg2+ (Fig. 3A). By using increasing Mg2+ concentrations, we found that the mgtC::Kan mutant is deficient for growth in Mg2+ concentrations below 60 μM (Fig. 3B); that is strikingly similar to what is found with mgtC mutants of S. enterica serovar Typhimurium (data not shown) and M. tuberculosis (5). This defect appears specific to Mg2+ since neither 1 mM CaCl2 nor 1 mM MnCl2 could restore growth (data not shown). On the other hand, growth of the mgtC::Kan mutant in low-Mg2+ medium was fully restored by complementation with the pBBR1-mgtBC plasmid (Fig. 3A). These data show that the mgtC gene of B. suis is required for growth in low-Mg2+ medium. MgtB, however, is not required for growth in low-Mg2+ medium both in B. melitensis (12) and S. enterica serovar Typhimurium (2), suggesting that a magnesium transporter(s) other than MgtB is functional in such conditions.
FIG. 3.
The mgtC::Kan mutant of B. suis is unable to grow in low-Mg2+ NCE liquid medium. A. OD600 reached by wild-type (WT), mgtC::Kan mutant, and mgtC::Kan/pBBR1-mgtBC strains at 24 h after inoculation with medium containing 10 μM or 10 mM MgCl2. B. Growth (OD600 at 24 h postinoculation) of mgtC::Kan mutant in NCE liquid medium supplemented with increasing Mg2+ concentrations. Values are the means of three independent experiments plus standard errors.
Mg2+ partially rescues the growth defect of the B. suis mgtC mutant in macrophages.
To test whether the macrophage growth defect of the mgtC mutant was linked to its inability to grow in a low-Mg2+ environment, we supplemented the tissue culture medium with 25 mM MgCl2 2 h after internalization of wild-type or mgtC::Kan bacteria. The addition of Mg2+ to the tissue culture medium had no significant effect on the wild-type strain, but it significantly improved the intracellular growth of mgtC::Kan bacteria, and therefore partially rescued the growth defect of the mgtC mutant (Fig. 4) in a manner similar to that described for an S. enterica serovar Typhimurium mgtC mutant (2). This result suggests that the Brucella-containing vacuole is indeed a Mg2+-limiting environment.
FIG. 4.
Effect of the addition of Mg2+ to tissue culture medium on the intramacrophage growth of B. suis mgtC mutant. MgCl2 (25 mM) was added to the RPMI 1640 medium 2 h after bacterial internalization. Values are the means of three independent experiments plus standard errors. WT, wild type.
Conclusion.
MgtC appears to be a remarkable factor since, to our knowledge, it provides the only clear example of an acquired intramacrophage growth factor shared by three unrelated intracellular pathogens, B. suis, S. enterica serovar Typhimurium, and M. tuberculosis. It suggests that while each pathogen has evolved specific mechanisms to modulate host cell biology, they also encounter environments with some striking similarities and have developed common virulence strategies. In agreement with MgtC phylogenetic analysis (3), we propose that the MgtC proteins from these pathogens share a similar function, most likely providing the ability to grow in a low-Mg2+ environment. The present data strengthen the hypothesis that MgtC-like proteins encoded by other pathogens that replicate within macrophages, such as Burkholderia and Yersinia species, will be found to be important for intracellular growth (3). The findings also give new information concerning the microenvironment of the Brucella-harboring phagosome, which has been shown to be poor in nutrients and characterized by low oxygen tension (9). Our results strongly suggest that the Brucella-harboring phagosome also constitutes a Mg2+-deprived environment. We propose that the acquisition of mgtC is a key evolutionary event in the adaptation of intracellular pathogens to a low-Mg2+ niche.
Acknowledgments
We thank Gisèle Bourg-Blossier and Safia Ouahrani-Bettache for technical assistance.
This work is funded by the Inserm (Avenir program), the European Community (QLK2-CT-2001-01200), La Région Languedoc-Roussillon, and the Université de Montpellier I (BQR). A.-B.B.-P. is supported by the Inserm Avenir program and the CANAM.
Editor: J. B. Bliska
REFERENCES
- 1.Amer, A. O., and M. S. Swanson. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5:56-61. [DOI] [PubMed] [Google Scholar]
- 2.Blanc-Potard, A. B., and E. A. Groisman. 1997. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16:5376-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc-Potard, A. B., and B. Lafay. 2003. MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens: evidence from molecular phylogeny and comparative genomics. J. Mol. Evol. 57:479-486. [DOI] [PubMed] [Google Scholar]
- 4.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 5.Buchmeier, N., A. B. Blanc-Potard, S. Ehrt, D. Piddington, L. Riley, and E. A. Groisman. 2000. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 35:1375-1382. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 7.Hou, Y. M. 1999. Transfer RNAs and pathogenicity islands. Trends Biochem. Sci. 24:295-298. [DOI] [PubMed] [Google Scholar]
- 8.Kölher, S., S. Michaux-Charachon, F. Porte, M. Ramuz, and J. P. Liautard. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 11:215-219. [DOI] [PubMed] [Google Scholar]
- 9.Kölher, S., V. Foulongne, S. Ouarhani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler, S., J. Teyssier, A. Cloeckaert, B. Rouot, and J. P. Liautard. 1996. Participation of the molecular chaperone DnaK in intracellular growth of Brucella suis within U937-derived phagocytes. Mol. Microbiol. 20:701-712. [DOI] [PubMed] [Google Scholar]
- 11.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 12.Lestrate, P., R. M. Delrue, I. Danese, C. Didembourg, B. Taminiau, P. Mertens, X. De Bolle, A. Tibor, C. M. Tang, and J. J. Letesson. 2000. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol. Microbiol. 38:543-551. [DOI] [PubMed] [Google Scholar]
- 13.Maloy, S. R. 1990. Experimental techniques in bacterial genetics. Jones and Bartlett Publishers, Boston, Mass.
- 14.Moncrief, M. B., and M. E. Maguire. 1998. Magnesium and the role of MgtC in growth of Salmonella typhimurium. Infect. Immun. 66:3802-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayan, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roop, R. M., II, B. H. Bellaire, M. W. Valderas, and J. A. Cardelli. 2004. Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 52:621-630. [DOI] [PubMed] [Google Scholar]
- 18.Smith, R. L., M. T. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology 144:1835-1843. [DOI] [PubMed] [Google Scholar]
- 19.Snavely, M. D., C. G. Miller, and M. E. Maguire. 1991. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266:815-823. [PubMed] [Google Scholar]
- 20.Tao, T., M. D. Snavely, S. G. Farr, and M. E. Maguire. 1995. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 177:2654-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]