Abstract
Mycobacterium tuberculosis contains five genes, rpfA through rpfE, that bear significant homology to the resuscitation-promoting factor (rpf) gene of Micrococcus luteus, whose product is required to resuscitate the growth of dormant cultures of M. luteus and is essential for the growth of this organism. Previous studies have shown that deletion of any one of the five rpf-like genes did not affect the growth or survival of M. tuberculosis in vitro. In conjunction with the results of whole-genome expression profiling, this finding was indicative of their functional redundancy. In this study, we demonstrate that the single deletion mutants are phenotypically similar to wild-type M. tuberculosis H37Rv in vivo. The deletion of individual rpf-like genes had no discernible effect on the growth or long-term survival of M. tuberculosis in liquid culture, and the ability to resuscitate spontaneously from a nonculturable state in a most probable number assay was also unaffected for the three strains tested (the ΔrpfB, ΔrpfD, and ΔrpfE strains). In contrast, two multiple strains, KDT8 (ΔrpfA-mutation ΔrpfC ΔrpfB) and KDT9 (ΔrpfA ΔrpfC ΔrpfD), which lack three of the five rpf-like genes, were significantly yet differentially attenuated in a mouse infection model. These mutants were also unable to resuscitate spontaneously in vitro, demonstrating the importance of the Rpf-like proteins of M. tuberculosis in resuscitation from the nonculturable state. These results strongly suggest that the biological functions of the five rpf-like genes of M. tuberculosis are not wholly redundant and underscore the potential utility of these proteins as targets for therapeutic intervention.
The majority of individuals infected with Mycobacterium tuberculosis harbor a clinically latent infection in which the organism is able to persist or remain dormant within an otherwise healthy individual for prolonged periods of time (15). In a relatively small proportion of these individuals, the infection may reactivate to cause active disease. Since it is estimated that one-third of the world's population is latently infected with M. tuberculosis, the implications of reactivation tuberculosis for the global burden of disease are alarming (5). The molecular mechanisms by which M. tuberculosis persists or remains dormant and reactivates are poorly understood (12, 15) and may be attributed to the host immune response and/or the physiology of the organism itself.
M. tuberculosis encodes a family of five proteins that bear significant similarity to the resuscitation promoting factor (Rpf) of Micrococcus luteus, which is a secreted protein essential for growth of this organism (9, 10). Studies on the growth stimulation and reactivation of cultures of M. luteus, Mycobacterium smegmatis, Mycobacterium bovis BCG, and M. tuberculosis by M. luteus Rpf and other Rpf-like proteins strongly suggest that one or more of the M. tuberculosis Rpf-like proteins may play similar roles in mycobacterial growth and/or reactivation of latent infection (8, 10, 13). However, it was shown recently that deletion of any one of the five rpf-like genes of M. tuberculosis had no effect on the growth or survival of this organism either in vitro (4, 16) or in a mouse model of infection (16). These observations confirmed that the biological functions served by the individual rpf-like genes are nonessential and suggested that they might be partially or even wholly redundant. To further explore the extent of redundancy within this multigene family in terms of the function of its members in controlling growth and/or dormancy and in the reactivation of M. tuberculosis, we describe the construction of multiple rpf-like mutant strains by the sequential deletion of one or two additional rpf-like genes in a ΔrpfA mutant background and the growth characteristics of the resulting strains.
MATERIALS AND METHODS
Construction of multiple-deletion mutant strains.
The strains and plasmids used in this study are shown in Table 1. Allelic exchange mutagenesis was carried out by two-step selection using the previously described knockout vectors pRPFBΔ2, pRPFCΔ2, and pRPFDΔ2, which harbor in-frame deletions in their respective rpf-like genes (4). Multiple mutants lacking two and three rpf-like genes were constructed using a parallel and sequential approach, with the M. tuberculosis ΔrpfA mutant as the single-mutation progenitor strain. This strain was electroporated with 5 μg of UV-pretreated pRPFBΔ2 or pRPFCΔ2 and plated onto 7H10 agar containing hygromycin, kanamycin, and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). In each case, a blue, single-crossover recombinant was streaked onto 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase (OADC) and containing X-Gal, grown, and serially diluted and plated onto 7H10 agar containing X-Gal and 2% sucrose. Sucrose-resistant colonies that were white and Kans were selected for genotypic analysis. Chromosomal DNA extraction and Southern blotting were carried out to identify and confirm the genotypes of the double mutant strains KDD6 (ΔrpfA ΔrpfB) and KDD7 (ΔrpfA ΔrpfC) using previously described methods (4). KDD7 was then used as the progenitor for producing two mutant strains lacking three rpf-like genes by electroporation with either pRPFBΔ2 or pRPFDΔ2, followed by two-step selection, as described above. The triple mutants thus generated were designated KDT8 (ΔrpfA ΔrpfC ΔrpfB) and KDT9 (ΔrpfA ΔrpfC ΔrpfD).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| H37Rv | Laboratory strain of M. tuberculosis (ATCC 25618) | Laboratory stock |
| ΔrpfA mutant | Single mutant of M. tuberculosis H37Rv carrying an internal in-frame deletion in rpfA | 4 |
| ΔrpfB mutant | Single mutant of M. tuberculosis H37Rv carrying an internal in-frame deletion in rpfB | 4 |
| ΔrpfD mutant | Single mutant of M. tuberculosis H37Rv carrying an internal in-frame deletion in rpfD | 4 |
| ΔrpfE mutant | Single mutant of M. tuberculosis H37Rv carrying an internal in-frame deletion in rpfE | 4 |
| KDD6 (ΔrpfA ΔrpfB) | Double rpf-like mutant; derivative of the ΔrpfA strain carrying an internal in-frame deletion in rpfB | This study |
| KDD7 (ΔrpfA ΔrpfC) | Double rpf-like mutant; derivative of the ΔrpfA strain carrying an internal in-frame deletion in rpfC | This study |
| KDT8 (ΔrpfA ΔrpfC ΔrpfB) | Triple rpf-like mutant; derivative of KDD7 carrying an internal in-frame deletion in rpfB | This study |
| KDT9 (ΔrpfA ΔrpfC ΔrpfD) | Triple rpf-like mutant; derivative of KDD7 carrying an internal in-frame deletion in rpfD | This study |
| Plasmids | ||
| pRPFBΔ2 | Knockout vector for constructing deletion mutation in rpfB | 4 |
| pRPFCΔ2 | Knockout vector for constructing deletion mutation in rpfC | 4 |
| pRPFDΔ2 | Knockout vector for constructing deletion mutation in rpfD | 4 |
Log phase growth and stationary phase survival of wild-type and mutant strains.
The levels of log phase growth and stationary phase survival of the wild type and the double and the triple mutant strains were compared as previously described (4). In brief, the strains were inoculated into Middlebrook 7H9 medium containing glycerol and 0.05% Tween 80 and supplemented with albumin-dextrose-catalase (ADC) and grown as stirred cultures at 37°C. The cultures were inoculated to an initial density of 105 to 106 CFU/ml. Initially samples were taken every 2 to 3 days and then every week, for 8 weeks, once stationary phase had been reached. CFU were enumerated by plating duplicate samples of three serial dilutions on Middlebrook 7H10 medium supplemented with OADC and scoring colonies after ca. 28 days' growth.
Long-term viability in Sauton's medium without oxygen.
In previous studies, we found that the precise state of the inoculum is important for the production of nonculturable mycobacterial cells during long-term oxygen starvation (13, 14). Therefore, to ensure that the inocula used in this study were as standardized as possible, they were prepared by two passages, as follows. The wild type and the rpf deletion mutant strains were inoculated into 40 ml of Sauton's medium containing 0.05% Tween 80 and supplemented with ADC. The initial cultures were grown with shaking at 150 rpm at 37°C for 2 weeks and then subcultured by adding 200 μl into 40 ml of supplemented Sauton's medium with 0.05% Tween 80 and growing to an optical density at 600 nm (OD600) of 1.8 to 2. Finally, 1 ml of the second-round cultures was inoculated into 200 ml Sauton's medium supplemented with ADC and containing 0.05% Tween 80 in sealed 750-ml flasks and grown with shaking at 200 to 250 rpm at 37°C for 4 months. Samples were removed aseptically and without oxygen input, and CFU were enumerated as previously described (13).
Resuscitation of nonculturable cells.
Three-and-a-half-month-old bacterial cultures, aged as described above, were serially diluted in growth medium, and duplicate 100-μl samples were plated in triplicate on agar-solidified, ADC-supplemented Sauton's medium (without Tween 80). Plates were incubated at 37°C, and CFU were enumerated after 2 months' growth, with the limit of detection being 5 CFU/ml. Resuscitation and most probable number (MPN) assays were performed by adding 10 of the serially diluted samples (100 μl) to replicate 10-ml plastic screw-cap tubes, each containing 2 ml of Sauton's medium supplemented with ADC. The tubes were incubated at 37°C without shaking for 2 months. The number of tubes with visible bacterial growth was recorded, and MPN values were calculated using standard statistical methods (3).
Infection of mice.
C57BL/6JCit (B6) mice were injected intravenously with 104 CFU/mouse of mid-log-phase individual single or triple rpf-like mutants of M. tuberculosis in 200 μl phosphate-buffered saline. The preparation of filtered, clump-free mid-log-phase mycobacterial suspensions has been described elsewhere (11). The numbers of bacilli in the inoculum were estimated directly by microscopic examination of filtered mycobacterial suspensions in a conventional hemocytometer and equalized for all mycobacterial strains prior to performing the infections. Enumeration of bacilli in the inocula by plating threefold serial dilutions on Dubos agar (Difco) and scoring microcolonies and visible colonies after 3 and 20 days' incubation at 37°C, respectively, confirmed that the accuracy of CFU count estimation by microscopy was >93%. To assess mycobacterial loads in spleens and lungs, 0.1 ml of serial 10-fold dilutions of sterile whole-organ homogenates was plated onto Dubos agar and colonies were counted after 18 to 20 days of incubation at 37°C.
RESULTS
Construction of multiple rpf-like mutant strains.
To begin to unravel the collective biological function of the five rpf-like genes in M. tuberculosis, we set about the construction of mutants lacking two or three of their rpf-like genes by using a sequential strategy. No information was available at the outset that could meaningfully inform or guide the order in which the five genes should be deleted. Since ΔrpfA was the first of the five single rpf-like mutant strains to be constructed in our laboratory (4), this strain was selected arbitrarily as the host for the parallel construction of two double mutants, KDD6 (ΔrpfA ΔrpfB) and KDD7 (ΔrpfA ΔrpfC). Similarly, since KDD7 was constructed before KDD6, the former was selected arbitrarily as the genetic background for deletion of additional rpf-like genes, resulting in the production of strains KDT8 and KDT9, which lacked either rpfB or rpfD in addition to rpfA and rpfC. However, subsequent gene expression studies revealed that (i) rpfC is probably the most highly expressed of the five rpf-like genes in vitro (10, 16), (ii) the loss of RpfC affected the expression of the largest number of genes in log-phase cultures of M. tuberculosis (4), and (iii) whereas the differential gene expression profile of the ΔrpfC mutant overlapped considerably with those of the ΔrpfB, ΔrpfD, and ΔrpfE mutants, the profile of the rpfA mutant was noticeably different, distinguishing this deletion mutant from the others (4). These findings validated the use of the double mutant strain lacking rpfA and rpfC (KDD7) as the preferred genetic background for the deletion of additional rpf-like genes. All of the multiple-mutation strains were genotypically characterized by Southern blot analysis (4) to confirm the rpf-like gene deletion and to verify the integrity of the preexisting deletion(s) in their corresponding progenitor strains (data not shown). As described previously, the in-frame deletion alleles were constructed in such a way as to minimize possible polar effects on downstream genes (4). However, since the single and mutant strains were not complemented genetically, the possibility that second-site mutations were introduced inadvertently during their construction cannot be completely excluded.
Effects of the rpf mutations on growth and stationary phase survival.
To ascertain whether the growth and stationary phase survival of M. tuberculosis was affected by the loss of more than one rpf-like gene, the mutant and wild-type strains were grown in stirred liquid cultures in Middlebrook 7H9 medium and maintained in stationary phase for 8 weeks. Culturability was assessed periodically over a 12-week time course by CFU enumeration as previously described (4). The strains displayed variable growth initially (data not shown), but this was not reproducible from one experiment to the next and probably represents different tendencies to grow as aggregates or clumps, which later disperse. As observed for the single mutant strains (4), the presence of multiple rpf-like mutations had little or no effect on stationary phase survival under the conditions tested (data not shown).
Effects of loss of multiple rpf genes on long-term viability and resuscitation.
To assess the ability of nonculturable cells of various rpf-like mutants to resuscitate spontaneously in liquid medium, the method that had been shown previously to produce nonculturable but spontaneously resuscitatable cells of the Academia strain of M. tuberculosis (13) was applied to H37Rv and its rpf-like deletion mutant derivatives. In a control experiment in which CFU were assessed as a function of time in the Sauton's medium/sealed-flask culture model, all of the strains attained a maximum cell density in stationary phase of ca. 108 CFU/ml (data not shown). By comparison, the maximum cell density reached in parallel cultures in which the flasks were not sealed, thus allowing free air exchange with the atmosphere, was ca. 1010 CFU/ml (data not shown). Therefore, in the sealed-flask model, oxygen was gradually depleted as a result of bacterial respiration. The long-term viability of H37Rv, one of the single mutants (ΔrpfB), and both of the triple mutants (KDT8 and KDT9), grown in Sauton's medium with gradual oxygen depletion, was then determined after 110 days' incubation. Under these conditions, all strains revealed almost identical behavior in extended stationary phase with a decrease in CFU to below the detectable limit (5 CFU/ml), as observed previously for the Academia strain of M. tuberculosis (13). Zhu et al. (17) had similarly found significantly more cells in 3-month-old cultures of M. tuberculosis H37Rv (microscopically determined) than viable bacteria, i.e., those that were able to form colonies on solid medium (6.9 × 107 versus 1.5 × 105 CFU).
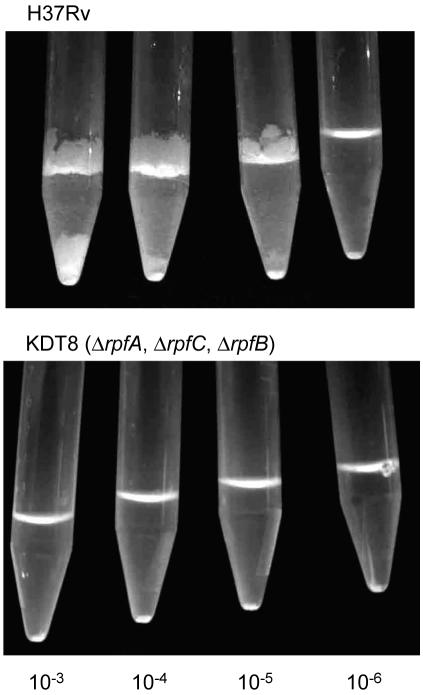
To estimate numerically the resuscitation of nonculturable cells, resuscitation of 3.5-month-old cultures with undetectable CFU (≤5 CFU/ml) was carried out in capped test tubes in Sauton's medium supplemented with ADC using an MPN assay. In this assay, the bacteria were diluted to such an extent that, statistically speaking, the samples used to inoculate the replicate tubes would contain no initially viable cells, thus allowing the number of resuscitatable cells to be estimated numerically (6, 13). In the present case, the initial number of CFU was, in any event, below the limit of detection (5 CFU/ml). As was found for the Academia strain (13), wild-type H37Rv demonstrated growth in large dilutions (Fig. 1, 10−3, 10−4, and 10−5 dilutions). Similar results were obtained with the single mutant, the ΔrpfB strain (data not shown). An estimation of the viability of H37Rv in Sauton's medium by the MPN assay led to an increase in the number of viable cells in comparison with CFU (Table 2), indicating the presence of nonculturable cells in the populations and their ability to resuscitate spontaneously in liquid medium. Similar results were obtained with resuscitation of the ΔrpfB single mutant (Table 2). In contrast, the triple mutant, KDT8, which lacks rpfA, rpfC, and rpfB, and KDT9, which lacks rpfA, rpfC, and rpfD, produced clumps that were barely visible to the naked eye after 4 weeks of incubation. Over the next 2 months of incubation, these clumps remained unchanged or even disappeared (Fig. 1). The bacteria in these clumps were unable to show normal growth. Since the MPN assay requires that unlimited growth must occur in tubes accepted as positive, these tubes were scored as negative. The triple mutants therefore displayed very low viability in the MPN assay, suggesting that the nonculturable cells of these two strains could not spontaneously resuscitate (Table 2).
FIG. 1.
Resuscitation of nonculturable M. tuberculosis strains in the MPN assay. Serially diluted samples of nonculturable cells prepared as described in Materials and Methods were employed to inoculate multiple tubes for the MPN assay. Representative tubes for successive dilutions are shown for strains H37Rv and KDT8.
TABLE 2.
Resuscitation of stationary phase cultures of M. tuberculosis rpf deletion mutants after maintenance in shaking sealed tubes for 3.5 monthsa
| Strain | CFU per ml | MPN per mlb
|
||
|---|---|---|---|---|
| MPN | 95% Confidence limits
|
|||
| Lower | Higher | |||
| H37Rv | <5 | 5.4 × 105 | 1.5 × 105 | 1.7 × 106 |
| ΔrpfB | <5 | 5.4 × 105 | 1.5 × 105 | 1.7 × 106 |
| KDT8 (ΔrpfA ΔrpfC ΔrpfB) | <5 | <103* | 6.8 × 103 | |
| KDT9 (ΔrpfA ΔrpfC ΔrpfD) | <5 | <103* | 6.8 × 103 | |
The starting cell density of all cultures was ∼104 CFU/ml (OD600 < 0.1). After 9 days of incubation, the cultures reached a density of 108 CFU/ml (OD600 ≈2.0). After 24 days, the CFU values had dropped to 2×106 CFU/ml (OD600 ≈0.2) and, after 100 days, were <5 CFU/ml (OD600 ≈0.2).
Where small aggregates were observed after the 3.5-month oxygen starvation period, the aggregates did not contain more than four cells (determined by microscopy). However, a significant difference in CFU versus MPN would not be expected to arise were there significant aggregation, since one aggregate represents one propagule when introduced onto solid medium (CFU) or into liquid medium (MPN). Aggregation might cause some slight underestimation of both CFU and MPN compared with the total count (by microscopy) but would not affect the ratio of MPN to CFU. *, Significantly different from the wild type at a P of <0.001.
In analogous experiments (results not shown), resuscitation of the wild type and three single and two triple mutants was assessed after the strains were held in stationary phase without oxygen input for 2.5 months. However, in this case, the residual viability of the strains after the prolonged incubation period was significantly higher, e.g., 5 × 103 and 7 × 104 for the ΔrpfD and ΔrpfE mutants, respectively. Nevertheless, resuscitation of these single mutants was observed (differences of up to 2 orders of magnitude between CFU and MPN). The other strains also displayed the same general trend in resuscitation behavior observed in the 3.5-month oxygen starvation experiment; i.e., resuscitation was observed for the wild type and the ΔrpfB mutant but not the triple mutants (data not shown).
Growth and survival of the single and triple rpf-like mutants in B6 mice.
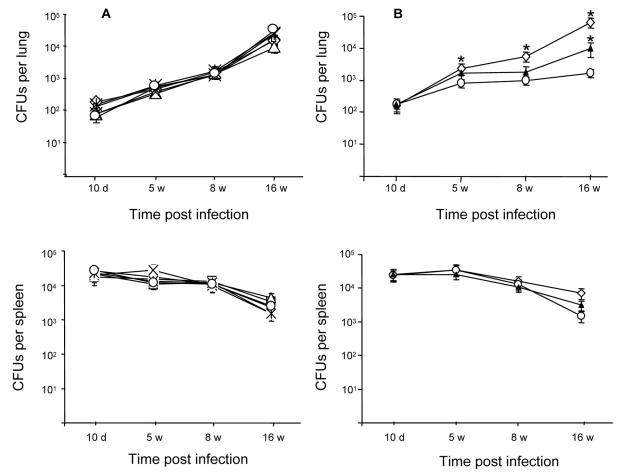
The in vivo phenotypes of the five single mutants and the two triple mutants (KDT8 and KDT9) were examined in the C57BL/6JCit (B6) mouse model (Fig. 2). The proliferation of the five single mutant strains in the lungs of infected mice was indistinguishable from that of their parental wild type, reaching ca. 105 CFU at 16 weeks postinfection (Fig. 2A). Moreover, all of the single mutant strains persisted as well as the wild-type strain in the spleens of the infected animals. In contrast however, infection of B6 mice with the triple mutants, KDT8 and KDT9, revealed that these strains were significantly attenuated in this mouse model, as evidenced by the 1- to 1.5-log reduction in lung CFU at week 16 (Fig. 2B). The triple mutant strains were also cleared significantly more rapidly from the spleens of the infected mice over the 16-week time course of the experiment (P = 0.045 and 0.028 for KDT9 and KDT8, respectively).
FIG. 2.
Growth of M. tuberculosis H37Rv and rpf-like mutant strains in the organs of C57BL/6JCit (B6) mice. (A) Single rpf-like mutants. Diamond, wild type; triangle, ΔrpfA; X, ΔrpfB; asterisk, ΔrpfC; circle, ΔrpfD; square, ΔrpfE. (B) Triple rpf-like mutants. Open diamond, wild type; open circle, KDT8 (ΔrpfA ΔrpfC ΔrpfB); filled triangle, KDT9 (ΔrpfA ΔrpfC ΔrpfD). Mice were infected with 104 bacilli of M. tuberculosis by the intravenous route, and bacillary loads in the lungs and spleen were determined by CFU assessment over a 16-week infection period as described in Materials and Methods. The top graph in each panel represents the bacillary growth in the lungs, whereas the bottom graph represents growth in the spleens of the infected animals. Each point represents the mean for four mice per group, and the error bars denote the standard deviations. The lung bacillary loads that differ significantly from those of the wild type are denoted by an asterisk above the relevant data points (P < 0.05 to 0.01). The P values of the spleen bacillary loads that differ significantly from those of the wild type are provided in the text.
DISCUSSION
The five rpf-like genes of M. tuberculosis were recently shown to be individually dispensable for growth in vitro (4, 16) and in vivo (16). To determine whether these genes play a collective role in controlling growth and/or dormancy and reactivation of M. tuberculosis, we constructed four derivatives of the rpfA-deficient mutant of the H37Rv strain (ΔrpfA) (4) in which additional rpf-like genes had been deleted in a sequential fashion. Deletion of one or two additional rpf-like genes from the ΔrpfA strain in the particular combinations investigated in this study had no adverse effect on the growth and long-term survival of M. tuberculosis in rich or minimal medium in vitro.
Recently, Shleeva et al. (13) described the formation of a homogeneous culture of nonculturable cells of the Academia strain of M. tuberculosis with a CFU value close to zero following growth in Sauton's medium containing ADC and Tween 80 and extended maintenance in stationary phase (4 to 5 months) without oxygen input. However, the MPN count for these cells was 105 organisms per ml, which was further increased by one log using supernatant from an actively growing culture, possibly as a result of the presence of one or more Rpf-like proteins in the supernatant. In this study, we employed the same assay system to investigate whether the triple mutants KDT8 and KDT9, which show attenuation of virulence (see below), are able to resuscitate spontaneously. Wild-type H37Rv and its derivatives KDT8 and KDT9 formed nonculturable cells under the conditions previously applied to the Academia strain. In this assay system, the wild type showed resuscitation from 0 to 105 cells per ml. After 4 months of starvation without oxygen, microscopic observations revealed that all the cultures tested were apparently homogeneous with very little tendency to form aggregates compared with cultures at the beginning of stationary phase (data not shown). Small aggregates were sometimes observed, but they did not contain more than four cells, thus excluding aggregation as a possible explanation for the 5-log difference between the CFU and MPN values for the wild-type strain (Table 2). The ΔrpfB mutant showed resuscitation similar to that of the wild type. This finding, together with the observed resuscitation of the ΔrpfD and ΔrpfE mutants, suggests that individually, rpf-like genes may be dispensable for resuscitation under the conditions tested.
In contrast to the behavior of the wild type and single mutants, the triple mutants displayed very poor resuscitation in this assay system (Fig. 1 and Table 2). After 4 weeks, the triple mutant samples showed barely visible clumps that were unable to sustain further growth. In this respect, their behavior resembled that observed for nonculturable M. luteus cells, which were found to undergo an only limited number of divisions in the absence of Rpf (7). It is tempting to speculate that the failure of the triple mutants to resuscitate suggests that the Rpf-like proteins of M. tuberculosis play an important collective role in promoting resuscitation from a nonculturable state. However, on the basis of the available data, we cannot exclude the possibility that the failure of these mutants to resuscitate is due to poor survival during prolonged incubation under oxygen-depleted conditions. In order to distinguish between these possibilities, it will be important to assess whether the triple mutants can be rescued from the nonculturable state by an exogenous source of Rpf-like proteins, such as supernatant from an actively growing culture of wild-type M. tuberculosis (13). Such studies are currently in progress.
In the present study, the deletion of any one of the five rpf-like genes was shown to have no effect on the in vivo growth of M. tuberculosis H37Rv in B6 mice. This result is in full agreement with the lack of an in vivo growth phenotype of single rpf deletion mutants of the Erdman strain of M. tuberculosis in C57BL/6 mice (16). In contrast, however, both triple rpf-like mutants were significantly attenuated in vivo, as assessed by monitoring changes in CFU recovered from the lungs and spleens of B6 mice over a 16-week infection period. Of the two triple mutants, KDT8 (ΔrpfA ΔrpfC ΔrpfB) was more attenuated than the other, KDT9 (ΔrpfA ΔrpfC ΔrpfD), suggesting that the loss of rpfB had a more pronounced effect on virulence than the loss of rpfD in a background that is deficient in rpfA and rpfC. Since the in vivo phenotype of KDD7 (the double mutant progenitor of KDT8 and KDT9) was not assessed, we cannot, however, rule out the possibility that deletion of rpfD has no effect at all on virulence.
Our ability to interpret the in vivo phenotype of the triple mutant strains is limited by the fact that the precise mechanism by which Rpf-like proteins control bacterial growth is still not known. On the basis of structural predictions, it has been suggested that the conserved Rpf-like domain is lysozyme-like (1). This suggestion is particularly interesting in light of the suspected role of these proteins in cell wall modification and cell detachment (M. Young and A. S. Kaprelyants, unpublished observations). It is therefore possible that the ability of the triple rpf-like mutant strains of M. tuberculosis to grow in mouse tissue is restricted by cell wall alterations and/or defects caused by depleted Rpf-like activity, which render the bacilli more susceptible to killing by the host immune response. However, the fact that both triple mutants also displayed a resuscitation defect in the MPN assay invites speculation that there is a correlation between attenuation in vivo and defects in spontaneous resuscitation of nonculturable cells in vitro. Although the precise nature of this relationship is presently unclear, one intriguing possibility is that the harsh environment encountered in mouse tissue induces the bacteria to enter into a state in vivo that is analogous to the nonculturable state provoked by starvation without oxygen input in vitro. Defects in the culturability of bacilli recovered from the mouse organs may thus have contributed to the observed attenuation of the triple mutant strains.
The data reported herein provide compelling evidence to suggest that the redundancy in biological function of the multigene Rpf system of M. tuberculosis is not complete. However, many important questions regarding the extent of functional overlap between the various Rpfs remain, which will require further investigation. Firstly, if Rpf-like proteins do indeed possess lysozyme-like enzymatic activity, could the five M. tuberculosis proteins have different targets, with the specificity being determined by the nonhomologous parts of the protein? Secondly, the extent to which the effects of progressive rpf-like gene loss on virulence are additive is presently unknown. Nonetheless, it is interesting to note that Mycobacterium leprae, which is considered to contain a minimal essential mycobacterial gene set as a result of convergent evolution (2), contains only three rpf-like genes, which are the homologues of rpfA (ML2151, encoding a homologue of the N-terminal, Rpf-like domain of RpfA), rpfB (ML0240) and rpfC (ML2030), which correspond to the three genes deleted in the mutant strain of M. tuberculosis that is most attenuated for growth in vivo. Finally, it will be of considerable interest to investigate the effects of the loss of multiple rpf-like genes on the expression of the remaining rpf-like genes. Given the regulatory interdependence between the various rpf-like genes revealed by previous transcriptome and quantitative, real-time reverse transcription-PCR analysis of the single mutant strains (4), the expression levels of the remaining rpf-like genes may be significantly altered. This work is currently in progress, together with studies aimed at establishing whether M. tuberculosis can maintain viability in the absence of all five of its rpf-like genes.
Acknowledgments
This work was supported by grants from the GlaxoSmithKline Action TB Initiative, the Technology for Human Resource in Industry Programme (THRIP) of the National Research Foundation of South Africa, the Medical Research Council of South Africa, and the UK BBSRC. V.M. and A.S.A. were also supported by International Research Scholar's grants from the Howard Hughes Medical Institute. M.O.S., A.S.A., and A.S.K. thank the Programme “Molecular and Cellular Biology,” the Russian Academy of Sciences, the RFBR (grant 03-04-89044), the ISTC (projects 2201 and 1879), and the CRDF (project 2412).
We thank Ken Duncan for advice and encouragement; Paul van Helden for generously providing laboratory space and facilities; Bhavna Gordhan, Bavesh Kana, Stephanie Dawes, and Edith Machowski for helpful discussions; and Helena Boshoff and Eric Rubin for critically reviewing the manuscript.
Editor: J. N. Weiser
REFERENCES
- 1.Cohen-Gonsaud, M., N. H. Keep, A. P. Davies, J. Ward, B. Henderson, and G. Labesse. 2004. Resuscitation-promoting factors possess a lysozyme-like domain. Trends Biochem. Sci. 29:7-10. [DOI] [PubMed] [Google Scholar]
- 2.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honoré, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 3.de Man, J. C. 1975. The probability of most probable numbers. Eur. J. Appl. Microbiol. 1:67-78. [Google Scholar]
- 4.Downing, K. J., J. C. Betts, D. I. Young, R. A. McAdam, F. Kelly, M. Young, and V. Mizrahi. 2004. Global expression profiling of strains harbouring null mutations reveals that the five rpf-like genes of Mycobacterium tuberculosis show functional redundancy. Tuberculosis 84:167-179. [DOI] [PubMed] [Google Scholar]
- 5.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 6.Kaprelyants, A. S., G. V. Mukamolova, and D. B. Kell. 1994. Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent medium at high dilution. FEMS Microbiol. Lett. 115:347-352. [Google Scholar]
- 7.Mukamolova, G. V., A. S. Kaprelyants, and D. B. Kell. 1995. Secretion of an antibacterial factor during resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase. Antonie Leeuwenhoek 67:289-295. [DOI] [PubMed] [Google Scholar]
- 8.Mukamolova, G. V., A. S. Kaprelyants, D. I. Young, M. Young, and D. B. Kell. 1998. A bacterial cytokine. Proc. Natl. Acad. Sci. USA 95:8916-8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukamolova, G. V., O. A. Turapov, K. Kazarian, M. Telkov, A. S. Kaprelyants, D. B. Kell, and M. Young. 2002. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol. Microbiol. 46:611-621. [DOI] [PubMed] [Google Scholar]
- 10.Mukamolova, G. V., O. A. Turapov, D. I. Young, A. S. Kaprelyants, D. B. Kell, and M. Young. 2002. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol. Microbiol. 46:623-635. [DOI] [PubMed] [Google Scholar]
- 11.Nikonenko, B. V., M. M. Averbakh, Jr., C. Lavebratt, E. Schurr, and A. S. Apt. 2000. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuber. Lung Dis. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 12.Parrish, N. M., J. D. Dick, and W. R. Bishai. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107-112. [DOI] [PubMed] [Google Scholar]
- 13.Shleeva, M. O., K. Bagramyan, M. V. Telkov, G. V. Mukamolova, M. Young, D. B. Kell, and A. S. Kaprelyants. 2002. Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology 148:1581-1591. [DOI] [PubMed] [Google Scholar]
- 14.Shleeva, M., G. V. Mukamolova, M. Young, H. D. Williams, and A. S. Kaprelyants. 2004. Formation of ‘non-culturable’ cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation. Microbiology 150:1687-1697. [DOI] [PubMed] [Google Scholar]
- 15.Stewart, G. R., B. D. Robertson, and D. B. Young. 2003. Tuberculosis: a problem with persistence. Nat. Rev. Microbiol. 1:97-105. [DOI] [PubMed] [Google Scholar]
- 16.Tufariello, J. M., W. R. Jacobs, Jr., and J. Chan. 2004. Individual Mycobacterium tuberculosis resuscitation-promoting factor homologues are dispensable for growth in vitro and in vivo. Infect. Immun. 72:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu, W., B. B. Plikaytis, and T. M. Shinnick. 2003. Resuscitation factors from mycobateria: homologs of Micrococcus luteus proteins. Tuberculosis 83:261-269. [DOI] [PubMed] [Google Scholar]