Abstract
Trichomonas vaginalis secretes putrescine that is readily detected in vaginal secretions. We wanted to examine the effect of decreased putrescine synthesis by inhibition of ornithine decarboxylase (ODC) on T. vaginalis. One reason is because inhibition of Tritrichomonas foetus ODC results in growth arrest, destruction of hydrogenosomes, and decreased amounts of hydrogenosomal enzymes. Treatment of T. vaginalis T016 with ≥20 mM 1,4-diamino-2-butanone (DAB) to inhibit ODC resulted in growth arrest, which was reversed by addition of exogenous putrescine. No similar reversal of growth arrest was achieved with the polyamines spermine or spermidine or with iron. Electron microscopic examination of control versus DAB-treated trichomonads did not reveal any adverse effects on the number and integrity of hydrogenosomes. Further, the adhesins AP65, AP51, and AP33 mediating binding to immortalized vaginal epithelial cells (VECs) share identity to enzymes of the hydrogenosome organelle, and there was no difference in amounts of adhesins between control versus DAB-treated T. vaginalis parasites. Likewise, similar patterns and extent of fluorescence were evident for the prominent AP65 adhesin. Surprisingly, DAB treatment increased by 4- to 20-fold above untreated trichomonads handled identically the level of adherence mediated by adhesins. Interestingly, the enhanced attachment to VECs was reversed by exogenous putrescine added to DAB-treated trichomonads. Equally noteworthy was that DAB-treated T. vaginalis with enhanced adherence did not possess the previously reported ability to kill host cells in a contact-dependent fashion mediated by cysteine proteinases, and total cysteine proteinase activity patterns were identical between control and DAB-treated trichomonads. Overall, these data suggest that polyamine metabolism and secreted putrescine are linked to host cell adherence and cytotoxicity.
The polyamines putrescine, spermine, and spermidine are ubiquitous small cations found in almost all living species and are essential for growth and function of normal cells (51). Polyamines have multiple functions that include stabilization of DNA, rRNA, and tRNA (28); association with acidic phospholipids (47); and regulation of membrane-associated enzymes (47). Polyamines have been shown to also have free radical scavenger properties (25), thus attributing an antioxidant function to these molecules (25, 51). It has been suggested that polyamines prevent glycation of proteins (25). More recently, it has been shown that polyamines regulate apoptosis (51). Indeed, it is now appreciated that polyamines are bivalent regulators of cellular function and are involved in cell growth and cell death depending on environmental cues (51).
Trichomonas vaginalis is the number one, nonviral sexually transmitted disease that affects more than 250 million people worldwide (52). Adverse consequences to women with trichomonosis include enhanced risk for human immunodeficiency virus transmission, especially among minority communities, thereby making this a health disparities disease (32, 48). Other sequelae resulting from infection are cervical cancer (50) and bad pregnancy outcomes (18). Unlike most eukaryotic cells, T. vaginalis is unable to de novo synthesize spermine and obtains this molecule from the host through an antiporter system coupled to the secretion of two molecules of putrescine for each molecule of spermine (57). T. vaginalis makes ornithine by the arginine dihydrolase pathways, and this leads to the production of ATP via carbamate kinase (53). Putrescine is synthesized by the trichomonad enzyme ornithine decarboxylase (ODC) (54, 55) and is present in large amounts in secretions of patients (17). Upon cure of patients, amounts of putrescine and other diamines in vaginal secretions were undetectable and equal to that seen for normal women (17), suggesting that parasites are the primary source of putrescine during infection.
Incorporated spermine is back-converted to spermidine inside the hydrogenosomes (57). The polyamine metabolism can yield ∼10% of T. vaginalis' energy during growth in a complex medium, but the contribution in vivo of the arginine dihydrolase pathway to energy production may be greater (53). Finally, the role of polyamines, especially that of secreted putrescine, in trichomonad and/or host cell biology during infection and in relation to parasite virulence and host pathogenesis is unclear but would appear to require attention.
Inhibition of Tritrichomonas foetus ODC reduced the amounts of putrescine, spermine, and spermidine (46). Not surprisingly, this led directly to arrest of cell growth that was reversible upon addition of putrescine (46). Remarkably, inhibition of the Tritrichomonas foetus ODC induced destruction of the hydrogenosome organelles that are responsible for the oxidative decarboxylation of pyruvate for energy generation (30, 37). Key hydrogenosome enzymes were downregulated in expression, as evidenced by immunocytochemical experiments (46). The present study reinforced the idea that the function of hydrogenosome organelles was linked with polyamine metabolism. Indeed, the enzyme polyamine oxidase involved in polyamine metabolism is found within the fraction enriched for hydrogenosome (57).
It has been previously shown that polyamine synthesis in T. vaginalis can be inhibited by polyamine analogues (16, 21, 40, 46, 53, 54, 56, 57) but, unlike Tritrichomonas foetus, the effects of inhibition on T. vaginalis properties, especially the virulence attributes, remain undefined. These findings with Tritrichomonas foetus are noteworthy with respect to T. vaginalis biology for important reasons. The T. vaginalis adhesins AP65, AP51, and AP33 share identity with the hydrogenosome enzymes decarboxylating malic enzyme, β-succinyl coenzyme A synthetase (β-SCS), and α-SCS, respectively (2-6, 9, 15, 20, 41). Therefore, if inhibition of T. vaginalis ODC also leads to destruction of hydrogenosomes, it is possible that this would decrease amounts of the adhesin-enzymes (AP65, AP51, and AP33) that are compartmentalized outside the organelles and placed on the parasite surface where the proteins have adhesin function (24). We tested, therefore, whether inhibition of polyamine metabolism led to degradation of the T. vaginalis hydrogenosomes, downregulated expression of the adhesin-enzymes, and decreased adherence and contact-dependent cytotoxicity (4, 13, 29) to immortalized vaginal epithelial cells (VECs). We show here that similar to what was found with Tritrichomonas foetus, the inhibition of polyamine metabolism led to arrest of growth of T. vaginalis organisms. However, there was no evidence of an adverse effect on the number and integrity of hydrogenosome organelles. We found an unexpected increased level of adherence to VECs mediated by the adhesins and, in contrast to earlier reports, a complete absence of contact-dependent host cell killing previously attributable to cysteine proteinases (11, 12, 26, 27). Our findings suggest an important role for polyamine metabolism and the secretion of putrescine in the biology of host parasitism by T. vaginalis, perhaps through modulation of VEC adherence and tissue cytopathology.
MATERIALS AND METHODS
Parasite and host cells.
T. vaginalis isolate T016 was grown at 37°C in Trypticase-yeast-maltose (TYM) medium supplemented with 10% heat-inactivated horse serum, as described previously (19). Growth kinetic experiments were performed with different concentrations of 1,4-diamino-2-butanone (DAB; Sigma Chemical Co., Saint Louis, Mo.) added to the medium before inoculation with parasites. Growth inhibition rescue experiments were performed by adding 40 mM exogenous putrescine, 50 μM spermine or 50 μM spermidine after 18 h of growth in DAB (Fig. 1B). The amount of putrescine (Sigma) was based on published reports on Tritrichomonas foetus (46), and >50 and >25 μM concentrations of spermine and spermidine, respectively, were toxic to the parasites as well as to the viable host cells used in cytotoxicity assays described below. For some experiments, TYM-horse serum medium was supplemented with 250 μM ferrous ammonium sulfate (Sigma), and iron-depleted parasites were obtained by cultivation in medium depleted of iron with 50 μM 2,2-dipyridyl (Sigma) (33, 34). Only mid-logarithmic-phase parasites were used throughout. Primary immortalized MS-74 vaginal epithelial cells (VECs) have been used recently (24) and were grown at 37°C in a 7.5% CO2 atmosphere in Dulbecco modified Eagle medium (DMEM; Gibco/Invitrogen, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (24). These VECs were washed and used in the absence of serum for both adherence and cytotoxicity assays described below. For monitoring the numbers of organisms, parasites were enumerated by using a Neubauer hemocytometer chamber.
FIG. 1.
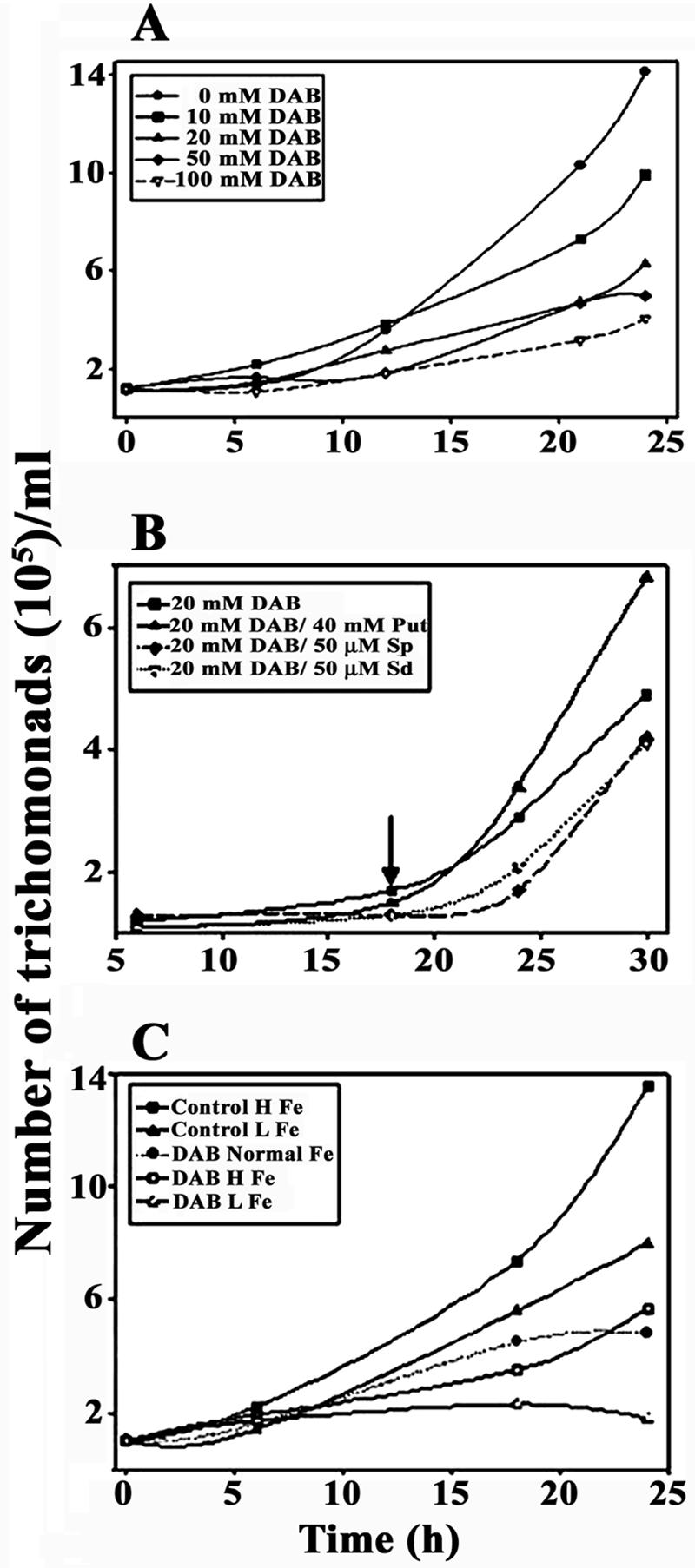
T. vaginalis growth is arrested by DAB, and putrescine restores growth to DAB-treated parasites. (A) TYM-serum medium was inoculated with mid-logarithmic-grown organisms at a density of 105 per ml in the presence of different concentrations of DAB, as indicated in the insert. Numbers of organisms were enumerated at different times of growth. (B) Trichomonads treated with 20 mM DAB and grown as in panel A for 18 h were supplemented with or without 40 mM putrescine, 50 μM spermine, or 50 μM spermidine. (C) Trichomonads were grown in high (H)- or low (L)-iron medium in the absence (control H and L Fe) or presence of 20 mM DAB. In this case the DAB-treated parasites were grown in normal medium (normal Fe) or in a high-iron (H Fe) or low-iron (L Fe) medium, as indicated in the inset and as detailed in Materials and Methods.
Transmission electron microscopy.
Briefly and as before (6, 24), trichomonads were fixed overnight at room temperature in 2.5% (vol/vol) glutaraldehyde (Sigma) in 0.1 M cacodylate (Sigma) buffer (pH 7.2). The cells were then washed twice in phosphate-buffered saline (PBS). Postfixation was performed for 30 min in 1% OsO4 (Sigma) in cacodylate buffer containing 5 mM CaCl2 and 0.8% potassium ferricyanide in the dark. Cells were washed in PBS, dehydrated in acetone, and embedded in Epon. Ultrathin sections were stained with uranyl acetate for 20 min and lead citrate for 5 min and observed in a JEOL 1210 electron microscope operating at 80 kV.
Cytotoxicity assay.
Immortalized MS-74 VECs were seeded onto individual wells of a MICROTEST 96 (Becton Dickinson, Franklin Lakes, N.J.) at an initial cell density of 5 × 104 per well and grown overnight in DMEM-10% heat-inactivated fetal bovine serum to form a confluent monolayer. Then, mid-logarithmic-phase of growth parasites grown for 18 h in the presence or absence of 20 mM DAB were washed and suspended in DMEM-TYM (2:1 [vol/vol]) without serum, and 2.5 × 105 trichomonads were added to each well of washed VECs. After incubation for 2 h at 37°C in a CO2 incubator, the wells were washed in cold PBS to remove parasites, and remaining VECs were fixed with 2% paraformaldehyde and stained with 0.13% crystal violet. The extent of cell killing was measured at an absorbance of 570-nm of solubilized dye, as detailed before (4, 10).
Substrate gel electrophoresis.
A total of 107 parasites were solubilized in 1% deoxycholate (12, 39), and protein equivalent to 4 × 104 trichomonads were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (31) with 10% acrylamide copolymerized with 0.2% gelatin, as before (39). After electrophoresis, the gel was incubated in 2.5% Triton X-100 for 45 min before the gel was placed overnight in activation buffer consisting of 100 mM sodium acetate and 1 mM dithiothreitol (pH 4.5). The proteinase activity indicated by the clear areas in the gel was visualized after the gel was stained with Coomassie brilliant blue R-250 (39).
Adherence assay.
T. vaginalis organisms at 18 h of growth were metabolically labeled with calcein by using the Vybrant Cell Adhesion Assay Kit (Molecular Probes, Eugene, Oreg.) for no less than 30 min according to the manufacturer's protocol. Then 4 × 105 parasites were added to confluent monolayers of 3% glutaraldehyde-fixed MS-74 VECs (24) on individual wells of a Costar Stripwell plate (Corning, Inc., Corning, N.Y.) 96-well microtiter plate. After 45 min of incubation at 37°C, wells were washed with warm PBS. Attached cells were lysed with 200 μl of 0.1% Triton X-100, and the extent of parasite binding was obtained by measuring the fluorescence with the VersaFluor Fluorometer (Bio-Rad Laboratories, Hercules, Calif.). Standard curves for both control and DAB-treated cells were obtained to determine the number of attached cells, and controls were always normalized to 100% for comparative purposes. Where indicated in antibody inhibition experiments, assays were performed as described by us earlier (24), and parasites were preincubated for 30 min with rabbit anti-AP65 immunoglobulin G (IgG). This anti-AP65 IgG was used because recent studies have shown this protein to be a prominent adhesin of T. vaginalis (24, 38). For some experiments 40 mM putrescine prepared as a 1 M stock solution in TYM without serum was added to labeled DAB-treated or untreated organisms incubated for 30 min prior to addition to the MS-74 VEC monolayers. Adherence experiments were also performed with organisms pretreated for 30 min with 1 mM TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone; Sigma), as we have described previously (13).
Ligand assay and immunoblotting.
Parasites grown in TYM-serum medium in the presence or absence of 20 mM DAB for 18 h and numbering 2 × 107 were lysed as previously described (14, 24, 34), and solubilized protein was incubated overnight with 2 × 106 HeLa cells or MS-74 VECs fixed with 3% glutaraldehyde (24). HeLa cell-bound trichomonad proteins were released by boiling and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) as described above. Proteins was blotted onto nitrocellulose by using a Trans-Blot Semi-Dry System (Bio-Rad), and membranes probed with monoclonal antibodies (MAbs) 12G4 and F5-2 to the trichomonad adhesins AP65 and AP33, respectively (20, 21, 24). For total protein immunoblots in experiments involving iron, proteins were precipitated with 10% trichloroacetic acid (3); protein samples equivalent to 7.5 × 105 parasites were electrophoresed, and gels were stained for visualization. Duplicate samples were electrophoresed and blotted. The ligand assay and immunoblotting were also performed with enriched membrane preparations of control, untreated and DAB-treated parasites. Membranes were derived from 8 × 109 parasites exactly as described by us before (1). Membranes were solubilized under the same conditions as organisms for the ligand assay, and 4 × 107 cell equivalents were electrophoresed for immunoblotting experiments.
Fluorescence detection of AP65.
Fluorescence was performed with control, untreated and 20 mM DAB-treated parasites grown in high-iron medium. A total of 2 ml containing 2 × 105 trichomonads in high-iron medium with serum was added to individual wells of 24-well plates with round glass coverslips. After 21 h of growth at 37°C, wells were washed twice with PBS, followed by fixation for 1 h at room temperature of trichomonads attached to the glass coverslips with 500 μl of 4% paraformaldehyde containing 0.25% glutaraldehyde (both from Sigma). Coverslips were then washed twice in PBS and treated with 1 mg of sodium bromohydrate (Sigma)/ml in PBS for 10 min. After blocking for 1 h at room temperature with 50 mM glycine plus 1% bovine serum albumin in PBS, parasites were again washed in PBS prior to adding 250 μl containing a 1:100 dilution of hybridoma supernatant with MAb 12G4 to AP65 prepared in 1% horse serum in PBS. After overnight incubation at 4°C, wells were washed twice and allowed to dry. The secondary fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma) diluted 1:100 in 1% horse serum in PBS was added, and coverslips were incubated for 90 min at 37°C. Coverslips were finally washed in distilled, deionized water prior to examination by fluorescence microscopy.
RESULTS
DAB arrests T. vaginalis growth that is reversed by exogenous putrescine.
Tritrichomonas foetus treated with 20 mM DAB reduced by ∼90% the amounts of intracellular putrescine and arrested parasite growth (46). In order to establish the effects of DAB on T. vaginalis isolate T016, we first examined different amounts of DAB on parasite growth kinetics. As seen in Fig. 1A, there was a concentration-dependent inhibition of growth. Complete growth arrest was seen at 100 mM DAB, but amounts of ≥50 mM at times affected overall parasite motility and viability. The concentration of 20 mM DAB was chosen for subsequent experiments since 100% of the organisms retained motility, and after 18 h of growth in DAB, trichomonads could be rescued by addition to the culture medium of 40 mM putrescine, as seen in Fig. 1B. No similar reversal of growth inhibition was achieved by the addition of spermine or spermidine, two polyamines that are a part of polyamine metabolism of and taken up by T. vaginalis (53). Importantly, 40 mM DAB was needed for growth inhibition similar to that seen for isolate T016 for other fresh clinical isolates, showing that optimization is required for other isolates for similar studies.
We also monitored the growth of DAB-treated organisms in iron-replete and iron-restricted medium. Not unexpectedly, high-iron parasites had elevated cell numbers, and low-iron trichomonads had lower levels of growth similar to DAB-treated organisms in normal medium (Fig. 1C). High-iron DAB-treated parasites did not have an increased rate of growth above that of DAB-treated organisms, suggesting that, regardless of the iron status, intact polyamine metabolism is required for optimal growth by T. vaginalis.
DAB has no effect on hydrogenosomes and on the amounts of T. vaginalis adhesins.
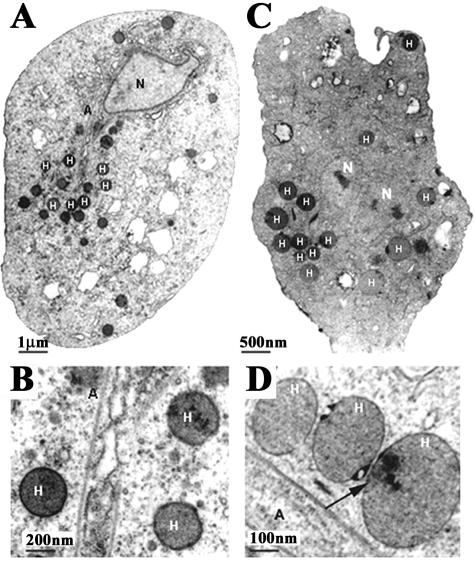
Since DAB inhibition of ODC of Tritrichomonas foetus destroyed hydrogenosomes, it was important to examine whether the decreased cell growth seen in DAB-treated T. vaginalis resulted from damage to hydrogenosomes. The integrity of T. vaginalis hydrogenosomes was visualized by transmission electron microscopy (Fig. 2). No significant changes were observed in the overall architecture of treated (Fig. 2C) versus control (Fig. 2A) trichomonads. Likewise, the number and nature of hydrogenosomes were similar regardless of treatment (Fig. 2B and D). This suggests that inhibition of polyamine metabolism in T. vaginalis has different consequences from that seen for Tritrichomonas foetus (46).
FIG. 2.
Transmission electron microscopy showing comparative thin sections and ultrastructure of control (A and B) versus 20 mM DAB-treated (C and D) T. vaginalis isolate T016. The hydrogenosomes (H), axostyle (A), and nucleus (N) are readily visible as indicated. All of the structures present a normal appearance. A higher magnification of T. vaginalis to visualize hydrogenosomes in routine preparations is shown in panels B and D. Notice the normal appearance of all cell structures, including the hydrogenosomes (H), in the DAB-treated organisms (C and D), although, on occasion, there were electron-dense spots in the hydrogenosomal matrix (panel D, arrow).
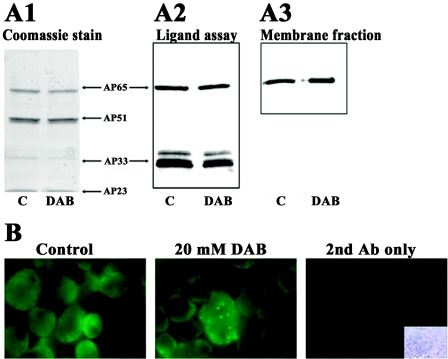
We next wanted to determine whether DAB treatment affected synthesis of the T. vaginalis AP65, AP51, and AP33 adhesins that are also enzymes of hydrogenosomes (2, 20, 41). SDS-PAGE and immunoblots of HeLa cell-binding proteins of a ligand assay were probed with the MAb 12G4 to AP65 and MAb F5.2 to AP33 (Fig. 3A2) and, as can be seen, almost identical amounts of protein bands were detected for equivalent numbers of control (lane C) and DAB-treated (lane DAB) parasites. The multiple banding in the AP33 area (Fig. 3A2) is the result of protease digestion of this adhesin, as shown previously (21). The stained duplicate gel (Fig. 3A1) also has the proteins AP51 and AP23 in addition to AP65 and AP33 (14, 34). Further, a ligand assay, followed by SDS-PAGE and immunoblotting, was performed by using enriched membranes from control (untreated) and 20 mM DAB-treated parasites. Blots with MAb 12G4 gave identical intensities of protein bands (Fig. 3A3). Identical results were obtained with the immortalized MS-74 VECs used in the ligand assay as described previously (24). Finally, we performed total protein immunoblotting with the same MAbs of T. vaginalis organisms grown in iron-replete or normal medium in the presence or absence of DAB. Not unexpectedly, higher amounts of the adhesins were seen for both the control and DAB-treated trichomonads grown in iron-replete medium compared to the normal grown organisms (data not shown). Here, too, no difference in the amounts of adhesins was seen between control and DAB-treated parasites. These results indicate that inhibition of polyamine metabolism has no effect on the upregulation by iron of transcription and translation of the adhesin genes.
FIG. 3.
Trichomonads treated with 20 mM DAB express identical amounts of adhesins as control, untreated parasites. (A) Panel A2 shows immunoblots of a HeLa cell-binding proteins from a ligand assay (Materials and Methods) with AP65 and AP33 detected with MAbs 12G4 and F5.2, respectively (21, 24). The left panel (A1) has duplicate Coomassie brilliant blue-stained gels after the ligand assay as in the right panel and shows the other two AP51 and AP23 adhesins (14, 34). Multiple bands for AP33 in panel A2 are due to partial degradation by the trichomonad cysteine proteinases, as described previously (21). Panel A3 shows immunoblot results for AP65 as in A2 of a ligand assay performed by using enriched membranes from identical cell equivalents of control, untreated and 20 mM DAB-treated parasites. (B) Immunofluorescence was performed with nonpermeabilized trichomonads with MAb 12G4, which reacts with surface AP65, as demonstrated recently (24, 38). No fluorescence was obtained in the absence of anti-AP65 MAb but in the presence of fluorescein-conjugated secondary goat anti-mouse IgG, as shown in the far right panel. The inset (bottom right) in the far right panel shows the bright-field microscopy picture of the cells used in the secondary antibody control.
Finally, immunofluorescence experiments (Fig. 3B) with MAb 12G4 to AP65 (24) showed identical intensity of signals for both control (untreated) organisms and 20 mM DAB-treated trichomonads, reaffirming the presence of equal amounts of AP65 regardless of inhibition of polyamine synthesis. No fluorescence was obtained in control experiments without the anti-AP65 MAbs.
Inhibition of polyamine metabolism enhances adherence mediated by adhesins of T. vaginalis to MS-74 VECs.
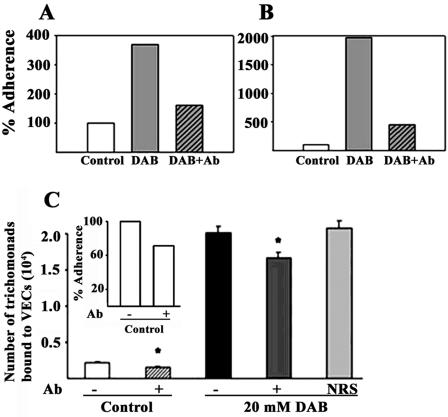
Since DAB-treated and control, untreated organisms had identical amounts and functional host cell binding adhesins, we next performed a typical adherence assay to measure the levels of parasite binding to host cells. To our surprise, treatment with 20 mM DAB enhanced adherence to the immortalized human MS-74 VECs by ca. 4- to 20-fold, as shown in two representative experiments (Fig. 4A and B, respectively). The increase in adherence was reproducible but varied among experiments. Since AP65 has been recently determined to be a prominent adhesin (24), additional adherence assays were done with duplicate DAB-treated parasites pretreated with rabbit anti-AP65 IgG and NRS IgG antibodies. Figure 4C illustrates another representative experiment with triplicate samples. The data are presented as the number of parasites bound to the VEC monolayer for statistical analysis. The asterisk indicates that the inhibition by rabbit anti-AP65 IgG antibody was significant (P < 0.001) for both control, untreated and 20 mM DAB-treated organisms. As shown for the DAB experiment (right side), there was no inhibition of adherence with the NRS IgG control antibody, and NRS was without effect in the control parasites (not shown). The insert illustrates the percent adherence levels between control, untreated (−) versus antibody-treated (+) trichomonads to better show the extent of inhibition by antibody. As expected based on our earlier study (24, 38), the anti-AP65 antibodies decreased levels of parasites bound to VECs, indicating that the enhanced adherence in DAB-treated parasites was still mediated by the adhesins.
FIG. 4.
(A and B) Two representative experiments of comparative adherence assays of 20 mM DAB-treated versus control, untreated T. vaginalis handled identically. The adherence assay was performed as described in Materials and Methods. Parasites treated with 20 mM DAB (gray bars) showed ∼4-fold (A)- to 20-fold (B)-increased levels of adherence compared to control, untreated parasites (open bars). Inhibition of adherence was achieved by addition of 50 μg of rabbit anti-AP65 IgG (Ab, hatched bars) to 20 mM DAB-treated trichomonads prior to addition to host cell monolayers. Each graph represents the average from quintuplet samples in respective experiments. The controls were normalized to 100% for comparison. The value for adherence in the samples in each experiment did not vary by >5% of the average. (C) Data from a representative experiment with triplicate samples. The data are presented as the number of parasites bound to the VEC monolayer for statistical analysis. The asterisk indicates that the inhibition by rabbit anti-AP65 IgG antibody (75 μg) was significant (P < 0.001) for both control, untreated and 20 mM DAB-treated organisms. NRS refers to normal rabbit serum used identically in inhibition experiments performed simultaneously and, as shown for the DAB experiment (right side), there was no inhibition of adherence. In separate adherence experiments not shown here, NRS was without effect in the control parasites. The inset illustrates the percent adherence levels between control, untreated (−) versus antibody-treated (+) trichomonads, and results were normalized to 100% for control organisms for comparison. Ab, antibody.
Exogenous putrescine reverses the enhanced adherence of DAB treatment.
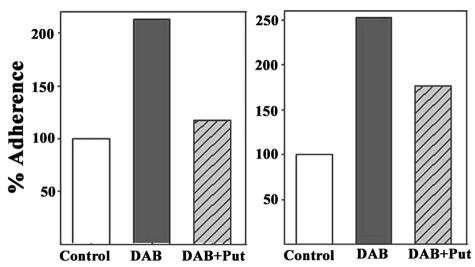
It was of interest whether the addition of 40 mM putrescine to DAB-treated trichomonads used to rescue growth arrest had an effect on the elevated levels of adherence. Indeed, as indicated in Fig. 5, exogenous putrescine (40 mM) pretreatment of DAB-treated organisms prior to the adherence assay decreased levels of adherence. No similar effect was evident upon pretreatment of the VECs (data not shown). These results reinforce the notion that polyamine metabolism and secretion of putrescine affects the true levels of VEC recognition and binding as a consequence of deficient putrescine production. Further, the data suggest that the decreased adherence results from putrescine interacting with the parasite and not the host cell surface.
FIG. 5.
Two representative experiments showing a return to decreased levels of adherence of the 20 mM DAB-treated trichomonads supplemented with 40 mM putrescine. The 20 mM DAB-treated parasites were preincubated with 40 mM putrescine (bar labeled DAB+Put) before addition to cell monolayers in the adherence assay. The open bars represent control, untreated trichomonads normalized to 100% for comparison. The shaded bars represent 20 mM DAB-treated parasites (labeled DAB), and hatched bars represent of 20 mM DAB-treated organisms supplemented with 40 mM putrescine (DAB+Put). Each graph represents the average from quintuplet samples in respective experiments. Statistical analysis showed that the individual samples did not vary >5% from the average.
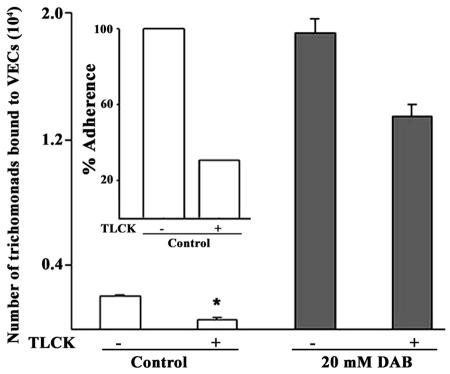
Proteinase activity is unnecessary for adherence by DAB-treated parasites.
Earlier we showed that cysteine proteinase activity was required for optimal adherence to epithelial cells (13, 26, 36). Therefore, it was important to determine whether proteinase activity was again necessary for adherence by DAB-treated trichomonads. As seen in Fig. 6, only a slight decrease by ∼20% in adherence was evident in DAB-treated organisms incubated with 1 mM TLCK (gray bars labeled 20 mM DAB). In contrast, ≥70% inhibition of adherence was seen in control parasites without DAB incubated with TLCK (clear bars labeled Control), as before (13), which was statistically significant (asterisk, P < 0.001). The inset graph is included to better illustrate the extent of inhibition of adherence by TLCK-treated control parasites. These data suggest that there is a diminished role for proteinase activity in the absence of polyamine metabolism and putrescine secretion.
FIG. 6.
Representative experiment with quintuplicate samples showing that cysteine proteinases are not required for adherence of 20 mM DAB-treated (shaded bars labeled DAB) versus control, untreated (open bars labeled control) trichomonads. The data are presented as numbers of trichomonads bound to VECs for statistical analysis, along with error bars. The inset shows results for the control, untreated parasites as the percent adherence to better illustrate the extent of decrease in adherence by TLCK-treated organisms, and results were normalized to 100% for control organisms for comparison as was done in Fig. 4C. A 1 mM TLCK pretreatment of control parasites inhibited adherence by ≥70% (□) and by ∼20% in 20 mM DAB-treated organisms (░⃞). In this experiment, 20 mM DAB treatment resulted in an ∼9-fold increase in the level of adherence compared to control, untreated trichomonads.
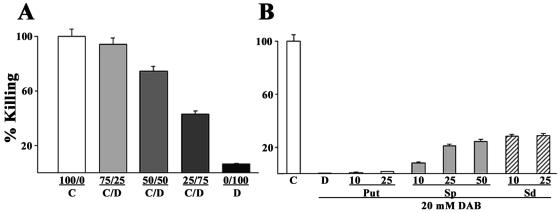
DAB treatment inhibits contact-dependent cytotoxicity.
We next reasoned that the rate and extent of contact-dependent host cell killing as described before (10, 12, 27, 29) and mediated by cysteine proteinases would be augmented in the DAB-treated organisms with elevated adherence levels. The results of Fig. 7A show the dramatic 90% decreased level of killing of MS-74 VECs for the DAB-treated (column D) compared to control, untreated trichomonads (column C). Altering the ratio of untreated to DAB-treated parasites (columns C/D) in the suspension added to VEC monolayers showed decreasing levels of cytotoxicity with increasing numbers of DAB-treated parasites. Lastly, the addition of exogenous putrescine to the parasite suspension prior to addition to VECs had no effect in elevating cytotoxicity levels (Fig. 7B). However, both spermine and spermidine at sublethal concentrations (≤50 and 25 μM, respectively) did produce some increased killing of VECs. Greater amounts of spermine and spermidine were toxic to both parasite and MS-74 VECs. These data suggest that a fully functional polyamine metabolism is required for optimal host cytotoxicity.
FIG. 7.
Contact-dependent cytotoxicity of levels of immortalized human MS-74 VECs by T. vaginalis. (A) Cytotoxicity results using different ratios of untreated and 20 mM DAB-treated parasites (columns C/D). The results were compared to the control, untreated (100/0, column C) and 20 mM DAB-treated (0/100, column D) organisms. The results are averages of three experiments, each with seven samples. (B) Results obtained with 20 mM DAB-treated parasites supplemented with millimolar amounts of putrescine (Put) and micromolar amounts of spermine (Sp) and spermidine (Sd). The numbers underneath the bars represent the amounts added. The cytotoxicity levels were compared to those of the control, untreated (column C) and 20 mM DAB-treated (column D) organisms, and the controls were normalized to 100% for comparison. The results are the average of three experiments, each with seven samples.
Cysteine proteinase patterns remain unchanged by DAB.
Given the absence of cytotoxicity, we tested for qualitative and quantitative differences in patterns of cysteine proteinases after inhibition of polyamine synthesis. One dimensional SDS-PAGE with gelatin copolymerized with acrylamide as a substrate gave no detectable changes in the overall expression pattern and activity as shown in Fig. 8. The arrows point to the electrophoretic mobilities of proteinase activities reported to be involved in cytotoxicity (11, 12, 27).
FIG. 8.
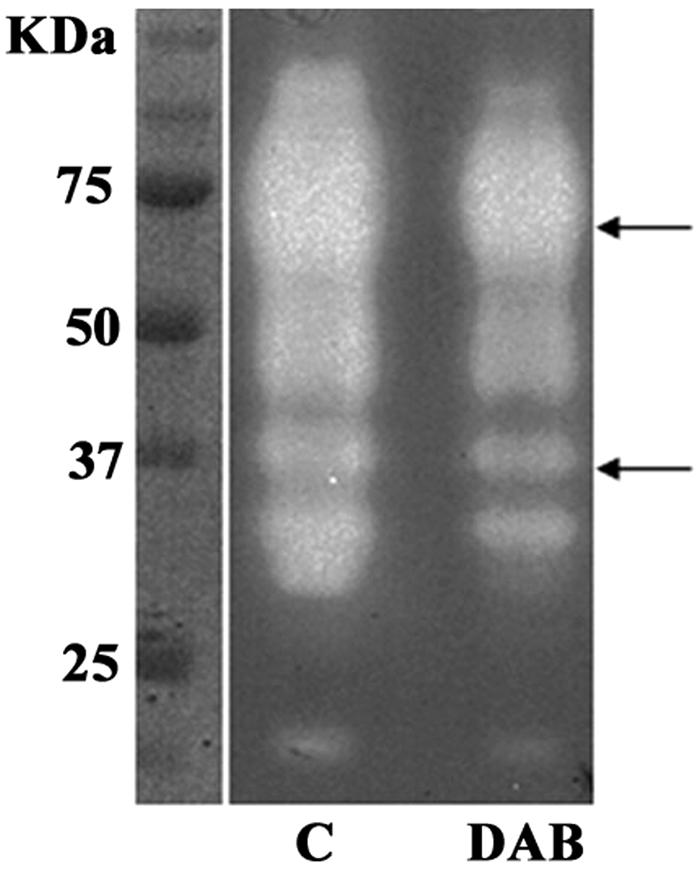
One-dimensional substrate SDS-PAGE analysis of T. vaginalis T016 grown in the absence (lane C) or presence (lane DAB) of 20 mM DAB. Clear regions of the gel represent proteinase digestion of gelatin as substrate copolymerized with acrylamide. Arrows indicate two regions of proteinase activity shown to be involved in contact-dependent cytotoxicity (11, 12, 27).
DISCUSSION
The polyamines putrescine, spermine, and spermidine are ubiquitous small positively charged molecules important for cell growth and differentiation (51). Unlike most eukaryotic cells, T. vaginalis lacks de novo synthesis of and is dependent upon the host for spermine, which is then back-converted to spermidine (57). Although trichomonad polyamine metabolism and adverse effects of inhibition of ODC in Tritrichomonas foetus have been characterized (46), this area of investigation for T. vaginalis is lacking and was the focus of our investigation. We wanted to determine for T. vaginalis if decreased amounts of putrescine by inhibition of ODC affected hydrogenosomes (46) and properties associated with little or no production of hydrogenosome proteins, as recently demonstrated for the nonadherent, drug-resistant MR100 trichomonads (24).
Growth arrest by treatment of T. vaginalis with DAB to inhibit ODC (46) was the only property that was affected and in common with Tritrichomonas foetus inhibition of polyamine metabolism. Polyamines modulate iron uptake in mammalian cells through the formation of polyamine-iron complexes incorporated via the polyamine transport system (22). However, the addition of spermine and spermidine to iron-replete medium of DAB-treated trichomonads (Fig. 1C) failed to restore growth (data not shown). Further and as expected (8, 14, 34), growth-arrested, DAB-treated parasites placed in iron-replete medium increased the amounts of adhesins as detected by MAbs in immunoblots. This suggests that transcription and translation of the adhesin genes per se is unlinked to polyamine metabolism essential for growth of T. vaginalis (33). These data also suggest that products of polyamine metabolism are not involved in any way in the uptake of iron and in iron-mediated upregulation of expression of adhesins (34). No change was observed in the synthesis of the adhesins that have identity to hydrogenosomes enzymes, as evidenced by the amount of protein bound to host cells in a ligand assay. Both stained total proteins and immunoblots gave similar if not identical amounts of adhesins (Fig. 3). Likewise, the patterns and extent of fluorescence on the surface were similar between control, untreated and DAB-treated parasites (Fig. 3).
In contrast to Tritrichomonas foetus inhibition of putrescine synthesis, there was no obvious deleterious effect on T. vaginalis hydrogenosomes (Fig. 2). Further, it would appear that polyamines are not involved in modulating protein expression of T. vaginalis organisms, as has been shown for mammalian cells (28, 49). It was remarkable and unexpected that DAB inhibition of ODC increased levels of adherence up to 20-fold (Fig. 4 and 5) while simultaneously lacking previously described contact-dependent killing of host cells (Fig. 7). The fact that pretreatment of DAB-treated parasites with polyclonal rabbit antibody to AP65, a prominent adhesin (24, 38), decreased adherence (Fig. 4) suggests strongly the involvement of adhesins in the increased ability to bind host cells. Likewise, that exogenous putrescine added to DAB-treated organisms immediately lowered adherence more in line with the untreated, control trichomonads (Fig. 5) indicates a direct involvement of putrescine with the T. vaginalis surface. Remarkably, the addition of both putrescine and anti-AP65 antibody to DAB-treated organisms produced no enhanced decreased adherence compared to putrescine alone (Fig. 5). Although we do not know exactly the reason for this, it is likely that the amount of putrescine (40 mM) is in excess, and upon immediate coating of the parasite surface there is diminished accessibility by antibody to the adhesins. The amount of putrescine that was used in these experiments was chosen to be consistent with the experiments performed in Fig. 5 and because this was the amount that rescued the parasites in growth experiments (Fig. 1B). It is conceivable, therefore, that in vivo the secreted putrescine may coat the parasite surface, thereby further attracting host macromolecules, the net result of which is masking adhesins for VEC recognition. These data may indicate that during infection, trichomonads are in effect less efficient at target cell attachment, albeit retaining the ability to be cytotoxic. The advantage that this affords to the survival of the parasite is discussed below.
At physiological pH, polyamines are referred to as super-cations (51). Thus, at the pH range of 4.0 to 7.0 of women infected with T. vaginalis (7), polyamines such as putrescine and spermine, which are transported from the host secretions, likely readily bind to the trichomonal surface, and this may promote the uptake of spermine through the antiporter (53, 57). Indeed, intracellular spermine is concentrated against a concentration gradient (53), which would be facilitated through polyamine interactions with the parasite surface. A consequence of putrescine and spermine on parasites may also be the binding of host macromolecules present in vaginal secretions, further coating the parasite surface. This may account for the ability of trichomonads to coat the surface with numerous loosely and avidly associated host proteins, as has been demonstrated previously by us (43-45). Equally noteworthy is the fact that a trichomonad cysteine proteinase activity is required for optimal adherence to epithelial cells (13). This earlier report established that proteinase action upon the parasite, but not host cell, surface increased adherence, and it was hypothesized that the proteinase was required for unmasking of the adhesins for more efficient epithelial cell recognition. The idea that polyamines on T. vaginalis promote host protein coating, therefore, has merit and would be consistent with a recent study showing that MAbs to a 62-kDa proteinase decrease parasite adherence to epithelial cells (26), possibly by blocking the unmasking of host proteins covering the adhesins. This notion is further reinforced by the fact that proteinase activity is not required for optimal adherence in DAB-treated parasites, since inhibition of proteinases with TLCK had a minimal effect on adherence compared to control organisms, as shown in Fig. 6 and previously by us (13).
As mentioned above, equally noteworthy was the inhibition of cytotoxicity mediated by adherence (10, 12, 29). Interestingly, previous work showed decreased cytotoxicity in T. vaginalis treated with α-difluoromethylornithine, another ODC inhibitor, but the precise mechanism was undefined (16). Numerous reports have shown a role for the trichomonad cysteine proteinases in killing host cells (11, 12, 27). The absence of qualitative and quantitative differences in the complex proteinase patterns (Fig. 8), including the proteinases with electrophoretic mobilities purported to be involved in cytotoxicity (11, 12, 27), questions whether the proteinases play a direct role in cell killing. One possible explanation for these results is that other unknown cidal factors linked to polyamine metabolism and that require proteinase processing are not being synthesized in DAB-treated parasites. Importantly, the secretion of putrescine in concert with the presence of proteinases has been found to be prerequisite for efficient mucolytic activity, a property that may be required for parasite penetration of mucus for colonization of the epithelium (35, 42). Thus, this finding shows that putrescine and proteinase(s) together through an unknown mechanism may be required for cytotoxicity but not for adherence. This observation on the absence of cytotoxicity, despite the synthesis of the trichomonad proteinases and elevated adherence, may now allow us to develop alternative strategies to examine for the factors induced by polyamine metabolism that directly lead to host cytopathology.
To our knowledge, we show for the first time that polyamine metabolism of T. vaginalis is directly linked to the capacity of the parasite to adhere and kill VECs. We present evidence for the association between polyamine metabolism, particularly putrescine synthesis and secretion, and less efficient VEC binding, which, paradoxically, allows for contact-dependent host cell killing (10, 29) for nutrient acquisition (43-45). The greatly increased levels of adherence achieved by abolishing putrescine synthesis would clearly not be favorable to survival of the parasite in vivo, since this might produce a more vigorous host response that would limit infection. Spermine acquisition requires putrescine synthesis and secretion into vaginal fluids in order to have intact polyamine metabolism. In this way, the ability to lyse host cells, albeit with less-than-optimal adherence, is complemented, and among the many nutrients available through host cell lysis may be spermine. Indeed, spermine concentrations in women undergo distinct cyclic changes during the menstrual cycle (23), necessitating a mechanism for spermine acquisition that relies on the cytotoxic ability at the expense of better adherence. Altogether, this complex, orchestrated interactions between polyamines, host proteins, and macromolecules, and the parasite surface permits successful host parasitism that in turn ensures the non-self-limiting nature of infection. In conclusion, future research targeting polyamine metabolism of T. vaginalis for increasing our knowledge of trichomonal virulence and pathogenesis and for development of novel chemotherapeutics should receive increased attention.
Acknowledgments
This study was supported by Public Health Service grants AI43940 and AI45429 from the National Institutes of Health.
Members of the laboratory are also acknowledged for their suggestions and discussion of our work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alderete, J. F., P. Demes, A. Gombosova, M. Valent, A. Janoska, H. Fabusova, L. Kasmala, G. E. Garza, and E. C. Metcalfe. 1987. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect. Immun. 55:1037-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. F., J. Engbring, C. M. Lauriano, and J. L. O'Brien. 1998. Only two of the Trichomonas vaginalis triplet AP51 adhesins are regulated by iron. Microb. Pathog. 24:1-16. [DOI] [PubMed] [Google Scholar]
- 3.Alderete, J. F., and G. E. Garza. 1988. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect. Immun. 56:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderete, J. F., and G. E. Garza. 1985. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect. Immun. 50:701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderete, J. F., M. W. Lehker, and R. Arroyo. 1995. The mechanisms and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parasitol. Today 11:70-74. [Google Scholar]
- 6.Alderete, J. F., K. W. Millsap, M. W. Lehker, and M. Benchimol. 2001. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell. Microbiol. 3:359-370. [DOI] [PubMed] [Google Scholar]
- 7.Alderete, J. F., E. Newton, C. Dennis, and K. A. Neale. 1991. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin. Med. 67:469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alderete, J. F., J. Nguyen, V. Mundodi, and M. W. Lehker. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263-271. [DOI] [PubMed] [Google Scholar]
- 9.Alderete, J. F., J. L. O'Brien, R. Arroyo, J. A. Engbring, O. Musatovova, O. Lopez, C. Lauriano, and J. Nguyen. 1995. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 17:69-83. [DOI] [PubMed] [Google Scholar]
- 10.Alderete, J. F., and E. Pearlman. 1984. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br. J. Vener. Dis. 60:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Sanchez, M. E., L. Avila-Gonzalez, C. Becerril-Garcia, L. V. Fattel-Facenda, J. Ortega-Lopez, and R. Arroyo. 2000. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 28:193-202. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo, R., and J. F. Alderete. 1995. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch. Med. Res. 26:279-285. [PubMed] [Google Scholar]
- 13.Arroyo, R., and J. F. Alderete. 1989. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect. Immun. 57:2991-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arroyo, R., J. Engbring, and J. F. Alderete. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6:853-862. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo, R., J. Engbring, J. Nguyen, O. Musatovova, O. Lopez, C. Lauriano, and J. F. Alderete. 1995. Characterization of cDNAs encoding adhesin proteins involved in Trichomonas vaginalis cytoadherence. Arch. Med. Res. 26:361-369. [PubMed] [Google Scholar]
- 16.Bremner, A. F., G. H. Coombs, and M. J. North. 1987. Antitrichomonal activity of alpha-difluoromethylornithine. J. Antimicrob. Chemother. 20:405-411. [DOI] [PubMed] [Google Scholar]
- 17.Chen, K. C., R. Amsel, D. A. Eschenbach, and K. K. Holmes. 1982. Biochemical diagnosis of vaginitis: determination of diamines in vaginal fluid. J. Infect. Dis. 145:337-345. [DOI] [PubMed] [Google Scholar]
- 18.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 19.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43:488-490. [PubMed] [Google Scholar]
- 20.Engbring, J. A., and J. F. Alderete. 1998. Three genes encode distinct AP33 proteins involved in Trichomonas vaginalis cytoadherence. Mol. Microbiol. 28:305-313. [DOI] [PubMed] [Google Scholar]
- 21.Engbring, J. A., and J. F. Alderete. 1998. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiology 144:3008-3011. [DOI] [PubMed] [Google Scholar]
- 22.Gaboriau, F., A. Kreder, N. Clavreul, J. P. Moulinoux, J. G. Delcros, and G. Lescoat. 2004. Polyamine modulation of iron uptake in CHO cells. Biochem. Pharmacol. 67:1629-1637. [DOI] [PubMed] [Google Scholar]
- 23.Gilad, V. H., R. Halperin, Z. Chen-Levy, and G. M. Gilad. 2002. Cyclic changes of plasma spermine concentrations in women. Life Sci. 72:135-141. [DOI] [PubMed] [Google Scholar]
- 24.Garcia, A. F., T. H. Chang, M. Benchimol, D. J. Klumpp, M. W. Lehker, and J. F. Alderete. 2003. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol. Microbiol. 47:1207-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gugliucci, A. 2004. Polyamines as clinical laboratory tools. Clin. Chim. Acta 344:23-35. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez, H., I. Sariego, G. Garber, R. Delgado, O. Lopez, and J. Sarracent. 2004. Monoclonal antibodies against a 62 kDa proteinase of Trichomonas vaginalis decrease parasite cytoadherence to epithelial cells and confer protection in mice. Parasite Immunol. 26:119-125. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Gutierrez, R., J. Ortega-Lopez, and R. Arroyo. 2003. A 39-kDa cysteine proteinase CP39 from Trichomonas vaginalis, which is negatively affected by iron may be involved in trichomonal cytotoxicity. J. Eukaryot. Microbiol. 50:696-698. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi, K., and K. Kashiwagi. 2000. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 271:559-564. [DOI] [PubMed] [Google Scholar]
- 29.Krieger, J. N., J. I. Ravdin, and M. F. Rein. 1985. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect. Immun. 50:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulda, J. 1999. Trichomonads, hydrogenosomes and drug resistance. Int. J. Parasitol. 29:199-212. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, M. Alary, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 33.Lehker, M. W., and J. F. Alderete. 1992. Iron regulates growth of Trichomonas vaginalis and the expression of immunogenic trichomonad proteins. Mol. Microbiol. 6:123-132. [DOI] [PubMed] [Google Scholar]
- 34.Lehker, M. W., R. Arroyo, and J. F. Alderete. 1991. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J. Exp. Med. 174:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehker, M. W., and D. Sweeney. 1999. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex. Transm. Infect. 75:231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendoza-Lopez, M. R., C. Becerril-Garcia, L. V. Fattel-Facenda, L. Avila-Gonzalez, M. E. Ruiz-Tachiquin, J. Ortega-Lopez, and R. Arroyo. 2000. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 68:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller, M. 1993. The hydrogenosome. J. Gen. Microbiol. 139:2879-2889. [DOI] [PubMed] [Google Scholar]
- 38.Mundodi, V., A. S. Kucknoor, D. J. Klumpp, T. H. Chang, and J. F. Alderete. 2004. Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Mol. Microbiol. 53:1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neale, K. A., and J. F. Alderete. 1990. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect. Immun. 58:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.North, M. J., B. C. Lockwood, and A. F. Bremner, Coombs, G. H. 1986. Polyamine biosynthesis in trichomonads. Mol. Biochem. Parasitol. 19:241-249. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien, J. L., C. M. Lauriano, and J. F. Alderete. 1996. Molecular characterization of a third malic enzyme-like AP65 adhesin gene of Trichomonas vaginalis. Microb. Pathog. 20:335-349. [DOI] [PubMed] [Google Scholar]
- 42.Paget, T. A., and S. L. James. 1994. The mucolytic activity of polyamines and mucosal invasion. Biochem. Soc. Trans. 22:394S. [DOI] [PubMed] [Google Scholar]
- 43.Peterson, K. M., and J. F. Alderete. 1982. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect. Immun. 37:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson, K. M., and J. F. Alderete. 1984. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol. Biochem. Parasitol. 12:37-48. [DOI] [PubMed] [Google Scholar]
- 45.Peterson, K. M., and J. F. Alderete. 1984. Trichomonas vaginalis is dependent on uptake and degradation of human low density lipoproteins. J. Exp. Med. 160:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reis, I. A., M. P. Martinez, N. Yarlett, P. J. Johnson, F. C. Silva-Filho, and M. A. Vannier-Santos. 1999. Inhibition of polyamine synthesis arrests trichomonad growth and induces destruction of hydrogenosomes. Antimicrob. Agents Chemother. 43:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuber, F. 1989. Influence of polyamines on membrane functions. Biochem. J. 260:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabor, C. W., and H. Tabor. 1976. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu. Rev. Biochem. 45:285-306. [DOI] [PubMed] [Google Scholar]
- 50.Viikki, M., E. Pukkala, P. Nieminen, and M. Hakama. 2000. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 39:71-75. [DOI] [PubMed] [Google Scholar]
- 51.Wallace, H. M., A. V. Fraser, and A. Hughes. 2003. A perspective of polyamine metabolism. Biochem. J. 376:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. 1995. An overview of selected curable sexually transmitted diseases, p. 2-27. In Global program on AIDS, World Health Organization, Geneva, Switzerland.
- 53.Yarlett, N., and C. J. Bacchi. 1994. Parasite polyamine metabolism: targets for chemotherapy. Biochem. Soc. Trans. 22:875-879. [DOI] [PubMed] [Google Scholar]
- 54.Yarlett, N., and C. J. Bacchi. 1988. Effect of dl-α-difluoromethylornithine on polyamine synthesis and interconversion in Trichomonas vaginalis grown in a semi-defined medium. Mol. Biochem. Parasitol. 31:1-9. [DOI] [PubMed] [Google Scholar]
- 55.Yarlett, N., B. Goldberg, M. A. Moharrami, and C. J. Bacchi. 1993. Trichomonas vaginalis: characterization of ornithine decarboxylase. Biochem. J. 293:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarlett, N., B. Goldberg, M. A. Moharrami, and C. J. Bacchi. 1992. Inhibition of Trichomonas vaginalis ornithine decarboxylase by amino acid analogs. Biochem. Pharmacol. 44:243-250. [DOI] [PubMed] [Google Scholar]
- 57.Yarlett, N., M. P. Martinez, B. Goldberg, D. L. Kramer, and C. W. Porter. 2000. Dependence of Trichomonas vaginalis upon polyamine backconversion. Microbiology 146:2715-2722. [DOI] [PubMed] [Google Scholar]