Abstract
Objective
MSCs and Platelet-Rich Plasma are the main focus in the study of new regenerative treatments aimed to reverse Osteoarthritis (OA). However, extracellular vesicles (EVs) present several advantages to cell-based treatments. Thus, the aim of this study was to compare and evaluate the regenerative potential of MSC-derived EVs (cEVs) and platelet-derived EVs (pEVs) in an OA cartilage rat model.
Design
OA in vivo model was established through injection of 6 mg MIA in the rat knee joints. After 14 and 21 days, OA knee joints were treated with 1 × 1010 particles of pEVs or cEVs. At day 28, the animals were sacrificed, plasma was collected to quantify CTX-II and knee joints were excised to be evaluated by Cone Beam Computed Tomography (CBCT). After decalcification, histology was used to determine the OARSI score and to visualize collagen and glycosaminoglycan content.
Results
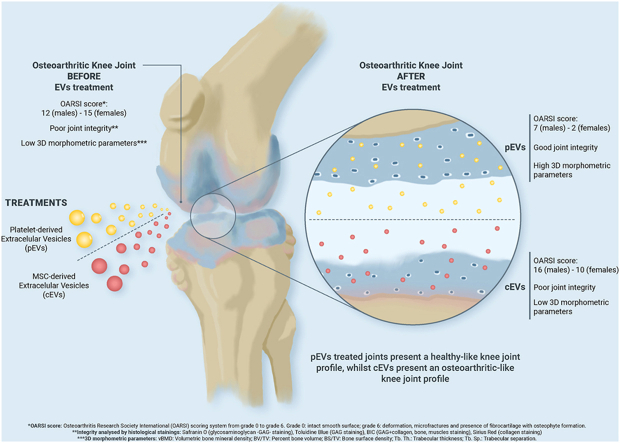
pEVs and cEVs samples did not show significant differences per se but they did in terms of regenerative effects on OA knee joints. pEVs-treated knee joints showed better subchondral bone integrity in CT-analysed parameters when compared to cEVs or OA group, showing similar values to the healthy control group. Moreover, OARSI score indicated that pEVs showed a greater OA reversion in knee joints, especially in female rats, and so indicated the analysed histological images.
Conclusions
pEVs are proposed as a viable regeneration treatment for OA since they are not only capable of exerting their regenerative potential on osteoarthritic cartilage, but also outperform cEVs in terms of efficacy, particularly in females.
Significance statement
Osteoarthritis (OA) is one of the most age-related diseases. It is estimated that 500 million people suffer from OA worldwide, representing the principal cause of chronic disability in adults. In the present study we evaluated the therapeutic effect of extracellular vesicles (EVs) from different sources (platelet lysate and human umbilical cord mesenchymal stromal cells) in an in vivo rat model. Our results demonstrate that platelet-derived EVs (pEVs) induce an OA reversion in knee joints, thus evidencing the therapeutic potential of pEVs as cell-free regenerative agents for OA treatment.
The translational potential of this article
Platelet-derived extracellular vesicles (pEVs) offer a promising cell-free therapy option for OA treatment. Their production could be easily standardized and reproduced without extensive platelet harvesting and amplification, thus paving the way for their clinical translation.
Keywords: Extracellular vesicles, Knee OA in vivo model, Mesenchymal stromal cells, Osteoarthritis, Platelet lysate
Graphical abstract
1. Introduction
Osteoarthritis (OA) is a chronic joint disease that causes articular cartilage deterioration and bone structure alteration. It's associated with chronic pain, inflammation, and disability, affecting patients' quality of life as they age [1]. The articular cartilage is avascular and elastic and contains chondrocytes that produce the extracellular matrix, including the main proteins aggrecan and type II collagen. Damage to the Extracellular Matrix (ECM) is a hallmark of OA and leads to joint instability, as cartilage has limited self-healing ability [2]. Hence, regenerative treatments for OA have been in the spotlight.
Knee OA treatments include surgical options like Autologous Chondrocyte Implantation (ACI) and non-surgical therapies such as platelet-rich plasma (PRP) or hyaluronic acid injections [3,4]. However, they only offer palliative relief and do not modify the disease course. ACI has low efficacy, it is invasive, and it is not cost-effective since the success rate are variable. PRP has patient-to-patient variability and no standardized protocol [5,6]. Cell-based therapies using mesenchymal stem cells (MSCs) show potential for tissue repair but have limitations such as low engraftment, high cost, and the need for surgeries [7,8].
Extracellular vesicles (EVs) are cell-derived membranous bodies with a powerful regenerative potential that can emulate the beneficial effects of cell-based therapies, avoiding their drawbacks. EVs oversee cell-to-cell communication and their regenerative effects have been widely proven in different diseases and injuries [9]. Their origin may vary, but MSCs are the main source used until now with proven effects. MSC-derived EVs (cEVs) have shown relevant therapeutic roles in inflammatory, respiratory, or neurodegenerative diseases [10,11]. Their effect has also been proved on regulating cartilage renovation while interfering with chondrocyte destruction and improving osteochondral repair [12]. However, the use of MSCs as EVs source presents some limitations for their clinical translation arising from their cell culture conditions, such as the high-cost cell maintenance or the use of exogenous reagent for cells growth that may cause adverse effects. Moreover, MCSs present decreased regenerative potential and reduced number with donor age [13,14]. Therefore, other non-MSC sources are also being investigated. As mentioned before, PRP is being used as a possible treatment for OA [4], thus, constituting an alternative as EV source [5].
Platelet-derived EVs (pEVs) have proven effect in different diseases and injuries in the regenerative field [15]. pEVs have shown a variety of effects in in vitro studies such as osteogenic potential on human umbilical cord MSCs (hUC-MSCs) [16] or, in ex vivo OA-induced model with cartilage explants where pEVs improved the collagen content and decreased the glycosaminoglycan (GAG) degradation in cartilage over time [17]. In vitro studies with pEVs derived from PRP promoted chondrocyte proliferation and migration, while in vivo OA models also treated with PRP-derived EVs showed improvement in OA progression [18,19].
Hence, here we aimed to evaluate the therapeutic use of pEVs as a potential cell-free treatment compared to cEVs in a validated animal model of OA, since MSCs, as EV source, are the cell-based treatment par excellence and cEVs are the most extended EVs used in tissue regeneration studies. Thus, we hypothesize that pEVs and cEVs, will present at least, similar effects in terms of regenerative potential for OA.
2. Materials and methods
2.1. Ethics committee approval and considerations
Human umbilical cord-MSCs and platelet lysate (PL) used for EV isolation were obtained from IdISBa Biobank, with the approval of the Ethics Committee (IB 1995/12 BIO) after ethical approval of the project by the CEI-IB (IB 3656118 PI). The in vivo study was approved and registered by the Ethical Committee of Animal Experimentation of the Balearic Islands (CEEA-125-09-19).
2.2. Human platelet lysate processing
Buffy coats were obtained from the IdISBa Biobank with the Ethics Committee approval (IB 1995/12 BIO) after ethical approval of the project by the CEI-IB (IB 3656118 PI). From these buffy coats, platelet concentrates were prepared following the conventional procedure used in blood banks with minor modifications. Briefly, six fresh buffy coats containing 25–40 % of residual plasma were pooled without any consideration of blood group, gender and/or age. When it was possible, buffy coats with platelet concentration above 200 × 109 platelets/l were used. Buffy coats from donors who had taken non-steroidal anti-inflammatories (NSAIDs) were excluded.
Selected buffy coats bags were washed with 0.9 % NaCl, centrifuged at 650×g 10 min and then, leukocytes were filtrated to finally obtain a platelet concentrate. Platelet concentration was determined and adjusted at 1,200-1,800x10 [9] platelets/l. To obtain PL, at least, three freeze/thaw cycles (−80 °C/37 °C) were performed to lyse more than 80 % of platelets. Then, a centrifugation at 5,050×g for 20 min at room temperature discarded cell debris and supernatant was filtered by 40 μm pore size membrane (Haemonetics, Fajardo, Puerto Rico USA). Then, microbiological control assays were performed by using the BACT ALERT system for aerobic and anaerobic growth. Finally, the whole batch was aliquoted in 50 ml tubes and stored at −20 °C until use.
Then, to eliminate small cell debris, PL was centrifuged at 1,500×g for 15 min at 4 °C and then at 10.000×g for 30 min at 4 °C. supernatant was filtered through 0.8 μm pore size membrane (Sartorius, Germany) for large cell debris elimination and then through 0.2 μm porous membrane (Sartorius). Afterwards, PL that was to be used for EV isolation by size exclusion chromatography (SEC) was aliquoted in 5 ml aliquots.
2.3. MSCs culture, EV production and conditioned medium processing
To obtain EVs from hUC-MSC, cells were cultured to obtain conditioned media as previously described [17] to produce cEVs. Once conditioned media were processed, a 5 mL aliquot was injected into the SEC system.
2.4. Size exclusion chromatography (SEC)
PL-derived EVs (pEVs) and hUC-MSC-derived EVs (cEVs) were isolated by SEC as described in previous studies [17]. Five millilitres’ aliquots were eluted with PBS. Enriched EV fraction was collected for each sample according to the chromatogram obtained at 280 nm UV absorbance.
2.5. Total protein quantification
Total protein quantification of EV samples was performed by reading absorbance at λ = 280 nm with the NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
2.6. Nanoparticle tracking analysis (NTA) and purity ratio
Nanosight NS300 (Malvern Instruments, Malvern, UK) was used to determine EV concentration (particle/mL). Settings were adjusted according to the manufacturer's manual and samples were diluted 1/1000. Purity ratio was calculated as described by Webber et al. [20].
2.7. Osteoarthritic animal model
Thirty healthy 8-week-old Wistar rats (15 males −330 g ± 7- and 15 females −191 g ± 10 g-) were used as knee OA model as described by others [21]. The left knee joint was settled as OA group in all rats by injecting 6 mg of monoiodoacetate (MIA, Sigma–Aldrich)/25 μL in sterile saline through the patellar tendon. The right knee joint of 6 males and 6 females were settled as control group and injected with 25 μL sterile saline. After 14 days, animals were equally distributed into three groups of 5 males and 5 females, and treated through the patellar tendon as follows: OA (OA-model injected with 25 μL sterile saline), pEVs (OA-model treated with 1 × 1010 pEVs) and cEVs (OA-model treated with 1 × 1010 cEVs). Healthy right knee joints were injected with 25 μL sterile saline and settle as control group. At day 21, treatments were re-administered. OA model treatment was conducted in a blinded fashion by the operator.
2.8. Samples collection
At day 28, animals were euthanized, legs were disarticulated at the hip joint and stored in 4 % paraformaldehyde for 1 week at 4 °C to further processed them to clear the entire knee joint for cone beam computed tomography (CBCT) and histological preparation. During hindlimbs disarticulation, blood was collected in EDTA tubes (Microvette® CB 300 EDTA potassium, Sarstedt) to obtain plasma that was stored at −80 °C until use. All animals survived and were included in further analysis; none was excluded from any experiment.
2.9. CBCT and CTan analysis
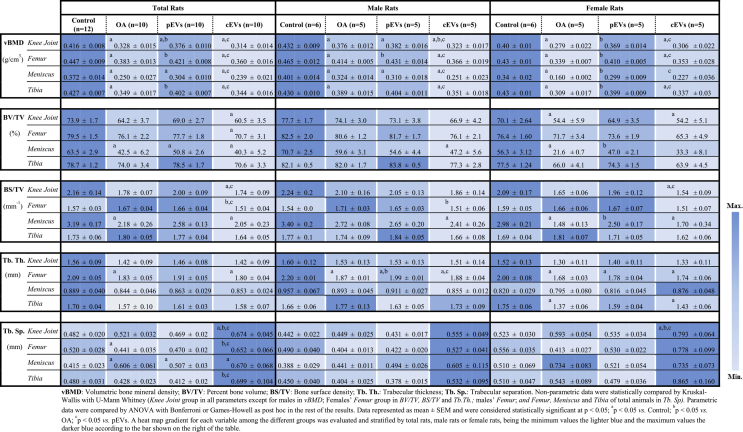
Hyperion X9 CBCT Scanner (MyRay, Cefla s.c., Italy) was used to scan fixed knee joints. Settings fixed at ultra-high resolution CBCT examination, field of view 11 × 8 cm with an X-ray source of 90 kV and 3 mA with a pixel size of 80 μm. The entire subchondral bone area was considered for the evaluation. Femur, tibia and meniscus were reconstructed, and 3D-analysis was performed independently using CTan software (Skyscan, Aartselaar, Belgium) (Table 1) previously calibrated.
Table 1.
CTan parameter to analyse knee joint after CBCT scanning.
| vBMD | Volumetric bone mineral density | Bone density within a mixed bone-soft tissue region |
|---|---|---|
| BV/TV | Percent bone volume | Ratio of the segmented bone volume to the total volume of the volume of interest (VOI) |
| BS/TV | Bone surface density | Ratio of the segmented bone surface to the VOI |
| Tb.Th | Trabecular thickness | Mean thickness of trabeculae |
| Tb.Sp | Trabecular separation | Mean distance between trabeculae |
2.10. Histology
After CBCT, knee joints were decalcified and embedded with paraffin to slice in 6 μm tissue sections for staining of collagen fibers (Sirius Red F3BA), proteoglycans (Safranin O and toluidine blue) or cartilage-bone-inflammation (Bone-Inflammation Cartilage -BIC-). Images were taken at 50x under polarized light for Sirius Red F3BA staining or under bight field for the other stains.
BIC-stained images at 200x and 500x were evaluated to determine the inflammation grade inducing synovitis at the synovia membrane, according to previous studies [22,23].Safranin O-stained images at 500x were evaluated according to the Osteoarthritis Research Society International (OARSI) scoring system [24,25] (Table 2). Images were analyzed independently and by 3 different blinded operators.
Table 2.
OARSI scoring system.
| STAGE (% OA Involvement in surface, area or volume) |
||||||
|---|---|---|---|---|---|---|
| GRADE (OA depth progression into cartilage) | S0 No OA |
S1 <10 % |
S2 10–25 % |
S3 25–50 % |
S4 >50 % |
|
| G0 | Intact, uninvolved cartilage. | — | — | — | — | — |
| G1 | Irregular but intact surface. | — | 1 | 2 | 3 | 4 |
| G2 | Superficial fibrillation. | — | 2 | 4 | 6 | 8 |
| G3 | Vertical fissures. | — | 3 | 6 | 9 | 12 |
| G4 | Partial loss of cartilage not extending to the tidemark. | — | 4 | 8 | 12 | 16 |
| G5 | Partial loss of cartilage beyond the tidemark but not till bone. | — | 5 | 10 | 15 | 20 |
| G6 | Bone loss, remodelling, deformation. | — | 6 | 12 | 18 | 24 |
| Score = grade x stage | ||||||
2.11. ELISA CTX-II
Cross Linked C-Telopeptide of Type II Collagen (CTX-II) was evaluated from serum samples by the commercially available ELISA kit and according to the manufacturer's instructions (Abbexa, United Kingdom).
2.12. Statistical analysis and data availability
Statistical analyses were performed using IBM SPSS software version 25. The sample size was 10 knee joints rats for each OA treated or not treated group (5 males and 5 female) and 12 knee joints for control group (6 males and 6 females). Shapiro–Wilks test was performed to indicate parametric or non-parametric distribution. For non-parametric results, Kolmogorov–Smirnov test with U-Mann Whitney and parametric results were analyzed by ANOVA test with Bonferroni post-hoc. GraphPad Prism software version 7.0 was used to represent the results. All data was deposited in the figshare repository (http://figshare.com/) https://doi.org/10.6084/m9.figshare.22360123.
3. Results
3.1. pEVs and cEVs characterization
Same batch of pEVs and cEVs used in this work have previously been used in a previous study on an ex vivo OA model [26]. All 16,17,27the EVs specificities and vesicular nature markers were confirmed following the criteria of MISEV2018 guidelines [9] as can be consulted in the EV-TRACK knowledgebase (EV-TRACK ID: EV230588) [28]. Particle concentration and size were evaluated by NTA, showing pEVs a concentration of 6.0 × 1011 particles/mL and a purity of 8.9 × 108 particles/μg of protein, and cEVs a concentration of 6.7 × 1010 particles/mL with a purity of 9.66 × 108 particles/μg of protein.
3.2. Effects of EVs treatments on the subchondral bone in OA rats
CTan analysis of the subchondral bone of knee joints is shown in Table 3 coloured following a heatmap gradient to facilitate comparison. Results evidence that pEVs treated group presents a similar pattern to Control group in almost every parameter evaluated. Especially in female rats. vBMD was significantly decreased in the OA group when compared to control both in male and female rats. Treatment with pEVs managed to achieve vBMD values significantly higher than the OA group although control values were not fully recovered. In contrast, no recovery was observed for the cEVs-treated group. Furthermore, the BV/TV, BS/TV, and trabecular thickness show similar pattern to vBMD, that is, lower values were observed in OA joints that were partially recovered in the pEVs treated group. For trabecular separation, higher values were observed after OA induction, achieving partial recovery in the pEVs-treated group.
Table 3.
Knee joint 3D morphometric analysis by CTan.
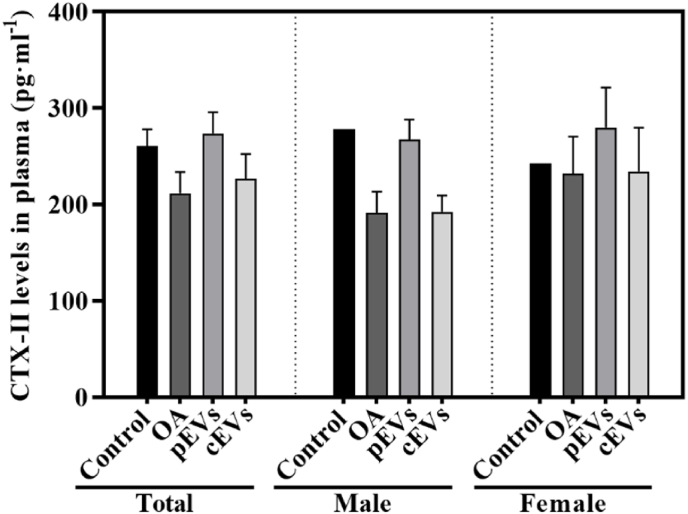
3.3. Collagen degradation: CTX-II plasma levels
CTX-II is one of the molecular components derived from the collagen degradation pathway that is used to evaluate collagen degradation during the OA process. As shown in Fig. 1, non-significant differences were found between groups, neither when stratifying by gender.
Fig. 1.
CTX-II plasma levels. CTX-II plasma levels measured the day of the sacrifice of the animals. Results are shown as total animals or sorted by gender. Data represents the mean value ± SEM. For statistical analysis. Total and Female groups presented a parametric distribution and were analysed by ANOVA with Bonferroni as post hoc for parametric results. Male group presented non-parametric distribution and was analysed by Kruskal–Wallis with U-Mann Whitney. The sample size was 10 plasma samples for each OA treated or not treated group (5 males and 5 female) and 12 plasma samples for control group (6 males and 6 females).
3.4. OARSI and synovitis scores
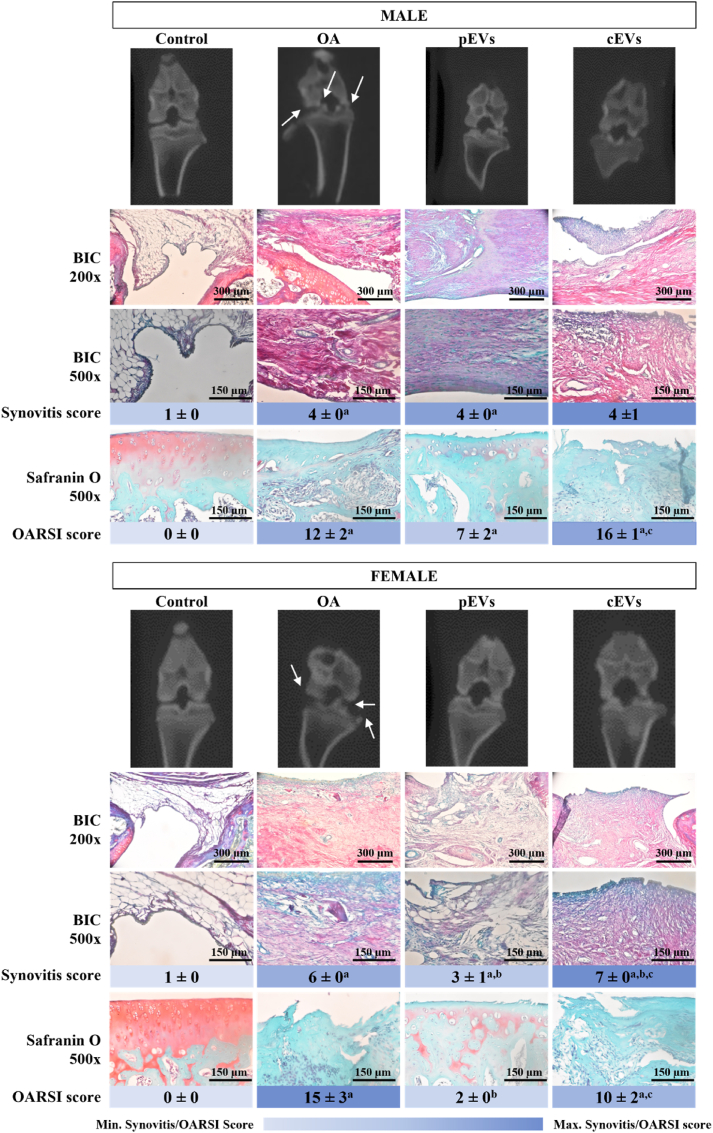
OARSI scoring allows a consensual classification of the different OA stages using images taken at tibial plateau to assure a plane perpendicular to the growth plate in frontal sections. Following the indications of previous studies [25] differences in OA progression were found between males and females (Fig. 2). Despite the more severe OA process in females, treatment with pEVs achieved a better improvement in females than in males. Such an effect could not be seen in the cEVs treatment group, in which males and females displayed the same OA progression stage. Moreover, pEVs do not only present a better recovery than cEVs but also compared to the OA group, reaching a score near to the control group, which do not present OA induction. In line with these results, pEVs also presented a lower inflammatory ratio when compared to the OA group, especially in females. However, no major differences were observed in males between treatments although OA and pEVs were significantly more inflamed than the control group or the cEVs group.
Fig. 2.
Representative images for 2D CBCT, Synovitis and OARSI scores for osteoarthritic rat knee joints after EVs treatment. 2D representative CBCT images represented with arrow to indicate osteophyte formation. Synovitis score obtained after the analysis of BIC staining images by four independent observers. BIC staining marks different structures at the same time: GAG and collagen in purple-pink, bone in red-pink and muscle in green and allow to observe inflammatory cells infiltration at a magnification of 200× and 500x. OARSI score obtained after grading and staging by three independent observers. Safranin O staining is the indicated stain to perform the OARSI score analysis. Red colour indicates GAG presence. Pictures were taken at a magnification of 500×. Deep blue in OARSI score indicates higher OA grade while pale blue indicates a lower OA grade if any. The sample size was 10 knee joints rats for each OA treated or not treated group (5 males and 5 female) and 12 knee joints for control group (6 males and 6 females). Synovitis scores were statistically analysed by ANOVA with Games-Howell as post hoc for males. Data represented as mean ± SEM and were considered statistically significant at p < 0.05: OA group ap = 0.004 vs. Control; pEVs group ap < 0.001 vs. Control. Females were statistically analysed by Kruskal Wallis with U-Mann Whitney and considered statistically significant at p < 0.05: OA group ap = 0.029 vs. Control; OA group ap < 0.0001 vs. Control; pEVs group ap = 0.019 vs. Control and bp = 0.010 vs. OA; cEVs group ap = 0.004 vs. Control, bp = 0.028 vs. OA and cp < 0.001 pEVs. OARSI scores were statistically analysed by ANOVA with Bonferroni as post hoc. Data represented as mean ± SEM and were considered statistically significant at p < 0.05 for males: OA group ap < 0.0001 vs. Control; pEVs group ap = 0.0042 vs. Control; cEVs group ap < 0.0001 vs. Control and cp = 0.0174 pEVs. And for females, results were also considered statistically significant at p < 0.05: OA group ap < 0.0001 vs. Control; pEVs group bp = 0.0003 vs. OA; cEVs group ap < 0.0001 vs. Control and cp = 0.0065 pEVs.
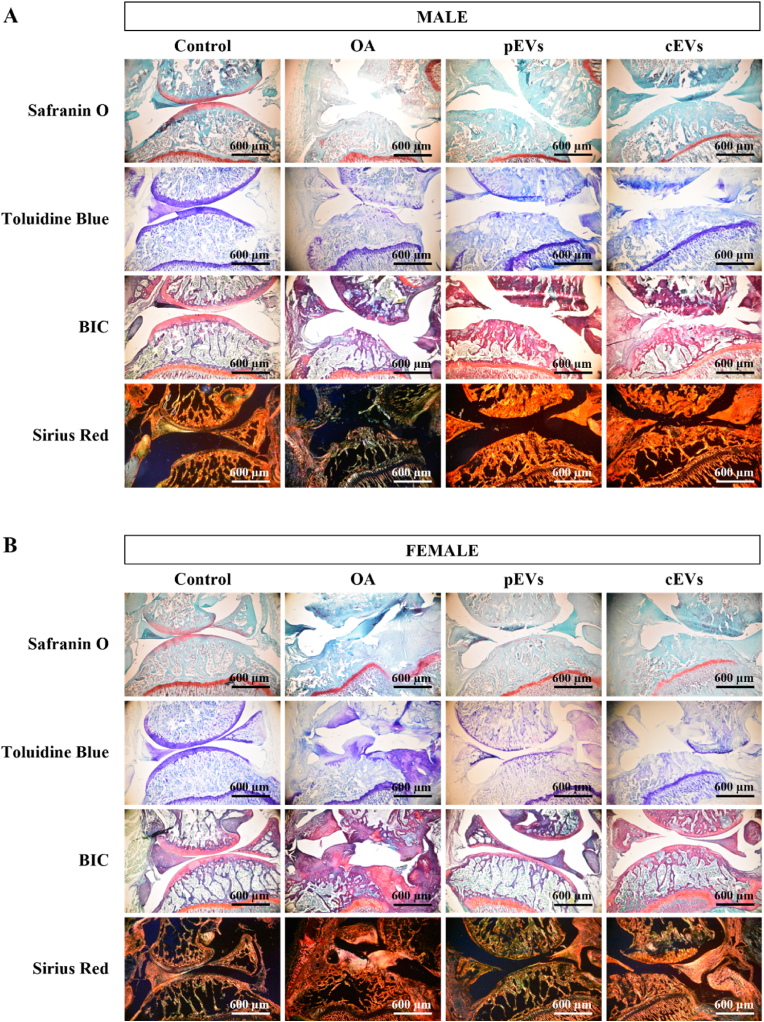
3.5. Histology of OA knee joints treated with EVs
Histological images in Fig. 3 agree with the results obtained for the subchondral bone microstructure presented in Table 3. In general, OA group presented a poor state in males and females, showing cartilage thinning and atrophy due to chondrocyte death, cartilage fibrillation and osteophyte presence in some cases, while control group showed a perfect knee joint with intact structures and great content of GAG and collagen. pEVs treated group presented slightly better results in cartilage matrix (toluidine blue and safranin O staining) rather than in cEVs, in males and females. In terms of collagen content, cEVs showed a higher presence of large collagen fibers (yellow in the Sirius Red F3BA staining) in females when compared to pEVs. For the BIC staining, pEVs showed similar appearance to the control group with the adequate presence of GAG and collagen (purple-pink) and bone (red-pink). However, cEVs did not present those purple tones, either in males or females, meaning a lack of GAG and collagen. As for OA group, there was an uneven distribution of GAG and collagen with an odd joint structure, especially in females and if compared with control group, indicating a massive effect of OA process in the OA group.
Fig. 3.
Histological analysis of decalcified osteoarthritic rat knee joints after EVs treatment. Images of knee joint in male (A) and female (B) rats. Safranin O staining dye in red GAG components in the ECM are stained in red for Safranin O stained samples and in purple in for Toluidine Blue stained samples. BIC staining mark different structures at the same time: GAG and collagen in purple-pink, bone in red-pink and muscle in green. Collagen stained with Sirius Red F3BA visualized under polarized light microscopy reveals larger collagen fibers as bright yellow or the thinner ones as orange, reticular fibers are detected as green (50). Pictures were taken at a magnification of 50×. The sample size was 10 knee joints rats for each OA treated or not treated group (5 males and 5 female) and 12 knee joints for control group (6 males and 6 females).
4. Discussion
Our results highlight that EVs derived from different cell sources exert different regenerative effects on an osteoarthritic environment. MSCs have been long studied in the regenerative field including osteochondral diseases, as well as cEVs. However, in the present OA animal model pEVs demonstrated a better outcome in the regeneration of cartilage joint after an OA process, especially in females.
EVs derived from different sources did not present major differences once characterized in terms of particle concentration, size or membrane markers by conventional methods [16,17,27]. However, their effect as a regenerative treatment on osteoarthritis in vivo model revealed that their regenerative potential differs. cEVs have been studied as an alternative to MSCs used in the regenerative field. Although EVs derived from MSCs have been shown to promote a beneficial effect in OA treatment [29,30], it is worth mentioning that different kinds of MSCs present different proteome and secretome profiles [31,32]. Therefore, EVs derived from different MSC sources could induce different effects. This could explain why our treatment with cEVs derived from umbilical cord MSCs did not replicate results reported in other studies, based on adipose MSC-derived EVs, bone marrow MSC-derived EVs and synovial MSC-derived EVs. Indeed, adipose MSC-derived EVs showed a higher ability to induce cartilage and bone regeneration than the other two [29].
OA is a progressive disease that causes cartilage loss, bone and fibrocartilage substitution, and osteophyte presence [25]. The need for effective regenerative treatments is crucial, and the disease stage when treatments are applied is decisive in determining if the OA process can be reversed [33]. OA induction by MIA injection in rats is a standardized protocol to obtain a bona fide OA. As OARSI score indicated in this study, OA was reached and EVs partially restored knee joint integrity, especially with pEVs treatment, suggesting that the source from which EVs are derived -PL vs. MSCs-can play a role in the recovery of cartilage recovery in an OA model. Moreover, males and females responded differently, especially in CTan analysis and OARSI score. Despite women have a higher prevalence of OA than men [34], most animal studies of OA have been performed in males, and those that have included both sexes have confirmed sex differences in development of OA, but, contrary to our results, higher OA development is observed in male mice in several murine OA models [[35], [36], [37], [38]], while previous studies in rats have found that old females are most vulnerable to OA [39]. This sex dimorphism could be due to differences in anatomical joint size, animal weight, hormones, and biomechanical processes intrinsic to animal sex affecting OA progression [40]. It is worth reminding that used animals were of the same age, with males being heavier than females. Females were more susceptible to OA damage, resulting in more severe OA [41]. Here, we observed higher OA development in females along with better treatment outcomes, possibly due to the fact that males do not develop OA in the same way as females due to the sex-related factors, as mentioned before, and probably the stage of OA that males reach cannot be perceived in the same way as in females. OA also causes changes in body weight and affects the ability of the damaged knee to withstand weight-bearing loads, which can affect OA progression [42].
Safranin O and toluidine blue are excellent staining techniques to identify the degradation of GAG in cartilaginous tissue. In presence of OA, it is recommended to perform both, since safranin O is not a sensitive indicator of GAG content in tissue with inflammation and severe cartilage loss [43]. However, safranin O is the staining recommended by OARSI to clearly identify the OA stage of osteoarthritic tissue [24,25]. Other stains to consider are BIC and Sirius Red F3BA, which allow to analyze the bone-cartilage state and the collagen presence. Sirius Red F3BA images showed a higher presence of large collagen fibers (yellow-orange) in those groups treated with pEVs or cEVs.
OARSI score allowed to classify the OA severity in the osteoarthritic knee joints. As previously mentioned, safranin O images were analysed by three different observers to reassure objective classification. Results showed, as expected, that OA is correctly achieved with the OA animal model via MIA intra-articular injection. Moreover, the pEVs treatment showed not only a better knee joint integrity compared to the OA group, but also when compared to the cEVs treated group. Thus, indicating that pEVs interact in the cartilage regeneration pathways to improve the OA state at the knee joint. Other scoring system could be used such as Mankin or Collins, however, these systems present less score points for OA classification and are intented for severe OA, while OARSI score can be used for milder or earlier OA stages [25]. Besides, inflammation was evaluated through the synovitis score, our findings indicate that females presented a reduced inflammation rate when exposed to pEVs.
Type II collagen degradation during OA releases CTX-II, which is quantifiable and has been proposed as an effective cartilage degradation biomarker [44]. However, in this study, the OA group had lower levels of plasma CTX-II compared to the control group, and pEVs had similar levels to the control group. This result differs from other studies that measured CTX-II in urine and found higher levels in OA patients. Luo et al. [45] noted that CTX-II fragments’ sequences detected from urine or blood differ, leading to different sensitivity in CTX-II quantification. This suggests that CTX-II detection from plasma has limitations and other sample sources, such as urine or synovial fluid, should be explored.
Histologic analysis showed that the in vivo model developed OA, and EVs treatment partially reversed the degenerative process, especially in females. Surprisingly, pEVs not only could improve the osteoarthritic joint but also surpass the beneficial effects that have been reported in the bibliography for cEVs used as a treatment in OA and other regenerative studies [46,47], highlighting the potential use of platelets as a source of EVs for regenerative medicine, particularly in the treatment of OA.
Limitations of the in vivo model should be also considered. Even though the male-female intrinsic variation, the rat model is one of the most extended OA in vivo models. This animal model is affordable, easy to manage and of sufficient size to perform different techniques. However, the small size of tissue samples in these models also mean that they may have anatomical and histological differences when compared to humans. This implies the use of a more complex in vivo model when aiming a translation to clinics of a potential new treatment. Moreover, apart from the MIA intra-articular model, other strategies can also be considered, such as a surgical knee joint destabilization or spontaneous models induced by aging and genetically modified animals to develop OA [48]. Published work has used pain behaviour tests to evaluate OA models [38,49] or in response to drug administration. Unfortunately, we did not make such determinations in our work, which is a limitation of our study.
Clinical translation of pEVs may be easier than that of cEVs since their MSC source presents several hurdles in the translation from bench to bedside as therapy, such as the large-scale production and consequent loss of potency due to prolonged in vitro expansion of cells to obtain more EVs, which may cause the loss of clonal and differentiation capacity of the source, whilst pEVs paves the way as cell-free therapy overcoming any of these challenges [50]. pEVs obtention do not need large-scale platelet harvesting and platelets as a source do not lose their properties over time. The use of platelets and, therefore, pEVs, as non-MSC sources, present also advantages that overcome the inconveniences with other platelet concentrates such as PRP, which is currently used as autologous therapy in OA but even so, it is not suitable for all patients. pEV obtention could be easily standardized, their preparation is reproducible, and it is possible their storage for their use off-the-shelf in clinics [5,6]. Moreover, it should be considered that OA lacks effective regenerative treatments, and clinical trials with new Disease- or Structure-modifying OA drugs (DMOADs or SMOADs) have failed [51]. Furthermore, other therapies available imply painful, surgical, expensive, and time-consuming processes for the patient and/or for the treatment production [52].
As a future perspective, further studies, such as the multiomics approach in EV content and composition, should be performed to elucidate the mechanism of action underlying the differential effect of pEVs and cEVs on OA.
5. Conclusions
Cell therapies present different drawbacks that lead to consider the use of alternative non-MSC sources in OA regeneration. cEVs present also some disadvantages in terms of clinical translation, so here, pEVs are presented as a feasible regenerative treatment for OA. Our results in an OA in vivo model in rats comparing pEVs and cEVs show a regenerative potential of pEVs on osteoarthritic cartilage, with a particular tendency towards positive effects in females, showing comparatively higher efficacy when compared to cEVs. All in all, here we postulate pEVs to be considered as a cell-free regenerative treatment for OA, which lacks in regenerative treatments, and for sure, these results will benefit to broad the research field of new regenerative therapies.
Author contributions
M.A.F.-G., M.A.-R., M.M. and J.M.R. contributed to the conception and design. J.C. and A.G. provided platelet lysate and cartilage explants. M.A.F.-G., M.A.-R. and C.R.-M. produced EV samples, performed the different assays, acquired and analysed data. A.T.S. performed the animal supervision and MIA and EV treatment injections. M.A.F.-G., M.A.-R., M.M. and J.M.R. contributed to interpretation of the results. M.A.F.-G. wrote the main manuscripts text and M.A.F.-G., M.A.-R., C.R.-M, A.T.S., J.C., A.G., M.M. and J.M.R reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Role of the funding source
This research was funded by Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, co-funded by the ESF European Social Fund and the ERDF European Regional Development Fund (MS16/00124; CP16/00124), PROGRAMA JUNIOR del proyecto TALENT PLUS, construyendo SALUD, generando VALOR (JUNIOR01/18), financed by the sustainable tourism tax of the Balearic Islands; the Direcció General d’Investigació and Conselleria d’Investigació, Govern Balear (FPI/2046/2017); the Mecanisme de Recuperació i Resiliència, intended to execute research projects of «Noves polítiques públiques per a un mercat de treball dinàmic, resilient i inclusiu», collected in Pla de Recuperació, Transformació i Resiliència, financed by European Union-Next Generation EU and driven by SOIB and Conselleria de Fons Europeus, Universitat i Cultura i la Conselleria de Model Econòmic, Turisme i Treball (NG0421) and the grant SYN20/03 from IdISBa.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Aina Arbós (IUNICS) for her contribution in the histology staining, María Tortosa (IdISBa) for her assistance with the animal care and ADEMA School of Dentistry for the access to their facilities. The authors are grateful to Illustrate Science (http://www.illustrate-science.com) for assistance with the Graphical Abstract.
Contributor Information
Maria Antònia Forteza-Genestra, Email: maria.forteza@ssib.es.
Miquel Antich-Rosselló, Email: miquel.antich1@estudiant.uib.es.
Carmen Ráez-Meseguer, Email: carmen.raez@uib.es.
Anna Tomàs Sangenís, Email: anna.tomas@ssib.es.
Javier Calvo, Email: jcalvo@fbstib.org.
Antoni Gayà, Email: agaya@fbstib.org.
Marta Monjo, Email: marta.monjo@uib.es.
Joana Maria Ramis, Email: joana.ramis@uib.es.
References
- 1.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 2004;12(Suppl A):S31–S33. doi: 10.1016/j.joca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol Nature Publishing Group. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 3.Roseti L., Desando G., Cavallo C., Petretta M., Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells Multidisciplinary Digital Publishing Institute. 2019;8(11):1305. doi: 10.3390/cells8111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Y., Lin L., Wang Z., Wang K., Xiao L., Lin W., et al. Research trends of platelet-rich plasma therapy on knee osteoarthritis from 2011 to 2021: a review. Medicine. 2023;102(2) doi: 10.1097/MD.0000000000032434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques L.F., Stessuk T., Camargo I.C.C., Sabeh Junior N., Santos L Dos, Ribeiro-Paes J.T. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets Platelets. 2015;26(2):101–113. doi: 10.3109/09537104.2014.881991. [DOI] [PubMed] [Google Scholar]
- 6.Yin W.-J., Xu H.-T., Sheng J.-G., An Z.-Q., Guo S.-C., Xie X.-T., et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in treating rabbit knee osteoarthritis. Med Sci Monit. 2016 doi: 10.12659/MSM.898218. ;22:1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res J Orthop Res. 2019;37(6):1229–1235. doi: 10.1002/jor.24343. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Liao L., Wang S., Tan J. Cell therapy with autologous mesenchymal stem cells-how the disease process impacts clinical considerations. Cytotherapy Cytotherapy. 2013;15(8):893–904. doi: 10.1016/j.jcyt.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 9.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles Taylor and Francis Ltd. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams A.M., Dennahy I.S., Bhatti U.F., Halaweish I., Xiong Y., Chang P., et al. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma J Neurotrauma. 2019;36(1):54–60. doi: 10.1089/neu.2018.5711. [DOI] [PubMed] [Google Scholar]
- 11.Guo H., Su Y., Deng F. Effects of mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and future perspectives. Stem Cell Rev Rep Stem Cell Rev Rep. 2021;17(2):440–458. doi: 10.1007/s12015-020-10085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You B., Zhou C., Yang Y. vol. 85. Ageing Res Rev Elsevier; 2023. MSC-EVs alleviate osteoarthritis by regulating microenvironmental cells in the articular cavity and maintaining cartilage matrix homeostasis. [DOI] [PubMed] [Google Scholar]
- 13.Savkovic V., Li H., Seon J.-K., Hacker M., Franz S., Simon J.-C. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9(6):469–488. doi: 10.2174/1574888x09666140709111444. [DOI] [PubMed] [Google Scholar]
- 14.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol [Internet] Elsevier Ltd. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Antich-Rosselló M., Forteza-Genestra M.A., Monjo M., Ramis J.M. Platelet-derived extracellular vesicles for regenerative medicine. Int J Mol Sci. 2021;22(16):8580. doi: 10.3390/ijms22168580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antich-Rosselló M., Forteza-Genestra M.A., Calvo J., Gayà A., Monjo M., Ramis J.M. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Joint Res. 2020;9(10):667–674. doi: 10.1302/2046-3758.910.BJR-2020-0111.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forteza-Genestra M.A., Antich-Rosselló M., Ramis-Munar G., Calvo J., Gayà A., Monjo M., et al. 2023. Comparative effect of platelet and mesenchymal stromal cells derived extracellular vesicles on human cartilage explants using an ex vivo inflammatory osteoarthritis model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X., Wang L., Ma C., Wang G., Zhang Y., Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res J Orthop Surg Res. 2019;14(1):470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Wang X., Chen J., Qian D., Gao P., Qin T., et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J Nanobiotechnology BioMed Central Ltd. 2022;20(1):56. doi: 10.1186/s12951-022-01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webber J., Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2(1):1–6. doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida T., Kubota S., Kojima S., Kuboki T., Nakao K., Kushibiki T., et al. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19(8):1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 22.Krenn V., Morawietz L., Burmester G.R., Kinne R.W., Mueller-Ladner U., Muller B., et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 23.Krenn V., Morawietz L., Häupl T., Neidel J., Petersen I., König A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198(5):317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 24.Gerwin N., Bendele A.M., Glasson S., Carlson C.S. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–S34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Pritzker K.P.H., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell K., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Forteza-Genestra M.A., Antich-Rosselló M., Ramis-Munar G., Calvo J., Gayà A., Monjo M., et al. 2023. Comparative effect of platelet and mesenchymal stromal cells derived extracellular vesicles on human cartilage explants using an ex vivo inflammatory osteoarthritis model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forteza-Genestra M.A., Antich-Rosselló M., Ortega F.G., Ramis-Munar G., Calvo J., Gayà A., et al. Labeling of extracellular vesicles for monitoring migration and uptake in cartilage explants. J Vis Exp. 2021;(176) doi: 10.3791/62780. [DOI] [PubMed] [Google Scholar]
- 28.Deun J Van, Mestdagh P., Agostinis P., Akay Ö., Anand S., Anckaert J., et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen T.H., Duong C.M., Nguyen X.-H. Than UTT. Mesenchymal stem cell-derived extracellular vesicles for osteoarthritis treatment: extracellular matrix protection, chondrocyte and osteocyte physiology, pain and inflammation management. Cells Multidisciplinary Digital Publishing Institute (MDPI) 2021;10(11) doi: 10.3390/cells10112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohd Noor N.A., Abdullah Nurul A., Ahmad Mohd Zain M.R., Wan Nor Aduni W.K., Azlan M. Extracellular vesicles from mesenchymal stem cells as potential treatments for osteoarthritis. Cells Multidisciplinary Digital Publishing Institute. 2021;10(6):1287. doi: 10.3390/cells10061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mianehsaz E., Mirzaei H.R., Mahjoubin-Tehran M., Rezaee A., Sahebnasagh R., Pourhanifeh M.H., et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther BioMed Central. 2019;10(1):340. doi: 10.1186/s13287-019-1445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodama J., Wilkinson K.J., Otsuru S. vol. 17. Bone Rep Elsevier Inc.; 2022. (MSC-EV therapy for bone/cartilage diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummer D., McAdam J., Seay R., Ferrando A., Bridges S.L., Singh J.A., et al. Osteoarthritis progression: mitigation and rehabilitation strategies. Frontiers in rehabilitation sciences Frontiers Media SA. 2021;2 doi: 10.3389/fresc.2021.724052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage W.B. Saunders. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.van Osch GJ, van der Kraan PM, Vitters EL, Blankevoort L, van den Berg WB. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage. 1993;1(3):171–177. doi: 10.1016/s1063-4584(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 36.Mahr S., Menard J., Krenn V., Müller B. Sexual dimorphism in the osteoarthritis of STR/ort mice may be linked to articular cytokines. Ann Rheum Dis. 2003;62(12):1234. doi: 10.1136/ard.2002.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma H.L., Blanchet T.J., Peluso D., Hopkins B., Morris E.A., Glasson S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15(6):695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Hwang H.S., Park I.Y., Hong J.I., Kim J.R., Kim H.A. Comparison of joint degeneration and pain in male and female mice in DMM model of osteoarthritis. Osteoarthritis Cartilage. 2021;29(5):728–738. doi: 10.1016/j.joca.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Ro J.Y., Zhang Y., Tricou C., Yang D., Silva JT da, Zhang R. Age and sex differences in acute and osteoarthritis-like pain responses in rats. J Gerontol: Series A. 2020;75(8):1465–1472. doi: 10.1093/gerona/glz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contartese D., Tschon M., Mattei M De, Fini M. Sex specific determinants in osteoarthritis: a systematic review of preclinical studies. Int J Mol Sci Multidisciplinary Digital Publishing Institute. 2020;21(10):3696. doi: 10.3390/ijms21103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peshkova M., Lychagin A., Lipina M., Matteo B Di, Anzillotti G., Ronzoni F., et al. Gender-related aspects in osteoarthritis development and progression: a review. Int J Mol Sci. 2022;23(5) doi: 10.3390/ijms23052767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagi C.M., Zakur D.E., Berryman E., Andresen C.J., Wilkie D. Correlation between μCT imaging, histology and functional capacity of the osteoarthritic knee in the rat model of osteoarthritis. J Transl Med BioMed Central. 2015;13(1):276. doi: 10.1186/s12967-015-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldstein W., Perino G., Gilbert S.L., Maher S.A., Windhager R., Boettner F. OARSI osteoarthritis cartilage histopathology assessment system: a biomechanical evaluation in the human knee. J Orthop Res John Wiley and Sons Inc. 2016;34(1):135–140. doi: 10.1002/jor.23010. [DOI] [PubMed] [Google Scholar]
- 44.Spil WE van, DeGroot J., Lems W.F., Oostveen J.C.M., Lafeber F.P.J.G. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18(5):605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y., He Y., Karsdal M., Bay-Jensen A.-C. Serological CTX-II does not measure the same as urinary CTX-II. Osteoarthr Cartil Open. 2020;(3) doi: 10.1016/j.ocarto.2020.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maumus M., Jorgensen C., Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie Biochimie. 2013;95(12):2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Burke J., Hunter M., Kolhe R., Isales C., Hamrick M., Fulzele S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin Transl Med Clin Transl Med. 2016;5(1):27. doi: 10.1186/s40169-016-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen D., Shen J., Zhao W., Wang T., Han L., Hamilton J.L., et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. Sichuan University. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piel M.J., Kroin J.S., Wijnen Aj Van Kc R., Im H.J. Pain assessment in animal models of osteoarthritis. Gene Elsevier. 2014;537(2):184–188. doi: 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson J., Wu Y.W., Blyth C., Lichtfuss G., Goubran H., Burnouf T. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol Elsevier Ltd. 2021;39(6):598–612. doi: 10.1016/j.tibtech.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Latourte A., Kloppenburg M., Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16(12):673–688. doi: 10.1038/s41584-020-00518-6. [DOI] [PubMed] [Google Scholar]
- 52.Roseti L., Desando G., Cavallo C., Petretta M., Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells. 2019;8(11):1305. doi: 10.3390/cells8111305. [DOI] [PMC free article] [PubMed] [Google Scholar]