Abstract
As a prelude to development of broader-spectrum vaccines for dental caries, we explored the immune potential of constructs combining epitopes from mutans streptococcal glucosyltransferases (GTF) and glucan binding protein B (GbpB). Two diepitopic peptide constructs were synthesized in a multiple antigenic peptide (MAP) format. Both constructs contained SYI, a 20-mer GbpB peptide that included a sequence having major histocompatibility complex class II binding characteristics. One diepitopic construct (SYI-CAT) also contained a 22-mer sequence from the catalytic domain of GTF. Another diepitopic construct (SYI-GLU) contained a 22-mer sequence from the glucan binding domain of GTF. To assess the ability of each construct to induce antibody reactive with GbpB and GTF native proteins, rats were injected subcutaneously with SYI-CAT, SYI-GLU, or the constituent monoepitopic constructs. Only the SYI-CAT construct induced significant levels of serum immunoglobulin G (IgG) and IgA antibody to both pathogenesis-associated proteins. Also, immunization with SYI-CAT significantly (P < 0.001) enhanced the antibody response to the CAT peptide. Experiments then compared experimental dental caries after immunization with SYI-CAT, SYI, or CAT MAP constructs, followed by infection with Streptococcus mutans strain SJr. Dental caries were lower in each peptide-immunized group than in the sham-injected group. The level of protection after SYI-CAT immunization was similar to that after immunization with constituent MAP constructs. In another experiment, rats were infected with Streptococcus sobrinus strain 6715 under an identical protocol. Significant protection was observed on buccal surfaces in both SYI-CAT and CAT construct-immunized, but not in the SYI construct-immunized, groups. Thus, addition of the GbpB-derived SYI peptide to the GTF-derived CAT peptide construct not only enhanced the immunological response to CAT and GTF epitopes, but also extended the protective effect of the construct to include both S. mutans and S. sobrinus.
The molecular pathogenesis of mutans streptococci (MS) is dependent on the ability of these cariogenic microorganisms to accumulate on dental surfaces (4). This process proceeds from the extracellular synthesis of glucans from sucrose by glucosyltransferase (GTF) enzymes secreted by MS. Glucan binding associated with cell surface proteins mediates attachment of MS to synthesized glucans (2), as does GTF, the C-terminal third of which contains multiple glucan binding domains (1, 9, 13). Collectively, these interactions result in bacterial accumulation.
Since both GTF and glucan binding proteins (Gbp) are essential to the pathogenicity of MS, these proteins have been pursued as components of potential dental caries vaccines. Native GTF enzymes from Streptococcus mutans and Streptococcus sobrinus (reviewed in references 8 and 14), as well as GbpB (10, 15) from S. mutans (17), have been effective as vaccines in experimental models for dental caries. Synthetic peptide constructs derived from either the catalytic domain (CAT) or the glucan binding domain (GLU) of mutans streptococcal GTF have been shown to induce protective immunity to experimental infection with cariogenic mutans streptococci (21). Identification of S. mutans GbpB-derived synthetic peptides associated with protein activity was more problematic since functional domains of this protein have yet to be resolved. However, since GbpB is exceptionally immunogenic (17), its sequence was explored for peptides which had the potential to be presented on the surfaces of antigen-presenting cells in the context of major histocompatibility complex (MHC) class II molecules to T lymphocytes in the process leading to antibody formation (16). Immunization of rats with a peptide construct, SYI, whose sequence had been identified through bioinformatic analysis to have this potential, gave rise to protective immunity from S. mutans-associated dental caries.
The value of subunit vaccines lies not only in concentrating the immune response to relevant epitopes, but also in permitting the combination in one vaccine of such epitopes from different regions on the native protein or, indeed, including multiple pathogenesis-associated components in the construct. We have shown previously that immunization with a diepitopic multiple antigenic peptide (MAP) construct that contained peptides from two distinct GTF domains (catalytic and glucan binding activities) enhanced the level of protection from dental caries caused by S. sobrinus, compared to the level of protection by immunization with either construct alone (24). In addition to the presence of complementary GTF functional domains, one of these peptides (GLU) contained a T-cell epitope (18, 22) which was thought to be responsible for some of the improvement in immune response to the B-cell epitope within the other peptide (CAT) component (19, 24) of the diepitopic construct of this subunit GTF vaccine.
Additional studies of subunit dental caries vaccines containing epitopes from more than one pathogenic component, for example, chimeric proteins (26) or fusion DNA vaccines (3), each containing epitopes from S. mutans GTF and an adhesin (PAc or AgI/AgII), have reported protective immunity in rats infected with S. mutans. Others (25) have demonstrated the formation of antibody that modified both adhesion and glucan synthesis in the sera of animals immunized with an adhesin-GTF fusion protein. However, this approach has not been pursued by combining GTF epitopes with those of S. mutans GbpB. Thus, we designed diepitopic synthetic peptide constructs containing sequences from each native protein that had previously been shown individually to give rise to protective responses. Our first aim was to explore the potential for immunological enhancement of immune responses to functional epitopes by including a putative T-cell epitope in the constructs containing peptides from GTF and GbpB. A 20-mer peptide, SYI, was selected based on MHC class II binding characteristics and the ability to induce caries-protective activity after immunization (16). This GbpB peptide was combined with either CAT- or GLU-GTF peptides to form SYI-CAT and SYI-GLU diepitopic constructs. Our second aim was to explore the ability of immunization with the diepitopic construct that gave the broader immune response to GTF and GbpB native protein to protect rats from the cariogenic effects of infection with S. mutans or S. sobrinus.
MATERIALS AND METHODS
Peptides.
Three monoepitopic peptide constructs were synthesized by AnaSpec, San Jose, Calif., as MAPs, using the stepwise solid-phase method of Merrifield (11) on a core matrix of lysines, yielding macromolecules with four identical peptides (20). One monoepitopic peptide construct (SYI) was based on a predicted MHC class II epitope that was contained within the sequence 113-KSNAATSYINAIINSKSVSD-132 of S. mutans GbpB (16). A second monoepitopic peptide construct (CAT) was based on the sequence DANFDSIRVDAVDNDVDADALLQI, containing an aspartate necessary for the catalytic activity of S. mutans GTF-B (residues 442 to 464) and S. sobrinus GTF-I (residues 444 to 466) (19). This sequence is the same in GTF-B and GTF-I. A third monoepitopic peptide construct (GLU) is identical to sequence residues 1303-TAGQTIKGQKLYFKANGQQVKG-1324 of S. sobrinus GTF-I (18). This sequence contains a region that is thought to be associated with glucan binding (13). Two sequences with >80% homology with GLU are found in the C-terminal third of S. mutans GTF-B (18).
Two diepitopic peptide constructs were also synthesized. One of these, SYI-CAT, contained two SYI peptides and two CAT peptides on the same lysine backbone. Another, SYI-GLU, contained two SYI peptides and two GLU peptides on the same backbone. The purity of all peptide constructs was assessed at >90%, using high-pressure liquid chromatography, amino acid analysis, and molecular weight determination by mass spectrometry.
Proteins.
GbpB was purified from S. mutans strain SJ by ion-exchange chromatography on MONO-Q HR 5/5 (Pharmacia) in the presence of urea as described previously (16). Bacteria were cultivated overnight at 37°C under anaerobic conditions in a sucrose-free defined medium containing 1% glucose. GbpB prepared in this manner migrates to a position corresponding to 59 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (18).
GTF from S. sobrinus strain 6715 was obtained by affinity and gel permeation chromatographies. After bacterial growth at 37°C under anaerobic conditions in a glucose-containing defined medium, enzymes were isolated from culture medium by affinity chromatography on Sephadex G-100 (Pharmacia) with 3 M guanidine HCl as the eluting solvent. This GTF-rich pool was then subjected to fast protein liquid chromatography on Superose 6 (Pharmacia) with 6 M guanidine HCl for elution. The S. sobrinus GTF obtained after gel filtration on Superose 6 contained a mixture of GTF isozymes, including GTF-I and GTF-S, but was essentially free of other proteins. Approximately 90% of the glucan synthesized by this GTF was water insoluble (19).
ELISA.
Serum immunoglobulin G (IgG) and salivary IgA antibody levels were determined by enzyme-linked immunosorbent assay (ELISA). Polystyrene microtiter plates (Flow Laboratories) were coated with 2.5 μg/ml of monoepitopic or diepitopic peptide, or with 0.5 μg/ml of S. mutans GbpB or S. sobrinus GTF. Antibody activity was then measured by incubation with a 1:200 or 1:2,000 dilution of sera or with a 1:4 dilution of saliva. Plates were then developed for IgG antibody with rabbit anti-rat IgG, followed in sequence by alkaline phosphatase goat anti-rabbit IgG (Biosource Inc.) and p-nitrophenylphosphate (Sigma Chemical Co., St. Louis, Mo.). For measurement of salivary IgA antibody, a mouse monoclonal antibody to rat α chain (Zymed, South San Francisco, Calif.) was used, followed by sequential incubation with biotinylated goat anti-mouse IgG (Zymed), avidin-alkaline phosphatase (ICN Biomedicals, Inc., Aurora, Ohio), and p-nitrophenylphosphate. Reactivity was recorded as absorbance (A405) by a microplate reader (Biotek Instruments, Winooski, Vt.). Data are reported as ELISA units (EU), which were calculated relative to levels in reference sera from rats immunized twice with the respective peptide or native protein. Reference serum dilutions of 1/1,000 for GbpB, 1/2,000 for GTF, 1/200 for CAT, 1/400 for SYI, 1/200 for SYI-CAT, and 1/800 for GLU, which produced an A405 of approximately 0.8, were considered 100 EU.
Experiment 1.
Sprague-Dawley CD strain 40-day-old female rats (Charles River Laboratories, Wilmington, Mass.) were used for injection. Six groups of seven rats each were injected subcutaneously (s.c.) in the vicinity of the salivary glands in order to maximize immune responses for the purposes of comparison. Separate groups were immunized with the CAT, GLU, SYI, SYI-CAT, or SYI-GLU construct. Monoepitopic constructs were given in doses of 30 μg; diepitopic constructs were given in 60-μg doses. Dose differences between diepitopic and monoepitopic constructs represented an effort to equalize the dose of the peptide in the monoepitopic construct with that of the respective peptide in the diepitopic construct. A sham-immunized group was immunized with phosphate-buffered saline (PBS). An additional two groups (four rats per group) were immunized with S. mutans GbpB (6 μg/dose) or S. sobrinus GTF (6 μg/dose). The initial injection included 0.04 ml of complete Freund adjuvant (FA) (Difco Laboratories, Detroit, Mich.) per site; one subsequent injection, given 21 days later, included 0.04 ml of incomplete FA per site. Samples for antibody analysis were collected 42 and 63 days after the initial injection; the results of the 63-day sample are reported herein.
For collection of serum and saliva samples, rats were first momentarily anesthetized with a gas mixture of 50% carbon dioxide and 50% oxygen and then anesthetized by intraperitoneal injection of a mixture (0.65 ml/kg of body weight) of 3 parts ketamine (Ketaset,100 mg/ml; Fort Dodge Laboratories, Ft. Dodge, Iowa) and 7 parts xylazine (Rompun, 20 mg/ml; Bayer Corp., Shawnee Mission, Kans.). Saliva secretion was stimulated by s.c. injection of 0.6 ml of carbachol (containing 0.1 mg/ml of saline; Sigma Chemical Co.) per kilogram of rat weight. After saliva collection, rats were awakened by injection s.c., first with 0.1 ml/kg of atropine sulfate (0.4 mg/ml; American Pharmaceutical Partners, Inc., Los Angeles, Calif.) and then with yohimbine (Yobine, 2.0 mg/ml; Lloyd Laboratories, Shenandoah, Iowa) at a volume equal to 1.4 times that used for anesthesia. Blood was collected from the tail vein during anesthesia. Sera from coagulated and centrifuged blood were stored frozen at −20°C until measurement of antibody activity. Clarified saliva was stored at −70°C.
Experiment 2 (S. mutans infection).
Four groups (n = 12 to13/group) of 24-day-old Sprague-Dawley female rats were singly caged. Rats were injected s.c. in the salivary gland vicinity with adjuvant (0.04 ml/site) and either PBS (sham), SYI-CAT (60 μg/dose), SYI (30 μg/dose), or CAT (30 μg/dose). Antigen was incorporated with complete FA. Rats were reinjected 13 days later with PBS or peptides at the same dose in incomplete FA. Nine days after the second injection, serum and saliva samples were collected under anesthesia as described above. Thirteen days after the second injection, rats were placed in tubs (six rats per tub), given cariogenic diet 2000 (56% confectioners' sugar), and orally infected with approximately 108 streptomycin-resistant S. mutans strain SJ32 cells for 3 consecutive days. Rats were again singly caged after the infection protocol was completed and continued on diet 2000 for the duration of the experiment. Blood and saliva were collected under anesthesia 64 days after initial infection, followed by CO2 asphyxiation. In preparation for the scoring of dental caries, rat skulls were defleshed by dermastide beetles, followed by a rinse with 70% ethanol.
Antibody inhibition of glucan synthesis.
Preinfection sera from experiment 2 (collected 22 days after initial immunization) were evaluated for their ability to inhibit water-insoluble glucan synthesis catalyzed by S. sobrinus GTF, using a filter assay. This GTF preparation contained a mixture of GTF-I and GTF-S. S. sobrinus GTF-I and S. mutans GTF-B had complete homology with the CAT peptide construct in the respective catalytic region. Twenty microliters of diluted sera (1:20 dilutions in 0.02 M sodium PBS and 0.2% sodium azide [PBSA], pH 6.5) were preincubated with 0.036 μg of GTF for 2 h at 37°C in a total volume of 0.04 ml of PBSA. Then, 0.05 mg of sucrose and 40 nCi of [14C]sucrose (approximately 100,000 cpm) were added to 0.1 ml of PBSA in the absence of primer (21). Incubation proceeded for 3 h at 37°C, after which water-insoluble glucan was collected by passage through Whatman GF/F glass fiber filters. Water-insoluble glucan collected on filters was washed and retained radioactivity, determined as previously reported (21). Under the conditions of this assay, approximately 900 cpm were incorporated into water-insoluble glucan in the presence of sham-immune sera. The percentage of inhibition of enzyme activity was calculated using the mean sham incorporation counts per minute values as the 100% incorporation levels. Positive control sera from rats immunized with S. sobrinus GTF, which were obtained from experiment 1, were also included in the assay.
Experiment 3 (S. sobrinus infection).
The protocol for experiment 3 was essentially identical to that of experiment 2 except that, following immunization and serological sampling, all animals were infected with streptomycin-resistant S. sobrinus strain 6715. Animals were immunized as described above when rats were 24 and 37 days old. Rats were then bled and salivated under anesthesia 7 days later and were infected with S. sobrinus strain 6715 9 days after the second injection, using the infection protocol followed for experiment 2. Infection continued for 70 days prior to experiment termination.
All experimental protocols were pursued in The Forsyth Institute's animal facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, only after approval by the Institutional Animal Care and Use Committee at The Forsyth Institute.
Bacterial recoveries.
The presence of mutans streptococcal infection was assessed at the end of each experiment. After systematic swabbing of teeth, sonication, and plating appropriate dilutions on mitis salivarius agar alone and on mitis salivarius agar with 0.2 mg/ml of streptomycin sulfate, plates were incubated for 48 h at 37°C in 80% N2, 10% CO2, 10% H2. Streptomycin-resistant S. mutans strain SJr or S. sobrinus strain 6715 colony-forming units were then enumerated microscopically.
Caries assessment.
The extent and depth of carious lesions in all rat molar teeth (caries score) were microscopically evaluated by a modified method of Keyes and Jordan (7). Caries scores were determined separately on smooth and on occlusal dental surfaces.
Statistical analysis.
The differences in the median values among the treatment groups were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey pairwise multiple-comparison test when data were normally distributed. Alternatively, data were analyzed by Kruskal-Wallis one-way ANOVA on ranks, followed by Dunn's multiple-comparison procedure when nonparametric distributions were encountered.
RESULTS
Experiment 1 (induction of immune response to S. sobrinus GTF and S. mutans GbpB).
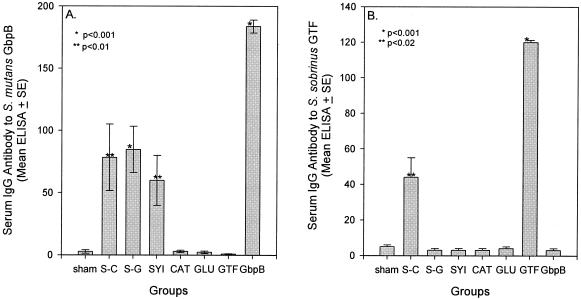
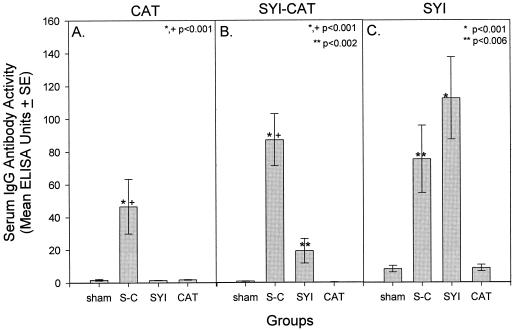
The IgG antibody reactivities to GTF and GbpB pathogenesis-associated proteins of mutans streptococci were measured in sera (day 63) from groups of rats immunized with monoepitopic or diepitopic peptide constructs or with the native proteins. Figure 1A displays the mean IgG antibody levels to S. mutans GbpB among groups of sera assayed in ELISA. Both diepitopic peptide constructs SYI-CAT and SYI-GLU induced significant levels of serum IgG antibody that were reactive with GbpB. Mean IgG antibody levels to GbpB were similar in the two diepitopic peptide-immunized groups and were somewhat, but not significantly, higher than antibody to GbpB induced by immunization with monoepitopic SYI. No other peptide induced a detectable serum IgG response to this antigen.
FIG. 1.
Experiment 1 serum IgG antibody levels to S. mutans GbpB (A) and S. sobrinus GTF (B) in rats immunized with peptide constructs (seven rats per group) or native proteins (four rats per group), indicated beneath the bars. S-C and S-G indicate groups immunized with the diepitopic constructs SYI-CAT and SYI-GLU, respectively. Sera, diluted 1:200, were assayed by ELISA 63 days after initial immunization. One hundred EU was equivalent to reference serum dilutions of 1/1,000 for GbpB and 1/2,000 for GTF. Bars indicate means ± 1 standard error of the mean. Asterisks indicate group data with levels significantly higher than levels in the sham-immunized group, determined by the Kruskal-Wallis ANOVA on ranks and Dunn's pairwise multiple-comparison method. EU for GpbB and GTF are not comparable.
Figure 1B displays the mean serum IgG antibody levels to S. sobrinus GTF among the eight groups. Of all the peptide constructs tested, only the diepitopic peptide SYI-CAT induced a significant serum IgG antibody response that was reactive with GTF. Presentation of CAT within the context of SYI, a peptide containing a putative T-cell epitope, enhanced the formation of antibody to S. sobrinus GTF since the monoepitopic CAT-immunized rats did not demonstrate IgG antibody to this antigen at the peptide concentration used for immunization and detection. Thus, the SYI-CAT diepitopic peptide construct induced a more broadly reactive immune response to GbpB and GTF and was selected for use in the following protection experiments.
Experiment 2 (immunization and S. mutans infection).
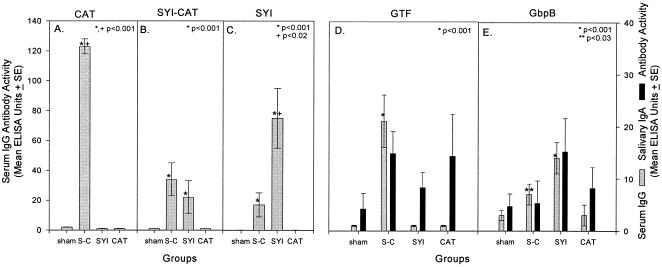
To determine whether protection could be induced by immunization with the diepitopic versus monoepitopic peptide construct, groups of weanling rats were immunized s.c. on day 0 (at 24 days of age) and on day 13 with SYI-CAT or with the component peptide constructs. The IgG responses to peptides and native proteins are shown in Fig. 2 for sera taken prior to infection (day 22). Immunization with the diepitopic peptide construct significantly (P < 0.001) enhanced the detectable serum IgG response to CAT (Fig. 2A). However, the serum IgG response to SYI-CAT itself (Fig. 2B) was not significantly different from the response induced by monoepitopic SYI. In contrast, the SYI-immunized group induced a significantly higher level of antibody to SYI (Fig. 2C) than did the diepitopic peptide construct, although the SYI-CAT-immunized group also demonstrated a significant level of SYI-reactive antibody.
FIG. 2.
Preinfection serum IgG antibody levels to monoepitopic CAT (A), diepitopic SYI-CAT (B), and monoepitopic SYI (C) peptide constructs and to S. sobrinus GTF (D) and S. mutans GbpB (E) native proteins (12 rats per group) in experiment 2. S-C indicates the group immunized with the diepitopic construct SYI-CAT. Preinfection salivary IgA antibody levels are also shown for the two native proteins. Immunizing antigens are indicated on the abscissa beneath the bars. Sera were diluted 1:200 and 1:2,000 for analysis of antibody to peptides and native proteins, respectively. Salivas were diluted 1:4 before assay. Antibody-containing fluids were assayed by ELISA 22 days after initial immunization. Bars indicate means ± 1 standard error of the mean. One hundred EU was equivalent to reference reagent dilutions of 1:1,000 (sera) or 1:8 (saliva) for GbpB and 1:2,000 (sera) or 1:4 (saliva) for GTF. Asterisks indicate immunized group data with levels significantly higher than the levels of the sham-immunized group, determined by the Kruskal-Wallis ANOVA on ranks and Dunn's pairwise multiple-comparison method. The plus symbol indicates the statistical comparison between SYI-CAT- and SYI-immunized groups. EU for different peptide constructs, native proteins, and antibody classes are not comparable.
The serum IgG antibody responses to native GTF and GbpB proteins observed in experiment 2 (22 days after initial immunization) (Fig. 2D and E) essentially paralleled those observed by ELISA analyses 63 days after initial immunization during experiment 1 (Fig. 1). The diepitopic peptide construct induced significant levels of serum IgG antibody to the GTF native protein. Again, this result represented a significant enhancement of the mean anti-GTF response induced by the CAT construct, indicating that the SYI epitope(s) provided additional help for the response. Also, as seen in the previous experiment, both monoepitopic and diepitopic peptide constructs contained SYI-induced statistically significant levels of antibody to the S. mutans GbpB native protein. Similar patterns of response were observed when the levels of serum IgA responses to GTF and GbpB were measured (not shown).
The mean levels of IgA antibody to native GTF and GbpB proteins in saliva samples collected on day 22 were modest and did not reach significance (Fig. 2). However, as with the serum IgG antibody levels, saliva from the group immunized with the diepitopic peptide construct had the highest mean IgA antibody level to GTF, while saliva from the group immunized with the SYI peptide construct demonstrated the highest mean IgA antibody reactivity with GbpB.
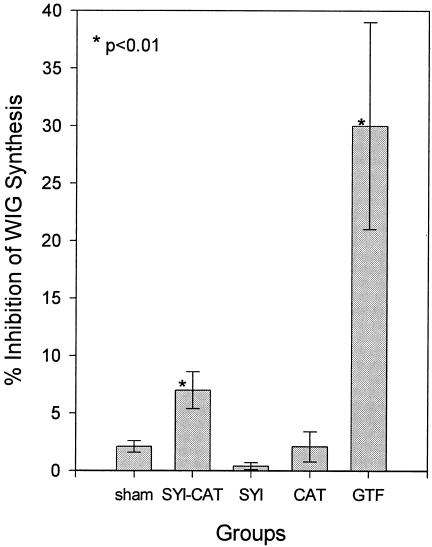
The preinfection sera from experiment 2 were also analyzed for their ability to inhibit GTF-mediated synthesis of water-insoluble glucan from sucrose (Fig. 3). Paralleling the ELISA observations, sera from the diepitopic SYI-CAT-immunized group showed low but significant (P < 0.01) inhibition of GTF activity compared to other peptide- or sham-immunized groups.
FIG. 3.
Mean activity of serum-mediated inhibition of water-insoluble glucan (WIG) synthesis from sucrose, catalyzed by S. sobrinus GTF in experiment 2. Bars indicate means ± 1 standard error of the mean. Rat sera used for assay (8 to 12 rats per group) were obtained 22 days after initial immunization. Positive control sera (4) from rats immunized with S. sobrinus GTF were obtained from experiment 1. Inhibition is expressed as a percentage of the mean 14C incorporation by the sham-immunized group. Asterisks indicate group inhibition levels that were significantly different from those of the sham-immunized group, determined by one-way ANOVA and the Tukey pairwise multiple-comparison test.
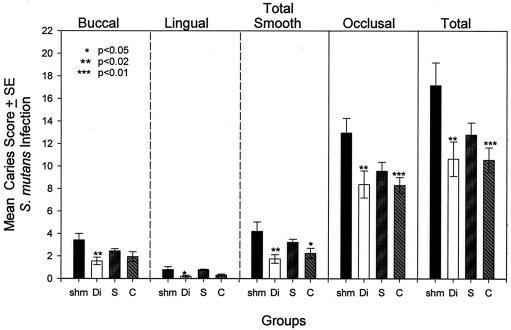
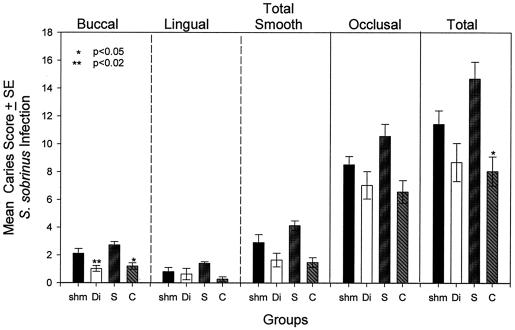
After 64 days of S. mutans infection, dental caries was evaluated on buccal, lingual, and occlusal molar surfaces (Fig. 4). Groups that were immunized with both the diepitopic SYI-CAT and the monoepitopic CAT peptide constructs demonstrated significantly lower caries scores on smooth surfaces. All peptide-immunized rats had lower occlusal caries scores than the sham-immunized group, reductions which reached statistical significance in the diepitopic-peptide-immunized and SYI-immunized groups. However, despite the significant serological improvement in antibody levels to GTF observed by immunization with SYI-CAT, no enhancement of protection was observed by immunization with the two peptides conjoined in the same construct.
FIG. 4.
Dental caries in experiment 2 after infection with S. mutans SJr. The buccal, lingual, total smooth (buccal plus lingual), occlusal, and total molar caries scores from the four immunized groups (11 to 12 rats per group) are shown by bars in the respective panels. Bars indicate means ± 1 standard error of the mean. Asterisks indicate groups that have significantly lower caries scores than those found in the sham-immunized group (shm). The level of significance, determined by one-way ANOVA and the Tukey pairwise multiple-comparison test, is indicated in the left-most panel. The labels on the abscissa indicate sham (shm)-, SYI-CAT (Di)-, SYI (S)-, and CAT (C)-immunized groups.
Experiment 3 (S. sobrinus infection).
A second immunization and infection experiment was performed to analyze the protective effect of diepitopic versus monoepitopic peptide immunization in rats infected with S. sobrinus 6715. Since S. mutans GbpB does not have a correlate among S. sobrinus glucan-binding proteins, responses induced by SYI in the monoepitopic or diepitopic context should not influence infection or disease caused by S. sobrinus. Rats (n = 48), separated into four equal groups, were immunized under the identical protocol used for experiment 2. Blood collected 20 days after the initial immunization with SYI-CAT, SYI, or CAT revealed levels of serum IgG antibody to immunizing peptides (Fig. 5) that were very similar in pattern to those observed in experiment 2 (Fig. 2). Dental caries on molar surfaces was measured after 70 days of S. sobrinus strain 6715 infection (Fig. 6). Groups immunized with the diepitopic SYI-CAT and the monoepitopic CAT peptide constructs had mean levels of caries on all surfaces which were lower than those found in sham-immunized rats, attaining statistical significance in buccal (both SYI-CAT and CAT) and total (CAT) caries comparisons. As expected, the level of caries in the group immunized with the S. mutans-associated SYI peptide was not affected by the immunization, since S. sobrinus does not express GbpB (the median total caries score of the SYI group [12.9] was similar to the median score of the sham group[12.0]). Consequently, both the SYI-CAT- and CAT-immunized groups had significantly (P < 0.05) lower total smooth and total overall caries scores than the SYI-immunized group.
FIG. 5.
Preinfection serum IgG antibody levels to monoepitopic CAT (A), diepitopic SYI-CAT (B), and monoepitopic SYI (C) peptide constructs (12 rats per group) in experiment 3. Immunizing antigens are indicated on the abscissa beneath the bars. Sera were diluted 1:200 for analysis of antibody to peptides. Sera were assayed by ELISA 20 days after initial immunization. Bars indicate means ± 1 standard error of the mean. Asterisks indicate immunized group data with levels significantly higher than the levels of the sham-immunized group, determined by Kruskal-Wallis ANOVA on ranks and Dunn's pairwise multiple-comparison method. The plus symbol indicates the statistical comparison between SYI-CAT- and SYI-immunized groups. EU for different peptide constructs and native proteins are not comparable.
FIG. 6.
Dental caries in experiment 3 after infection with S. sobrinus 6715. The buccal, lingual, total smooth (buccal plus lingual), occlusal, and total molar caries scores from the four immunized groups (11 to 12 rats per group) are shown. Bars indicate the mean caries scores for the respective surface(s) ± 1 standard error of the mean. Asterisks indicate groups that have significantly lower caries scores than those found in the sham-immunized group (shm). The level of significance, determined by one-way ANOVA and the Tukey pairwise multiple-comparison test, is indicated in the left-most panel. The labels on the abscissa indicate sham (shm)-, SYI-CAT (Di)-, SYI (S)-, and CAT (C)-immunized groups.
DISCUSSION
The hypothesis that the addition of a putative T-cell epitope to a MAP construct containing CAT would significantly enhance the response to CAT, as previously demonstrated for GTF-derived peptides (22-24), was confirmed in the context of the GbpB-derived SYI peptide. The monoepitopic MAP-CAT peptide construct has previously been shown to induce a modest serum IgG antibody response to itself (18, 23, 24) that was sufficient for protection (22, 24). Previously we showed that addition of a T-cell epitope-containing GTF-derived peptide (GLU) to a MAP construct greatly enhanced the serum antibody response to CAT (24). A similar enhancement of serum antibody to CAT was observed in the present study after addition of the GbpB-derived SYI peptide, which appears to contain MHC class II binding properties with empirical indications of a T-cell epitope (16). A hypothetical explanation for this phenomenon is that mature specific B cells recognize the B-cell epitope on the CAT portion of the diepitopic construct and/or the B-cell epitope on SYI. These B cells (and other professional antigen-presenting cells) then present the T-cell epitope peptide (presumably from the SYI portion of the construct), in association with MHC class II molecules, to specific T cells, resulting in the production of B-cell-activating cytokines and an enhanced CAT response. Since the SYI peptide has already been shown to contain characteristics reflective of B- and T-cell epitopes, the diepitopic SYI-CAT construct did not enhance serum antibody levels to SYI above those induced by the monoepitopic SYI construct alone (Fig. 2 and 5).
The effect of the diepitopic construct on the immune response to mutans streptococcal GTF was more striking. The contribution of SYI in SYI-CAT not only potentiates the response to CAT, but more significantly, it strongly enhances the portion of that antibody response that reacts with (Fig. 1 and 2) and inhibits (Fig. 3) GTF. The CAT peptide sequence contains an aspartate which, when in the context of S. mutans and S. sobrinus GTF sequences, has been demonstrated by molecular genetic and biochemical approaches to be critical to the catalytic properties of GTF (6, 12). The B-cell epitope within the CAT peptide subsumes this aspartate (12); thus, antibody raised to CAT is expected to, and has previously been demonstrated (20, 25) to, interfere with GTF function. Thus, this diepitopic construct, containing a putative T-cell epitope unrelated to GTF, appears to significantly enhance the serum IgG immune response to this important epitope.
Previously we observed an approximately twofold increase in salivary IgA antibody level to GTF when rats were immunized with the GTF-based dipeptide CAT-GLU, rather than with the monoepitopic CAT peptide (24). In the present experiment, only a modest salivary IgA response to GTF was detected when CAT was presented in the context of the diepitopic SYI-CAT construct. Zhang and coworkers (26) attempted to enhance the mucosal response to the immunologically weak S. mutans salivary binding receptor component by intranasally immunizing with this domain, presented as a chimeric protein with a sequence reflecting most of the glucan binding domain. They observed a 10-fold increase in salivary IgA response to the SBR domain, although the response to the native AgI/AgII adhesin was not reported. The difference in ability to increase the mucosal responses may be related to the significant difference in the relative sizes of the synthetic peptide and the protein chimera or to the route of immunization. However, both studies reinforce the ability to broaden and, in some cases, to enhance the immune response to multiple mutans streptococcal virulence antigens.
Interestingly, addition of the GTF-irrelevant SYI peptide to the diepitopic construct containing the GTF-sequence-based GLU peptide showed no enhancement of a serum IgG response to GTF (Fig. 1). This lack of enhancement was observed despite the fact that SYI-GLU induced high levels of serum IgG and salivary IgA antibody to GLU (data not shown). These responses were more consistently elevated than those induced by the respective monoepitopic MAP antigens. Thus, the diepitopic construct SYI-GLU, which contained T- and B-cell epitopes on both peptide components, improved responses to constituent peptide epitopes but not necessarily to GTF. The lack of a detectable improvement in antibody reactive with GTF is perhaps because the cross-reactive epitopes may be masked in the MAP construct, resulting in a response of low affinity and/or amount. The lack of detectable antibody to GTF in most animals immunized with the GLU construct may be a consequence of using a lower dose of peptide than was used in previous experiments (18, 24).
Groups of rats immunized with monoepitopic SYI or CAT constructs and infected with S. mutans strains had lower levels of molar caries than sham-immunized rats (Fig. 4), as previously observed in experiments using higher doses (50 μg) of the various peptide constructs (17, 22, 24). The protective responses to S. mutans infection were presumably based on antibody to epitopes on GbpB (via SYI) and GTF-B (via CAT), which are native proteins that have been associated with different aspects of the molecular pathogenesis of S. mutans-mediated dental caries. The specificity of protection was confirmed in the S. sobrinus infection experiment, since lower caries scores were observed only in the groups immunized with CAT-containing constructs which share a sequence with GTFs of both mutans streptococcal species. The modest protection achieved in rats immunized with CAT occurred despite the inability to detect serum IgG or salivary IgA antibody to the CAT construct alone or to GTF in most CAT-immunized rats prior to infection. Antibody to CAT could, however, be detected at the end of the experiment (data not shown). As indicated above, the CAT peptide construct appears to be less immunogenic than SYI or GLU, and the use of a lower peptide-immunizing dose presumably influenced antibody detection. Nonetheless, caries reductions following CAT immunization underscore the functional significance of amino acid residues with the respective GTF sequences.
Although the SYI-CAT diepitopic construct induced protective responses after infection with cariogenic mutans streptococci, the extent of protection was similar to that observed with the monoepitopic construct(s). Other workers have recently examined the effect of multivalent caries vaccines coding for or containing major adhesin (AgI/AgII) and glucan binding (GTF) domains. By using the percentage of S. mutans cells per total number of streptococci as the measure of protection, Zhang and coworkers (26) observed a significant reduction after immunization with the chimeric SBR-GLU construct but saw no significant reductions after immunization with constituent 42-kDa SBR or 33.5-kDa GLU proteins. Previously, Jespersgaard et al. (5) demonstrated caries-protective effects with the GLU polypeptide alone. Guo and coworkers (3) found that s.c. immunization with fusion DNA vaccines coding for both the GLU of GTF and the adhesin domains of AgI/AgII gave marginally better protection from enamel caries than did a DNA vaccine containing only the adhesin domains. Variations in the model system, including animal species, infection regimens, antibiotic suppression, dietary sucrose levels, and age of infection limit the comparability of these three approaches. The semiquantitative disease data obtained in these experimental models can present a challenge for reliable measurement of degrees of protection, in that significant differences in in vitro measures of antibody levels may not always be accompanied by commensurate differences in measures of disease.
In summary, the present studies have shown that the serological and functional inhibitory responses to an epitope associated with the catalytic activity of GTF can be significantly enhanced by epitopes from a peptide (SYI) from another virulence component that, by itself, can induce only a specific protective immune response to S. mutans. Conversely, the addition of the GTF-derived CAT epitope to the GbpB-derived SYI peptide broadens the resulting protective immune response to include S. sobrinus. Taken together, the combination of epitopes associated with function or immunogenicity from more than one mutans streptococcal virulence component through recombinant DNA vaccine, chimeric protein, or synthetic peptide approaches is a promising approach in the design of more-effective dental caries vaccines.
Acknowledgments
This work was supported by Public Health Service grants DE-06153 and DE-04733 from the National Institute of Dental and Craniofacial Research and by a student (J.R.) research grant from Harvard Medical School.
Editor: J. D. Clements
REFERENCES
- 1.Abo, H., T. Matsumura, T. Kodama, H. Ohta, K. Fukui, K. Kato, and H. Kagawa. 1991. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase). J. Bacteriol. 173:989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 14:89-99. [DOI] [PubMed] [Google Scholar]
- 3.Guo, J. H., R. Jia, M. W. Fan, Z. Bian, Z. Chen, and B. Peng. 2004. Construction and immunogenic characterization of a fusion anti-caries DNA vaccine against PAc and glucosyltransferase I of Streptococcus mutans. J. Dent. Res. 83:266-270. [DOI] [PubMed] [Google Scholar]
- 4.Hamada, S., and H. D. Slade. 1980. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersgaard, C., G. Hajishengallis, Y. Huang, M. W. Russell, D. J. Smith, and S. M. Michalek. 1999. Protective immunity against Streptococcus mutans infection in mice after intranasal immunization with the glucan-binding region of S. mutans glucosyltransferase. Infect. Immun. 67:6543-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato, C., Y. Nakano, M. Lis, and H. K. Kuramitsu. 1992. Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem. Biophys. Res. Commun. 189:1184-1188. [DOI] [PubMed] [Google Scholar]
- 7.Keyes, P. H., and H. V. Jordan. 1964. Periodontal lesions in the Syrian hamster. III. Findings related to an infectious and transmissible component. Arch. Oral Biol. 9:377. [DOI] [PubMed] [Google Scholar]
- 8.Koga, T., T. Oho, Y. Shimazaki, and Y. Nakano. 2002. Immunization against dental caries. Vaccine 20:2027-2044. [DOI] [PubMed] [Google Scholar]
- 9.Lis, M., T. Shiroza, and H. K. Kuramitsu. 1995. Role of the C-terminal direct repeating units of the Streptococcus mutans glucosyltransferase-S in glucan binding. Appl. Environ. Microbiol. 61:2040-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattos-Graner, R. O., S. Jin, W. F. King, T. Chen, D. J. Smith, and M. J. Duncan. 2001. Cloning of the Streptococcus mutans gene encoding glucan binding protein B and analysis of genetic diversity and protein production in clinical isolates. Infect. Immun. 69:6931-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merrifield, R. B. 1963. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85:2149-2154. [Google Scholar]
- 12.Mooser, G., S. Hefta, R. J. Paxton, J. E. Shively, and T. D. Lee. 1991. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J. Biol. Chem. 266:8916-8922. [PubMed] [Google Scholar]
- 13.Mooser, G., and C. Wong. 1988. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect. Immun. 56:880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, D. J. 2002. Dental caries vaccines: prospects and concerns. Crit. Rev. Oral Biol. Med. 13:335-349. [DOI] [PubMed] [Google Scholar]
- 15.Smith, D. J., H. Akita, W. F. King, and M. A. Taubman. 1994. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 62:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, D. J., W. F. King, L. A. Barnes, Z. S. Peacock, and M. A. Taubman. 2003. Immunogenicity and protective immunity induced by synthetic peptides associated with putative immunodominant regions of Streptococcus mutans glucan-binding protein B. Infect. Immun. 71:1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, D. J., and M. A. Taubman. 1996. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect. Immun. 64:3069-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, D. J., M. A. Taubman, C. J. Holmberg, J. W. Eastcott, W. F. King, and P. Ali-Salaam. 1993. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect. Immun. 61:2899-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, D. J., M. A. Taubman, W. F. King, S. Eida, J. R. Powell, and J. Eastcott. 1994. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect. Immun. 62:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam, J. P. 1988. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 85:5409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taubman, M. A., C. J. Holmberg, and D. J. Smith. 1995. Immunization of rats with synthetic peptide constructs from the glucan-binding or catalytic regions of mutans streptococcal glucosyltransferase protects against dental caries. Infect. Immun. 63:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taubman, M. A., C. J. Holmberg, D. J. Smith, and J. W. Eastcott. 1995. T and B cell epitopes from peptide sequences associated with glucosyltransferase function. Clin. Immunol. Immunopathol. 76:s95. [Google Scholar]
- 23.Taubman, M. A., D. J. Smith, C. J. Holmberg, and J. W. Eastcott. 2000. Coimmunization with complementary glucosyltransferase peptides results in enhanced immunogenicity and protection against dental caries. Infect. Immun. 68:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taubman, M. A., C. J. Holmberg, and D. J. Smith. 2001. Diepitopic construct of functionally and epitopically relevant complementary peptides enhances immunogenicity, reactivity with glucosyltransferase, and protection from dental caries. Infect. Immun. 69:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, H., Y. Nakano, Y. Yamashita, T. Oho, and T. Koga. 1997. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect. Immun. 65:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, P., C. Jespersgaard, L. Lamberty-Mallory, J. Katz, Y. Huang, G. Hajishengallis, and S. M. Michalek. 2002. Enhanced immunogenicity of a genetic chimeric protein consisting of two virulence antigens of Streptococcus mutans and protection against infection. Infect. Immun. 70:6779-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]