Significance
This study shows that ancient trees, which are rare and threatened vestiges in remote European high-mountain regions, have a unique physiology that is linked to their role in the ecosystem. By exploring the physiology of ancient mountain pine trees from five populations occurring in remote areas in the Pyrenees, we show that specific longevity traits are linked to their unique ecological functions, serving specific roles in protecting lichen species in remote European high-mountain regions.
Keywords: biodiversity, ecological functions, longevity, tree ageing, tree senescence
Abstract
Mature forests and their extremely old trees are rare and threatened ancient vestiges in remote European high-mountain regions. Here, we analyze the role that extremely long-living trees have in mature forests biodiversity in relation to their singular traits underlying longevity. Tree size and age determine relative growth rates, bud abortion, and the water status of long-living trees. The oldest trees suffer indefectible age-related constraints but possess singular evolutionary traits defined by fitness adaptation, modular autonomy, and a resilient metabolism that allow them to have irreplaceable roles in the ecosystem as biodiversity anchors of vulnerable lichen species like Letharia vulpina. We suggest that the role of ancient trees as unique biodiversity reservoirs is linked to their singular physiological traits associated with longevity. The set of evolutionarily plastic tools that can only be provided by centuries or millennia of longevity helps the oldest trees of mature forests drive singular ecological relationships that are irreplaceable and necessary for ecosystem dynamics.
Climate, topography, and fire events have modulated the adaptive evolution and diversification of pine species at the global scale (1). The remaining primary forests in Europe cover only 0.7% of the total forest area, with most occurring in mountainous or boreal regions. Among them, the highest parts of the Pyrenees remain as one of the regions with the highest likelihood of containing primary forests at the continental scale. Although scarce, rare primary forests possess important conservation values and their distributions have been modulated historically by anthropogenic disturbances (2). European mountains, such as the Pyrenees, hold several ecological gradients influenced by old land use practices (3). The ecological benefits of mature forests are explained by their singular physiological attributes. Mature stands display diminished branch densities, a higher diameter at breast height (DBH), and greater bark crevices, with singular age-related morphological features (4, 5). An important fraction of extremely long-living old trees in mature forests presents several dead modules and may ultimately contribute (as decaying elements) to the carbon sequestration in subalpine poor soils (6). Old-growth forests, such as those of the Pyrenees, harbor trees with extreme longevities that are unique treeline past vestiges with the potential to be ecologically unique (5, 7, 8). Forest management results in homogeneous compositions and forest structures that negatively reduce the hosting biodiversity capacity (9).
Very old trees are of special interest in ecosystem dynamics. Forests with extended longevities provide unique and different niches for forest specialists, as different decay- and age-related stages provide different habitat-colonizing options that affect the occurrence of communities. Ancient trees are essential elements that play crucial ecological and biodiversity roles in forests, with most of them rapidly declining in many threatened parts of the world (8, 10–12). The oldest living trees are extremely valuable historical standards for studying the climate sensitivity of forest growth and the dynamics occurring over millennia and under the constraints of climate change (13–18). Tree decay determines lichen communities in several worldwide ecosystems and climate zones. The chemical properties of the decaying wood of old conifer trees modulate epiphytic lichen and bryophyte communities, while senescence is a key modulator enhancing lichen species richness (19, 20). Forest age heterogeneity determines plant diversity in mature forests (21). Hence, ancient trees provide ecosystem benefits to old-growth forests and should be protected before they disappear in order to protect these hotspots of biodiversity and resilience (22).
Longevity provides crucial intrinsic benefits for the functioning of mature ecosystems in many of the endangered old forests around the world (12). Survival has been diversified through the development of several diverse evolutionary strategies depending on tree size (23). Among tree species, longevity is promoted by several genetic-dependent and environmental factors that balance their developmental and aging strategies (24). The long lifespans of the oldest living trees entail complex mechanisms that minimize and ultimately postpone the death of the whole organism, with the idea of a continuous slow growth being the most effective strategy in preventing senescence being generally accepted (8). Tree death and living cycles are defined by life-history events, where trade-offs between growth and survival mechanisms are driven by stress tolerance and resilience strategies (8, 25). All organs are prepared to face physiological and morphological trade-offs (25), which results in efficient strategies that allow long-living trees to overcome survival constraints over centuries or even millennia (26). Despite this, these plastic growth– and stress tolerance–related mechanisms are not enough to finally escape death, which still occurs (27, 28). In this context, meristem indeterminacy, the protective mechanisms of stem cells, the absence of meristem and genetic senescence, the low occurrence of fixed somatic mutations, and the whole-plant plastic and adaptive mechanisms serve to defy aging by postponing and delimiting death to independent modules (5, 27–32). Although evolution has provided several ways to almost attain immortality, anthropogenic disturbances threaten the distribution and occurrence of the oldest trees in the world (33, 34).
In this research article, we use physiological and ecological tools to reveal some of the mechanisms underlying high-mountain-forest dynamics. We show that ancient mountain pine trees have evolved unique singular morphological and physiological characteristics that make them unique reservoirs of biodiversity. Specifically, we show that the ability to survive for several centuries has enabled these trees to develop specific traits that are essential for harboring a huge number of species in high-mountain European ecosystems. Therefore, the oldest trees of high-mountain forests sustain mature forests dynamics by exerting essential ecological functions.
Results and Discussion
Age and Size Regulate Growth Capacity and Stress Dynamics at the Spatiotemporal Scale in Mature Forests.
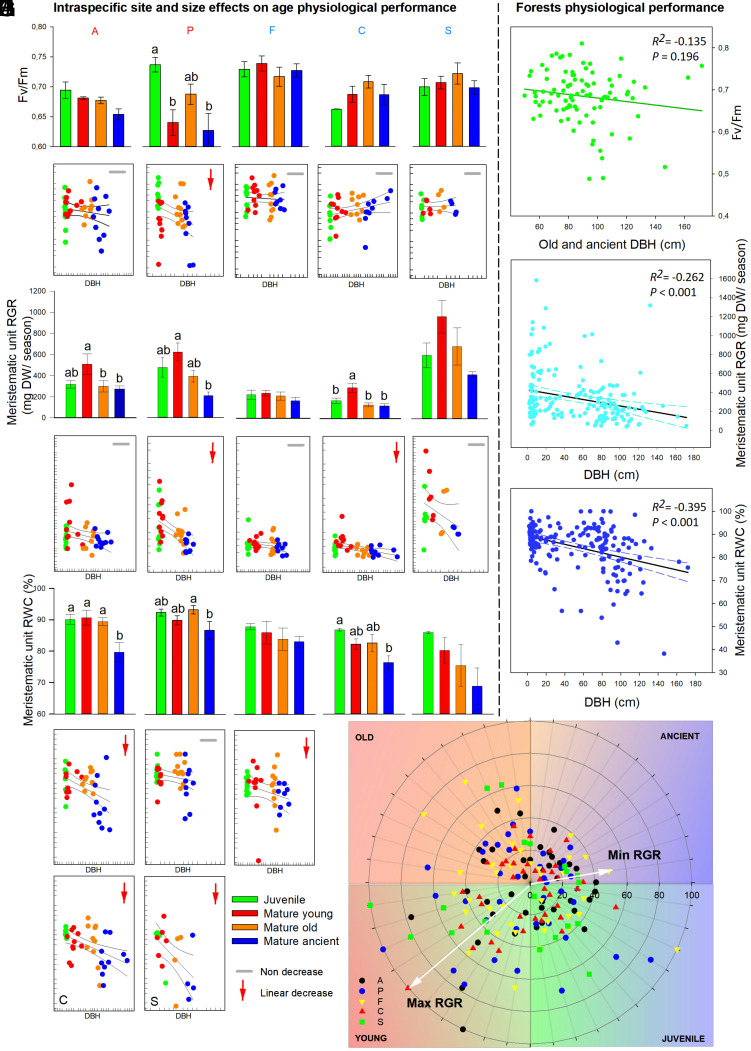
Most of the mature mountain pine (Pinus uncinata) stands remain in small and remote portions representing the last primary forests of the high treeline in south European mountain ranges. These environments are home to some of the oldest European standing trees and have escaped the effects of human activity for centuries, going unnoticed during different historical periods as authentic past vestiges (SI Appendix, Fig. S1). To discern the uniqueness of the oldest trees in these protected areas, we studied the irreplaceable role of these trees according to their physiological status and contribution to biodiversity after several centuries of continuous growth and death. According to our results, longevity has impacted both the physiology and the ecosystem services of the oldest organisms in these high-mountain ecosystems that are subjected to harsh environmental conditions. Developmental stage, size, age, and physiological resilience over centuries have affected the amount of stress we observed among the meristematic and somatic tissues of the oldest trees in mature forests (Figs. 1 and 2). Basic parameters relating to growth capacity, photosynthesis, and hydric conditions differed with tree size and age. Photosynthetic performance, which we determined as the maximal efficiency of photosystem II (Fv/Fm), did not significantly decrease when the trees entered the mature developmental stage (Fig. 1 A, B, and G). Fv/Fm was only observed to be decreased in the mature ancient trees from one of the most irradiated south-facing forests, while tree populations from north-facing forests showed no changes (Fig. 1 A, B, and G). Hence, Fv/Fm cannot be used as a generalized aging stress marker in mature forests. After snowmelt, ancient and old trees showed limited relative growth rates (RGR) during the regrowth season (Fig. 1 C, D, and H). The RGR decreased with age, exhibiting a lower meristematic bud activation to break winter dormancy, resulting in a clear age-related limitation in forming new aerial structures. Thus, as the oldest trees age, they maintain a suboptimal growth capacity and slow down metabolic activation of buds during the growth season, enabling them to maintain feasible growing structures during centuries or even millennia. From a hydraulic point of view, age is an even more important decay-related trait among mature ancient high-mountain trees. The relative water content (RWC) of the most recent-year meristematic unit (bud opened and growing) was clearly reduced in the oldest trees (Fig. 1 E, F, and I). These results highlight the importance of the variations in both meristematic hydric conditions and growth rates over centuries once trees become older, possibly explaining a relevant cause of death in the oldest trees of mature forests. This is inferred from the lower growth capacity of the less abundant but oldest fraction of the trees in mature forests, with the most abundant trees (established as mature young trees) presenting the highest growth capacity in these forests (Fig. 1J).
Fig. 1.
Physiological traits of long-living trees correlate with DBH. (A–F) Means and linear correlations of the physiological traits of somatic and meristematic tissues for each forest depending on the tree DBH. Maximum efficiency of PSII (A and B, Fv/Fm), relative growth rate (RGR) of the meristematic unit (C and D, mg DW/season of growth), and relative water content (RWC) of the meristematic unit (E and F, %). (G–I) Linear correlations between DBH and physiological traits. The RGR of the meristematic unit negatively correlated with the tree DBH (H, N = 185 studied trees) as well as the RWC of the meristematic unit (I, N = 182 studied trees). (J) Spider plot presenting the RGR of the high-mountain trees according to their developmental stage (N = 178 studied trees). Mature young trees presented the highest RGR values, while mature ancient trees presented the lowest (J). Data show mean ± SE of each studied physiological parameter. Differences in letters indicate significant differences between the developmental stage based on one-way ANOVA (P = 0.05) and Tukey’s test comparing the effect of the tree DBH on the physiological traits studied. Bars sharing at least one letter show no significant differences between them (A, C, and E). Dots correspond to a linear correlation for the individual trees from each studied forest (B, D, and F). Colors represent different developmental stages: green = juvenile trees; red = mature young trees; orange = mature old trees; blue = mature ancient trees (A–F). Trend lines and dashed lines represent correlation coefficients ± 95% CIs. A, P, F, C, and S indicate the forests studied, while the blue-colored letters indicate north-facing forests and the red-colored letters indicate south-facing forests. Linear correlations were set as significant with P < 0.05 and very significant with P < 0.01.
Fig. 2.
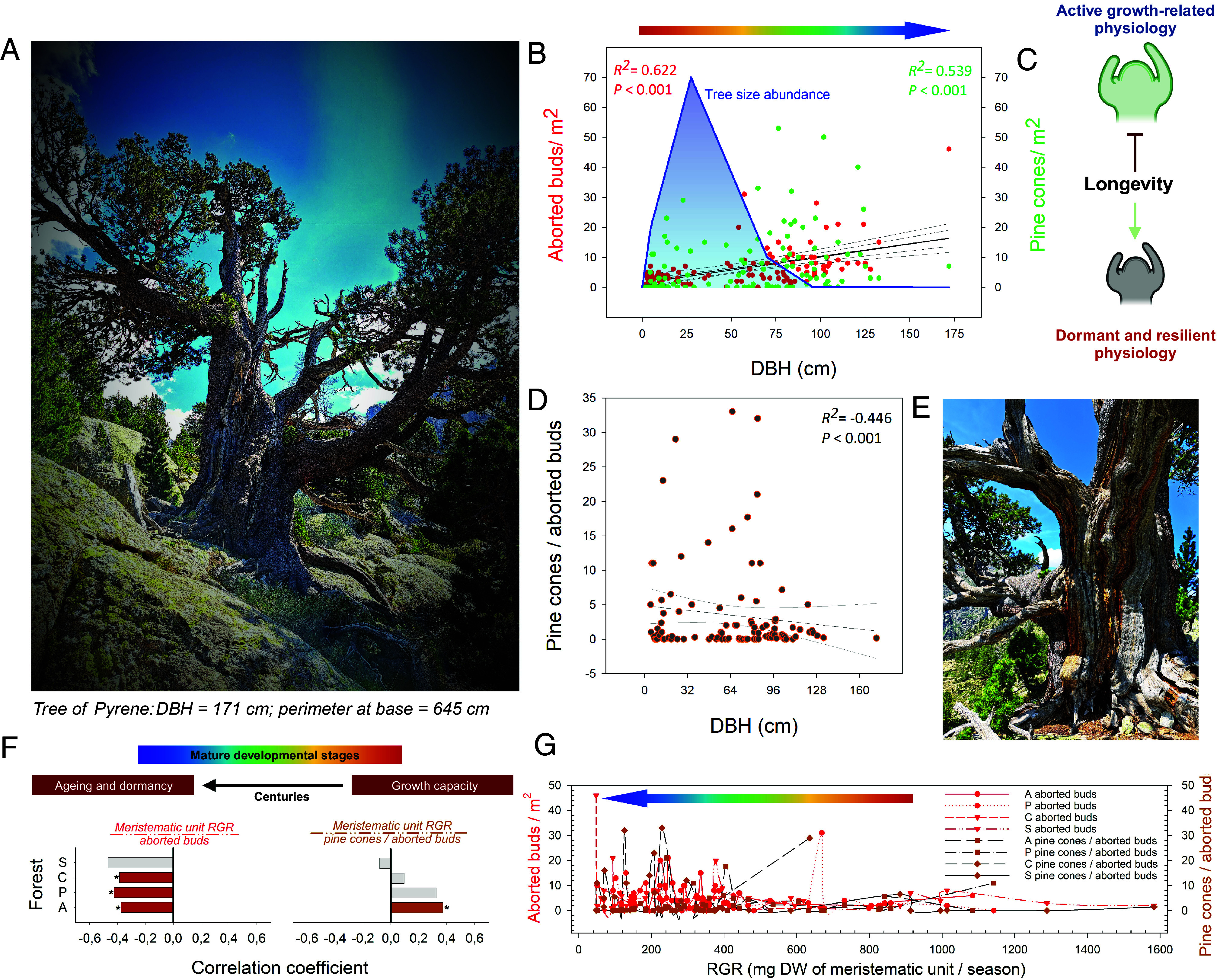
Growth/dormancy dynamics throughout the lifespan of long-living trees. (A and E) Images of the Tree of Pyrene, the tree with the largest DBH described to date for this species, which was identified during our study. (B and D) Linear correlations between the DBH and the dormancy-related physiological and aging traits. The number of aborted buds/m2 and the number of pine cones/m2 positively correlated with the tree DBH (B, red, corresponding to aborted buds: N = 136 studied trees; green, corresponding to pine cones: N = 136 studied trees). The pine cone/aborted bud ratio negatively correlated with the tree DBH and was established as an aging ratio for long-living trees (D, N = 136 studied trees). Aborted buds were not studied in forest F because the samplings performed in previous studies might limit the validity of the results. (C) The balance between growth and dormancy changed with tree aging, as physiological dormancy and resilience became more frequent in the last living period of extremely old trees. (F) (Left) Correlation between the RGR and the number of aborted buds in the studied forests. Asterisks indicate significant linear correlations. (F) (Right) Correlation between the RGR and the pine cone/aborted bud ratio in the studied forests. Asterisks indicate significant linear correlations. (G) Relationship between the RGR and aborted buds (red dots) and between the RGR and the pine cone/aborted bud ratio in all the studied forests. Linear correlations were set as significant with P < 0.05 and very significant with P < 0.01.
As observed in the new meristematic units, the RWC of tree needles abruptly decreased with age (SI Appendix, Fig. S2 A–C). Furthermore, the RWC of meristematic aerial tissues seemed to display a close relationship with two opposite growth-related traits: the RGR and the number of aborted buds per m2 of photosynthetic surface (SI Appendix, Fig. S2 E and F). Lower RWC values were associated with both lower RGR values and higher amounts of aborted tree buds. Hence, the meristematic water status can be used as a key trait to determine the growth capacity/incapacity to form new structures as trees age in mature forests. Furthermore, tree size influenced the differences observed between the RWC of the somatic and meristematic tissues. Most ancient and old trees showed an enhanced RWC in the somatic tissues compared to the meristematic tissues, contrary to that observed in the younger trees, which showed a higher RWC in the meristematic tissues than in the somatic ones (SI Appendix, Figs. S2 G and H and S3). Interestingly, it appeared that the meristematic tissues of the oldest trees were more physiologically stressed and less active than the meristematic tissues of the vast majority of forest trees. This can be viewed as a defined fitness adaptation to overcome the hypothetical structural constraints that higher growth rates would exert during several centuries of continuous growth. Therefore, slow growth rates are a key developmental adaptation that promotes and ensures long life expectancies in perennials, while also maintaining the meristematic tissue capacity to grow over time (35). In this context, old mountain pine trees have the capacity to act as carbon sinks despite having a lifespan exceeding several centuries. Although it has been postulated that climate change will produce growth benefits in high-mountain cold forests (16), we express a certain degree of concern for the oldest trees of these forests. Snow persistence plays a crucial role in spring metabolic reactivation and favors slow growth rates due to low soil temperatures (36). With a warmer climate, shorter snow cover duration and higher soil temperatures may favor forest growth at the expense of longevity. Thus, there might be a negative effect of enhanced growth on longevity. Increased demanding growth conditions in very old trees may result in lower stress tolerance and a reduced defense capacity, leading to higher mortality rates. This would lead to the loss of the irreplaceable ecosystem functions of these very old trees (see below). Moreover, under an extended period of current climate change conditions, the survival of ancient high-mountain trees, which mostly grow on steep rocky areas and possess exposed roots, will be negatively affected because of drought stress due to an early snowmelt, as seen in water-stressed substrates (37). The key role of roots in sustaining extreme longevities and their sensitivity to climate change should be further explored, as drought stress negatively affects root production in mature forests (38). In this work, we highlight the important role that exposed roots have in tissue water content and photosynthetic capacity in the oldest trees (SI Appendix, Fig. S4). The exposure of the main tree roots decreased the RWC of meristematic units in old and ancient trees, leading to physiological damage and a lower photosynthetic capacity. Thus, the root dynamics in ancient high-mountain trees may not tolerate prolonged drought seasons, resulting in slow but irreparable root damage that could cause mortality. Considering the monospecific presence of P. uncinata in the altitudinal range of the Pyrenean mature treeline forests, the highest mature mountain pine forests might be vulnerable to drought events (39). Furthermore, the oldest high-mountain trees, with large diameters, might be at more risk of death because of environmental factors (SI Appendix, Fig. S4).
Ancient Trees: Enhancing Stress Tolerance and Resilience Mechanisms at the Expense of Growth.
P. uncinata trees possess a great set of physiological tools to defy aging, presenting considerable phenotypic plasticity and unique evolutionary morphological adaptations (5). These physiological traits were majestically reflected in the largest and most likely oldest individual ever described for this species, the Tree of Pyrene, which was identified here and named in reference to the mythological legend of Pyrene and the creation of the Pyrenees (Fig. 2 A and E). We observed that this tree, as well as the other older and larger trees, presented higher numbers of aborted buds per m2 (Fig. 2B). Thus, as the mature old and mature ancient trees age, meristem abortion becomes more frequent. Moreover, despite showing larger numbers of pine cones per surface, the oldest trees exhibited an accentuated decrease in the ratio of cones to aborted buds. Therefore, longevity causes changes in the behavior of trees (Fig. 2 B–G). Once trees reach the mature stage, they prioritize growth and establishment over cone production, with higher growth rates usually connected to lower longevities and fewer resources invested in stress tolerance and damage prevention. These complex trade-offs between growth and defensive strategies can be affected by environmental and biogeographic variables (40). We noted that old and ancient trees, which were the nearest to natural terminal decay, showed more dormant and resilient physiologies, promoting the mechanisms for stress tolerance and resilience at the expense of growth (Fig. 2 C, F, and G). This resulted in large, twisted, and plastic modular structures, allowing a slow but continuous growth over centuries. The dormant physiologies enable the death of the whole tree to be postponed for as long as possible. Thus, we suggest that theoretically, despite being a complex random selective process, trees that reach extraordinarily advanced ages might have experienced greater stress tolerance and resilience during their younger years, presenting lower growth rates. The role of meristematic tissues is therefore decisive when considering longevity. Although very old trees maintain meristematic indeterminacy with powerful protective strategies, allowing them to cope with age-related constraints (41, 42), we should be cautious when talking about generalized senescence at the whole-plant level.
We observed that long-living trees in high-mountain mature forests presented a loss of vitality and dysfunction in the meristematic units, which may be linked to their lower hydric status resulting from longevity-related physiological constraints (Figs. 1 and 2, and SI Appendix, Figs. S2 and S4). These results indicate a correlation between the overall water status of the tree and meristematic tissue mortality, demonstrating a process of dehydration-driven decay. These higher rates of mortality were only found in mature old and ancient trees. Therefore, we hypothesize that long-living trees reach a critical RWC value in their meristematic tissues that affects the capacity of the whole tree to grow and cope with age-related physiological constraints, leading to a slow but continuous decay that culminates in the death of the whole tree. As pointed out by (43), our results support the idea that hydraulic failures in vascular tissues drive the irreversible dehydration of tissues that leads to their death, independently of their function. This would make sense in long-living trees that show a different degree of decay depending on each modular structure. Ancient trees show clear physiological deterioration that involves the meristematic water content, meristematic growth, and the abortion of meristematic tissues (Figs. 1 and 2, and SI Appendix, Figs. S2–S4). Altogether, this may be a sign of senescence at the modular level in the long-living trees showing more age-related constraints, eventually leading to the death of the different tree modules. We suggest that tree death occurs once this sequence of senescence events at the modular level interferes with and prevents growth at the organism level at any point of the life of the tree.
Irreplaceable Role of Old and Ancient Trees in the Ecosystem.
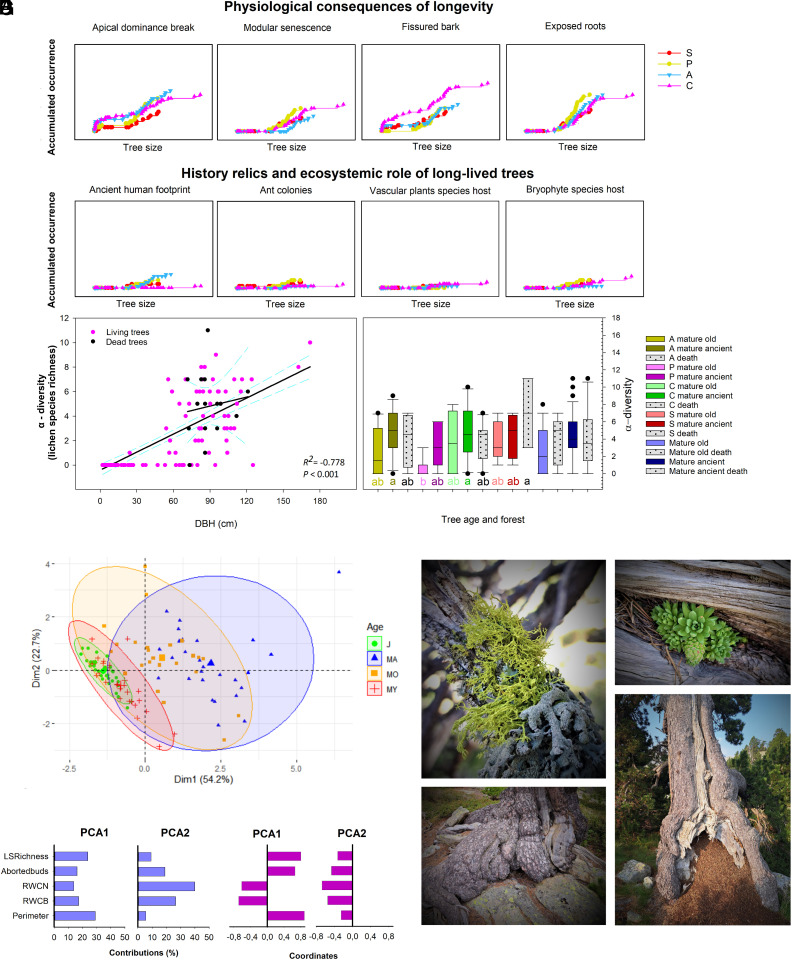
Linking the unique physiology of ancient trees to their effects on high-mountain-forest biodiversity, we observed that only ancient trees act as unique hosts of other living species (Fig. 3). Singular longevity-associated physiological traits have irreplaceable ecosystem functions in mature forests (Fig. 3 A–C), enabling the establishment of other species. We noted the occurrence and loss of apical dominance, as well as the presence of entire large dead modules, fissured or stripped bark, and exposed roots as specific aging traits in the old and ancient mature trees (Fig. 3A). This led to the presence of several ant colonies inside the main dead modules of the oldest trees as well as the occurrence of several vascular plant and bryophyte species, which were not found in most of the forest composed of younger trees (Fig. 3B). Lichen species benefited the most from the presence of both living and dead old and ancient trees (Fig. 3 C and D). Tree aging and a larger DBH were found to be essential for lichen species richness across the mature forests, although almost no differences were observed in species richness between the forests, with age having a more significant effect on α-biodiversity than climate, altitude or the treeline biogeographic pattern. We noted that ancient trees, which possessed a unique combination of heterogeneous surfaces and microhabitats formed from wear and tear over time, behaved differently from the other forest trees. In this context, the DBH and number of aborted buds strongly correlated with lichen species richness, which, along with the water status of both somatic and meristematic tissues, explained more than 75% of the observed differences in the forests (Fig. 3 E and F). Ancient trees were home to vulnerable lichen species like Letharia vulpina (Fig. 3 G, Upper left), whose presence is described here to occur preferentially in ancient trees. Among the forests and valleys studied, we observed L. vulpina growing specifically in some of the oldest ancient trees, including the Pyrene tree. This is a crucial finding when considering the rareness of extremely old trees and the associated threatened life forms. L. vulpina is a well-known common species in other continents, altitudes and latitudes; however, its distribution is extremely limited and it is almost absent from the high-mountain treeline ranges in south Europe. The existence of well-preserved habitats harboring extremely old trees is therefore mandatory to maintain extremely valuable species. Therefore, these irreplaceable roles of the oldest trees (living or dead) in mature forests are essential and it is imperative to maintain them to protect the unique ecosystems and biodiversity that would be lost without these rare trees (Fig. 3G).
Fig. 3.
Physiological and ecological consequences of attaining extraordinarily advanced ages. (A) Accumulated occurrence of longevity-related physiological traits with the increase in tree DBH. The presence of an apical dominance break, modular senescence, fissured or stripped bark, and exposed roots were longevity traits found in most of the mature old and ancient trees. (B) Accumulated occurrence of ancient human footprints and coexisting organisms with an increasing DBH. Red= forest S; yellow = forest P; blue = forest A; pink = forest C. (C) Linear correlation between tree DBH and lichen species richness (α-diversity), including long-living dead trees. Lichen species richness positively correlated with the DBH of living trees (C, N = 121 studied trees). (D) α-Diversity present in each developmental stage in living and dead mature old and ancient trees. The highest diversity values were recorded at mature ancient living trees of forests A and C, as well as in dead mature ancient trees of forest S. Differences in letters indicate significant differences in size based on one-way ANOVA (P = 0.05) and Tukey’s test comparing the effect of size and the DBH on lichen species richness between the studied forests. Bars sharing at least one letter show no significant differences between them. (E) Principal component analysis (PCA) of the variables linking tree physiological traits to the ecosystem functions of extremely old trees. Biplot representing individual trees resulting from the PCA. Colors represent the different developmental stage, while the colored ellipses represent the 95% CIs (green = juvenile trees; red = mature young trees; orange = mature old trees; and blue = mature ancient trees). (F) Bar plots for the contributions and coordinates for each variable in the PCA. LSPRichness, lichen species richness; Abortedbuds, number of aborted buds/m2; RWCN, somatic tissue RWC; RWCB, meristematic unit RWC; perimeter, DBH. (G) Photographs illustrating the irreplaceable functions of the oldest trees in the ecosystem. Upper left: a vulnerable rare lichen species, Letharia vulpina, which was described in the mature forests studied, growing only on some of the oldest ancient trees; Upper right: extremely old trees harboring vascular plants, such as Sempervivum montanum; Bottom left: complete exposure of the main roots of the oldest roots provide microhabitats and wet substrates for bryophytes and certain lichen species; Bottom right: the oldest trees of the high-mountain mature forests have faced harsh environmental conditions for several centuries, which have directly affected their morphological trunk structures, producing scars and bark crevices that some ant colonies take advantage of to create their own habitat. Linear correlations were set as significant with P < 0.05 and very significant with P < 0.01.
Limits of Tree Life and Death: The Link between Physiology and Ecology.
To explore the relationship between the degree of tree decay and lichen species dynamics, we investigated whether changes in several physiological traits in the oldest trees affected the degree of lichen coverage under cold stress and nonstress environmental conditions (SI Appendix, Figs. S5 and S6). We carried out two samplings (under snow and under nonstress conditions) in mature old and mature ancient trees in one of the studied forests to determine how decayed trees responded under stress conditions and whether lichen coverage can be a useful tool to define tree decay. The study of 37 metabolic markers in needles (somatic tissues) and buds (meristematic tissues) suggested that some markers related to stress, growth, and dormancy correlated linearly with lichen dynamics and the degree of canopy cover of old and ancient trees (SI Appendix, Fig. S5 A–E). Therefore, they could be used as markers of tree decay in similar mature forests. Higher amounts of lichen coverage (CLBP) correlated positively with the gibberellin A4 (GA4, a growth hormone) content and with the α- and γ-tocopherol content per unit of chlorophyll in the meristematic tissues under cold and nonstress conditions, respectively (SI Appendix, Fig. S5 A, D and E). On the other hand, a decrease in the contents of the photoprotective pigments (carotenoids and anthocyanins) was observed with an increased lichen presence under nonstress conditions (SI Appendix, Fig. S5 A, D, and E). Meanwhile, we found an increase in the dormancy index (the ABA/GA7 ratio) under nonstress conditions, with a similar pattern observed in the somatic tissues (SI Appendix, Fig. S5 A, D, and E). We conducted a principal component analysis (PCA) to test the relevance of the proposed markers of decay to explain the variance in our data. Overall, the first two components explained 80% of the variance, with lichen coverage and the photoprotective pigments the most relevant for component 1 and the tocopherol content per unit of chlorophyll the most relevant for component 2 (SI Appendix, Fig. S5 B and C). Therefore, we suggest that the significant variations in the stress, growth, and dormancy markers are explanatory of the level of tree decay, which could be estimated by quantifying the level of lichen coverage in these environments (SI Appendix, Fig. S5E). Thus, changes in growth and dormancy along with different stress markers are characteristic of the trees with larger amounts of lichen coverage (a marker of decay).
The line between life and death is unclear in extremely old trees with large structures that apparently can no longer sustain life. Trees vitally depend on their growth capacity and on a vast series of environmental factors as well as complex and correlated physiological traits involving growth and size. Slow growth is a well-known optimal way of maximizing growth durability and defense mechanisms. Nevertheless, even if meristematic tissues can confer a hypothetical infinite growth, size constraints can reduce growth in large old trees (44). Interestingly, existing trade-offs throughout the lifespan of trees determine tree performance and decay, which, we found, had an essential role in the ecosystem of mature forests (Fig. 4 A–D). As shown in Figs. 1–3, we observed clear correlations between age, size, growth/dormancy, and water content of newly formed tissues in the meristematic unit. Trees with higher growth rates presented a higher water content and smaller size. Meanwhile, the oldest trees of the mature forests exhibited a clear trade-off toward dormancy and resilience, explained by lower water contents, less growth, and more aborted buds (Fig. 4 A–C). Considering the complex modular structures that entail the coexistence of large amounts of dead modules alongside small living parts in the oldest and largest trees, the functions that their physiologies provide to the ecosystem are irreplaceable (Fig. 4 D, F, and G). Trees possess evolutionary tools such as phenotypic plasticity that are crucial for longevity in many ways (45). Modularity, understood as the capacity to grow and die through independent aerial and underground structures, provides the required autonomy to avoid age-related physiological deterioration and attain long life expectancies, which is crucial for the ecosystem. We observed that the heterogeneous structures of the oldest trees supported a large number of species, especially lichens, which could not be found in the younger trees of the forests (Fig. 4D). Without the set of evolutionary plastic tools that can only be obtained through a long life (Fig. 4E) and considering the importance of dead ancient trees to the ecosystem (Fig. 2), the presence of the ancient oldest trees is irreplaceable and necessary for high-mountain ecosystem dynamics. Decaying ancient and old mature trees were observed to show more developmental difficulties and increased meristem abortion as well as large lichen species richness in high-mountain mature forests (Fig. 4F). We observed that deteriorating heterogeneous structures, the combination of living and dead modules, and the presence of bark crevices and exposed roots all enhanced species richness. Thus, the growth-decay dichotomy cannot be understood without a natural decay process that, in some cases, is favored and sustained by ancient trees over decades. The role of extremely old trees with deteriorating physiologies in the ecosystem can be understood as a cyclic phenomenon that starts with the presence of long-living old and ancient trees, continues with the near-death decaying trees, and goes beyond with the death of the ancient trees (Fig. 4 E and G). This is especially important for high-mountain ecosystem preservation, as most of these forests have been disturbed, with only few remote and fragile small areas that harbor ancient trees.
Fig. 4.
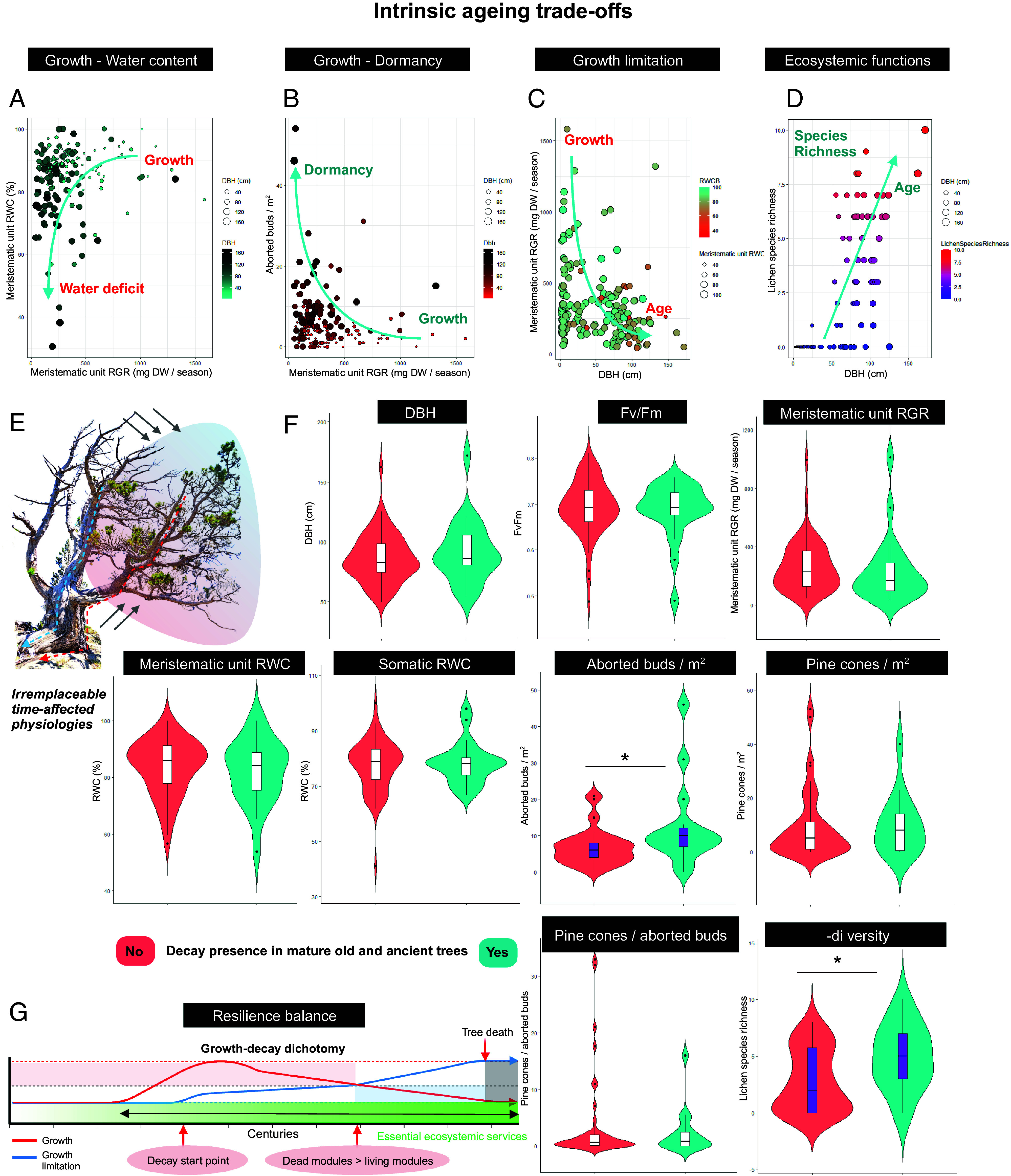
Intrinsic aging trade-offs in high-mountain mature forests. (A–D) Scatter plots showing the trade-offs among the different physiological, morphological, and ecosystem variables associated with growth capacity. (A) Trees with a higher RWC in newly formed tissues also showed a higher RGR. (B) Trees with a higher number of aborted buds/m2 showed a lower RGR during the growth season. (C and D) Size and age were found to be determining factors for decreased growth and increased lichen species richness. (A, B, and D) Dot size correlates with the tree DBH. (C) Dot size correlates with meristematic unit RWC. (E) Almost all ancient trees and most old trees have a unique ecological role in high-mountain forests due to their singular physiological traits. Long-living trees possess a vast set of dead, decaying, and living modules with different physiological properties that provide several heterogeneous microhabitats to high-mountain mature forests. In the image, the red dashed line delimits the living tree modules (red area) with the decaying modules (blue dashed line and blue area). Gray lines indicate the progress of decay. (F) Violin plots showing the differences between various physiological traits and the decay occurring in mature old and ancient trees (N = 87 old and ancient trees). Long-living trees with clear signs of structural decay showed a higher number of aborted buds/m2 (P = 0.001) and an increased lichen species α-diversity (P = 0.001). Asterisks and purple bars indicate significant differences in physiological traits between decayed and nondecayed old and ancient trees. (G) The indefectible growth-decay dichotomy delimits the maximum lifespans of trees and is determined by the extension of the modular growth capacity. Tree death results when growth limitation (blue time surface) exceeds growth capacity (red time surface) turning in a final indefectible tree death (gray surface). Essential ecosystemic services (described as green surface) increase with growth limitation and still occur during a vast extended period of time once whole tree death occurs. Linear correlations were set as significant with P < 0.05 and very significant with P < 0.01.
The Singular Spatiotemporal Scale of Whole-tree Aging, a Sustained Long-living Tool for the Environment.
Tree lifespan is highly influenced by intraspecific traits, such as size and mechanisms for stress tolerance, and external factors, such as environmental and edaphic factors (24). However, the complex basis explaining the extraordinary lifespans of the oldest trees is still far from being fully understood. In this work, we provide an essential view on the spatiotemporal dynamics of high-mountain mature forests that serve an essential function for biodiversity. In these forests, old and ancient trees play a key role as irreplaceable reservoirs for biodiversity, a function that is linked to their unequaled morphological and physiological features obtained from several centuries of nonperturbed growth (Fig. 5). The oldest trees, which are rare but sustain the integrity of mature forests, suffer indefectible physiological consequences of remaining alive for hundreds of years. Nonetheless, to a certain extent, ancient trees can take advantage of the constraints resulting from longevity to adapt and reorganize their structures so that they can extend their lifespan. Aging involves large changes in cell and tissue physiology, which can also be extrapolated at the whole-individual level. The largest and oldest trees display autonomous modules with independent vascular and growth dynamics. This results in huge, plastic, and resilient organisms that can overcome severe structural damages or extended modular senescence (SI Appendix, Figs. S1 and S4). As tree aging extends over centuries, a clear physiological deterioration occurs. At the whole-organism level, death and exposed roots increase, with hydric deficits in key tissues determining the sustainability of the growth capacity. Therefore, ancient trees are subject to fitness adaptation by overcoming their physiological limits and by deploying crucial trade-offs to favor stress resilience and dormancy over growth (Fig. 5). It is necessary to point out that the effect of the spatiotemporal scale on aging depends on intraindividual and interlocational traits. Nevertheless, organisms cannot fully defy death for millennia. Despite not being immortal, old and ancient trees in high-mountain mature forests, either alive or dead, are necessary biodiversity anchors harboring rare and vulnerable species that can even in some cases be unique to that particular ecosystem. Thus, the singular physiological traits of old and ancient trees provide unique functions to the ecosystems of some of the last remaining primary forests in Europe.
Fig. 5.
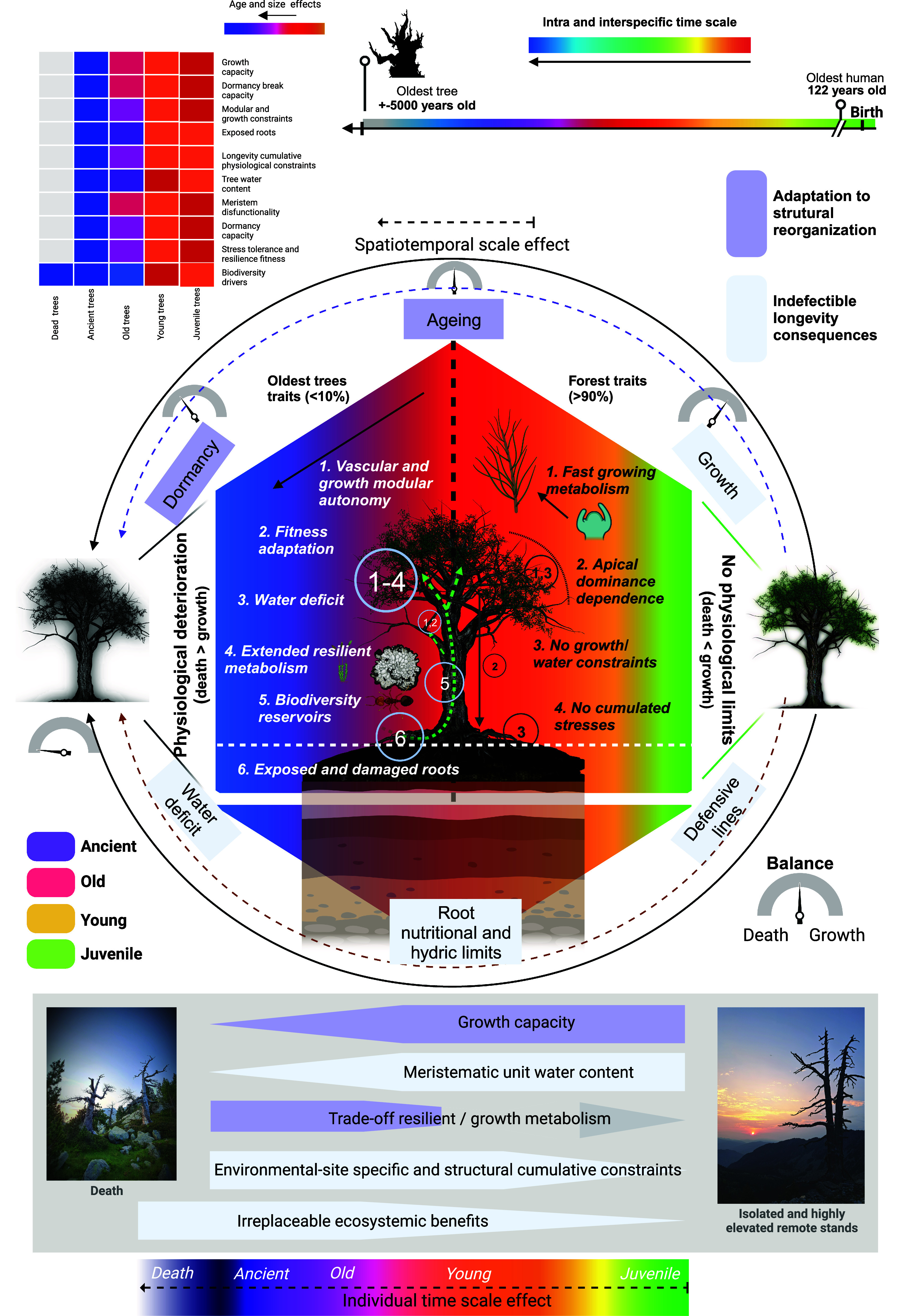
The spatiotemporal scale of tree aging and the role of longevity in high-mountain ecosystems. Longevity is characterized by a complex network of spatiotemporal traits that affect the link between the physiology of long-lived trees and their ecological functions. Most of the trees composing mature forests are extremely young compared to the oldest trees. During their juvenile and early mature developmental stages, growth is not threatened by intrinsic physiological limitations. Contrary to that, the oldest trees must adapt to the wear and tear of aging and they do so by adopting singular structures, acting as essential biodiversity anchors at high-mountain mature forests. Once trees enter to the mature old developmental stage, a slow but continuous loss of growth capacity starts with a distinct severity in each tree module. Chromatic changes reflect traits defining the time scale effect over centuries. Green surface represents juvenile traits, orange to red surface represents mature young traits, red to pink surface represents mature old traits, and purple to blue surface represents mature ancient traits, which precede death (gray surface). Both dashed and solid arrows represent time linearity of the physiological tree aging occurring over centuries. Chromatic grid summary of age and size effects toward the physiology of long-living trees and the ecosystem (at the Upper left part). The ecological benefits of the singular physiological traits of ancient trees persist with the whole tree death and define an important conservative value of high mature forests harboring extremely old trees.
Conclusion
In this work, we thoroughly disclose how ancient trees enhance stress tolerance and resilience mechanisms at the expense of growth by using a number of sophisticated morphological, biochemical and physiological adaptations. Most importantly, we found that tree longevity plays a crucial role in the conservation of biodiversity in the treeline of high-mountain forests. Linking the unique physiology of ancient trees to their effects on high-mountain-forest biodiversity, we observed that only ancient trees act as unique hosts of other living species, such as the lichen L. vulpina. It appears that singular longevity-associated physiological traits, such as loss of apical dominance, as well as the presence of entire large dead modules, fissured or stripped bark, and exposed roots have irreplaceable ecosystem functions in mature forests, enabling the establishment of other species. Lichen species benefited the most from the presence of both living and dead old and ancient trees, and a larger DBH and tree aging were found to be essential for lichen species richness across the mature forests. This study not only reveals that ancient trees display singular traits that enable them to attain extreme longevities, but it also shows that they are also unique reservoirs of biological diversity in the treeline of high-mountain ecosystems.
Materials and Methods
Study Area.
Complex climate–anthropogenic interactions have historically modulated forest dynamics in the montane, subalpine, and alpine Pyrenean belts, particularly affecting the treeline. These areas have undergone different intensities of grazing and logging depending on the valleys, the historical period, climate phases, and topographic constraints such as isolation (18, 46). The central Pyrenean treeline is dominated by mountain pine (P. uncinata Ram.) forests that represent a particular threatened belt area considered an important reservoir for species diversity (47). The conformation and treeline dynamics vary highly depending on the biogeographical and climate conditions, with low air temperatures especially crucial for determining the tree growth range (48, 49). As a result of the cessation of human activity, forests have taken over many old grasslands and the density of most of the protected areas has increased, changing the landscape and, in turn, affecting the biodiversity and ecosystem dynamics (50).
The study was conducted in several high-mountain P. uncinata mature stands covering 120 ha of protected areas, mostly from the treeline (up to 2,400 m a.s.l.), from the Aigüestortes i Estany de Sant Maurici National Park, the Alt Pirineu Natural Park, and the Cadí-Moixeró Natural Park. This vast area comprises different types of mountain soils and different valleys that have different historical, climate, and anthropogenic pressures (SI Appendix, Fig. S1). The studied mature forests were named according to their location as: A: Amitges; P: Peguera Valley; F: Ferrera Valley; C: Corticelles and Cometes Valley; S: Son Valley (Figs. 1–3 and SI Appendix, Figs. S2–S4). Due to their high elevation and isolation (one of the least densely populated areas of the Iberian Peninsula with the highest mountains), these habitats and forests are characterized by several seriously endangered species. Two of the studied forests are key habitats for two highly threatened species, the western capercaillie (Tetrao urogallus) and the Eurasian brown bear (Ursus arctos arctos).
Mature Stands and Longevity Traits.
A representative pool of six mature forests was selected to maximize the heterogeneity of tree ages and both the biogeographic and topographic traits (SI Appendix, Fig. S1). Five of the six mature stands were in mature treelines remote areas, located on rocky steep substrates in the elevated areas of isolated valleys. The studied trees included several declared monumental trees. In each studied valley, a comprehensive search of the trees with the highest number of longevity-related traits was conducted. Age estimation was undertaken according to the method in refs. 5 and 8, except for ancient trees, which were considered to be the trees with the largest DBH in each forest (SI Appendix, Materials and Methods, Mature Stands and Longevity Traits S1).
Biodiversity Characterization.
Field annotations of the qualitative and quantitative presence of vascular plants and bryophyte species were performed during field samplings. The presence of ant colonies and ancient human footprints on tree trunks (ancient shepherds were used to obtain pine resin by stripping and cutting the tree bark) was recorded as well. To determine lichen species richness, in situ observations plus detailed visual analyses of the photogrammetric data collected from the canopy of the oldest trees of the stands were used to identify the lichen genera and species. In the forest with more lichen presence, the lichen abundance characterization was performed in a total of six mature ancient trees and six mature old trees that were also sampled to perform biochemical analyses (SI Appendix, Materials and Methods, Biodiversity Characterization S1).
Growth and Physiological Traits.
Samples of somatic tissues (40 needles, at least 1 y old) and newly formed aerial shoots (3 to 5 meristematic units) were obtained from each of the studied trees at the end of the growing season (early summer). The RGR was obtained by determining the dry weight (DW) of the complete meristematic unit. The fresh (FW) and turgid (TW) weights of the meristematic unit were also recorded to obtain the RWC. Aborted buds were not considered when calculating the RWC value of meristematic units. At the same time, Fv/Fm was measured and the somatic RWC was recorded from the samples obtained from the same part of the canopy as that used to establish the RGR. Therefore, the RWC was calculated as 100 × (FW-DW)/(TW-DW). The needle mass was calculated by dividing the weight of all the needles sampled by the total number of needles (weight of total needles/ number of needles). The number of aborted buds per m2, which appeared as nongrowing dry and dark structures, was recorded, while the numbers of mature and immature pine cones from the same area were obtained. The occurrence of exposed roots was confirmed in old and ancient trees by the presence of at least one section of one main root exposing ¾ of its diametric section, while lightning damage was considered to have occurred if scars were found on one of the main trunks or branches of the trees. To determine the occurrence of an apical dominance break as well as the presence of modular senescence and fissured bark, we followed the same procedures as those in ref. 5.
Decay Markers.
At the physiological level, the contents of total chlorophyll (Chl), total carotenoid (Car), and total anthocyanin (Ant), as well as the Car/Chl and Ant/Chl ratios, and the contents of tocopherol (Vit-E) and lipid hydroperoxides (LOOH), among other markers, were used to assess the physiological stress associated with tree decay in the somatic (needle) and meristematic (bud) tissues. Growth hormones and vigor indexes were used as markers to indicate no decay, while stress-related phytohormones were used as markers of physiological decay (5). At the morphological level, the presence of modular senescence, fissured tree bark, and exposed roots were used as signs of decay.
Biochemical Analyses.
Focusing on tree decay, random terminal buds and needles from six mature ancient and six mature old mountain pine trees were collected at midday on two dates with different environmental conditions affecting tree performance: prior to the arrival of cold weather (6 October 2021) and after the very first cold and snowy weeks when there were 15 to 20 cm of accumulated snow (20 November 2021). The needles and buds from each tree were collected and immediately placed in liquid nitrogen in situ and transported to the laboratory, where they were analyzed after being stored at −80°C. All samples were collected at midday (between 12:00 and 14:00 local time). Biochemical procedures are available in SI Appendix, Materials and Methods, Biochemical Analyses S1.
Data Treatment.
All used data are provided as a supplementary archive (Dataset S1). Statistical analyses were performed using R and SigmaPlot, while graphic design was performed using SigmaPlot and BioRender.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
We thank the Aigüestortes i Estany de Sant Maurici National Park, the Alt Pirineu Natural Park, and the Cadí-Moixeró Natural Park for facilitating the research tasks inside their protected areas. We express our special gratitude to Oriol Grau. We appreciate the assistance of Antonio Gómez Bolea and Néstor Hladun for their help with lichen identification. We are also grateful to the Serveis Cientificotècnics of the University of Barcelona for their technical assistance and Michael Maudsley for English correction of the manuscript. This work was funded by the Spanish Government (Grant PID2019-10335GB-I00) and the Catalan Government (Grant 2021 SGR 00675 and ICREA Academia award to S.M.-B.).
Author contributions
O.P. and S.M.-B. designed research; O.P. performed research; S.M.-B. contributed new reagents/analytic tools; O.P. analyzed data; and O.P. and S.M.-B. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Jin W. T., et al. , Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. U.S.A. 118, e2022302118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabatini F. M., et al. , Where are Europe’s last primary forests? Divers. Distrib. 24, 1426–1439 (2018). [Google Scholar]
- 3.Ferré A., et al. , “From vegetation mapping to a prediction for landscape evolution in the catalan Pyrénées” in Colloques Phytosociologiques, XXIX, Stelvio '70 (2013). [Google Scholar]
- 4.Bäcklund S., Jönsson M., Strengbom J., Frisch A., Thor G., A pine is a pine and a spruce is a spruce—The effect of tree species and stand age on epiphytic lichen communities. PLoS ONE 11, E0147004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasques O., Munné-Bosch S., Physiological mechanisms underlying extreme longevity in mountain pine trees. Plant Physiol. 191, 974–985 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., et al. , Characteristics and intrinsic influencing factors of log humification depend on wood traits in a subalpine forest. Catena 221, 106788 (2023). [Google Scholar]
- 7.Wagner C., Schram L. J., McMullin R. T., Hunt S. L., Anand M., Lichen communities in two old-growth pine (Pinus) forests. Lichenologist 46, 697–709 (2014). [Google Scholar]
- 8.Cannon C. H., Piovesan G., Munné-Bosch S., Old and ancient trees are life history longevity winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 8, 136–145 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Fouédjeu L., et al. , The socio-ecological legacies of centuries-old charcoal making practices in a mountain forest of the northern Pyrenees. For. Ecol. Manage. 502, 119717 (2021). [Google Scholar]
- 10.Jarman S. J., Kantvilas G., Epiphytes on an old Huon pine tree (Lagarostrobos franklinii) in Tasmanian rainforest. N. Z. J. Bot. 33, 65–78 (1995). [Google Scholar]
- 11.Gilhen-Baker M., Roviello V., Beresford-Kroeger D., Roviello G. N., Old growth forests and large old trees as criticial organisms connecting ecosystems and human health: A review. Environ. Chem. Lett. 20, 1529–1538 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenmayer D. B., Laurance W. F., Franklin J. F., Global decline in large old trees. Science 338, 1305–1306 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Camarero J. J., et al. , Recent and intense dynamics in a formerly static Pyrenean treeline. Arct. Antarct. Alp. Res. 47, 773–783 (2015). [Google Scholar]
- 14.Camarero J. J., et al. , Back to the future: The responses of alpine treelines to climate warming are constrained by the current ecotone structure. Ecosystems 20, 683–700 (2017). [Google Scholar]
- 15.Galván J. D., et al. , Drought-induced weakening of growth-temperature associations in high-elevation Iberian pines. Glob. Planet Change 124, 95–106 (2015). [Google Scholar]
- 16.Granda E., et al. , Aged but withstanding: Maintenance of growth rates in old pines is not related to enhanced water-use efficiency. Agric. For. Meteorol. 242, 43–54 (2017). [Google Scholar]
- 17.Büngten U., et al. , Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat. Commun. 10, 2171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangüesa-Barreda G., et al. , Climate-human interactions contributed to historical forest recruitment dynamics in Mediterranean subalpine ecosystems. Glob. Change Biol. 26, 4988–4997 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Bade C., Jacob M., Leuschner C., Hauck M., Chemical properties of decaying wood in an old-growth spruce forest and effects on soil chemistry. Biogeochemistry 122, 1–13 (2015). [Google Scholar]
- 20.Ansaldo D., et al. , Tree decay modulates the functional response of lichen communities in Patagonian temperate forests. Sci. Total Environ. 771, 145360 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Dittrich S., et al. , Separating forest continuity from tree age effects on plant diversity in the ground and epiphyte vegetation of a Central European mountain spruce forest. Flora 208, 238–246 (2013). [Google Scholar]
- 22.Piovesan G., Cannon C. H., Liu J., Munné-Bosch S., Ancient trees: Irreplaceable conservation resource for ecosystem restoration. Trends Ecol. Evol. 37, 1025–1028 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Johnson D. J., et al. , Climate sensitive size-dependent survival in tropical trees. Nat. Ecol. Evol. 2, 1436–1442 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Groover A., “Age-related changes in tree growth and physiology” in Encyclopedia of Life Sciences (John Wiley & Sons, Ltd., Chichester, England, 2017), 10.1002/9780470015902.a0023924. [DOI]
- 25.Piovesan G., Biondi F., On tree longevity. New Phytol. 231, 1318–1337 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Piovesan G., et al. , Tree growth patterns associated with extreme longevity: Implications for the ecology and conservation of primeval trees in Mediterranean mountains. Anthropocene 26, 100199 (2019). [Google Scholar]
- 27.Munné-Bosch S., Limits to tree growth and longevity. Trends Plant Sci. 23, 985–993 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Munné-Bosch S., Long-lived trees are not immortal. Trends Plant Sci. 25, 846–849 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Flanary B. E., Kletetschka G., Analysis of telomere length and telomerase activity in tree species of various life-spans, and with age in the bristlecone pine Pinus longaeva. Biogerontology 6, 101–111 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Mencuccini M., Oñate M., Peñuelas J., Rico L., Munné-Bosch S., No signs of meristem senescence in old Scots pine. J. Ecol. 201, 555–565 (2014). [Google Scholar]
- 31.Schmid-Siegert E., et al. , Low number of fixed somatic mutations in a long-lived oak tree. Nat. Plants 3, 926–929 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Munné-Bosch S., Do perennials really senesce? Trends Plant Sci. 13, 216–220 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Liu J., et al. , Age and spatial distribution of the world’s oldest trees. Conserv. Biol. 36, E13907 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Guo W. Y., et al. , High exposure of global tree diversity to human pressure. Proc. Natl. Acad. Sci. U.S.A. 119, e2026733119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales M., Munné-Bosch S., Secret of long life lies underground. New Phytol. 205, 463–467 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Sanmiguel-Vallelado A., et al. , Snow dynamics influence tree growth by controlling soil temperature in mountain pine forests. Agric. For. Meteorol. 296, 108205 (2021). [Google Scholar]
- 37.Sanmiguel-Vallelado A., et al. , Detecting snow-related signals in radial growth of Pinus uncinata mountain forests. Dendrochronologia 57, 125622 (2019). [Google Scholar]
- 38.Zwetsloot M. J., Bauerle T. L., Repetitive seasonal drought causes substantial species-specific shifts in fine-root longevity and spatio-temporal production patterns in mature temperate forest trees. New Phytol. 231, 974–986 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Grossiord C., Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytol. 228, 42–49 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Vázquez-González C., Sampedro L., Rozas V., Zas R., Climate drives intraspecific differentiation in the expression of growth-defence trade-offs in a long-lived pine species. Sci. Rep. 10, 10584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentz B. J., Hood S. M., Hansen E. M., Vandygriff J. C., Mock K. E., Defense traits in the long-lived Great Basin bristlecone pine and resistance to the native herbivore mountain pine beetle. New Phytol. 213, 611–624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., et al. , Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. U.S.A. 117, 2201–2210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantova M., Herbette S., Cochard H., Torres-Ruiz J. M., Hydraulic failure and tree mortality: From correlation to causation. Trends Plant Sci. 27, 335–345 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Peñuelas J., A big issue for trees. Nature 437, 965–966 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Borges R. M., Phenotypic plasticity and longevity in plants and animals: Cause and effect? J. Biosci. 34, 605–611 (2009). [DOI] [PubMed] [Google Scholar]
- 46.García-Ruiz J. M., et al. , Transhumance and long-term deforestation in the subalpine belt of the central Pyrenees: An interdisciplinary approach. Catena 195, 104744 (2020). [Google Scholar]
- 47.Wielgolaski F. E., Hofgaard A., Holtmeier F. K., Sensitivity to environmental change of the treeline ecotone and its associated biodiversity in European mountains. Clim. Res. 73, 151–166 (2017). [Google Scholar]
- 48.Feuillet T., et al. , Spatial dynamics of alpine tree lines under global warning: What explains the mismatch between tree densification and elevational upward shifts at the tree line ecotone? J. Biogeogr. 47, 1056–1068 (2020). [Google Scholar]
- 49.Leunda M., et al. , Ice cave reveals environmental forcing of long-term Pyrenean tree line dynamics. J. Ecol. 107, 814–828 (2019). [Google Scholar]
- 50.Ameztegui A., et al. , Forest expansion in mountain protected areas: Trends and consequences for the landscape. Landsc. Urban Plan. 216, 104240 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All study data are included in the article and/or supporting information.