Abstract
Microbial fuel cells (MFCs) are promising for generating renewable energy from organic matter and efficient wastewater treatment. Ensuring their practical viability requires meticulous optimization and precise design. Among the critical components of MFCs, the membrane separator plays a pivotal role in segregating the anode and cathode chambers. Recent investigations have shed light on the potential benefits of membrane-less MFCs in enhancing power generation. However, it is crucial to recognize that such configurations can adversely impact the electrocatalytic activity of anode microorganisms due to increased substrate and oxygen penetration, leading to decreased coulombic efficiency. Therefore, when selecting a membrane for MFCs, it is essential to consider key factors such as internal resistance, substrate loss, biofouling, and oxygen diffusion. Addressing these considerations carefully allows researchers to advance the performance and efficiency of MFCs, facilitating their practical application in sustainable energy production and wastewater treatment. Accelerated substrate penetration could also lead to cathode clogging and bacterial inactivation, reducing the MFC's efficiency. Overall, the design and optimization of MFCs, including the selection and use of membranes, are vital for their practical application in renewable energy generation and wastewater treatment. Further research is necessary to overcome the challenges of MFCs without a membrane and to develop improved membrane materials for MFCs. This review article aims to compile comprehensive information about all constituents of the microbial fuel cell, providing practical insights for researchers examining various variables in microbial fuel cell research.
Keywords: Microbial fuel cell, Microorganism, Electrogenesis, Electrochemical process, Electricity production
1. Introduction
Microbial fuel cells (MFCs) are devices harnessing the catabolic activity of microorganisms to generate electricity from organic materials. Safeguarding freshwater resources is essential to meet the growing needs of communities, especially amid the water crisis and resource scarcity. Both industrial and domestic wastes harbor a concealed reservoir of water and energy. Domestic wastewater and wastewater from food processing industries in the United States alone are estimated to contain approximately 17 GW of energy [1]. The increasing global population and industrialization have resulted in challenges concerning worldwide access to water and energy resources. [2], Kober et al. (2019) highlighted that the increasing population and industrialization are causing challenges in ensuring global access to water and energy resources [3]. The increasing demand for energy and decreased fossil fuel supply could result in a global energy crisis with significant environmental and human health impacts [4,5]. Fossil fuels, nuclear energy, and renewable energy are the main energy sources [6]. Lee et al. (2014) noted a recent upswing in interest regarding renewable energy systems. They underscored the urgency of addressing environmental pollution, focusing on water pollution and the associated challenges and pollutants in discharged wastewater [7]. Gupta et al. (2021) highlighted that freshwater availability could decrease by up to 40% in the next decade [8]. Nie et al. (2020) emphasized that the advancement and integration of new technologies for environmentally friendly bioenergy production face substantial challenges, including rising levels of greenhouse gases, societal and economic instability, and various other obstacles [9]. In summary, MFCs present a promising solution for generating electricity from organic materials. They should be considered part of a larger effort to address the water and energy crisis while reducing environmental pollution (see Table 3, Table 4, Table 5).
Table 3.
Some Exoelectrogenic archaea.
| Euryarchaeota [33] |
|---|
|
|
|
|
Table 4.
Some exoelectrogenesis eukaryotes.
| Ascomycota [33] |
|---|
|
|
|
|
Table 5.
Electrotrophic bacteria.
| Protobacterium | Firmicutes | Actinobacteria | other things |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
2. Methodology
Examining microbial fuel cells encompasses an analysis of the anode, cathode, and membrane. Table 1 provides an overview of various batteries constructed using these materials. Subsequently, a brief exploration of energy storage in microbial fuel cells, specifically focusing on Vespa's microorganism, a pivotal component in these cells. The discussion delves into electrogenesis and electrotrophic microorganisms, spanning bacteria, archaea, eukaryotes, and electrotrophs, particularly within the context of electrogenesis. The study also extends to the coexistence and utilization of multiple microorganisms within the cell (see Table 6) (see Table 2).
Table 1.
Some investigated microbial fuel cells of different sizes.
| Anode volume (L) | Structure of MFC | type of action | Andes type | Cathode type | Cathode catalyst material | Type of incoming effluent | Input rate COD (kg/m^3(day)) | Removal percentage COD(%) | power per unit area of the cathode)mW. m−2 ( | Power per unit volume)mW.m−2 ( | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.9 | Cylindrical multi-anode-cathode | Closed and continuous | Carbon cloth a and Carbon cloth b | Carbon cloth | Pt | Domestic wastewater | 4.55 | 75 %21 | 28/87 | 55/2 | [10] |
| 1.5 | Cylindrical single chamber, multi-anode-cathode | closed | Graphite rod and carbon cloth | Carbon cloth | Pt | Domestic wastewater | – | 229 | 2/1 | [11] | |
| 20 | Cylindrical single chamber, multi-anode-cathode | Continuous | Graphite rod and Carbon cloth | Carbon cloth | Pt,Cu–MnO2 orCo-MnO2 | Domestic wastewater | 0.66۰ 0.19 | 1200 | 0.9 | [12] | |
| 5.7 | Single-chamber, multi-anode-cathode | Continuous | Graphite felt | Carbon cloth | Pt | Domestic wastewater | 0.43_0.32 | %(65–80) | 149 | 0/53 | [13] |
| 1.47 | Cylindrical single chamber air cathode | Continuous | Graphite felt | Carbon cloth | MnO2 | Swine wastewater c | 1.2_4.9 | %83.3 | 3.226 | 14/38 | [14] |
| 10 | Cylindrical single chamber air cathode | Continuous | Graphite felt | Carbon cloth | MnO2 | Malt wastewater | 1.06 | %87.1 | 0.097 | 6 | [15] |
| 2 | Air cathode single chamber stack | Continuous | carbon brush d | Carbon cloth | Pt and activated carbon | Domestic wastewater | %68.3 | [16] | |||
| 4 | Cylindrical single chamber air cathode | Continuous | carbon brush | Carbon cloth | Activated carbon with and without Pt | Domestic wastewater | %(65–70) | [17] | |||
| 1000 | Special mode stack | Continuous | carbon brush | Carbon cloth | Pt | Domestic wastewater | 0.014 | 14.5 | 0.116 | [18] | |
| 90 | Single chamber multi-anode-cathode stack | Continuous | carbon brush | Do not rust steel mesh | Activated carbon | Malt wastewater | 0. 27and 0.55 | %87.6 | [19] | ||
| 96 | Air cathode cylindrical stack | Continuous | carbon brush | Carbon cloth | Activated carbon | Domestic wastewater | %78.8 | 17.25 | 1.35 | [20] | |
| 1.4 | Air cathode single chamber | Closed and continuous | carbon brush | Do not rust steel mesh | Activated carbon | Domestic wastewater | [21] | ||||
| 192 | Air cathode cylindrical stack | Continuous | carbon brush | Carbon cloth | Activated carbon | Domestic wastewater | 0.31 | %37.6 | 10.74 | 0.84 | [22] |
| 18.8 | Cylindrical stack with air cathode | Continuous | carbon brush | Carbon cloth | – | Malt wastewater | 0.39_0.18 | %94.5 | 1.61 | 0.44 | [23] |
| 1.6 | Air cathode single chamber | closed | Graphite fiber with titanium core | Carbon cloth | Pt | Domestic wastewater | – | – | – | 6.8 | [23] |
| 7.5 | Two compartment stack | Continuous | carbon brush | Carbon cloth | Pt and activated carbon | Domestic wastewater | 0.31 | 83 14% | – | 2–10 | [24] |
| 20 | Two compartment stack | Continuous | Carbon cloth | Carbon cloth | Pt | Domestic wastewater | – | – | – | 11 | [25] |
| 1 | Cylindrical stack with air cathode | Continuous | Carbon cloth | Carbon cloth | Pt | Domestic wastewater | 0.34 | 43% | – | 6/5 | [26] |
| 50 | Two compartment stack | closed | Carbon brush | Carbon cloth | Pt and activated carbon | Domestic wastewater | 0.79 | 95% | – | 43.7_38.2 | [27] |
| 72 | Special mode stack - multi-anode and cathode | Closed and continuous | Granular carbon with a titanium core | Carbon cloth | – | Domestic wastewater | 1.3_5.2 | 87 %10 | 44–59 | 25.6–42.1 | [28] |
| 1 | Multi anode stack with a single chamber air cathode | Continuous | Carbon brush | Carbon cloth | Pt and activated carbon | Domestic wastewater | – | – | 96_ 318 | 3.2–10.6 | [29] |
| 1000 | Multiple anodes and cathodes | stack | Activated carbon | Activated carbon | – | Municipal sewage | – | %(70–90) | 0.42_3.64 | 7–60 | [30] |
| 1000 | Multiple anodes and cathodes | stack | Activated carbon | Activated carbon | – | Municipal wastewater | – | %(80–90) | 7.58 | 125 | [30] |
| 0.5 | two compartment MFC | closed | carbon cloth | carbon cloth | – | Vegetable oil industry wastewater | – | %80-90 | 6119 | – | [31] |
Carbon cloth.
Graphite rod.
Swine wastewater.
carbon brush.
Table 6.
Electrotrophic eukaryotes.
Table 2.
List of several important electrogenesis bacteria.
| Protobacteria | Firmicutes | other groups |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
Different types of microbial fuel cell architectures are elucidated in the final segment. Information on the types of microorganisms and their corresponding power densities, drawing from previous studies, is gathered in Table 7.
Table 7.
Several types of microorganisms and their power.
| power density (mW.m−2) | microorganism | Reference |
|---|---|---|
| 2096.5 ± 11.8 | Pseudomonas aeruginosa MTCC 2474 | [45] |
| 20 | Acetobacter aceti | [46] |
| 610 | Citrobacter sp. LAR-1 | [47] |
| 204.5 | Citrobacter freundii Z7 | [48] |
| 10.5 | Bacillus subtilis | [48] |
| 1606 | E.coli (DH5α) | [49] |
| 2142 | bacterias | [50] |
| 19 | Clostridium butyricum | [46] |
| 1270 ± 30 | Escherichia coli | [51] |
| 1460 | Shewanella oneidensis MR-1 | [52] |
| 42 | Enterobacter cloacae | [46] |
| 3118.9 | Escherichia coli | [53] |
| 1421 | E.coli (ATCC 11,775) | [54] |
| 2420 | Escherichia coli | [55] |
| 188 | Escherichia coli BL21 | [46] |
| 3800 | Escherichia coli DH5α | [46] |
| 530 | Geobacter sulfurreducens PCA | [46] |
| 3900 | Geobacter sulfurreducens KN400 | [46] |
| 1850 ± 20 | E.coli(K12) | [56] |
| 2143 | Mixed culture of bacteria | [57] |
| 1624 | Escherichia coli (ATCC 11,775) | [58] |
| 310 | Klebsiella aerogenes | [46] |
| 410 | Klebsiella pneumoniae | [46] |
| 92 | Lysinibacillus sphaericus D-8 | [46] |
| 85 | Lysinibacillus sphaericus VA5 | [46] |
| 1296 | Geobacter sp. | [59] |
| 1008 | Escherichia coli | [60] |
| 2668 | E.coli ATCC 25,922 | [61] |
| 89 | Ochrobactrum anthropi | [46] |
| 269 | Proteus vulgaris | [46] |
| 3000 | Shewanella putrefaciens | [46] |
| 2720 ± 60 | Rhodopseudomonas palustris DX-1 | [62] |
| 1926 ± 21.4 | Chlorella vulgaris AG30007 UTEX 0000265 | [63] |
| 39 | Pseudomonas aeruginosa KRP1 | [46] |
| 53 | Pseudomonas aeruginosa | [46] |
| 33 | Rhodoferax ferrireducens | [46] |
| 4400 | Shewanella putrefaciens | [46] |
| 760 | Candida melibiosica | [46] |
| 509 | Tolumonas osonensis | [46] |
| 20 | Haloferax volcanii | [46] |
| 119 | Natrialba magadii | [46] |
| 46 | Pyrococcus furiosus | [46] |
| 3220 | Anaeromusa spp | [64] |
3. Applications of MFC technology
3.1. Electricity production
The progress made in microbial fuel cell (MFC) technology has brought forth a pioneering method for efficiently generating bio-electricity utilizing microorganisms and biomass as renewable sources. Comparable to conventional fuel cells, MFCs consist of an anode and a cathode, often separated by a selectively permeable membrane. Sometimes, a membrane and salt bridge are employed to ensure adequate segregation. These membranes facilitate the passage of ions, while MFCs incorporate enzymatic catalysts derived from microorganisms or isolated proteins. By harnessing the metabolic capabilities of microorganisms, MFCs facilitate the direct conversion of the chemical energy stored within biomass into electrical energy. This transformative process represents a significant advancement in energy conversion technology, offering substantial potential for sustainable electricity production [1].
Research findings on electron recovery efficiency (Coulombic efficiency) have been reported with varying results. Bond and Lovely Lovely [65] Chaudhuri et al. (2003) reported an electron recovery efficiency of 95%, aligning with findings by Chaudhuri and Lovely [66]. reported 80%, and Robi et al.(2004) [67] reported 89% for the electron recovery efficiency from the substrate. Although power generation in microbial fuel cells is still deficient, very high coulombic efficiency (90%) during oxidation with platinum catalysts has also been reported. These values represent the decomposed substrate's conversion rate into electrons [67]. Potter's seminal work in 1911 is often acknowledged as the earliest successful endeavor in harnessing electric current through bacterial activity [1]. Calignano et al. (2015) articulated a fundamental concept: microorganisms dwelling within a microbial cell break down organic compounds (oxides), generating electrons. These electrons navigate through a series of respiratory enzymes within the cell, ultimately producing adenosine triphosphate (ATP) energy [68]. Subsequently, these electrons are transferred to electron acceptors, such as oxygen, facilitating the conversion of these acceptors into water through the interaction of electrons and protons. Notably, electron acceptors, including oxygen, nitrate, and sulfate, effortlessly diffuse into the microbial cell, acting as recipients for the transferred electrons [1].
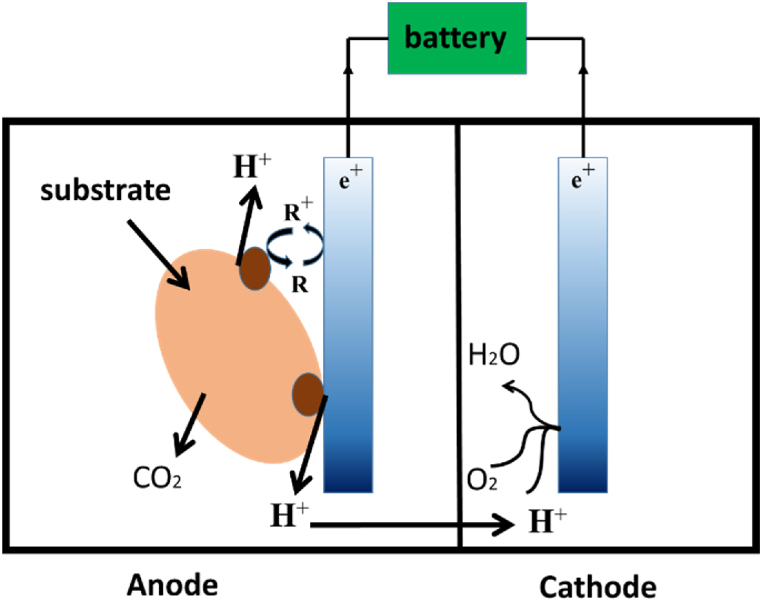
In Fig. 1, a simplified schematic of microbial fuel cells illustrates the process: microorganisms are transferred to the anode electrode, facilitating electron transfer to the external circuit. Simultaneously, H+ is released, traversing the intermediary layer to reach the cathode. Here, it combines oxygen from the existing molecule and an electron derived from the external circuit, generating water. In essence, the microbial fuel cell operates according to this mechanism.
Fig. 1.
A technique of microbial fuel cell operation.
3.2. Sewage treatment
Microbial Fuel Cells (MFCs) have surfaced as a promising wastewater and waste treatment technology. This is owing to their unique capability to generate electricity concurrently with treating effluents and residues containing organic compounds. Lu et al. (2004) highlighted that Microbial Fuel Cells (MFCs) were initially introduced for wastewater treatment back in 1991. Since then, they have demonstrated the capability to generate up to 50% of the energy needed in conventional wastewater treatment methods. The significant energy savings primarily stem from eliminating aeration and solid sludge burial, which collectively constitute half of the operational expenses in aerobic treatment procedures. Additionally, MFCs contribute to a substantial reduction of 50–90% in the production of solids for disposal, thereby positioning them as an eco-friendly alternative [67].
B.E. Logan et al. noted a significant concern in Microbial Fuel Cell (MFC) systems. The issue of substantial power degradation emerges prominently, with a noticeable decline in electricity production as the scale of these systems increases [69]. B.E. Logan et al. (2007) highlighted two strategies to address the challenge of large-scale power degradation in Microbial Fuel Cell (MFC) systems. One approach involves enlarging the reactor, but this comes with the drawback of increasing the distance between the anode and cathode. This, in turn, raises internal resistance and diminishes electricity production. Alternatively, connecting multiple MFC reactors appears to be a more viable method for constructing large-scale systems. However, it has drawbacks, including voltage return and challenging operational aspects [69]. Another constraint facing Microbial Fuel Cells (MFCs) is their limited electricity production. Despite exhibiting high power density in smaller dimensions (less than 50 ml), larger cells (exceeding 2 L) demonstrate a lower power density, typically below 30 (W/m^3) [69]. Nonetheless, MFCs are a promising technology for generating electricity and treating wastewater and waste simultaneously. Their potential to substantially cut costs in comparison to aerobic treatment processes makes MFCs an attractive prospect for the future of sustainable wastewater treatment.
3.3. Biosensors
Another application of microbial fuel cells is to use them as a sensor to analyze pollutants and control and monitor processes. The proper correlation between the coulombic efficiency of microbial fuel cells and the amount of wastewater pollution makes these cells suitable sensors for online measurement of biological oxygen concentration and demand. To accurately measure the COD value of liquid flow, it is necessary to calculate its coulombic efficiency. Recently, a microbial fuel cell with an electrical energy density of 3-kW hours per cubic meter per day has been reported. This amount of energy is suitable for feeding low-power sensors [68].
3.4. Biological recycling
The functionality of the battery in the recycling process hinges on two crucial elements: the anode and the cathode. Typically, the cathode is the primary location for recycling nitrate and uranium. The anode, composed of conductive and non-corrosive carbon material surrounding an insoluble substrate such as chitin, undergoes a gradual breakdown facilitated by bacteria. This complexity arises as bacteria employ nitrate and uranium as electron acceptors, yielding U(IV) and NO2 from gaseous NO. The gradual degradation in the anode results in a current flow toward the cathode. Consequently, nitrate and U(IV) can undergo reduction by acquiring electrons from the cathode. Additionally, the potential for chemical oxidation in the anode exists, especially concerning pollution induced by oil and gasoline [1].
3.5. Biological hydrogen
Morris, J.M. et al. (2007) conveyed that researchers have discovered the potential of utilizing microbial cells for electrolysis and hydrogen production [70]. In the typical operation of a microbial cell under standard conditions, the H+ derived from the anodic-to-cathodic reaction combines with oxygen to generate water. However, we can shift the output to produce hydrogen rather than electricity through slight modifications in the microbial cell. This generated hydrogen can find application in various ways, including as a fuel source for hydrogen fuel cells.Reimers, C.E. et al. (2001) articulated that generating hydrogen from protons and electrons produced by microbial metabolism in a microbial fuel cell faces thermodynamic challenges. Researchers have successfully introduced an external potential within the microbial fuel cell (MFC) circuit to overcome this hurdle. This strategic intervention allows the electrons and protons generated during the anodic reaction to combine within the cathode, forming hydrogen. Notably, the theoretical potential for MFC operation stands at 110 mV, significantly lower than the 1210 mV required for direct water electrolysis at a neutral pH. This shift towards hydrogen production eliminates the need for oxygen in the cathode, thereby enhancing the efficiency and practicality of MFCs. These findings underscore the potential of MFCs as a promising alternative for hydrogen generation, with reduced energy requirements compared to conventional electrolysis methods [71].
4. Types of materials used in MFC
Microbial fuel cells (MFCs) comprise three key elements: the anode, cathode, and, in certain instances, a membrane. Advances in anode technology, notably the introduction of brush electrodes and graphite fibers, mark significant progress. However, the widespread adoption of MFCs faces a notable challenge due to the elevated cost of membranes and their adverse impact on increasing internal resistance. The cathode is pivotal in MFCs, necessitating a catalyst for oxygen reduction. Recent research has uncovered promising alternatives to precious metals, exploring transition metals and other cost-effective metal compounds as viable cathode catalysts [1]. These advancements are crucial for enhancing the efficiency and lowering the cost of Microbial Fuel Cells (MFCs), ultimately facilitating their practical utilization in diverse fields, such as wastewater treatment and energy production.
4.1. Anodes
Concerning the characteristics of the anode material, key attributes include high conductivity, non-corrosiveness, a high surface-to-volume ratio (indicative of a high specific area value), high porosity, absence of deposition, cost-effectiveness, ease of manufacturing, and scalability. Notably, the paramount feature among these considerations is electrical conductivity, a distinguishing factor from other biofilm reactants. Moreover, for effective electrical connections, bacteria must adhere to the materials. Adding coatings to these materials also influences the bacteria's ability to transfer electrons to the surface through nanowires, mediators, or direct contact. Consequently, the utilization of numerous metals appears improbable.
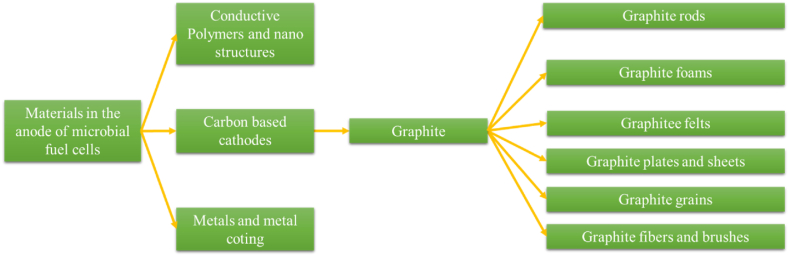
As depicted in Fig. 2, microbial fuel cells comprise three primary groups of ingredients, specifically electrodes, that are responsible for facilitating the transfer of electrons released from microorganisms to the external circuit of the cell. Carbon-based compounds have emerged as highly favored among these materials due to their exceptional efficiency. Various studies have explored the effectiveness of different structures of these graphite-based materials concerning their interaction with microorganisms in electron transfer processes.
Fig. 2.
Materials used in the anode.
Kaewkannetra. et al. (2011) highlighted the growing significance of Microbial Fuel Cells (MFCs) as an alternative energy source. This is attributed to their capacity to directly convert organic matter into electrical energy through the action of microorganisms. The anode, a critical component of MFCs, has witnessed substantial development in recent years. Notably, using graphite sheets as electrodes in MFCs has demonstrated promising outcomes, achieving a maximum power density of 1771 (mW m−2) when applied to treating cassava factory wastewater [72]. Ishii, Y. et al. (2017) highlighted the enhanced wastewater treatment achieved through the utilization of felt graphite macrostructures as vaned anodes in continuous-flow cassette electrode Microbial Fuel Cells (MFCs). This improvement is attributed to the interaction between microbes and the organic components of wastewater, facilitated by the vertical placement of blades (vanes) in the anode [73].Metal-based materials, specifically three-dimensional porous structures comprising titanium oxide nanosheets, hold promise as anode materials. This is attributed to their corrosion resistance and cost-effectiveness [[59], [74], [75]].
Zhou J. et al. (2018) noted that nitrogen-doped porous carbons have shown remarkable results, achieving an impressive maximum power density of 2777.7 (mW m−2) [76]. Hybrid composite materials like RGO/SnO2-CC have been designed to enhance the biocompatibility of the anode, yielding a power density of 1624 (mW m−2). This represents a substantial increase 4.8 times higher than that achieved with the pristine electrode [[58], [77], [78]]. Singh S. et al. (2016) conveyed that in Direct Carbon Microbial Fuel Cells (DCMFCs), electrodes crafted from nickel nanoparticles dispersed on carbon micro-nanofibers (ACFs/CNFs) exhibit significant potential, demonstrating a reported power density of 1145 (mW m−2) [51]. Zhang C. et al. (2016) highlighted the utilization of carbon felts coated with reduced graphene oxides and manganese (RGO-MnO2-CF) to enhance the efficiency of extracellular electron transfer and the adhesion of electrogenic bacteria. This approach yielded a notable power density of 2065 (mW m−2) [79].
Hou et al. (2015) noted that an anode comprised of a stainless steel composite felt and fiber (SSFF) coating on a graphite thin film has been documented to yield a power density of 2143 (mW m−2). This surpasses the performance of CC-MFC modified with graphene.
Zheng S. et al. (2015) communicated that utilizing cost-effective materials, such as the uncoated 3D stainless steel mesh with carbon black (SSM/CB), has enhanced electrical properties and microbial adhesion. This improvement translates to a higher power density of 80 ± 3215 (mW m−2) [80].
Lai, M.-F et al. (2018) reported a recent development involving a 3D laminated FZ (zinc/carbon-coated metal wire composite), demonstrating effectiveness and efficiency as an anode in Microbial Fuel Cells (MFCs). This innovative anode is associated with an electrical voltage of 291 (mV) and achieves an impressive 81% removal of Chemical Oxygen Demand (COD) [81].
In the realm of Microbial Fuel Cells (MFCs) aimed at electricity generation and wastewater treatment, Filtration composite anodes have proven to be successful. These anodes combine carbon fiber and carbon tissue, providing a distinctive filtration capability. This attribute improves mass transfer and fosters the proliferation of microbial populations, resulting in increased electricity production and enhanced removal of chemical oxygen demand (COD). Using Filtrason composite anodes in MFCs showcases their potential for efficient and effective electricity generation and wastewater treatment, offering promising avenues for future research and practical implementation [82,83].
The strides in developing anode materials collectively offer a positive trajectory for improving Microbial Fuel Cells (MFCs) efficiency and cost-effectiveness in sustainable energy generation and wastewater treatment applications. These advancements lay the foundation for notable enhancements in MFC performance, facilitating a more effective conversion of organic matter into electrical energy alongside wastewater treatment. Consequently, these developments carry significant potential for propelling the field of MFCs forward and contributing to the transition toward sustainable energy production and eco-friendly wastewater treatment systems.
4.2. Cathode
Presently, the efficiency of microbial fuel cells faces a significant obstacle rooted in the performance of the cathode, posing a formidable challenge for the widespread adoption of this technology. As a result, the design and materials utilized in the cathode have emerged as a crucial focus in microbial fuel cell research. The cathode serves as the site of the electrochemical reaction involving electrons, protons, and oxygen within a three-phase interface (solid catalyst, air, and water) [1]. Wei J. et al. (2011) emphasized that, unlike the anode, the cathode necessitates a catalyst to facilitate the chemical reaction. The commonly employed cathodes in laboratory microbial fuel cells include air-cathodes and dissolved oxygen-aqueous air-cathodes. Air-cathodes comprise a permeable layer exposed to the air, a conductive material, and, if required, a catalytic layer. Blue air cathodes are fashioned with conductive materials and, when necessary, coated with a catalytic layer [83]. Various forms of carbon, including sheets, cloth, brushes, felts, granular activated carbon, and graphite in plate and granular forms, as well as stainless steel mesh, have been employed as cathode electrodes. Stainless steel mesh is a more cost-effective alternative than carbon cloth or paper. Researchers have recently incorporated carbon nanotubes as cathodes in air-cathode microbial fuel cells to construct a three-dimensional electrode network, enhancing the specific area and catalytic reaction efficiency. This cathode type has exhibited a higher maximum power density compared to fabric carbon.
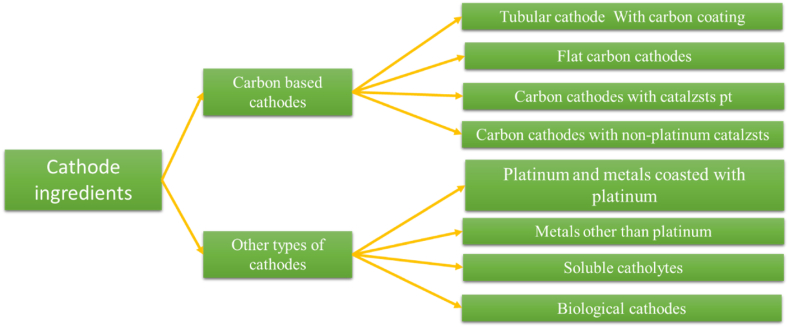
While platinum-coated cathodes remain widely used for their superior efficiency, recent studies have demonstrated that more economical catalysts, such as potassium permanganate, can offer even greater efficiency at a significantly lower cost [83]. The materials used in the cathode are illustrated in Fig. 3.
Fig. 3.
Materials used in the cathode.
In this investigation, we delved into the impact of different cathode materials on both the anode and wastewater treatment in microbial fuel cells (MFCs). Our findings underscored the significance of the oxygen reduction reaction (ORR) process in influencing the overall performance of MFCs. Oxidants such as ferricyanide and permanganate were employed to enhance the ORR process [84,85].
Furthermore, using oxygen as an oxidant in the cathode chamber has been suggested as a viable approach to enhance MFC performance during the oxygen reduction reaction (ORR) process [86,87]. Cathode is an essential factor in MFCs. While platinum-based electrodes have been used as ORR catalysts, they are associated with disadvantages such as high cost, biofouling, and surface poisoning of microorganisms [88,89]. An, J. et al. (2011) emphasized pursuing low-cost materials with elevated catalytic activity and resistance to biofouling formation, including carbon structures, metal complexes, metal oxides, and conductive polymers. These materials have been proposed as means to enhance the power output of Microbial Fuel Cells (MFCs). Additionally, the chemical activation of electrode materials, such as chemically treated graphite, has demonstrated improvements in the oxygen reduction reaction (ORR) process, leading to increased power densities [90].
Feng et al. (2012) conveyed that carbon structures have exhibited superior performance among electrode materials. For instance, heteroatom-doped carbon derived from alfalfa leaves has showcased enhanced cathodic catalytic activity and performance in comparison to conventional Pt/C cathodic catalysts [91].
Li et al. (2015) indicated that an efficient cathode, composed of polyvinylidene fluoride, carbon fiber fabric, and catalytic elements C, Mn, Fe, and O, was prepared and employed for wastewater treatment. The outcomes demonstrated high efficacy in removing COD, ammonium, and total phosphate [92]. Li et al. (2017) discussed the development of a coupled, cost-effective membrane-microbial Fuel Cell (MFC) bioreactor system featuring a poly(vinylidene fluoride) (PVDF)-coated carbon fiber fabric cathode membrane for wastewater treatment. The system demonstrated high COD and ammonium removal efficiency, effectively eliminating tetracycline hydrochloride by generating active oxidant species such as OH [93]. Chen et al. (2015) conveyed that various alternatives for anode and cathode materials have been put forth to enhance the performance and long-term operational stability of Microbial Fuel Cells (MFCs). An example includes using Ni nanoparticles dispersed on ACFs/CNFs as electrode materials for both the anode and cathode [94]. Divyapriya et al. (2017) indicated that a GO/MnO hybrid nanocomposite on carbon cloth has been employed in the cathode to activate the oxygen reduction reaction (ORR) process during wastewater treatment in Microbial Fuel Cells (MFCs). This application has led to high power densities and efficient removal of chemical oxygen demand (COD [95]. Appropriately choosing appropriate cathode materials is crucial for microbial fuel cells' performance and long-term stability (MFCs). Cost-effective and efficient alternatives have been suggested to boost power output and optimize the efficiency of wastewater treatment.
Peera et al. (2021) emphasized the crucial role of cathode catalysts as a significant component in Microbial Fuel Cells (MFCs), vital in enhancing MFC power density. The effectiveness of a cathode electrocatalyst is contingent upon its capacity to efficiently and durably facilitate the Oxygen Reduction Reaction (ORR). ORR at the cathode can occur through a direct four-electron transfer, leading to the reduction of O2 to H2O or a two-electron pathway, resulting in the formation of H2O2 as a product. The electrocatalyst's nature profoundly influences the cathode's reduction process [96].
Ter Heijne et al. (2010) added that electrocatalysts synthesized from noble metals typically exhibit a predominant four-electron process. In contrast, those derived from non-noble metals can follow either a direct four-electron route or a combined two and four-electron mechanism. An ideal ORR catalyst should possess several characteristics: i) high catalytic activity, ii) strong stability, iii) notable selectivity, iv) resilience against poisoning (particularly relevant in single-chamber MFCs), and v) cost-effectiveness. The catalytic activity of an ORR catalyst is dependent on factors such as morphology [97]. Logan et al. (2006) highlighted that the electrochemical surface area, electronic conductivity, and porosity are crucial factors influencing the stability of catalysts. The stability of a catalyst is closely linked to graphitization and interactions between metal nanoparticles and the catalyst support. An optimal catalyst should facilitate a direct four-electron reduction process. In the specific context of single-chamber Microbial Fuel Cells (MFCs), the cathode catalyst must withstand pollutants present in wastewater, such as sulfides, chlorides, alcohols, and gaseous CO2. Additionally, biofilm formation presents a challenge in single-chamber MFCs, obstructing the cathode catalyst and reducing catalytic activity by increasing the overpotential for the Oxygen Reduction Reaction (ORR). This issue is less pronounced in dual-chambered MFCs due to the direct exposure of the cathode catalyst to atmospheric air, resulting in higher efficiencies [98].
Encompassing catalyst synthesis strategies, (ORR) performance, catalyst stability, and economic considerations, various materials have been explored as efficient ORR catalysts. These include carbon-based electrocatalysts, heteroatom-doped carbon catalysts (metal-free), and transition metal-based catalysts. Additionally, we delineate the technical obstacles and future challenges in commercializing Microbial Fuel Cells (MFCs) [[99], [100], [101], [102], [103], [104]].
Carbon-based materials, independently or in conjunction with transition metals such as Fe, Co, Ni, Mn, and heteroatom-doped variants, emerge as economical electrocatalysts. They boast advantageous physical properties such as high surface area, porosity, and electronic conductivity, making them well-suited for electrochemical ORR [[105], [106], [107], [108], [109]]. Several carbon nanomaterials, including.
-
•
carbon black,
-
•
activated carbon
-
•
graphite, graphene
-
•
carbon nanotubes
-
•
carbon nanofibers
-
•
biomass-derived carbons
they have been investigated as potential cathode catalysts.
4.3. Membrane
Li et al. (2011) noted that in microbial fuel cells, anodes and cathodes are physically separated by separators. However, it's worth noting that a membrane is not an absolute necessity, as water can conduct protons within the system. Research has indicated that a microbial fuel cell without a membrane can generate more power than one with a membrane (Nafion) connected to the cathode. This suggests that, in certain cases, the membrane may have the opposite effect on power generation [110]. Despite this, selecting an appropriate membrane is crucial and should consider factors such as internal resistance, substrate loss, biofouling, and oxygen diffusion [[111], [112], [113]]. The absence of separators between the anodic and cathodic chambers enhances substrate and oxygen penetration, resulting in lower Coulombic efficiency and reduced bioelectrocatalytic activity by anode microorganisms. Nevertheless, the use of separators comes with its own limitations, such as the slow transfer of protons from the anode to the cathode, creating a pH gap that diminishes the stability and biochemical efficiency of the system. Additionally, it contributes to increased internal resistance and cost [110]. Peera et al. (2021)emphasized that in an ideal scenario, a membrane should effectively restrict the transfer of oxygen and substrate at a low cost while facilitating proton passage. However, the intricacies of microbial fuel cell systems and the limitations associated with various materials pose challenges in identifying the perfect membrane that fulfills all these criteria [96].
Significant efforts have been devoted to improving the efficiency of microbial fuel cells (MFCs), placing particular emphasis on optimizing membranes in recent research. Despite notable progress in membrane development, challenges persist, specifically concerning proton transfer limitations and the risk of oxygen leakage and penetration. These factors contribute to elevated internal resistance and reduced MFC performance, limiting their practical applicability [110]. For large-scale wastewater treatment with suspended solids, the high initial cost of the membrane, membrane clogging, and the need for replacement limit the use of microbial fuel cells. Removing the membrane and using a substitute increases microbial fuel cells' acceptability and economic feasibility [83].
While single-compartment microbial fuel cells without membranes exhibit higher power density, they encounter two significant challenges. Firstly, the permeation of substrates from the cathode reduces coulombic efficiency compared to systems using a membrane. Secondly, the absence of a membrane increases the distance between the anode and cathode, negatively impacting anaerobic microbe activities on the anode, reducing coulombic efficiency, and increasing internal resistance. Additionally, accelerated substrate penetration results in bacterial clogging and inactivation, further diminishing the efficiency of microbial fuel cells. Despite these challenges, a membrane is essential to ensure stable and effective performance in microbial fuel cells. However, it introduces its own set of problems. Developing cost-effective, novel membranes that minimize oxygen penetration without significantly impacting internal resistance or power density is crucial for sustainable membrane advancement. The efficiency of a membrane can undergo significant changes based on factors such as type, thickness, surface conditions, membrane shape, and operational conditions, including electrolyte solution composition and current density.
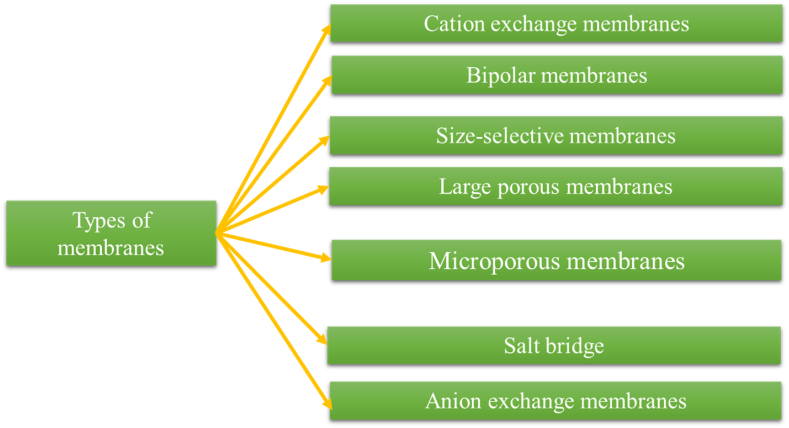
In recent years, extensive research has been conducted on different types of membranes for microbial fuel cells (MFCs). These include cation exchange membranes, anion exchange membranes, bipolar membranes, microfiltration membranes, ultrafiltration membranes, salt bridges, glass fibers, and porous fabrics. These membranes have been thoroughly studied to determine their suitability for MFC applications. Based on their purification properties, these membranes can be categorized into three primary groups: cation exchange membranes, size-selective separators, and salt bridges. Fig. 4 provides an illustrative representation of the various membrane types utilized in microbial fuel cells.
Fig. 4.
Materials used in the membrane.
Tiwari et al. (2018) discussed a recent study that explored the utilization of a cost-effective proton exchange membrane (PEM) in microbial fuel cells (MFCs) for energy generation. The membrane in question was composed of poly(vinyl alcohol) (PVA), Nafion, and Brusilicate [114]. Vickers, N.J. et al. (2017) reported on a membrane that demonstrated enhanced organic matter decomposition in wastewater, improving the rate from 54.4% to 64.25%. Another method involved synthesizing a membrane for microbial fuel cells (MFCs) using a semi-fluorinated sulfonated polytriazole with cross-linked polyvinyl alcohol. This synthesis resulted in improved control over water absorption, alterations in membrane dimensions, and enhanced oxidative stability [115].
Alternative materials like sulfonated polymers have been suggested as membrane separators in Microbial Fuel Cells (MFCs) to improve Nafion membrane performance. Furthermore, ceramics and fibers can serve as ion exchangers in MFCs. For instance, a membrane composed of natural flax fibers coated with polyvinyl acetate grafted onto polyvinylidene fluoride (PVAc-g-PVDF) has been devised for wastewater treatment [116].
Sulfonated polyether ether ketone (SPEEK) membranes have been used in MFCs to remove phenol and acetone from wastewater while generating electricity [117]. Zhao and colleagues reported that composite membranes comprising sulfonated poly(ether ether ketone) (SPEEK) and nanofillers exhibited superior performance to Nafion series membranes. Separators made from SPEEK and graphene oxide-modified SPEEK (GO-SPEEK) achieved efficiencies of 82.65% and 83.01%, respectively, for removing chemical oxygen demand (COD) [118]. In a recent study, PEM in MFCs for domestic and dairy wastewater treatment produced 55.2% more power density than Nafion 117 [119]. Polyether sulfone (PES) has also been used as a desirable PEM in MFCs due to its improved chemical and thermal properties and mechanical stability. A mixed SPES-PES membrane was prepared through solvent-free phase separation techniques, which significantly increased the antifouling performance of PEM and reduced its hardness [120]. Additionally, Fe3O4 was added to PES to create an advanced organic-mineral hybrid nanocomposite membrane [121]. Vickers et al. (2017) and colleagues highlighted using sulfonated polystyrene (SPS) and sulfonated polyvinylidene fluoride (PVDF) to enhance power density and achieve a COD removal percentage of up to 94%. Porous sulfonated PVDF membranes exhibit comparable performance to Nafion membranes, attributed to heightened sulfonation within the porous structure. This results in increased ion exchange capacity, selectivity, reduced electrical resistance, and oxygen permeability [115]. Furthermore, environmentally friendly biopolymer such as chitosan and cellulose has been successfully used as a substitute for PEM in MFCs [122]. Table 1summarizes some tested fuel cells' production power, building type, and anode and cathode.
4.4. Catalysts in microbial
In general, the buildings used in the micro fuel cell can be divided into SIX categories, which are shown in Fig. 5.
Fig. 5.
storage system.
In Table 1, each fuel cell is used to compare the power density. It is important to emphasize that the Coulombic efficiency (CE) and polarization curve provide a more accurate insight into battery performance than power density alone. Columbian efficiency quantifies the effectiveness of charge transfer in facilitating electrochemical reactions in a system using an organic substrate.
Assessing MFC performance commonly involves employing various techniques, with Polarization Studies being a key method. These studies thoroughly examine fuel cell performance, specifically regarding power generation. The current cut-off method is primarily utilized to assess the overall internal resistance of microbial fuel cells. Electrochemical Impedance Spectroscopy (EIS) analysis is instrumental in identifying different components of internal resistance in electrochemical systems, including solution, charge transfer, and diffusion. Cyclic voltage (CV) measurements are applied to investigate the oxidation or reduction mechanisms occurring at the electrode surface. Collectively, these techniques form a robust framework for evaluating fuel cells' and electrochemical systems' performance and efficiency. Additional details can be found in Appendix (A).
5. Using a storage system in MFC
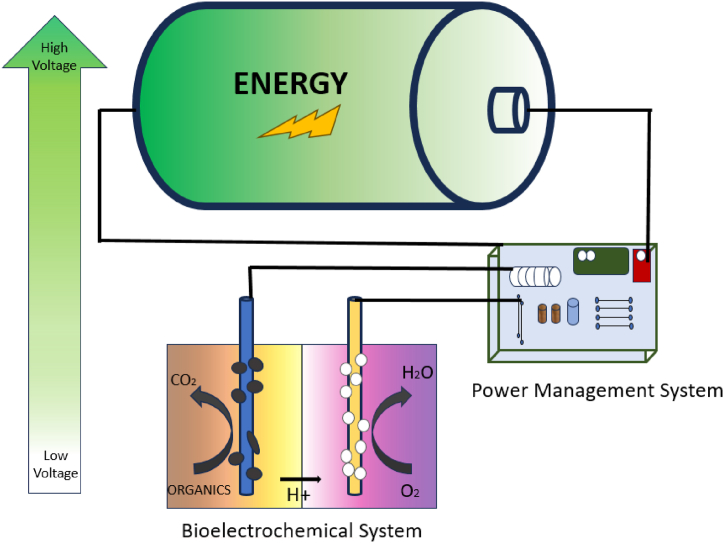
With the advancement of technology related to extracting energy from biological materials, the question arises of how to use the obtained energy optimally. The main reason for this question is the output voltage fluctuations from microbial fuel cells, which necessitates a control system to convert the fluctuating voltages into a uniform output, Figure)2–10(. Dong Y. et al. (2015) highlighted that in numerous studies, the focus has primarily been examining the efficiency of Microbial Fuel Cells (MFCs) in electricity generation. In alternative studies, the harnessed energy has been directed towards powering minimal electronic devices with low energy consumption, including wireless sensors, or utilized for charging batteries and capacitors [19].
Wang H. et al. (2015) discussed a primary challenge associated with utilizing energy from MFCs, namely the issue of low output power and voltage. This limitation hampers the efficiency and effectiveness of employing MFC-generated energy in electronic devices through standard power conversion circuits. In addressing this concern, various studies have explored strategies to boost voltage, consequently enhancing output power efficiency. These approaches include the utilization of a capacitor, pump, and step-up converter [123]. Two notable instances showcasing the effective utilization of the output energy from microbial fuel cells have been documented in Refs. [19,20]. For example, Santoro et al. (2018) [29] reported that a 90-L stack fueled with beer effluent initiated a DC pump with a 3–6 V voltage range. In one of the recent studies, a supercapacitor was employed as an energy storage source for a 28-cell stack with a volume of 1 L [29]. It can be seen in the schematic of storage in a microbial fuel cell in Fig. 5.
6. Design MFC
The design of various Microbial Fuel Cells (MFCs) has garnered significant attention due to the growing demand for environmentally friendly bioenergy production from degradable waste resources. However, the high construction cost and relatively slow microbial activity, compared to other technologies, have hindered the large-scale commercialization of this technology. MFCs employing a trielectrode arrangement are commonly employed in electrochemical analysis [124]. This arrangement comprises three electrodes: the cathode, functioning as the working electrode, typically made of glassy carbon or occasionally platinum, to accommodate the microbial consortium. The counter electrode conducts electricity, and the reference electrode, usually composed of silver or silver in potassium chloride, is employed to monitor the delivered current, often with a cyclic voltammeter [125].
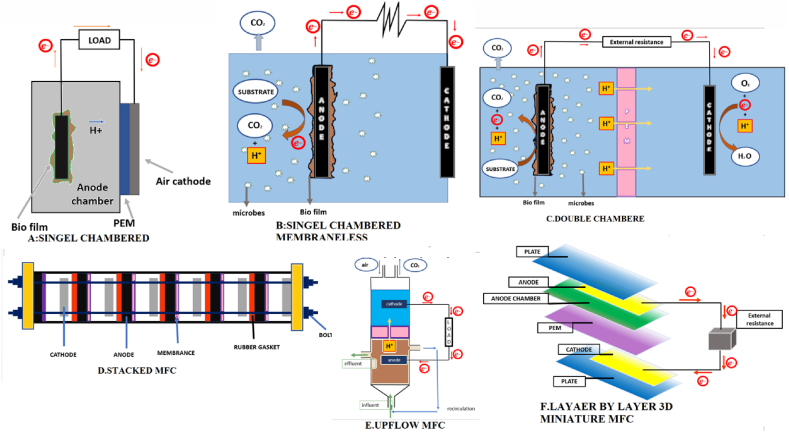
6.1. Singel chamber memberane MFC
Two-chambered Microbial Fuel Cells (MFCs) are known for their intricate design, which makes scaling up difficult. Despite this complexity, they can be operated in either batch or continuous mode. On the other hand, single-compartment MFCs offer a more straightforward and economical design. These MFCs comprise only one chamber, housing the cathode and anode components. They are called anodic chambered microbial fuel cells with an air cathode compartment. The cathode is porous and positioned on the adjacent wall of the cathodic chamber, allowing protons to move through it. Notably, in this setup, there is no proton exchange membrane.
In contrast, single-chambered membrane MFCs possess a proton exchange membrane between the anode chamber and the air cathode. This membrane-less design is similar to the single-chambered MFC but lacks the proton exchange membrane, making it simpler and potentially more cost-effective [1]. Intensive research is underway to enhance bioenergy production using innovative Microbial Fuel Cells (MFCs) approaches. Achieving commercial viability necessitates careful consideration of factors such as architectural design, cost-effectiveness, scalability, improved performance, and seamless integration with wastewater treatment technologies [125].
Focusing on system architecture, rather than microbial composition, is crucial for efficient bioenergy generation in MFC technology [98]. Mixed microbial communities have shown promising results in pollutant removal and power density compared to single-culture inoculation [126]. However, single-chambered MFC designs suffer from disadvantages like straightforward acidification due to microbial activity, which can be mitigated using buffer solutions or short-period alkaline mediation [127,128]. Researchers have explored different MFC configurations, such as single-chamber and dual-chamber systems, with varying substrate types [129]. Additionally, integrating photosynthetic microbes in one-chambered MFCs holds promise for bioenergy production with the assistance of sunlight [130].
Nishio K. et al. (2013) discussed a study that emphasized the advantages of employing co-cultures of Chlamydomonas reinhardtii and G. sulfurreducens in photosynthetic Microbial Fuel Cells (MFCs), demonstrating an integrated approach to bioenergy generation [131].
6.2. Double chamber MFC
Prathiba S. et al. (2022) discussed the operation of two-chambered Microbial Fuel Cells (MFCs) in batch mode, utilizing synthetically defined substrates like acetate or glucose for bioenergy generation. Currently, these MFCs are predominantly employed in laboratory settings. A typical two-chambered MFC, as illustrated in Fig. 6 comprises a cathodic compartment and an anodic compartment separated by a Proton Exchange Membrane (PEM) or, in some designs, a salt bridge. Bacterial communities, often containing carbon-based substances, inhabit the anodic compartment, facilitating oxidation as the primary energy source. The PEM's function is to enable proton transfer from the anodic chamber to the cathodic compartment while regulating the entry of oxygen from the cathode to the anode side. Recent advancements in MFC design have introduced various shapes, including cylindrical, rectangular, and miniature, addressing the practical needs of MFC applications [132].
Fig. 6.
General schematic of microbial fuel cell building designs.
Most two-chambered MFCs are operated in fed-batch and batch modes and can help run autonomous sensors for long-term operations in small spaces. However, a major drawback of this system is that the energy required to pump the fluid is considerably higher than the power output, making it more suitable for wastewater processing rather than its primary function as power generation. The most commonly employed and economically designed two-chambered MFCs follow an "H" form, with dual containers linked through a conduit containing a separator, usually a cation exchange membrane like Ultrex or Nafion, and sometimes a basic salt bridge [125,126].
The key advantage of the double-chambered structure over the single-chambered system lies in the ability to control cathodic compartment action through prompt oxygen supply, increased flow rate, and the addition of suitable mediators, ultimately improving MFCs' efficacy for power generation [127]. However, H-type MFCs face limitations in terms of lower bioenergy production due to high internal resistance. As a result, such MFC designs are primarily chosen for fundamental research, evaluating microbial activity, and investigating new electrode or separator materials [133]. An intriguing approach involves the implementation of photosynthetic microbes, such as blue-green algae and cyanobacteria, as biocatalysts in double-chambered MFCs, which has garnered significant attention. These photosynthetic microbes can be utilized on both the cathodic and anodic sides, offering unique possibilities for bioenergy production [134].
6.3. Upflow MFC
A study was conducted to investigate bioenergy generation using specially designed wastewater in an up-flow Microbial Fuel Cell (MFC). The system utilized flat graphite electrodes and an anion exchange membrane, operated in fed-batch and continuous modes. Fed-batch operation and various recirculation modes were explored to enhance MFC performance and mass transfer rates. It was observed that reducing hydraulic retention time led to increased bioenergy production in continuous mode [135].
Marotta M. et al. (2002) conducted a study utilizing an up-flow MFC design devoid of a membrane. The configuration featured a plexiglass cylinder with two compartments, cathode and anode chambers, each equipped with corresponding electrodes, glass wool, and beads. The feed was introduced at the base of the anode chamber, and the effluent stream continuously traversed through the cathodic chamber, as illustrated in Fig. 6. Oon Y et al. (2015) addressed the COD removal rate in their study, yet encountered a drawback of oxygen penetration from the cathode to the anodic chamber due to unfavorable reactions at the cathode side. To overcome this limitation, a hybrid structure combining an up-flow MFC model within a wetland system was devised. This integrated system aimed to fulfill both wastewater treatment and bioenergy generation objectives, ultimately achieving a notable maximum power density of 6.12 (mW m−2) [136]. Integrating MFC and constructed wetlands offers economic feasibility for wastewater treatment and bioenergy production. Up-flow MFCs possess high cell density and improved mass transfer efficiency, making them a practical option for treating discharges and organic wastes [137].
6.4. Stacked MFC
Asensio et al.(2017) conducted a study exploring the improvement of Microbial Fuel Cells (MFCs) through stacking. The findings revealed that parallel connections Fig. 6 resulted in elevated current densities and power outputs. The research affirmed the direct correlation between the removal of Chemical Oxygen Demand (COD) and bioenergy generation with the surface area of the stacked electrodes. However, a significant challenge persists in adapting MFC systems for large-scale bioenergy production with a continuous supply of wastewater [138].
Stacked MFCs can be configured with MFCs connected in series or parallel circuits. This arrangement does not affect Coulombic efficiency but enhances the battery's energy. The high current was obtained in parallel connections Fig. 6. In series connections, higher voltage was achieved in the stacked MFCs. Designing an effective microbial fuel cell is a crucial development, considering various factors such as the direction of the stack (horizontal or vertical), electrode type, reactor shape, assembly method, and modulation [127].
Gajda J. et al. (2020) explored the utilization of human urine as a substrate for microbial fuel cells, devising stacked modules, cascade modules (with a three-module configuration), and individual units employing cost-effective materials like ceramics. The outcomes showcased maximum power outputs of 21.4 mW, 75 mW, and 1.56 mW from the respective units or modules over one year and seven months. Specific units demonstrated distinct advantages, such as higher Chemical Oxygen Demand (COD) removal in the three-module system and lower power loss compared to membrane modules (conventional modules), emphasizing the suitability of stacked modules with ceramic membranes as separators [139].
Proposed methods to suppress and control voltage reversal in stacked MFC models by balancing system kinetics using developed electroactive microbes or altering the circuit mode [140].
Junyeong An et al. (2015) researched stacked Microbial Fuel Cells (MFCs) and identified that voltage reversal is effectively regulated by implementing a threshold resistance. Ensuring that the current density remains below the critical value [141].
6.5. Lazer bz lazer 3D miniature MFC
Wang et al. (2013) highlighted a recent shift in research focus toward micro-designed Microbial Fuel Cells (MFCs) owing to their significant potential for bioenergy generation. Micro-designed MFCs present several advantages, such as enhanced surface area, reduced electrode distance, and faster reaction times [142]. Di Lorenzo et al. (2016) illustrated this with a study utilizing small-scale, single-chambered air cathode Microbial Fuel Cells (MFCs) created through rapid prototyping and 3D printing [143]. Chouler et al. (2016) [144] developed an effective small-scale MFC with an innovative air cathode design, layer-by-layer arrangement, and inlet and outlet holes, utilizing urine as a substrate for power generation. They found that increasing the electrode length in these miniature MFCs resulted in higher power density. Developing miniature MFCs with volumes of a few milliliters necessitates crafting micro-scale counterparts of MFC components. The chambers of micro-scale MFCs are crafted using diverse carbon materials such as graphite felt, microsized reticulated vitreous carbon, and plastic tubes or sheets as electrodes. These micro-designed MFCs hold significance for powering self-sustained sensor devices in restricted spaces and can be customized to various commercial shapes [145,146]. Yang et al. (2016) highlighted that compact MFCs equipped with 3D electrodes demonstrate a heightened reaction rate attributed to the augmented surface area-to-volume ratio and improved mass transfer rate [147]. However, smaller MFCs present challenges, such as higher resistance and limited proton transfer through commercial Proton Exchange Membranes. To overcome these obstacles, researchers are exploring improved electrode designs that provide more contact surfaces, particularly suitable for more viscous media or substrates, and facilitate biofilm formation. Additionally, spacer arrangements are being considered to optimize performance [148,149].
7. Microorganisms for MFC performance
Microorganisms constitute a diverse group of living organisms, with some possessing the unique capability to produce and transfer electrons. Exoelectrogens, exemplified by iron-reducing bacteria like Geobacter sulfurreducens, can generate significant electricity at moderate temperatures. Additionally, various microorganisms, ranging from common yeasts to extreme thermophiles, can produce high current concentrations, albeit at a lower frequency than exoelectrogens. Electrotrophic microorganisms also serve as electron acceptors for innovative cathode-based reactions, although their current concentrations are generally lower than those of exoelectrogens. The wide range of microorganisms that can generate active electrons across diverse environments holds promising potential for advancing electrochemical technologies. These technologies include microbial fuel cells for electricity generation and microbial electrolysis cells for producing hydrogen or methane.
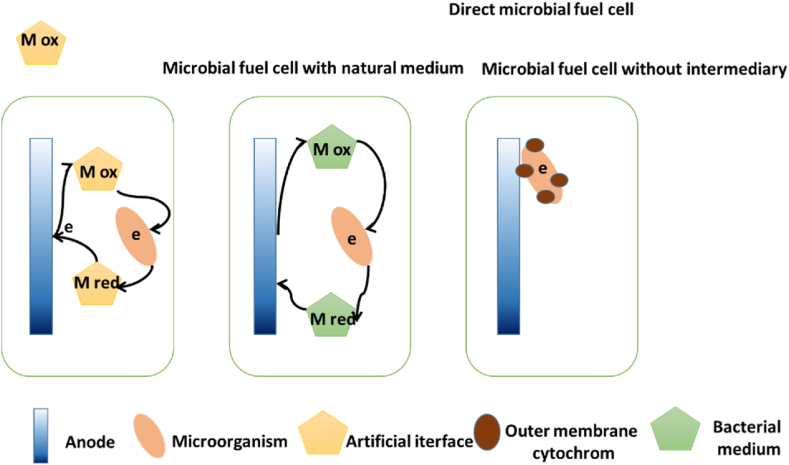
Michael Potter is widely recognized for his pioneering work in exploring electricity production by microorganisms, particularly with a focus on Escherichia coli and Saccharomyces cerevisiae. However, subsequent research has shown that these microorganisms have limited capabilities in transferring electrons to carbon electrodes over extended periods. As a result, they are not widely acknowledged as exoelectrogenic organisms. Environmental chemicals, such as those present in yeast extract containing specific B vitamins and flavins, may offer adequate electron shuttles to generate current in Potter's experiments [150]. Researchers have recently reported relatively high electricity concentrations associated with Saccharomyces cerevisiae yeast extract, but pure E. coli cultures typically generate very low currents [151,152]. The second era in microbial fuel cell (MFC) development relied on exogenous intermediates, but their toxic nature and high cost hindered practical application. The direct generation of current characterizes the third modern era of MFCs without exogenous intermediates [[152], [153], [154]].
In 1999, Kim and colleagues demonstrated the direct flow production of biosensors from pure cultures of the microorganism Shewanella. Microorganisms that can directly transfer electrons without using additional intermediaries or electronic nanowires have been of scientific interest for over a century. Modern biochemical systems focus on these microorganisms as they provide efficient energy conversion pathways for various applications [155]. These systems are currently undergoing diverse stages of advancement, employing various technologies. Among them are microbial fuel cells (MFCs) utilized for electricity generation, wastewater treatment, and detection of toxic chemicals. Furthermore, microbial electrolysis cells (MECs) electrochemically produce hydrogen or methane gas. Another technology known as microbial electrosynthesis cells (METs) can be applied for desalination or producing chemicals like hydrogen peroxide [156,157].METs employ microorganisms capable of either transferring electrons to a solid electrode (known as exoelectrogens) or extracting electrons from the electrode, like electrotrophs that receive electrons. Microorganisms employ diverse mechanisms for electron transfer, such as direct contact of cytochromes on the cell surface or the utilization of self-produced mediators like flavins. These mediators facilitate the shuttle of electrons between the cell and the anode, enabling effective electron transfer [[158], [159], [160]]. Additionally, conducting pili can transport long-range electron electrons, transferring electrons to the anode. These mechanisms have been thoroughly reviewed in recent studies [160,161], so we will not detail them here.
Koch et al. (2016) conveyed that in microbial fuel cells (MFCs), the cathode often utilizes the spontaneous reduction of oxygen to generate current. This procedure usually involves catalysts like platinum or activated carbon, although alternative materials such as cobalt, manganese, and others have also been employed [162]. Among mixed cultures, the highest current densities observed thus far have been attributed to the predominance of Deltaproteobacteria, specifically Geobacter species. Nonetheless, numerous other microorganisms demonstrate the ability to transfer electrons to the anode.
Electrons originating from the cathode play a crucial role in facilitating non-living and microbial chemical reactions, particularly in oxygen reduction. The occurrence of these reactions can vary depending on specific conditions and the nature of the chemicals involved. While some reactions are spontaneous, others necessitate an additional energy source.
For instance, in microbial electrolysis cells (MECs) equipped with a bioanode, hydrogen production can occur spontaneously if the catholyte exhibits a highly acidic pH. While any metal can function as an electron acceptor for spontaneous current generation, producing hydrogen at neutral or alkaline catholyte pH values necessitates an additional voltage input. Moreover, diverse electron acceptors, such as nitrate, sulfate, and various metals, can be cultivated at the cathode, expanding the range of potential reactions.Significant findings indicate the occurrence of direct electron transfer (DIET) between exoelectrogenic and electrotrophic microorganisms, both in controlled laboratory settings and bioreactors like anaerobic digesters. This review comprehensively encompasses the astonishing range of electroactive microorganisms, including bacteria, archaea, and eukaryotes. Noteworthy examples include Geobacter sulfurreducens, Shewanella oneidensis MR-1, and recently engineered microorganisms [163]. depicts an overview of how microorganisms transfer electrons [164].
Fig. 7 illustrates the transfer of electrons from microorganisms to the electrode. As described in the text, microorganisms can transfer electrons to the electrode surface through three mechanisms: 1) direct contact between their intracellular redox-active biomolecules and the electrode, 2) electron shuttling via two chemicals capable of redox cycling that are mobile in the electrolyte and able to collide with the electrode, and 3) production of conductive nanowires that directly connect the cell to the electrode.
Fig. 7.
Electron transfer by microorganisms.
7.1. Exo-electrogenic microorganisms
Pure culture experiments have demonstrated that microorganisms from all three domains of life, including bacteria from the Firmicutes, Actinobacteria, and Proteobacteria phyla, archaea such as the hyperthermophile Pyrococcus furiosus, and eukaryotes such as Saccharomyces cerevisiae, are exoelectrogenic [165]. Despite the growing interest in microbial fuel cells (MFCs), there is still a lack of standardized methodologies for measuring and comparing their performance, which makes it difficult to compare results across different studies. The power densities generated by MFCs are typically lower than those of conventional chemical fuel cells, and the amount of electricity generated by microorganisms can vary significantly depending on the growth conditions and experimental parameters [166].
A wide range of experimental conditions and reactor setups are used in MFC research, from simple two-chamber systems with aerated catholytes to more complex single-chamber air-cathode designs [167,168]. Using membranes and large electrode distances can further reduce the current density in MFCs, as high internal resistance limits electron production in typical two-compartment H-type cells with salt bridge-type membranes [166,167].
Exoelectrogenic bacteria, particularly Geobacter sulfurreducens, are the most commonly studied microorganisms in MFC research [169]. Under neutral pH and moderate temperature conditions in a buffered environment with carbonate or phosphate-acetate buffer, G. sulfurreducens is usually dominant when the inoculum is obtained from sediments or sewage [167,169]. However, recent studies have shown an increase in the abundance of Proteiniphilum acetatigenes and a decrease in the abundance of Geobacter spp. as MFC electricity production increases [170]. It should be noted that P. acetatigenes has not been shown to be exoelectrogenic. G. sulfurreducens KN 400 has been shown to produce one of the highest power densities, 3900 (mW m−2), in complex organic substrates such as dairy, domestic, potato, and wine juices [171].
Another model of electrogenic bacteria is Shewanella oneidensis, which can transfer electrons through direct contact of outer membrane cytochromes with a surface [[172], [173], [174]]. However, S. oneidensis is rarely abundant in MFCs due to its inability to metabolize anaerobic acetate. Escherichia coli strains have also been shown to generate power concentrations comparable to those of Geobacter or Shewanella strains (up to 3800 (mW m−2) under certain conditions [146,175]. However, the lack of purity of the tests performed on this species is a major concern in their investigation.
In conclusion, despite the progress made in MFC research, much remains to be learned about the mechanisms underlying exoelectrogenic microorganisms' electricity generation and the optimization of MFC performance. Standardized methodologies for measuring and comparing MFC performance, as well as a better understanding of the microbial ecology and diversity in MFCs, will be crucial for realizing the full potential of this promising technology.
7.2. Exoelectrogenic archaea
Archaea, a group of single-celled microorganisms, exhibit the remarkable capability of producing electricity through exoelectrogenic electron transfer. Notably, hyperthermophilic archaea can generate electrical current at elevated temperatures, while methanotrophic archaea can do so under moderate temperature conditions. However, in comparison to bacteria, the power generation and current density achieved by archaea have been less extensively documented. For instance, research has demonstrated that hyperthermophilic P. furiosus can produce 225 (mW m−2) in an H-structured fuel cell utilizing ferricyanide catholyte at a temperature of 90 °C. These findings shed light on the electrical potential of archaea and their role in electricity generation [176].
Additional hyperthermophilic microorganisms, including F. placidus and Geoglobus Ahangari [145]., have demonstrated their capability to generate significant electrical currents in microbial electrolysis cells (MECs). Specifically, F. placidus exhibits a remarkable current concentration of 680 (mA m−2) at 85 °C, while Geoglobus Ahangari achieves a current concentration of 570 (mA m−2) at 80 °C. These findings emphasize the substantial electrical potential of hyperthermophiles and their suitability for MEC applications [176].In contrast, G. sulfurreducens PCA generated 1900 (mA m−2) in the same reactors [177]. Despite these findings, the mechanism underlying archaea's exoelectrogenic electron transfer, particularly in Arci, remains largely unknown.
7.3. Exo-electrogenic eukaryotes
In addition to bacteria and archaea, some eukaryotes can produce electric currents. Fungi, especially those belonging to the Saccharomycetaceae family, such as S. cerevisiae (brewer's or baker's yeast), have been shown to possess this ability. Studies have reported power outputs ranging from 20 to 70 (mW. m−2) for S. cerevisiae in fuel cells [178]. Other species, such as Candida sp. and IR11, have also been shown to produce power outputs of 26 (mW m−2) and 21(mW.m−2), respectively [179,180]. Furthermore, an impressive power output of 720 (mW m−2) was attained by employing Candida melibiosica in conjunction with a nickel nanostructured carbon anode and a ferricyanide catholyte. This significant achievement highlights the potential of Candida melibiosica as an efficient microorganism for electricity generation, particularly when paired with advanced electrode materials and appropriate catholyte solutions [181]. Exploration of electron transport mechanisms involving the secretion of endogenous intermediates into the solution has been conducted for specific species, including Blastobotrys adeninivorans. The investigation focuses on understanding the unique pathways through which Blastobotrys adeninivorans facilitate electron transfer, shedding light on the intricate dynamics of microbial electron transport systems [182].but there is also evidence for direct electron transport from the cell surface. In contrast to bacteria and archaea, the complex structure and branched eukaryotic cells of yeasts may have contributed to the advances in using yeast as biocatalysts in MFCs. Nevertheless, additional research is imperative to unravel the intricacies of electron transport and elucidate the precise components of the yeast cell membrane implicated in exogenous electron transfer. Subsequent studies should emphasize the production of these components in the absence of yeast extract, as it would provide a more comprehensive understanding of the underlying mechanisms and enhance the practical applications of exogenous electron transport in yeast.
Also, to end the discussion about electrogenesis microorganisms, it can be mentioned that ammonia-oxidizing exoelectrogens, electroactive extremophiles, and cable bacteria are among the latest known electrogenesis microorganisms [183].
7.4. Electrotrophic microorganisms
Electrotrophs, microorganisms capable of receiving electrons from their food source, have been studied extensively for their potential in microbial batteries. Bacterial bio-cathodes, well-known for reducing oxygen in metals, have shown increased electricity production when placed in seawater beds with a carbon cathode and a magnesium alloy anode [182,184]. Cao et al. (2009) discussed electrotrophic activity in biochemical cells, noting that Geobacter metallireducens was the first to demonstrate the conversion of nitrate to nitrite in pure cultures [184]. Hongying Li et al. (2018) highlighted the emergence of the betaproteobacteria Alkaligenes faecalis as a promising candidate for in-depth investigation. While several studies have showcased the ability of biocathodes to aid in nitrate reduction, only a limited number have employed pure cultures for comprehensive analysis. Additionally, various strains of Desulfopila have shown potential in this context, prompting further exploration to unravel their specific contributions and mechanisms in nitrate reduction processes [185].and Desulfovibrio has been shown to reduce sulfate, with Desulfovibrio Pakosi capable of producing H2 gas at very negative potentials (−900 mV) [185,186].
Doyle et al. (2018) conducted a pioneering study focusing on pure cultures, utilizing Lepsiella pneumoniae to illustrate electrotrophic oxygen reduction. This groundbreaking research showcased the effective reduction of manganese oxides accumulated on the cathode surface by Lepsiella pneumoniae. Furthermore, the microorganism could reduce oxygen using stainless steel electrodes. These findings illuminate the unique electrochemical capabilities of Lepsiella pneumoniae, offering valuable insights for future investigations in this field [186].
Biocathodes have garnered significant attention in industrial food production and biofuel synthesis. By harnessing electricity, these biocathodes facilitate the reduction of carbon dioxide into complex, multi-carbon organic molecules through a process known as microbial electrosynthesis. Initial investigations unveiled the capability of acetogenic bacteria, including Spermosa and Clostridium, to accept electrons from cathodes and effectively convert carbon dioxide into acetate. These early findings highlight the potential applications of biocathodes in sustainable and efficient production processes, offering promising avenues for future research in this domain [187,188]. Pham et al. (2008) highlighted that several strains have demonstrated the production of diacetate from electric current, although the current density has generally remained relatively low (usually <10 (mA m−2). Archaea have also been explored for their potential to generate hydrogen and methane through electrochemical processes, offering valuable insights for future investigations [188].
7.5. Electricity production by cultivating several microorganisms
Cooperative electricity production holds promise by harnessing the collective potential of microorganisms to amplify electricity generation. Utilizing co-cultures of exoelectrogenic microorganisms alongside other bacterial species can synergistically enhance current generation through chemical removal or producing substrates conducive to electricity generation. This approach demonstrates the potential for enhanced and efficient electricity generation through microbial cooperation, opening avenues for further exploration and application in this field.
For instance, Geobacter sulfurens can remove oxygen in co-cultures with Escherichia coli, maintaining anaerobic conditions. In another example, co-cultures of Astobacter aesti and Gluconobacter rosaceus exhibited a distinct growth pattern. Both species, capable of producing electricity separately, are attached to the membrane through a combination of substrate and periplasmic production granules of cytochrome c and ubiquinone. Interestingly, these diverse cultures working together produced electricity at the highest rate of bed removal (140 mW m−3) compared to individual cultures. Electrogens can also utilize the breakdown products of other cells to generate currents, further enhancing cooperative electricity production. These findings suggest that microorganisms' synergistic effects can be applied in various sustainable electricity generation scenarios [189].
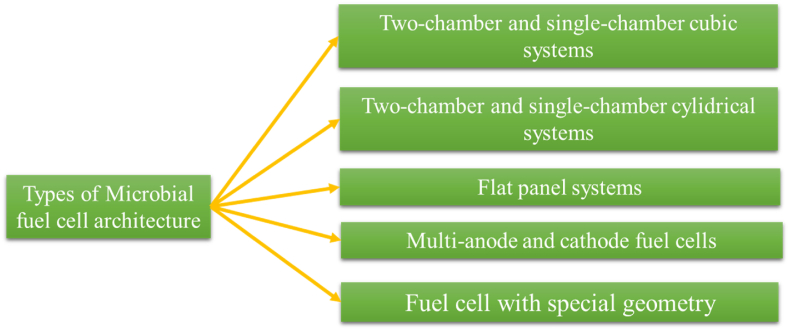
8. The architecture of fuel cells of MFC
Developing microbial fuel cells presents numerous challenges, including identifying materials and architectures that optimize coulombic power and efficiency while minimizing cost. Achieving scalability in design is also a major challenge. Various materials are used in fuel cells, and determining how to connect and arrange them in the final design and selecting the appropriate reactor architecture is crucial. Air cathodes have been the preferred choice for many researchers, as they are expected to be used in larger systems. Optimization studies have explored chemical reduction with ferricyanide or permanganate to maximize power output. However, the power produced may not be proportional to bacterial size. Two-chamber systems with high internal resistance have been used to determine the effect of specific substrates or microbial populations on production power. Still, their effect on overall microbial fuel cell performance is poorly understood. Laboratory studies have generated a variety of potential architectures based on research objectives, but a practical, efficient, cost-effective design remains elusive. Research focuses on developing scalable and economical systems, including brush electrodes, graphite fiber, and tubular cathodes. The ultimate goal is to create a microbial fuel cell design that is both high-performing and economically feasible and uses readily available materials [1].
Several designs of microbial fuel cells (MFCs) have been proposed recently, ranging from single-chamber to two-chamber configurations. Notably, single-chamber systems with an air cathode, where the electrode integrates all components into a single layer, exhibit the most practical potential for wastewater treatment applications. The simplicity of this design, coupled with its cost-effectiveness and high power production, positions it favorably. This model features a minimal distance between the cathode and anode, resulting in significantly lower internal resistance than alternative models. In this study, the objective was to compile and categorize the various components of microbial fuel cells. For this purpose, Fig. 8 delineates microbial fuel cells in terms of the constituent parts that have been examined in previous studies.
Fig. 8.
A view of the building types of microbial fuel cells.
9. Concluding remarks and future perspectives
ترجمه بیش از حد طولای است و د.
This comprehensive review article thoroughly examines the latest developments in materials, methodologies, structural innovations, and microbial components pertinent to Microbial Fuel Cell (MFC) technology. Despite the significant promise of MFCs in sustainable electricity generation and wastewater treatment, their practical application on a larger scale poses substantial challenges. In this context, this review identifies key areas for future research and outlines specific recommendations to overcome these challenges. These areas encompass materials, methodologies, structural considerations, and the utilization of microorganisms in MFC technology. By addressing these aspects, we aim to facilitate the progression of MFCs towards becoming viable and sustainable green energy solutions.
-
•Material innovations: one critical avenue for improving MFC technology involves the development of innovative materials. This review underscores the need to:
-
a.Engineer low-cost, high-performance anode materials, including metal oxides and conductive polymers, to replace expensive metals and enhance MFC efficiency.
-
b.Investigate cathode materials, such as heteroatom-doped carbons and transition metal complexes, as alternatives to costly noble metal catalysts.
-
c.Design proton exchange membranes with high conductivity are cost-effective and exhibit antifouling properties.
-
a.
-
•Methodological improvements and the refinement of methodologies are pivotal in furthering MFC technology. Our recommendations include:
-
a.Optimization of reactor configurations to mitigate internal resistance and enhance mass transfer. Notably, stacked and cascaded MFCs exhibit potential for scalability. b. Integration of MFCs into existing wastewater treatment infrastructure for simultaneous electricity generation and bioremediation. c. Development of standardized protocols to facilitate comparing results across different research studies.
-
a.
-
•Structural innovations enhance the practicality of MFCs for real-world applications; we emphasize the importance of structural innovation. This entails:
-
a.The design of compact and portable single-chamber MFCs featuring air-cathodes.
-
b.Utilizing 3D printing techniques to fabricate miniature MFCs suitable for remote sensing and robotics deployment.
-
c.The engineering of modular and stackable MFC systems tailored for large-scale electricity generation.
-
a.
-
•Harnessing Microorganisms and microbial components plays a crucial role in MFCs, and exploring their potential is vital. Recommendations in this domain include:
-
a.Exploration of cooperative microbial communities and metabolic engineering techniques to augment electricity generation.
-
b.Investigation into extremophiles and their robust bioelectrochemical systems, particularly in high-temperature MFCs.
-
c.Elucidating direct interspecies electron transfer mechanisms among electroactive microbes can significantly enhance MFC efficiency.
-
a.
In conclusion, overcoming the challenges associated with scaling up MFC technology while simultaneously improving performance and reducing costs is paramount. The suggestions delineated in this review aim to provide clear guidance for future researchers, ultimately advancing MFCs toward becoming sustainable and practical green energy solutions.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Payam Jalili: Conceptualization. Amirhosein Ala: Writing – review & editing, Writing – review & editing. Parham Nazari: Writing – review & editing, Writing – original draft. Bahram Jalili: Investigation. Davood Domiri Ganji: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Bahram Jalili, Email: b.jalili@iau-tnb.ac.ir.
Davood Domiri Ganji, Email: ddg_davood@yahoo.com.
Appendix(A).
A polarization diagram, commonly referred to as a polarization curve within the field of electrochemistry, serves as a pivotal graphical tool. Its primary purpose lies in the meticulous examination and comprehension of the electrochemical dynamics inherent to electrodes or electrochemical cells. This critical representation affords researchers and engineers a profound understanding of how the electrode's potential, also known as voltage, responds to varying current densities during electrochemical reactions.
Experimental Setup: To fashion a polarization diagram, an electrode, typically designated as the working electrode, is submersed within an electrolyte solution. Simultaneously, a reference electrode assumes the role of measuring the electrode potential, while a counter electrode completes the electrochemical cell arrangement.
Voltage versus Current Density: The crux of this experimentation revolves around the acquisition of data. This involves meticulously recording the electrode potential, quantified in volts, as a function of current density. Current density, often expressed in units such as milliamperes per square centimeter (mA/cm2), represents the magnitude of electric current traversing the electrode's surface area.
Plotting: The amassed dataset is subsequently translated into a graphical representation, with current density usually aligned along the x-axis and electrode potential (voltage) plotted on the y-axis. This graph captures the electrode potential's nuanced variations as the current density is systematically altered.
Characteristics of the Curve: The resultant curve on the graph, aptly termed the polarization curve, conventionally manifests in three distinctive regions:
-
1.
Cathodic Region: Manifesting at lower current densities, this region is characterized by a relatively stable electrode potential. It delineates the electrode's behavior during instances when reduction reactions, such as the reduction of oxygen or other chemical species, predominate.
-
2.
Transitional Region: As the current density escalates, a discernible departure from stability emerges. This transitional zone frequently harbors crucial insights into mixed electrochemical reactions.
-
3.
Anodic Region: At elevated current densities, the polarization curve exhibits a precipitous change in potential. This region underscores the electrode's response when oxidation reactions, such as the oxidation of metal ions, exert their dominance.
Analytical Utility: Within the realm of scientific inquiry, polarization curves serve as invaluable tools. They empower scientists and engineers to extract comprehensive insights into an array of electrochemical processes. Such analyses extend to the assessment of material corrosion rates, the efficiency of electrochemical reactions, and the intricate kinetics governing reactions transpiring at the electrode interface [190].
Polarization diagrams wield substantial significance across diverse scientific domains, including corrosion science, electroplating, fuel cell advancement, and battery research. These diagrams furnish essential insights into the operational dynamics of electrodes and electrochemical systems, furnishing the requisite knowledge to fine-tune and oversee these processes for multifarious applications.
The polarization curve serves as a fundamental tool for the assessment of microbial fuel cell (MFC) performance and efficiency in scientific research. By systematically measuring voltage across a range of current levels, one can deduce critical parameters such as the internal resistance, power output, and the overall electrochemical behavior of the MFC. This analytical approach empowers researchers to fine-tune MFC designs, electrode materials, and operational parameters, leading to increased energy yield from microbial processes. The meticulous examination of polarization curves is imperative for advancing the understanding and optimization of MFCs, which hold substantial potential for sustainable bioenergy generation and wastewater treatment applications in the realm of scientific exploration and innovation. Figure 9 shows an example of polarization diagram.
Fig. 9.
Power density, and polarization curves during the experiment for the OC (black lines), SMFC-1 (blue lines) and SMFC-2 (red lines). Power and density are shown for day 0 (a), day 46 (b), and day 96 (c). Polarization curves are shown for day 0 (d), day 46 (e), and day 96 (f). The dashed lines in (d–f) are the linear portion of the polarization curves used to determine the internal resistance [191].
References
- 1.Kim J.R., Zuo Y., Regan J.M., Logan B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnology and bioengineering. 2008;99(5):1120–1127. doi: 10.1002/bit.21687. [DOI] [PubMed] [Google Scholar]
- 2.Stern P.C., Sovacool B.K., Dietz T. Towards a science of climate and energy choices. Nature Climate Change. 2016;6(6):547–555. [Google Scholar]
- 3.Kober T., Schiffer H.-W., Densing M., Panos E. Global energy perspectives to 2060–WEC's world energy scenarios 2019. Energy Strategy Reviews. 2020;31 [Google Scholar]
- 4.Dincer I. Renewable energy and sustainable development: a crucial review. Renewable and sustainable energy reviews. 2000;4(2):157–175. [Google Scholar]
- 5.Rahimnejad M., et al. A novel microbial fuel cell stack for continuous production of clean energy. International journal of hydrogen energy. 2012;37(7):5992–6000. [Google Scholar]
- 6.Akdeniz F., Çağlar A., Güllü D. Recent energy investigations on fossil and alternative nonfossil resources in Turkey. Energy Conversion and Management. 2002;43(4):575–589. [Google Scholar]
- 7.Lee D.-J., Liu X., Weng H.-L. Sulfate and organic carbon removal by microbial fuel cell with sulfate-reducing bacteria and sulfide-oxidising bacteria anodic biofilm. Bioresource technology. 2014;156:14–19. doi: 10.1016/j.biortech.2013.12.129. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S., Srivastava P., Patil S.A., Yadav A.K. A comprehensive review on emerging constructed wetland coupled microbial fuel cell technology: potential applications and challenges. Bioresource Technology. 2021;320 doi: 10.1016/j.biortech.2020.124376. [DOI] [PubMed] [Google Scholar]
- 9.Nie J., et al. vol. 707. Science of the Total Environment; 2020. (Bioremediation of Water Containing Pesticides by Microalgae: Mechanisms, Methods, and Prospects for Future Research). [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre O., et al. Full-loop operation and cathodic acidification of a microbial fuel cell operated on domestic wastewater. Bioresource technology. 2011;102(10):5841–5848. doi: 10.1016/j.biortech.2011.02.098. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D., et al. Power recovery with multi-anode/cathode microbial fuel cells suitable for future large-scale applications. International Journal of Hydrogen Energy. 2010;35(16):8683–8689. [Google Scholar]
- 12.Jiang D., et al. A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. International Journal of Hydrogen Energy. 2011;36(1):876–884. [Google Scholar]
- 13.Yu J., et al. Electricity generation and microbial community in a submerged-exchangeable microbial fuel cell system for low-strength domestic wastewater treatment. Bioresource technology. 2012;117:172–179. doi: 10.1016/j.biortech.2012.04.078. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang L., et al. Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresource technology. 2012;106:82–88. doi: 10.1016/j.biortech.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang L., Yuan Y., Wang Y., Zhou S. Long-term evaluation of a 10-liter serpentine-type microbial fuel cell stack treating brewery wastewater. Bioresource Technology. 2012;123:406–412. doi: 10.1016/j.biortech.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F., et al. In situ investigation of tubular microbial fuel cells deployed in an aeration tank at a municipal wastewater treatment plant. Bioresource technology. 2013;136:316–321. doi: 10.1016/j.biortech.2013.02.107. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F., et al. Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environmental science & technology. 2013;47(9):4941–4948. doi: 10.1021/es400631r. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., et al. A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresource technology. 2014;156:132–138. doi: 10.1016/j.biortech.2013.12.104. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y., et al. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresource technology. 2015;195:66–72. doi: 10.1016/j.biortech.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Ge Z., Wu L., Zhang F., He Z. Energy extraction from a large-scale microbial fuel cell system treating municipal wastewater. Journal of Power Sources. 2015;297:260–264. [Google Scholar]
- 21.He W., et al. Microbial fuel cells with an integrated spacer and separate anode and cathode modules. Environmental Science: Water Research & Technology. 2016;2(1):186–195. [Google Scholar]
- 22.Ge Z., He Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: treatment, energy, and cost. Environmental Science: Water Research & Technology. 2016;2(2):274–281. [Google Scholar]
- 23.Lu M., et al. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. Journal of Power Sources. 2017;356:274–287. [Google Scholar]
- 24.Clauwaert P., Mulenga S., Aelterman P., Verstraete W. Litre-scale microbial fuel cells operated in a complete loop. Applied microbiology and biotechnology. 2009;83:241–247. doi: 10.1007/s00253-009-1876-0. [DOI] [PubMed] [Google Scholar]
- 25.Dekker A., et al. Analysis and improvement of a scaled-up and stacked microbial fuel cell. Environmental science & technology. 2009;43(23):9038–9042. doi: 10.1021/es901939r. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.R., et al. Increasing power recovery and organic removal efficiency using extended longitudinal tubular microbial fuel cell (MFC) reactors. Energy & Environmental Science. 2011;4(2):459–465. [Google Scholar]
- 27.Liang P., Wei J., Li M., Huang X. Scaling up a novel denitrifying microbial fuel cell with an oxic-anoxic two stage biocathode. Frontiers of Environmental Science & Engineering. 2013;7:913–919. [Google Scholar]
- 28.Wu S., et al. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water research. 2016;98:396–403. doi: 10.1016/j.watres.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Santoro C., et al. Ceramic Microbial Fuel Cells Stack: power generation in standard and supercapacitive mode. Scientific Reports. 2018;8(1):1–12. doi: 10.1038/s41598-018-21404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang P., et al. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Research. 2018;141:1–8. doi: 10.1016/j.watres.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Gupta M., Gupta H., Kharat D. Adsorption of Cu (II) by low cost adsorbents and the cost analysis. Environmental technology & innovation. 2018;10:91–101. [Google Scholar]
- 32.Jiang Y., et al. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. International Journal of Hydrogen Energy. 2013;38(8):3497–3502. [Google Scholar]
- 33.Cheng S., Xing D., Call D.F., Logan B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environmental science & technology. 2009;43(10):3953–3958. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- 34.Gregory K.B., Bond D.R., Lovley D.R. Graphite electrodes as electron donors for anaerobic respiration. Environmental microbiology. 2004;6(6):596–604. doi: 10.1111/j.1462-2920.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 35.Clauwaert P., Verstraete W. Methanogenesis in membraneless microbial electrolysis cells. Applied microbiology and biotechnology. 2009;82:829–836. doi: 10.1007/s00253-008-1796-4. [DOI] [PubMed] [Google Scholar]
- 36.Sato K., Kawaguchi H., Kobayashi H. Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. Energy Conversion and Management. 2013;66:343–350. [Google Scholar]
- 37.Jiang X., et al. Electrochemical study of enhanced nitrate removal in wastewater treatment using biofilm electrode. Bioresource technology. 2018;252:134–142. doi: 10.1016/j.biortech.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 38.Bergel A., Féron D., Mollica A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochemistry Communications. 2005;7(9):900–904. [Google Scholar]
- 39.Yates M.D., Cusick R.D., Logan B.E. Extracellular palladium nanoparticle production using Geobacter sulfurreducens. ACS Sustainable Chemistry & Engineering. 2013;1(9):1165–1171. [Google Scholar]
- 40.Dexter S., Gao G. Effect of seawater biofilms on corrosion potential and oxygen reduction of stainless steel. Corrosion. 1988;44(10):717–723. [Google Scholar]
- 41.Hasvold Ø., et al. Sea-water battery for subsea control systems. Journal of Power Sources. 1997;65(1–2):253–261. [Google Scholar]
- 42.Debuy S., Pecastaings S., Bergel A., Erable B. Oxygen-reducing biocathodes designed with pure cultures of microbial strains isolated from seawater biofilms. International Biodeterioration & Biodegradation. 2015;103:16–22. [Google Scholar]
- 43.Cordas C.M., Guerra L.T., Xavier C., Moura J.J. Electroactive biofilms of sulphate reducing bacteria. Electrochimica Acta. 2008;54(1):29–34. [Google Scholar]
- 44.Clauwaert P., et al. Biological denitrification in microbial fuel cells. Environmental science & technology. 2007;41(9):3354–3360. doi: 10.1021/es062580r. [DOI] [PubMed] [Google Scholar]
- 45.Jayapriya J., Ramamurthy V. Use of non-native phenazines to improve the performance of Pseudomonas aeruginosa MTCC 2474 catalysed fuel cells. Bioresource Technology. 2012;124:23–28. doi: 10.1016/j.biortech.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 46.Logan B.E., Rossi R., Ragab A.a., Saikaly P.E. Electroactive microorganisms in bioelectrochemical systems. Nature Reviews Microbiology. 2019;17(5):307–319. doi: 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- 47.Liu L., et al. Isolation of Fe (III)-reducing bacterium, Citrobacter sp. LAR-1, for startup of microbial fuel cell. International Journal of Hydrogen Energy. 2016;41(7):4498–4503. [Google Scholar]
- 48.Huang J., et al. Exoelectrogenic bacterium phylogenetically related to Citrobacter freundii, isolated from anodic biofilm of a microbial fuel cell. Applied biochemistry and biotechnology. 2015;175:1879–1891. doi: 10.1007/s12010-014-1418-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen X., et al. Porous carbon with defined pore size as anode of microbial fuel cell. Biosensors and Bioelectronics. 2015;69:135–141. doi: 10.1016/j.bios.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Hou J., Liu Z., Yang S., Zhou Y. Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells. Journal of Power Sources. 2014;258:204–209. [Google Scholar]
- 51.Singh S., Modi A., Verma N. Enhanced power generation using a novel polymer-coated nanoparticles dispersed-carbon micro-nanofibers-based air-cathode in a membrane-less single chamber microbial fuel cell. international journal of hydrogen energy. 2016;41(2):1237–1247. [Google Scholar]
- 52.Zhou L., et al. Isolation of a facultative anaerobic exoelectrogenic strain LZ-1 and probing electron transfer mechanism in situ by linking UV/Vis spectroscopy and electrochemistry. Biosensors and bioelectronics. 2017;90:264–268. doi: 10.1016/j.bios.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 53.Tang H., et al. Iron-embedded nitrogen doped carbon frameworks as robust catalyst for oxygen reduction reaction in microbial fuel cells. Applied Catalysis B: Environmental. 2017;202:550–556. [Google Scholar]
- 54.Mehdinia A., Ziaei E., Jabbari A. Multi-walled carbon nanotube/SnO2 nanocomposite: a novel anode material for microbial fuel cells. Electrochimica Acta. 2014;130:512–518. [Google Scholar]
- 55.Tao Y., et al. Hierarchically three-dimensional nanofiber based textile with high conductivity and biocompatibility as a microbial fuel cell anode. Environmental science & technology. 2016;50(14):7889–7895. doi: 10.1021/acs.est.6b00648. [DOI] [PubMed] [Google Scholar]
- 56.Modi A., Singh S., Verma N. In situ nitrogen-doping of nickel nanoparticle-dispersed carbon nanofiber-based electrodes: its positive effects on the performance of a microbial fuel cell. Electrochimica Acta. 2016;190:620–627. [Google Scholar]
- 57.Hou J., et al. A comparative study of graphene-coated stainless steel fiber felt and carbon cloth as anodes in MFCs. Bioprocess and biosystems engineering. 2015;38:881–888. doi: 10.1007/s00449-014-1332-0. [DOI] [PubMed] [Google Scholar]
- 58.Mehdinia A., Ziaei E., Jabbari A. Facile microwave-assisted synthesized reduced graphene oxide/tin oxide nanocomposite and using as anode material of microbial fuel cell to improve power generation. International journal of hydrogen energy. 2014;39(20):10724–10730. [Google Scholar]
- 59.Yamashita T., Yokoyama H. Molybdenum anode: a novel electrode for enhanced power generation in microbial fuel cells, identified via extensive screening of metal electrodes. Biotechnology for biofuels. 2018;11(1):1–13. doi: 10.1186/s13068-018-1046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirubaharan C.J., Santhakumar K., Senthilkumar N., Jang J.-H. Nitrogen doped graphene sheets as metal free anode catalysts for the high performance microbial fuel cells. International journal of hydrogen energy. 2015;40(38):13061–13070. [Google Scholar]
- 61.Zhang Y., et al. A graphene modified anode to improve the performance of microbial fuel cells. Journal of Power Sources. 2011;196(13):5402–5407. [Google Scholar]
- 62.Cheng S., Regan J. Electricity generation by Rhodopseudomonas palustris. Environmental Science and Technology. 2008;42(11):4146–4151. doi: 10.1021/es800312v. [DOI] [PubMed] [Google Scholar]
- 63.Cui Y., et al. Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy conversion and management. 2014;79:674–680. [Google Scholar]
- 64.Borole A.P., et al. Integrating engineering design improvements with exoelectrogen enrichment process to increase power output from microbial fuel cells. Journal of Power Sources. 2009;191(2):520–527. [Google Scholar]
- 65.Bond D.R., Lovley D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Applied and environmental microbiology. 2003;69(3):1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaudhuri S.K., Lovley D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nature biotechnology. 2003;21(10):1229–1232. doi: 10.1038/nbt867. [DOI] [PubMed] [Google Scholar]
- 67.Liu H., Logan B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environmental science & technology. 2004;38(14):4040–4046. doi: 10.1021/es0499344. [DOI] [PubMed] [Google Scholar]
- 68.Calignano F., Tommasi T., Manfredi D., Chiolerio A. Additive manufacturing of a microbial fuel cell—a detailed study. Scientific reports. 2015;5(1) doi: 10.1038/srep17373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh S.-E., Logan B.E. Voltage reversal during microbial fuel cell stack operation. Journal of Power Sources. 2007;167(1):11–17. [Google Scholar]
- 70.Morris J.M., Jin S. Feasibility of using microbial fuel cell technology for bioremediation of hydrocarbons in groundwater. Journal of Environmental Science and Health, Part A. 2007;43(1):18–23. doi: 10.1080/10934520701750389. [DOI] [PubMed] [Google Scholar]
- 71.Reimers C.E., Tender L.M., Fertig S., Wang W. Harvesting energy from the marine sediment− water interface. Environmental science & technology. 2001;35(1):192–195. doi: 10.1021/es001223s. [DOI] [PubMed] [Google Scholar]
- 72.Kaewkannetra P., Chiwes W., Chiu T. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel. 2011;90(8):2746–2750. [Google Scholar]
- 73.Ishii Y., Miyahara M., Watanabe K. Anode macrostructures influence electricity generation in microbial fuel cells for wastewater treatment. Journal of bioscience and bioengineering. 2017;123(1):91–95. doi: 10.1016/j.jbiosc.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 74.Iannaci A., et al. Power generation using a low-cost sulfated zirconium oxide based cathode in single chamber microbial fuel cells. Journal of Alloys and Compounds. 2017;693:170–176. [Google Scholar]
- 75.Yin T., et al. Preparation of vertically oriented TiO2 nanosheets modified carbon paper electrode and its enhancement to the performance of MFCs. ACS applied materials & interfaces. 2015;7(1):400–408. doi: 10.1021/am506360x. [DOI] [PubMed] [Google Scholar]
- 76.Zhou J., et al. Magnetic multi-porous bio-adsorbent modified with amino siloxane for fast removal of Pb (II) from aqueous solution. Applied Surface Science. 2018;427:976–985. [Google Scholar]
- 77.Herrero-Hernández E., Smith T., Akid R. Electricity generation from wastewaters with starch as carbon source using a mediatorless microbial fuel cell. Biosensors and Bioelectronics. 2013;39(1):194–198. doi: 10.1016/j.bios.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 78.Guo X., et al. Influence of packing material characteristics on the performance of microbial fuel cells using petroleum refinery wastewater as fuel. Renewable Energy. 2016;87:437–444. [Google Scholar]
- 79.Zhang C., et al. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosensors and Bioelectronics. 2016;81:32–38. doi: 10.1016/j.bios.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 80.Zheng S., et al. Binder-free carbon black/stainless steel mesh composite electrode for high-performance anode in microbial fuel cells. Journal of Power Sources. 2015;284:252–257. [Google Scholar]
- 81.Lai M.-F., Lou C.-W., Lin J.-H. Improve 3D electrode materials performance on electricity generation from livestock wastewater in microbial fuel cell. International Journal of Hydrogen Energy. 2018;43(25):11520–11529. [Google Scholar]
- 82.Zhou Y., et al. Development of a novel membrane‐less microbial fuel cell (ML‐MFC) with a Sandwiched Nitrifying chamber for efficient wastewater treatment. Electroanalysis. 2018;30(9):2145–2152. [Google Scholar]
- 83.Wei J., Liang P., Huang X. Recent progress in electrodes for microbial fuel cells. Bioresource technology. 2011;102(20):9335–9344. doi: 10.1016/j.biortech.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 84.Li W., et al. Characteristics of self-alkalization in high-rate denitrifying automatic circulation (DAC) reactor fed with methanol and sodium acetate. Bioresource technology. 2014;154:44–50. doi: 10.1016/j.biortech.2013.11.097. [DOI] [PubMed] [Google Scholar]
- 85.Lu M., Li S.F.Y. Cathode reactions and applications in microbial fuel cells: a review. Critical Reviews in Environmental Science and Technology. 2012;42(23):2504–2525. [Google Scholar]
- 86.Noori M.T., Ghangrekar M., Mukherjee C. V2O5 microflower decorated cathode for enhancing power generation in air-cathode microbial fuel cell treating fish market wastewater. International journal of hydrogen energy. 2016;41(5):3638–3645. [Google Scholar]
- 87.Tang H., et al. Metal–organic‐framework‐derived dual metal‐and nitrogen‐doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Advanced science. 2016;3(2) doi: 10.1002/advs.201500265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rozendal R.A., et al. Towards practical implementation of bioelectrochemical wastewater treatment. Trends in biotechnology. 2008;26(8):450–459. doi: 10.1016/j.tibtech.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Pei S., et al. Co/CoO nanoparticles/Ag nanowires/nitrogen codoped electrospun carbon nanofibers as efficient electrocatalysts for oxygen reduction. Int. J. Electrochem. Sci. 2016;11:8994–9006. [Google Scholar]
- 90.An J., Jeon H., Lee J., Chang I.S. Bifunctional silver nanoparticle cathode in microbial fuel cells for microbial growth inhibition with comparable oxygen reduction reaction activity. Environmental science & technology. 2011;45(12):5441–5446. doi: 10.1021/es2000326. [DOI] [PubMed] [Google Scholar]
- 91.Feng Y., et al. Effects of sulfide on microbial fuel cells with platinum and nitrogen-doped carbon powder cathodes. Biosensors and Bioelectronics. 2012;35(1):413–415. doi: 10.1016/j.bios.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 92.Li Y., Liu L., Yang F., Ren N. Performance of carbon fiber cathode membrane with C–Mn–Fe–O catalyst in MBR–MFC for wastewater treatment. Journal of Membrane Science. 2015;484:27–34. [Google Scholar]
- 93.Li Y., Liu L., Yang F. Destruction of tetracycline hydrochloride antibiotics by FeOOH/TiO2 granular activated carbon as expanded cathode in low-cost MBR/MFC coupled system. Journal of membrane science. 2017;525:202–209. [Google Scholar]
- 94.Chen R., Ruan X., Liu W., Stefanini C. A reliable and fast hydrogen gas leakage detector based on irreversible cracking of decorated palladium nanolayer upon aligned polymer fibers. International Journal of Hydrogen Energy. 2015;40(1):746–751. [Google Scholar]
- 95.Divyapriya G., Nambi I.M., Senthilnathan J. An innate quinone functionalized electrochemically exfoliated graphene/Fe3O4 composite electrode for the continuous generation of reactive oxygen species. Chemical Engineering Journal. 2017;316:964–977. [Google Scholar]
- 96.Peera S.G., et al. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. International Journal of Hydrogen Energy. 2021;46(4):3056–3089. [Google Scholar]
- 97.Ter Heijne A., Strik D.P., Hamelers H.V., Buisman C.J. Cathode potential and mass transfer determine performance of oxygen reducing biocathodes in microbial fuel cells. Environmental science & technology. 2010;44(18):7151–7156. doi: 10.1021/es100950t. [DOI] [PubMed] [Google Scholar]
- 98.Logan B.E., Regan J.M. Electricity-producing bacterial communities in microbial fuel cells. TRENDS in Microbiology. 2006;14(12):512–518. doi: 10.1016/j.tim.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Rabaey K., Clauwaert P., Aelterman P., Verstraete W. Tubular microbial fuel cells for efficient electricity generation. Environmental science & technology. 2005;39(20):8077–8082. doi: 10.1021/es050986i. [DOI] [PubMed] [Google Scholar]
- 100.Liu H., Cheng S., Huang L., Logan B.E. Scale-up of membrane-free single-chamber microbial fuel cells. Journal of Power Sources. 2008;179(1):274–279. [Google Scholar]
- 101.Peera S.G., Lee T.G., Sahu A.K. Pt-rare earth metal alloy/metal oxide catalysts for oxygen reduction and alcohol oxidation reactions: an overview. Sustainable Energy & Fuels. 2019;3(8):1866–1891. [Google Scholar]
- 102.Peera S.G., et al. Catalytic activity of Pt anchored onto graphite nanofiber-poly (3, 4-ethylenedioxythiophene) composite toward oxygen reduction reaction in polymer electrolyte fuel cells. Electrochimica Acta. 2013;108:95–103. [Google Scholar]
- 103.Peera S.G., Arunchander A., Sahu A. Platinum nanoparticles supported on nitrogen and fluorine co-doped graphite nanofibers as an excellent and durable oxygen reduction catalyst for polymer electrolyte fuel cells. Carbon. 2016;107:667–679. [Google Scholar]
- 104.Peera S.G., et al. Nitrogen and fluorine co-doped graphite nanofibers as high durable oxygen reduction catalyst in acidic media for polymer electrolyte fuel cells. Carbon. 2015;93:130–142. [Google Scholar]
- 105.Shaik G.P., Kwon H.-J., Lee T.G. Highly efficient Co@ NCS nanosheet electrocatalyst for oxygen reduction reaction: an environment-friendly, low-cost and sustainable electrocatalyst. Materials Research Bulletin. 2020;128 [Google Scholar]
- 106.Peera S.G., Arunchander A., Sahu A. Cumulative effect of transition metals on nitrogen and fluorine co-doped graphite nanofibers: an efficient and highly durable non-precious metal catalyst for the oxygen reduction reaction. Nanoscale. 2016;8(30):14650–14664. doi: 10.1039/c6nr02263d. [DOI] [PubMed] [Google Scholar]
- 107.Akula S., et al. Simultaneous Co-doping of nitrogen and fluorine into MWCNTs: an in-situ conversion to graphene like sheets and its electro-catalytic activity toward oxygen reduction reaction. Journal of The Electrochemical Society. 2017;164(6):F568. [Google Scholar]
- 108.Arunchander A., et al. Simultaneous co-doping of N and S by a facile in-situ polymerization of 6-N, N-dibutylamine-1, 3, 5-triazine-2, 4-dithiol on graphene framework: an efficient and durable oxygen reduction catalyst in alkaline medium. Carbon. 2017;118:531–544. [Google Scholar]
- 109.Peera S.G., Balamurugan J., Kim N.H., Lee J.H. Sustainable synthesis of Co@ NC core shell nanostructures from metal organic frameworks via mechanochemical coordination self‐assembly: an efficient electrocatalyst for oxygen reduction reaction. Small. 2018;14(19) doi: 10.1002/smll.201800441. [DOI] [PubMed] [Google Scholar]
- 110.Li W.-W., Sheng G.-P., Liu X.-W., Yu H.-Q. Recent advances in the separators for microbial fuel cells. Bioresource technology. 2011;102(1):244–252. doi: 10.1016/j.biortech.2010.03.090. [DOI] [PubMed] [Google Scholar]
- 111.Ghasemi M., et al. Sulfonated poly ether ether ketone with different degree of sulphonation in microbial fuel cell: application study and economical analysis. International journal of hydrogen energy. 2016;41(8):4862–4871. [Google Scholar]
- 112.Hernández-Fernández F., et al. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Processing Technology. 2015;138:284–297. [Google Scholar]
- 113.Laali K.K., et al. Novel fluorinated curcuminoids and their pyrazole and isoxazole derivatives: synthesis, structural studies, Computational/Docking and in-vitro bioassay. Journal of Fluorine Chemistry. 2018;206:82–98. [Google Scholar]
- 114.Tiwari B., Ghangrekar M. Electricity production during distillery wastewater treatment in a microbial fuel cell equipped with low cost PVA-nafion-borosilicate membrane. J. Clean Energy Technol. 2018;6(2):155–158. [Google Scholar]
- 115.Vickers N.J. Animal communication: when i'm calling you, will you answer too? Current biology. 2017;27(14):R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 116.Zhang S., Bao R., Lu J., Sang W. Simultaneous sulfide removal, nitrification, denitrification and electricity generation in three-chamber microbial fuel cells. Separation and Purification Technology. 2018;195:314–321. [Google Scholar]
- 117.Wu H., et al. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Bioresource technology. 2018;260:130–134. doi: 10.1016/j.biortech.2018.03.133. [DOI] [PubMed] [Google Scholar]
- 118.Zhao Z.-y., et al. A critical review of factors affecting the wind power generation industry in China. Renewable and Sustainable Energy Reviews. 2013;19:499–508. [Google Scholar]
- 119.Ayyaru S., Letchoumanane P., Dharmalingam S., Stanislaus A.R. Performance of sulfonated polystyrene–ethylene–butylene–polystyrene membrane in microbial fuel cell for bioelectricity production. Journal of Power Sources. 2012;217:204–208. [Google Scholar]
- 120.Zinadini S., et al. High power generation and COD removal in a microbial fuel cell operated by a novel sulfonated PES/PES blend proton exchange membrane. Energy. 2017;125:427–438. [Google Scholar]
- 121.Di Palma L., et al. Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. European Polymer Journal. 2018;99:222–229. [Google Scholar]
- 122.Srinophakun P., et al. Application of modified chitosan membrane for microbial fuel cell: roles of proton carrier site and positive charge. Journal of Cleaner Production. 2017;142:1274–1282. [Google Scholar]
- 123.Wang H., Park J.-D., Ren Z.J. Practical energy harvesting for microbial fuel cells: a review. Environmental science & technology. 2015;49(6):3267–3277. doi: 10.1021/es5047765. [DOI] [PubMed] [Google Scholar]
- 124.Aelterman P., et al. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environmental science & technology. 2006;40(10):3388–3394. doi: 10.1021/es0525511. [DOI] [PubMed] [Google Scholar]
- 125.Angelaalincy M.J., et al. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Frontiers in Energy Research. 2018;6:63. [Google Scholar]
- 126.Do M., et al. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: a mini review. Science of the Total Environment. 2018;639:910–920. doi: 10.1016/j.scitotenv.2018.05.136. [DOI] [PubMed] [Google Scholar]
- 127.Flimban S.G., Kim T., Ismail I.M.I., Oh S.-E. 2018. Overview of Microbial Fuel Cell (MFC) Recent Advancement from Fundamentals to Applications: MFC Designs, Major Elements, and Scalability. [Google Scholar]
- 128.Yang N., Ren Y., Li X., Wang X. Effect of short-term alkaline intervention on the performance of buffer-free single-chamber microbial fuel cell. Bioelectrochemistry. 2017;115:41–46. doi: 10.1016/j.bioelechem.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 129.Kim J.R., Jung S.H., Regan J.M., Logan B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresource technology. 2007;98(13):2568–2577. doi: 10.1016/j.biortech.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 130.Bradley R.W., Bombelli P., Rowden S.J., Howe C.J. Biological photovoltaics: intra-and extra-cellular electron transport by cyanobacteria. Biochemical society transactions. 2012;40(6):1302–1307. doi: 10.1042/BST20120118. [DOI] [PubMed] [Google Scholar]
- 131.Nishio K., Hashimoto K., Watanabe K. Light/electricity conversion by defined cocultures of Chlamydomonas and Geobacter. Journal of bioscience and bioengineering. 2013;115(4):412–417. doi: 10.1016/j.jbiosc.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 132.Prathiba S., Kumar P.S., Vo D.-V.N. Recent advancements in microbial fuel cells: a review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere. 2022;286 doi: 10.1016/j.chemosphere.2021.131856. [DOI] [PubMed] [Google Scholar]
- 133.Pandit S., Sengupta A., Kale S., Das D. Performance of electron acceptors in catholyte of a two-chambered microbial fuel cell using anion exchange membrane. Bioresource technology. 2011;102(3):2736–2744. doi: 10.1016/j.biortech.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 134.Rosenbaum M., He Z., Angenent L.T. Light energy to bioelectricity: photosynthetic microbial fuel cells. Current opinion in biotechnology. 2010;21(3):259–264. doi: 10.1016/j.copbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 135.Lay C.-H., Kokko M.E., Puhakka J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy. 2015;91:235–241. [Google Scholar]
- 136.Oon Y.-L., et al. Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresource technology. 2015;186:270–275. doi: 10.1016/j.biortech.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 137.Marotta M., et al. Degradation of dental plaque glucans and prevention of glucan formation using commercial enzymes. Process Biochemistry. 2002;38(1):101–108. [Google Scholar]
- 138.Asensio Y., et al. Towards the scale-up of bioelectrogenic technology: stacking microbial fuel cells to produce larger amounts of electricity. Journal of Applied Electrochemistry. 2017;47:1115–1125. [Google Scholar]
- 139.Gajda J., Thiel B., Zimmermann T. Monatsschrift Kinderheilkunde; 2020. Helpful Psychosocial Support for Parents in Pediatric Oncology: A Qualitative Study from an Interdisciplinary Perspective in Germany; pp. 1–7. [Google Scholar]
- 140.Kim B., Mohan S.V., Fapyane D., Chang I.S. Controlling voltage reversal in microbial fuel cells. Trends in biotechnology. 2020;38(6):667–678. doi: 10.1016/j.tibtech.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 141.An J., Sim J., Lee H.-S. Control of voltage reversal in serially stacked microbial fuel cells through manipulating current: significance of critical current density. Journal of Power Sources. 2015;283:19–23. [Google Scholar]
- 142.Wang H., Ren Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnology advances. 2013;31(8):1796–1807. doi: 10.1016/j.biotechadv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Di Lorenzo M., et al. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosensors and Bioelectronics. 2014;62:182–188. doi: 10.1016/j.bios.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 144.Chouler J., et al. Towards effective small scale microbial fuel cells for energy generation from urine. Electrochimica acta. 2016;192:89–98. [Google Scholar]
- 145.Fan Y., Hu H., Liu H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. Journal of Power Sources. 2007;171(2):348–354. [Google Scholar]
- 146.Ringeisen B.R., et al. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environmental science & technology. 2006;40(8):2629–2634. doi: 10.1021/es052254w. [DOI] [PubMed] [Google Scholar]
- 147.Yang Y., et al. Microfluidic microbial fuel cells: from membrane to membrane free. Journal of Power Sources. 2016;324:113–125. [Google Scholar]
- 148.Li X.M., Cheng K.Y., Wong J.W. Bioelectricity production from food waste leachate using microbial fuel cells: effect of NaCl and pH. Bioresource Technology. 2013;149:452–458. doi: 10.1016/j.biortech.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 149.Qian F., Baum M., Gu Q., Morse D.E. A 1.5 μL microbial fuel cell for on-chip bioelectricity generation. Lab on a Chip. 2009;9(21):3076–3081. doi: 10.1039/b910586g. [DOI] [PubMed] [Google Scholar]
- 150.Potter M.C. vol. 84. 1911. Electrical effects accompanying the decomposition of organic compounds; pp. 260–276. (Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character). 571. [Google Scholar]
- 151.Logan B.E. John Wiley & Sons; 2008. Microbial Fuel Cells. [Google Scholar]
- 152.Logan B.E., Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science. 2012;337(6095):686–690. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- 153.El-Naggar M.Y., et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proceedings of the National Academy of Sciences. 2010;107(42):18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pirbadian S., et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proceedings of the National Academy of Sciences. 2014;111(35):12883–12888. doi: 10.1073/pnas.1410551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Von Canstein H., Ogawa J., Shimizu S., Lloyd J.R. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Applied and environmental microbiology. 2008;74(3):615–623. doi: 10.1128/AEM.01387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu S., Jangir Y., El-Naggar M.Y. Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochimica Acta. 2016;198:49–55. [Google Scholar]
- 157.Lovley D.R. Syntrophy goes electric: direct interspecies electron transfer. Annual review of microbiology. 2017;71:643–664. doi: 10.1146/annurev-micro-030117-020420. [DOI] [PubMed] [Google Scholar]
- 158.Lovley D.R. Happy together: microbial communities that hook up to swap electrons. The ISME journal. 2017;11(2):327–336. doi: 10.1038/ismej.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Light S.H., et al. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature. 2018;562(7725):140–144. doi: 10.1038/s41586-018-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reguera G. Harnessing the power of microbial nanowires. Microbial biotechnology. 2018;11(6):979–994. doi: 10.1111/1751-7915.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bretschger O., et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Applied and environmental microbiology. 2007;73(21):7003–7012. doi: 10.1128/AEM.01087-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koch C., Harnisch F. Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem. 2016;3(9):1282–1295. [Google Scholar]
- 163.Yang W., Kim K.-Y., Saikaly P.E., Logan B.E. The impact of new cathode materials relative to baseline performance of microbial fuel cells all with the same architecture and solution chemistry. Energy & Environmental Science. 2017;10(5):1025–1033. [Google Scholar]
- 164.Das D. Springer-Verlag GmbH; Auflage: 2018. Microbial Fuel Cell. [Google Scholar]
- 165.Qu Y., Feng Y., Wang X., Logan B.E. Use of a coculture to enable current production by Geobacter sulfurreducens. Applied and environmental microbiology. 2012;78(9):3484–3487. doi: 10.1128/AEM.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oh S.-E., Logan B.E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Applied microbiology and biotechnology. 2006;70:162–169. doi: 10.1007/s00253-005-0066-y. [DOI] [PubMed] [Google Scholar]
- 167.Oliot M., et al. Separator electrode assembly (SEA) with 3-dimensional bioanode and removable air-cathode boosts microbial fuel cell performance. Journal of Power Sources. 2017;356:389–399. [Google Scholar]
- 168.Yang W., Logan B.E. Immobilization of a metal–nitrogen–carbon catalyst on activated carbon with enhanced cathode performance in microbial fuel cells. ChemSusChem. 2016;9(16):2226–2232. doi: 10.1002/cssc.201600573. [DOI] [PubMed] [Google Scholar]
- 169.Holmes D., et al. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microbial ecology. 2004;48:178–190. doi: 10.1007/s00248-003-0004-4. [DOI] [PubMed] [Google Scholar]
- 170.Kiely P.D., Regan J.M., Logan B.E. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Current opinion in biotechnology. 2011;22(3):378–385. doi: 10.1016/j.copbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 171.Lovley D.R. Bug juice: harvesting electricity with microorganisms. Nature reviews microbiology. 2006;4(7):497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 172.Kiely P.D., Rader G., Regan J.M., Logan B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresource Technology. 2011;102(1):361–366. doi: 10.1016/j.biortech.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 173.Kiely P.D., et al. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresource technology. 2011;102(1):388–394. doi: 10.1016/j.biortech.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 174.Yi H., et al. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosensors and Bioelectronics. 2009;24(12):3498–3503. doi: 10.1016/j.bios.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 175.Rosenbaum M., Cotta M.A., Angenent L.T. Aerated Shewanella oneidensis in continuously fed bioelectrochemical systems for power and hydrogen production. Biotechnology and bioengineering. 2010;105(5):880–888. doi: 10.1002/bit.22621. [DOI] [PubMed] [Google Scholar]
- 176.Jones S. New electricigens get wired. Nature Reviews Microbiology. 2006;4(9) 642-642. [Google Scholar]
- 177.Subramanian P., Pirbadian S., El-Naggar M.Y., Jensen G.J. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proceedings of the National Academy of Sciences. 2018;115(14):E3246–E3255. doi: 10.1073/pnas.1718810115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reguera G., et al. Extracellular electron transfer via microbial nanowires. Nature. 2005;435(7045):1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 179.Myers C.R., Myers J.M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. Journal of bacteriology. 1992;174(11):3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marsili E., et al. Shewanella secretes flavins that mediate extracellular electron transfer. Proceedings of the National Academy of Sciences. 2008;105(10):3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Call D.F., Logan B.E. Lactate oxidation coupled to iron or electrode reduction by Geobacter sulfurreducens PCA. Applied and environmental microbiology. 2011;77(24):8791–8794. doi: 10.1128/AEM.06434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hunt K.A., et al. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. Journal of bacteriology. 2010;192(13):3345–3351. doi: 10.1128/JB.00090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang L., et al. Boosting current generation in microbial fuel cells by an order of magnitude by coating an ionic liquid polymer on carbon anodes. Biosensors and Bioelectronics. 2017;91:644–649. doi: 10.1016/j.bios.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 184.Cao X., et al. A mini-microbial fuel cell for voltage testing of exoelectrogenic bacteria. Frontiers of Environmental Science & Engineering in China. 2009;3:307–312. [Google Scholar]
- 185.Li H., et al. Power output of microbial fuel cell emphasizing interaction of anodic binder with bacteria. Journal of Power Sources. 2018;379:115–122. [Google Scholar]
- 186.Doyle L.E., Marsili E. Weak electricigens: a new avenue for bioelectrochemical research. Bioresource technology. 2018;258:354–364. doi: 10.1016/j.biortech.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 187.Rabaey K., Boon N., Höfte M., Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environmental science & technology. 2005;39(9):3401–3408. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- 188.Pham T.H., et al. Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer. Applied microbiology and biotechnology. 2008;77:1119–1129. doi: 10.1007/s00253-007-1248-6. [DOI] [PubMed] [Google Scholar]
- 189.Kiely P.D., et al. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera. Applied microbiology and biotechnology. 2010;88:371–380. doi: 10.1007/s00253-010-2757-2. [DOI] [PubMed] [Google Scholar]
- 190.Goel S. Microfluidic microbial fuel cell: on-chip automated and robust method to generate energy. Microbial Fuel Cell: A Bioelectrochemical System that Converts Waste to Watts. 2018:229–247. [Google Scholar]
- 191.Algar C.K., Howard A., Ward C., Wanger G. Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens. Scientific reports. 2020;10(1) doi: 10.1038/s41598-020-70002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.