Abstract
A search for an immunomodulating agent from mycobacteria was carried out using Mycobacterium leprae. The antigenicity of each fraction of the bacterial membrane, which contains the most antigenic components of M. leprae, was assessed by using sera from paucibacillary leprosy. N-terminal sequencing of the serum-reactive protein and functional assessment of the membrane fractions using monocyte-derived dendritic cells (DCs) identified major membrane protein II (MMP-II) as one of the efficient T-cell-activating candidates. Purified MMP-II stimulated DCs from healthy individuals to produce interleukin-12 p70 and up-regulated the surface expression of major histocompatibility complex class I and II, CD86, and CD83 molecules. Also, there was an increase in the percentage of CD83+ cells in the DC population. Furthermore, MMP-II-pulsed DCs expressed their derivatives on their surfaces. Using Toll-like receptor 2 (TLR-2)-dependent receptor constructs, we found that TLR-2 signaling was involved in DC maturation induced by MMP-II. Taken together, MMP-II can be recognized as an immunomodulating protein in terms of activation of antigen-presenting cells and innate immunity.
Mycobacterial infection is a major public health risk world-wide, and around one-third of the world's population is estimated to be latently infected. Mycobacterium tuberculosis, the causative agent of human tuberculosis, accounts for 8 to 10 million new active cases and 2 million deaths annually (12, 41). Nontuberculoid mycobacterial infections of immunocompromised individuals, such as human immunodeficiency virus type 1 (HIV-1)-infected patients (8, 28), evoke serious concern, and Mycobacterium leprae induces a chronic progressive peripheral nerve injury which leads to systemic deformity (16, 37). The sole immunomodulating agent currently available for human use against mycobacterial diseases is Mycobacterium bovis bacillus Calmette-Guérin (BCG). However, its protective effect against mycobacterial infection is unconvincing, especially to elderly people, and thus, its use is limited (3). Various efforts are currently being made to develop immunomodulating agents, but convincing protection against mycobacterium-induced diseases has not been achieved to date. The development of other useful molecules as immunomodulating agents is greatly desired. In this study, we attempted to find such antigenic molecules in M. leprae subfractions.
M. leprae is the causative agent of human leprosy, for which a broad disease spectrum is clinically observed (34). Most individuals infected with M. leprae do not manifest leprosy, but a few manifest the disease, depending on their immunological status. The representative spectra are the tuberculoid, or paucibacillary (PB), leprosy and the lepromatous, or multibacillary (MB), leprosy. In the former disease spectrum, localized skin and nerve lesions are observed, and T cells act chiefly to localize bacterial spread and, thus, disease lesions (20, 31, 36). In contrast, in the latter disease spectrum, such cell-mediated immune responses are not efficiently evoked; rather, T cells show M. leprae antigen (Ag)-specific anergic responses. In both types of leprosy, the protective effect of antibody (Ab) is not observed. These observations indicate that the bacterial component Ags capable of modulating immune responses should be identified. Previously it has been demonstrated that monocyte-derived dendritic cells (DCs), which are the most potent antigen-presenting cells (APCs), are capable of stimulating both memory and naïve CD4+ and CD8+ T cells (14, 15, 21). Also, we reported that DCs played a central role in stimulating T cells (10, 18, 22); however, macrophages stimulated T cells less efficiently. Furthermore, we showed that among subcellular components of M. leprae, the cell membrane fraction was quite antigenic and contained molecules which stimulated DCs to produce interleukin-12 (IL-12) p70 (22). However, the molecules associated with DC activation have not been elucidated. For identification of an APC-associated immunomodulator, the following issues should be addressed: (i) the ability of the immunogen to activate APCs, including both DCs and macrophages; (ii) the ability of the immunogen to be processed and presented on the surfaces of these APCs, because mycobacteria are intracellular parasitic pathogens. In this context, we fractionated the M. leprae-derived cell membrane fraction, screened the fractions for such a protein by using DCs as APCs, and subsequently evaluated the newly identified molecule, major membrane protein II (MMP-II), in terms of innate immunity.
MATERIALS AND METHODS
Preparation of cells and bacteria.
Peripheral blood was obtained with informed consent from healthy individuals who were positive for purified protein derivative (PPD) due to M. bovis BCG vaccination. We are aware that PPD-negative individuals would help to provide full information for these experiments, however, in Japan, such individuals are not available for study, because M. bovis BCG vaccination is compulsory for children (0 to 4 years old). Moreover, PPD-negative individuals in the Japanese population are those who do not respond to BCG vaccination, and therefore, it is likely that they suffer from an unknown human disease or immune insufficiency. Therefore, these individuals cannot be used as controls for our experiments. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and cryopreserved in liquid nitrogen until use, as previously described (23). Macrophages were differentiated by culturing plastic-adherent CD14+ monocytes with RPMI 1640 medium containing 20% of fetal calf serum (FCS) and 1% penicillin G (Katayama Chemical, Osaka, Japan) in the presence of 5 ng/ml of macrophage colony-stimulating factor (R & D Systems, Abingdon, United Kingdom), as previously described (40). For preparation of the monocytes, CD3+ T cells were removed from either freshly isolated heparinized blood or cryopreserved PBMCs using immunomagnetic beads coated with an anti-CD3 monoclonal antibody (MAb) (Dynabeads 450; Dynal, Oslo, Norway). The CD3− fraction of the PBMCs was plated on collagen-coated plates and cultured for 60 min at 37°C. The non-plastic-adherent cells were then removed by extensive washing, and the remaining adherent cells were used as monocytes and precursors of macrophages and DCs (23). Monocyte-derived DCs were differentiated from the plastic-adherent cells as described previously (23, 24). Briefly, the plastic-adherent cells were cultured in 3 ml of RPMI 1640 medium containing 10% FCS for 5 days in the presence of 50 ng of recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) (Pepro Tech EC Ltd., London, England) and 10 ng of rIL-4 (Pepro Tech) per ml. rGM-CSF and rIL-4 were supplied every 2 days, and 400 μl of medium was replaced as described previously (24). In some cases, DCs unpulsed or pulsed with Ags were further treated with a soluble form of CD40 ligand (CD40L) (Pepro Tech) to obtain fully matured DCs capable of efficiently activating T cells. The purity of DCs obtained was 90.5% as judged by the expression of CD1a. Since M. leprae cannot be cultivated or grown in vitro, M. leprae (Thai-53) was obtained from an armadillo liver that had been previously infected with M. leprae. The isolated bacteria were counted by Shepard's method (35) and were frozen at −80°C until use. The viability of M. leprae was assessed by using a fluorescent diacetate/ethidium bromide test (17).
Fractionation of M. leprae protein and N-terminal sequencing.
The fractionation of the mycobacterial proteins into cell wall, membrane, and cytosolic fractions was carried out according to previous reports (13, 18, 22). Briefly, the mycobacterial suspension was mixed with zirconium beads in the presence of protease inhibitors at a ratio of approximately 1:1 (vol/vol) and homogenized using Beads Homogenizer, model BC-20 (Central Scientific Commerce, Tokyo, Japan), at 1,500 rpm for 90 s three to four times. The beads were separated, and the suspension was centrifuged at 10,000 × g for 30 min. The supernatant was then further ultracentrifuged at 100,000 × g for 1 h. The resulting pellet was suspended in phosphate-buffered saline, washed twice, and taken as the membrane fraction. For the identification of M. leprae antigenic molecules, the membrane fraction was further fractionated using a fast protein liquid chromatography system (Amersham Bioscience, New Jersey). Four hundred micrograms of protein was run on a Superose 12 column (Amersham Bioscience) in 50 mM Tris-HCl, 0.5 M NaCl, and 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) at a flow rate of 0.5 ml/min. Fractions were collected, concentrated, buffer exchanged to 50 mM Tris-HCl using Microcon centrifuge filter units YM-3 (Millipore, Bedford, MA), and run on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The gel was stained with “Daiichi” silver stain (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions. Western blotting was performed using PB patients' pooled sera, at a dilution of 1:25, which had been preadsorbed with the M. leprae cytosolic fraction. Alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) (Biosource, Camarillo, CA) was used as the secondary Ab, and detection was performed by using the nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolylphosphate (BCIP) reagent (Calbiochem, San Diego, CA). N-terminal peptide sequencing of the protein which reacted to the sera was performed at the Center for Instrumental Analysis, Hokkaido University.
Analysis of cell surface Ags.
The expression of cell surface Ags on DCs was analyzed using FACScalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). Dead cells were eliminated from the analysis by staining with propidium iodide (Sigma Chemical Co., St. Louis, MO), and 104 live cells were analyzed. For analysis of cell surface Ags, fluorescein isothiocyanate-conjugated MAbs against HLA-ABC (G46-2.6; PharMingen, San Diego, CA), HLA-DR (L243; PharMingen), CD86 (FUN-1; PharMingen), and CD83 (HB15a; Immunotech, Marseille, France) were used. A murine MAb against MMP-II was raised by immunizing a mouse with purified MMP-II. The optimal concentrations of MAbs were determined in advance.
Identification and purification of MMP-II.
The MMP-II gene was PCR amplified from M. leprae chromosomal DNA and cloned into an Escherichia coli expression vector. Briefly, the MMP-II gene was inserted into the expression plasmid pET28 (Novagen Inc., Madison, WI) and transformed into E. coli strain ER2566 (New England Biolabs Inc., Beverly, MA). The expressed protein was eluted using Whole Gel Eluter (Bio-Rad Laboratories, Hercules, CA) and used for all experiments. The amount of lipopolysaccharide (LPS) in the purified MMP-II protein was determined by using a Limulus amebocyte lysate assay (Whittaker Bioproducts, Walkersville, MD) and found to be less than 70 pg per mg of MMP-II, a level that did not affect the maturation of DCs.
Assessment of APC function of DCs pulsed with cell membrane fractions.
The ability of DCs pulsed with various fractions of the M. leprae cell membrane to stimulate autologous T cells was assessed using an autologous stimulator-T-cell mixed reaction as previously described (10, 24). The Ag-pulsed DCs were treated with 50 μg/ml of mitomycin C, washed extensively to remove extracellular Ags, and used as a stimulator. T cells were prepared as follows: freshly thawed PBMCs were depleted of major histocompatibility complex (MHC) class II+ cells by using magnetic beads coated with a MAb to MHC class II Ag (Dynabeads 450; Dynal) and were further treated with beads coated with either a CD4 or a CD8 MAb to select T cells negatively as previously reported (10). The purity of CD4+ T cells or CD8+ T cells was more than 98%. The supernatant of the stimulator-T-cell mixture was collected on day 4 of coculture, and the level of gamma interferon (IFN-γ) produced was measured by an Opt EIA Human ELISA Set (BD PharMingen International).
Assessment of cytokine production.
Levels of the following cytokines were measured: tumor necrosis factor alpha (TNF-α), IL-10, and IL-12 p70 produced from either macrophages or DCs by stimulation with MMP-II for 24 h in the presence or absence of a soluble form of CD40L (Pepro Tech). The murine MAb against TLR-2 (clone 2392; IgG1) with neutralizing activity was obtained from Genentech (San Francisco, CA). The optimal concentration of the anti-Toll-like receptor 2 (anti-TLR-2) Ab was determined in advance. The concentrations of IL-12 p70, IL-10, and TNF-α were quantified using the Opt EIA Human ELISA Set enzyme assay kits, available from BD PharMingen International.
Cell transfection and luciferase assay.
Human embryonic kidney cells (HEK293) were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% FCS, 50 mg/ml penicillin/streptomycin, and nonessential amino acids (Invitrogen, Carlsbad, CA) at 37°C in a humidified incubator with 5% CO2. The cDNA of human TLR-2 was PCR amplified using a human spleen cDNA library (BD Biosciences, San Jose, CA) and inserted into pCIneo (Promega, Madison, WI). HEK293 cells (2 × 104) were transiently transfected with a mixture of plasmids—200 ng of pCIneo hTLR2, 25 ng of p5×NF-κB-luc (Stratagene, La Jolla, CA), and 10 ng of pRL-TK-Renilla luciferase plasmid (Promega)—using the FuGENE 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN), as previously described (38). Thirty-six hours after transfection, cells were treated with various amounts of glutathione S-transferase (GST), MMP-II, or peptidoglycan (PGN) as a positive control (for TLR-2-dependent luciferase activity) for a further 6 h. The cells were lysed in 70 μl of 1× passive lysis buffer (Promega), and luciferase activity in 10 μl of the cell lysate was measured using the Promega Dual-Luciferase Reporter Assay System according to the protocol provided by the manufacturer. Data were expressed as fold induction relative to the activity of Renilla luciferase, which is an internal control for transfection efficiency.
Statistical analysis.
Student's t test was applied to demonstrate statistically significant differences.
RESULTS
Identification of M. leprae-derived antigenic molecules.
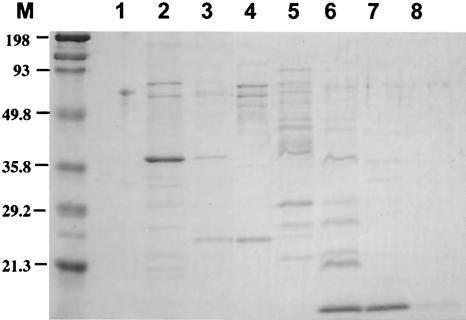
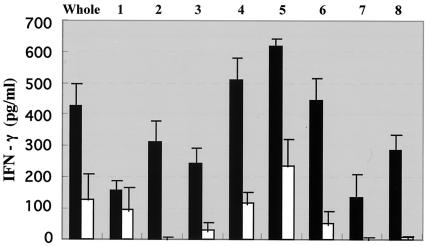
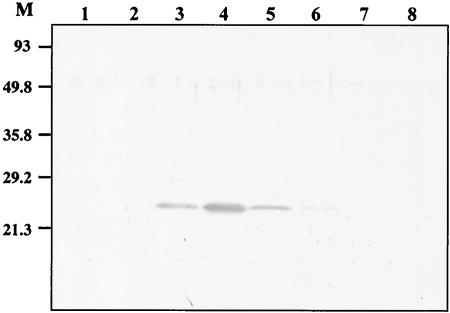
The cell membrane fraction from M. leprae was found to be the most T-cell stimulating (10, 22), although it may also contain some inhibitory molecules (10). The M. leprae-derived cell membrane fraction was solubilized and further fractionated using a gel filtration column to search for the antigenic molecules. Figure 1 shows the silver staining of each fraction, which revealed several proteins. Then we pulsed healthy donor-derived DCs with each of these fractions individually and examined the antigenicity of each fraction by monitoring IFN-γ production by DC-stimulated CD4+ and CD8+ T cells derived from PPD-positive healthy individuals (Fig. 2). Among the eight fractions, fractions 4 through 6 seemed to be efficient at stimulating cytokine production by CD4+ T cells, and fractions 4 and 5 appeared to be involved in the activation of both CD4+ and CD8+ T cells. Thus, using these two fractions of cell membrane, we identified one of the antigenic molecules. The pooled PB leprosy sera, preadsorbed with M. leprae cytosol fractions, were used for Western blot analysis of the fractions (not shown). N-terminal sequencing of the serum-reactive bands common to fractions 4 and 5 identified MMP-II as one of the candidates. The presence of MMP-II in fractions 4 and 5 was further confirmed by Western blotting using a MAb against MMP-II (Fig. 3). For the purification of the protein, the MMP-II gene was amplified by PCR from the genomic DNA of M. leprae, and MMP-II protein was subsequently expressed in E. coli by using the T7 expression system (pET-28). The expressed protein was confirmed to be MMP-II by Western blot analysis (not shown), by comparison to purified MMP-II, used as a positive control (donated by P. J. Brennan, Colorado University).
FIG. 1.
M. leprae membrane fractions were separated into eight fractions by gel filtration as described in Materials and Methods. Then 3 μg of each fraction was run on a 12% SDS-polyacrylamide gel, and silver staining of the gel was performed.
FIG. 2.
IFN-γ production by T cells stimulated with membrane fraction-pulsed DCs. The responder CD4+ and CD8+ T cells (105/well) were stimulated for 4 days with autologous DCs which had been previously stimulated with 10 μg/ml of various membrane fractions of M. leprae obtained by gel filtration. Solid bars, IFN-γ production from CD4+ T cells; open bars, IFN-γ production from CD8+ T cells.
FIG. 3.
Western blot of M. leprae membrane fractions. Five micrograms of various membrane fractions of M. leprae was run on a 12% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane, which was further probed with an anti-MMP-II MAb. An alkaline phosphatase-conjugated anti-mouse IgG Ab was used as the secondary Ab, and the protein was detected with NBT/BCIP reagent.
Antigenicity of M. leprae-derived MMP-II.
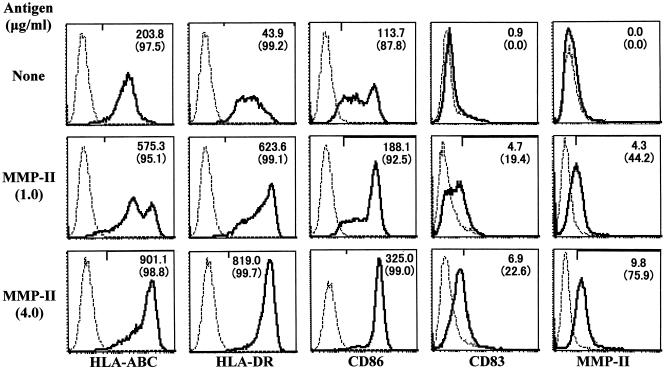
The ability of MMP-II to evoke cellular immunity was assessed using DCs and macrophages as APCs. Previously we demonstrated that the cytosol fraction from M. leprae was less efficient at the induction of DC maturation and that the whole cell membrane fraction partially induced DC maturation (22). In contrast, when immature DCs were pulsed with MMP-II, they up-regulated the expression levels of HLA-ABC, HLA-DR, CD86, and CD83 Ags on the surfaces of DCs in an Ag dose-dependent manner, and the percentage of CD83+ cells was found to increase significantly (Fig. 4). The expression of MMP-II on the surfaces of MMP-II-pulsed DCs was revealed using a MAb to MMP-II (Fig. 4). The functional aspects of MMP-II in terms of APC activation were assessed by measuring production of cytokines, such as IL-12 p70, IL-10, and TNF-α, by APCs (Tables 1 and 2). The bioactive form of IL-12 was released from DCs by pulsing MMP-II in the absence of CD40L, and the cytokine production level was enhanced by copulsing DCs with CD40L and MMP-II (Table 1). Obviously IL-12 was not produced from DCs by stimulation with the amount of LPS estimated to be present in 4 μg/ml of MMP-II. Furthermore, DCs produced TNF-α in the presence or absence of CD40L, but they did not produce any significant amount of IL-10 due to MMP-II stimulation. These results suggested that MMP-II could activate DCs and induce their maturation. Macrophages derived from monocytes did not produce IL-12 p70 by stimulation with MMP-II, but they produced TNF-α and IL-10 (Table 2), which are found predominantly in granulomatous mycobacterium-infected lesions. These results indicate that MMP-II also activated macrophages, but macrophages and DCs seem to have distinct functional roles. All cytokines were produced in an Ag dose-dependent fashion.
FIG. 4.
Expression of various molecules on DCs pulsed with MMP-II. Monocyte-derived DCs from healthy individuals (PPD positive) were pulsed with the indicated dose of MMP-II. DCs were gated and analyzed. Solid curves, isotype-matched control IgG; broken curves, the indicated MAb. The number in the top right corner of each panel represents the difference in mean fluorescence intensity between the control IgG and the test MAb. The number in parentheses is the percentage of positive cells. Results of one experiment representative of three separate experiments are shown.
TABLE 1.
Cytokine production from DCs stimulated with MMP-IIa
| DC stimulation (dose) | Concn (pg/ml) of the following cytokine:
|
|||||
|---|---|---|---|---|---|---|
| IL-12 p70
|
TNF-α
|
IL-10
|
||||
| CD40L (−) | CD40L (+) | CD40L (−) | CD40L (+) | CD40L (−) | CD40L (+) | |
| None | 2.6 ± 0.2*,† | 17.7 ± 0.4‡,§ | 2.6 ± 0.4¶,∥ | 15.4 ± 3.8**,†† | 2.0 ± 0.1 | 3.4 ± 0.1 |
| MMP-II (1 μg/ml) | 51.2 ± 0.5* | 782.0 ± 8.7‡ | 345.4 ± 9.9¶ | 345.7 ± 19.3** | 2.5 ± 0.3 | 1.7 ± 0.1 |
| MMP-II (4 μg/ml) | 404.0 ± 9.8† | 1624.0 ± 11.0§ | 773.8 ± 11.1∥ | 747.3 ± 18.7†† | 2.2 ± 0.1 | 2.8 ± 0.3 |
| LPS (0.3 pg/ml) | 2.8 ± 0.3 | 18.7 ± 0.6 | 5.0 ± 1.3 | 36.0 ± 9.2 | 2.0 ± 0.3 | 3.0 ± 0.2 |
Monocyte-derived DCs (105/well) were stimulated for 24 h with the indicated dose of MMP-II in the absence [CD40L (−)] or presence [CD40L (+)] of a soluble form of CD40L (1.0 μg/ml). The DCs were also stimulated for 24 h with 0.3 pg/ml of LPS, which is estimated to be present in 4 μg/ml of MMP-II. Results representative of more than three separate experiments are shown. Assays were done in triplicate, and results are expressed as means ± standard deviations. Titers with the same symbols are statistically compared by Student's t test, as follows: *, P < 0.001; ‡, P < 0.001; ¶, P < 0.0005; **, P < 0.001; †, P < 0.001; §, P < 0.001; ∥, P < 0.0001; ††, P < 0.0005.
TABLE 2.
Cytokine production from macrophages stimulated with MMP-IIa
| Macrophage stimulation (dose) | Concn (pg/ml) of the following cytokine:
|
||
|---|---|---|---|
| IL-12 p70 | TNF-α | IL-10 | |
| None | 2.0 ± 0.2 | 19.3 ± 1.8*,† | 45.3 ± 8.8‡,§ |
| MMP-II (1.0 μg/ml) | 2.1 ± 0.3 | 122.2 ± 6.8* | 149.9 ± 20.3‡ |
| MMP-II (4.0 μg/ml) | 1.9 ± 0.2 | 568.6 ± 12.4† | 561.2 ± 31.9§ |
| LPS (0.3 pg/ml) | 1.8 ± 0.1 | 10.0 ± 2.0 | 15.6 ± 3.2 |
Monocyte-derived macrophages (105/well) were stimulated for 24 h in the presence of a soluble form of CD40L (1.0 μg/ml) with the indicated dose of MMP-II. Results representative of more than three separate experiments are shown. Assays were done in triplicate, and results are expressed as means ± standard deviations. Titers with the same symbols are statistically compared by Student's t test, as follows: *, P < 0.001; ‡, P < 0.005; †, P < 0.0005; §, P < 0.001.
Involvement of TLR-2 in activation of DCs.
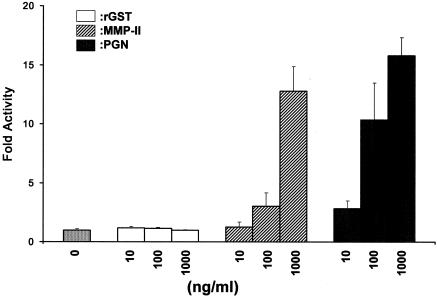
In order to elucidate the mechanism by which MMP-II activates DCs, we examined the relationship of MMP-II and TLR-2, because TLR-2 is reported to be highly associated with induction of innate immunity against mycobacterial infection (1, 4, 30). When HEK293 cells that had been cotransfected with pCIneo TLR-2, p5×NF-κB-luc, and pRL-Tk-Renilla luciferase were pulsed with MMP-II, significant levels of luciferase activity were induced in an Ag dose-dependent manner, levels comparable to those induced by PGN, a well-defined TLR-2-associated bacterial Ag (Fig. 5). Similar results were also obtained using M. leprae-derived MMP-II. Such changes were not induced by rGST, a negative-control protein. Furthermore, when the surface TLR-2 Ag on DCs was masked by an antagonistic Ab to TLR-2, IL-12 p70 production by DCs stimulated with MMP-II was significantly, though partially, suppressed (Table 3). The isotype-matched control IgG did not affect IL-12 p70 production by MMP-II-stimulated DCs. As expected, the TLR-2-antagonistic Ab did not suppress IL-12 p70 production by DCs stimulated with LPS (a ligand for TLR-4) (not shown). These results indicate that MMP-II might use TLR-2 as its ligand on APCs, resulting in stimulation of DCs.
FIG. 5.
NF-κB-dependent reporter gene activity of the TLR2 transfectant was measured after stimulation with or without 10, 100, or 1,000 ng/ml of rGST, MMP-II, or PGN, as described in Materials and Methods. Data are expressed as fold induction relative to the activity of Renilla luciferase, which is an internal control for transfection efficiency in the dual-luciferase reporter assay. Results of one experiment representative of two separate experiments are shown. Assays were done in triplicate, and the results are expressed as means ± standard deviations.
TABLE 3.
Effect of the TLR-2-antagonistic Ab on IL-12 p70 production by DCsa
| DC stimulation (dose) | IL-12 p70 production (pg/ml) in the presence of:
|
||||
|---|---|---|---|---|---|
| TLR-2-antagonistic Ab at the following concn (μg/ml):
|
Control IgG at the following concn (μg/ml):
|
||||
| 0 | 5.0 | 10.0 | 5.0 | 10.0 | |
| MMP-II (1.0 μg/ml) | 603.1 ± 11.0*† | 491.2 ± 10.2* | 178.1 ± 8.8† | 658.2 ± 11.3 | 675.9 ± 10.7 |
| MMP-II (4.0 μg/ml) | 1,210.2 ± 20.0‡,§ | 949.0 ± 9.3‡ | 805.3 ± 7.9§ | 1,290.3 ± 12.4 | 1,403.8 ± 31.5 |
Monocyte-derived immature DCs (106/ml) were treated with the indicated dose of a TLR-2-antagonistic MAb or an isotype-matched control IgG and were subsequently stimulated for 24 h with MMP-II in the presence of CD40L (1.0 μg/ml). Results representative of more than three separate experiments are shown. Assays were done in triplicate, and results are expressed as means ± standard deviations. Titers with the same symbols were statistically compared by Student's t test, as follows: *, P < 0.0001; †, P < 0.0005; ‡, P < 0.0001; §, P < 0.0001.
DISCUSSION
Leprosy is a broad-spectrum disease (34). One representative manifestation is PB leprosy. Studies on clinical specimens of the skin lesions indicate that the infection is localized and the spread of M. leprae is suppressed as a consequence of activation of cellular immune responses (20, 31, 36). On the other hand, MB leprosy usually manifests widespread infection due to the lack of an efficient response to M. leprae components. The mechanisms leading to the broad spectrum are not fully understood yet, but these observations suggest the presence of an Ag with immunomodulating activities that modify the immune responses in vivo. So far, however, such Ags have not been identified. Previously we evaluated the APC function of professional APCs and found that DCs were superior to macrophages in activating T cells (10). When we examined the DC-mediated antigenicity of subcellular components of M. leprae for identification of immunomodulating molecules, we found that the cell membrane fraction was more suitable than other fractions (22). Therefore, the M. leprae membrane fraction was size fractionated, and each fraction was examined for its T-cell-stimulating ability by using DCs as APCs. Two of the fractions with high activity were examined by reaction to PB leprosy sera, and subsequently the N terminus of the reactive protein was sequenced. As a result, MMP-II was identified as one of the antigenic cell membrane proteins, and the result was confirmed by Western blotting of the various fractions using an anti-MMP-II antibody (Fig. 3).
MMP-II was originally identified from M. leprae as a major native protein in 1990 (13) and was recognized as being identical to mycobacterial bacterioferritin (32). Purification of MMP-II by reverse-phase chromatography revealed a large molecular mass of 380 kDa and a ferroxidase center residue. MMP-II contains 1,000 to 4,000 atoms of iron per molecule of protein (32). A homology search on the mycobacterial nucleotide database revealed that MMP-II is conserved among M. leprae, M. tuberculosis, and M. avium. The percent homology at the amino acid level is about 86% among these species. The previous studies reported that MMP-II was recognized in vivo by B and T cells. Sera from patients were reported to have higher IgG titers to MMP-II, regardless of the clinical type of leprosy, than sera from healthy individuals (7). Also, T cells from leprosy of both the PB and the MB type were stimulated by MMP-II to proliferate and to produce both IFN-γ and IL-5 (29). However, tuberculosis patients or individuals who have had contact with leprosy patients have not been examined yet. Also, the influence of MMP-II on the innate immune response has not yet been clarified.
MMP-II stimulated DCs to produce TNF-α and a bioactive form of IL-12 (IL-12 p70) (Table 1) and induced their maturation, as observed by their phenotypic changes (Fig. 4). Further, MMP-II also stimulated macrophages to produce TNF-α and IL-10 (Table 1). These cytokines were produced by stimulation with either MMP-II derived from M. leprae (not shown) or MMP-II overexpressed in E. coli (Table 1). DCs and macrophages play distinct roles in the host defense against mycobacterial infection (9). DCs are central to the initiation of Ag-specific T-cell responses (6, 27, 36), and in our preliminary experiments, DCs pulsed with purified MMP-II stimulated both CD4+ and CD8+ T cells from PPD-positive healthy individuals to produce IFN-γ (not shown). The activated form of macrophage is involved in the formation of tuberculoid granulomatous lesions (5, 9). These results indicate that MMP-II might contribute to the immune regulation of host cells against mycobacteria. Then we investigated what could be the MMP-II ligand that is expressed on APCs. TLR-2 is associated mainly with innate immunity and has been shown to recognize the molecular pattern of pathogens (4, 11, 18, 26, 33). In mycobacterial infection, it has been reported that a 19-kDa lipoprotein isolated from M. tuberculosis ligated TLR-2 (4, 19), and the M. leprae 33-kDa lipoprotein could be another candidate participating in the TLR-2-associated innate immune system (19). In our study using the TLR-2 reporter assay with HEK293 cells, we found that TLR-2 is likely to be involved in the recognition of MMP-II in spite of the fact that MMP-II lacks the triacylated region. This finding surprised us, but a similar ligation of protein to TLR-2 has also been reported for neisserial porins HSP60 and HSP70, which have no posttranslational modification of acylation (2, 25, 39). IL-12 production by MMP-II-stimulated DCs was partially inhibited by a TLR-2-antagonistic Ab, which indicates that other receptors are also involved in signals leading to IL-12 production.
The data in this report, taken together, indicate that MMP-II has an immunomodulating activity and contributes to the activation of innate immunity. Further study should be pursued to evaluate its host defense-associated activity against leprosy and other mycobacterial infections that pose a world-wide threat.
Acknowledgments
We appreciate the valuable comments of S. H. E. Kaufmann on the manuscript. We are grateful to N. Makino for the preparation of the manuscript. We acknowledge K. Takahashi, Hokkaido University, for N-terminal sequencing of the protein. We also thank the Japanese Red Cross Society for kindly providing PBMCs from healthy donors. Purified MMP-II was provided by P. J. Brennan (Colorado State University) through NIH contract 1-AI-25468, “Leprosy Research Support.”
This work was supported in part by funds from the health science research grants Research on Emerging and Re-emerging Infectious Diseases and Grant-in-Aid for Research on HIV/AIDS from the Ministry of Health, Labour and Welfare of Japan.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Asea, A., M. Rehli, E. Kabingu, J. A. Boch, O. Bare, P. E. Auron, M. A. Stevenson, and S. K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70. J. Biol. Chem. 277:15028-15034. [DOI] [PubMed] [Google Scholar]
- 3.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Davis, J. M., H. Clay, J. L. Lewis, N. Ghori, P. Herbomel, and L. Ramakrishnan. 2002. Real-time visualization of Mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17:693-702. [DOI] [PubMed] [Google Scholar]
- 6.Demangel, C., U. Palendira, C. G. Feng, A. W. Heath, A. G. Bean, and W. J. Britton. 2001. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect. Immun. 69:2456-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande, R. G., M. B. Khan, D. A. Bhat, and R. G. Navalkar. 1995. Immunoaffinity chromatographic isolation of a high molecular weight seroreactive protein from Mycobacterium leprae cell sonicate. FEMS Immunol. Med. Microbiol. 11:163-169. [DOI] [PubMed] [Google Scholar]
- 8.Falkinham, J. O. 1996. Epidemiology of infection by nontuberculosis mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto, K., Y. Maeda, H. Kimura, K. Suzuki, A. Masuda, M. Matsuoka, and M. Makino. 2002. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect. Immun. 70:5167-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hertz, C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 12.Huebner, R. E., and K. G. Castor. 1995. The changing face of tuberculosis. Annu. Rev. Med. 46:47-55. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, S. W., B. Rivoire, V. Mehra, B. R. Bloom, and P. J. Brennan. 1990. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265:14065-14068. [PubMed] [Google Scholar]
- 14.Inaba, K., J. P. Metlay, M. T. Crowley, and R. M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 16.Job, C. K. 1989. Nerve damage in leprosy. Int. J. Lepr. Other Mycobact. Dis. 57:532-539. [PubMed] [Google Scholar]
- 17.Katoch, V. M., K. Katoch, U. Ramanathan, V. D. Sharma, C. T. Shivannavar, A. K. Datta, and V. P. Bharadwaj. 1989. Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining. Int. J. Lepr. Other Mycobact. Dis. 57:615-621. [PubMed] [Google Scholar]
- 18.Kimura, H., Y. Maeda, F. Takeshita, L. E. Takaoka, M. Matsuoka, and M. Makino. 2004. Upregulation of T-cell-stimulating activity of mycobacteria-infected macrophages. Scand. J. Immunol. 60:278-286. [DOI] [PubMed] [Google Scholar]
- 19.Krutzik, S. R., M. T. Ochoa, P. A. Sieling, S. Uematsu, Y. W. Ng, A. Legaspi, P. T. Liu, S. T. Cole, P. J. Godowski, Y. Maeda, E. N. Sarno, M. V. Norgard, P. J. Brennan, S. Akira, T. H. Rea, and R. L. Modlin. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9:525-532. [DOI] [PubMed] [Google Scholar]
- 20.Lewinsohn, D. M., M. R. Alderson, A. L. Briden, S. R. Riddell, S. G. Reed, and K. H. Grabstein. 1998. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J. Exp. Med. 187:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 22.Maeda, Y., M. Gidoh, N. Ishii, C. Mukai, and M. Makino. 2003. Assessment of cell mediated immunogenicity of Mycobacterium leprae-derived antigens. Cell. Immunol. 222:69-77. [DOI] [PubMed] [Google Scholar]
- 23.Makino, M., and M. Baba. 1997. A cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells. Scand. J. Immunol. 45:618-622. [DOI] [PubMed] [Google Scholar]
- 24.Makino, M., S. Shimokubo, S. Wakamatsu, S. Izumo, and M. Baba. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 73:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 26.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 27.Mittal, A., and I. Nath. 1987. Human T cell proliferative responses to particulate microbial antigens are supported by populations enriched in dendritic cells. Clin. Exp. Immunol. 69:611-617. [PMC free article] [PubMed] [Google Scholar]
- 28.Montessori, V., P. Phillips, J. Montaner, L. Haley, K. Craib, E. Bessuile, and W. Black. 1996. Species distribution in human immunodeficiency virus-related mycobacterial infections: implications for selection of initial treatment. Clin. Infect. Dis. 22:989-992. [DOI] [PubMed] [Google Scholar]
- 29.Ohyama, H., S. Matsushita, F. Nishimura, N. Kato, K. Hatano, S. Takashiba, and Y. Murayama. 2002. T cell responses to major membrane protein II (MMP II) of Mycobacterium leprae are restricted by HLA-DR molecules in patients with leprosy. Vaccine 20:475-482. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira, R. B., M. T. Ochoa, P. A. Sieling, T. H. Rea, A. Rambukkana, E. N. Sarno, and R. L. Modlin. 2003. Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect. Immun. 71:1427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 32.Pessolani, M. C., D. R. Smith, B. Rivoire, J. McCormick, S. A. Hefta, S. T. Cole, and P. J. Brennan. 1994. Purification, characterization, gene sequence, and significance of a bacterioferritin from Mycobacterium leprae. J. Exp. Med. 180:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Toll-like receptor (TLR) 2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 34.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 35.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 36.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 37.Stoner, G. L. 1979. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet ii:994-996. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 39.Vabulas, R. M., P. Ahmad-Nejad, C. da Costa, T. Miethke, C. J. Kirschning, H. Hacker, and H. Wagner. 2001. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 276:31332-31339. [DOI] [PubMed] [Google Scholar]
- 40.Wakamatsu, S., M. Makino, C. Tei, and M. Baba. 1999. Monocyte-driven activation-induced apoptotic cell death of human T-lymphotropic virus type I-infected T cells. J. Immunol. 163:3914-3919. [PubMed] [Google Scholar]
- 41.World Health Organization. 2001. Global plan to stop the spread of tuberculosis. Wkly. Epidemiol. Rec. 76:335-336. [Google Scholar]