Abstract
Bartonella quintana is a louse-borne gram-negative bacillus that remains a poorly characterized cause of bacteremia, fever, and infective endocarditis. Due to the link with pediculosis, B quintana transmission is tied to poverty, conflict, overcrowding, and inadequate water access to maintain personal hygiene. Although these risk factors may be present globally, we argue that a substantial burden of undocumented B quintana infection occurs in Africa due to the high prevalence of these risk factors. Here, we describe the neglected burden of B quintana infection, endocarditis, and vector positivity in Africa and evaluate whether B quintana meets criteria to be considered a neglected tropical disease according to the World Health Organization.
Keywords: Africa, culture-negative bacterium, infective endocarditis, louse
The presence of Bartonella quintana human infection and lice positivity throughout the African continent indicates a hidden burden of illness. Here, we describe how B quintana meets criteria to be considered a neglected tropical disease.
Graphical Abstract
Graphical Abstract.
Bartonella quintana is a louse-borne gram-negative bacillus that remains a poorly characterized cause of bacteremia, fever, and infective endocarditis (IE) [1]. B quintana is primarily transmitted via the inoculation of infected body louse feces into abraded human skin and mucous membranes [1]. Once in the human host, the bacterium infects erythrocytes, causing chronic bacteremia [1]. Due to the link with pediculosis, B quintana transmission is tied to poverty, conflict, overcrowding, and inadequate water access to maintain personal hygiene [1, 2]. Although these risk factors may be present globally, we argue that a substantial burden of undocumented B quintana infection occurs in Africa due to the high prevalence of these risk factors.
The first description of B quintana infection was reported among World War I soldiers, causing a syndrome known as trench fever [3]. In the 1990s, B quintana was determined to cause bacteremia and IE among houseless individuals living in cities of high-income countries, leading to the designation “urban trench fever” [1]. While many studies of B quintana focus on urban trench fever in Europe and the United States, some of the first documented epidemics of B quintana occurred in Africa, with Ethiopian and Tunisian outbreaks occurring in 1946 and 1961, respectively [4–6]. Contemporary descriptions of B quintana acquired in Africa are predominantly cases of IE among African inhabitants immigrating to high-income countries outside Africa and diagnosed there.
IE is B quintana's most severe clinical syndrome. B quintana IE predominantly affects the aortic valve, causing valvular destruction with large vegetations prone to embolization [1, 7]. Before valvular damage occurs, most cases of B quintana IE are associated with months of nonspecific symptoms [1]. Consequently, cases of B quintana IE are diagnosed late, after heart failure and embolization occur, if diagnosed at all. Mortality rates due to B quintana IE exceed 10%, even with recommended treatment involving antimicrobials and valvular replacement surgery [1]. In 2023, Bartonella test positivity was added as a major update to the modified Duke criteria for IE [8].
This update reflects the complexity of diagnosing Bartonella infections, including those caused by B quintana. Species within the Bartonella genus, including B quintana, are difficult to culture from blood and are typically not identified via routine 5-day incubation [1]. The bacillus is thus designated a culture-negative bacterial pathogen and a major cause of culture-negative IE (CNIE) [1]. As with the 8 other Bartonella species known to cause IE, B quintana infection is primarily diagnosed via serologic and molecular techniques [9]. Serology, such as indirect immunofluorescent antibody testing (IFA), identifies current or previous Bartonella infection but may be limited by cross-reactivity with other pathogens and by its inability to identify Bartonella to the species level, unless cross-adsorption procedures are used, which are restricted to reference laboratories [1, 10]. Serologic positivity of different tests with different pathogen antigens may not exclude possible coexposure to multiple pathogens [11]. Speciating B quintana necessitates molecular techniques such as polymerase chain reaction (PCR) with species-specific primers [1, 12]. For cases of IE, sensitivity is highest when testing is performed on explanted cardiac tissue rather than blood samples [1, 9]. The reliance on molecular techniques performed on invasive tissue samples undermines the feasibility of diagnosing B quintana in African settings that have limited access to cardiovascular surgery and where 1.3% of the biologic laboratories on the continent perform bacteriologic culture and antimicrobial susceptibility testing [13, 14]. Nevertheless, the number of cases of B quintana IE acquired throughout the African continent indicate hidden transmission (Figure 1, Supplementary Appendices 1 and 2). Here, we describe the neglected burden of B quintana infection in Africa and evaluate whether B quintana meets criteria to be considered a neglected tropical disease (NTD).
Figure 1.
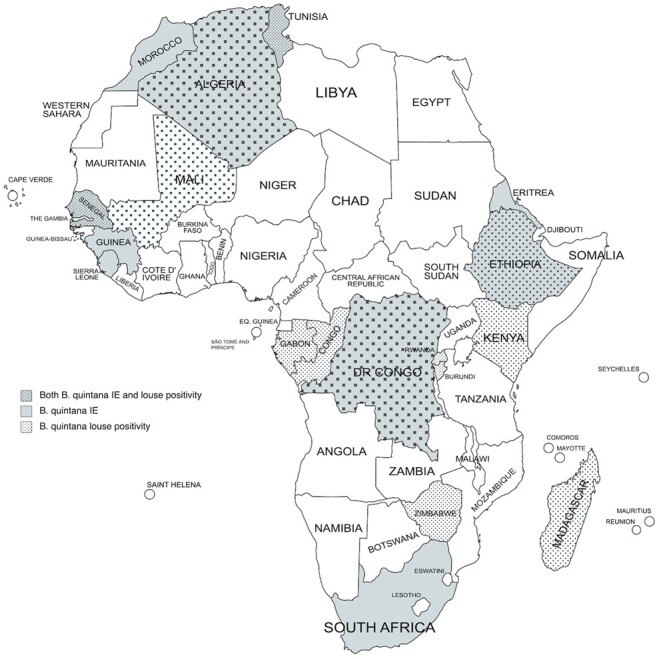
Map of Bartonella quintana infective endocarditis and louse positivity on the African continent. References for this map are available in Supplementary Appendices 1 and 2. IE, infective endocarditis.
B quintana in Sub-Saharan Africa
The first documented outbreak of B quintana in sub-Saharan Africa occurred in Ethiopia in 1946, and the Horn of Africa continues to be endemic for B quintana [4]. In 2017, among 5 Ethiopian children undergoing cardiovascular surgery for IE in Israel, B quintana was the main agent in 4 of 5 patients [15]. In 2023, an additional Ethiopian case of B quintana endocarditis was added to this series [16]. While the sample size remains small, B quintana was the predominant cause of IE in this cohort despite testing for alternate etiologies [15]. In 2021, B quintana endocarditis was diagnosed in an Eritrean man living in the Netherlands after presenting with fatigue, renal failure, and weight loss [17]. One year later, an additional case of B quintana endocarditis was reported in a 31-year-old Eritrean woman soon after immigrating to Canada [7].
B quintana is common in louse vectors in the Horn of Africa. B quintana is the most common pathogen in Ethiopian lice, as identified in >9% of 65 head lice pools [18]. While body louse transmission of B quintana is well established, entomologic and epidemiologic studies suggest that head lice may occasionally transmit B quintana, especially in resource-poor contexts, as highlighted by a recent review article and a B quintana outbreak associated with head lice in Senegal [19, 20]. In a study of 271 head and 424 body lice collected from 134 Ethiopian individuals, B quintana was identified in 6.7% and 12.7% of those with lice, respectively [21]. B quintana positivity in Ethiopian lice and the pathogen's presence among cases of IE acquired in Ethiopia and Eritrea suggest a burden of undiagnosed B quintana infection in the Horn of Africa.
Regions of sub-Saharan Africa outside the Horn of Africa have long been known to harbor B quintana. Cases of B quintana were recognized during a 1995 outbreak of epidemic typhus in Burundi [22]. IE in the region was previously characterized by high rates of CNIE, but recent results from the Tygerberg Endocarditis Cohort suggest that B quintana may be the predominant cause CNIE in South Africa. The Tygerberg Endocarditis Cohort is a study of patients with endocarditis treated at Tygerberg Academic Hospital, Cape Town, South Africa [23]. This study included 140 patients, of which 65 were recruited prospectively. In the prospective arm, patients with suspected IE were subjected to a set protocol for organism detection, including serology for Bartonella species and routine tissue PCR at the time of surgery [23]. This study identified B quintana as the most common cause of CNIE and the second-most common cause of IE after Staphylococcus aureus [23]. This finding supports the notion that B quintana may be a major contributor to the high rates of CNIE in Southern Africa.
In 2022, an outbreak of febrile illness due to B quintana occurred in the Senegalese village of Ndiop [20]. Of the 228 patients whose blood sample was tested for B quintana, 4.4% (n = 10) were positive by real-time PCR [20]. Previously, 3 cases of B quintana IE acquired in Senegal were diagnosed in Europe, including a pediatric case of a 13-year-old girl and a fatal case of a 50-year-old man [24, 25]. In 2019, B quintana IE was reported in a 37-year-old school teacher after immigrating from the Democratic Republic of Congo to the United States [26]. Here too, the authors maintained that the infection was acquired in the Democratic Republic of Congo. In 2023, there was a case of B quintana IE in an 11-year-old patient from Rwanda who underwent valve replacement surgery in Israel [16]. These descriptions exemplify cases of B quintana endocarditis acquired in Africa but diagnosed and treated outside the African continent.
The sub-Saharan African presence of infection due to Bartonella species and B quintana was demonstrated in a 2021 multicenter study of febrile illness in African children: Bartonella species were among the first- and second-most common origins of bacterial DNA found in the blood of patients in Madagascar and Burkina Faso who were febrile, as identified in 2.5% and 2% of all acute fever cases, respectively [27]. Of the 9 Bartonella cases from Madagascar, 4 were speciated to B quintana [27]. No other Bartonella species were identified [27]. While this study did not identify all Bartonella-associated febrile illness to the species level, the only Bartonella cases that underwent sequencing were identified as B quintana, supporting the notion that Bartonella species—and B quintana specifically—likely remain common and underrecognized causes of fever in sub-Saharan Africa.
B quintana in North Africa
Many of the first cases of B quintana IE were acquired in North Africa, and recent cohorts from the region identify B quintana as a common cause of CNIE. The first African case of B quintana IE was reported in 1996 in a 34-year-old Algerian farmer, only 3 years after the first publication on B quintana IE in 1993 [5, 28]. As the Algerian farmer denied houselessness, alcohol use, and ectoparasitosis, this description provided the first suggestion that risk factors for B quintana “may be different in western Europe or North America and in Africa” [28]. Subsequently, 14 other Algerian cases of B quintana IE were described, and 12 cases were identified from Tunisia, leading the authors to conclude that the disease “seems to be very common in Tunisia” [29, 30]. While specific risk factors for B quintana infection in North Africa have not been explicitly studied, the majority of B quintana IE from Algeria and Tunisia was among individuals with “poor living conditions” who lived in crowded households “of at least 10 persons” [29, 30]. This suggests that overcrowding and poverty are risk factors for B quintana infection, independent of houselessness and alcohol use disorder.
Estimating the Hidden Burden of B quintana in Africa
The available evidence on human B quintana infection acquired in Africa is disproportionately based on cases of IE treated in high-income countries outside Africa and regions within Africa with access to cardiovascular services. This reflects bias due to the unequal availability of cardiovascular surgery and molecular diagnostics [14, 31]. Estimating the burden of B quintana in Africa is limited by the compound difficulties of identifying B quintana infection microbiologically and diagnosing endocarditis clinically. Estimating B quintana prevalence is problematic as those who are infected may have subclinical disease and may not seek care. These difficulties are exacerbated by the fact that the disease is not notifiable.
Studies of houseless populations in high-income countries suggest that >15% of individuals had prior B quintana infection and 5% to 14% had B quintana bacteremia [1, 32]. Applying these figures to African jurisdictions where B quintana IE has been reported would suggest a burden of undiagnosed infection, even if the risk would be several fold lower in this context. Considering that 20% of patients with B quintana bacteremia may develop IE, a substantial number of B quintana IE may be overlooked [1, 33]. This issue is complicated by the chronicity of B quintana bacteremia, which may persist well over a year despite minimal symptoms [6, 34].
While large studies of heart failure and IE in Africa are scant, the existing data suggest a hidden burden of CNIE, possibly consistent with B quintana [14, 31]. In a cross-sectional study of 106 Ethiopian children admitted with acute heart failure, IE was the most common cardiac etiology [35]. In a systematic review of IE in Africa, half of the cases were culture negative [14, 23]. While direct evidence of B quintana IE in large prospective African studies is limited to the Tygerberg Endocarditis Cohort and the studies described here, it is possible that many of the cases of CNIE are due to undiagnosed B quintana. This systematic review also characterizes IE in Africa as a disease of young patients, a finding reflected in the cases of B quintana IE described earlier [14].
Does B quintana Meet Criteria to Be Considered a Neglected Tropical Disease?
NTDs are a diverse grouping of diseases united by their association with poverty and their disproportionate burden in tropical and subtropical areas [36]. The World Health Organization (WHO) has 4 criteria for designating a condition as an NTD (Table 1) [36]. Existing evidence suggests that B quintana may meet these criteria, though additional research is needed.
Table 1.
WHO Criteria for Classifying a Condition as a Neglected Tropical Disease and Applicability to Bartonella quintana
| WHO Criteria for Neglected Tropical Disease Classification | Applicability to B quintana |
|---|---|
| 1. Disproportionately affect populations living in poverty and cause important morbidity and mortality—including stigma and discrimination. |
|
| 2. Primarily affects populations living in tropical and subtropical areas. |
|
| 3. Is immediately amenable to broad control, elimination, or eradication by applying ≥1 of the 5 public health strategies adopted by the Department for Control of Neglected Tropical Diseases. |
|
| 4. Is relatively neglected by research—specifically, resource allocation is not commensurate with the magnitude of the problem. |
|
Abbreviation: WHO, World Health Organization.
B quintana Disproportionately Affects Populations Experiencing Poverty
B quintana causes morbidity and mortality among populations experiencing houselessness, displacement, and lack of access to running water to maintain personal hygiene [1, 2]. Within Africa, B quintana positivity in lice is correlated to country gross domestic product [2]. Countries with lower gross domestic product have a higher prevalence of lice with B quintana, though specific risk factors for B quintana infection in the African context have not been thoroughly investigated [2]. B quintana IE causes morbidity in the form of heart failure, renal dysfunction, and embolization [1]. As access to cardiovascular surgery is limited in many African contexts, mortality due to B quintana IE may be simultaneously amplified and underreported. Stigma occurs via the association with ectoparasitosis. Individuals with pediculosis may be shunned due to fears of transmission, pruritis, and the development of skin abrasions. As illustrated by the cases presented here, B quintana acquired in Africa disproportionately affects a younger population, for an additional economic burden on families, although cohort studies that incorporate an economic analysis are needed to better elucidate the cost of B quintana infection in Africa.
B quintana Primarily Affects Populations Living in Tropical and Subtropical Areas
While B quintana was historically described as being localized to Northern Europe, the disproportionate burden of B quintana infection in Africa has long been postulated, including a gradient of increasing B quintana infection from Northern Europe to African countries [29]. This likely reflects an economic gradient rather than a climactic one [2]. The preponderance of B quintana cases reported from high-income countries in nontropical areas likely reflects the combined availability of cardiovascular surgery and molecular diagnostics to speciate B quintana, rather than a large burden of disease, as indicated by the cases of B quintana endocarditis from northern high-income countries where the pathogen was determined to be acquired in Africa [26, 37–40]. The largest description of Bartonella endocarditis is a French study where B quintana was detected in 48 (53%) cases [39]. A recent systematic review of B quintana endocarditis identified 105 (62.9%) cases to be acquired in a high-income country [40]. More studies are needed to determine the true burden of B quintana infection in low- and middle-income countries, including those on the African continent.
B quintana Is Immediately Amenable to Broad Control
B quintana infection may be eliminated by applying a combination of 4 of the 5 public health strategies adopted by the WHO for control of NTDs [36]. Oral antimicrobials, such as doxycycline, may be used as chemotherapy to prevent B quintana disease in areas with elevated B quintana infection, as occurs with mass drug administration for other neglected infections, though studies involving B quintana are lacking [41]. Intensified case management and household contract tracing may use existing technology, such as IFA, to screen people for infection prior to endocarditis-related morbidity and mortality. Vector control is feasible by using existing pediculicidal agents, such as permethrin and ivermectin, and interventions to improve access to water to maintain personal hygiene. The latter may be integrated into existing WASH interventions (water, sanitation, and hygiene) that are known to be indispensable components of NTD control.
Research on B quintana Is Neglected
All aspects of B quintana research are neglected. B quintana epidemiology is poorly described, with most studies focusing on houseless populations in high-income countries, as exemplified by studies of B quintana bacteremia among houseless persons in the United States and France [1, 5, 6, 42]. Seroprevalence studies in Africa are lacking. No large studies describe the prevalence of B quintana infection among people living on the African continent and presenting with fever, heart failure, or symptoms of embolization. Diagnosis of B quintana is complex, relying on expensive equipment and specialized personnel. IFA, the main serologic test for B quintana, has low throughput and necessitates a fluorescent microscope, which may not be available in laboratories in certain low-resource settings. Interpretation of IFA requires specific training. Many of the diagnostic companies that produce commercial B quintana IFA kits do not distribute to certain African countries where B quintana is endemic [18]. Isolating B quintana from blood culture samples necessitates specific techniques, such as lysis centrifugation, freeze and thaw, subculturing, and prolonged incubation up to 45 days [1, 43]. If growth occurs, the pathogen is excluded from many commercial databases for interpreting results based on matrix-assisted laser desorption and ionization time-of-flight mass spectrometry, requiring the inclusion of additional spectra limited to specific international reference laboratories [44, 45]. PCR from whole blood has limited sensitivity as compared with its performance on invasive tissue samples, and there is no consensus on the best molecular target to use [46, 47]. Even in high-income countries, B quintana testing is often centralized in reference laboratories. B quintana serology and PCR are not available in most African countries, despite the capacity of many national laboratories to perform these tests. Rapid diagnostic tests, such as immunochromatographic tests, do not exist, and none are in development. Treatment for B quintana is largely based on a small open trial of gentamicin and doxycycline vs placebo for treatment of chronic bacteremia [48]. As gentamicin is associated with significant toxicity, drug-level monitoring is recommended but is rarely available in low-resource settings where the infection is endemic. American resources recommend treatment with rifampin and doxycycline based on limited data [49]. There are no published or registered randomized controlled trials comparing the efficacy of different antimicrobial regimens.
Public Health Recommendations
While B quintana may meet all 4 WHO NTD criteria to be considered for elimination, the current state of research on B quintana is so severely deficient that further research is needed to confidently define the condition as an NTD (Table 2). We believe that a few modest measures may substantially improve awareness of this neglected disease. We advocate for B quintana to be considered a national notifiable disease to ensure that data on existing cases are reported. We suggest making B quintana testing available at national reference laboratories capable of performing serology and PCR, as this would use existing infrastructure and trained personnel to avoid significant financial costs. We suggest that initial surveillance testing occur in batch among patients at high risk with an elevated pretest probability of B quintana infection, such as those with CNIE. For countries without capacity to perform B quintana testing, we propose that samples be sent to regional reference laboratories with dedicated B quintana diagnostic capacity, as occurs with other infections [50]. We encourage the development of immunochromatographic tests to facilitate surveillance in low-resource settings. Last, we advocate for randomized controlled trials to identify safer alternatives to gentamicin. Early detection and treatment of subclinical B quintana infection may prevent avoidable morbidity, mortality, and cost.
Table 2.
Key Bartonella quintana Knowledge Gaps and Associated WHO Criteria for Classifying a Condition as a Neglected Tropical Disease
| WHO Criteria for Neglected Tropical Disease Classification | Key Knowledge Gaps |
|---|---|
| 1. Disproportionately affect populations living in poverty and cause important morbidity and mortality—including stigma and discrimination. |
|
| 2. Primarily affects populations living in tropical and subtropical areas. |
|
| 3. Is immediately amenable to broad control, elimination, or eradication by applying ≥1 of the 5 public health strategies adopted by the Department for Control of Neglected Tropical Diseases. |
|
| 4. Is relatively neglected by research—specifically, resource allocation is not commensurate with the magnitude of the problem. |
|
Abbreviations: LMICs, low- and middle-income countries; WHO, World Health Organization.
The inverse care law states that the availability of care “tends to vary inversely with the need of the population served” [51]. B quintana predominantly affects individuals experiencing substantial need, with a concealed burden on the African continent. Recognizing that B quintana meets many NTD criteria is a first necessary step.
Supplementary Material
Contributor Information
Carl Boodman, Section of Infectious Diseases, Department of Internal Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Unit of Neglected Tropical Diseases, Clinical Sciences Department, Institute of Tropical Medicine, Antwerp, Belgium.
Noah Fongwen, Diagnostics Access, Africa Centres for Disease Control and Prevention, Addis Ababa, Ethiopia.
Alfonso J Pecoraro, Division of Cardiology, Department of Medicine, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa.
Adane Mihret, Microbiology Department, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Hiwot Abayneh, Microbiology Department, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Pierre-Edouard Fournier, French Reference Center for Rickettsioses, Q Fever and Bartonelloses, Institut Hospitalier Universitaire, Marseille, France.
Nitin Gupta, Department of Infectious Diseases, Kasturba Medical College, Manipal, India.
Johan van Griensven, Unit of Neglected Tropical Diseases, Clinical Sciences Department, Institute of Tropical Medicine, Antwerp, Belgium.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. C. B.’s salary is supported by the University of Manitoba's Clinical Investigator Program. No other funding source is associated with this publication.
Author contributions . C. B.: conceptualization, methodology, investigation, writing (original draft), project administration. N. F.: writing (review and editing). A. J. P.: writing (review and editing), supervision. A. M.: writing (review and editing), methodology, resources/investigation. H. A.: writing (review and editing), investigation. P.-E. F.: writing (review and editing), supervision. N. G.: writing (review and editing), project administration. J. v. G: conceptualization, writing (review and editing), supervision.
Patient consent statement : This study exclusively uses existing published data and thus does not include factors necessitating patient consent or ethical committee approval.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis J 2006; 12:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sangaré AK, Boutellis A, Drali R, et al. . Detection of Bartonella quintana in African body and head lice. Am Soc Trop Med Hyg 2014; 91:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graham JHP. A note on a relapsing febrile illness of unknown origin. Lancet 1915; 186:703–4. [Google Scholar]
- 4. Vinson JW. In vitro cultivation of the rickettsial agent of trench fever. Bull World Health Organ 1966; 35:155–64. [PMC free article] [PubMed] [Google Scholar]
- 5. Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis 2000; 31:131–5. [DOI] [PubMed] [Google Scholar]
- 6. Foucault C, Barrau K, Brouqui P, Raoult D. Bartonella quintana bacteremia among homeless people. Clin Infect Dis 2002; 35:684–9. [DOI] [PubMed] [Google Scholar]
- 7. Boodman C, Wuerz T, Lagacé-Wiens P, et al. . Serologic testing for Bartonella in Manitoba, Canada, 2010–2020: a retrospective case series. CMAJ Open 2022; 10:E476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowler VG Jr, Durack DT, Selton-Suty C, et al. . The 2023 Duke-ISCVID criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis 2023; 77:518–26.37138445 [Google Scholar]
- 9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev 2017; 30:709–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boodman C, Gupta N. Schrödinger's cat paradox: Bartonella serology cannot be used to speciate Bartonella endocarditis. Open Forum Infect Dis 2023; 10:ofad436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiel N, Baker M, Lipton B, Fuller L, Breitschwerdt EB, Rabinowitz P. Risk factors for Bartonella seroreactivity among veterinary workers in the Pacific Northwest. Vector Borne Zoonotic Dis 2023; 23:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCormick D, Rassoulian-Barrett S, Hoogestraat D, et al. . Bartonella spp infections identified by molecular methods, United States. Emerg Infect Dis J 2023; 29:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. African Society for Laboratory Medicine . Mapping Antimicrobial Resistance and Antimicrobial Use Partnership. 2023. Available at: https://aslm.org/what-we-do/maap/. Accessed 2 October 2023.
- 14. Noubiap JJ, Nkeck JR, Kwondom BS, Nyaga UF. Epidemiology of infective endocarditis in Africa: a systematic review and meta-analysis. Lancet Glob Health 2022; 10:e77–86. [DOI] [PubMed] [Google Scholar]
- 15. Tasher D, Raucher-Sternfeld A, Tamir A, Giladi M, Somekh E. Bartonella quintana, an unrecognized cause of infective endocarditis in children in Ethiopia. Emerg Infect Dis 2017; 23:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato S, Shapira L, Tasher D, Maruyama S, Giladi M. Molecular epidemiology of Bartonella quintana endocarditis in patients from Israel and Eastern Africa. BMC Infect Dis 2023; 23:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plantinga NL, Vos RJ, Georgieva L, Roescher N. Bartonella quintana as a cause for prosthetic valve endocarditis and post-sternotomy mediastinitis. Access Microbiol 2021; 3:000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cutler S, Abdissa A, Adamu H, Tolosa T, Gashaw A. Bartonella quintana in Ethiopian lice. Comp Immunol Microbiol Infect Dis 2012; 35:17–21. [DOI] [PubMed] [Google Scholar]
- 19. Feldmeier H. Head lice as vectors of pathogenic microorganisms. Trop Med Health 2023; 51:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammoud A, Louni M, Fenollar F, et al. . Bartonella quintana transmitted by head lice: an outbreak of trench fever in Senegal. Clin Infect Dis 2023; 76:1382–90. [DOI] [PubMed] [Google Scholar]
- 21. Angelakis E, Diatta G, Abdisa A, et al. . Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg Infect Dis 2011; 17:2357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raoult D, Ndihokubwayo JB, Tissot-Dupont H, et al. . Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 1998; 352:353–8. [DOI] [PubMed] [Google Scholar]
- 23. Pecoraro AJK, Pienaar C, Herbst PG, et al. . Causes of infective endocarditis in the Western Cape, South Africa: a prospective cohort study using a set protocol for organism detection and central decision making by an endocarditis team. BMJ Open 2021; 11:e053169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barbe KP, Jaeggi E, Ninet B, et al. . Bartonella quintana endocarditis in a child. N Engl J Med 2000; 342:1841–2. [DOI] [PubMed] [Google Scholar]
- 25. Thiam M, Fall PD, Gning SB, Grinda JM, Mainardi JL. Bartonella quintana infective endocarditis in an immunocompetent Senegalese man. Rev Med Interne 2002; 23:1036–7. [DOI] [PubMed] [Google Scholar]
- 26. Mohammadian M, Butt S. Endocarditis caused by Bartonella quintana, a rare case in the United States. IDCases 2019; 17:e00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marks F, Liu J, Soura AB, et al. . Pathogens that cause acute febrile illness among children and adolescents in Burkina Faso, Madagascar, and Sudan. Clin Infect Dis 2021; 73:1338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mainardi JL, Drancourt M, Roland JM, et al. . Bartonella (Rochalimaea) quintana endocarditis in an Algerian farmer. Clin Microbiol Infect 1996; 1:275–6. [DOI] [PubMed] [Google Scholar]
- 29. Znazen A, Rolain J-M, Hammami N, Kammoun S, Hammami A, Raoult D. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg 2005; 72:503–7. [PubMed] [Google Scholar]
- 30. Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis 2005; 11:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pecoraro AJ, Herbst PG, Doubell AF. Infective endocarditis in Africa: an urgent call for more data. Lancet Glob Heal 2022; 10:e8–9. [DOI] [PubMed] [Google Scholar]
- 32. McCormick D, Rowan S, Pappert R, et al. . Bartonella seroreactivity among persons experiencing homelessness during an outbreak of Bartonella quintana in Denver, Colorado, 2020. Open Forum Infect Dis 2021; 8:ofab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spach DH, Kanter AS, Dougherty MJ, et al. . Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med 1995; 332:424–8. [DOI] [PubMed] [Google Scholar]
- 34. Kostrzewski J. The epidemiology of trench fever. Bull Int Acad Pol Sci Let Cl Med 1949; 7:233–63. [PubMed] [Google Scholar]
- 35. Gebremariam S, Moges T. Pediatric heart failure, lagging, and sagging of care in low income settings: a hospital based review of cases in Ethiopia. Cardiol Res Pract 2016; 2016:7147234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. Recommendations for the adoption of additional diseases as neglected tropical diseases. 2017. Available at: https://cdn.who.int/media/docs/default-source/ntds/strategic-and-advisory-group-on-neglected-tropical-diseases-(stag-ntds)/tenth-ntdstag-report-2017.pdf?sfvrsn=9ec99065_2&download=truedate. Accessed 15 October 2023.
- 37. Barbe KP, Jaeggi E, Ninet B, et al. . Bartonella quintana endocarditis in a child. N Engl J Med 2000; 342:1841–2. [DOI] [PubMed] [Google Scholar]
- 38. Thiam M, Fall PD, Gning SB, Grinda JM, Mainardi JL. Bartonella quintana infective endocarditis in an immunocompetent Senegalese man. Rev Med Interne 2002; 23:1035–7. [DOI] [PubMed] [Google Scholar]
- 39. Edouard S, Nabet C, Lepidi H, Fournier P-E, Raoult D. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol 2015; 53:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boodman C, Gupta N, Nelson C, van Griensven J. Bartonella quintana endocarditis: a systematic review of individual cases. Clin Infect Dis 2023. doi:10.1093/cid/ciad706 [DOI] [PubMed] [Google Scholar]
- 41. Karthikeyan K. Mass drug administration in neglected tropical diseases: beyond elimination. Lancet Glob Health 2023; 11:e813–4. [DOI] [PubMed] [Google Scholar]
- 42. Jackson LA, Spach DH. Emergence of Bartonella quintana infection among homeless persons. Emerg Infect Dis 1996; 2:141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maggi RG, Richardson T, Breitschwerdt EB, Miller JC. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species within human clinical samples. J Microbiol Methods 2020; 176:106022. [DOI] [PubMed] [Google Scholar]
- 44. El Hamzaoui B, Laroche M, Almeras L, Bérenger J-M, Raoult D, Parola P. Detection of Bartonella spp in fleas by MALDI-TOF MS. PLoS Negl Trop Dis 2018; 12:e0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El Hamzaoui B, Laroche M, Parola P. Detection of Bartonella spp in Cimex lectularius by MALDI-TOF MS. Comp Immunol Microbiol Infect Dis 2019; 64:130–7. [DOI] [PubMed] [Google Scholar]
- 46. Wolf LA, Cherry NA, Maggi RG, Breitschwerdt EB. In pursuit of a stealth pathogen: laboratory diagnosis of bartonellosis. Clin Microbiol Newsl 2014; 36:33–9. [Google Scholar]
- 47. Agan BK, Dolan MJ. Laboratory diagnosis of Bartonella infections. Clin Lab Med 2002; 22:937–62. [DOI] [PubMed] [Google Scholar]
- 48. Foucault C, Raoult D, Brouqui P. Randomized open trial of gentamicin and doxycycline for eradication of Bartonella quintana from blood in patients with chronic bacteremia. Antimicrob Agents Chemother 2003; 47:2204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spach DH. Bartonella quintana infections: clinical features, diagnosis, and treatment. 2023. Available at: https://www.uptodate.com/contents/bartonella-quintana-infections-clinical-features-diagnosis-and-treatment. Accessed 16 October 2023.
- 50. World Health Organization . WHO H5 reference laboratories. 2023. Available at: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system/h5-reference-laboratoriesdate. Accessed 20 October 2023.
- 51. Hart JT. The inverse care law. Lancet 1971; 297:405–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.