Abstract
Immunoglobulin A1 (IgA1) protease, an enzyme that selectively cleaves human IgA1, may be a virulence factor for pathogenic organisms such as Neisseria gonorrhoeae. Host protection from the effects of IgA1 protease includes antibody-mediated inhibition of IgA1 protease activity, and it is believed that the relative balance between IgA1 protease and inhibitory antibodies contributes to the pathogenesis of disease caused by IgA1 protease-producing organisms. We have examined the levels of these two opposing factors in genital tract secretions and sera from women with uncomplicated infection with N. gonorrhoeae. When IgA1 in cervical mucus was examined by Western blotting, no evidence of cleavage fragments characteristic of IgA1 protease activity was seen in gonococcus-infected or control patients. Cleavage fragments typical of IgA1 protease were detected, however, after the addition of exogenous IgA1 protease to cervical mucus. Degraded IgA1 was detected in some vaginal wash samples, but the fragment pattern was not typical of IgA1 protease activity. All N. gonorrhoeae isolates from the infected patients produced IgA1 protease in vitro. All but two serum samples and 16 of 65 cervical mucus samples displayed inhibitory activity against gonococcal IgA1 protease, but there was no significant difference in the level of inhibitory activity between gonococcus-infected and noninfected patients in either cervical mucus or serum. There was no difference in the levels of IgA1 protease-inhibitory activity in serum or cervical mucus collected from patients at recruitment and 2 weeks later. These results suggest that cleavage of IgA1 by gonococcal IgA1 protease within the lumen of the female lower genital tract is unlikely to be a significant factor in the pathogenesis of infections by N. gonorrhoeae.
Immunoglobulin A1 (IgA1) proteases are postproline endopeptidases that were initially recognized by their ability to specifically cleave human IgA1, but not IgA2, at the hinge region (28). Cleavage of human IgA1 by IgA1 protease generates specific Fabα and Fcα fragments [or, in the case of secretory IgA1 (S-IgA1), (Fcα)2.J.SC fragments]. Apart from the great apes, which have IgA1 resembling that found in humans, no other species has IgA that is cleavable by these enzymes, and until recently, no other mammalian substrates for them were known. IgA1 protease is produced by a variety of organisms, including the pathogenic species of Neisseria, Haemophilus influenzae, Streptococcus pneumoniae, and some oral streptococci and periodontal pathogens, as well as Ureaplasma urealyticum, and it has been implicated as a virulence factor in the pathogenesis of human mucosal infections caused by them (for a recent review, see reference 17). Cleavage of intact IgA1 (or S-IgA1) results in the loss of Fcα-mediated secondary effector functions, such as inhibition of adherence (7, 15), despite the retention of antigen-binding activity by the Fabα fragments (25). Moreover, it has been hypothesized that Fabα fragments may simultaneously block the binding of intact (functional) antibodies of IgA or other isotypes to bacterial epitopes (15) and thereby inhibit, for example, complement activation and bacteriolysis (13, 31). IgA1 protease activity can be inhibited by antibodies (6, 19), and antibodies to IgA1 protease and inhibitory antibodies have been detected in serum and secretions from patients infected with IgA1 protease-producing organisms (3, 5). It has been suggested that the pathogenesis of infections with IgA1 protease-producing organisms is dependent, in part, upon the balance between the relative levels of IgA1 protease and neutralizing antibody.
Neisseria meningitidis and Neisseria gonorrhoeae, but not closely related nonpathogenic Neisseria species, produce IgA1 protease, suggesting that it serves as a virulence factor for these bacteria (26). Similar considerations apply to pathogenic and nonpathogenic species of Haemophilus (16). However, experimental investigation of the role of IgA1 proteases as virulence factors is complicated by the fact that, other than great apes, no animal models are available because of the specificity of IgA1 proteases for human and ape IgA1. Furthermore, N. gonorrhoeae is exclusively a human pathogen. Vaginal washes from patients infected with N. gonorrhoeae have been found to contain IgA1 protease activity demonstrable in vitro (2). Fallopian tube organ culture experiments showed that an IgA1 protease-deficient mutant of N. gonorrhoeae was not defective in its ability to colonize (4). However, it has been pointed out that in the absence of the relevant substrate for IgA1 protease, i.e., IgA1, and especially IgA1 antibody against the organism, it is not possible to draw definitive conclusions as to the role of the enzyme in pathogenesis. Furthermore, there are no reports relating the levels and activities of IgA1 protease and IgA1 protease-inhibitory antibodies to the development of IgA1 antigonococcal antibodies during human infection with N. gonorrhoeae. Therefore, in this study we have examined the role of IgA1 protease and inhibitory activity against IgA1 protease in naturally acquired N. gonorrhoeae infections in humans.
MATERIALS AND METHODS
Patients.
Female patients attending the Jefferson County Department of Health Sexually Transmitted Diseases (STD) Clinic (Birmingham, Ala.) were recruited into the study, and informed consent was obtained from each patient prior to enrollment. Patients attending the clinic, but not infected with N. gonorrhoeae, were enrolled as controls. Presumption of infection was based on clinical criteria, including Gram-stained smears, and infection was confirmed by routine culture methods (see below). The median age of infected patients was 23 (range, 17 to 57) years, and that of patients not infected with N. gonorrhoeae was 25.5 (range, 19 to 36) years. Where possible, samples of cervical mucus, vaginal wash, and blood were obtained from the patients during three visits to the STD Clinic spaced at approximately 2-week intervals following their initial visit. Samples were collected from 51 patients (20 infected and 31 not infected with N. gonorrhoeae), but not all specimens were obtained from every patient. All infected patients were given appropriate antibiotic treatment during their first visit and were tested for infection at subsequent visits; no patient remained infected after treatment.
Sample collection and handling.
Cervical mucus (∼0.05 to 0.5 ml) was mechanically collected with a sterile swab and dispersed in an approximately equal volume of sterile phosphate-buffered saline (PBS) containing a 2× protease inhibitor cocktail (final composition: 10 mM EGTA, 150 mM NaN3, 0.01% [wt/vol] leupeptin [Sigma Chemical Co., St. Louis, Mo.], 0.02 M Pefabloc [Boehringer Mannheim, Indianapolis, Ind.]). The suspended mucus was treated with Nonidet P-40 (final concentration, 0.01%; Sigma), then diluted one-fifth (or up to a maximum of 2 ml) with PBS, vortexed, stored overnight at 4°C, finally centrifuged at 1,500 × g for 10 min at 4°C, then aliquoted, and stored at −70°C.
Vaginal wash samples were collected by instilling 10 ml of PBS and recovering approximately 8 ml by aspiration; 0.1 volume of the above-described protease inhibitor cocktail at a 10× concentration was then added. The wash sample was centrifuged at 2,000 × g for 10 min at 4°C. The supernatant was treated with Nonidet P-40, aliquoted, and stored at −70°C.
Peripheral venous blood was collected in a Vacutainer tube and allowed to clot, and the serum was recovered by centrifugation. Nonidet P-40 was added as described above, and aliquots were stored at −70°C.
IgA1 antibody and Ig measurement.
Cervical mucus and vaginal wash were tested for total IgA1 concentrations by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well plates were initially coated with human-absorbed goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, Ala.) (diluted 1:1,000) followed by overnight incubation with mouse anti-human IgA1 (Sigma) diluted 1:2,000. Duplicate serial dilutions of sample were incubated overnight. Bound Igs were detected with peroxidase-conjugated goat anti-human IgA (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) incubated for 4 h. The levels of IgA1 antibodies to N. gonorrhoeae MS11 were estimated in the same samples. For use as antigen, N. gonorrhoeae MS11 (kindly provided by Mogens Kilian) was taken from frozen stocks and cultured on chocolate agar plates (Becton Dickinson, Cockeysville, Md.) at 37°C in a 5% CO2–air atmosphere. Bacteria were scraped from plate cultures showing confluent growth and resuspended in 1 ml of PBS, and optical density at 590 nm (OD590 was measured. The concentration of each bacterial preparation was estimated by comparison to a previously determined standard ratio of bacterial cell concentration and OD590. Bacterial preparations were subsequently fixed with 0.5% formaldehyde at 4°C overnight. Whole, formaldehyde-treated N. gonorrhoeae cells were used to coat plates at a concentration of 5 × 106 CFU/well, followed by duplicate serial dilutions of sample incubated overnight. Bound IgA1 was detected by incubation with mouse anti-human IgA1 (Sigma) followed by incubation with peroxidase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates) for 4 h.
For all assays, color development used a substrate consisting of o-phenylenediamine and H2O2 in citrate-phosphate buffer (pH 4.0); development was stopped with sulfuric acid after 15 min, and absorbance was read at 490 nm in a Vmax microplate reader (Molecular Devices Corp., Menlo Park, Calif.) interfaced to a Macintosh computer for data retrieval. Standard curves were determined for each plate and type of assay from serial dilutions of human Ig calibrator at appropriate concentrations (The Binding Site Ltd., Birmingham, United Kingdom). Unknowns were interpolated on standard curves generated by a computer program with four-parameter logistic algorithms as described elsewhere (30), and parallelism between unknown and standard dilution curves was demonstrated over the range of unknown dilutions used for calculation.
Preparation and assay of IgA1 protease.
Crude preparations of IgA1 protease from N. gonorrhoeae MS11 were used for all assays and prepared as previously described (12). N. gonorrhoeae MS11 was streaked from frozen culture onto a chocolate agar plate (Becton Dickinson) and incubated overnight at 37°C. Growing bacteria were removed from the plate in 1 ml of sterile PBS by scraping with a sterile glass rod, and 0.1 ml of this suspension was spread on a chocolate agar plate that had been covered with a sterile dialysis membrane (molecular weight cutoff, 12,000 to 14,000; Spectrum, Houston, Tex.). The inoculated plates were subsequently incubated for 2 days at 37°C. When grown, the bacteria and secreted proteins were collected from the membrane on each plate by scraping with a sterile glass rod in 1 ml of sterile PBS. The bacterial suspension was centrifuged at 2,000 × g for 20 min, and the supernatant containing the IgA1 protease was sterilely filtered through a 0.22-μm-pore-size filter. Aliquots of IgA1 protease were frozen at −20°C until needed.
IgA1 protease activity was measured by the method described by Reinholdt (29). Briefly, serial dilutions of the crude IgA1 protease were prepared in 25 μl of PBS, and IgA1(κ) myeloma protein (25 μl) was added as substrate (final concentration, 10 μg/ml). After incubation at 37°C overnight, 150 μl of 0.01 M bathocuprone disulfonate (Sigma) was added to all wells to prevent further enzyme activity. Cleavage of IgA1 was measured by ELISA for IgA1 as described above, with the exception that bound IgA1 was detected with peroxidase-labeled anti-κ light chain antibodies (Dako). A dose-response curve of IgA1 cleavage was generated, and the dilution resulting in 50% cleavage of the IgA1 substrate was calculated (29). One unit of IgA1 protease activity is defined as the activity of IgA1 protease causing 50% cleavage under the conditions of the assay.
IgA1 protease inhibition assays.
The levels of IgA1 protease-inhibitory activity in samples of serum and cervical mucus were quantified by the method of Reinholdt (29), which was developed to overcome the problem that most biological fluids contain IgA1 that may serve as substrate for the protease. The principle of this assay is that sample dilutions are first incubated on ice with a fixed amount of IgA1 protease, to allow complexation of any antibody with the enzyme. Then sufficient substrate (IgA1 myeloma protein) is added, and residual IgA1 left after incubation is assayed by ELISA, in which IgA1 is captured by mouse anti-human IgA1 antibody and detected by means of labeled anti-light chain antibody. Cleaved Fcα1 fragments are also captured and therefore compete against intact IgA1 but, lacking Fab arms, are not detected. In practice, serial dilutions of cervical mucus (from 1:2) or serum (from 1:20) samples were prepared on ice in quadruplicate in microtiter plates by using PBS-Tween (PBST) containing 1 mM Ca2+. Two of the four rows of wells then received 25 μl of IgA1 protease diluted to 1 U of activity. The two remaining rows received 25 μl of PBST with Ca2+. Two additional wells received protease and PBST with Ca2+. Following incubation on ice for 1 h, all wells received 25 μl of substrate [monomeric myeloma IgA1(κ), 20 μg/ml, in PBST]. After incubation at 37°C overnight, 150 μl of 0.01 M bathocuprone disulfonate (Sigma) was added to all wells to prevent further enzyme activity. Cleavage of IgA1 was measured by ELISA for IgA1 (as described above) with the exception that bound IgA1 was detected with peroxidase-labeled anti-κ and anti-λ light chain antibodies (Dako). The IgA1 protease inhibition titer was defined as the dilution of sample that resulted in 50% inhibition of IgA1 cleavage. Samples without measurable inhibitory activity were arbitrarily assigned a titer corresponding to 0.1.
Western blots.
Cervical mucus samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7% acrylamide gel) under nonreducing conditions and then transferred to nitrocellulose by Western immunoblotting (11). In some experiments, cervical mucus (final IgA1 concentration, 24 μg/ml) was incubated at 37°C with 10 U of IgA1 protease in a final volume of 60 μl prior to electrophoresis and blotting. Replicate blots were probed with mouse anti-human IgA1 (Sigma) followed by horseradish peroxidase-conjugated anti-mouse IgG or horseradish peroxidase-conjugated antibody to human secretory component (SC; Dako Corp.). The binding site of the IgA1-specific monoclonal antibody used to probe these blots is not positively known; however, it is believed to be located within the CH2 or CH3 domains. Bands were revealed by development with 3-amino-9-ethylcarbazole–H2O2 substrate.
IgA1 protease production by N. gonorrhoeae isolates.
Clinical isolates of N. gonorrhoeae from infected patients were maintained frozen at −80°C until required. For measurement of protease production, strains were streaked onto chocolate agar plates and incubated for 1 to 2 days at 37°C and 5% CO2. A single isolated colony of N. gonorrhoeae was picked with a sterile loop, transferred to 200 μl of medium (18) in a 96-well plate, and incubated for 24 h at 37°C and 5% CO2. Bacterial growth was confirmed by monitoring the OD590 spectrophotometrically. Two replicates of 20 μl of medium from each N. gonorrhoeae strain were incubated overnight with 1 μg of IgA1(κ) myeloma protein in a total volume of 100 μl, then transferred to ELISA plates prepared for total IgA1 measurement, and incubated at room temperature overnight. Samples consisting of IgA1 protease from N. gonorrhoeae MS11, with and without the addition of IgA1 myeloma protein, were included as controls for each assay. Negative controls consisted of wells containing IgA1 myeloma protein but no protease. Bound intact IgA1 was detected with peroxidase-conjugated goat anti-human κ light chain antibodies (Dako) incubated for 4 h. Color development and absorbance readings were as described above for total Ig measurement. IgA1 protease was deemed to be present if the OD of the intact IgA1 was more than 2 standard deviations below the mean OD of IgA1 myeloma protein in the negative controls.
Statistics.
Statistical tests were performed with the Instat statistics program for Macintosh computers. The Kruskal-Wallis and Mann-Whitney U tests were used to test for differences between groups. P < 0.05 was considered significant.
RESULTS
IgA1 and antigonococcal IgA1 antibodies in infected patients.
If IgA1 proteases are to have a role in the pathogenesis of gonococcal infections, there needs to be an appropriate substrate for their enzymic activity. One relevant substrate is IgA1 and, in particular, IgA1 antibody against gonococcal antigens. IgA1 was detectable in almost all samples of cervical mucus and vaginal wash from patients, whether infected with N. gonorrhoeae or not (Table 1). IgA1 antibodies to gonococci were detected in some samples of cervical mucus and vaginal wash, as well as in all serum samples obtained from these patients (Table 2). Serum IgA1 antibodies were significantly higher in gonococcus-infected than in uninfected patients at both visit 1 and visit 2. IgA1 antibodies were found in approximately one-third of cervical mucus samples regardless of whether the subject was infected with N. gonorrhoeae. IgA1 antibody levels in vaginal wash samples were highly variable, and no statistically significant differences were discernible. Thus, currently infected subjects appear to have an appropriate substrate upon which gonococcal IgA1 protease may act in the form of IgA1 in genital tract secretions, and some patients also had IgA1 antibodies to N. gonorrhoeae.
TABLE 1.
Total IgA1 concentrations in genital secretions
| Sample | Median IgA1 concn in μg/ml [(range)/n]
|
|||
|---|---|---|---|---|
| Visit 1
|
Visit 2
|
|||
| Noninfected | N. gonorrhoeae infected | Noninfected | N. gonorrhoeae infected | |
| Cervical mucus | 50.2 (0.9–1,150.0)/31 | 49.1 (1.1–630.0)/17 | 59.5 (3.1–287.1)/15 | 20.3 (0–211.2)/13 |
| Vaginal wash | 4.6 (0.2–227.0)/29 | 3.7 (0.6–39.4)/16 | 6.4 (0.1–107.5)/14 | 2.5 (0.4–10.1)/10 |
TABLE 2.
IgA1 antigonococcal antibodies in patients with and without gonococcal infection
| Sample | Specific IgA1 antibody/total IgA1, median (range)a; no. of antibody-positive patientsb/n
|
|||
|---|---|---|---|---|
| Visit 1
|
Visit 2
|
|||
| Noninfected | N. gonorrhoeae infected | Noninfected | N. gonorrhoeae infected | |
| Cervical mucus | 0 (0–7,947); 7/30 | 0 (0–4,498); 7/17 | 0 (0–3,610); 5/15 | 0 (0–11,210); 5/13 |
| Vaginal wash | 0 (0–33,667); 10/29 | 0 (0–10,702); 7/16 | 0 (0–1,017); 5/14 | 1,879 (0–7,765); 6/10 |
| Serum | 32 (1–236); 31/31 | 139 (20–502)c; 19/19 | 63 (23–157); 16/16 | 133 (12–485)c; 13/13 |
Nanograms of antibody per milligram of IgA1.
Patients with detectable antibody levels (≥2 ng/ml).
P < 0.05 compared with noninfected patients at same visit (Mann-Whitney U test).
Cleavage fragments of IgA1 in cervical mucus and vaginal wash.
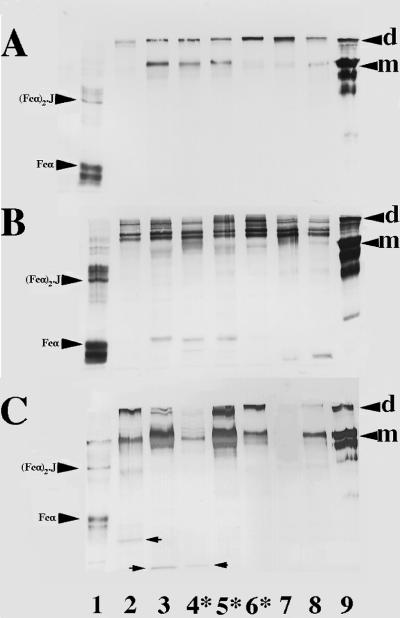
We examined whether protein fragments resulting from IgA1 protease digestion were present in cervical mucus from women infected with gonococci as well as noninfected subjects. Samples obtained from 49 patients at visit 1, from 24 patients at visit 2, and from 13 patients at visit 3 were analyzed by Western blotting for the presence of IgA1. Examples of IgA1 Western blots of cervical mucus and vaginal wash are presented in Fig. 1. Myeloma IgA1 protein and the same myeloma IgA1 protein digested with exogenous IgA1 protease (from N. gonorrhoeae MS11) were used as negative and positive controls, respectively, on every blot. Dimeric IgA1 and three bands of IgA1 that did not contain J chain (170, 160, and 150 kDa) were present in the nondigested myeloma protein. After digestion of the same myeloma protein with IgA1 protease, there was a decrease in the band densities corresponding to the intact IgA1 forms and the appearance of (Fcα)2.J and two to three bands of Fcα1.
FIG. 1.
IgA1 immunoblots of cervical mucus (A), cervical mucus after the addition of exogenous IgA1 protease (B), and vaginal wash samples (C) from patients infected with N. gonorrhoeae (first visit) or 2 weeks after treatment (second visit). Samples were electrophoresed in the presence of sodium dodecyl sulfate on 7.5% acrylamide gels under nonreducing conditions, transferred to nitrocellulose, and probed with mouse anti-human IgA1. Lane 1, IgA1 protease-digested IgA1 myeloma protein; lane 2, patient 31, second visit; lane 3, patient 32, second visit; lane 4, patient 33, first visit; lane 5, patient 34, first visit; lane 6, patient 35, first visit; lane 7, patient 33, second visit (panels A and B only; blank in panel C); lane 8, patient 36, first visit; lane 9, uncleaved IgA1 myeloma protein. Dimeric (d) and monomeric (m) IgA1 in the myeloma negative control and Fcα and (Fcα)2.J in the myeloma positive control are indicated. Arrows indicate IgA1 fragments in vaginal washes. All visit 1 samples shown were infected with gonococci (indicated by asterisks). All visit 2 samples are from patients who were infected at visit 1.
Monomeric and dimeric IgA1 were present in both cervical mucus and vaginal wash from infected and noninfected patients. Parallel blots of patient samples probed with antibody to SC showed the presence of SC in the dimeric IgA1 bands. There was no evidence of IgA1 fragments characteristic of cleavage by IgA1 protease in any cervical mucus sample (Fig. 1A). In comparison, the band intensities of monomeric and polymeric IgA1 in cervical mucus decreased after incubation with exogenous IgA1 protease (Fig. 1B). In the same treated samples, IgA1 bands with molecular weights corresponding to those of Fcα1 and (Fcα)2.J as well as multiple bands of IgA1 with molecular weights greater than that of undigested monomeric IgA1 were generated. Similar weak IgA1 fragments were detected in three vaginal washes (Fig. 1C). Two of these samples were from patients who had been successfully treated for gonococcal infection 2 weeks previously (lanes 2 and 3), while the third sample was from an infected patient (lane 4). In all cases, the fragments observed in these samples were of lower molecular weights than those found in the positive controls.
IgA1 protease production by patients’ isolates of N. gonorrhoeae.
Because of the lack of evidence for IgA1 cleavage in samples of cervical mucus, we tested whether the patients’ clinical isolates of N. gonorrhoeae could produce IgA1 protease in vitro. All of 20 strains were able to produce IgA1 protease in liquid culture conditions in vitro.
IgA1 protease-inhibitory activity in serum and cervical mucus.
Antibodies as well as neutralizing antibodies to IgA1 protease have been found previously in mucosal secretions and serum (3, 5); the lack of digested IgA1 fragments in cervical mucus could be due to inhibition of IgA1 protease activity resulting from such antibodies. The levels of IgA1 protease inhibition in both cervical mucus and serum were therefore measured. As the total IgA1 concentrations in cervical mucus are highly variable and might consequently affect the apparent level of inhibition of IgA1 protease, IgA1 protease-inhibitory activity was also expressed relative to the concentration of total IgA1 in serum and cervical mucus. Inhibition of IgA1 protease activity was detected in sera from all but two patients (Table 3). In contrast, only 16 of 65 samples of cervical mucus from visits 1 and 2 were positive for IgA1 protease inhibition (Table 4). There was no difference in the level of IgA1 protease-inhibitory activity between gonococcus-infected and control patients in either cervical mucus or serum. There was no difference in the levels of IgA1 protease-inhibitory activity in either serum or cervical mucus at visit 1 compared to visit 2.
TABLE 3.
Level of IgA1 protease inhibition in serum from patients with and without gonococcal infection
| Subject group | Visit no.a | Inhibition level, median (range) | Relative inhibition level,b median (range) | No. of patients with positive inhibition titers/n |
|---|---|---|---|---|
| Noninfected | 1 | 178 (0.1c–854) | 60 (0–362) | 22/23 |
| 2 | 226 (84–1,135) | 62 (13–291) | 14/14 | |
| N. gonorrhoeae infected | 1 | 165 (0.1–1,514) | 50 (0–397) | 12/13 |
| 2 | 210 (60–1,268) | 101 (13–510) | 13/13 |
Visit 1, at time of treatment; visit 2, 2 weeks after treatment.
Ratio of inhibition level to total IgA1 concentration (milligrams per milliliter).
Levels below that detectable were assigned a value of 0.1.
TABLE 4.
Levels of IgA1 protease inhibition in cervical mucus from patients with and without gonococcal infection
| Subject group | Visit no.a | Inhibition level, median (range) | Relative inhibition level,b median (range) | No. of patients with positive inhibition titers/n |
|---|---|---|---|---|
| Noninfected | 1 | 0.1c (0.1–70) | 10.5 (0.5–5,454.5) | 9/26 |
| 2 | 0.1 (0.1–25) | 9.3 (0.6–1,707.0) | 3/11 | |
| N. gonorrhoeae | 1 | 0.1 (0.1–24) | 4.6 (0.2–158.6) | 3/17 |
| infected | 2 | 0.1 (0.1–12.6) | 4.9 (0.5–417.2) | 1/11 |
Visit 1, at time of treatment; visit 2, 2 weeks after treatment.
Ratio of inhibition level to total IgA1 concentration (milligrams per milliliter).
Levels below that detectable were assigned a value of 0.1.
DISCUSSION
IgA1 protease has been implicated as a virulence factor for a variety of pathogenic organisms, on account of its ability to cleave human IgA1 into Fab and Fc fragments. At the simplest level, this would result in loss of IgA1 function by severing the antigen-binding part of the molecule from the secondary effector functions of the Fc region. However, the unique consequences of IgA1 protease activity are not always appreciated; for example, it is sometimes suggested that IgA2, which is resistant to IgA1 protease and may be as abundant as IgA1 in secretions, would remain intact and functional. Such arguments, however, overlook several points, the most important of which is that IgA1 protease action leaves intact Fabα fragments which retain full antigen-binding activity (25) and therefore are able to block epitopes on pathogens against recognition by other specific immune defense factors, including IgA2 antibodies (15). Other less exquisitely specific proteases produced by some bacteria (e.g., Pseudomonas aeruginosa, Porphyromonas gingivalis, or Proteus mirabilis) can completely break down IgA and other Igs (9, 14, 24) but do not achieve this effect. Secondly, the antigen specificities of IgA1 and IgA2 are different: IgA1 antibodies tend to recognize protein antigens, whereas IgA2 antibodies are usually directed against carbohydrate determinants (34). Thus, elimination of IgA1 antibody function does not necessarily leave intact an equivalent activity of IgA2 antibody. In particular, our analysis of IgA subclass antibodies to gonococcal antigens in human gonorrhea has revealed a paucity of IgA2 antibodies, whereas IgA1 antibody responses occur, albeit at modest levels (9a). Indeed, the development of an IgA1 antibody response is an essential prerequisite for the ability of IgA1 proteases to fulfill their postulated role as virulence factors (17). Furthermore, molecular and biochemical analyses of IgA1 proteases themselves reveal that the same enzymatic activity has evolved independently in at least three separate groups of human pathogens (for a review, see reference 17); this is a compelling argument that IgA1 proteases serve as important virulence factors, even if the mechanism of action is incompletely understood.
Knowledge of the respective levels of IgA1 antibodies to the pathogen, IgA1 protease activity, and inhibitory activity against IgA1 protease may be crucial in comprehending the role of IgA1 proteases in pathogenesis. IgA1 is normally present in all mucosal secretions including those from the genital tract. In addition, IgA antibodies to N. gonorrhoeae have been previously detected by fluorescence microscopy in female genital secretions (27, 33). We quantitatively measured the levels of IgA1 antibodies to N. gonorrhoeae in genital tract secretions and serum by ELISA. The variation of gonococcal surface proteins makes it difficult to select any single antigen that will be appropriate for measurement of immune responses to gonococci. Therefore, instead of attempting to monitor responses to a single (variable) antigen, we chose to examine the antibody levels to whole fixed bacteria. IgA1 antibodies in secretions detectable against N. gonorrhoeae MS11 did not appear to be affected by current infection. In contrast, serum IgA1 antibodies, although present at low levels as a percentage of total IgA1, were higher in infected than in noninfected women. Western blot analysis of cervical mucus and vaginal wash samples indicated that multiple forms of S-IgA1 as well as IgA1 from systemic sources were present in most samples. Thus, as expected from previous reports, we have shown that a relevant substrate for gonococcal IgA1 protease was present at the site of infection, albeit at low levels.
Whether IgA1 protease is active in genital secretions during gonococcal infection has been difficult to demonstrate. Blake et al. (2) reported that vaginal washings from gonococcus-infected women were able to cleave exogenous IgA1 in vitro in a manner suggestive of IgA1 protease activity. Despite the presence of substrate, and contrary to our expectations, we did not detect any evidence of IgA1 cleavage by gonococcal IgA1 protease in cervical mucus or vaginal wash samples. This result is similar to the recently reported lack of IgA1 cleavage in saliva from infants infected with oral bacteria capable of producing IgA1 protease (32). Some IgA1 degradation was detected in vaginal washings, although the fragment patterns were not typical of gonococcal IgA1 protease activity. This affect may be due to the α-chymotrypsin- and trypsin-like protease activity noted by Blake et al. (2).
The positive identification of IgA1 protease-derived IgA1 fragments in the complex mixtures of IgA1 found in mucosal secretions can be difficult. Polymeric IgA1 contains four or more potential cleavage sites; therefore, four or more IgA1 fragments containing the CH2 or CH3 domains (recognized by the IgA1-specific monoclonal antibody) may be detected on Western blots from the complete and incomplete IgA1 protease cleavage of polymeric IgA1. The cleavage of monomeric IgA1 by IgA1 protease produces only Fcα; however, changes in carbohydrate residues could cause variation in the apparent size of the band. In addition, digestion of IgA1 by other proteases and the further digestion of cleavage products from IgA1 protease may compound this problem. There was no evidence in cervical mucus, however, of the multiple fragments that could be generated from polymeric IgA1, nor were Fcα fragments of any size detected. Moreover, the molecular weight of the IgA1 in the cervical mucus matched the predicted size of intact (not cleaved) IgA1. This was especially important since the molecular mass of (Fcα)2.J.SC (210 kDa) is similar to that of monomeric IgA1 (170 kDa), with which it could potentially be confused. After the addition of exogenous IgA1 protease to the cervical mucus, however, there were loss of intact IgA1 and the production of multiple IgA1 fragments with molecular weights greater than that of monomeric IgA1 and smaller IgA1 fragments with molecular weights similar to those seen in cleaved myeloma protein. It is possible that the absence of IgA1 fragments originating from the IgA1 protease in cervical mucus may have been due to complete digestion of these fragments by other proteases. This is an unlikely explanation, however, since the IgA1 in most cervical mucus and vaginal wash samples showed no evidence of this type of degradation by nonspecific proteases. The lack of detectable IgA1 protease activity was not due to any inability of the infecting N. gonorrhoeae strains to produce IgA1 protease. All clinical isolates of gonococci examined here were capable of producing IgA1 protease, confirming previous reports of the ubiquitous nature of IgA1 protease secretion by gonococcal isolates in vitro (22, 26). The absence of IgA1 protease cleavage products in cervical mucus does not negate the possibility that cell-associated gonococcal IgA1 protease might cleave IgA1 antibody already bound to the organisms in vivo and that such activity may have a role in pathogenesis. In this context, fragmented IgA1 has been found in dental plaque (1), suggesting that IgA1 protease produced by streptococci can cleave IgA1 in the bacterial deposits, even if not in free saliva (32). Given the lack of alternative explanations, we suggest that IgA1 protease activity in the lumen of the female genital tract is low (at best) during uncomplicated cervical gonococcal infections.
Antibodies to IgA1 protease and IgA1 protease-inhibitory antibodies have been previously found in mucosal secretions and serum when IgA1 protease-producing bacteria were present (3, 5). We therefore examined the possibility that the lack of IgA1 protease activity in cervical mucus from gonococcus-infected patients was due to the presence of such inhibitory antibodies. Quantifying IgA1 protease-inhibitory activity has previously been problematic, especially in secretions, since IgA1 can function as a substrate as well as an inhibitor if it contains antibody activity. Using a novel assay that was developed to overcome this difficulty and allow measurement of IgA1 protease activity in secretions and serum (29), we found low levels of inhibitory activity against IgA1 protease produced by N. gonorrhoeae MS11 in a small proportion of cervical mucus samples. It is possible that lack of inhibitory activity was due, in part, to inadequate antigenic cross-reactivity between the IgA1 protease produced by a patient’s infecting strain and the MS11 protease, as gonococcal IgA1 protease is known to display limited antigenic heterogeneity similar to that shown by the meningococcal enzyme (21, 23). However, as the IgA1 protease produced by MS11 is cross-reactive with IgA1 proteases produced by other strains of N. gonorrhoeae as well as N. meningitidis (21), this possibility seems unlikely. Furthermore, in contrast to cervical mucus, higher levels of inhibitory activity were found in virtually all serum samples. The source of inhibitory antibodies to IgA1 protease in serum from infected and noninfected women is unknown. Serum antibodies to IgA1 protease have been shown to be generated during asymptomatic carriage of N. meningitidis (3). It is possible that the putative inhibitory antibody was induced by exposure to N. meningitidis, which commonly colonizes the nasopharynx without giving rise to overt infection, as meningococci produce IgA1 proteases that closely resemble those of gonococci (21). Furthermore, the control subjects, taken from the same population of clients at the Jefferson County STD Clinic, although not infected with N. gonorrhoeae at the time of examination in this study, may have experienced previous episodes of gonococcal infection and so could have retained antibody responses to IgA1 protease. As the antibody response to genital infection with N. gonorrhoeae is surprisingly low in both local secretions and serum (9a), it is likely therefore that the low level of inhibitory activity against IgA1 protease in cervical mucus is related to the weak immune response to this organism in general.
In this report, we have examined whether IgA1 protease plays a role in the pathogenesis of infections with N. gonorrhoeae. The effects of bacterial IgA1 proteases in such infections remain controversial. The large body of indirect evidence (reviewed in reference 17), as well as the potential benefits to a pathogenic organism provided by the cleavage of IgA1, strongly suggests that IgA1 protease is a virulence factor. Despite this, however, there is little direct evidence supporting a significant role of these enzymes in the pathogenesis of mucosal infections, in part because of the lack of suitable animal models for experimental investigation. The results presented here suggest that IgA1 protease does not play a significant role in the pathogenesis of uncomplicated gonococcal infections within the lumen of the lower female genital tract. Nevertheless, all of the clinical isolates of N. gonorrhoeae infecting these patients produced IgA1 protease in vitro. These apparently contradictory results may be explained by two linked hypotheses. First, N. gonorrhoeae may not be present in the lumen or on the mucosal surface in sufficiently high numbers but rather may colonize a subepithelial niche. This hypothesis is supported by the lack of significant local immune or cytokine responses in women infected with N. gonorrhoeae, although low levels of antibody and cytokine responses to N. gonorrhoeae were detected in serum (10). Therefore, the lack of detectable IgA1 protease activity in cervical mucus, in addition to the lack of local host responses, may simply be due to small numbers of bacteria at that site. Secondly, N. gonorrhoeae may require IgA1 protease for survival within as well as outside the host tissues. In this regard, it is interesting to note that the three organisms most commonly responsible for bacterial meningitis produce IgA1 protease (H. influenzae, N. meningitidis, and S. pneumoniae) (17). As these are invasive pathogens, the cleavage of systemic IgA1 may play an as yet unknown role in their pathogenic mechanisms. In addition, the recent evidence that the lysosomal-phagosomal protein LAMP-1 is cleaved by IgA1 protease and that this may contribute to intracellular gonococcal survival (8, 20) affords a new perspective on this enzyme as a virulence factor. We therefore suggest that IgA1 protease produced by N. gonorrhoeae may have more profound effects as a virulence factor when this organism is present in the subepithelium rather than in the lumen or on the mucosal surface.
ACKNOWLEDGMENTS
We thank Carol Blalock, Annalee Hughes, and Sharon Davis for the recruitment of patients and for clinical procedures in the Jefferson County STD Clinic.
This study was supported by US-PHS grants AI34970 and AI28147.
REFERENCES
- 1.Ahl T, Reinholdt J. Detection of immunoglobulin A1 protease-induced Fabα fragments on dental plaque bacteria. Infect Immun. 1991;59:563–569. doi: 10.1128/iai.59.2.563-569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake M, Holmes K K, Swanson J. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhea. J Infect Dis. 1979;139:89–92. doi: 10.1093/infdis/139.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Brooks G F, Lammel C J, Blake M S, Kusecek B, Achtman M. Antibodies against IgA1 protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J Infect Dis. 1992;166:1316–1321. doi: 10.1093/infdis/166.6.1316. [DOI] [PubMed] [Google Scholar]
- 4.Cooper M D, McGee Z A, Mulks M H, Koomey J M, Hindman T L. Attachment to and invasion of human fallopian tube mucosa by an IgA1 protease-deficient mutant of Neisseria gonorrhoeae and its wild-type parent. J Infect Dis. 1984;150:737–744. doi: 10.1093/infdis/150.5.737. [DOI] [PubMed] [Google Scholar]
- 5.Devenyi A G, Plaut A G, Grundy F J, Wright A. Post-infectious serum antibodies inhibit IgA1 proteinases by interaction with the cleavage site specificity determinant. Mol Immunol. 1993;30:1243–1248. doi: 10.1016/0161-5890(93)90039-e. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert J V, Plaut A G, Longmaid B, Lamm M E. Inhibition of bacterial IgA proteases by human secretory IgA and serum. Ann N Y Acad Sci. 1983;409:625–634. doi: 10.1111/j.1749-6632.1983.tb26904.x. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G, Nikolova E, Russell M W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck C R, Meyer T F. The lysosomal/phagosomal membrane protein h-lamp-1 is a target of the IgA1 protease of Neisseria gonorrhoeae. FEBS Lett. 1997;405:86–90. doi: 10.1016/s0014-5793(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 9.Heck L W, Alarcon P, Kulhavy R, Morihara K, Russell M W, Mestecky J. Degradation of immunoglobulin A (IgA) proteins by Pseudomonas aeruginosa elastase. J Immunol. 1990;144:2253–2257. [PubMed] [Google Scholar]
- 9a.Hedges, S. R., et al. Unpublished data.
- 10.Hedges S R, Sibley D, Mayo M S, Hook III E, Russell M W. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 11.Heegaard N H H, Bjerrum O J. Immunoblotting—general principles and procedures. In: Bjerrum O J, Heegaard N H H, editors. Handbook of immunoblotting of proteins. 1. Technical descriptions. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 1–25. [Google Scholar]
- 12.Higerd T B, Virella G, Cardenas R, Koistinen J, Fett J W. New method for obtaining IgA-specific protease. J Immunol Methods. 1977;18:245–249. doi: 10.1016/0022-1759(77)90178-8. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis G A, Griffiss J M. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J Immunol. 1991;147:1962–1967. [PubMed] [Google Scholar]
- 14.Kilian M. Degradation of immunoglobulins A1, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981;34:757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilian M, Mestecky J, Schrohenloher R E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979;26:143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V G. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Knapp J S, Rice R J. Neisseria and Branhamella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 324–340. [Google Scholar]
- 19.Kobayashi K, Fujiyama Y, Hagiwara K, Kondoh H. Resistance of normal serum and secretory IgA to bacterial IgA proteases: evidence for the presence of enzyme-neutralizing antibodies in both serum and secretory IgA, and also in serum IgG. Microbiol Immunol. 1987;31:1097–1106. doi: 10.1111/j.1348-0421.1987.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson S R, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 21.Lomholt H, Kilian M. Antigenic relationships among immunoglobulin A1 proteases from Haemophilus, Neisseria, and Streptococcus species. Infect Immun. 1994;62:3178–3183. doi: 10.1128/iai.62.8.3178-3183.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomholt H, Lind I, Kilian M. Neisseria gonorrhoeae IgA1 proteases share epitopes recognized by neutralizing antibodies. Vaccine. 1995;13:1213–1219. doi: 10.1016/0264-410x(95)00057-8. [DOI] [PubMed] [Google Scholar]
- 23.Lomholt H, Poulsen K, Kilian M. Comparative characterization of the iga gene encoding IgA1 protease in Neisseria meningitidis, Neisseria gonorrhoeae and Haemophilus influenzae. Mol Microbiol. 1995;15:495–506. doi: 10.1111/j.1365-2958.1995.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 24.Loomes L M, Senior B W, Kerr M A. A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect Immun. 1990;58:1979–1985. doi: 10.1128/iai.58.6.1979-1985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansa B, Kilian M. Retained antigen-binding activity of Fabα fragments of human monoclonal immunoglobulin A1 (IgA1) cleaved by IgA1 protease. Infect Immun. 1986;52:171–174. doi: 10.1128/iai.52.1.171-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulks M H, Plaut A G. IgA protease production as a characteristic distinguishing pathogenic from harmless Neisseriaceae. N Engl J Med. 1978;299:973–976. doi: 10.1056/NEJM197811022991802. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly R J, Lee L, Welch B G. Secretory IgA antibody responses to Neisseria gonorrhoeae in the genital secretions of infected females. J Infect Dis. 1976;133:113–125. doi: 10.1093/infdis/133.2.113. [DOI] [PubMed] [Google Scholar]
- 28.Plaut A G, Gilbert J V, Artenstein M S, Capra J D. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme that cleaves human immunoglobulin A. Science. 1975;190:1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- 29.Reinholdt J. A method for titration of inhibiting antibodies to bacterial immunoglobulin A1 proteases in human serum and secretions. J Immunol Methods. 1996;191:39–48. doi: 10.1016/0022-1759(95)00286-3. [DOI] [PubMed] [Google Scholar]
- 30.Russell M W, Brown T A, Radl J, Haaijman J J, Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986;87:87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- 31.Russell M W, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fabα fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–2249. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 32.Smith D J, King W F, Gilbert J V, Taubman M A. Structural integrity of infant salivary immunoglobulin A (IgA) in IgA1 protease-rich environments. Oral Microbiol Immunol. 1998;13:89–96. doi: 10.1111/j.1399-302x.1998.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 33.Tapchaisri P, Sirisinha S. Serum and secretory antibody responses to Neisseria gonorrhoeae in patients with gonococcal infections. Br J Vener Dis. 1976;52:374–380. doi: 10.1136/sti.52.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee J R, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric-IgA2 subclass antibody responses. J Immunol. 1990;144:3770–3778. [PubMed] [Google Scholar]