Abstract
Legionella pneumophila, an intracellular pathogen causing a severe pneumonia, possesses distinct lipolytic activities which have not been completely assigned to specific enzymes so far. We cloned and characterized a gene, plaC, encoding a protein with high homology to PlaA, the major secreted lysophospholipase A of L. pneumophila and to other hydrolytic enzymes belonging to the GDSL family. Here we show that L. pneumophila plaC mutants possessed reduced phospholipase A and lysophospholipase A activities and lacked glycerophospholipid:cholesterol acyltransferase activity in their culture supernatants. The mutants' reduced phospholipase A and acyltransferase activities were complemented by reintroduction of an intact copy of plaC. Additionally, plaC conferred increased lysophospholipase A and glycerophospholipid:cholesterol acytransferase activities to recombinant Escherichia coli. Furthermore, PlaC was shown to be another candidate exported by the L. pneumophila type II secretion system and was activated by a factor present in the bacterial culture supernatant dependent on the zinc metalloprotease. Finally, the role of plaC in intracellular infection of Acanthamoeba castellanii and U937 macrophages with L. pneumophila was assessed, and plaC was found to be dispensable. Thus, L. pneumophila possesses another secreted lipolytic enzyme, a protein with acyltransferase, phospholipase A, and lysophospholipase A activities. This enzyme is distinguished from the previously characterized phospholipases A and lysophospholipases A by its capacity not only to cleave fatty acids from lipids but to transfer them to cholesterol. Cholesterol is an important compound of eukaryotic membranes, and an acyltransferase might be a tool for host cell modification to fit the needs of the bacterium.
Legionella pneumophila is a gram-negative bacterium which is found in freshwater environments, where it associates with amoebae. The inhalation of Legionella-containing aerosols can lead to an acute pneumonia, Legionnaires' disease. In the human lung the bacteria are able to replicate within alveolar macrophages and epithelial cells, causing tissue damage and eventually leading to lung failure. Some of the factors which promote intracellular replication are exported by or depend on other factors exported by the type II secretion system Lsp or the type IVB secretion system Dot/Icm (6, 22, 32, 39, 40). The type II secretion system is responsible for translocation of a variety of hydrolytic activities, because L. pneumophila type II secretion mutants show considerably reduced protease, acid phosphatase, lipase, lysophospholipase A (LPLA), phospholipase A (PLA), p-nitrophenylphosphorylcholine (p-NPPC) hydrolase, and nuclease activities in their culture supernatants (22, 39). Accordingly, substrates of the type II secretion system include the major zinc metalloprotease ProA or Msp, the major acid phosphatase Map, the lipase LipA, and the major secreted LPLA PlaA (3, 4, 17, 22). Studies with knockout mutants indicate that none of these proteins are essential for infection of amoebae or macrophages (3, 18, 47). However, the loss of one might easily be compensated for by enzymes fulfilling similar functions. Thus, the type II secreted factors necessary for intracellular replication are still unknown. Substrates of the type IVB secretion system may also include hydrolytic enzymes, because one recently identified substrate, SidB, shows homology to lipases and possesses three paralogs, SdbA to SdbC, in the Legionella genome (28). Although the major secreted lipase, PLA, and LPLA activities of L. pneumophila are dependent on the type II protein secretion system, there is evidence that some secreted hydrolytic enzymes might be exported differently, as L. pneumophila mutants lacking a functional type II secretion system still retain residual secreted lipolytic activities (3, 17, 39).
Phospholipases are hydrolytic enzymes which cleave ester bonds in phospholipids. These enzymes are categorized into four groups, groups A to D. PLAs cleave fatty acid residues from the sn-1 or sn-2 position of the glycerol backbone. LPLAs, a subgroup of the first group, hydrolyze phospholipids which have only one fatty acid residue. Phospholipases B cleave both fatty acid residues from the sn-1 and sn-2 positions. Phospholipases C (PLC) and D are phosphodiesterases that release phosphomonoesters and the alcohol, respectively. There is evidence that especially PLA and PLC are involved in bacterial pathogenesis. For example, ExoU, a type III secreted cytotoxin of Pseudomonas aeruginosa, has been recently identified as a PLA and LPLA (37, 43, 48). Furthermore, the PLCs of Listeria monocytogenes are virulence factors important for releasing the bacterium into the cytoplasm, where it can replicate (21), and are also necessary for cell-to-cell spread of the bacteria (44). L. pneumophila possesses several lipolytic enzymes, including a lipase LipA, a secreted LPLA PlaA, and a cell-associated PLA/LPLA PlaB (4, 18, 19). Additionally, L. pneumophila has been reported to export PLA and acyltransferase activities into its culture supernatant, which have not been assigned to any specific gene so far (16, 18). A screen of the L. pneumophila genome for putative lipolytic enzymes identified two more homologs of PlaA (18). The major secreted LPLA PlaA belongs to the family of GDSL hydrolases, which include lipases, PLAs, hemolysins, and acyltransferases (49). The proteins of this family possess five successive conserved blocks in which block I and block V contain the putative catalytic active serine, glycine, and histidine residues (49). The best-characterized homolog of PlaA is the glycerophospholipid:cholesterol acyltransferase (GCAT) SatA of Aeromonas salmonicida, a major secreted toxin (10, 26). A. salmonicida SatA was shown to form high-molecular-weight complexes with lipopolysaccharide which are toxic to fish (26). Additionally, SatA is known to be activated by a serine protease (23). Since the Legionella genome encodes two more proteins with the GDSL motif (18), we aimed to characterize them further. Here we demonstrate that one of these proteins, designated PlaC, possesses PLA and LPLA activities and is the major secreted GCAT of L. pneumophila. Furthermore, we show that PlaC is another candidate for type II secretion and that its activity depends on the major secreted zinc metalloprotease.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The L. pneumophila sg1 strain 130b was used for mutagenesis of the plaC gene and later served as the wild-type control. The plaA mutant, a 130b derivative, is defective in the major secreted LPLA activity (Table 1). The lspDE and proA mutants are two further derivatives of 130b; the lspDE mutant is defective in the type II secretion system, and the proA mutant contains a knockout of the zinc metalloprotease gene (Table 1). L. pneumophila was routinely grown on buffered charcoal-yeast extract (BCYE) agar for 2 or 3 days at 37°C (15) and subsequently was cultured in buffered yeast extract (BYE) broth at 37°C with shaking at 350 rpm. Bacterial growth was checked by determining the optical density at 660 nm (OD660) with a Beckman spectrophotometer DU520 (Beckman Coulter, Unterschleißheim, Germany) after inoculation to an OD660 of 0.2 to 0.4. Escherichia coli strain DH5α, the host for new recombinant plasmids, was grown in Luria-Bertani (LB) agar or broth (5). In LB broth, E. coli was grown to an OD660 of 1.0 to 1.1, and isopropyl β-d-thiogalactopyranoside (Sigma Chemicals, Munich, Germany) was added to a final concentration of 1 mM in order to induce a Ptac or Plac promoter on the respective vector and bacterial growth was continued until the OD660 was 2.0 to 2.1. When appropriate, media were supplemented with antibiotics at final concentrations suitable for L. pneumophila or E. coli, as follows: kanamycin, 25 μg/ml for L. pneumophila and 50 μg/ml for E. coli; chloramphenicol, 6 μg/ml for L. pneumophila and 30 μg/ml for E. coli; and ampicillin, 100 μg/ml for E. coli.
TABLE 1.
Overview of the L. pneumophila strains used in this study
| L. pneumophila strain | Characteristic(s) | Antibiotic selection marker(s) | Reference |
|---|---|---|---|
| 130b | Wild type | Strain ATCC BAA-74 | |
| plaC2 (from 130b) | plaC knockout | Kmr | This study |
| plaC4 (from 130b) | plaC knockout | Kmr | This study |
| plaC5 (from 130b) | plaC knockout | Kmr | This study |
| plaC8 (from 130b) | plaC knockout | Kmr | This study |
| 130b(pMMB2002) | Cmr | This study | |
| plaC5(pMMB2002) | Kmr, Cmr | This study | |
| plaC5(pMY7) | plaC complementation | Kmr, Cmr | This study |
| ΔlspDE (from 130b) | Type II secretion mutant | Kmr | 39 |
| ΔproA (from 130b) | Zinc metalloprotease mutant | Kmr | 34 |
| ΔplaA (from 130b) | Lysophospholipase A mutant | Kmr | 18 |
Preparations of culture supernatants and cell lysates.
Culture supernatants for assessment of hydrolytic activities were obtained at the end of exponential growth (i.e., at an OD660 of 2.0 to 2.1) by centrifugation for 5 min at 5,000 × g. Cell lysates were produced as described previously (18, 19). In short, bacteria from the late exponential phase were pelleted by centrifugation as described above and then lysed by addition of 1/20 volume of the original culture volume of 10 mg/ml of lysozyme and 1 μl/ml of Triton X-100 in 40 mM Tris-HCl pH 7.5 (25°C) at 37°C for 30 min. After repeated passage through a 26-gauge needle, the lysates were finally resuspended in one-quarter of the original culture volume of 40 mM Tris-HCl (pH 7.5) (25°C) for E. coli lysates or were resuspended in the original culture volume of Tris-HCl and diluted 1:10 in Tris-HCl for L. pneumophila lysates. Culture supernatants and cell lysates either were tested immediately for enzymatic activities or were stored overnight at 4°C.
DNA techniques and sequence analysis.
PCR was carried out using a T-gradient thermocycler (Biometra, Göttingen, Germany) and Taq DNA polymerase (New England Biolabs, Frankfurt am Main, Germany), Platinum Taq DNA polymerase (Invitrogen, Karlsruhe, Germany), or Pfu DNA polymerase (Fermentas GmbH, St. Leon-Rot, Germany). E. coli DH5α was employed for propagation of recombinant plasmid DNA. The following vectors were used: pGEMTeasy (backbone in plasmids pBH1, pBH5, and pAF10; Promega, Mannheim, Germany), pBCKS+ (backbone in plasmid pMY2; Stratagene, Heidelberg, Germany), and pMMB2002 (backbone in plasmid pMY3 and pMY7) (Table 2) (40). Foreign DNA was introduced into bacterial strains by electroporation by means of a Cell-Porator from Life Technologies (Paisley, Scotland) used according to the manufacturer's specifications. Electroporation of E. coli (L. pneumophila) was carried out using 200 DC volts (400 DC volts), 4 kΩ (4 kΩ), and 330 μF (330 μF). Both strands of plasmid DNA were sequenced by using a BigDye terminator cycle sequencing mixture (Applied Biosystems, Darmstadt, Germany) and an automated DNA sequencer at the sequencing facility of the Robert Koch-Institut. Primers were purchased from Tib Molbiol (Berlin, Germany). Nucleotide and translated protein sequences were analyzed using the DNASTAR package, the pedant website (http://pedant.gsf.de/), the SignalP program (36), and the L. pneumophila genome project website (http://genome3.cpmc.columbia.edu/∼legion.) (12). Nucleotide sequences were also analyzed for promoters using the web-based program BPROM (www.softberry.com). Sequence database searches as well as protein alignments were performed by the BLAST algorithm (1).
TABLE 2.
Overview of plaC plasmid constructs used in this study
| Plasmid | Construct | Size of insert (kbp) | Antibiotic selection marker(s) | Primers used for amplification |
|---|---|---|---|---|
| pAF10 | pGEMTeasy + plaCa,b | 1.559 | Ampr | gdsl2a1, gdsl2b1 |
| pBH1 | pGEMTeasy + plaC | 1.383 | Ampr | gdsl2c1, gdsl2e1 |
| pMY2 | pBCKS + plaC | 1.383 | Cmr | Derived from pBH1 |
| pBH5 | pGEMTeasy + plaC::Kmra,b | 2.375 | Ampr, Kmr | Derived from pAF10 |
| pMY3 | pMMB2002 + plaC | 1.383 | Cmr | Derived from pMY2 |
| pMY7 | pMMB2002 + plaCb | 1.559 | Cmr | gdsl2a1, gdsl2b1 |
The cloned plaC sequence contains a point mutation at base pair position 30, where guanine is mutated to adenine.
Contains a putative promoter region.
Southern hybridization.
Chromosomal DNA from L. pneumophila 130b wild type and plaC mutants, obtained by means of an E.Z.N.A. bacterial DNA kit from Peqlab (Erlangen, Germany), was digested with AvaI and AvaII and subjected to electrophoresis, and fragments were transferred to a nylon membrane (Roche Diagnostics, Mannheim, Germany) by means of a Bio-Rad vacuum blotter (Bio-Rad, Munich, Germany). The DNA probe, generated with primers gdsl2c1 (5′-TTATGATCCAAAACAACAGG-3′) and gdsl2e1 (5′-GAGGATCAATTTAGACAACTT-3′), containing the complete L. pneumophila plaC gene, was used as a plaC-specific probe. Likewise a kanamycin resistance gene probe was generated from pET28b (Novagen, San Diego, Calif.) using primers kan1 (5′-GACGCTCAGTGGAACGAAAAC-3′) and kan2 (5′-ATGTGCGCGGAACCCCTATT-3′). All reagents needed for labeling and detection of the DNA probes were obtained from Roche Diagnostics (Mannheim, Germany). Detection was carried out by means of disodium 3-(4-methospiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenylphosphate (CSPD), the chemiluminescent substrate for alkaline phosphatase. Hybridization was performed at 42°C overnight. Membranes were washed twice for 5 min at room temperature in low-stringency buffer (2× SSC, 0.1% sodium dodecyl sulfate [SDS] [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]), followed by 15 min at 65°C in high-stringency buffer (0.1× SSC, 0.1% SDS). Hybridization and detection were carried out according to the manufacturer's instructions.
Gene cloning and Legionella mutant construction.
Primers gdsl2c1 and gdsl2e1, based on the sequence found in the L. pneumophila Philadelphia-1 database (http://genome3.cpmc.columbia.edu/∼legion.) (12), were used to amplify the plaC gene from strain 130b DNA. The resulting 1,383-bp PCR product begins 2 bp upstream of plaC and ends 79 bp after the stop codon. To assist with ascribing function to plaC, several recombinant plasmids were derived from pBH1, a plaC-containing plasmid, which was generated by cloning the PCR product obtained with primers gdsl2c1 and gdsl2e1 into plasmid pGEMTeasy. Next, the plaC gene from pBH1 was subcloned into the vector pBCKS by using SpeI and SacII, yielding pMY2. pMY2 was then used to clone the plaC gene into pMMB2002 after digestion with KpnI and SacI, resulting in pMY3 (Table 2). For disruption of the plaC gene, a 1,559-bp fragment was amplified from strain 130b using primers gdsl2a1 (5′-TTAACGGCATATTGGGTGAA-3′), which binds 148 bp upstream from the plaC open reading frame (ORF), and gdsl2b1 (5′-TGCTTAAAAACCGCTCTGGA-3′), which binds 110 bp after the end of the plaC ORF, and cloned into pGEMTeasy, resulting in pAF10. Next, pAF10 was restricted with HindIII and ligated with a kanamycin resistance gene (Kmr) cassette amplified from pET28b using primers kan1 and kan2, yielding pBH5 (Table 2). Plasmids were isolated from E. coli by alkaline lysis using either a Miniprep kit from QIAGEN (Hilden, Germany) or a Midiprep kit from Bio-Rad. To generate an L. pneumophila plaC mutant, plasmid pBH5 was brought into strain 130b, and the Kmr cassette was introduced into the chromosomal plaC gene by natural transformation and homolog recombination as described previously (18). PCR and Southern blot analysis were used to examine Kmr legionellae for the presence of the plaC mutation (42). For trans complementation of the plaC mutant, the PCR product obtained with primers gdsl2a1 and gdsl2b1 by amplification with Pfu polymerase was cloned into pMMB2002 restricted with SmaI, yielding pMY7. After ligation, pMY7 was directly transformed into an L. pneumophila plaC mutant by electroporation.
Enzymatic assay for lipolytic activities.
Enzymatic activities were detected as described previously (18, 19). In short, different lipids were incubated with the same volume of bacterial culture supernatant or cell lysate in a mixture containing 6.7 mM substrate (1,2-dipalmitoylphosphatidylcholine [DPPC], 1,2-dipalmitoylphosphatidylglycerol [DPPG], 1-monopalmitoyllysophosphatidylcholine [MPLPC], 1-monopalmitoyllysophosphatidylglycerol [MPLPG], or 1-monopalmitoylglycerol [1-MPG]), 3 mM NaN3, 0.5% (vol/vol) Triton X-100, and 40 mM Tris-HCl (pH 7.5) (25°C). Incubations with bacterial products were performed at 37°C with continuous agitation at 100 rpm for overnight incubations and at 170 rpm for various shorter times, as indicated below for specific experiments. Free fatty acids (FFA) were determined by using an NEFA-C-kit (WAKO Chemicals, Neuss, Germany) according to the instructions of the manufacturer. Depending upon the nature of the experiment, BYE broth, LB broth, or 40 mM Tris-HCl (pH 7.5) (25°C) was incubated, treated like the cultures, and subsequently used as a negative control. When culture supernatants or cell lysates were assessed for GCAT activity, 50 μl per ml of cholesterol in ethanol (10 mg/ml) was added to a DPPG mixture prior to sonication. All lipids, including standards for thin-layer chromatography (TLC), were obtained from Sigma Chemicals or Avanti Polar Lipids, Inc. (Alabaster, Ala.). Prior to incubation, the lipid substrates were vortexed for 15 min at 37°C and then exposed to ultrasonication with a probe (Sonoplus; Bandelin, Berlin, Germany) three times for 15 s at cycle 4 (10%) with the power set to 65%. In order to activate PlaC in bacterial culture supernatants and cell lysates, 100-μl reaction mixtures (containing the lipid suspension and the bacterial product) were incubated with 20 μl of culture supernatant from L. pneumophila plaC mutants or partly purified zinc metalloprotease. Partly purified protease was obtained by the following method (17). L. pneumophila culture supernatant was concentrated 10-fold by ultrafiltration (exclusion pore size, 30 kDa; Millipore, Schwalbach, Germany) and subjected to anion-exchange chromatography using a Resource Q column material (Amersham Biosciences, Freiburg, Germany), followed by gel filtration using a prepacked HiLoad 26/60 Superdex 200 column (Amersham Biosciences) of protease-active fractions. Gel filtration fractions which contained only protease, as estimated by SDS-polyacrylamide gel electrophoresis and silver staining, with a protein concentration of approximately 2 μg/ml were stored at −20°C. ZnCl2 at a final concentration of 12 mM was added to the fractions prior use in the activation assays.
Lipid extraction and thin-layer chromatography.
For the detection of distinct apolar lipids, including cholesterol esters, reaction mixtures of lipids and cholesterol with culture supernatants or cell lysates and corresponding negative controls were subjected to lipid extraction (7). The lower chloroform phase was subsequently used for separation of lipids by TLC. For detection of cholesterol esters, silica gel plates (Merck, Darmstadt, Germany) were developed in tanks containing a petroleum ether-diethyl ether-glacial acetic acid (90:10:1, vol/vol/vol) solvent mixture (29). For visualization, the silica gel plates were then stained with naphthol blue black (Aldrich, Milwaukee, Wis.) (18, 38).
Intracellular infection of U937 cells and Acanthamoeba castellanii amoebae.
A. castellanii amoebae and U937 (CRL-1593.2; American Type Culture Collection, Manassas, Va.), a human cell line that differentiates into macrophage-like cells upon treatment with phorbol esters (80 nM phorbol-12-mystrate-13-acetate [P-8139; Sigma Chemicals, Munich, Germany]; incubation for 36 to 48 h), were used as hosts for in vitro infection by L. pneumophila (13, 33). The amoebae and the cell line were maintained and infected as previously described (13, 27, 33). To assess intracellular growth of L. pneumophila, wells containing amoebae or U937 cells at concentrations of 105/ml and 106/ml, respectively, were infected with wild-type bacteria or isogenic mutants at multiplicities of infection of 0.01 for amoebae and 1 for U937 cell infections (zero time). U937 macrophages were incubated for 2 h with the added bacteria in plain RPMI, and then monolayers were washed three times with plain RPMI to remove unbound bacteria and were incubated with RPMI containing 10% (vol/vol) fetal calf serum (PAA, Linz, Austria). At various times, coincubations of U937 cells and legionellae were treated with 10% (wt/vol) saponin (Sigma Chemicals, Munich, Germany) to lyse the host cells, and serial dilutions were plated on BCYE agar.
Nucleotide accession number.
The L. pneumophila 130b plaC sequence has been deposited in the GenBank database at the National Center for Biotechnology Information under accession number AY745197.
RESULTS
Identification of a new Legionella gene that codes for a putative lipolytic enzyme.
Since L. pneumophila secretes PLA and acyltransferase activities encoded by unidentified genes, we sought to identify potential candidates coding for these activities. To do this, the PlaA sequence was used in a BLAST search against the L. pneumophila genome (http://genome3.cpmc.columbia.edu/) (12). In this way, we identified two paralogs of PlaA containing the GDSL motif (18). One of these paralogs, designated PlaC for PLA protein C, is encoded by a 1,302-bp ORF and was predicted to represent a 433-amino-acid protein with a molecular mass of about 49,8 kDa and an isoelectric point of 5.27. Moreover, the PlaC protein was predicted to have a signal sequence of 22 amino acids at its N terminus and might therefore be secreted by the sec system and additionally might be a candidate for a type II secreted protein. PlaC showed 26% identity and 46% similarity to the paralog PlaA and 22% similarity and 40% identity to SatA of A. salmonicida, an orthologous GDSL protein with acyltransferase, PLA, and LPLA activities (10). The closest homolog of PlaC as evaluated by the expect value is a putative phospholipase/lecithinase/hemolysin from Nostoc punctiforme (24% identity, 40% similarity) also belonging to the GDSL family. In addition, PlaC contained all five conserved blocks characteristic of members of the GDSL family (Fig. 1). Noticeably, an alignment of PlaC with L. pneumophila PlaA and A. salmonicida SatA showed that the distance between the first two blocks in the PlaC sequence is more than twice as large as the distance in the other two sequences (Fig. 1). As PlaC seemed to be a potentially type II secreted hydrolytic enzyme, because of its high homology to lipolytic proteins and its predicted signal peptide, we were interested in further characterization. Next, we examined the genetic plaC locus. Two uncharacterized genes flank plaC (Fig. 2). Interestingly, ORF1 to ORF3 surrounding plaC encoded proteins which showed homology to different transferases. The downstream ORF1, which is oriented in the same direction as plaC, possesses homology to a putative glucosamine-fructose-6-phosphate aminotransferase of P. aeruginosa (gi 15600742 [46]). The oppositely oriented upstream ORF2 displays homology to a putative sulfur transferase of Microbulbifer degradans (gi 48862923). ORF3 is homologous to a putative thiopurine methyltransferase of Photobacterium profundum (CAG20091). A gene encoding a predicted transcriptional accessory protein is located farther downstream (ORF4), and this protein shows homology to the Tex protein of Bordetella pertussis involved in toxin expression (20) (Fig. 2). We suspect that plaC and ORF1 do not form an operon as they are separated by 367 bp and because there are −10 and −35 promoter sequences in the intergenomic region. Thus, plaC most probably is transcribed monocistronically.
FIG. 1.
Partial alignment of amino acid sequences involving conserved blocks I to V from PlaC and PlaA of L. pneumophila 130b and SatA from A. salmonicida. The amino acid sequences from L. pneumophila PlaC and PlaA, as well as from A. salmonicida GCAT, were aligned using the program MEGALIGN (DNASTAR). Putative active site amino acids are underlined. The numbers under uppercase delta indicate the numbers of amino acids between blocks.
FIG. 2.
plaC locus in L. pneumophila and recombinant E. coli. The upper line represents the L. pneumophila Philadelphia-1 chromosome region that contains the plaC gene, along with the location of the relevant HindIII restriction site which was utilized for introduction of a Kmr cassette. The dotted line below it illustrates the region from 130b which was sequenced. The arrows below this line depict the relative locations, sizes, and orientations of plaC and neighboring ORFs. The lines at the bottom represent the segments of Legionella DNA that were cloned into plasmid vectors. Plasmid pBH5 contained a Kmr gene cassette.
Isolation of L. pneumophila plaC mutants.
To investigate the enzymatic activities of PlaC in L. pneumophila and its importance for bacterial infection, knockout mutants with mutations in the plaC gene were generated. pBH5, which contained the plaC gene disrupted by a Kmr cassette (Table 2), was used to introduce a Kmr cassette into the chromosomal plaC gene of strain 130b by allelic exchange, taking advantage of the natural competence of L. pneumophila when it is grown at 30°C (18, 45). Four independent plaC mutants (plaC2, plaC4, plaC5, plaC8) were obtained. The mutation in the plaC gene was confirmed by PCR and Southern blot analysis (data not shown). In the following experiments, results are given mostly for plaC5, but plaC2 and plaC4 behaved similarly. plaC8 differed from the other clones regarding colony morphology and intracellular multiplication in host cells, which might have been due to a second-site mutation in a gene or genes other than plaC (data not shown).
Lipolytic activities of L. pneumophila plaC mutants.
L. pneumophila possesses several secreted as well as cell-associated lipolytic activities (2, 4, 16, 17, 18, 19). As PlaA, the major secreted LPLA of L. pneumophila, is a paralog of PlaC, we tested the L. pneumophila plaC mutants for loss of lipolytic activities. Lipolytic activities were determined by measuring the amounts of free fatty acids released from substrates. Accordingly, culture supernatants and cell lysates of the plaC mutants were incubated with diacylphospholipids (DPPC and DPPG, substrates for PLA activity), lysophospholipids (MPLPC and MPLPG, substrates for LPLA activity), and a nonphospholipid (1-MPG, a substrate for lipase activity) and monitored for the release of FFA. The culture supernatants of the plaC mutants liberated less FFA from all the lipids tested (Fig. 3), implying that PlaC contributes to the exported PLA, LPLA, and lipase activities of L. pneumophila. That an LPLA can also cleave nonphospholipids and thereby show lipase activity has been shown previously for PlaA, because the L. pneumophila plaA mutant exhibited a 50%-reduced capacity to cleave 1-MPG (17, 18). On the other hand, the cell lysates of the wild type and plaC mutants released comparable amounts of FFA from the substrates (data not shown). In conclusion, our data show that PlaC is a PLA and LPLA which is transported into the Legionella culture supernatant.
FIG. 3.
Lipolytic activities of L. pneumophila wild type and a plaC mutant. Culture supernatants of the wild type and a plaC mutant were incubated with DPPC, DPPG, MPLPC, MPLPG, or 1-MPG for 5 h at 37°C. The release of FFA was quantified. Data are expressed as differences between the amount of FFA released by culture supernatants and the amount released by BYE broth. The results represent the means and standard deviations of duplicate cultures and are representative of three independent experiments. For all substrates, the lipolytic activities were significantly different from the wild-type activities (P < 0.01 for DPPC, DPPG, MPLPC and MPLPG, P < 0.05 for 1-MPG; Student's t test).
Since PlaC showed homology to SatA from A. salmonicida, we wanted to determine whether it is responsible for the GCAT activity observed in culture supernatants of L. pneumophila (18). In previous experiments, it was established that DPPG is the preferred substrate of L. pneumophila GCAT (18). In the presence of GCAT, a fatty acid residue from a phospholipid donor molecule is transferred to cholesterol, resulting in the formation of a cholesterol ester which can be detected, for example, by TLC. Indeed, whereas the culture supernatant of the L. pneumophila wild type incubated with DPPG and cholesterol showed GCAT activity, the culture supernatants of the plaC mutants lacked GCAT activity (Fig. 4A), indicating that plaC is essential for the secreted GCAT activity of L. pneumophila. Interestingly, under the same reaction conditions, the plaC mutant also generated reduced amounts of an additional unidentified spot (Fig. 4A). Since this spot occurs only when cholesterol is added to the lipid reaction mixture, it likely represents an unknown cholesterol derivative (data not shown). Unlike the culture supernatants, the bacterial cell lysates of both the wild type and plaC mutants, under the reaction conditions used, did not possess GCAT activity (data not shown). Thus, PlaC not only contributes to PLA and LPLA activities but is also the major secreted GCAT of L. pneumophila.
FIG. 4.
TLC analysis of GCAT activity of L. pneumophila wild type and different mutants. Culture supernatants of wild-type strain 130b (duplicate supernatants) and plaC mutants plaC5, plaC2, plaC4, and plaC8 were incubated with a mixture of DPPG and cholesterol for 23 h at 37°C, and then lipids were extracted and applied to a TLC (A). Culture supernatants of the wild type harboring the empty pMMB2002 vector [130b(pMMB)], the plaC mutant plaC5 harboring the empty pMMB2002 vector [plaC5(pMMB)], and plaC5 harboring pMY7 [plaC5(pMY7)] were incubated with a mixture of DPPG and cholesterol for 23 h at 37°C, and then lipids were extracted and applied to a TLC (B). Culture supernatants of wild-type strain 130b, a lspDE mutant (ΔlspDE), a proA mutant (ΔproA), plaC mutant plaC5, and a plaA mutant (ΔplaA) were either incubated with a mixture of DPPG and cholesterol or added to a lipid mixture incubated with supernatant from a plaC mutant (plus sign) or partly purified ProA (asterisk) for 20 h at 37°C, and then lipids were extracted and applied to a TLC (C). An apolar solvent mixture was employed for separation of the apolar lipids, particularly the cholesterol ester. A mixture of BYE and the lipids was also incubated and served as a negative control (lane N). For qualitative identification of the lipid spots, lanes St containing lipid standards were used. Abbreviations: CholE, cholesterol ester; TPG, tripalmitoylglycerol; Chol, cholesterol.
Lipolytic activities of trans-complemented plaC mutants.
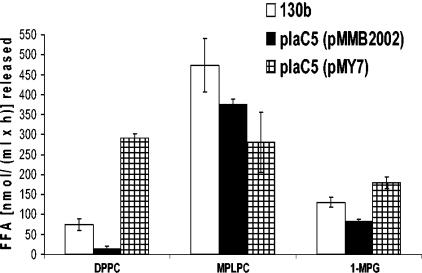
Next we assessed whether the introduction of plaC in trans could restore the missing lipolytic activities of the L. pneumophila plaC mutants, particularly the missing GCAT activity. To that end, culture supernatants of the wild type and the plaC mutant harboring either the empty pMMB2002 vector or pMY7 (Table 2) were assessed for GCAT activity by incubation with cholesterol and DPPG. As expected, the wild type showed GCAT activity, as indicated by the formation of the cholesterol ester, which was missing in the plaC mutant harboring the empty pMMB2002 vector (Fig. 4B). In contrast, the plaC mutant harboring pMY7 with intact plaC showed GCAT activity, confirming that plaC is indeed responsible for the secreted GCAT activities of L. pneumophila (Fig. 4B). Next, culture supernatants of the wild type as well as the plaC mutant harboring pMMB2002 or pMY7 were assessed for PLA, LPLA, and lipase activity by monitoring the release of FFA from the respective substrates. Again, the culture supernatant of plaC5 harboring the empty pMMB2002 vector showed a reduced capacity to cleave FFA from DPPC, MPLPC, and 1-MPG compared to the wild type (Fig. 5). In contrast, plaC5 harboring pMY7 exhibited a considerable increase in the release of FFA from DPPC and 1-MPG, verifying that plaC contributes to the secreted PLA and lipase activities of L. pneumophila (Fig. 5). The LPLA activity could not be restored by trans complementation (Fig. 5). It is possible that the overexpression of plaC might result in the formation of partially folded PlaC with restricted activities. Taken together, our complementation data demonstrate that plaC contributes to L. pneumophila PLA and lipase activities and is essential for the GCAT activity of L. pneumophila.
FIG. 5.
Lipolytic activities of L. pneumophila wild type, a plaC mutant, and a complemented strain. Culture supernatants of the wild type, plaC mutant plaC5 harboring the empty pMMB2002 vector, and plaC5 harboring pMY7 were incubated with DPPC, MPLPC, or 1-MPG for 5 h at 37°C. The release of FFA was quantified. Data are expressed as differences between the amount of FFA released by culture supernatants and the amount released by BYE broth. The results represent the means and standard deviations of duplicate cultures and are representative of three independent experiments. For all substrates except MPLPC, the lipolytic activities were significantly different from those of the plaC5 mutant (P < 0.01; Student's t test).
Secretion and activation of PlaC by L. pneumophila.
Since the L. pneumophila plaC mutants showed reduced lipolytic activities in their culture supernatants and since the PlaC protein possessed a predicted signal sequence and might therefore be a type II secreted protein, we examined a type II secretion mutant (lspDE) for secretion of PlaC as represented by its GCAT activity. The culture supernatant of the type II secretion mutant showed considerably decreased acyltransferase activity (Fig. 4C). This indicates either that PlaC is exported by the type II system or that its activity is dependent on a type II secreted effector. Since the lspDE mutant also lacks the type II secreted zinc metalloprotease (Msp or ProA) which has been shown to contribute to the PLA and LPLA activities of L. pneumophila culture supernatants (2, 18, 22, 39), we also tested the culture supernatant of an isogenic proA mutant for GCAT activity. We found that culture supernatants of the proA mutant had also lost GCAT activity, suggesting that ProA is directly or indirectly involved in PlaC activation (Fig. 4C). To exclude the possibility that the lack of GCAT activity in the culture supernatant of the lspDE mutant was simply due to the absence of ProA, we coincubated the culture supernatant of the lspDE mutant with (i) the supernatant of a plaC mutant which still contained protease activity and other type II secreted activities but lacked GCAT activity and (ii) partially purified chromatography fractions containing ProA. For both incubations, the wild type showed GCAT activity, whereas the type II secretion mutant did not show GCAT activity, confirming that PlaC itself is a candidate for a type II secreted effector (Fig. 4C). In contrast, the GCAT activity could be recovered from culture supernatants of the proA mutant by incubation with the culture supernatant of a plaC mutant or partially purified ProA (Fig. 4C). In conclusion, it was shown that PlaC is another candidate for export by the type II secretion system of L. pneumophila and is dependent on the presence of the zinc metalloprotease.
Enzymatic activities of E. coli clones harboring the plaC gene.
L. pneumophila plaC mutants showed reduced PLA, LPLA, and lipase activities and a loss of acyltransferase activity in their culture supernatants. Since L. pneumophila possesses several lipolytic enzymes which may influence each other, we investigated the enzymatic activities of PlaC in recombinant E. coli clones. To do this, the recombinant E. coli harboring pMY2 (Table 2) was assayed for PLA, LPLA, lipase, and acyltransferase activities in both culture supernatant and cell lysates. Indeed, compared to the clone containing the empty pBCKS vector, the cell lysates from the E. coli clone harboring pMY2 showed an increased capacity to cleave fatty acids from MPLPC and MPLPG, indicating that PlaC, like PlaA, is an LPLA (Fig. 6). There was a small increase in the amount of FFA released from DPPC, which was not consistently reproducible in additional experiments; since no increased FFA release could be observed for DPPG, it is not yet clear whether PlaC also expresses PLA activity in E. coli. No increased release of fatty acids from any of the substrates tested could be detected in the culture supernatant, indicating that PlaC is not exported into the culture supernatant by E. coli (data not shown).
FIG. 6.
Lipolytic activities of recombinant E. coli containing L. pneumophila plaC. Fourfold-concentrated cell lysates of E. coli containing pBCKS or its derivative pMY2 were mixed with DPPG, DPPC, MPLPC, MPLPG, and 1-MPG. After 23 h of incubation at 37°C, the release of FFA was quantified. Data are expressed as differences between the amount of FFA released by cell lysate and the amount released by Tris-HCl buffer. The results represent the means and standard deviations of duplicate cultures and are representative of three independent experiments. For all substrates except DPPG the lipolytic activities were significantly different from those of E. coli carrying the empty vector (P < 0.01; Student's t test).
Additionally, we wanted to assess whether GCAT activity could also be detected in E. coli clones harboring plaC. To do this, culture supernatants and cell lysates of the E. coli clones were assayed for acyltransferase activity. E. coli clones containing only pBCKS or pMMB2002 did not show acyltransferase activity in their culture supernatants and cell lysates. In contrast, GCAT activity could be detected in cell lysates but not in culture supernatants of E. coli clones containing plaC in pMMB2002 (pMY3) (Fig. 7). E. coli clones carrying the plaC gene in the pBCKS vector (pMY2) did not show GCAT activity in their culture supernatants or in bacterial lysates (Fig. 7). As described above, we discovered that in L. pneumophila the GCAT activity of PlaC is dependent on a factor secreted by L. pneumophila, which could either be the zinc metalloprotease ProA itself or a factor dependent on ProA. We therefore additionally investigated the effect of culture supernatant of the plaC mutant (showing no GCAT activity but showing protease activity) on the acyltransferase activity of plaC cloned into E. coli. In order to stimulate PlaC activity, culture supernatants and cell lysates of E. coli clones harboring plaC, as well as the respective clones harboring the empty vector, were coincubated with culture supernatant of an L. pneumophila plaC knockout mutant, DPPG, and cholesterol. Under these conditions, the cell lysates but not the culture supernatants of recombinant E. coli clones containing pMY2 or pMY3 both showed increased GCAT activities, confirming that plaC actually codes for an acyltransferase (Fig. 7 and data not shown). Taken together, our data corroborate the conclusion that plaC confers LPLA and GCAT activity on E. coli and that the latter activity can be enhanced by a factor present in L. pneumophila culture supernatant.
FIG. 7.
Acyltransferase activities of recombinant E. coli DH5α containing L. pneumophila plaC after addition of putative PlaC activating factors of L. pneumophila. Fourfold-concentrated cell lysates of E. coli containing pBCKS [DH5α(pBCKS)] or its derivative pMY2 [DH5α(pMY2)], as well as pMMB2002 [DH5α(pMMB)] or its derivative pMY3 [DH5α(pMY3)], were incubated with a mixture of DPPG and cholesterol or added to the lipid mixture incubated with supernatant from a plaC mutant (plus sign) for 20 h at 37°C, and then lipids were extracted and applied to a TLC. A mixture of Tris-HCl and the lipids was also incubated and served as a negative control (lanes N and N+). Lanes St contained lipid standards used for qualitative identification of the lipid spots. The observations depicted here were made on two more occasions. I and II indicate duplicate cultures. The arrows indicate the presence of cholesterol ester or the unknown substance related to cholesterol. Abbreviations: CholE, cholesterol ester; TPG, tripalmitoylglycerol; Chol, cholesterol.
Role of plaC during intracellular infection of A. castellanii amoebae and U937 macrophages.
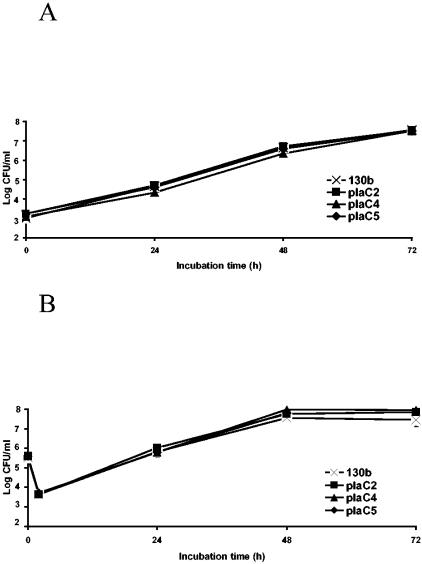
Since phospholipases are also known as virulence factors that support intracellular survival of bacteria, we wanted to assess the importance of PlaC for infection of amoebae and macrophages. To determine whether plaC is essential for L. pneumophila to infect its natural host, A. castellanii amoebae were inoculated with wild-type L. pneumophila and plaC mutants, and the CFU were counted at various times. The plaC mutants showed the same increase in CFU during the whole infection period as the wild type (Fig. 8A); namely, both the plaC mutants and the L. pneumophila wild type multiplied about 100,000- to 1,000,000-fold by 72 h postinoculation. Additionally, we investigated the ability of the plaC mutants to infect U937 macrophages. Again, the L. pneumophila wild type and plaC mutants displayed comparable replication rates (Fig. 8B). In summary, our studies show that plaC is not essential for infection of and replication within A. castellanii amoebae and U937 macrophages.
FIG. 8.
Intracellular infection by wild-type and plaC mutant L. pneumophila. Strains 130b and plaC mutants plaC5, plaC2, and plaC4 were used to infect monolayers of A. castellanii amoebae (A) and cultures of U937 macrophages (B) at multiplicities of infection of 0.01 and 1, respectively. At various times postinoculation, the numbers of bacteria were quantified by plating aliquots on BCYE agar. The results represent the means and standard deviations of triplicate samples and are representative of three independent experiments.
DISCUSSION
In the present investigation, we identified the gene for the major secreted glycerophospholipid:cholesterol acyltransferase of L. pneumophila designated plaC. We demonstrated that PlaC possesses PLA, LPLA, lipase, and GCAT activities and is a candidate for a type II exported effector. Furthermore, we showed that PlaC requires activation which is either directly or indirectly dependent on the zinc metalloprotease ProA. This study presents evidence that PlaC is closely related to A. salmonicida SatA. Both proteins display GCAT activity as well as PLA and LPLA activities (10). PlaC has only weak phospholipase A activity when it is expressed in E. coli. The difference in substrate specificity between plaC cloned into E. coli and plaC in L. pneumophila may be caused by activating proteins, chaperones, or cofactors present in Legionella but not in E. coli. Furthermore, A. salmonicida SatA has been shown to possess broad substrate specificity, accepting a variety of phospholipids as acyl donors but preferring short-chain or unsaturated fatty acids (9). The preferred acyl acceptor of A. salmonicida SatA is cholesterol, but other steroids with a planar ring system and β-hydroxyl group at the 3 position are suitable as well; even aliphatic alcohols were found to act as acyl acceptors (9). Whether PlaC accepts a similar variety of substrates has yet to be determined. Moreover, A. salmonicida SatA requires activation by the AspA serine protease (23, 50). L. pneumophila PlaC also requires an unidentified factor which enhances its activity. We have shown that this activating factor is present in the L. pneumophila culture supernatant and is dependent on the zinc metalloprotease ProA. However, L. pneumophila ProA does not belong to the family of serine proteases, like A. salmonicida AspA. Since A. salmonicida SatA and PlaC are homologous to each other on the protein level and show similar substrate preferences, we favor the hypothesis that a serine protease is the more likely activating factor of PlaC which can be processed by ProA. Apart from ProA, no other proteases have been characterized on the molecular level for L. pneumophila so far. Nevertheless, a BLAST search of the L. pneumophila genome with the AspA protease of A. salmonicida showed that L. pneumophila indeed possesses a putative serine protease with homology to A. salmonicida AspA (25% identity, 42% similarity).
PlaC belongs to the family of GDSL hydrolases comprising plant and prokaryotic lipases, phospholipases, acyltransferases, and hemolysins. So far no crystal structure of a GDSL enzyme has been solved, but the structures of some GDSL-like enzymes, such as the Streptomyces scabies esterase which forms a Ser-His dyad (51) and the mammalian platelet-activating factor acetylhydrolase PAF-AH(Ib) which forms a Ser-His-Asp triad (25), have been elucidated. It was shown for the Aeromonas hydrophila GCAT, which also is a member of the GDSL family, that the active site serine belonging to a catalytic triad is located in block I near the N terminus and is embedded in the conserved motif G-X-S-X-S (8, 24, 25). The other two members of the active site were proposed to be aspartic acid in block III located in the motif G-X-N-D and histidine in block V located in the motif F-X-D-X-X-H-P (8). However, elucidation of the crystal structure of several GDSL-like enzymes, including the above-mentioned platelet-activating factor PAF-AH(Ib) and Aspergillus aculeatus rhamnogalacturonan acetylesterase (25, 35), revealed that the active site aspartic acid is located in block V in close proximity to the histidine residue rather than in block III. An alignment of PlaC with SatA of A. salmonicida showed that the PlaC sequence not only contains all five conserved blocks but also possesses the three members of the putative catalytic triad in blocks I and V embedded in the respective conserved motifs (Fig. 1). Interestingly, both PlaC and A. salmonicida GCAT possess the conserved aspartic acid motif in block III, whereas PlaA lacks this motif. In contrast to SatA of A. salmonicida and PlaC, which are acyltransferases with additional PLA and LPLA activity, PlaA possesses only LPLA activity and lacks GCAT activity (18). The conserved aspartate has been found to stabilize the conformation of the enzyme by participating in several hydrogen bonds (35). However, since it is present in most of the diverse GDSL enzymes and is missing only in some (e.g., the esterase of S. scabies and the phospholipase from Vibrio vulnificus [14, 18]), it is difficult to predict its effect on the enzymatic activity without data on the acyltransferase activity.
Furthermore, we noticed that the distance between blocks I and II in the PlaC sequence is more than twice as large as the distance in most GDSL enzymes (Fig. 1) (8, 14, 18). In the case of rhamnogalacturonan acetylesterase of A. aculeatus, the oxyanion of the transition state is stabilized by hydrogen bonds from the main chain NH groups of the active site serine in block I, the conserved glycine residue in block II, and the side chain amide of the conserved asparagine in block III (35). Thus, a large distance between blocks I and II, like that present in PlaC, may allow entrance of a larger molecule and might indicate that PlaC could be able to hydrolyze substrates more voluminous than phospholipids and lysophospholipids. Another possibility is that the loop formed between blocks I and II could be involved in the multimerization of the enzyme, as in the case of PAF-AH(Ib), in which the N-terminal α helix preceding the first β strand is involved in dimer formation of the enzyme (25). The possibility that PlaC might be a multimeric enzyme is suggested by the fact that after gel filtration of L. pneumophila culture supernatant, the GCAT activity eluted at a considerably higher molecular mass than the molecular mass of the monomer (data not shown). A. hydrophila GCAT is also found in a high-molecular-weight complex (>500 kDa), as estimated by gel filtration. This complex originates from association of the enzyme with lipopolysaccharide-containing outer membrane vesicles rather than from a multimeric form (29, 30, 31).
Moreover, we have shown that PlaC is not required for intracellular infection of A. castellanii and U937 macrophages. Likewise, it was shown that an A. salmonicida satA mutant does not display attenuated virulence. In spite of being a potent toxin, the enzyme is dispensable for establishment of lethal acute furunculosis in Atlantic salmon (50). Since L. pneumophila possesses at least three GDSL enzymes (18), it is possible that the loss of one of these enzymes might be compensated for by one or both of the others. There are other indications which link GCAT activity to bacterial virulence. For instance, the GCAT of Staphylococcus aureus and Staphylococcus epidermidis, termed FAME, is a virulence factor which neutralizes bactericidal fatty acids by binding them to cholesterol (11). Another putative acyltransferase belonging to the GDSL family is SseJ of Salmonella enterica serovar Typhimurium, which is a type III translocated virulence factor and localizes with the Salmonella-containing vacuolar membrane (41). SseJ is considered to be necessary for modification of the vacuolar membrane, which is enriched in cholesterol (41). Likewise, PlaC might be a way for L. pneumophila to modify host cell membranes as cholesterol is present in eukaryotic membranes but not in the membrane of the bacterium.
In summary, we have shown that PlaC is a new candidate for a type II exported protein which is dependent on the zinc metalloprotease for its activity. PlaC displays PLA, LPLA, and lipase activities and is essential for the GCAT activity of L. pneumophila. Finally, it was shown that PlaC is dispensable for intracellular infection. Identification of the activating factor of PlaC and the combined role of PlaC and PlaA during intracellular infection are subjects for further investigation. GCAT activity in gram-negative bacteria has been described previously only for the family Vibrionaceae, in particular Aeromonas sp., and S. aureus is the only member of gram-positive bacteria for which GCAT activity has been reported (30). Thus, PlaC is the first GCAT of a gram-negative bacterium that does not belong to the family Vibrionaceae and the first GCAT to be described for an intracellular pathogen.
Acknowledgments
We thank Lucy Tompkins for providing the isogenic L. pneumophila proA mutant. We are thankful to Horst Emmel and Julia Tesch at the sequencing facility of the Robert Koch-Institut for performing the sequencing. We acknowledge Kerstin Rydzewski for skillful technical assistance and thank Shanta Banerjee for helpful suggestions concerning the manuscript.
A. Flieger was supported by grant FL359/1-1 from Deutsche Forschungsgemeinschaft.
Editor: J. N. Weiser
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon, V., S. Kurtz, A. Flieger, B. Neumeister, and N. P. Cianciotto. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragon, V., S. Kurtz, and N. P. Cianciotto. 2001. Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect. Immun. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragon, V., O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. Wiley, New York, N.Y.
- 6.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 7.Bligh, E. G., and Dyer, W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 8.Brumlik, M. J., and J. T. Buckley. 1996. Identification of the catalytic triad of the lipase/acyltransferase from Aeromonas hydrophila. J. Bacteriol. 178:2060-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley, J. T. 1982. Substrate specificity of bacterial glycerophospholipid:cholesterol acyltransferase. Biochemistry 21:6699-6703. [DOI] [PubMed] [Google Scholar]
- 10.Buckley, J. T., L. N. Halasa, and S. MacIntyre. 1982. Purification and partial characterization of a bacterial phospholipid:cholesterol acyltransferase. J. Biol. Chem. 257:3320-3325. [PubMed] [Google Scholar]
- 11.Chamberlain, N. R., and S. A. Brueggemann. 1997. Characterisation and expression of fatty acid modifying enzyme produced by Staphylococcus epidermidis. J. Med. Microbiol. 46:693-697. [DOI] [PubMed] [Google Scholar]
- 12.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 13.Cianciotto, N. P., and B. S. Fields. 1992. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc. Natl. Acad. Sci. USA 89:5188-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalrymple, B. P., D. H. Cybinski, I. Layton, C. S. McSweeney, G. P. Xue, Y. J. Swadling, and J. B. Lowry. 1997. Three Neocallimastix patriciarum esterases associated with the degradation of complex polysaccharides are members of a new family of hydrolases. Microbiology 143:2605-2614. [DOI] [PubMed] [Google Scholar]
- 15.Edelstein, P. H. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flieger, A., S. Gong, M. Faigle, M. Deeg, P. Bartmann, and B. Neumeister. 2000. Novel phospholipase A activity secreted by Legionella species. J. Bacteriol. 182:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flieger, A., S. Gong, M. Faigle, S. Stevanovic, N. P. Cianciotto, and B. Neumeister. 2001. Novel lysophospholipase A secreted by Legionella pneumophila. J. Bacteriol. 183:2121-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flieger, A., B. Neumeister, and N. P. Cianciotto. 2002. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect. Immun. 70:6094-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flieger, A., K. Rydzewski, S. Banerji, M. Broich, and K. Heuner. 2004. Cloning and characterization of the gene encoding the major cell-associated phospholipase A of Legionella pneumophila, plaB, exhibiting hemolytic activity. Infect. Immun. 72:2648-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs, T. M., H. Deppisch, V. Scarlato, and R. Gross. 1996. A new gene locus of Bordetella pertussis defines a novel family of prokaryotic transcriptional accessory proteins. J. Bacteriol. 178:4445-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldfine, H., T. Bannam, N. C. Johnston, and W. R. Zuckert. 1998. Bacterial phospholipases and intracellular growth: the two distinct phospholipases C of Listeria monocytogenes. Symp. Ser. Soc. Appl. Microbiol. 27:7S-14S. [DOI] [PubMed] [Google Scholar]
- 22.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67:3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilton, S., and J. T. Buckley. 1991. Action of a microbial lipase/acyltransferase on phospholipid monolayers. Biochemistry 30:6070-6074. [DOI] [PubMed] [Google Scholar]
- 24.Hilton, S., and J. T. Buckley. 1991. Studies on the reaction mechanism of a microbial lipase/acyltransferase using chemical modification and site-directed mutagenesis. J. Biol. Chem. 266:997-1000. [PubMed] [Google Scholar]
- 25.Ho, Y. S., L. Swenson, U. Derewenda, L. Serre, Y. Wei, Z. Dauter, M. Hattori, T. Adachi, J. Aoki, H. Arai, K. Inoue, and Z. S. Derewenda. 1997. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature 385:89-93. [DOI] [PubMed] [Google Scholar]
- 26.Lee, K. K., and A. E. Ellis. 1990. Glycerophospholipid:cholesterol acyltransferase complexed with lipopolysaccharide (LPS) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida: LPS stabilizes and enhances toxicity of the enzyme. J. Bacteriol. 172:5382-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liles, M. R., P. H. Edelstein, and N. P. Cianciotto. 1999. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31:959-970. [DOI] [PubMed] [Google Scholar]
- 28.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacIntyre, S., and J. T. Buckley. 1978. Presence of glycerophospholipid:cholesterol acyltransferase and phospholipase in culture supernatant of Aeromonas hydrophila. J. Bacteriol. 135:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacIntyre, S., T. J. Trust, and J. T. Buckley. 1979. Distribution of glycerophospholipid-cholesterol acyltransferase in selected bacterial species. J. Bacteriol. 139:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre, S., T. J. Trust, and J. T. Buckley. 1980. Identification and characterization of outer membrane fragments released by Aeromonas sp. Can. J. Biochem. 58:1018-1025. [DOI] [PubMed] [Google Scholar]
- 32.Marra, A., S. J. Blander, M. A. Horwitz, and H. A. Shuman. 1992. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. USA 89:9607-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat, J. F., and L. S. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12:693-705. [DOI] [PubMed] [Google Scholar]
- 35.Molgaard, A., S. Kauppinen, and S. Larsen. 2000. Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Struct. Fold Des. 8:373-383. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 38.Plekhanov, A. Y. 1999. Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal. Biochem. 271:186-187. [DOI] [PubMed] [Google Scholar]
- 39.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossier, O., S. R. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72:310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Albert, J., X. J. Yu, C. R. Beuzon, A. N. Blakey, E. E. Galyov, and D. W. Holden. 2002. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol. Microbiol. 44:645-661. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone, B. J., and Y. A. Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 47.Szeto, L., and H. A. Shuman. 1990. The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect. Immun. 58:2585-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura, M., T. Ajayi, L. R. Allmond, K. Moriyama, J. P. Wiener-Kronish, and T. Sawa. 2004. Lysophospholipase A activity of Pseudomonas aeruginosa type III secretory toxin ExoU. Biochem. Biophys. Res. Commun. 316:323-331. [DOI] [PubMed] [Google Scholar]
- 49.Upton, C., and J. T. Buckley. 1995. A new family of lipolytic enzymes? Trends Biochem. Sci. 20:178-179. [DOI] [PubMed] [Google Scholar]
- 50.Vipond, R., I. R. Bricknell, E. Durant, T. J. Bowden, A. E. Ellis, M. Smith, and S. MacIntyre. 1998. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 66:1990-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei, Y., J. L. Schottel, U. Derewenda, L. Swenson, S. Patkar, and Z. S. Derewenda. 1995. A novel variant of the catalytic triad in the Streptomyces scabies esterase. Nat. Struct. Biol. 2:218-223. [DOI] [PubMed] [Google Scholar]