Highlights
-
•
Overall, 8127 measles cases and 84 deaths were reported in the 2022 outbreak affecting all counties in Liberia.
-
•
The overall case fatality rate was 1.0% but varied from 0.0% to 10.6% across districts.
-
•
The outbreak is probably caused by suboptimal first dose MCV coverage (79%, 2022) in Liberia.
Keywords: Measles-containing vaccine; Outbreak; Vaccination, Liberia
Abstract
Background
Liberia reported a large outbreak of measles involving all the counties in 2022. We conducted a descriptive analysis of the measles surveillance data to understand the trend of the outbreak and guide further policy action to prevent future outbreaks.
Methods
We analyzed the measles surveillance data from Epi week 1 to 51, 2022. All the laboratory-confirmed cases, clinically compatible and epidemiologically linked cases were included in the analysis, the variables of interest included the patient's age, sex, place of residence, measles classification, measles vaccination status, and outcome. We cleaned and analyzed the data using R version 4.2.0 and Arc GIS Pro. The demographic characteristics of the cases were presented, the progression of the cases was presented in Epicurve and the spatial distribution and the case fatality rate (CFR) of the case were presented at the district level using the Arc GIS Pro.
Results
The median age of the cases was 4 years (interquartile range: 2-8 years). Children under five years of age constituted 60% of the cases (4836/8127), and females accounted for 52% (4204/8127) of the cases. Only 1% (84/8127) of the cases had documentary evidence of receiving at least one dose of measles-containing vaccine (MCV). Only 3 out of 92 health districts in the country did not report a case of measles during the period under review. The overall cases fatality rate was 1% however CFR of up to 10% were reported in some districts.
Conclusion
The outbreak of measles involved almost all the districts of the country, exposing a possible nationwide suboptimal immunization coverage for MCV. The high CFR reported in some districts needs further investigation.
Introduction
Measles is a highly contagious, epidemic-prone, vaccine-preventable disease spread from person to person through respiratory droplets. The measles virus is a spherical, non-segmented, enveloped, negative-sense single-stranded RNA virus that belongs to the Morbillivirus genus within the Paramyxovirus family [1]. The virus is exclusively found in humans with an incubation period of 7-21 days. The disease, which commonly affects children aged under 5 years, is often self-limiting manifesting as fever, cough, coryza, and body rash. It can, however, sometimes result in severe sequelae, including death, especially among malnourished and immunocompromised individuals [2].
Two doses of measles-containing vaccines (MCV) are recommended for everyone, given at least 6 months apart (typically at age 9 and 15 months) within the first 2 years of life for most low-resourced countries, including Liberia [3,4]. The vaccine is effective and confers lifelong immunity against the infection. The measles vaccination program targets coverage of 95% of the eligible population to achieve herd immunity. This target has not been achieved in most low- and middle-income countries, especially in Sub-Saharan Africa, including Liberia. A multi-country assessment of national immunization coverage and case surveillance data throughout 15 West African countries revealed that the first dose coverage for the first dose of MCV ranged from 45% in 2001 to 66% in 2019. Only seven out of 15 countries have launched the second dose of MCV since 2015 [5]. These challenges were further compounded by the impact of the COVID-19 pandemic on routine health services, including vaccination. A report on the effect of the pandemic on routine health services in Liberia documented a 47% decrease in routine immunization outreach events conducted in the first 6 months of 2020 [6].
Routine immunization services by the health facilities are provided as scheduled services at the facilities and as scheduled outreach community services. The Expanded Program on Immunization (EPI) and partners also provide supplemental immunization activities like campaigns aimed at augmenting routine immunization activities. Liberia introduced the first dose of measles vaccination into its national immunization schedule in 1978 and the second dose in 2019. Every child in Liberia is expected to receive the first dose of MCV at 9 months of age and the second dose at 15 months of life [3,4]. However, only 61%, 58%, and 79% of eligible children have received first dose of MCV in 2020, 2021, and 2022, respectively, according to the World Health Organization/United Nations Children's Emergency Fund estimates of national immunization coverage [7]. This estimated coverage, which varies widely at the sub-national levels [5], is below what is required to provide adequate herd immunity to prevent measles outbreaks. The last measles vaccination campaign targeting children under 60 months of age in Liberia was conducted in 2015.
Liberia reported an outbreak of measles in December 2021, which involved all the counties, with an unusually high number of deaths reported, especially in some counties. We conducted an epidemiological characterization of the outbreak, using the measles surveillance data to understand the factors responsible for the outbreak and make policy adjustments to prevent future outbreaks.
Methods
Study setting
Liberia, with an estimated population of 5.3 million people, is divided into 15 administrative sub-national units called counties [5]. These are further subdivided into 93 health districts. The health system is three-tiered and managed by different administrative arms of the government. The routine immunization program in Liberia is managed by the EPI unit of the Ministry of Health. The EPI unit is responsible for the procurement, storage, and distribution of the vaccines in the national immunization schedule to the over 850 health facilities that provide immunization services across the 15 counties in the country.
Study design
We conducted a descriptive analysis of the reported measles cases captured through the Integrated Disease Surveillance and Response (IDSR) system from Epi week 1 (January 1) to 51 (December 25) of 2022.
Measles surveillance
Measles surveillance in Liberia is managed by the National Public Health Institute of Liberia (NPHIL). The NPHIL uses the IDSR framework for monitoring and responding to the disease outbreak. Measles cases are detected in communities and at health facilities and reported immediately through districts and counties to the national level. Data are reviewed, and decisions are taken to quickly respond and limit the spread of the disease.
A suspected case of measles is defined as any person with fever and maculopapular (non-vesicular) generalized rash and cough, coryza or conjunctivitis, or any person in whom a clinician suspects measles. A confirmed case is a suspected case with laboratory confirmation (positive IgM antibody) or epidemiological link to confirmed cases in an outbreak. If one confirmed case among five total suspected cases or three cases are confirmed in a district within a month is observed, an outbreak is declared, and all suspected cases are then line-listed at the health facility level, and data are shared with district, county, and national levels. A case is considered epidemiologically linked when a patient has or had exposure to a probable or confirmed case, whereas a clinically compatible case is a clinical case of measles that has not been linked epidemiologically to a laboratory-confirmed or another epidemiologically linked case of measles.
During the outbreak, the Government of Liberia, with support from partners, initiated a number of public health response actions, including fast-tracking a joint action plan for a nationwide reactive measles vaccination campaign, active case search and case line listing, isolation and case management, intensified routine immunization across the country, and enhanced risk communication and community engagement throughout the country with emphasis on affected communities and health facilities.
Data source and management
The Division of Infectious Disease and Epidemiology of NPHIL provided an electronic version of the IDSR weekly epidemiological data. A Microsoft Excel file line list was used to extract the IDSR weekly epidemiological measles data from Epi week 1 (January 1) to 51 (December 25) of 2022. The age, sex, date of symptom onset, and outcome (dead/alive) variables were included on the spreadsheet after the data were extracted into Microsoft Excel file format.
Data analysis
All laboratory-confirmed cases, clinically compatible and epidemiologically linked, were included in the analysis. The suspected cases that were tested and were negative for measles and rubella (non-measles discarded) were excluded from the analysis. The data were cleaned and analyzed using R statistical software version 4.2.0. The demographic and clinical characteristics of the cases were presented. The median age and interquartile range of the age were estimated. The ages were grouped into intervals of 5 years (pediatric population) and 10 years (adult population). The incidence of measles in each county was calculated and presented in a chart. The overall case fatality rate (CFR) was calculated by dividing the total number of deaths by the number of measles cases. The CFR was also calculated by districts, age group, and sex. The timeline of the outbreak was depicted by an epidemiologic curve using epidemiological weeks. The overall and county-specific attack rate of the outbreak was calculated. To identify the hotspots, the spatial distribution of the cases and deaths was plotted per district using ArcGIS Pro version 3.0.3.
Ethical considerations
Ethical approval for the study was obtained from the Atlantic Center for Research and Evaluation (ACRE) Institutional Review Board (approval number 22-08-336). To ensure confidentiality, all identifiers, such as names and addresses, were eliminated prior to analysis. The information obtained was stored on a web server accessible to only authorized members of the research team.
Results
A total of 8772 cases were reported; however, only 8127 cases were included in the analysis. The median age of cases was 4 years (interquartile range: 2-8). The youngest age was a 14-day-old neonate, and the oldest case was a 67-year-old female. Children under 5 years of age constituted 60% of all the cases (4836/8127). Females accounted for 52% (4204/8127) of the cases (Table 1).
Table 1.
Demographic characteristics of measles cases, Liberia 2022.
| Characteristics | Frequency (n = 8,127) | Percent |
|---|---|---|
| Age (years) | ||
| 0-4 | 4,836 | 59.5 |
| 5-9 | 1,527 | 18.8 |
| 10-14 | 872 | 10.7 |
| 15-24 | 572 | 7.0 |
| 25-34 | 185 | 2.3 |
| 35-44 | 95 | 1.2 |
| >45 | 40 | 0.5 |
| Median (IQR) = 4 (2-8) | ||
| Sex | ||
| Female | 4,204 | 51.7 |
| Male | 3,923 | 48.3 |
| Place of case detection | ||
| Community | 1,582 | 19.5 |
| Hospital | 6,545 | 80.5 |
| Vaccination status | ||
| Vaccinated (card) | 84 | 1.0 |
| Vaccination (history) | 3,611 | 44.4 |
| Not vaccinated | 4,432 | 54.6 |
| County of residence | ||
| Montserrado | 4,264 | 52.4 |
| Grand Bassa | 749 | 9.2 |
| Nimba | 737 | 9.1 |
| Margibi | 603 | 7.4 |
| Bong | 376 | 4.6 |
| Maryland | 320 | 3.9 |
| Grand Kru | 217 | 2.7 |
| Lofa | 196 | 2.4 |
| Sinoe | 175 | 2.2 |
| Grand Gedeh | 143 | 1.8 |
| Grand Cape Mount | 136 | 1.7 |
| Bomi | 80 | 1.0 |
| Rivercess | 80 | 1.0 |
| River Gee | 33 | 0.4 |
| Gbarpolu | 19 | 0.2 |
| Outcome | ||
| Dead | 84 | 1.0 |
| Alive | 8,043 | 99.0 |
IQR = interquartile range.
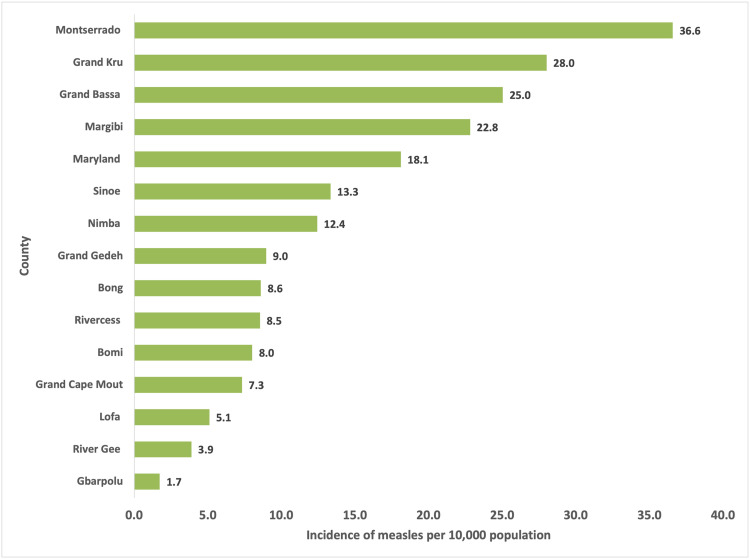
Only 52.4% (n = 4264) of the cases were reported from Montserrado County, which hosts the country's capital, whereas River Gee and Gbarpolu Counties reported less than 50 cases each during the period (Table 1). The incidence of measles during the period was found to be the highest in Montserrado County (36.6 cases per 10,000 population) and the lowest in Gbarpolu (1.7 cases per 10,000 population, Figure 1). Approximately 1% of cases had documentation of a prior dose of an MCV, although 44.4% reported prior vaccination but did not have documentation. The overall CFR was 1%. The CFR, however, varied by sex, county, and age, mostly among the 0-4 years (1.61%) and Bomi County (6.25%, Table 2).
Figure 1.
The incidence of measles by county of residence, 2022.
Table 2.
Case fatality rate among the measles cases.
| Variable | Cases | Dead | Case fatality rate |
|---|---|---|---|
| Age (years) | |||
| 0-4 | 4,836 | 78 | 1.61 |
| 5-9 | 1,527 | 3 | 0.20 |
| 10-14 | 872 | 3 | 0.34 |
| 15-24 | 572 | 0 | 0.00 |
| 25-34 | 185 | 0 | 0.00 |
| 35-44 | 95 | 0 | 0.00 |
| >45 | 40 | 0 | 0.00 |
| Sex | |||
| Male | 3,923 | 46 | 1.17 |
| Female | 4,204 | 38 | 0.90 |
| County | |||
| Bomi | 80 | 5 | 6.25 |
| Grand Cape Mount | 136 | 3 | 2.21 |
| Montserrado | 4,264 | 66 | 1.55 |
| Rivercess | 80 | 1 | 1.25 |
| Grand Bassa | 749 | 7 | 0.93 |
| Margibi | 602 | 1 | 0.17 |
| Nimba | 737 | 1 | 0.14 |
| Bong | 376 | 0 | 0.00 |
| Maryland | 320 | 0 | 0.00 |
| Grand Kru | 217 | 0 | 0.00 |
| Lofa | 196 | 0 | 0.00 |
| Sinoe | 175 | 0 | 0.00 |
| Grand Gedeh | 143 | 0 | 0.00 |
| River Gee | 33 | 0 | 0.00 |
| Gbarpolu | 19 | 0 | 0.00 |
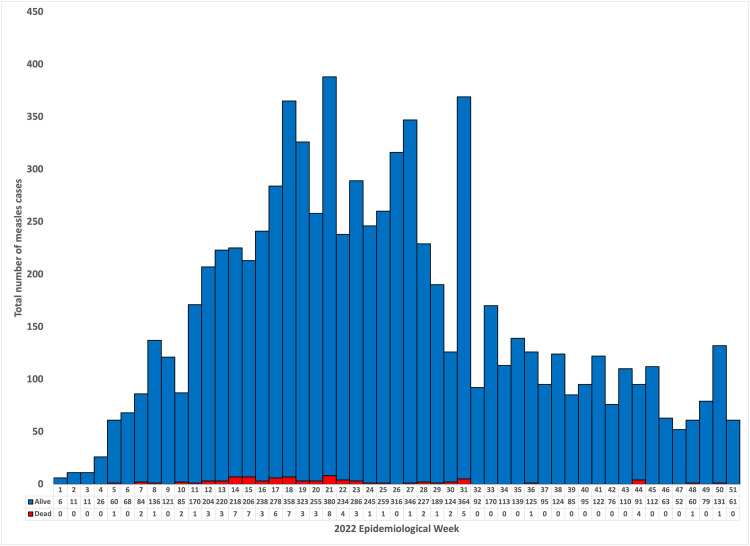
The outbreak started in the first epidemiological week (2022), with cases peaking during week 21. The outbreak saw a consistent increase in cases up to week 21, with subsequent sporadic peaks in weeks 23, 27, and 31. After the 31st epidemiological week, cases continued to decrease (Figure 2).
Figure 2.
Epicure of measles cases by epidemiological week, 2022.
The spatial distribution of measles cases and deaths by district of residence over epidemiological weeks shows that most cases were found in Montserrado County, followed by counties in southern, northern, and central regions (including Nimba, Grand Bassa, and Margibi Counties). From epidemiological week 15 to 51, cases were identified eventually throughout the remainder of the country´s districts and counties (Figure 3).
Figure 3.
Spatial distribution of the measles cases by district of residence and epidemiological week.
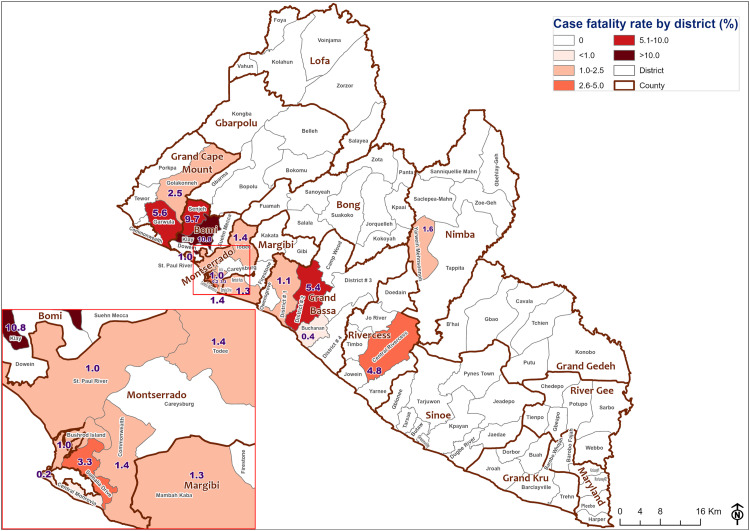
Measles case fatality was reported in several counties, and the highest CFR was reported in values from Bomi, Grand Cape Mount, and Montserrado (Figure 4).
Figure 4.
Measles case fatality rate by district at epidemiological week 51, 2022.
Discussion
Liberia recorded a very large outbreak of measles in 2022, involving almost all the health districts reporting at least one case during the period under review. The magnitude and spread of the outbreak suggest a possible suboptimal measles vaccine coverage throughout the country. The suboptimal coverage could have resulted from COVID-19 pandemic-related impacts on routine health services provision, underlying gaps in immunization program coverage, or both. The negative effect of the COVID-19 pandemic on routine health services, including immunization delivery in Liberia, has been documented previously. Vaccination rates have been reported below optimal levels in the African region in conjunction with fewer routine immunization campaigns conducted because of the COVID-19 pandemic [5,6].
Most measles cases reported in Liberia during the period involved children under 5 years of age. Although measles may affect people of any age in any community, it is most severe in children under 5 years of age. Most cases and deaths occurred among children under 5 years of age in our study. The high morbidity and mortality in this age group are consistent with reports from Nigeria and other countries [8], [9], [10], [11].
Only 1% of cases had documentation of a prior dose of an MCV, although 46% reported prior vaccination but did not have documentation. This vaccination coverage is low when compared with World Health Organization standards for reaching and maintaining 95% of two doses of the measles vaccine through regular immunization programs and supplemented by additional immunization actions to eradicate measles [12]. Results from earlier studies conducted in Ethiopia, South Africa, and Nigeria confirm that intermittent cases of the disease occur when coverage drops below the 95% coverage threshold necessary for eradication [13], [14], [15]. Our findings also document Liberia´s challenges with accurately recording vaccination delivery and/or maintaining quality records. The high number of vaccinations by history may be indicative that vaccination information in routine health care services is self-reported without evidence, and under such circumstances, it may be difficult to monitor the completion of vaccination doses and schedules. Similar observations have been reported in other countries, including Brazil, Mongolia, and Japan [16,17].
The overall CFR in this study is 1%, which is comparable to outbreak investigation findings from the Oromia region of Ethiopia's Ginir and Artuma districts of 0.5% and 2.6%, respectively [18,19]. However, research in Nigeria showed a 7-year CFR of 4.8% in 2018, which was in line with the CFR reported from earlier studies in Nigeria, which ranged from 3% to 5% and might reach as high as 10% for developing nations [8,20,21]. A CFR of 7% was also found in earlier research conducted in India [22]. The low overall CFR need to be taken with caution given the variability of CFR across the different counties. Bomi, Grand Cape Mount, and Montserrado counties recorded the highest CFR. Higher CFR in certain counties may represent better detection or, more plausibly, greater gaps in community awareness, clinician knowledge, or supportive clinical treatment. Additional studies could assist in delineating between these possible explanations.
The highest incidence of measles was observed in Montserrado County, which is home to Monrovia, the nation's capital. Given the stark differences in income, social services, infrastructure, and other opportunities between Monrovia and other regions of the nation, this may be attributable to greater population density brought on by both rural-to-urban and inter-urban migration [23]. Similar results in previous research showed that measles cases were less common in rural than urban areas [24,25].
This study's seasonal trend showed an increase in measles cases as the dry season, which starts in April, transitioned into the rainy season, which concludes in October. Although several earlier studies in Africa indicated that measles cases soared during the dry season, significant seasonal variations in measles incidence have also been observed during a number of significant outbreaks of the disease amid various environmental factors and variations in population size [26,27].
The Ministry of Health, through the EPI unit, as part of the response to the outbreak, conducted a reactive vaccination with MCV initially at hotspots counties but later nationwide as the vaccines became more available. This was in addition to sending out a rapid response team to contain the outbreak and working with partners and the NPHIL to conduct operational research to understand the drivers of the outbreak.
The limited nature of the variables included in the surveillance database made it difficult to explore the likely factors (malnutrition, complications, etc) associated with fatality among the cases. In addition, the vaccination status of cases was largely self-reported, which may not be accurate. Most of the time, there was no proof of vaccination cards.
This study provides critical information about the Liberian measles outbreak of 2022, which significantly had the largest number of cases in recent memory [28], [29], [30]. In urban and rural areas of the country, especially among children under 5 years of age, measles has continued to be a serious health challenge. In addition, there is disturbingly little proof of measles vaccination by card, which has serious ramifications for maintaining immunization schedules and dosages and, ultimately, monitoring vaccine coverage. Therefore, it is advised that efforts to conduct a reactive measles campaign at the national and sub-national levels be stepped up and that measles surveillance, regular immunization, and additional immunization actions to reach every community be given top priority. In addition, there is a need for caregivers to get enough knowledge regarding the significance and safekeeping of vaccination records, as well as adequate training for health care professionals and vaccine providers on correct documentation of vaccination records.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval for the study was obtained from the ACRE Institutional Review Board (approval number 22-08-336). To ensure confidentiality, all identifiers, such as names and addresses, were eliminated prior to analysis. The information obtained was stored on a web server accessible to only authorized members of the research team.
Acknowledgments
The authors acknowledge the support from the US CDC, AFENET, and NPHIL. The ideas in this work are, however, those of the individual authors and do not represent the official position of the respective institutions.
Author contributions
Conceptualization: MA, CDU, and RI
Design: CDU and MA
Methodology: CDU, MA, JG, and RI
Administration: MA, RI, and JM
Data Management and Analysis: CDU and GA
Interpretation: CDU, BS, MA, and RI
Initial drafting: CDU, BS, and MA
Revision and approval: BS, CDU, RWJ, JG, GA, MA, JM, and RI
Footnotes
Bode Shobayo and Chukwuma David Umeokonkwo contributed equally.
References
- 1.Hübschen JM, Gouandjika-Vasilache I, Dina J. Measles. Lancet. 2022;399:678–690. doi: 10.1016/S0140-6736(21)02004-3. [DOI] [PubMed] [Google Scholar]
- 2.Misin A, Antonello RM, Di Bella S, Campisciano G, Zanotta N, Giacobbe DR, et al. Measles: an overview of a re-emerging disease in children and immunocompromised patients. Microorganisms. 2020;8 doi: 10.3390/MICROORGANISMS8020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sbarra AN, Rolfe S, Nguyen JQ, Earl L, Galles NC, Marks A, et al. Mapping routine measles vaccination in low- and middle-income countries. Nature. 2020;589:415–419. doi: 10.1038/s41586-020-03043-4. 589:7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Measles vaccines: WHO position paper - April 2017. Wkly Epidemiol Rec. 2017;92:205–227. [Google Scholar]
- 5.Wariri O, Nkereuwem E, Erondu NA, Edem B, Nkereuwem OO, Idoko OT, et al. A scorecard of progress towards measles elimination in 15 west African countries, 2001–19: a retrospective, multicountry analysis of national immunisation coverage and surveillance data. Lancet Glob Health. 2021;9:e280–e290. doi: 10.1016/S2214-109X(20)30481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babalola OJ, Sesay HW, Blebo LS, Whesseh FK, Umeokonkwo CD, Adewuyi PA, et al. The influence of first wave of COVID-19 outbreak on routine healthcare services, Liberia, August 2020: a mixed study approach. BMC Health Serv Res. 2022;22:1–11. doi: 10.1186/S12913-022-08074-3. 22:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Measles vaccination coverage 2023, https://immunizationdata.who.int/pages/coverage/MCV.html?CODE=LBR&ANTIGEN=MCV1&YEAR=; (accessed December 24, 2023).
- 8.Saleh J-EA. Trends of measles in Nigeria: A systematic review. Sahel Med J. 2016;19:5. doi: 10.4103/1118-8561.181887. [DOI] [Google Scholar]
- 9.Faruk AS, Adebowale AS, Balogun MS, Taiwo L, Adeoye O, Mamuda S, et al. Temporal trend of measles cases and impact of vaccination on mortality in Jigawa State, Nigeria, 2013–2017: a secondary data analysis. Pan Afr Med J. 2020;35(Suppl. 1):13. doi: 10.11604/PAMJ.SUPP.2020.35.1.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shorunke FO, Adeola-Musa O, Usman A, Ameh C, Waziri E, Adebowale SA. Descriptive epidemiology of measles surveillance data, Osun state, Nigeria, 2016–2018. BMC Public Health. 2019;19:1–8. doi: 10.1186/S12889-019-8012-6/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim BS, Usman R, Mohammed Y, Datti Z, Okunromade O, Abubakar AA, et al. Burden of measles in Nigeria: a five-year review of casebased surveillance data, 2012–2016. Pan Afr Med J. 2019;32(Suppl. 1):5. doi: 10.11604/PAMJ.SUPP.2019.32.1.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donadel M, Scobie HM, Pastore R, Grabovac V, Batmunkh N, O'Connor S, et al. Comprehensive vaccine-preventable disease surveillance in the western Pacific region: A literature review on integration of surveillance functions, 2000–2021. Glob Health Sci Pract. 2022;10 doi: 10.9745/GHSP-D-22-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousif M, Hong H, Malfeld S, Smit S, Makhathini L, Motsamai T, et al. Measles incidence in South Africa: a six-year review, 2015–2020. BMC Public Health. 2022;22:1647. doi: 10.1186/S12889-022-14069-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsegaye G, Gezahegn Y, Tesfaye A, Mulatu G, Bulcha GG, Berhanu N. Measles outbreak investigation in Guradamole District of bale zone, south Eastern Ethiopia, 2021. Infect Drug Resist. 2022;15:669–683. doi: 10.2147/IDR.S343270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ori PU, Adebowale A, Umeokonkwo CD, Osigwe U, Balogun MS. Descriptive epidemiology of measles cases in Bauchi State, 2013–2018. BMC Public Health. 2021;21:1311. doi: 10.1186/s12889-021-11063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarenko C, San Pedro A, Paiva NS, Santos JPCD, Medronho RA, Gibson G. Measles resurgence in Brazil: analysis of the 2019 epidemic in the state of São Paulo. Rev Saude Publica. 2022;56:50. doi: 10.11606/S1518-8787.2022056003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki F, Miyoshi M, Ikeda T, Nishijima H, Saikusa M, Itamochi M, et al. Nationwide molecular epidemiology of measles virus in Japan between 2008 and 2017. Front Microbiol. 2019;10:1470. doi: 10.3389/FMICB.2019.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalil FS, Gemeda DH, Bedaso MH, Wario SK. Measles outbreak investigation in Ginnir district of Bale zone, Oromia region, Southeast Ethiopia, May 2019. Pan Afr Med J. 2020;36:20. doi: 10.11604/PAMJ.2020.36.20.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tariku MK, Misikir SW. Measles outbreak investigation in Artuma Fursi Woreda, Oromia Zone, Amhara Region, Ethiopia, 2018: A case control study. BMC Res Notes. 2019;12:1–6. doi: 10.1186/S13104-019-4806-Y/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poletti P, Parlamento S, Fayyisaa T, Feyyiss R, Lusiani M, Tsegaye A, et al. The hidden burden of measles in Ethiopia: how distance to hospital shapes the disease mortality rate. BMC Med. 2018;16:1–12. doi: 10.1186/S12916-018-1171-Y/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sbarra AN, Mosser JF, Jit M, Ferrari M, Ramshaw RE, O'Connor P, et al. Estimating national-level measles case–fatality ratios in low-income and middle-income countries: an updated systematic review and modelling study. Lancet Glob Health. 2023;11:e516–e524. doi: 10.1016/S2214-109X(23)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzeyie KA, Lowang D, Dikid T, Wangsu W, Tamir T. Working Group*. Measles outbreak investigation at Indo-Myanmar border, Longding District, Arunachal Pradesh, India, 2017. Indian J Public Health. 2021;65(Suppl):S23–S28. doi: 10.4103/ijph.IJPH_1067_20. [DOI] [PubMed] [Google Scholar]
- 23.Ngafuan RF. The “Overcrowding” of Monrovia and its link to Rural-Urban Migration in Liberia: causes, Consequences and Solutions. Perspective 2010, https://www.theperspective.org/2010/0614201001.html; (accessed January 1, 2024).
- 24.Goodson JL, Masresha BG, Wannemuehler K, Uzicanin A, Cochi S. Changing epidemiology of measles in Africa. J Infect Dis. 2011;204(Suppl. 1):S205–S214. doi: 10.1093/INFDIS/JIR129. [DOI] [PubMed] [Google Scholar]
- 25.Garba FM, Usman R, Umeokonkwo C, Okolocha EC, Yahaya M, Abdulqadir I, et al. Descriptive epidemiology of measles cases in Zamfara State-Nigeria, 2012–2018. J Interv Epidemiol Public Health. 2022;5 doi: 10.37432/jieph.2022.5.4.69. [DOI] [Google Scholar]
- 26.Bharti N, Tatem AJ, Ferrari MJ, Grais RF, Djibo A, Grenfell BT. Explaining seasonal fluctuations of measles in Niger using nighttime lights imagery. Science. 2011;334:1424–1427. doi: 10.1126/SCIENCE.1210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari MJ, Djibo A, Grais RF, Bharti N, Grenfell BT, Bjornstad ON. Rural-urban gradient in seasonal forcing of measles transmission in Niger. Proc Biol Sci. 2010;277:2775–2782. doi: 10.1098/RSPB.2010.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagbe T, Williams GS, Rude JM, Flomo S, Yeabah T, Fallah M, et al. Lessons learned from detecting and responding to recurrent measles outbreak in Liberia post Ebola-Epidemic 2016–2017. Pan Afr Med J. 2019;33(Suppl. 2):7. doi: 10.11604/pamj.supp.2019.33.2.17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masresha BG, Luce R, Weldegebriel G, Katsande R, Gasasira A, Mihigo R. The impact of a prolonged Ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014–2015. Pan Afr Med J. 2020;35(Suppl. 1):8. doi: 10.11604/PAMJ.SUPP.2020.35.1.19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobway F, Whesseh FT, Umeokonkwo CD, Babalola OJ, Lawubah J, Seysay HW, et al. Measles outbreak investigation in Buah health district. Liberia: Grand Kru County, 2020. Journal of Interventional Epidemiology and Public Health. 2023;6 [Google Scholar]