Abstract
Recent evidence suggests that pregnancy-associated malaria (PAM), associated with maternal anemia and low birth weight, results from preferential sequestration of parasitized red blood cells (pRBC) in the placenta via binding of variant surface antigens (VSA) expressed on the surface of pRBC to chondroitin sulfate A (CSA). The VSA mediating CSA binding (VSACSA) and thus sequestration of pRBC in the placenta are antigenically distinct from those that mediate pRBC sequestration elsewhere in the body, and it has been suggested that VSACSA are relatively conserved and may thus constitute an attractive target for vaccination against PAM. Using flow cytometry, levels of antibody to VSA and VSACSA expressed on the surface of red blood cells infected with Plasmodium falciparum isolates were measured during pregnancy and lactation in Ghanaian primigravid women enrolled in a trial of maternal vitamin A supplementation. Antibody responses to VSACSA were detected within the first trimester of pregnancy and increased with increasing duration of pregnancy, and they seemed to be isolate specific, indicating that different CSA-adherent parasite lines express antigenically distinct VSA and thus may not be as antigenically conserved as has been previously suggested. Levels of anti-VSACSA were not significantly associated with placental malarial infection determined by histology, indicating that primary immune responses to VSACSA may not be sufficient to eradicate placental parasitemia in primigravidae.
Pregnant women, especially those in their first and second pregnancies, are at increased risk of pregnancy-associated malaria (PAM) and its associated complications, including severe maternal anemia, stillbirth, and low birth weight (17, 33). Increased susceptibility to PAM is attributed to generalized suppression of cell-mediated immune responses (23, 27) and/or preferential sequestration of parasitized red blood cells (pRBC) in the placenta (2, 11). Placental pRBC bind to complex polysaccharides (e.g., chondroitin sulfate A [CSA] and hyaluronic acid) in the placental intervillous space; binding is mediated by erythrocyte variant surface antigens (VSA) encoded by highly polymorphic, multigene families in the parasite genome, members of which also mediate binding (via numerous cell surface receptors) to vascular endothelium throughout the body (reviewed in reference 15). Parity-dependent development of immunity to PAM has been linked to acquisition, during first and second pregnancies, of anti-VSA antibodies (Abs) thought to block pRBC sequestration in the placenta (12, 31). The VSA mediating sequestration of pRBC in the placenta are antigenically distinct from those that mediate pRBC sequestration elsewhere in the body (4, 16, 22, 31), and it has been suggested that these VSA are antigenically conserved (12) and may thus constitute an attractive target for vaccination against PAM (30).
The purposes of this study were to describe the acquisition of anti-VSACSA immunoglobulin G (IgG) during pregnancy and lactation in primigravid women, to investigate antigenic variation in VSA mediating CSA adhesion (VSACSA) between P. falciparum isolates, and to determine if anti-VSACSA IgG developing during a first pregnancy is able to prevent placental malarial infection as determined by histology.
MATERIALS AND METHODS
Study subjects.
The study subjects were women enrolled in a randomized, double-blind, placebo-controlled trial of vitamin A supplementation and immunity to PAM in primigravidae, the details of which are reported elsewhere (Cox et al., submitted). Women were recruited from antenatal clinics at Nkoranza District Hospital and three rural health clinics in the Brong Ahafo region of central Ghana during the period from March to June 2001. In this area malaria transmission is perennial and intense (6), and a recent survey (2003) indicated that the parasite prevalence was 55% in children under 5 (S. Owusu-Agyei, personal communication, 2005). Primigravid women were eligible for inclusion in the study if they were pregnant (as assessed by urine test or palpation), were residents of the study area, were in good health, and were less than approximately 24 weeks pregnant, as determined by fundal height measurements. However, as fundal height measurements are imprecise, gestational age was also determined by back-calculation from estimated gestational age at delivery; some women recruited into the study were thus found to have been more than 24 weeks pregnant at enrolment, but these women were not excluded. According to available health records, none of the women were suffering from clinical human immunodeficiency virus infection or tuberculosis, but diagnostic tests were not performed. The estimated prevalence of human immunodeficiency virus infection in pregnant Ghanaian women in the Brong Ahafo region for 2000 was 1.6% (18). All study women received a full treatment regimen of chloroquine at enrolment. Nine percent of women reported using a bed net. Individual informed consent was obtained from all study participants, and this study was approved by the Ethical Review Committee of the London School of Hygiene and Tropical Medicine and the Ministry of Health, Ghana.
Laboratory assays. (i) Sample collection.
Venous blood samples (4 ml) were collected into heparinized Vacutainers (Greiner) on three occasions from each woman: at enrolment (n = 46 in the placebo group, n = 48 in the vitamin A group), in late pregnancy (n = 34 in the placebo group, n = 45 in the vitamin A group), and at 6 weeks postpartum (n = 45 in the placebo group, n = 45 in the vitamin A group). At the same time, finger prick blood samples were obtained for determination of malarial parasitemia by microscopic examination of Giemsa-stained thick blood films.
(ii) Placental histology.
Local midwives were trained to collect placenta samples as described previously (8). In brief, samples (1 to 2 cm2) were cut from the maternal side of the placenta, approximately one-quarter of the distance from the center to the edge of the placenta, and placed in 60 ml of 10% formalin. Samples were stored for a maximum of 8 months before processing and embedding in paraffin wax. Sections that were 1 μm thick were stained with Harris hematoxylin and eosin Y (HD Supplies, United Kingdom) and modified Giemsa stain (HD Supplies). Sections containing formalin pigment were treated with picric acid for time-dependent removal of the pigment (3). All slides were read by two independent investigators. When there were discrepancies between readings, sections were restained and read again until agreement was reached. Placentas were classified according to previously published criteria (8).
(iii) P. falciparum lines.
Three genetically distinct isolates of P. falciparum were used. FCR3 is an established laboratory isolate (13), Busua was originally collected from a nonimmune Danish man presenting with uncomplicated malaria upon return from the coastal region of Ghana (32), and EJ-24 was isolated directly from the placenta of a Ghanaian woman in the Ashanti region of Ghana (26). The genotypic identities of all isolates were regularly confirmed by genotypic profiling at the polymorphic msp-1, msp-2, and glurp loci (28). To enrich for expression of VSA associated with sequestration in the placenta, CSA-adhering sublines were established and maintained by regular panning on CSA-coated petri dishes (Falcon; BD PharMingen) as described elsewhere (22, 24). EJ-24 bound spontaneously to CSA so did not need to be selected in vitro, but regular panning on CSA was carried out to ensure maintenance of the binding phenotype in culture. To ensure continued expression of VSACSA, experiments were always carried out with sublines that had been panned within the previous 10 days. Parasites were maintained in cultures of human type O erythrocytes (4% hematocrit) in RPMI 1640 (Invitrogen) supplemented with Albumax II (Life Technologies) and 2% nonimmune serum (isolates Busua and EJ-24), according to standard methods (9).
(iv) CSA adhesion assay.
Cultures containing approximately 1% late-stage trophozoites and schizonts were washed twice in RPMI 1640 before suspension in hypoxanthine-free RPMI 1640 with 10% nonimmune serum and cultured overnight. The following day, ring-stage parasites were labeled with [3H]hypoxanthine, cultured overnight (31), and purified by gelatin sedimentation. Flat-bottom, 96-well microtiter plates (Falcon; BD PharMingen) were coated with various concentrations of CSA (Sigma) (4 to 200 μg/ml; 100 μl/well) for 16 h at 5°C and blocked with 20 mg/ml of BSA in phosphate-buffered saline (PBS) (100 μl/well) for 30 min at room temperature. Samples containing 2 × 106 pRBC/well were incubated in triplicate wells for 1 h at room temperature, nonadherent pRBC were washed off with RPMI 1640, adherent pRBC were harvested onto glass fiber pads, and [3H]hypoxanthine activity was measured by liquid scintillation counting (Beckman Coulter).
(v) Flow cytometry.
Plasma levels of VSA-specific IgG were measured by flow cytometry as previously described (29). In brief, purified late-stage parasites were stained with ethidium bromide, sequentially incubated with human test plasma, goat anti-human IgG (Dako), and fluorescein isothiocyanate-conjugated rabbit anti-goat immunoglobulin (Dako), and analyzed by flow cytometry (EPICS XL-MCL; Coulter Electronics). The binding of anti-VSA IgG was quantified by determining the mean fluorescence index (MFI). For each parasite line, all test and control plasma samples were tested on the same day using parasites from the same batch. The negative controls included 11 samples from Danish non-malaria-exposed pregnant women, duplicate samples of pooled plasma from Danish non-malaria-exposed nonpregnant women, and pooled plasma from Ghanaian malaria-exposed men. Pooled plasma from multigravid, pregnant, Ghanaian women previously demonstrated to have high levels of anti-VSA IgG (22) was used as a positive control.
Statistical analysis.
Except where explicitly stated otherwise, analyses were performed on data obtained from plasma from the placebo group only. Flow cytometric data were analyzed by using WinMDI software and by statistical analyses performed with Stata 7 (Stata Corporation, Texas). The median levels of Ab binding for the study samples were compared with the levels for the pooled positive and negative control sera using single sample sign rank tests. The seroprevalence of anti-VSA IgG responses was determined using MFI values from (i) Ghanaian men's serum to set a cutoff (mean plus 2 standard deviations) for the presence of anti-VSACSA IgG, (ii) pregnant Danish women's serum to set a cutoff (mean plus 2 standard deviations) for the presence of malaria-specific IgG during pregnancy, and (iii) nonpregnant Danish women's serum to set a cutoff (mean plus 2 standard deviations) for the presence of malaria-specific IgG in postpartum samples. Subsequent statistical analyses of levels of Ab binding were performed with normalized MFI values (i.e., expressed as a percentage of the positive control serum). Analyses of levels of IgG over time were performed using linear regression with the inclusion of robust standard errors to allow for the repeated measurements for individuals. In order to increase the power of the analysis of the relationship between the levels of anti-VSACSA IgG and placental malarial infection (active infection versus past infection and active infection versus no infection), data from both the placebo and vitamin A-supplemented groups were used. The analysis was by ordinary linear regression with treatment group included as a covariate, thus controlling for any potential effect of vitamin A supplementation.
RESULTS
CSA-selected parasite lines exhibited increased levels of CSA adhesion compared to the nonselected parent lines.
Measurement of the adhesion of the selected and nonselected parasite lines to CSA in vitro confirmed that the CSA-selected lines had considerably higher levels of adhesion than the nonselected lines (ratio of CSA adhesion [× 10−3 cpm] for FCR3CSA to FCR3, 3.64 at 40 μg/ml of CSA; ratio of CSA adhesion [× 10−3 cpm] for BusuaCSA to Busua, 4.74 at 10 μg/ml of CSA). There was no appropriate control line for the placental line, EJ-24, but the binding of EJ-24 to CSA was 6.0-fold higher than the binding of EJ-24 to PBS. For comparison, unselected FCR bound equally to CSA and PBS, whereas the binding of FCRCSA to CSA was 6.6-fold higher than the binding to PBS.
Sex-specific recognition of CSA-adhering pRBC by anti-VSA IgG.
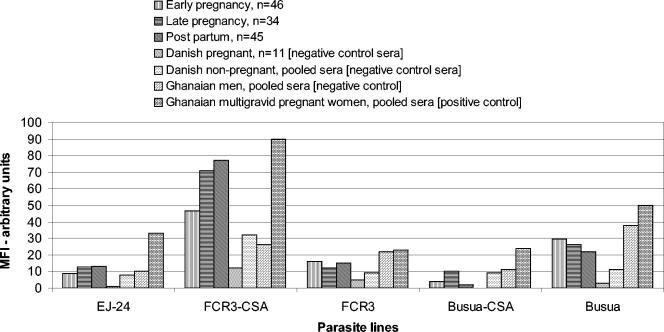
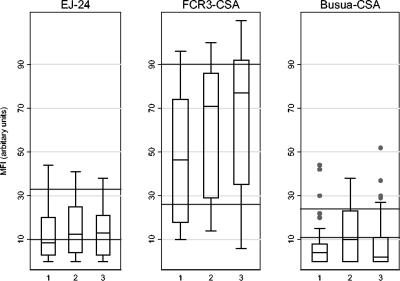
The median Ab binding to non-CSA adherent parasite lines was higher in the Ghanaian primigravid subjects and in the pooled Ghanaian male and multigravid control plasma than in the Danish plasma, but among the Ghanaians, anti-VSA IgG did not appear to differ by sex or parity (Fig. 1). Ab binding was higher in the positive control (pooled plasma from pregnant multigravid Ghanaian women) than in samples from primigravid Ghanaian women, in part as multigravids had been selected for particularly high levels of anti-VSA IgG. By contrast, the median Ab binding to CSA-adherent parasite lines varied significantly by sex (Fig. 1 and 2). Ab in pooled plasma from Ghanaian men bound less well to all three CSA-adherent parasite isolates than Ab from multigravid Ghanaian women bound. For the primigravid subjects, the median MFI for CSA-adherent parasites was significantly lower than that for the multigravid plasma pool (single sample sign rank test, P < 0.001 in all cases). For FCR3CSA there were highly significant increases in the median MFI between the primigravid subjects and the male control sera for all times (P < 0.001), while for EJ-24 the median was not significantly increased at late pregnancy and postpartum (P = 0.08 and P = 0.11). For BusuaCSA the median MFI for the primigravid subjects was lower (P < 0.01) at early pregnancy and postpartum but similar at late pregnancy compared to the Ghanaian men. Although the median levels of Ab binding to BusuaCSA were below or similar to the levels in Ghanaian men (Fig. 2), some of the primigravid subjects had levels of Ab binding that were greater than that in the multigravid positive control. In Fig. 1 and 2 the results are presented with background fluorescence removed; therefore, the relative level of IgG binding can be compared for the different parasite isolates. Thus, it appears either that pRBC infected with FCR3CSA express a greater number of sites for binding of anti-VSACSA IgG or that they express VSACSA that are antigenically distinct and more frequently recognized by antibody than the VSA expressed by other CSA-adherent isolates.
FIG. 1.
Comparison of plasma IgG recognition for CSA-adherent and non-CSA-adherent pRBC in test and control plasma.
FIG. 2.
Plasma IgG recognition of CSA-adherent pRBC in primigravid subjects over time. The lower horizontal line indicates the MFI of the negative control plasma from Ghanaian men (mean of duplicate tests of pooled plasma). The upper horizontal line indicates the MFI of the positive control plasma from multigravid pregnant Ghanaian women (mean of duplicate tests of pooled plasma). 1, enrolment at early pregnancy; 2, late pregnancy; 3, postpartum.
Development of anti-VSACSA IgG during the first pregnancy.
Primigravidae are expected to develop novel anti-VSACSA Ab specificities during gestation as a consequence of exposure (for the first time) to significant numbers of CSA-adherent parasites. However, a considerable proportion of women were already seropositive for anti-VSACSA Ab by the time of enrolment (median gestational age, 17 weeks; range, 5 to 26 weeks). More than 60% of women had antibodies to FCR3CSA at the time of enrolment and 40% had antibodies to EJ-24, but only 10% had antibodies to BusuaCSA (Table 1). This presumably reflects very rapid parasitization of the placenta in this high-transmission environment. In support of this, for all three of the CSA-adherent parasite lines and for the nonselected Busua line, the levels of anti-VSA IgG at enrolment were positively associated with the presence of peripheral parasitemia (as determined by Student's t test, for EJ-24, 71.3 versus 79.4 [P = 0.001]; for FCR3CSA, 50.1 versus 62.3 [P = 0.01]; for BusuaCSA, 60.8 versus 64.9 [P = 0.05]; for Busua, 49.7 versus 53.1 [P = 0.05]). This association was not present in late pregnancy or postpartum (data not shown).
TABLE 1.
Changes in plasma IgG over time in the placebo groupa
| Group or comparison | EJ-24
|
FCR3CSA
|
BusuaCSA
|
FCR3
|
Busua
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | % Positive | Mean | % Positive | Mean | % Positive | Mean | % Positive | Mean | % Positive | |
| At enrolment | ||||||||||
| Early pregnancy (n = 46) | 70.77 | 43 | 60.91 | 61 | 64.36 | 13 | 78.01 | 72 | 51.35 | 96 |
| Late pregnancy (n = 34) | 73.46 | 53 | 72.76 | 76 | 70.53 | 42 | 75.01 | 59 | 48.77 | 97 |
| Postpartum (n = 45) | 72.69 | 58 | 75.07 | 80 | 64.13 | 20 | 77.13 | 49 | 44.62 | 76 |
| Late vs early pregnancy | 0.20 | 0.001 | 0.02 | 0.32 | 0.02 | |||||
| Postpartum vs early pregnancy | 0.36 | <0.001 | 0.91 | 0.67 | <0.001 | |||||
| Postpartum vs late pregnancy | 0.60 | 0.39 | 0.001 | 0.38 | 0.005 | |||||
The mean levels of IgG are expressed as percentages of the mean value for duplicates of positive controls.
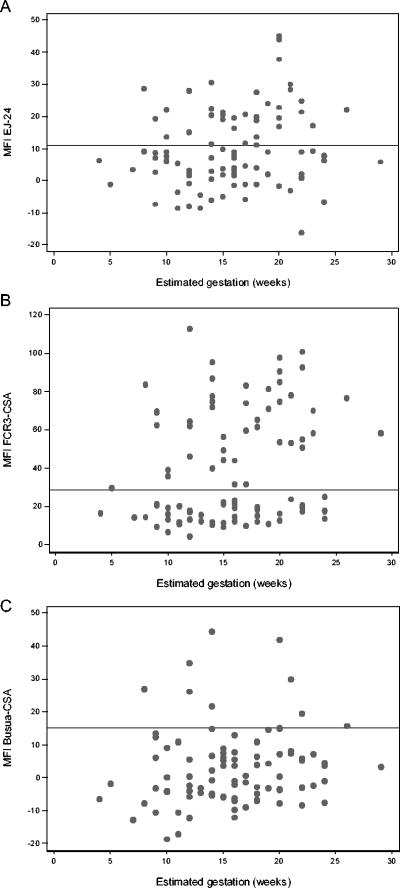
When Ab responses at enrolment were broken down by estimated gestational age (Fig. 3), it appeared that Ab secretion and hence exposure to placental parasites could begin as early as an estimated gestational age of 10 weeks. Even allowing for imprecision in gestational age estimates of ±2 weeks, this is earlier than previously reported (20, 31). Overall, there is a poor correlation between MFI and gestational age, indicating that IgG levels are not a function of gestational age per se but reflect the stochastic nature of exposure to parasites.
FIG. 3.
Association of anti-VSACSA IgG with estimated gestational age at enrolment (early pregnancy). Data are shown for both the placebo and vitamin A-supplemented groups. (A) MFI for EJ-24 against gestation in early pregnancy. Correlation coefficient (r) = 0.16 and P = 0.13. (B) MFI for FCR3CSA against gestation in early pregnancy. r = 0.23 and P = 0.03. (C) MFI for BusuaCSA against gestation in early pregnancy. r = 0.11 and P = 0.28. The solid horizontal line indicates the MFI plus 2 standard deviations for negative control plasma from malaria-exposed, Ghanaian men (cutoff for positive anti-VSA-CSA). The values are MFI minus the background fluorescence (mean for pRBC incubated in the absence of plasma). Gestational age was assessed by palpation or back-calculation from term deliveries if data were available. Estimates were assumed to be accurate to within ±2 weeks.
In late pregnancy (median gestational age, 31 weeks; range, 26 to 36 weeks), the mean levels of anti-VSACSA (expressed as a % of the level for positive control serum from multigravid pregnant women) were higher than those at enrolment for all of the CSA-adherent parasite lines, reaching statistical significance for FCR3CSA and BusuaCSA (P = 0.001 and P = 0.02, respectively) (Table 1), and with the exception of BusuaCSA, they remained high 6 weeks postpartum. In contrast, the mean levels of IgG to the non-CSA adherent lines decreased during pregnancy, reaching statistical significance for the Busua parasite line, and also for the Busua line they decreased significantly further postpartum, a finding similar to previous observations for a Cameroonian cohort (31).
FCR3CSA was the most frequently recognized of the three lines, and BusuaCSA was the least frequently recognized; accordingly, the median MFI was highest for FCR3CSA and lowest for BusuaCSA (Fig. 1 and 2).
Do different CSA-selected parasite lines express antigenically distinct VSA?
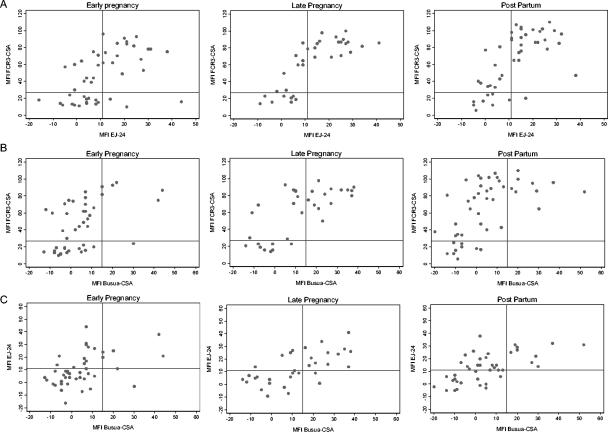
Marked variations between the three CSA-selected parasite lines in both the median MFI and the prevalence of Ab binding (Fig. 2, Table 1) suggest either that these parasite lines express different amounts of the same VSA on the pRBC surface or that they express antigenically distinct VSA variants. Indeed, both of these may occur. Thus, the VSA expressed by FCR3CSA may be commonly expressed by placental parasites (such that most primigravidae are likely to be exposed to it) and may also be expressed at a high level (giving rise to very high MFI in seropositive women [Fig. 1]). Conversely, the VSA expressed by BusuaCSA may be both rare and expressed at low levels (low seroprevalence, low MFI), while that of EJ-24 may be commonly expressed (high seroprevalence) (Table 1) but only at low levels (low MFI) (Fig. 2). Taken together, these observations suggest that the three lines express different VSA. To test this hypothesis further, we directly compared the MFI of individual plasma samples for binding to the three CSA-adherent lines of pRBC (Fig. 4). Although there appeared to be some correlation between the presence of anti-VSACSA IgG to one parasite line and the presence of anti-VSACSA IgG to another (which might reflect either concomitant exposure to two different VSA, cross recognition of similar VSA, or recognition of a conserved epitope present on both VSA), in each of the comparisons made there were significant proportions of women who were seropositive for one parasite line but seronegative for another, indicating clear differences in expression of B-cell epitopes between the three lines.
FIG. 4.
Correlations between levels of anti-VSACSA IgG for different parasite lines during pregnancy and postpartum. (A) Levels of anti-VSACSA IgG for FCR3CSA versus levels of anti-VSACSA IgG for EJ-24; (B) levels of anti-VSACSA IgG for FCR3CSA versus levels of anti-VSACSA IgG for BusuaCSA; (C) levels of anti-VSACSA IgG for EJ-24 versus levels of anti-VSACSA IgG for BusuaCSA. The solid horizontal line indicates the MFI of the negative control from malaria-exposed Ghanaian men (mean of duplicate tests of pooled plasma plus 2 standard deviations) (cutoff for positive anti-VSACSA IgG).
Levels of anti-VSACSA are not associated with placental malarial infection.
Of the 76 placenta samples collected, 66 (87%) showed evidence of malarial infection. Thirteen percent of the samples were classified as not infected (no evidence of parasites or hemozoin [malarial pigment] deposition), 8% were classified as having an active infection (parasites and/or pigment in the intervillous spaces, pigment in monocytes but not in fibrin), 36% were classified as having active chronic infections (same as for an active infection but including pigment in fibrin and/or the chorionic villous syncytiotrophoblast), and 43% were classified as having past chronic infections (parasites not present and pigment confined to fibrin). EJ-24 was the only parasite line for which there was evidence that suggested an association between placental malarial infection and anti-VSACSA IgG; when controlling for a possible effect of vitamin A supplementation, the levels were higher in women with past placental infection than in women with active placental infection at delivery (mean MFI expressed as a % of the positive control, 77.3% [95% confidence interval, 71.8 to 82.8%; n = 30] versus 71.8% [95% confidence interval, 66.6 to 77.0%; n = 26]; P = 0.069).
DISCUSSION
In this study we described the acquisition of IgG antibodies to CSA-binding parasites in primigravid women experiencing primary infections with this parasite phenotype and determined if these antibodies were associated with placental infection at delivery as determined by histology. In addition, the testing of three genetically distinct CSA-adherent parasite lines provided the opportunity to determine the extent of antigenic conservation of VSACSA between parasite isolates.
Our data clearly confirm previous data regarding sex-specific IgG recognition of pRBC that have been selected for in vitro binding to CSA (22, 31). Similarly, our data confirm previous observations that anti-VSACSA IgG responses are present in first pregnancies in women living in highly endemic areas (20, 31), in contrast to some earlier reports that primigravidae lack such Abs (12). The reported prevalence of such antibodies varies depending on the endemicity of the study area and may also depend on the techniques used for Ab detection (12, 16, 20, 22, 31). In this study, anti-VSACSA IgG (prevalence and mean MFI) increased with increasing duration of pregnancy, was positively associated with peripheral parasitemia at enrolment, and could be detected as early as an estimated gestational age of 10 weeks in some women. Even allowing for inevitable inaccuracies in determination of the gestational age, this is appreciably earlier than the 20 weeks reported for previous studies with primigravidae (31) but is in line with reports of increasing titers of anti-VSACSA IgG in multigravid women from 12 weeks of gestation (20). These results indicate that the placenta is capable of supporting parasite sequestration at a very early stage of pregnancy, a finding supported by a recent report of placental CSA expression and binding of pRBC from the second trimester onward and possibly as early as the latter part of the first trimester (1). This observation has important public health implications as it suggests that antimalarial drugs need to be given early in pregnancy (i.e., around the start of the second trimester) in order to have optimal effects.
The rapid seroconversion of primigravid women in this study may be explained by the very high intensity of malaria transmission in the study area and correspondingly high infection rates during pregnancy, as shown by the high proportion of placentas with evidence of malarial infection. These high rates of placental infection also reflect the fact that at the time of this study, chemoprophylaxis was not routinely being implemented.
Our data indicate that the three CSA-adherent parasite lines studied here express antigenically distinct VSACSA. This is at variance with the conclusion of an earlier study (12) which suggested that the CSA-binding ligand is antigenically conserved. Rather, our data suggest the existence of a number of multiple, antigenically distinct VSACSA recognized by similarly diverse repertoires of VSACSA-specific IgG. This conclusion, that VSACSA-binding ligands are antigenically diverse, is consistent with reports that the products of several different var genes (which encode VSA) have affinity for CSA (7, 10, 14, 21), However, the finding that one particular var gene (var2csa) has been found to be up-regulated in a placental isolate and in several isolates selected for CSA adhesion in vitro (26) implies that the repertoire of VSACSA may be relatively small. Immunologically significant, but limited, diversity in VSACSA would explain why susceptibility to PAM decreases over the first two or three pregnancies but is usually firmly established by the fourth or fifth pregnancy (5). Our data are consistent with a model in which parasites switch initially to a default gene (for example, the gene expressed by FCR3) and subsequently switch (e.g., as Abs develop to FCR3 VSACSA) to less commonly expressed genes (such as those expressed by Busua and EJ-24), as has been suggested to occur in children (19). Diversity in the antigens mediating placental sequestration poses a potential problem for the development of vaccines against pregnancy-associated malaria. However, some VSACSA serotypes (e.g., FCR3CSA) are clearly more commonly expressed than others (e.g., BusuaCSA), suggesting that a vaccine may need to include only a small number of commonly expressed serotypes in order to confer significant levels of protection against PAM. For example, recent data show that Abs to the product of the var2csa gene are associated with protection against low birth weight (25).
There are several potential explanations for the lack of a consistent association between plasma levels of anti-VSACSA IgG and placental malarial infection. First, measurements of plasma anti-VSACSA IgG were made at different times in the last trimester for different women and in some cases occurred several weeks prior to delivery and placental sampling. Second, so many of the women showed evidence of placental infection that there was only a small chance of observing significant differences between women with and without placental infection. Third, the antibodies present in plasma may have allowed one infection to be cleared but may not have been able to prevent reinfection by parasites expressing an antigenically variant VSACSA. In support of this, in Cameroon (31), it was reported that there was an association between levels of anti-VSACSA IgG and protection from placental infection, but only in multigravidae. Alternatively, although we have documented the development of anti-VSACSA antibodies very early in pregnancy, these Abs may not be capable of clearing an infection once it is established but may be able to prevent placental infection when they are part of a secondary immune response (i.e., during second and subsequent pregnancies).
In summary, we found that VSA expressed by placental parasites induced a primary, specific IgG response starting around the end of the first trimester of gestation. More importantly, our data suggest the existence of a repertoire of antigenically distinct VSACSA, each inducing a variant-specific IgG response. Further work is required to determine the extent of this diversity among placental parasite isolates.
Acknowledgments
This work was supported by MRC UK (G78/6363), the University of London Central Research Fund, the Nestle Foundation, the Commission of the European Communities (QLK2-CT-2001-01302, PAMVAC), the Danish Medical Research Council (SSVF 22-02-0571), a Denis Burkitt Study Award, a Jeremy Chadwick Traveling Fellowship, and the Parkes Foundation.
We thank the computer center staff at Kintampo Health Research Centre, especially Boniface Batosona and Seeba Amenga-Etego; Kofi Tchwum, who was the laboratory technician; and the field workers Kwasi Osei Owusu, Betty Baazing, Doris Asare, and Prosper Owusu. We also thank Maxwell Appawu (Parasitology Unit, NMIMR, University of Ghana) for assessment of malaria parasitemias and Kirsten Pihl and Anne Corfitz (Centre for Medical Parasitology, Copenhagen, Denmark) for excellent technical assistance with the flow cytometry analysis. We also express our immense appreciation for the cooperation of all of our study participants.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Agbor-Enoh, S. T., R. N. Achur, M. Valiyaveettil, R. Leke, D. W. Taylor, and D. C. Gowda. 2003. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infect. Immun. 71:2455-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, K. T., and M. Lanzer. 2002. Maternal malaria: Plasmodium falciparum sequestration in the placenta. Parasitol. Res. 88:715-723. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft, J., and A. Stevens (ed.). 1996. Theory and practice of histological techniques, 4th ed. Churchill Livingstone, Edinburgh, United Kingdom.
- 4.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 6.Browne, E. N., E. Frimpong, J. Sievertsen, J. Hagen, C. Hamelmann, K. Dietz, R. D. Horstmann, and G. D. Burchard. 2000. Malariometric update for the rainforest and savanna of Ashanti region, Ghana. Ann. Trop. Med. Parasitol. 94:15-22. [PubMed] [Google Scholar]
- 7.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, D. Parzy, L. H. Miller, J. Gysin, and A. Scherf. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulmer, J. N., F. N. Rasheed, N. Francis, L. Morrison, and B. M. Greenwood. 1993. Placental malaria. I. Pathological classification. Histopathology 22:211-218. [DOI] [PubMed] [Google Scholar]
- 9.Cranmer, S. L., C. Magowan, J. Liang, R. L. Coppel, and B. M. Cooke. 1997. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91:363-365. [DOI] [PubMed] [Google Scholar]
- 10.Degen, R., N. Weiss, and H. P. Beck. 2000. Plasmodium falciparum: cloned and expressed CIDR domains of PfEMP1 bind to chondroitin sulfate A. Exp. Parasitol. 95:113-121. [DOI] [PubMed] [Google Scholar]
- 11.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 12.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. (Letter.) [DOI] [PubMed] [Google Scholar]
- 13.Jensen, J. B., and W. Trager. 1978. Plasmodium falciparum in culture: establishment of additional strains. Am. J. Trop. Med. Hyg. 27:743-746. [DOI] [PubMed] [Google Scholar]
- 14.Khattab, A., J. Kun, P. Deloron, P. G. Kremsner, and M. Q. Klinkert. 2001. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J. Infect. Dis. 183:1165-1169. [DOI] [PubMed] [Google Scholar]
- 15.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 16.Maubert, B., N. Fievet, G. Tami, M. Cot, C. Boudin, and P. Deloron. 1999. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect. Immunol. 67:5367-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGregor, I. A., M. E. Wilson, and W. Z. Billewicz. 1983. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans. R. Soc. Trop. Med. Hyg. 77:232-244. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health of Ghana. 2001. Estimating National HIV prevalence in Ghana using sentinel surveillance data. National AIDS/STI Control Programme, Disease Control Unit, Ghana Ministry of Health, Accra, Ghana.
- 19.Nielsen, M. A., T. Staalsoe, J. A. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 168:3444-3450. [DOI] [PubMed] [Google Scholar]
- 20.O′Neil-Dunne, I., R. N. Achur, S. T. Agbor-Enoh, M. Valiyaveettil, R. S. Naik, C. F. Ockenhouse, A. Zhou, R. Megnekou, R. Leke, D. W. Taylor, and D. C. Gowda. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeder, J. C., A. F. Cowman, K. M. Davern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 23.Roberts, C. W., A. Satoskar, and J. Alexander. 1996. Sex steroids, pregnancy-associated hormones and immunity to parasitic infection. Parasitol. Today 12:382-388. [DOI] [PubMed] [Google Scholar]
- 24.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. Jensen, M. P. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 27.Smith, N. C. 1996. An immunological hypothesis to explain the enhanced susceptibility to malaria during pregnancy. Parasitol. Today 12:4-6. [DOI] [PubMed] [Google Scholar]
- 28.Snounou, G., X. Zhu, N. Siripoon, W. Jarra, S. Thaithong, K. N. Brown, and S. Viriyakosol. 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:369-374. [DOI] [PubMed] [Google Scholar]
- 29.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 30.Staalsoe, T., A. T. Jensen, T. G. Theander, and L. Hviid. 2002. Novel Plasmodium falciparum malaria vaccines: evidence-based searching for variant surface antigens as candidates for vaccination against pregnancy-associated malaria. Immunol. Lett. 84:133-136. [DOI] [PubMed] [Google Scholar]
- 31.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 32.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363:283-289. [DOI] [PubMed] [Google Scholar]
- 33.Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28-35. [DOI] [PubMed] [Google Scholar]