Abstract
Objectives
The objective of this umbrella review is to evaluate the efficacy and adverse effects of different teeth whitening techniques in-office (IO) and at-home (AH), regarding chromatic changes and teeth sensitivity.
Materials and methods
The search was carried out from several databases. The included studies were all systematic reviews with or without meta-analysis of RCT or quasi-RCT. The participants were patients that underwent external dental bleaching in permanent vital teeth. The interventions were in-office (IO) bleaching techniques and at-home (AT) bleaching techniques with different bleaching agents and concentrations.
Results
The search resulted in a total of 257 articles, and 28 SR were included in the qualitative analysis and nine in the quantitative analysis. There is no difference between in-office and at-home techniques in terms of color change (p = 0.95) and post-treatment sensitivity (p = 0.85). There is similarity risk and intensity of teeth sensitivity between AH and IO bleaching. IO bleaching with light-activated systems with low concentrations of bleaching agent showed similar results to IO bleaching techniques with high concentrated bleaching gels. With the application of the criteria of the AMSTAR 2 tool, the reviews were considered critically low to high.
Conclusions
There are no significant differences in terms of color change between the different bleaching techniques compared. Teeth sensitivity is always present regardless of the technique used. The use of light activation systems did not increase the intensity and risk of post-operative sensitivity.
Keywords: At-home dental bleaching, In-office dental bleaching, Efficacy, Umbrella review, Tooth bleaching, Dentin sensitivity
Clinical Relevance: Both bleaching techniques were efficacy in color change, and both presented the same risk for tooth sensitivity, even with light activation.
1. Introduction
In the last years, society has been placing a strong emphasis on the aesthetics and appearance of the individual, leading to an increased demand for treatments aimed at improving dental aesthetic. Dental aesthetic depends on the harmony between several factors, such as the tooth color, shape, dimensions, and symmetry. It is important to consider that individual preferences and personal expectations also play a significant role in patient satisfaction with their smile. Among other, patients may be unsatisfied with tooth coloration, dental misalignment, irregular tooth shape or size and the presence of diastema [1,2].
The tooth's color is determined by the optical properties of the enamel and dentin. However, it can also be influenced by the presence of extrinsic or intrinsic stains. Extrinsic stains result from the deposition of pigments on the tooth's outer surface due to poor oral hygiene, consumption of certain foods, and smoking. These pigments are known as chromophores and are the cause of acquired dental discoloration. They present as large organic compounds with double bonds or as metal complex compounds, the latter being less likely to be successfully whitened. Intrinsic stains are integrated into the tooth structure. They may emerge due to systemic conditions, such as genetic diseases affecting the hard tissues of the tooth such as, dentinogenesis imperfecta, amelogenesis imperfecta or local pre-eruptive or post-eruptive factors, for instance, age or the use of some drugs [1,[3], [4], [5], [6]].
Dental bleaching (DB) is the therapy that allows changing the color of the tooth structure through the interaction of different whitening products with the pigments present in the teeth [7]. Due to its low cost and ease of obtaining satisfactory results, it has been one of the most used treatments for dental discoloration [1].
Currently, there are various approaches to and variations of teeth whitening techniques, such as the use of different active agents at multiple concentrations, different prescription protocols for in-office (IO) or at-home (AH) use, the use of light activation systems and treatment done on the external surface, inside the tooth structure or a combined approach [4]. The main active agents used are hydrogen peroxide (HP) and carbamide peroxide (CP) [8]. Hydrogen peroxide (H2O2) is an unstable compound that decomposes into water and reactive oxygen radicals. It is highly soluble, producing an acidic solution with a pH that differs according to the concentration. Carbamide peroxide (CH6N2O3) is a stable structural complex that ultimately reacts with water and breaks down into its active components, HP and urea. Its structural stability leads to its slow degradation, allowing for a prolonged active whitening process compared to HP [5,7].
The mechanism that underlies tooth whitening using peroxide-based materials is a complex phenomenon. First, the whitening agent moves through the enamel and dentin to interact with the organic chromophores. Then, the interaction of the whitening agent with the stain molecule occurs. Oxygen radicals released by HP effectively react with the organic chromogens through an oxidizing process, which breaks the strong double bonds, destabilizing the chromogenic compound and ultimately reducing tooth discoloration. They convert the chains into simple structures or change their optical properties. These reactions will also yield products higher in polarity and lower in molecular weight than the original stain molecule [5,7]. The longer the exposure time to the whitening product and the higher its concentration, the more effective and faster the oxidation process and color modification will be [4].
As previously referred, dental bleaching can be performed in-office, at-home under dental supervision, or through over-the-counter products (OTC) that are available for free sale without a prescription [1,9]. Dentist-supervised techniques include both in-office and at-home techniques and allow the use of bleaching agents with higher concentrations to be used by the patient under professional supervision. This often means using less product, and fewer application cycles are required, decreasing the treatment duration [10]. In-office teeth whitening procedures are performed by a dentist in the dental office, using CP up to 16% or HP up to 6%. With this technique, a gel containing the whitening product is applied to the teeth after protecting the soft tissues, and the active agent may or may not be activated using a light system. Of notice, the evidence regarding the efficacy of light activation systems in accelerating the bleaching procedure is still limited [4,11,12]. At-home teeth whitening procedures are performed under the supervision of a dentist after evaluating the patient and creating a customized tray to hold the whitening product. Patients self-administer the treatment at home and have follow-up appointments. With this method, lower concentrations of the product (CP 10%–16%) during a variable period (from 2 to 8 h per day) are typically used. As advantages, it can be referred that at-home whitening has fewer reported side effects and is a more cost-effective solution [3,5,13]. Over-the-counter products are purchased and applied without professional supervision. These products are available in various forms, such as kits with whitening gel for application in a tray, toothpastes, mouthwashes, pens, brushes with whitening gel, whitening strips, and dental floss. They contain several concentrations of whitening agent (HP 0.1%–36%) and should be applied daily. However, these products present several adverse effects, such as dental sensitivity, gum irritation, or mucosal erosion [1,3,4,9]. Also, because non-customized trays are used, there is a very high risk of oral and gastric mucosal damage with these products [1,14].
Regardless of the technique, whitening therapies present numerous adverse effects on dental structure, which can be influenced by various factors, especially the technique, type and concentration of the active agent, and duration of treatment [5]. They can cause common adverse effects such as transitory hypersensitivity, gingival irritation, oral mucosal erosion, and taste alterations. Beyond these, research has found that dental bleaching can cause small defects on the surface and subsurface of enamel, making it more susceptible to damage, reducing its hardness, and increasing its roughness. Even more, hydrogen peroxide and its radicals can penetrate the dental pulp and cause genotoxicity, cytotoxicity, and inflammation in the pulp constituents [[14], [15], [16]].
Several original research on whitening therapies has been published, mainly evaluating the technique's effectiveness (chromatic alteration) and post-whitening sensitivity. However, the diverse methodologies and evaluations made it challenging to reach a definitive conclusion about the therapeutic effects. Also, several systematic reviews on this topic were also performed but the results are inconsistent, due to the different included studies in each one. This way, it is not possible to assert with certainty which is the best technique and the adverse effects of each one. Furthermore, since these are widely used therapies and accessible to the general public, it is fundamental to assess their effectiveness and the prevalence of side effects, namely post-treatment sensitivity, to provide the most appropriate care to patients. Therefore, it is essential to review the available evidence in the literature to draw reliable conclusions [6].
Considering this, this umbrella review aims to evaluate the efficacy and adverse effects of different teeth whitening techniques regarding chromatic changes and teeth sensitivity.
2. Materials and methods
2.1. Protocol registration
The review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with the number CRD42023418089.
2.2. Review question
This umbrella review was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17] The following research question was considered, following the PICO (Population, Intervention, Comparison, Outcome) framework: “In patients that underwent external dental bleaching in permanent vital teeth, are at-home as effective as in-office technique regarding the efficacy and post-operative sensitivity?” (Table 1).
Table 1.
PICO question.
| PICO | |
|---|---|
| Population | Patients that underwent external dental bleaching in permanent vital teeth |
| Intervention | Clinically supervised at-home bleaching technique |
| Comparison | In-office bleaching technique |
| Outcome | Efficacy and Post-Operative Sensitivity |
2.3. Eligibility criteria
The included studies were all systematic reviews (SR) of randomized controlled trials (RCT) and quasi-RCT, with or without meta-analysis. Other categories of literature reviews and other study types were excluded. Only studies conducted with adult human participants were included. The included participants performed external bleaching using in-office or at-home professional dental bleaching. Studies where the participants used OTC products were excluded. The outcomes of interest for this review were the efficacy in color change and the reported or assessed post-operative sensitivity following treatment.
2.4. Search strategy
A literature search was performed up to November 28, 2022. Controlled vocabulary (MeSH terms) and free keywords, defined following the concepts of the PICO question, were used for the search strategy. Searches were performed in PubMed (via MEDLINE), Cochrane Library, Excerpta Medica Database (Embase), Web of Science (all databases), and Epistemonikos. Articles published in english, portuguese, french and spanish were considered. No publication date restriction was established. When available the filter of systematic review was applied. The search strategies for each database are presented in appendix in Table S1.
2.5. Study selection and data collection
In the first phase, articles were selected based on title and abstract according to the eligibility criteria by two independent reviewers (MA and CMM). Afterward, the full-text articles were evaluated for potential inclusion. In cases of disagreement, a third evaluator (AP) was included in the eligibility process. A consensus was achieved for every disagreement.
From the included studies, the following data was extracted and summarized, whenever possible: authors and publication data, design of the included studies, registration number, number of studies included and their design, sample size, bleaching technique and agent, follow-up periods, outcomes, adverse effects, and other relevant observations.
2.6. Meta-analysis
Meta-analysis was performed in IBM SPSS, version 28, and analyzed at a 5% significance level, using risk ratios for qualitative data (computed through the number of events in each group) and standardized mean difference from quantitative data. Synthetic measures were obtained using the der Simonian-Laird estimation method a applying the random effects model due to the number of studies involved, even for outcomes from different studies which have presented a small amount of heterogeneity (evaluated through the Higgins and Thompson I2).
2.7. Quality assessment
The selected studies' qualitative assessment was performed using the Assessment of Multiple Systematic Reviews (AMSTAR 2) checklists. AMSTAR 2 is a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions. AMSTAR2 checklists contain sixteen questions that evaluate the methodology in the different studies and the Risk of Bias (ROB). This tool assists researchers in the identification of high, moderate, low, and critically low confidence of systematic reviews [18].
Two reviewers (MA and CMM) performed the quality assessment of the studies in duplicate and independently, categorizing them as: high quality if none or only one of the parameters is weak; moderate quality if more than one parameter is weak; and poor quality if there are several weak parameters or a major failure [18] Disagreements were resolved as described above.
2.8. Analysis of the degree of overlap
The analysis of the degree of overlap of selected studies between systematic reviews was performed through % Overlap, covered area (CA) and corrected covered area (CCA). The CCA was categorized as slight overlap (0–5); moderate overlap (6–10), high overlap (11–15) and very high overlap (>15) correlation with the number of included reviews and the primary publication [19].
3. Results
3.1. Study selection
A literature search resulted in a total of 257 articles (Appendix in Table S1). The included studies are characterized by being SR of RCT or quasi-RCT, with or without meta-analysis, that aim to compare the different bleaching techniques in-office or at-home in vital teeth.
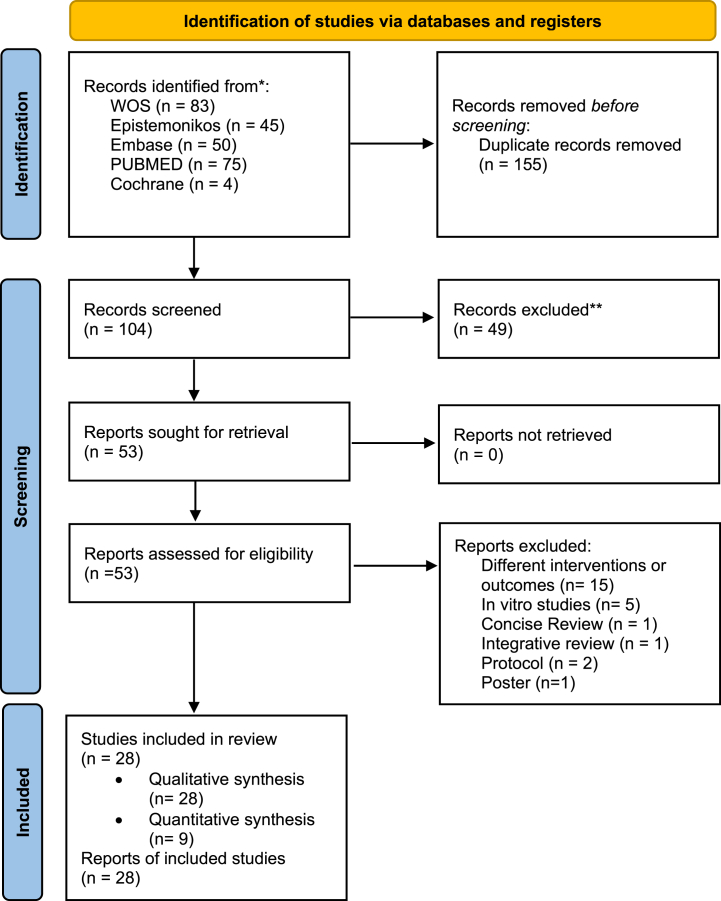
The initial database searches revealed 257 reviews. After the database screening and removal of duplicates, 104 reviews were identified. After the titles and abstracts evaluation, 53 reviews were selected for full-text analysis. After the inclusion and exclusion criteria were applied, a total of 28 studies were selected for inclusion. Twenty-eight SRs were included in the qualitative analysis and nine in the quantitative analysis (meta-analysis) (Fig. 1).
Fig. 1.
PRISMA Flow chart for included studies. (* appendix in Table S1; ** appendix in Table S2).
The reasons for exclusion of the excluded reviews are presented in appendix in Table S2.
3.2. Characteristics of the included reviews
The characteristics and results of the included reviews are presented in Table 2 and Table 3. This umbrella review included twenty-eight SR, which report 416 RCTs. Sixteen SRs were registered in PROSPERO [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] and 12 did not report any registration [6,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] For the quality assessment, twenty-one SRs used the Cochrane ROB tool [11,21,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35],39,42,43,[45], [46], [47]] one used Minors [44], one used Jadad scale [38], other used Risk of bias instrument [40], and 4 studies did not report any assessment [6,36,37,41].
Table 2.
The characteristics of the included systematic reviews.
| Author/Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| De Geus et al., 2016 [47] | SR/ MA | PROSPERO CRD42015015564 |
12 RCT | Cochrane | High/unclear risk (4) Low risk (8) |
557 10 to 30 patients/group |
27.5 | Males predominate (2) Females predominate (3) NR (7) |
AH: 10%, 16%, 20%, 32% CP, 2–10 h/day, 6–28 days IO: 25%, 35%, 38% HP, 20–45 min/session or 15–60 min/session, 1–3 session/ variable number |
1 day to 3 months |
| Dioguardi et al., 2022 [6] | SR | NR | 10 RCT | NR | NR | 468 19 to 96 patients/ group |
(18–70) | Female/male |
AH: 9.5%-16%CP; 1 h–8h/day; 14 days, 3 weeks IO: 25–38% HP; 3 × 10 min, 3 × 15 min, 4 × 15 min; 1 or 2 appointments (7 or 15 days interval), with/without light |
12–16 weeks, 3 and 6 months |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Wasserman et al., 2014 [36] | SR | NR | 8 RCT | NR | NR | 380 20-92 patients/group |
(18–73) | NR |
IO: 35% or 38% HP, 3 × 15 min, 15 min interval AH: 10% or 16% CP 2 h/night, 3 weeks 15% PC, 120 min, 10 days 6% HP; 2x/day, 30 min × 2s Meta tray: 28% CP, 20 min/10 days Opalescent: 10% CP, 6–8h/night x10 days 10% CP- 7.2 h or 7.3 h/day x 14 days |
≥1 year |
| Naidu et al., 2020 [37] | SR | NR | 1 RCT | NR | GRADE | 16 patients | (>18) | NR |
AH: 6% HP varnish, 1x/day; 10 min, 10 days IO: 6% HP varnish,1x/week/2weeks |
0 days, 14 days, 6 months |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Kielbassa et al., 2015 [38] | SR | NR | 18 RCT | Jadad scale | High bias (7) Moderate bias (7) Low bias (4) |
NR | NR | NR | AH and IO: HP-6%,15%,20%,25%,35%, 38%; CP- 8%,10%,15%,20% | ≤1 month > 1 month |
| Kothari et al., 2019 [39] | SR/ MA | NR | 2 RCT | Cochrane | Low risk (2) | 123 31- 92 patients/group |
18–30 | Female | 3 sessions: 6% HP or Nitrogen-doped titanium dioxide light activated bleaching agent, 35% HP 10% or 16% CP |
1 week 1 month |
| Alghulikah et al., 2021 [40] | SR | NR | 10 RCT | ROB instrument | Score: 34.6; 33.6; 28.8; 34.4; 31.4; 30.4; 33.2; 33; 33.6; 34 | 341 patients | (>18) | NR |
IO: HP 35% (20 min/ 40 min applications) CP: 10%, 20%, 35%, 37% |
1 week |
| Maran et al., 2020 [24] | SR/ MA | PROSPERO CRD42018108266 |
24 RCT | Cochrane | Low risk (5) Unclear risk (13) High risk (7) |
1126 patients | 24 (11–75) | NR |
IO: Low concentrate: 6–15% HP or 37% CP, 24–60 min; High: 35%–40%, 20%–30% HP. High: 35%–40% HP, 15–60 min, 1 to 3 clinical appointments with a interval of 7 days |
7–30 days 1 year |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Rosa et al., 2020 [25] | SR/ MA | PROSPERO CRD42017070562 |
20 RCT | Cochrane | GRADE: Moderate (color change in ΔE*, risk of TS, intensity of TS, GI) Very Low (color change in ΔSGU, risk of GI, patient satisfaction) |
1028 patients | 31.5 | Female/Male |
AH: 10%–35% CP or 7.5%–10%, 7–28 days, 30min-10 h IO: 15%–38% HP, 15–45min, 1–3 sessions |
4 weeks |
| Moran et al., 2021 [26] | SR/ MA | PROSPERO CRD42018095806 |
32 RCT | Cochrane | GRADE: Low risk (6) Unclear risk (23) High risk (3) |
NR | 18-78 (27.9 years) | Female/male |
IO: 6–38% HP; 35% CP; 3 × 15 min applications/session; 1–5 clinical session Light activation: halogen lamps, LED/laser, LED, metal-halide, violet light, laser, PACs |
1–30 days |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Casado et al., 2020 [27] | SR/ MA | PROSPERO CDR42018096591 |
5 RCT | Cochrane | Unclear Risk (5) | 136 16 to 40 patients/group |
(18–70) | Female/male | IO: 35%, 37%, 38% HPLight source: Halogen; LED, LED/Laser, PAC Light source time/session: 2 sessions/20 min; 3 sessions/15 min; 3 sessions/7 min; 3 sessions/20 min; 3 sessions/3 min; 3 sessions/30 min; 3 sessions/8 min | Immediately after dental bleaching |
| Kury et al., 2021 [28] | SR/ MA | PROSPERO CDR42020215279 |
5 RCT | Cochrane | High risk (2) Unclear (3) |
160 (20–44) patients/group | 26.8 to 53.7 | Male (55.5%) |
IO: High concentrated HP: 35–38% Low concentrated and light activated HP (6%); 1 or 2 sessions, 3 times (3x12 or 15 min) or 2 times (2 × 20min) |
1 week, 1 month or 84 days |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Niederman et al., 2000 [41] | SR/ MA | NR | 7 RCT | NR | NR | 170 patients | (>18) | NR |
AH: = White & Brite, Omni International; Rembrandt Lighten, Den-Mat; Proxigel, Reed and McCormick; Colgate Platinum, Colgate Palmolive; Opalesence, Ultradent Products, Inc. Overnight or <4hr/day >14 days or <7 |
1,2,3,6,12, 24 weeks |
| He et al., 2012 [42] | SR/ MA | NR | 11: RCT/quasi-RCT | Cochrane | Low risk (5) Moderate risk (4) High risk (2) |
462 patients | (>18) | NR | IO: 35% HP, 15 min x 3/ 2 sessions; 35% HP, 10 min x 2; 35% HP, 20 min x 3; 35% HP, 3.5 min x 2/ 2 sessions; 25% HP, 15 min x 3; 15% HP, 20 min x 3Non light, LED/Laser, Halogen light, LED, Halide Lamp, Nd:YAG, Short-arc plasma ligh | Immediate: 1 day Short-term effect: 1 week to 4 weeks Median-term effect: 12 weeks–24 weeks |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Maran et al., 2017 [22] | SR/ MA | PROSPERO CRD42016037630 |
21 RCT | Cochrane | GRADE: Moderate (Color change ΔE*, Risk of TS) Low (Color change ΔSGU, TS intensity) |
924 patients | 29.1 (18–70) | Females prevalent |
IO: 15%–38% HP, 3: 15 min application in each session, 1 session, 2 sessions or 3 sessions, with intervals between 7 and 10 days Light activation: halogen light, LEDs/lasers, LEDs, metal-halide light, laser, plasma arc lamp, Nd:YAG laser |
1 week to 1 month |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Hasson et al., 2006 [29] | SR | NR | 25 RCT | Cochrane | Moderate risk (4) High Risk of bias (21) |
528 patients | (18–80) | Male and Female |
AH: 10% CP gel (NUPRO Gold) in a tray 10% CP gel (Opalescence) applied in a tray 2 h/day 15% CP + F gel (Opalescence) applied in a tray 2 h/day 20% CP + F (Opalescence) gel in a tray 2 h/day 10% CP gel (Rembrandt Superior Plus) in tray, 20–30 min 10% CP gel (Nite White Excel) applied in a tray 1 × 2 h/day 10% CP gel (Nite White Excel 2) in a tray 2 h/day 10% CP gel (NUPRO Gold) applied in a tray, 4 h 15% CP (NUPRO Gold), in a tray 4 h 10% CP gel (Colgate Platinum Pro) applied in tray, 1 h ×2 daily 10% CP gel (Rembrandt Lighten) in tray, 1 h × 2 day 10% CP gel (Colgate Platinum Pro) in a tray, 1 hx2 daily for 14 days 10% CP (Rembrandt Gel Plus) in a tray, 1hx2 day for 14 days 7.5% HP gel (Day White 2) in a tray, 2 × 30 min/day 16% CP equivalent gel (Nite White Excel Mint) applied in a tray 10% CP gel custom mouthguard for up to 14 day 15% CP, gel, custom mouthguard o/n for up to 14 days 20% CP and 7.5% HP in a custom tray for 2 × 1h/day Colgate Platinum Pro 10% CP gel Rembrandt Lighten, 10% CP gel 5.5% HP in gel (Day White), 2 × 30 min/day 15% CP gel (Opalescence F), 2 × 30min/day |
NR |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Serraglio et al., 2015 [43] | SR/MA | NR | 9 RCT | Cochrane | GRADE: Low | 196 patients | (>18) | Female/ Male | AH: 10% CP in a tray system, 1 or 2x/ day; 40/120/480min, 14 days | NR |
| SoutoMaior et al., 2017 [30] | SR/ MA | PROSPERO CRD 42017060574 |
23 RCT | Cochrane | Low risk- participant blinding, personnel, outcome assessment, incomplete outcome, selective reporting items Unclear Risk- random sequence generation and allocation concealment items |
925 20 to 88 patients/group |
(>18) | Female (prevalent in 9 articles) Male |
15, 20, 25, 35, 38% HPWith/without light: halogen, short arc plasm lamp, metal halide lamp, LED, LED/Laser, sodium arc bulb lamp |
Immediate: 1 day Short-term: 1–4 weeks Medium-term: >4 weeks |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Pontes et al., 2020 [31] | SR/ MA | PROSPERO CRD42017064493 |
14 RCT | Cochrane | Low risk | 649 patients | 36.32 (13.9–31) | NR |
IO: Higher concentration of HP: 35%; Lower concentration of HP: 6%–20%; 3 × 15min, 1 session; 2 × 12 min, 3 × 16 min, 2x12 in 2 sessions, 1 × 40 min, 1 × 50 min, 1 × 120 min. Use of light (UV, LED) AH: 10% HP |
Sensitivity: 1 day to 1 week Color change: 24 h, 48 h, 72 h, 1w, 1 m, 6 m |
| Maran et al., 2019 [23] | SR/ MA | PROSPERO CRD42017078743 |
28 RCT | Cochrane | High RoB (5) Unclear (23 |
1048 patients | 30 (18–78) | Female prevalent |
IO: 6–38% HP; 3 × 15 min, 1–4 applications/session, 1–3 sessions with intervals between 7 and 14 days. Light activation: halogen lamps; laser source; LED, LED/ laser; metal-halide light; PAC |
NR |
| Luque-Martinez et al., 2016 [32] | SR/ MA | PROSPERO CRD42015008993 |
13 RCT | Cochrane | High risk (5) Low risk (8) |
744 16 to 114 patients/ group |
35.7 | Female and male |
AH: 5–35% CP; 2.5%–14% HP, 2–28 days, 1 h/day, 3 h/day, 1 or 2/day Bleaching trays with or without reservoirs |
No minimum follow-up |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Geus et al., 2018 [21] | SR/ MA | PROSPERO CRD42016029360 |
17 RCT | Cochrane | Unclear Risk (10) Low Risk (3) |
655 10 to 30 patients/group |
32.4 | Male prevalent | AH: bleaching trays with/without reservoirs, 12%,15%,16%,17%,20%,28% CP, 20 min to overnight, 7 days to 6 months | 2–3 weeks |
| Cardenas et al., 2019 [33] | SR/ MA | PROSPERO CRD42016036555 |
12 RCT | Cochrane | GRADE: Low quality (4) Very Low (3) |
243 patients | 29.7 (18–78) | Female prevalent |
IO: high-concentrate HP: 35% or 38%; 15–60 min, 1–4 clinical appointments, 6–15 days between sessions AH: 10% or 16%) CP or 4%HP, 7–21 days, 1–8 h/day |
Immediately after bleaching 1–2 weeks post- bleaching |
| Costacurta et al., 2019 [34] | SR/ MA | PROSPERO CRD42018090990 |
6 RCT | Cochrane | Low risk (1) Unclear Risk (5) GRADE: Moderate (tooth sensitivity and intensity); Low (Color change) |
281 patients | 26.2 (18–69) | Female and male | AH: 10% CP, 30% CP; impression trays with or without reservoirs, 1 h–7h/day, 10–21 days | 1 day to 13 weeks |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Rossi et al., 2022 [35] | SR | PROSPERO CRD 42021248093 |
3 RCT | Cochrane | No risk (2) Unclear risk (1) High risk (2) |
184 patients | 18 to 35 | NR | 10%, 35%, 37% CP; 17.5%, 35% HP AH: violet LED irradiation and IO 20 cycles of 1 min violet LED irradiation followed by a 30 s pause in a total 30 min/session or 20 min without interruption IO: gel in association with light - bleaching study with bleaching gel in association to light: 2 clinical session with a 7 day interval or 3 sessions with a 7 day interval or 9 sessions. |
7 days–21 days |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Fagogeni et al., 2021 [44] | SR | NR | 9 RCT | MINORS | Fair (7) Good (2) |
Central incisive (1) |
(14) | NR | 1 × 2 w (4 h/ day) External bleaching | NR |
| Alshammery, 2019 [45] | SR | NR | 12 RCT | Cochrane | NR | 398 patients 21- central or lateral incisive |
(>18) | NR |
IO: 35% HP with a 1-week break between sessions for 6 months 25% HP 44% CP gel with LED light activation followed by 35% CP, 14 days AH: 37% HP/135 min/without light activation and 72 min with activation 37.5% HP HP and CP for 3–8 weeks |
NR |
| Author/ Year | Study Design | Registration | No. of Trials and Design | ROB tool | Quality of evidence and number of studies | Sample | Mean age (range years) | Gender | Bleaching protocols and active principle | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Eachempati et al., 2018 [46] | SR | NR | 71 RCT (25) | Cochrane | Certainty of evidence: low to very low for all comparisons Unclear Risk of bias |
1398 | 18 to 70 | NR |
AH: 10% CP gel in tray; 5% CP gel in tray; 6–8 h/day for 1 week, 2 h/day for 2 weeks 6% and 3% HP gel 2x/day for 14 days. Tray: 10%,16%, 20%, 28% CP; 7.5%, 9%,6% HP 20%CP, 7.5%HP 4 h/day or 2x/day/1 h- 12 weeks 10% CP and 7.5% HP, 2 weeks 20% CP and 9% HP, 30 min/day/ 2 weeks 10%CP and 6% HP, 8 h–10 h/day, 2 weeks 3 h daily for 24 days for 3.5% HP 2 h daily for 28 days in 10% CP 3.5% HP, 3 h/day/4 weeks |
2 weeks to 6 months |
AH- at-home, CP- Carbamide peroxide; GRADE- Grading of Recommendations Assessment, Development and Evaluation; HP- Hydrogen peroxide; IO- in-office; MA - Meta-analysis; NR- Not reported; PROSPERO- International prospective register of systematic reviews.
Table 3.
The characteristics of the included systematic reviews.
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| De Geus et al., 2016 [47] | Shade guide units Spectrophotometer or colorimeter |
Color Change in ΔSGU: SDM: 0.184, CI: 0.66 to 0.29 (p = 0.451); Heterogeneity- Q- value: 10.7 (p = 0.01); Tau = 0.40; I2: 72%. No difference in the color change. High heterogeneity Color Change in ΔE*: SDM: 0.260; CI: 0.77 to 0.22 (p = 0.292); Heterogeneity- Q- value: 8.05 (p = 0.05); Tau = 0.38; I2: 62.8%. No difference in the color change measured with a spectrophotometer |
NR |
Risk of tooth sensitivity: Heterogeneity- Q- value: 32.7 (p < 0.001); Tau = 1.31; I2: 87.8%, OR: 2.186; 95% CI of 0.63–7.53 (p = 0.215). Lower chance of tooth sensitivity for the at-home bleaching protocol Intensity of tooth sensitivity: SDM: 0.823, CI: 0.42 to 2.09; Heterogeneity- Q- value: 91.8 (p < 0.001); Tau = 1.38; I2: 95.6%. No difference in the intensity of tooth sensitivity between the two bleaching protocols |
In an overall comparison of at-home and in-office, no differences were detected. This comparison, does not take into consideration variations in the protocol. |
| Dioguardi et al., 2022 [6] | Spectrophotometer | Higher concentration HP in IO gives evident results in a shorter exposure time. Short exposure time to a high concentration can be compensated for a lower concentration of the bleaching agent and longer exposure time Different bleaching techniques tested showed similar clinical efficiency and color stability over time. Light source accelerates the whitening process. LED is the most favorable light source. |
NR | High concentrated whitening agent caused dental sensitivity Low concentrations used in home treatment caused dental hypersensitivity in the patients Dental hypersensitivity present in the first days of application and tends to disappear over time. The various whitening techniques tested showed a similar degree of dental hypersensitivity. Laser whitening resulted in the sensitivity of the teeth. |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Wasserman et al., 2014 [36] | Spectrophotometer, Polarized light, Digital images Vita Shade guide Vita guide (patient) |
10% CP vs. 16% CP: No difference in color change. 28% CP vs. 10% CP: 1 year after treatment, color change: 3 units. 10% CP: higher effectiveness 1 year after treatment 10% CP vs Placebo: color change 7–14 days after treatment AH, 10%CP: no differences between before treatment and 3 months after treatment (color change) |
NR | NR | NR |
| Naidu et al., 2020 [37] | VCS |
0 day: AH, IO: A2 or darker 14 Days: AH: VCS: 2.8 shades (p < 0.05); IO: VCS: 2.5 shades (p < 0.05) |
NR | NR | NR |
| Kielbassa et al., 2015 [38] | NR | NR | NR |
During and after treatment: Patients using 7% HP experienced more BS to the 10% CP 7 days post-treatment: no significant difference Occurrence/incidence: 7 days (1–13 days) |
Etiology of BS: Reversible pulpitis. 10% CP used for 14 days has been shown to cause mild reversible histologic changes of the pulp tissues. |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Kothari et al., 2019 [39] | NR |
ΔE, over 1 month: 35% HP: 7.98 + 2.45 6%HP: 5.57 + 3.71. Similar changes after 1 week 6% HP: difference greater than 5 units of ΔE Similar effect 10 and 16 % CP |
NR | 35% HP: 6.80 + 17.16 6% HP: 3.53 + 9.10 Third session with smaller effects |
Measure OHRQoL:Overall OHRQoL (I-squared 82.1%): IO -Es (95%CL) 0.31 (0.05; 0.57) % Weight (20.33) AH- Es (95%CL) −0.05 (−0.12; 0.03) % weight 30.68 OHIP OHRQoL: IO-Es (95%CL) 0–31 (0.05; 0.57) % Weight (26.21) AH- Es (95%CL) −0.05 (−0.12; 0.03) % weight 39.80 |
| Alghulikah et al., 2021 [40] | NR | Better clinical outcome for color change with 37% HP No difference in color change with low, medium or high concentrate products for IO For moderate and severe dental discoloration IO whitening with high concentration HP |
NR | Less sensitivity with 37% CP Low and medium HP concentrate products for IO reduce risk and intensity of BS than high concentration HP 20% CP higher prevalence of sensitivity Single 40 min in-office bleaching agent had similar results and similar teeth sensitivity levels as 2 × 20 min |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Maran et al., 2020 [24] | Vita Classical Shade Guide Trubyte Bioform Color Ordered Shade Guide Vita bleached guide 3D master shade guide Spectrophotometer or chromameter |
Immediate color(ΔE*ab) Low/medium vs. high HP: MD = −0.52; 95% CI -1.37 to 0.33; p = 0.23 No statistical difference among groups Low vs. high HP: MD = −1.16; 95% CI -2.10 to −0.21; p = 0.02. For every 10 % increase in the concentrate HP, the ΔE*ab increases approximately 1.26 unitsImmediate color(ΔSGU/SGU with Vita Classical): MD = −0.42; 95% CI -0.96 to 0.12; p = 0.13, low, high heterogeneity, p < 0.01; I2 = 77 % Immediate color(ΔSGU/SGU with Vita Bleachedguide): MD = −0.36; 95 % CI -0.99 to 0.26; p = 0.26, very low certainty of the evidence; heterogeneity: p = 0.02; I2 = 64% Long-term color change (ΔE*ab): Subgroup low vs. high: MD = −2.39; 95% CI –3.65 to–1.14, p = 0.07. No difference among groupsLong-term color change (ΔSGU/SGU with Vita Classical): MD = −0.94; 95% CI -2.02 to 0.14; p = 0.09, low certainty of the evidence; heterogeneity: p = 0.98; I2 = 0% |
VAS NRS |
Risk of BS: the risk of BS was 33 % lower for the less concentrate HP products: RR = 0.67; 95% CI 0.51 to 0.86; p < 0.01, heterogeneity: p = 0.06; I2 = 42 % Intensity of BS: MD = −0.47; 95% CI -0.66 to −0.28; p < 0.01, moderate certainty of the evidence, Heterogeneity of the overall data: p < 0.01; I2 = 51 % |
PatientSatisfaction: different questionnaires, no differences between groups. |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Rosa et al., 2020 [25] | Vita Classical Shade Guide Photography Vita bleached guide 3D master shade guide Spectrophotometer Colorimeter or chromameter |
Color Change in ΔE*: SMD: 0.50 (95% CI -0.79 to −0.21, p = 0.61; I2 = 0% Color Change in ΔSGU: SMD: 0.39 (95% CI -1.16 to 0.37), p = 0.0002; I2 = 79% |
VAS NRS |
Risk of tooth sensitivity: RR: 0.78 (95% CI 0.65; 0.94), p = 0.98; I2 = 0% Intensity: SMD: 0,30 (95% CI -0.56; −0.04), p = 0.62; I2 = 0% |
Patient Satisfaction Gingival irritation |
| Moran et al., 2021 [26] | NR | NR | VAS NRS |
Risk of sensitivity: no significant differences (high concentration- 5 treatments); no significant differences (low concentration- 4 treatments) Intensity of tooth sensitivity: no significant differences (high concentration-7 treatments); no significant differences (low concentration- 3 treatments) |
NR |
| Casado et al., 2020 [27] | Vita Classical shade guide | MD: 2.22; CI: 6.36 to 1.93; p = 0.29, x2: 30,60; I2 = 97%; p < 0.00001 No significant differences between the use of laser and other light sources combined IO |
VAS |
Intensity: MD: 1.60, CI: 3.42 to −0.22; p = 0.09), (x2: 29.39; I2 = 90%; p < 0.0001. No significant difference. Laser did not lead to significantly lower dental sensitivity compared with the other light system Incidence: MD: 1.00, CI: 0.755 to 1.33, p = 1.00. No differences between group |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Kury et al., 2021 [28] | NR | Objective Δeab: SMD: 0.08 (95% CI-0.15; 0.89), p < 0.001, I2 = 91%, no differences in color outcomes promoted by single application protocol in comparison to the renewal. Subjective ΔSGU: SMD: 0.11 (95% CI-0.51; 0.29), heterogeneity moderate p = 0.03, I2 = 29%. No differences between after bleaching and 1 month after bleaching |
VAS Numeric rating scale from 0 to 4 |
Absolute Risk: RR: 0.07 (95% CI -0.16; 0.31), p = 0.03, I2 = 71% No differences observed in terms of prevalence |
NR |
| Niederman et al., 2000 [41] | Shade Guide | Whitening results in a significant mean change of 6, 4 shade guide units (p < 0.01), while the placebo control group exhibited little change (0.7. 0.6, p > 0.05) Active Group: Mean: 5.9 SD: 4.094, Min: 0.4, Max 17.5; p < 0.01 Control Group: Mean: 0.7 SD: 0.6 Min: 0, Max 1.5; p < 0.01 <4 h/day: Mean: 4.6,SD: 2.1, Min: 0.4, Max 7.1; p > 0.2 Overnight: Mean: 7.8, SD: 5.5, Min: 4.5, Max 17.5; p > 0.2 1 week: Mean: 4.9 SD: 1,6, Min: 3.7, Max 6; p > 0.2 2 week: Mean: 8.7,SD: 5.9, Min: 5.1, Max 17.5; p > 0.2 3 week: Mean: 4.4, SD: 2.2, Min: 0.4, Max 6.9; p > 0.2 |
NR | NR | The brand of bleaching agent had a significant effect on tooth whitening, but the daily application time and duration of treatment did not. Gingival indices nor plaque indices were adversely or favorably affected by bleaching |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| He et al., 2012 [42] | Visual measurements Instrumental measurements |
Immediate- High concentration: MD:0.39, 1.15 to 0.37, p = 0.32, I2 = 36%; Low concentration: MD:1.78, 2.30 to1.26, p < 0.00001, I2 = 44% Short-term effect- High concentration: MD:0.25, 0.47 to 0.96, p = 0.50, I2 = 18% Low concentration: MD:0.87 [0.23, 1.98], p = 0.12, I2 = 0% |
NR |
Incidence of tooth sensitivity: MD:3.53 [1.37, 9.10], p = 0.009, I2 = 12% Intensity of tooth sensitivity: MD:0.57 [0.21, 0.92], p = 0.002, I2 = 16% |
NR |
| Maran et al., 2017 [22] | NR |
Color Change in ΔE: IO bleaching with light (Bleach + light) vs. without light (Bleach): SMD 0.08, 95% CI -0.27 to 0.11, p = 0.39, p = 0.28; I2 = 17%, p < 0.00001, I2 = 87% Color Change in ΔSGU for in-office bleaching with light (Bleach + light) vs. without light (Bleach): SMD -0.35, 95% CI -0.84 to 0.13 |
NR |
Risk of Tooth Sensitivity: no significant difference was observed. SMD: 1.07 [95% CI 0.88 to 1.30], (p = 0.18; I2 = 30%) Intensity of Tooth Sensitivity: SMD: 0.12, 95% CI -0.53 to 0.76, p < 0.00001; I2 = 90% |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Hasson et al., 2006 [29] | Digital image Vita Shade Guide Chroma Meter Colour change value |
10% CP had statistically significant improvement when compared to 0% (p < 0.01) On study found a statistically significant shade change for the 10% CP gel applied in trays when compared to the placebo: MD: 6.29; 95% CI: 4.76 to 7.82 Colgate Platinum, MD: 3.61, 95% CI 2.45 to 4.77 Rembrandt, MD: 2.01 (95% CI 1.25 to 2.77) Vita Shade Guide: 15% CP gave statistically significantly greater shade lightning than 10% CP overnight: MD: 1.73; 95% CI 0.26 to 3.2 No significant difference between 10% and 15% CP (2 h/day).But statistically significant differences evident in compounded colour index between 10% and 20% and between 15% and 20% (2 h/day). MD: 1.42; 95% CI 0.45 to 2.39 and MD: 1.48; 95% CI 0.58 to 2.38 Colgate Platinum Pro was reported to give statistically significantly greater change in compounded colour index than Rembrandt Gel Plus. MD: 1.86 (95% CI 1.01 to 2.70, Chi2 = 0.31, df = 2, p = 0.86, I2 = 0%) |
NR | Two studies reported that there is no difference between HP and CP in causing tooth sensitivity 15% CP was reported to lead to slightly greater sensitivity than 10% CP 10% CP: 2.78 ± 2.44 15% CP: 4.19 ± 4.58 |
One study reported that CP caused more gingival sensitivity than HP Gingival irritation in 41%–62% of HP users (2 × 30 min) and 25%–41% sensitivity compared to 0%–12% irritation and 8%–24% sensitivity when CP was use overnight |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Serraglio et al., 2015 [43] | Digital image Shade guide: 16 shade tab |
Shade guide (ΔE): 10%CP- 982 min to achieve the defined the color change MD: 0.12; 95%; CI [−1.51; 1.75]; Z = 0.14; p = 0.89 Color change favoring by the increase of the contact time of the 10% CP with the dental structure |
Dichotomous variable |
Tooth sensitivity: 3.38 ± 1.66 14 cycles of 10 % CP gel application did not reveal any significant difference between groups (RR 1.13; 95 % CI [0.77–1.66]; Z = 0.63; p = 0.53 |
Gingival irritation: higher when the 10 % CP gel was applied on tray Patient acceptance was 1.46 ± 1.33 |
| SoutoMaior et al., 2017 [30] | Vita Classical shade guide Spectrophotometer |
Immediate: No significant differences between light and non-light bleaching. MD: 0.38; CI: 1.24 to 0.47; p = 0.38; x2 = :178.87; I2 = 94% Short-term: MD: 0.16; CI: 0.5 to 0.83; p = 0.63; x2 = 31.02; I2 = 65%, p = 0.001 Medium-term: MD: 0.23; CI: 1.07 to 0.62); p = 0.60; x2: 24.76; I2 = 84%; p < 0.00001 |
VAS |
Intensity of Tooth Sensitivity: significant difference in favor of light bleaching systems. MD: 2.19; CI: 3.85 to −0.53, p = 0.01; x2 = 325.81; I2 = 98%; p < 0.0001 Incidence of Tooth Sensitivity: MD:1.07; CI: 0.95 to 1.21; p = 0.28; x2 = 7.89; I2 = 11%; p = 0.33 |
NR |
| Pontes et al., 2020 [31] | Shade guide units Spectrophotometer |
Color Change (ΔE): significant difference between a high concentration of HP and low concentration. MD: 1.53; 95% CI: 2.99;-0.08; p < 0.0001; I2: 82%) Color Change (ΔSGU): no significant difference was found between a low concentration and a high concentration of HP. MD: 0.24; CI: 0.75; 1.23, p < 0.00001; I2: 89%) |
VAS Five-step scale five point verbal scale Sensitivity questionnaire |
OR: 0.38; 95% CI: 0.16; 0.92, p = 0.04; I2:56% Less tooth sensitivity when a lower concentration of HP was used |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Maran et al., 2019 [23] | Shade Guide Spectrophotometer or colorimeter Photography |
Color change in ΔE: Light free vs LED: MD -0.25 (−1.7; 0.83), p = 0.6769115 Light-free vs laser: MD -0.25 (−1.6; 0.59), p = 0.92722864LED/Laser vs Halogen lamp: MD 0.46 (−1.4; 2.6), p = 0.94003 Halogen Lamp vs LED: MD -0.38 (−2.4; 1.6) p = 0.7211394 Halogen lamp vs Laser: MD -0.34 (−2.3; 1.4), p = 0.3335832LED/Laser vs LED: MD -0.77 (−2.7; 0.64), p = 0.7016492 Laser/LED: MD 0.086 (−1.5; 1.3), p = 0.5337331 Color Change in ΔSGU: Light free vs LED: MD 0.16 (−0.69; 1.0), p = 0.4947526 Light-free vs LED/ laser: MD -0.15 (−0.54; 0.31), p = 0.7923538LED/Laser vs Halogen lamp: MD -0.38 (−0.92; 0.21), p = 0.8028486 Halogen Lamp vs LED: MD -0.081 (−0.98; 0.90) p = 0.5449775 Halogen lamp vs Laser: MD 0.74 (−0.40; 1.9), p = 2428786LED/Laser vs LED: MD 0.31 (−0.50; 1.1), p = 0.9565217 Laser vs LED: MD 0.82 (−0.51; 2.1), p = 0.5847076 |
NR | NR | NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Luque-Martinez et al., 2016 [32] | VITA shade guide Spectrophotometer or colorimeter Digital image Fluorosis scale |
ΔE: SMD: −0.42; 95 %, CI: 0.64 to −0.2, p = 0.27 ΔSGU: SMD: 0.13; 95 %, CI:−0.22 to 0.48, p = 0.009 |
NR | Tooth sensitivity: RR: 0.96, 95 % CI 0.76 to 1.20, p = 0.81 | Gingival irritation: 1.18 (95 % CI 0.62 to 2.23), no significant difference (p = 0.62) |
| Geus et al., 2018 [21] | Shade guide Spectrophotometer or colorimeter Photographs or digital images. |
Color Change in ΔSGU: SMD: 0.29, CI: 0.25 to 0.83 (p = 0.29); x2 test, p < 0.00001; I2 = 85%, no difference in the color Color Change in ΔE: SMD: 0.16, CI-0.38 to 0.06 (p = 0.16), x2 test, p = 0.56; I2 = 0% |
VAS NRS |
Risk of TS: OR: 0.41 (95% CI 0.20 to 0.84; p = 0.01), 2 people from the PC 10% will experience the event for 5 who will not, (x2 test, p = 0.09; I2 = 41%) Intensity of TS: SMD: 0.44 (95% CI -0.67 to −0.20; p = 0.0003), lower intensity of pain for CP 10% than CP with higher concentrations, (x2 test, p = 0.34; I2 = 12%) |
NR |
| Cardenas et al., 2019 [33] | Vita Classical Shade guide Vita Bleached guide 3DMastershade guide Spectrophotometer or colorimeter Photography |
Color change in ΔE*: Combined vs. in-office bleaching: MD: −0.63 (95% CI − 3.40 to 2.13), p = 0.65 Combined vs. at-home bleaching: MD: 0.19 (95% CI − 0.57 to 0.95), p = 0.62 Color change in ΔSGU: Combined vs. in-office bleaching: MD: −0.49 (95% CI − 0.87 to − 0.10), p = 0.01 Combined vs. at-home bleaching: MD: 0.10 (95% CI − 0.54 to 0.33), p = 0.64 |
VAS |
Risk of tooth sensitivity: Combined vs. in-office bleaching: RR: 0.98 (95% CI 0.80 to 1.22), p = 0.89 Combined vs. at-home bleaching: RR: 1.40 (95% CI 1.10 to 1.80), p = 0.007 Intensity of tooth sensitivity: Combined vs. at-home bleaching: MD: 1.40 (95% CI 0.18 to 2.63), p = 0.02 |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Costacurta et al., 2019 [34] |
VITA Classic shade guide VITA 3D Master shade guide Spectrophotometer VITA Lumin Vacuum shade guide |
Color Change: SMD: 0.12 (95% CI = −0.22 to 0.46; p = 0.49), no statistically significant difference on color, x2 = 7.09, p = 0.13; I2 = 44% ΔSGU: MD: 0.07, 95% CI: 0.41; 0.5) ΔE: MD: 0.70, 95% CI: 0.66; 2.06 |
VAS NRS |
Tooth sensitivity risk: RR: 0.93 (95% CI 0.73 to 1.19, p = 0.56, no statistically significant differences, (x2 = 0.76, p = 0.38; I2 = 0%) Pain intensity: 0.10 (95% CI = −0.36 to 0.16, p = 0.45), no statistically significant differences, (x2 = 1.35, p = 0.72; I2 = 0%) NRS: MD: 0.03 (95% CI -0.32; 0.38) VAS: MD: 0.10 (95% CI -0.55; 0.75) |
NR |
| Rossi et al., 2022 [35] | Spectrophotometer or colorimeter Vita Scale shade guide |
LED alone without association with bleaching agents could show a slight capability of bleaching VL has a lower capability of penetration through teeth, and it is believed that the LED mechanism when applied alone is restricted to the enamel surface, which could justify the limitation of bleaching when only violet LED was applied Higher concentrations of peroxide promote better whitening VL in association with lower concentrated bleaching peroxides could promote effective dental bleaching and showed similar results to higher concentrated peroxides bleaching gel |
VAS Neurosensory analysis of thermal sensation threshold |
VL associated with bleaching peroxides, sensibility was reported No study reported increased tooth sensibility when VL was associated with bleaching agents in comparison to bleaching agents without light Patients treated with VL + CP reduced risk and intensity of tooth sensitivity than only the HP group with the same clinical result VL did not influence self-reported tooth sensitivity. Its use increased tooth sensitivity when teeth were exposed to low temperature. |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Fagogeni et al., 2021 [44] | NR | Improvement of shade was noted, the patient was not fully satisfied with the shade | NR | NR | Regenerative endodontic treatment |
| Alshammery, 2019 [45] | Spectrophotometer Vita classical shade guide |
Studies have shown that the time taken for bleaching with hybrid light is lesser than LED alone. Shorter clinical time is required for bleaching with a hybrid light source. Light activation of hydrogen peroxide IO does not affect effectiveness of bleach. HP for bleaching does not prove beneficial as the color is not stable after 3 months. Photoactivation results in fast bleaching, color regression was observed in less than a year. |
VAS | Three studies used violet LED for light activation and presented no photosensitivity. Low-power laser demonstrates anti-inflammatory and biomodulation potentials. No scientific evidence of low-power laser effect on reducing post-bleaching sensitivity. Any association of light activation with in-office bleaching to sensitivity. Light activation used increased tooth sensitivity. |
NR |
| Author/ Year | Evaluation | Efficacy | Evaluation | Adverse effects | Relevant observations |
|---|---|---|---|---|---|
| Eachempati et al., 2018 [46] | NR |
CP gel in tray vs placebo (DENTIST): 5% CP gel - 2 weeks: MD: 4.56 [1.52; 7.59] 10% CP gel with desensitiser - 2 weeks: MD: 4.70 [3.28; 6.12] 0% CP gel - light shade - 2 weeks: MD 4.5 [4.04; 4.96] 10% CP gel - medium dark shade - 2 weeks: MD: 6.90 [6.35; 7.45] 10% CP gel - darker shade - 2 weeks: MD: 10.0 [9.44; 10.56] 10% CP gel - 6 months: MD: 6.74 [3.15; 14.40] HP gel vs placebo(DENTIST): 6% HP gel - 14 days: MD 3.08 [2.28; 3.88] CP tray versus CP tray(DENTIST): 10% CP tray vs 10% CP tray - 2 weeks: MD: 1.03 [0.90; 1.18] 5% CP vs 10% CP - 1 week: MD: 0.38 [-5.55; 4.79] 2 5% CP vs 10% CP - 2 weeks: MD: 0.41 [-2.17; 2.98] 10% CP Colgate Platinum vs 10% CP Rembrandt Lighten - 2 weeks: MD: 1.92 [-2.8;-1.03 10% CP vs 28% CP - 1 year follow-up: MD: 3.3 [-8.71; 2.11] 10% CP vs 16% CP - 2-year follow-up: MD: 1.2 [-0.35; 2.75] 30% CP vs 30% CP + KN - 10 days: MD: 0.3 [-8.28; 7.68] 16% CP vs 16% CP + KN + NaF - 4 weeks: MD -0.2 [-0.44; 0.04] 16% CP v 16% CP + ACP - 6 months: MD: 0.78 [0.37; 1.19] 10% CP Polanight vs 10% CP Opalescence - 2 weeks: MD: 1.46 [0.13; 2.79] 10% CP vs 15% CP - 2 weeks: MD: 1.65 [0.22; 3.08] 10% CP vs 15% CP - 2 weeks: MD: 2.22 [1.29; 3.15] 10% CP vs 10% CP + KN + NaF - 2 weeks: MD: 0.32 [-0.2; 0.84] CP tray versus CP tray (patient): 1 10% CP vs 17% CP - Patient-contentment - 3 weeks: MD: 2.6 [2.57; 2.63] CP tray versus HP tray: 20% CP vs 7.5% HP - 12 days: MD: 0.99 [-2.32,0.34] 10% CP vs 7.5% HP - 2 weeks: MD: 1 [-2.86; 0.86] 20% CP vs 9% HP - 2 weeks: MD: 0.58 [-8.01; 6.85] 10% CP vs 6% HP - 2 weeks - medium dark and light shade: MD: 2.22 [-2.63;-1.81] 10% CP vs 6% HP - 2 weeks - darker shade: MD: 4.3 [-5.02;-3.58] 20% CP vs 7.5% HP - 12 weeks: MD: 0.25 [-0.4;-0.1] 20% CP vs 7.5% HP - 12 weeks: MD: 0.25 [0.1; 0.4] |
NR | Tooth sensitivity: more prevalent with higher concentrations of active agents though the effects were mild and transient | Tooth whitening did not have any effect on oral health-related quality of life Patient-reported level of comfort and gastrointestinal sensitivity |
AH- at-home; CP- Carbamide peroxide; CI- confidence interval; HP- Hydrogen peroxide; IO- in-office; MD-mean difference; NRS- numeric rating scale; OR-odds ratio; OHRQoL- Oral Health Related Quality of Life; RR-risk ratio; SDM- Standard mean difference; VAS- visual analog scale; VCS- Vitapan Classical Shade Guide; ΔE-color difference measured with a objective instrument; ΔSGU- shade guide units.
The included patients ranged in age from 18 years old to 80 years old, in one review the patients age were 14 years old [44] and in six reviews the age parameter was not reported, but the population was adults. In two of the 28 reviews, most participants were male [23,28], in five reviews females predominated [22,23,30,33,39], and 12 studies did not report this information [25,31,[35], [36], [37], [38],[40], [41], [42],[44], [45], [46]].
Eleven reviews compared in-office bleaching techniques [[22], [23], [24],[26], [27], [28],30,33,39,40,42], eight reviews compared at-home bleaching techniques [21,29,32,34,41,43,44,46] and nine reviews compared in-office and at-home bleaching techniques [6,25,31,[35], [36], [37], [38],45,47].
Hydrogen peroxide and carbamide peroxide were used as the agent bleaching in 24 studies. Hydrogen peroxide was used as the agent bleaching in two studies [28,31]. Carbamide peroxide was used as the agent bleaching in two studies [34,43].
The product concentrations ranged from 5 to 44 % for CP with concentrations between and from 5.5% to 38% for HP, both for in-office and at-home techniques.
For at-home bleaching techniques, CP or HP were employed. The bleaching trays were used from 7 to 28 days, with a daily use time that varied from 30 min to 10 h. Bleaching trays with reservoirs were used in three studies [32,34,47].
The protocol of the in-office teeth bleaching techniques was varied. Active agent was applied 15–60 min in each clinical session. Variations with one to five applications per clinical session were reported. Different types of light activation were used in eleven studies [6,22,23,[26], [27], [28],30,31,35,42,45].
Bleaching efficacy was evaluated by subjective (Vita Classical shade guide, Trubyte Bioform Color Ordered Shade Guide, Vita bleached guide 3D master shade guide) and objective (spectrophotometer, colorimeter, chromameter, digital image, polarized image) tooth color change methods. The parameters for color change assessment were ΔE in 5 studies [23,36,39,43,47], ΔSGU and ΔE in 10 studies [21,22,24,25,28,[31], [32], [33], [34],47], and 5 studies did not report this information [26,38,40,44,46].
Teeth sensitivity was assessed by visual analog scale (VAS) in 12 studies [21,[24], [25], [26], [27], [28],30,31,[33], [34], [35],45], numeric rating scale (NRS) in five studies [21,[24], [25], [26],34], dichotomous variable (presence or absence of the event) in one study [43], sensitivity questionnaire in one study [31], and neurosensory analysis of thermal sensation in one study [35].
Patient satisfaction was evaluated in two studies [24,25] with different questionnaires, but they did not report significantly differences between groups.
Gingival irritation was evaluated in five studies [25,29,32,41,43] Serraglio et al., 2016 [43] concluded that gingival irritation was higher when 10 % CP gel was applied on tray [43]. Luque-Martinez et al., 2016 demonstrated that the use of tray-delivered CP gels or HP gel produce equal level of gingival irritation [32]. Oral Health Related Quality of Life (OHRQoL) was evaluated in 2 studies [39,46] Kothari et al., 2019 [39] concluded that DB appeared to impact some domains of OHRQoL positively and some negatively, while Eachempati et al., 2018 [46] concluded that DB did not have any effect on oral health-related quality of life.
Niederman et al., compared 5 different brands bleaching agent and concluded that the brand had a significant effect on dental bleaching. In this study, 93% of patients who underwent bleaching showed an improvement of 2 color units, compared to 20% in the placebo control group. The whitening agent brand had a significant effect on the tooth whitening, but the time of daily application and treatment duration did not. The whitening results were maintained for 6 months for 50% of participants. Neither gingival indices nor plaque indices were adversely or favorably affected by the bleaching procedures [41].
Fagogeni et al., 2020 evaluated the effectiveness of bleaching procedures used to treat discolored teeth after regenerative endodontic procedures [44] They concluded that whitening of discolored teeth after REPs is achievable, but depends on bleaching technique, material, and duration.
3.3. Meta-analysis
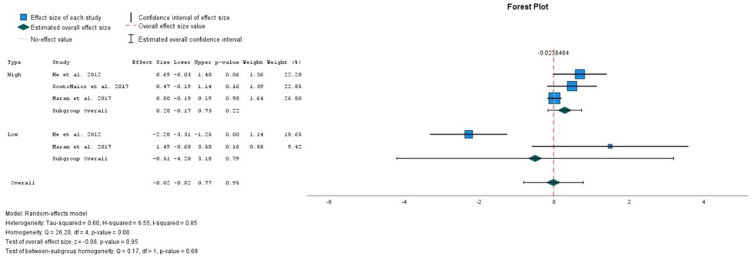
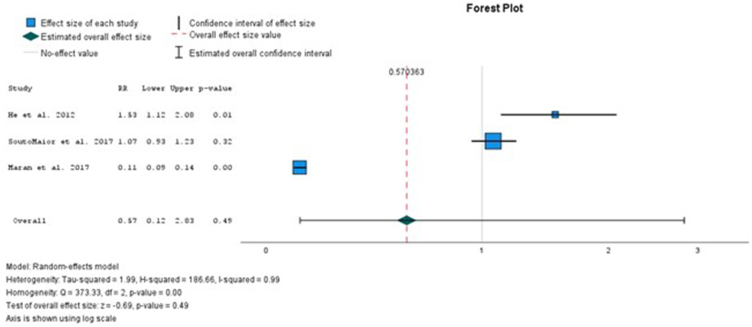
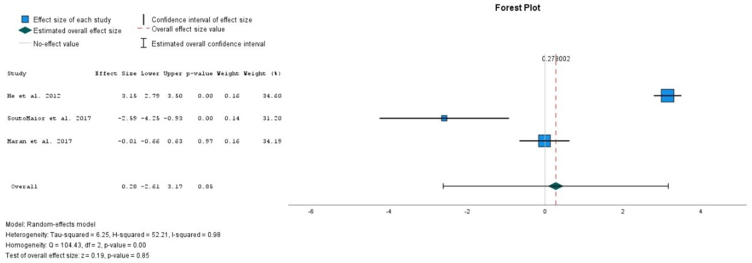
All the analysis presented high heterogeneity. In the overall comparison between the use of light and non-light for high and low concentrations of HP, there is no significative difference between the parameters evaluated (Figure 2). Also, in the overall comparison between the use of light and non-light, no significative increase in the risk ratio of sensitivity was detected (Figure 3). In the overall comparison between the use of light and non-light, no differences in the sensitivity intensity between the use of light or not was detected (Figure 4).
Fig. 2.
Comparison of bleaching efficacy between light versus non-light for high and low concentrations at the sort-term comparison.
Fig. 3.
Comparison between the risk ratio of sensitivity between light versus non-light.
Fig. 4.
Comparison between the sensitivity intensity between light versus non-light.
3.4. Quality assessment of the included reviews
The quality of the included reviews was evaluated using the AMSTAR2 tool, and the results are shown in Table 4.
Table 4.
Quality assessment of the included reviews, according to the AMSTAR2 tool.
| Author/ Year | PICO | Protocol | Inclusion Criteria | Comprehensive Search | Duplicate in Selection | Duplicate in Data Extraction | List of Excluded Studies | Description of Included Studies | Assessing Risk of Bias | Funding of Included Studies | Results of Statistical Combination | ROB Effect on the Statistical Combination | ROB in the Discussion | Discussion for the Heterogeneity | Publication Bias | Author's Funding and COF Reporting | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Geus et al., 2016 [47] | Yes | Yes | Yes | Yes | No | No | No | Partial Yes |
Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Dioguardi et al., 2022 [6] | Yes | No | Yes | Yes | No | No | No | Yes | No | No | No meta-analysis | No meta-analysis | Yes | Yes | No meta-analysis | Yes | Critically Low |
| Wasserman et al., 2014 [36] | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | No | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | No | Critically low |
| Naidu et al., 2020 [37] | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | No | No meta-analysis | No meta-analysis | No | Yes | No meta-analysis | No | Critically low |
| Kielbassa et al., 2015 [38] | No | No | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No meta-analysis | No meta-analysis | Yes | Yes | No meta-analysis | No | Low |
| Kothari et al., 2019 [39] | No | No | Yes | Partial Yes |
Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically Low |
| Alghulikah et al., 2021 [40] | Yes | No | Yes | Yes | No | No | No | Yes | No | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | No | Critically low |
| Maran et al., 2020 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Author/ Year | PICO | Protocol | Inclusion Criteria | Comprehensive Search | Duplicate in Selection | Duplicate in Data Extraction | List of Excluded Studies | Description of Included Studies | Assessing Risk of Bias | Funding of Included Studies | Results of Statistical Combination | ROB Effect on the Statistical Combination | ROB in the Discussion | Discussion for the Heterogeneity | Publication Bias | Author's Funding and COF Reporting | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosa et al., 2020 [25] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Moran et al., 2021 [26] | Yes | Yes | Yes | Yes | Yes | No | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Casado et al., 2020 [27] | Yes | Yes | Yes | Partial Yes |
Yes | No | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Moderate |
| Kury et al., 2021 [28] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | No | Yes | Low |
| Niederman et al., 2000 [41] | No | No | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No | No | Critically low |
| He et al., 2012 [42] | Yes | No | No | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | Moderate |
| Maran et al., 2017 [22] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Hasson et al., 2006 [29] | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | No | Low |
| Serraglio et al., 2015 [43] | No | No | No | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Low |
| SoutoMaior et al., 2017 [30] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Low |
| Pontes et al., 2020 [31] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | No | Yes | No | Low |
| Author/ Year | PICO | Protocol | Inclusion Criteria | Comprehensive Search | Duplicate in Selection | Duplicate in Data Extraction | List of Excluded Studies | Description of Included Studies | Assessing Risk of Bias | Funding of Included Studies | Results of Statistical Combination | ROB Effect on the Statistical Combination | ROB in the Discussion | Discussion for the Heterogeneity | Publication Bias | Author's Funding and COF Reporting | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maran et al., 2019 [23] | Yes | Yes | Yes | Yes | Yes | No | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Luque-Martinez et al., 2016 [32] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Partial Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Geus et al., 2018 [21] | Yes | Yes | Yes | Yes | Yes | No | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| Cardenas et al., 2019 [33] | Yes | Yes | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Costacurta et al., 2019 [34] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Low |
| Rossi et al., 2022 [35] | Yes | Yes | Yes | Yes | Yes | No | Partial Yes | Yes | Yes | No | No meta-analysis | No meta-analysis | Yes | Yes | No meta-analysis | Yes | Moderate |
| Fagogeni et al., 2021 [44] | No | No | Yes | Yes | Yes | Yes | Partial Yes | Partial Yes | Yes | No | No meta-analysis | No meta-analysis | No | No | No meta-analysis | Yes | Low |
| Alshammery, 2019 [45] | No | No | Yes | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No meta-analysis | No meta-analysis | Yes | No | No meta-analysis | No | Low |
| Eachempati et al., 2018 [46] | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Low |
PICO- Patient/Population, Intervention, Comparison and Outcome; ROB- Risk Of Bias.
The PICO question was present in 20 reviews and not present in eight reviews [29,38,39,41,[43], [44], [45], [46]] Successful registration was carried out in 15 reviews [[21], [22], [23], [24], [25], [26], [27], [28],[30], [31], [32], [33], [34], [35],47], but not carried out in 13 [6,29,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] The inclusion criteria were omitted in 2 reviews [42,43] The search was partial explained in three reviews [27,36,39], and fully explained in 25 reviews. Data selection was carried out in duplicate in 23 reviews and extraction were carried out in duplicate in 14 studies. The list of excluded studies is only presented in 3 reviews [29,41,46] The reason of excluded studies is presented in 16 reviews. The description of the included studies is just not presented in one study [41]. The risk of bias assessment was not performed in four reviews [6,36,40,41] The funding of included studies was only reported in four reviews [24,25,32,46] Eight reviews did not present a meta-analysis and consequently do not have results of statistical combination, ROB effects on the statistical combination and publication Bias. Discussion of possible causes of heterogeneity was presented in 20 reviews [6,[21], [22], [23], [24], [25], [26],28,30,[32], [33], [34], [35],[37], [38], [39],42,43,46,47] ROB was presented in 18 reviews [6,[21], [22], [23], [24], [25], [26], [27],[30], [31], [32], [33], [34], [35],38,43,45,47] Author's funding and the conflict of interests reporting were referred in 16 reviews [6,[21], [22], [23], [24], [25], [26], [27], [28],32,33,35,39,44,46,47] With the application of the AMSTAR 2 tool, six reviews were considered to be of critically low quality [6,36,37,[39], [40], [41]], 11 were considered to be of low quality [[28], [29], [30], [31],34,38,[43], [44], [45], [46], [47]], six were considered to be of moderate quality [[21], [22], [23],27,35,42], and five were considered to be of high quality [[24], [25], [26],32,33].
3.5. Analysis of the degree of overlap
The 28 systematic reviews included 416 studies, of which 214 overlapped in two or more systematic reviews (Appendix, Table S3). The % overlap was 13%, CA was 0.073 and CCA was 0.039. These values correspond to a slight overlap.
4. Discussion
This umbrella review aimed to compare the in-office and at-home bleaching techniques, regarding their efficacy and associated side-effects, mainly the post-operative sensitivity.
This is a widely explored dental topic and several systematic reviews with and without meta-analyses have been performed on it. However, due to variation on the bleaching protocols, products and concentrations used, or evaluation methods the obtained results are mixed.
Overall, the included SR showed that there is no difference in the color change outcomes between high (15%–44% for CP, and 6%–38% for HP) and low concentration (5%–15% for CP, and 5.5%–6% for HP) bleaching products. This can be explained since, although bleaching products at lower concentrations can have less immediate whitening capacity when compared to higher ones, they are often used for an extended duration, which results in similar levels of teeth bleaching [21,23,40,47] The studies by Geus et al., 2016 [47], Maran et al., 2017 [22], Dioguardi et al., 2022 [6], Kothari et al., 2019 [39], Alghulikah et al., 2021 [40], Maran et al., 2020 [24], Maran et al., 2019 [23], Geus et al., 2018 [21], Wasserman et al., 2014 [36] support this findings. In these studies, in-office bleaching with high concentrations of bleaching product with one or two sessions was compared to at-home bleaching with low concentrations during some days (one to two weeks). The low concentration was compensated by the repetitive daily at-home bleaching protocol, achieving the same results. However, a direct comparison between low and high concentrated products for the same application times was not available, which is a limitation of the included SRs.
There was also no reported difference in clinical efficiency between in-office and at-home techniques. This can be explained since, as previously discussed, the lower concentrations of at-home products are compensated for by prolonging the number of treatment days [6,36].
As mentioned above, the studies included present very different usage times, from 30 min to 10 h, which is called daytime or nighttime. Also, introduction, the whitening product, whether HP or CP, degrades in the tooth structure through a known mechanism of action. This means that the product, after degradation, becomes ineffective, and increasing exposure time is not a factor that will increase the treatment effectiveness. The increase in treatment time is related to the staining severity and should be performed in cases of more severe staining, such as tetracycline staining. The risk of side effects will also proportionally increase with increasing treatment times.
Considering the color change improvement outcome, the age of the participants was found to have an impact on the whitening degree, with color change being negatively affected by increasing age. Most of the studies evaluated color change in young patients; however, some studies included patients with large age-ranges, which can directly impact the obtained results and influence the results from different SRs [22,23,39].
Also, one of the limitations highlighted in the included SRs was the lack of standardization in protocols and methodologies, including color assessment, making direct comparisons challenging. Most of the SR use shade guides for color evaluation. This is a subjective measurement that is influenced by the individual evaluator's interpretation and depend on other variables, such as external light conditions, age, experience, eye fatigue, which may lead to inconsistencies [24,25,31] This way, the use of subjective measurements can lead to high bias and can help explain the heterogenicity observed between different SRs conclusions. Further studies with standardized protocols are required as well as studies that use precise, objective color assessment methods to reduce the potential influence of bias.
Most of the included SRs showed that both high concentrations and low concentrations of whitening agents caused dental hypersensitivity. These findings are supported by Dioguardi et al., 2022 [6], Moran et al., 2021 [26], Sensitivity is a common reaction during dental whitening and can vary from person to person. The contradictory results obtained can be explained by two main factors: first, in the primary studies included in the SRs the sensitivity was evaluated by diverse methodologies, which can origin different results. Second, most SR included studies that assessed the short-term sensitivity, since it is known that teeth sensitivity in most cases occurs immediately after dental bleaching and ceases two or three days after treatment [6,34].
Also, the included SR showed that there is a similar risk and intensity of teeth sensitivity between at-home and in-office bleaching techniques. This can be explained by the fact that most of the studies used at-home and in-office bleaching agents with potassium nitrate and sodium fluoride or because the wide variation in bleaching protocols and concentrations, as concluded by the studies by Geus et al., 2016 [47].
Although considering that the gums are exposed differently to the whitening product in an in-office or at-home whitening procedure (due to the use of gingival barriers in in-office treatments), there is no evidence of post-treatments differences regarding gingival irritation. This would be an important research topic in future clinical studies, also including a over-the-counter products, since the trays adaptation for such products is many times deficient.
The included SRs analyzed different types of light activation systems, including halogen lamps, lasers, LED/laser, and metal halides [6,22,23,27,30,42,45] Some of the SRs concluded that none of the light activation systems demonstrated to clearly improve the color change results. The heterogenicity of included light sources and bleaching protocols (including the concentration of product, number of sessions and duration of treatment) can explain the lack of positive results. Also, all the studies included in the referred SRs utilized high concentrations of the bleaching gel, so the additional benefit of the light source may not be significant in such cases. Opposite, Maran et al., 2017 [22], SoutoMaior et al., 2017 [30], Pontes et al., 2020 [31], and He et al., 2012 [42], concluded that light-activated systems with low concentrations of bleaching agent produced better immediate bleaching efficacy and showed similar results to higher concentrated peroxides bleaching gel, probably because heat and light sources that accelerate the decomposition of HP to form oxygen and perhydroxyl free radicals, increasing the bleaching efficacy [22,30] In this case, the lower concentrations of the bleaching agents used, benefits from the use of a light source, to increment their efficacy. Further studies on this topic should compare the same bleaching protocol (including type of agent and low and high concentrations), diverging only the light source tested, to achieve a definitive conclusion.
Considering the relationship between the use of light sources and bleaching-associated sensitivity, some of the SRs showed that there is no increase of the intensity and the risk of teeth sensibility with the use of light activation [22,23,26,27,45] One possible explanation for this result is the use of combination of light sources in most of the evaluated primary studies. This combination of light sources may prevent the laser light from being properly collimated [22,23]. Also, the layer formed by the bleaching gel can minimize the energy density and cause light reflection, which significantly reduces the absorption of light by the dental pulp, potentially reducing the teeth sensitivity [27] Additionally, the included SRs evaluated diverse light-sources, such as Violet LED. LED sources emit photons at a smaller wavelength and higher frequency compared to other sources. This characteristic results in less penetration into dental tissues, leading to decreased sensitivity. The lower penetration of violet LED light causes less molecular alteration and operates at a shallower depth, which helps preserve the dental pulp [35,45] On the contrary, He et al., 2012 [42], found a positive association of light activation with increased intensity and risk of tooth sensitivity. This SR included studies where long periods of light application were used, such as 60 min, which improves the probability of severe sensitivity occurred.
Alghulikah et al., 2021 [40], and Kury et al., 2021 [28], reported that there are no significant differences observed between the single application and the renewal application of in-office protocols in terms of tooth sensitivity and color change. Since most in-office techniques use high concentrations of the bleaching products, and the teeth have a bleaching limit, the additional product does not bring an additional benefit.
Eachempati et al., 2018 were unable to draw any conclusions regarding the at-home techniques because the available primary studies presented bleaching protocols with several variations making it impossible to compare the studies [46].
Two important limitations exist concerning the effect of dental bleaching on overall oral health-related quality of life. First, only two SRs evaluated the effect of DB on oral health-related quality of life [39,46] Second, it is essential to acknowledge that the assessment of oral health-related quality of life is a complex and multifaceted construct that may not have been fully captured. These SRs reported an increase in tooth color satisfaction and improvements in the aesthetic domain of oral health-related quality of life among the groups that underwent bleaching. It is important to note that the duration of these studies was relatively short, and it is unclear how the results might differ with longer-term follow-up. Due to the limited number of studies, short-term follow-up, and potential bias in the available research, further studies with larger sample sizes and longer-term assessments are needed to provide more reliable and comprehensive conclusions.
It is important to emphasize that this umbrella review included RCTs and quasi RCTs. However, even if these are controlled clinical studies, it is important to note a limitation due to high heterogeneity attributed to the difference in-office and at-home bleaching protocols and evaluation methods.
Also, most included SRs presents a low or critically low overall quality. This is mainly due to the absence of well-developed protocols, which increases the risk of bias. The discussion regarding the potential impacts of bias when interpreting and analyzing the results is not adequately performed in most cases. Also, the lack of justification for excluding individual studies and the assessment of the presence and impact of publication bias contributes to the low score. The results of this umbrella review should consider these limitations in the interpretation of results. Future research should carry out blinded RCTs with well-developed treatment and evaluation protocols to control possible sources of bias.
5. Conclusion
This umbrella review suggests that there is no difference between in-office and at-home techniques, and between high and low concentration bleaching in terms of color change. Sensitivity was observed for both modalities and high and low concentrations products. The light activation systems did not increase the intensity and risk of teeth sensibility. Further studies with long-term assessments are necessary to a comprehensive understanding of tooth sensitivity.
Ethics and consent to participate
Review or approval by an ethics committee was not needed for this study because this is an umbrella review, presenting only data already published.
Funding
No funding was obtained for this study.
Data availability statement
Data associated with this study has not been deposited in a publicly available repository. The data is included in the article and supplementary material.
CRediT authorship contribution statement
Maria Aidos: Writing – original draft, Formal analysis, Conceptualization. Carlos Miguel Marto: Writing – original draft, Validation, Methodology, Conceptualization. Inês Amaro: Visualization, Software. Mariangela Cernera: Investigation, Formal analysis. Inês Francisco: Visualization, Validation. Francisco Vale: Writing – review & editing, Validation, Resources. Manuel Marques Ferreira: Writing – review & editing, Supervision, Data curation. Bárbara Oliveiros: Visualization, Resources. Gianrico Spagnuolo: Supervision, Methodology. Eunice Carrilho: Writing – review & editing, Funding acquisition. Ana Coelho: Writing – original draft, Software. Anabela Baptista Paula: Writing – original draft, Project administration, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e25833.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Demarco F.F., Meireles S.S., Masotti A.S. Over-the-counter whitening agents: a concise review. Braz. Oral Res. 2009;23:64–70. doi: 10.1590/S1806-83242009000500010. [DOI] [PubMed] [Google Scholar]
- 2.Ellakany P., Fouda S.M., AlGhamdi M.A., Aly N.M. Influence of dental education on esthetics self-perception and shade selection. Int. J. Environ. Res. Publ. Health. 2022;19 doi: 10.3390/ijerph191811547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alqahtani M.Q. Tooth-bleaching procedures and their controversial effects: a literature review. Saudi Dent J. 2014;26:33–46. doi: 10.1016/j.sdentj.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joiner A. The bleaching of teeth: a review of the literature. J. Dent. 2006;34:412–419. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Alkahtani R., Stone S., German M., Waterhouse P. A review on dental whitening. J. Dent. 2020;100 doi: 10.1016/j.jdent.2020.103423. [DOI] [PubMed] [Google Scholar]
- 6.Dioguardi M., Quarta C., Spirito F., Sovereto D., Laneve E., Zerman N., Scarpa M., Cazzolla A.P., Pettini F., Cantore S., Lo Muzio L., Di Cosola M., Covelli M. Bleaching in vital teeth: a systematic review. J. Biol. Regul. Homeost. Agents. 2022;36 [Google Scholar]
- 7.Kwon S.R., Wertz P.W. Review of the mechanism of tooth whitening. J. Esthetic Restor. Dent. 2015;27:240–257. doi: 10.1111/jerd.12152. [DOI] [PubMed] [Google Scholar]
- 8.Faus-Matoses V., Palau-Martinez I., Amengual-Lorenzo J., Faus-Matoses I., Faus-Llacer V. Bleaching in vital teeth: combined treatment vs in-office treatment. J Clin Exp Dent. 2019;11:e754–e758. doi: 10.4317/jced.56079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ArtiS Naidu, Bennani V., Brunton J.M.A.P., Brunton P. Over-the-Counter tooth whitening agents: a review of literature. Braz. Dent. J. 2020;31:221–235. doi: 10.1590/0103-6440202003227. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard D., Wissen K. Home‐based chemically induced whitening (bleaching) of teeth in adults: a summary of a systematic review. Publ. Health Nurs. 2020;37:626–627. doi: 10.1111/phn.12713. [DOI] [PubMed] [Google Scholar]
- 11.Maran B.M., Burey A., de Paris Matos T., Loguercio A.D., Reis A. In-office dental bleaching with light vs. without light: a systematic review and meta-analysis. J. Dent. 2018;70:1–13. doi: 10.1016/j.jdent.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Lo Giudice R., Pantaleo G., Lizio A., Romeo U., Castiello G., Spagnuolo G., Lo Giudice G. Clinical and spectrophotometric evaluation of LED and laser activated teeth bleaching. Open Dent. J. 2016;10:242–250. doi: 10.2174/1874210601610010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasson H., Ismail A., Neiva G. Home-based chemically-induced whitening of teeth in adults. Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD006202. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg M., Grootveld M., Lynch E. Undesirable and adverse effects of tooth-whitening products: a review. Clin. Oral Invest. 2010;14:1–10. doi: 10.1007/s00784-009-0302-4. [DOI] [PubMed] [Google Scholar]
- 15.Tredwin C.J., Naik S., Lewis N.J., Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br. Dent. J. 2006;200:371–376. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 16.Naim S., Spagnuolo G., Osman E., Mahdi S.S., Battineni G., Qasim S.S.B., Cernera M., Rifai H., Jaafar N., Maalouf E., Zogheib C.M. Quantitative measurements of the depth of enamel demineralization before and after bleach: an in vitro study. BioMed Res. Int. 2022;2022:1–8. doi: 10.1155/2022/2805343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieper D., Antoine S.-L., Mathes T., Neugebauer E.A.M., Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014;67:368–375. doi: 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 20.de Geus J., Wambier L., Kossatz S., Loguercio A., Reis A. At-home vs in-office bleaching: a systematic review and meta-analysis. Operat. Dent. 2016;41:341–356. doi: 10.2341/15-287-LIT. [DOI] [PubMed] [Google Scholar]
- 21.de Geus J., Wambier L., Boing T., Loguercio A., Reis A. At-home bleaching with 10% vs more concentrated carbamide peroxide gels: a systematic review and meta-analysis. Operat. Dent. 2018;43:E210–E222. doi: 10.2341/17-222-L. [DOI] [PubMed] [Google Scholar]
- 22.Maran B.M., Burey A., de Paris Matos T., Loguercio A.D., Reis A. In-office dental bleaching with light vs. without light: a systematic review and meta-analysis. J. Dent. 2018;70:1–13. doi: 10.1016/j.jdent.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Maran B.M., Ziegelmann P.K., Burey A., de Paris Matos T., Loguercio A.D., Reis A. Different light-activation systems associated with dental bleaching: a systematic review and a network meta-analysis. Clin. Oral Invest. 2019;23:1499–1512. doi: 10.1007/s00784-019-02835-x. [DOI] [PubMed] [Google Scholar]
- 24.Maran B.M., Matos T. de P., de Castro A. dos S., Vochikovski L., Amadori A.L., Loguercio A.D., Reis A., Berger S.B. In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: a systematic review and meta-analysis. J. Dent. 2020;103 doi: 10.1016/j.jdent.2020.103499. [DOI] [PubMed] [Google Scholar]
- 25.da Rosa G., Maran B., Schmitt V., Loguercio A., Reis A., Naufel F. Effectiveness of whitening strips use compared with supervised dental bleaching: a systematic review and meta-analysis. Operat. Dent. 2020;45:E289–E307. doi: 10.2341/19-160-L. [DOI] [PubMed] [Google Scholar]
- 26.Moran B., Ziegelmann P., Berger S., Burey A., de Paris Matos T., Fernández E., Loguercio A., Reis A. Evaluation of tooth sensitivity of in-office bleaching with different light activation sources: a systematic review and a network meta-analysis. Operat. Dent. 2021;46:E199–E223. doi: 10.2341/20-127-L. [DOI] [PubMed] [Google Scholar]
- 27.Casado B., Pellizzer E., Souto Maior J., Lemos C., Vasconcelos B., Moraes S. Laser influence on dental sensitivity compared to other light sources used during in-office dental bleaching: systematic review and meta-analysis. Operat. Dent. 2020;45:589–597. doi: 10.2341/19-064-L. [DOI] [PubMed] [Google Scholar]
- 28.Kury M., Lins R.B.E., Resende B.D.A., Picolo M.Z.D., André C.B., Cavalli V. The influence of the renewal or the single application of the peroxide gel on the efficacy and tooth sensitivity outcomes of in‐office bleaching—a systematic review and meta‐analysis. J. Esthetic Restor. Dent. 2022;34(3):490–502. doi: 10.1111/jerd.12827. [DOI] [PubMed] [Google Scholar]
- 29.Hasson H., Ismail A., Neiva G. Home-based chemically-induced whitening of teeth in adults. Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD006202. [DOI] [PubMed] [Google Scholar]
- 30.SoutoMaior J., de Moraes S., Lemos C., Vasconcelos B. do E., Montes M., Pellizzer E. Effectiveness of light sources on in-office dental bleaching: a systematic review and meta-analyses. Operat. Dent. 2019;44:E105–E117. doi: 10.2341/17-280-L. [DOI] [PubMed] [Google Scholar]
- 31.Pontes M., Gomes J., Lemos C., Leão R., Moraes S., Vasconcelos B., Pellizzer E. Effect of bleaching gel concentration on tooth color and sensitivity: a systematic review and meta-analysis. Operat. Dent. 2020;45:265–275. doi: 10.2341/17-376-L. [DOI] [PubMed] [Google Scholar]
- 32.Luque-Martinez I., Reis A., Schroeder M., Muñoz M.A., Loguercio A.D., Masterson D., Maia L.C. Comparison of efficacy of tray-delivered carbamide and hydrogen peroxide for at-home bleaching: a systematic review and meta-analysis. Clin. Oral Invest. 2016;20:1419–1433. doi: 10.1007/s00784-016-1863-7. [DOI] [PubMed] [Google Scholar]
- 33.Cardenas A.F.M., Maran B.M., Araújo L.C.R., de Siqueira F.S.F., Wambier L.M., Gonzaga C.C., Loguercio A.D., Reis A. Are combined bleaching techniques better than their sole application? A systematic review and meta-analysis. Clin. Oral Invest. 2019;23:3673–3689. doi: 10.1007/s00784-019-03042-4. [DOI] [PubMed] [Google Scholar]
- 34.Costacurta A., Kunz P., Silva R., Wambier L., Cunha L., Correr G., Gonzaga C. Does the addition of potassium nitrate to carbamide peroxide gel reduce sensitivity during at‐home bleaching? Aust. Dent. J. 2020;65:70–82. doi: 10.1111/adj.12739. [DOI] [PubMed] [Google Scholar]
- 35.Rossi B., Morimoto S., Tedesco T.K., Cunha S.R., Horliana A.C.R.T., Ramalho K.M. Effectiveness of Violet LED alone or in association with bleaching gel during dental photobleaching: a Systematic Review. Photodiagnosis Photodyn. Ther. 2022;38 doi: 10.1016/j.pdpdt.2022.102813. [DOI] [PubMed] [Google Scholar]
- 36.Adriana I.W., Diana C., Mejía F.J., Milhen I.W., Silva A.M.C., Correa D.M.F., Ayala J.A.M. Efectividad y estabilidad del blanqueamiento dental, una revisión sistemática. Revista Salud Bosque. 2014;4(2):7–18. doi: 10.18270/rsb.v4i2.21. n.d. [DOI] [Google Scholar]
- 37.ArtiS Naidu, Bennani V., Brunton J.M.A.P., Brunton P. Over-the-Counter tooth whitening agents: a review of literature. Braz. Dent. J. 2020;31:221–235. doi: 10.1590/0103-6440202003227. [DOI] [PubMed] [Google Scholar]
- 38.A.M. Kielbassa, M. Maier, A.-K. Gieren, E. Eliav, Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem., Quintessence Int.. 46 (n.d.)881–897. 10.3290/j.qi.a34700.. [DOI] [PubMed]
- 39.Kothari S., Gray A.R., Lyons K., Tan X.W., Brunton P.A. Vital bleaching and oral-health-related quality of life in adults: a systematic review and meta-analysis. J. Dent. 2019;84:22–29. doi: 10.1016/j.jdent.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Alghulikah K., Alahmed A.A., Bin Muattish A.Z., Almazyad A.A., Almalki M.A., Almaliki O.A. The effectiveness of different clinical methods of application for in-office bleaching materials with hydrogen peroxide: a systematic review. J Pharm Res Int. 2021:311–317. doi: 10.9734/jpri/2021/v33i37A32013. [DOI] [Google Scholar]
- 41.Niederman R., Tantraphol M.C., Slinin P., Hayes C., Conway S. Effectiveness of dentist-prescribed, home-applied tooth whitening. A meta analysis. J. Contemp. Dent. Pract. 2000;1(4):20–36. (n.d.) [PubMed] [Google Scholar]
- 42.He L.-B., Shao M.-Y., Tan K., Xu X., Li J.-Y. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J. Dent. 2012;40:644–653. doi: 10.1016/j.jdent.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Serraglio C.R., Zanella L., Dalla-Vecchia K.B., Rodrigues-Junior S.A. Efficacy and safety of over-the-counter whitening strips as compared to home-whitening with 10 % carbamide peroxide gel—systematic review of RCTs and metanalysis. Clin. Oral Invest. 2016;20:1–14. doi: 10.1007/s00784-015-1547-8. [DOI] [PubMed] [Google Scholar]
- 44.Fagogeni I., Falgowski T., Metlerska J., Lipski M., Górski M., Nowicka A. Efficiency of teeth bleaching after regenerative endodontic treatment: a systematic review. J. Clin. Med. 2021;10:316. doi: 10.3390/jcm10020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aishammery S.A. Evaluation of light activation on in-office dental bleaching: a systematic review. J. Contemp. Dent. Pract. 2019;20:1355–1360. doi: 10.5005/jp-journals-10024-2676. [DOI] [PubMed] [Google Scholar]
- 46.Eachempati P., Kumbargere Nagraj S., Kiran Kumar Krishanappa S., Gupta P., Yaylali I.E. Home-based chemically-induced whitening (bleaching) of teeth in adults. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD006202.pub2. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Geus J., Wambier L., Kossatz S., Loguercio A., Reis A. At-home vs in-office bleaching: a systematic review and meta-analysis. Operat. Dent. 2016;41:341–356. doi: 10.2341/15-287-LIT. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has not been deposited in a publicly available repository. The data is included in the article and supplementary material.