Key Points
Question
What is the optimal volume of early moderate-to-vigorous physical activity (MVPA) postconcussion and its association with symptom burden?
Findings
In this cohort study including 267 children and youths MVPA volumes up to 259 minutes in the first week and up to 565 minutes throughout the first 2 weeks were associated with lower symptom burden at week 1 and week 2.
Meaning
These findings suggest that MVPA reduced symptoms up to a certain threshold for children and youths but appeared to offer no further benefit in symptom reduction beyond that point.
This cohort study investigates the association between cumulative moderate-to-vigorous-intensity physical activity throughout 2 weeks after a concussion and the symptom burden a 1 week, 2 weeks, and 4 weeks postinjury among youths in Canada.
Abstract
Importance
Determining the optimal volume of early moderate-to-vigorous-intensity physical activity (MVPA) after concussion and its association with subsequent symptom burden is important for early postinjury management recommendations.
Objectives
To investigate the association between cumulative MVPA (cMVPA) over 2 weeks and subsequent symptom burden at 1 week, 2 weeks, and 4 weeks postinjury in children and examine the association between cMVPA and odds of persisting symptoms after concussion (PSAC) at 2 weeks and 4 weeks postinjury.
Design, Setting, and Participants
This multicenter cohort study used data from a randomized clinical trial that was conducted from March 2017 to December 2019 at 3 Canadian pediatric emergency departments in participants aged 10.00 to 17.99 years with acute concussion of less than 48 hours. Data were analyzed from July 2022 to December 2023.
Exposure
cMVPA postinjury was measured with accelerometers worn on the waist for 24 hours per day for 13 days postinjury, with measurements deemed valid if participants had 4 or more days of accelerometer data and 3 or fewer consecutive days of missing data. cMVPA at 1 week and 2 weeks postinjury was defined as cMVPA for 7 days and 13 days postinjury, respectively. Multiple imputations were carried out on missing MVPA days.
Main Outcomes and measures
Self-reported postconcussion symptom burden at 1 week, 2 weeks, and 4 weeks postinjury using the Health and Behavior Inventory (HBI). PSAC was defined as reliable change on the HBI. A linear mixed-effect model was used for symptom burden at 1 week, 2 weeks, and 4 weeks postinjury with a time × cMVPA interaction. Logistic regressions assessed the association between cMVPA and PSAC. All models were adjusted for prognostically important variables.
Results
In this study, 267 of 456 children (119 [44.6%] female; median [IQR] age, 12.9 [11.5 to 14.4] years) were included in the analysis. Participants with greater cMVPA had significantly lower HBI scores at 1 week (75th percentile [258.5 minutes] vs 25th percentile [90.0 minutes]; difference, −5.45 [95% CI, −7.67 to −3.24]) and 2 weeks postinjury (75th percentile [565.0 minutes] vs 25th percentile [237.0 minutes]; difference, −2.85 [95% CI, −4.74 to −0.97]) but not at 4 weeks postinjury (75th percentile [565.0 minutes] vs 25th percentile [237.0 minutes]; difference, −1.24 [95% CI, −3.13 to 0.64]) (P = .20). Symptom burden was not lower beyond the 75th percentile for cMVPA at 1 week or 2 weeks postinjury (1 week, 259 minutes; 2 weeks, 565 minutes) of cMVPA. The odds ratio for the association between 75th and 25th percentile of cMVPA and PSAC was 0.48 (95% CI, 0.24 to 0.94) at 2 weeks.
Conclusions and Relevance
In children and adolescents with acute concussion, 259 minutes of cMVPA during the first week postinjury and 565 minutes of cMVPA during the second week postinjury were associated with lower symptom burden at 1 week and 2 weeks postinjury. At 2 weeks postinjury, higher cMVPA volume was associated with 48% reduced odds of PSAC compared with lower cMVPA volume.
Introduction
Concussion poses a significant burden on population health and the economy. Globally, 50 to 60 million people are estimated to have a traumatic brain injury (TBI) each year, with 75% to 90% of hospital cases being TBIs or concussions.1,2 Approximately 30% to 35% of concussion cases will demonstrate persisting symptoms after concussion (PSAC).3,4 PSAC significantly impairs daily activities and quality of life,5 underscoring the urgent need to generate and refine best evidence management protocols. Early engagement in physical activity has been associated with reduced symptom burden at 2 weeks postinjury, faster recovery, and a lower risk of developing PSAC.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 However, the optimal volume of daily physical activity for achieving recovery in the general pediatric population remains unclear.14
The reference standard protocol for identifying the optimal intensity of physical activity postconcussion is the Buffalo Concussion Treadmill Test. This graded exercise test is designed to assess an individual’s tolerance for physical exertion and to guide the development of an individualized physical activity plan based on heart rate thresholds.9,13 However, many primary care clinicians do not have the equipment, personnel expertise, or time to routinely conduct this protocol in children and adolescents. Further, many children and adolescents do not have access to such specialized clinics.
Current research is limited and contradictory regarding the optimal volume and intensity of physical activity during the first weeks postinjury. In youths with acute sport-related concussion (SRC), engaging in higher volumes (approximately 72.4 minutes per day) of moderate to vigorous intensity physical activity (MVPA) was associated with longer recovery compared with lower volumes (approximately 36.9 minutes per day) during the first 3 days postconcussion.10 In contrast, adolescents with a SRC recruited within 10 days of injury who performed 20 minutes of individualized, subsymptom aerobic activity (based on heart rate threshold) per day for 4 weeks had reduced recovery time and risk of PSAC.13 In adolescents with SRC, a higher threshold of prescribed exercise volume (more than 160 minutes per day) was associated with being asymptomatic at 1 month.7 Moreover, adolescents with SRC who engaged in more than 30 minutes per day of MVPA for 7 days reported symptom resolution sooner compared with those who recorded less.11 Further, those completing 3 exercise sessions and 135 minutes per week of physical activity were medically cleared to return to play faster (less than 28 days) than those who did minimal exercise (more than 28 days).8 Studies that have investigated the optimal volume of physical activity have primarily focused on individuals with PSAC,23,24,25,26 used small sample sizes,7,8,10,11,13 only included sport-related populations,7,8,9,10,11,13 or lacked objective measurements to assess the intensity of physical activity performed by patients.7,9
The lack of prospective studies with rigorous methods on stepwise physical activity guidelines, specifically for the general pediatric population with acute concussion, has made it challenging for clinicians to implement standardized recommendations. This study had 3 goals: (1) to investigate the association between cumulative MVPA (cMVPA) volume and total subsequent symptom burden at 1 week, 2 weeks, and 4 weeks postinjury; (2) to investigate the association between cMVPA volume and the risk of developing PSAC at 2 weeks and 4 weeks postinjury; and (3) to explore the association with cMVPA and subsequent cognitive and somatic symptoms at 1 week, 2 week, and 4 weeks postinjury. We hypothesized that increased cMVPA would be associated with decreased symptom burden at 1 week, 2 weeks, and 4 weeks postinjury, and decreased the odds of PSAC at 2 weeks and 4 weeks postinjury.
Methods
This cohort study was a planned secondary analysis of data collected from the Pediatric Concussion Assessment of Rest and Exertion study (PedCARE, NCT02893969),27 a multicenter clinical trial. Participants were recruited from March 2017 to December 2019 in 3 emergency departments (EDs) affiliated with the Pediatric Emergency Research Canada (PERC) network. The PedCARE study was approved by the ethics committees of all 3 participating institutions. All participants provided written informed assent or consent and parents provided consent. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
Youth aged 10.00 years to 17.99 years who sustained a concussion within 48 hours of presenting to the ED were included in this study. Concussions were defined based on the Zurich and Berlin consensus statement for SRC.28,29 An adapted version of the US Centers for Disease Control and Prevention tiered framework was used to confirm concussion in participants.30 Those with either 1 highest level of certainty signs (eg, retrograde or anterograde amnesia; loss of consciousness) or 2 higher level of certainty signs or symptoms (eg, nausea or vomiting; headache; dizziness) immediately or within 1 hour of injury were included. Exclusion criteria were as follows: Glasgow Coma Scale score of 13 or less; abnormality on brain imaging if completed; neurosurgical intervention, intubation, or intensive care unit admission; multisystem injury requiring hospitalization; severe preexisting neurological developmental delay resulting in communication difficulties; intoxication; absence of trauma history as primary event; previously enrolled; insurmountable language barrier; or inability to complete follow-ups.
Study Protocol
The study protocol and primary analyses have been published.6,27 Participants completed questionnaires collecting demographics, injury characteristics, personal health, and mental health history. Balance was assessed using the Balance Error Scoring System (BESS).31
The Health and Behavior Inventory (HBI)32,33 and retrospective HBI were used to measure the participant’s preinjury and postinjury symptom profile and severity. The HBI is a 20-item (score range 0 to 60, higher scores indicate increased number of symptoms or more severe symptoms) valid and reliable symptom rating scale recommended as a National Institutes of Health core common data element for concussion.34,35 The HBI queries symptoms in somatic (9 items; range 0 to 27) and cognitive (11 items; range 0 to 33) domains.36 Parents completed the retrospective HBI in the ED, and participants completed the HBI at 1 week, 2 weeks and 4 weeks postinjury via the Research Electronic Data Capture (REDCap)37,38 database or phone.
Next, participants were randomized to either the experimental (ie, start return-to-physical activity at 72 hours) or control group (ie, return to physical activity once asymptomatic). Participants received standardized instructions on return-to-learn and return-to-physical activity,28,39,40,41 concussion education, and were given an Actical accelerometer to provide valid and reliable estimates of physical activity.42 The accelerometer was worn on a belt around the waist at the right midaxillary line for 14 consecutive days (24 hours per day) post ED-visit, and collected data in 1 minute epochs (excluding aquatic activities).
Outcome Measures
Symptom burden was defined as the sum score of the 20 items on the HBI. The cognitive and somatic symptom scores were defined as the sum score of 11 and 9 items, respectively.
PSAC was determined with reliable change z scores.36 The reliable change score compares the parent’s retrospective rating of preinjury total symptoms with the child’s rating of symptoms reported during the 2 week and 4 week follow-up, based on formulae derived from regression analyses in children with orthopedic injuries. PSAC was defined by a reliable change z score of 1.65 or more, which indicates a greater-than-expected increase in symptoms based on retrospective parent preinjury ratings.
| Postinjury z score for total score = postinjury child total score – (6.352 + [0.476 × retrospective parent preinjury total score]) / 9.597 |
Data Processing
Data processing procedures have been published elsewhere.6 Time spent in each movement intensity was classified according to established intensity cut-offs for the accelerometer: vigorous physical activity, 6500 or more counts per minute; moderate physical activity, between 6499 and 1500 counts per minute; light physical activity, between 1499 and 100 counts per minute43; and sedentary behaviors, less than 100 counts per minute.44
cMVPA at 1 week was defined as the sum of MVPA for days 1 to 7 after the ED visit. cMVPA at 2 weeks and 4 weeks was defined as the sum of MVPA for days 1 to 13 after the ED visit. cMVPA (for week 1 and week 2) was used for the analysis of 2 weeks and 4 weeks postinjury.
Statistical Analysis
Based on Ledoux et al,6 no differences in mean MVPA were found between the experimental and control groups, and therefore they were combined in the current analysis. Data for MVPA per day were included if participants had at least 4 days of accelerometer data (with more than 8 hours per day of waking wear time) and 3 or fewer consecutive days of incomplete data.
We used multiple imputations using chained equations to account for missing estimator and outcome values.44 A estimated mean matching strategy was used to impute missing values from numeric variables (continuous or ordinal), and logistic regression was used for categorical variables. The imputation model included total HBI score, number of minutes of MVPA per day, and 56 additional variables selected from the PedCARE data set known to be potential confounders or predictors of symptom burden or PSAC (eTable 1 in Supplement 1). Finally, the imputation model included auxiliary variables associated with nonresponse, which were preselected using the algorithm of van Buuren et al.45
Modeling results using the multiple imputed data sets were combined into final estimates based on Rubin rules.45 The model estimates were assessed for the relative efficiency of imputation, and an efficiency of 95% or higher was sought.
A linear mixed-effects model was fitted with HBI score as dependent variable to assess the association between cMVPA in the previous week and symptom burden at 1 week, 2 weeks, and 4 weeks postinjury. Fixed effects were cMVPA, cMVPA at each follow-up time point, time, randomization group, age, sex, Predicting Persistent Postconcussive Problems in Pediatrics (5P) risk score,4 preinjury mental health and learning disability comorbidity (concatenated variable, including anxiety, depression, sleep disorder, other mental health disorder, learning disabilities, attention disorder, and other developmental disorder, coded as 1 or 0), and retrospective and ED HBI score. The random effect was participants. Contrasts were specified between the 75th vs 25th percentile to provide estimates of effect size associated with cMVPA.
Two multivariable logistic regression models were computed to investigate the association of cMVPA in the previous week and PSAC odds at 2 weeks and 4 weeks postinjury. Fixed factors were cMVPA, age, sex, 5P risk score, mental health and learning disability comorbidity, and ED HBI.
Exploratory linear mixed-effects analyses were fitted with the scores as dependent variables to assess the association between cMVPA in the previous week and cognitive and somatic HBI scores at 1 week, 2 weeks, and 4 weeks postinjury. For both models, we used nonimputed data (ie, HBI cognitive and somatic scores). Participants were excluded only if they lacked HBI scores at all time points. Fixed and random factors were the same as analysis 1.
For all analyses, a restricted cubic spline (3 knots) was applied to cMVPA and age to allow for nonlinearity. cMVPA was treated as a time-varying variable. Nonlinearity for all models was assessed by reviewing locally estimated scatterplot smoothing (LOESS) curves and if necessary, the application of a restricted cubic spline to compare the model fit with a linear term was conducted. For all analyses, 2-sided P < .05 was considered statistically significant, and mice version 3.16.0 and R version 4.0.5 (R Project for Statistical Computing) were used.46,47
Results
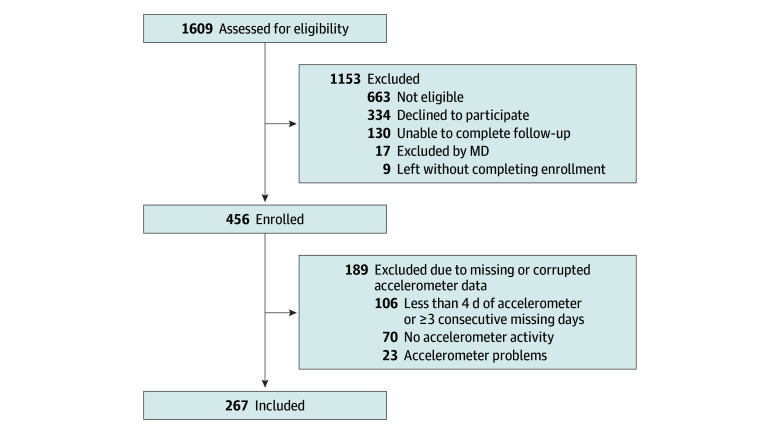
In this study, 267 of 456 children from the larger trial sample (119 [44.6%] female; median [IQR] age, 12.9 [11.5-14.4] years) were included in the analysis (Figure 1); 189 (41.4%) were lost due to missingness in the activity monitor data. Personal and injury characteristics are presented in Table 1. Median (IQR) cMVPA was 146.0 (90.8-264.2) minutes at 1 week postinjury and 380.5 (235.8-589.5) minutes at 2 weeks postinjury. Median (IQR) HBI scores were 18.0 (10.0-29.0) at 1 week postinjury, 14.0 (5.0-23.0) at 2 weeks postinjury, and 10.0 (1.5-19.0) at 4 weeks postinjury (eTable 2 in Supplement 1).
Figure 1. Flow Diagram.
MD indicates medical doctor.
Table 1. Personal Characteristics.
| Variable | Participants, No. (%) | |||
|---|---|---|---|---|
| Complete sample | Analytical sample | Missing, in analytical sample | Data without or insufficient accelerometer data | |
| Total, No. | 456 | 267 | NA | 189 |
| Age, median (IQR), y | 13.1 (11.5-14.8) | 12.9 (11.5-14.4) | 0 | 13.4 (11.8-15.5) |
| Sex | ||||
| Female | 201 (44.1) | 119 (44.6) | 0 | 82 (43.4) |
| Male | 255 (55.9) | 148 (55.4) | 0 | 107 (56.6) |
| 5P clinical risk score, median (IQR) | 7.0 (5.0-8.0) | 7.0 (5.0-8.0) | 1 (0.4) | 7.0 (5.0-8.0) |
| Time from head injury to triage, median (IQR), h | 3.0 (1.6-20.0) | 3.0 (1.6-20.0) | 3 (1.1) | 3.1 (1.6-19.7) |
| Personal migraine history | 25 (5.5) | 11 (4.1) | 0 | 14 (7.4) |
| Previous concussions | 150 (32.9) | 86 (32.2) | 0 | 64 (33.9) |
| Previous number of concussions, median (IQR) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 181 (67.8) | 1.0 (1.0-2.0) |
| Symptom duration of prior concussion of 1 week or more | 101 (22.1) | 52 (19.5) | 0 | 49 (25.9) |
| Parent-reported diagnostic history | ||||
| Learning disabilities | 52 (15.5) | 33 (14.9) | 46 (17.2) | 19 (16.5) |
| Attention-deficit hyperactivity disorder | 48 (14.3) | 33 (14.9) | 46 (17.2) | 15 (13.0) |
| Other developmental disorder | 20 (6.0) | 12 (5.4) | 46 (17.2) | 8 (7.0) |
| Anxiety | 60 (17.9) | 34 (15.4) | 46 (17.2) | 26 (22.6) |
| Depression | 20 (6.0) | 8 (3.6) | 46 (17.2) | 12 (10.4) |
| Sleep disorder | 13 (3.9) | 9 (4.1) | 46 (17.2) | 4 (3.5) |
| Other psychiatric disorder | 7 (2.1) | 4 (1.8) | 46 (17.2) | 3 (2.6) |
| Mechanism of injury | 1 (0.4) | |||
| Occupant in motor vehicle collision | 6 (1.3) | 4 (1.5) | 0 | 2 (1.1) |
| Pedestrian struck by auto | 1 (0.2) | 1 (0.4) | 0 | 0 |
| Bike struck by auto | 0 | 0 | 0 | 0 |
| Bike collision or fall while riding | 4 (0.9) | 2 (0.8) | 0 | 2 (1.1) |
| Fall from an elevation (including playground equipment) | 14 (3.1) | 9 (3.4) | 0 | 5 (2.7) |
| Fall downstairs | 8 (1.8) | 3 (1.1) | 0 | 5 (2.7) |
| Sport | 238 (52.4) | 144 (54.1) | 0 | 94 (50.0) |
| Fall from standing, walking, or running | 63 (13.9) | 37 (13.9) | 0 | 26 (13.8) |
| Ran into stationary object | 24 (5.3) | 16 (6.0) | 0 | 8 (4.3) |
| Other mechanism | 96 (21.1) | 50 (18.8) | 0 | 46 (24.5) |
| Loss of consciousness | 81 (17.8) | 48 (18.0) | 0 | 33 (17.5) |
| Duration of loss of consciousness | ||||
| <1 min | 60 (74.1) | 37 (77.1) | 0 | 23 (69.7) |
| 1-5 min | 18 (22.2) | 9 (18.8) | 0 | 9 (27.3) |
| >5 min | 3 (3.7) | 2 (4.2) | 0 | 1 (3.0) |
| Assessed in the ED | ||||
| Seizure | 4 (0.9) | 2 (0.7) | 0 | 2 (1.1) |
| Answers questions slowly | 204 (44.7) | 113 (42.3) | 0 | 91 (48.1) |
| Headache | 417 (91.4) | 241 (90.3) | 0 | 176 (93.1) |
| Sensitivity to noise | 254 (55.7) | 153 (57.3) | 0 | 101 (53.4) |
| Fatigue | 392 (86.0) | 230 (86.1) | 0 | 162 (85.7) |
| Balance error score tandem stance ≥4 | 51 (11.2) | 38 (14.3) | 1 (0.4) | 13 (6.9) |
| Retrospective HBI, median (IQR) | 13.0 (6.5-20.0) | 12.5 (6.0-19.0) | 1 (0.4) | 14.0 (7.0-20.0) |
| HBI at ED, median (IQR) | 23.0 (16.0-31.0) | 21.0 (15.0-31.0) | 1 (0.4) | 25.0 (18.0-31.2) |
| cMVPA, median (IQR) | ||||
| cMVPA week 1 | NA | 146.0 (90.8-264.2) | 99 (37.1)a,b | NA |
| cMVPA week 2 | NA | 380.5 (235.8-589.5) | 147 (55.1)a,c | NA |
Abbreviations: 5P, Predicting Persistent Postconcussive Problems in Pediatrics; cMVPA, cumulative moderate-to-vigorous-intensity physical activity; ED, emergency department; HBI, Health and Behavior Inventory; NA, not available.
Missing because of noncompliance or low wear time.
Missing at any day over the first week.
Missing at any day over the first 2 weeks.
cMVPA and Recovery Trajectory
Multiple imputation was carried out and pooled model results showed relative efficiencies of 99% and higher for all variables. LOESS curves based on observed and imputed data for HBI vs cMVPA at 1 week, 2 weeks, and 4 weeks postinjury are shown in the eFigure in Supplement 1. Participants with greater cMVPA had significantly lower HBI scores at 1 week (75th percentile [258.5 minutes] vs 25th percentile [90.0 minutes]; difference, −5.45 [95% CI, −7.67 to −3.24]) and 2 weeks postinjury (75th percentile [565.0 minutes] vs 25th percentile [237.0 minutes]; difference, −2.85 [95% CI, −4.74 to −0.97]) but not at 4 weeks postinjury (75th percentile [565.0 minutes] vs 25th percentile [237.0 minutes]; difference, −1.24 [95% CI, −3.13 to 0.64]) (P = .20) (Figure 2). Symptom burden was not lower beyond the 75th percentile for cMVPA at 1 week or 2 weeks postinjury (1 week, 259 minutes; 2 weeks, 565 minutes) of cMVPA. Past these thresholds, our estimated model suggested either no improvement or an increase in symptoms. Nonlinearity was significant P < .001. cMVPA percentiles for 1 week, 2 weeks, and 4 weeks postinjury are presented in Table 2.
Figure 2. Cumulative Moderate-to-Vigorous-Intensity Physical Activity (cMVPA) and Total Health Behavior Inventory (HBI) Score.
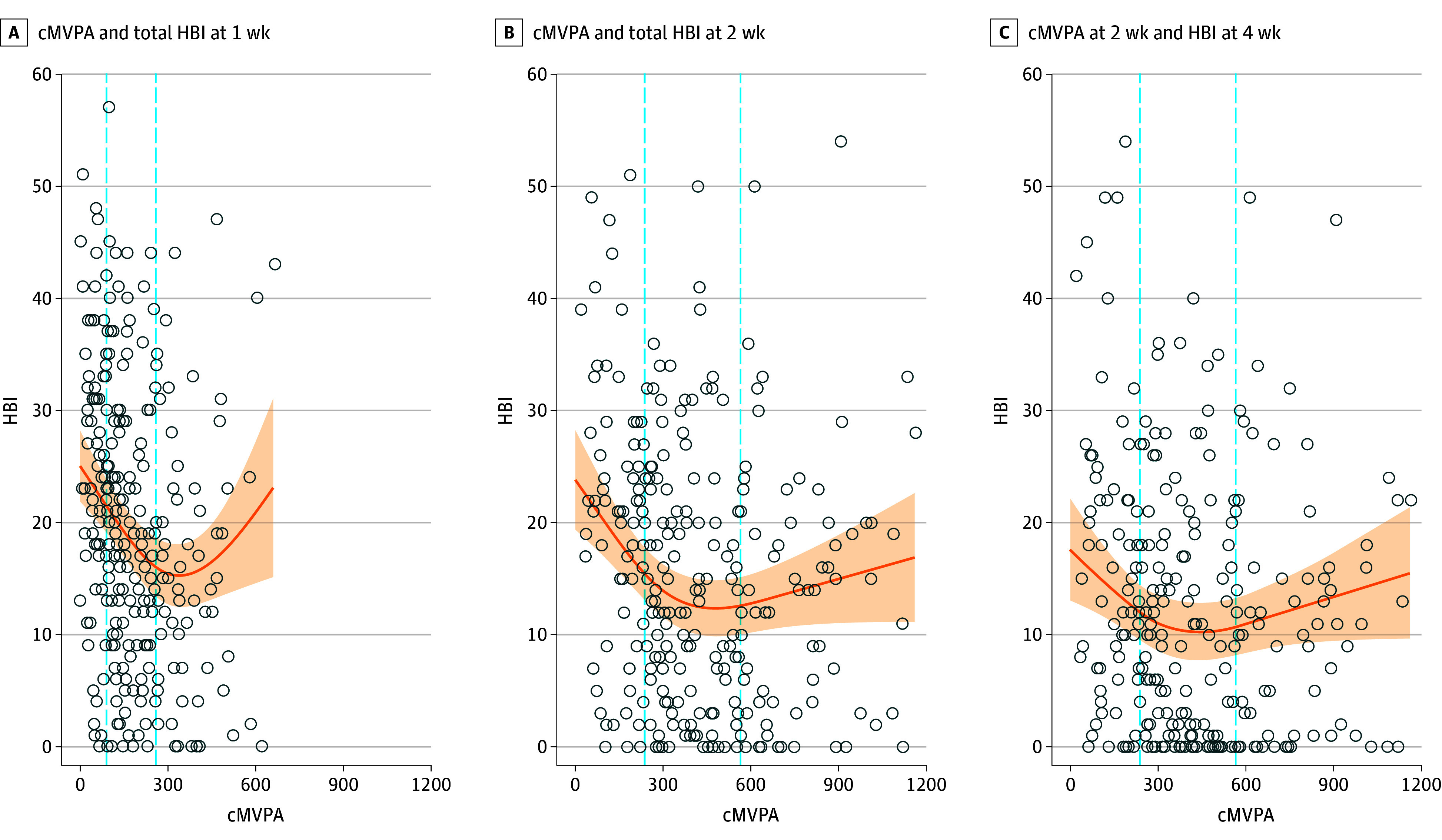
Curves are adjusted for randomized treatment group, age, sex, Predicting Persistent Postconcussive Problems in Pediatrics risk score, mental health and learning disability comorbidity, retrospective HBI score, and emergency department HBI score. Circles represent imputed data of the first imputation. Dashed lines represent the 25th and 75th quantiles of cMVPA. Shaded areas represent the 95% CIs.
Table 2. Cumulative Moderate-to-Vigorous Physical Activity Percentiles at 1 Week and 2 Weeks.
| Week | 10th percentile | 25th percentile | Median | 75th percentile | 90th percentile |
|---|---|---|---|---|---|
| 1 | 45.00 | 90.00 | 151.00 | 258.50 | 381.00 |
| 2a | 125.00 | 237.00 | 366.00 | 565.00 | 797.20 |
The same percentiles were used to estimate the 4 week outcome.
cMVPA and Odds of PSAC at 2 Weeks and 4 Weeks Postinjury
Additionally, 29 participants (10.9%) were reliably symptomatic at 2 weeks postinjury, and 19 participants (7.1%) were reliably symptomatic at 4 weeks postinjury. The P values for nonlinear fit were significant at 2 weeks postinjury (P <.001) and were not significant at 4 weeks postinjury. The effect size of cMVPA on PSAC was significant at 2 weeks postinjury (P = .001) but was not significant at 4 weeks postinjury. The estimated probability of PSAC at the 25th and 75th percentiles of cMVPA was 0.07 (95% CI, 0.03-0.14) vs 0.03 (95% CI, 0.01-0.09) at 2 weeks postinjury and 0.07 (95% CI, 0.03-0.14) vs 0.04 (95% CI, 0.02-0.12) at 4 weeks postinjury. When contrasting cMVPA at the 75th vs 25th percentile at 2 weeks and 4 weeks postinjury, the odds ratio for PSAC was 0.48 (95% CI, 0.24-0.94; P = .03) and 0.65 (95% CI, 0.30-1.37; P = .25), respectively.
cMVPA and Cognitive and Somatic Recovery Trajectory
Given that the interaction of time and cMVPA was not significant, it was removed from the model. Significant nonlinearity was found for both cognitive and somatic models. cMVPA was associated with reduced cognitive (F2, 271.9 = 4.23; P = .016) and somatic symptom burden (F2, 277.8 = 9.15; P < .001) (Figure 3). When comparing the weekly 25th and 75th percentiles of cMVPA for both symptom scores, the slope of the graph was significant for cognitive symptom burden at 1 week postinjury and for somatic symptom burden at 1 week, 2 weeks, and 4 weeks postinjury (eTable 3 in Supplement 1).
Figure 3. Cumulative Moderate-to-Vigorous-Intensity Physical Activity (cMVPA) and Health Behavior Inventory (HBI) Cognitive Score and Somatic Score.
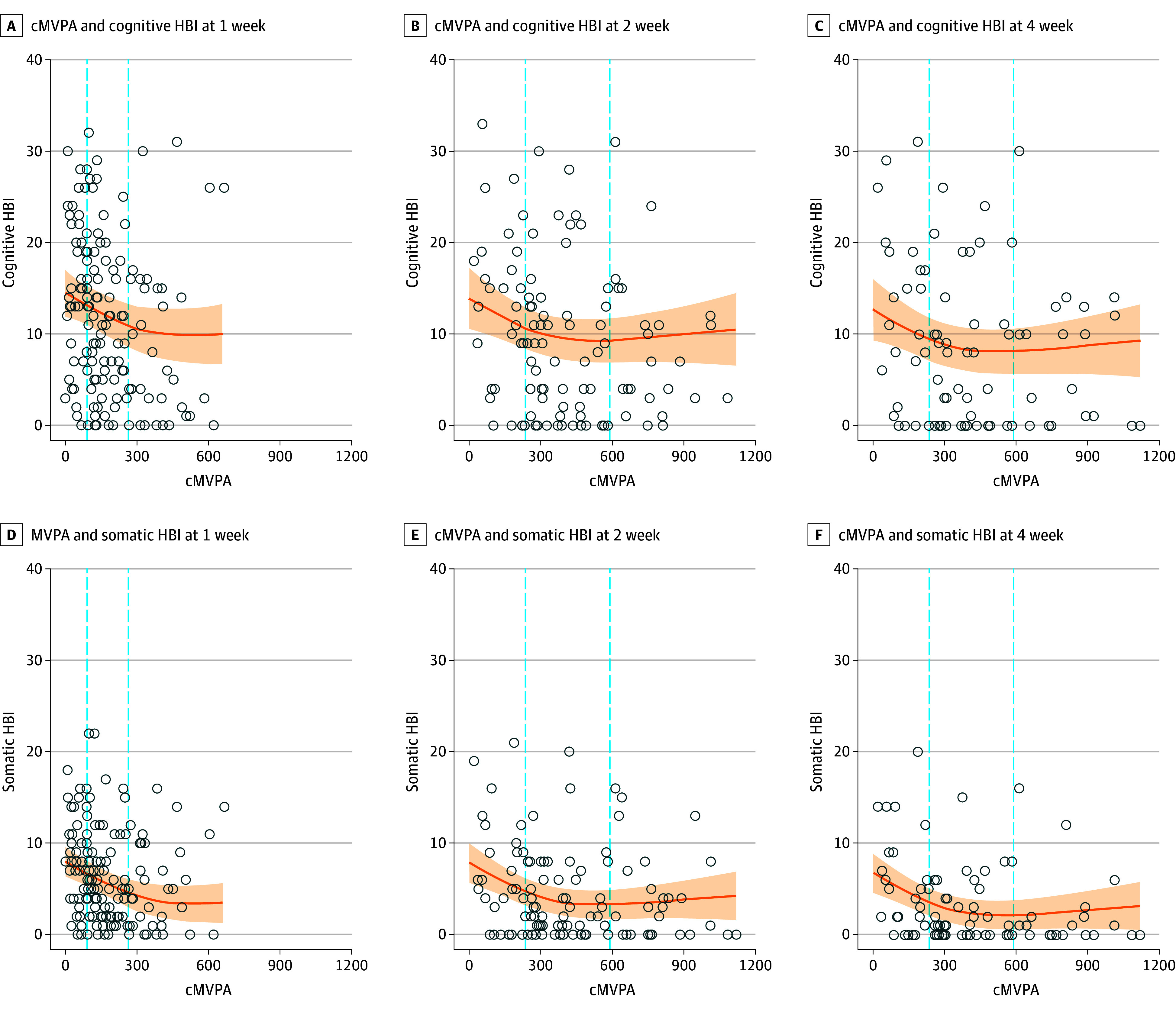
Curves are adjusted for randomized treatment group, age, sex, 5P risk score, mental health and learning disability comorbidity, retrospective HBI score, and emergency department HBI score. Circles represent imputed data of the first imputation. Dashed lines represent the 25th and 75th quantiles of cMVPA. Shaded areas represent the 95% CIs.
Discussion
Among children and adolescents with acute concussions, engaging in higher volumes of MVPA within the first week postinjury (259 vs 90 minutes) or within the first 2 weeks postinjury (565 vs 237 minutes) was associated with lower symptom burden. Beyond these thresholds, no clear benefit in symptom burden was apparent. The cMVPA does not appear to have a greater impact on reducing somatic symptoms vs cognitive symptoms. Higher volumes of cMVPA at 2 weeks postinjury (565 vs 237 minutes) was associated with 48% lower odds of PSAC.
Nationally representative physical activity data from Statistics Canada indicate that the mean daily minutes of MVPA is 65 minutes for children aged 6 to 11 years and 52 minutes for adolescents aged 12 to 17 years, which is much higher than the levels observed in our study population.48 This is not surprising and may be attributed to the injury itself, because adolescents may not have fully returned to their regular activities and may engage in more light or sedentary behaviors after concussion, as demonstrated in our previous research.49 Interestingly, we found that the association between cMVPA and symptom burden appeared to plateau after the 259 minute in the first week and exceeding 375 minutes of cMVPA during the first week may have a negative impact on recovery. The pattern of our data seemed to be consistent with a Goldilocks effect (optimal range), with higher symptom burden at both very low levels and very high levels of MVPA, but this could not be confirmed due to wider confidence intervals beyond the higher thresholds. Our data suggested that cMVPA was associated with reduced symptoms within a threshold and was no longer beneficial past these thresholds. Further investigation with a larger sample size is required to confirm the suspected Goldilocks effect.
A small sample size study on subacute SRC found that more than 30 minutes per day of MVPA between 7 to 14 days postinjury was associated with decreased time to symptom resolution.11 Other groups have found increased physical activity to be associated with faster symptom resolution,9 full resolution of symptoms at 4 weeks,7 faster medical clearance to return-to-play,8 and decreased PSAC risk.13 Our findings aligned with these studies and suggested that an even higher threshold of 259 minutes of cMVPA within the first week is beneficial. Consistent with the existing research,12,13,50 our results indicated that individuals who engaged in a moderate volume of MVPA had significantly lower odds of experiencing PSAC. In a large prospective observational study, where physical activity was based on self-report questionnaire, those who returned to physical activity within 7 days of concussion had a 24.6% risk of PSAC at 4 weeks postinjury compared with a 43.5% risk in those who had not returned to physical activity.12 The risk of PSAC in those who resumed full contact physical activity was 14.5% compared with 43.5% in those who did not resume full-contact physical activity. A recent randomized clinical trial involving adolescents within 10 days of SRC reported a 48% lower risk of PSAC at 4 weeks among those assigned to a prescribed exercise program (20 minutes per day) compared with those assigned to a placebo stretching program.13 Further aligning with previous studies, we found increased cMVPA volume at 2 weeks was associated with 48% lower PSAC odds.
The present study has several strengths, including a relatively large sample and objective evaluation of cMVPA. Unlike studies that relied on questionnaires to assess the volume of, or adherence to, physical activity, our study used accelerometer-based measurement on a 24-hour basis. This research was conducted across 3 Canadian centers on a variety of concussion mechanisms, enhancing the generalizability and external validity of our results. Additionally, we used a standardized definition of PSAC. This standardized approach ensured consistency in identifying and categorizing individuals with PSAC, enhancing the reliability of our results and facilitating comparisons with other studies.51
We would like to emphasize the importance and benefits of gradually returning to routine activities, including physical activity, following a concussion. Several studies have highlighted the positive impacts of prescribed physical activity in managing symptoms. Engaging in physical activity can contribute to the overall well-being of children and adolescents during their recovery process and reduce the odds of PSAC. However, our data also suggested that exceeding 300 minutes in the first week (equivalent to more than 43 minutes of MVPA per day) had no effect on symptoms and might even result in increased symptoms. In this study, some participants who engaged in a high volume of MVPA had no symptom improvement, suggesting that they may not be adequately adjusting their activity levels in response to their symptoms. In contrast to individuals who were avoiding physical activity, those who were overexercising might require a different clinical approach. As outlined in concussion guidelines,39,52 patients should gradually resume physical activity as long as their symptoms remain tolerable. This gradual approach ensures a safe and effective return to physical activity while minimizing the risk of exacerbating symptoms.
Limitations
This study has limitations. While the results provide valuable insights into the association between cMVPA and symptom burden, they do not establish a causal association and are potentially limited by reverse causation. The nature of the study design precludes establishing the directionality of the observed associations. Moreover, children and adolescents with worse symptoms or PSAC may have been less compliant at wearing the accelerometer or in MVPA participation, or conversely those less motivated to wear the accelerometer could differ in both activity levels and recovery. To mitigate this possibility, we adjusted for baseline symptom severity and 5P risk score. Future studies using RCT designs should investigate the association with cMVPA in the first 2 weeks postinjury and concussive symptom resolution.
The crude nature of the cMVPA metric provides no information on the temporality of accumulation during the designated time (1 week or 2 weeks postinjury). Furthermore, we have no information on cMVPA in weeks 3 and 4 postinjury, limiting our understanding of cMVPA variability in the past 2 weeks. Although our sample included participants with various mechanisms of injury, they were all recruited in the ED. This may introduce selection bias, as individuals who sought medical attention may differ from those who did not in terms of injury severity or mechanism of injury. Another limitation was the presence of missing activity monitor data. To address this issue, we used multiple imputation techniques to estimate missing values in participants with sufficient data. The imputation introduces some degree of uncertainty in the analysis, and the accuracy of the imputed values relies on the underlying assumptions made during the imputation process. However, when comparing the imputed data to complete case data, our results are consistent (eFigure in Supplement 1).
Conclusions
In children and adolescents with acute concussion, 259 minutes of cMVPA during the first week postinjury and 565 minutes of cMVPA during the second week postinjury were associated with lower symptom burden. At 2 weeks postinjury, higher cMVPA volume compared with lower volume was associated with 48% reduced odds of PSAC.
eFigure. LOESS Curve of Unadjusted Observed and Imputed Distribution With 95% CI of Cumulative Moderate to Vigorous Physical Activity (cMVPA) and Health and Behavior Inventory (HBI) at 1, 2, and 4 Weeks
eTable 1. Additional Variables Included in the Imputation Model
eTable 2. HBI Scores Through Time
eTable 3. Contrast for Exploratory Analysis
Data Sharing Statement
References
- 1.Maas AIR, Menon DK, Manley GT, et al. ; InTBIR Participants and Investigators . Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21(11):1004-1060. doi: 10.1016/S1474-4422(22)00309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor AM, Nigrovic LE, Saillant ML, et al. Trends in ambulatory care for children with concussion and minor head injury from eastern Massachusetts between 2007 and 2013. J Pediatr. 2015;167(3):738-744. doi: 10.1016/j.jpeds.2015.05.036 [DOI] [PubMed] [Google Scholar]
- 3.Chadwick L, Sharma MJ, Madigan S, Callahan BL, Owen Yeates K. Classification criteria and rates of persistent postconcussive symptoms in children: a systematic review and meta-analysis. J Pediatr. 2022;246:131-137.e2. doi: 10.1016/j.jpeds.2022.03.039 [DOI] [PubMed] [Google Scholar]
- 4.Zemek R, Barrowman N, Freedman SB, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. doi: 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
- 5.Novak Z, Aglipay M, Barrowman N, et al. ; Pediatric Emergency Research Canada Predicting Persistent Postconcussive Problems in Pediatrics (PERC 5P) Concussion Team . Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 2016;170(12):e162900. doi: 10.1001/jamapediatrics.2016.2900 [DOI] [PubMed] [Google Scholar]
- 6.Ledoux AA, Barrowman N, Bijelić V, et al. ; PERC PedCARE Concussion team . Is early activity resumption after paediatric concussion safe and does it reduce symptom burden at 2 weeks post injury: the Pediatric Concussion Assessment of Rest and Exertion (PedCARE) multicentre randomised clinical trial. Br J Sports Med. 2022;56(5):271-278. doi: 10.1136/bjsports-2021-105030 [DOI] [PubMed] [Google Scholar]
- 7.Howell DR, Hunt DL, Aaron SE, Meehan WP III, Tan CO. Influence of aerobic exercise volume on postconcussion symptoms. Am J Sports Med. 2021;49(7):1912-1920. doi: 10.1177/03635465211005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seehusen CN, Wilson JC, Walker GA, Reinking SE, Howell DR. More physical activity after concussion is associated with faster return to play among adolescents. Int J Environ Res Public Health. 2021;18(14):7373. doi: 10.3390/ijerph18147373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173(4):319-325. doi: 10.1001/jamapediatrics.2018.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lishchynsky JT, Rutschmann TD, Toomey CM, et al. The association between moderate and vigorous physical activity and time to medical clearance to return to play following sport-related concussion in youth ice hockey players. Front Neurol. 2019;10:588. doi: 10.3389/fneur.2019.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rademacher JG, Wingerson MJ, Smulligan KL, Little CC, Wilson JC, Howell DR. Early moderate to vigorous physical activity after concussion is associated with faster symptom resolution time. J Sport Rehabil. 2023:32(7):790-796. doi: 10.1123/jsr.2023-0022 [DOI] [PubMed] [Google Scholar]
- 12.Grool AM, Aglipay M, Momoli F, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316(23):2504-2514. doi: 10.1001/jama.2016.17396 [DOI] [PubMed] [Google Scholar]
- 13.Leddy JJ, Master CL, Mannix R, et al. Early targeted heart rate aerobic exercise versus placebo stretching for sport-related concussion in adolescents: a randomised controlled trial. Lancet Child Adolesc Health. 2021;5(11):792-799. doi: 10.1016/S2352-4642(21)00267-4 [DOI] [PubMed] [Google Scholar]
- 14.Baker B, Koch E, Vicari K, Walenta K. Mode and intensity of physical activity during the postacute phase of sport-related concussion: a systematic review. J Sport Rehabil. 2020;30(3):492-500. doi: 10.1123/jsr.2019-0323 [DOI] [PubMed] [Google Scholar]
- 15.Leddy JJ, Wilber CG, Willer BS. Active recovery from concussion. Curr Opin Neurol. 2018;31(6):681-686. doi: 10.1097/WCO.0000000000000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X, Gao B, Wang Z, et al. Therapeutic effect of aerobic exercise for adolescents after mild traumatic brain injury and sport-related concussion: a meta-analysis from randomized controlled trials. World Neurosurg. 2021;146:e22-e29. doi: 10.1016/j.wneu.2020.09.143 [DOI] [PubMed] [Google Scholar]
- 17.Powell C, McCaulley B, Scott Brosky Z, Stephenson T, Hassen-Miller A. The effect of aerobic exercise on adolescent post-concussion: a systematic review and meta-analysis. Int J Sports Phys Ther. 2020;15(5):650-658. doi: 10.26603/ijspt20200650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal A, Kolakowsky-Hayner SA, Ghajar J, Balamane M. The effect of physical exercise after a concussion: a systematic review and meta-analysis. Am J Sports Med. 2018;46(3):743-752. doi: 10.1177/0363546517706137 [DOI] [PubMed] [Google Scholar]
- 19.Langevin P, Frémont P, Fait P, Dubé MO, Bertrand-Charette M, Roy JS. Aerobic exercise for sport-related concussion: a systematic review and meta-analysis. Med Sci Sports Exerc. 2020;52(12):2491-2499. doi: 10.1249/MSS.0000000000002402 [DOI] [PubMed] [Google Scholar]
- 20.Art K, Ridenour C, Durbin S, Bauer M, Hassen-Miller A. The effectiveness of physical therapy interventions for athletes post-concussion: a systematic review. Int J Sports Phys Ther. 2023;18(1):26-38. doi: 10.26603/001c.68071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leddy JJ, Burma JS, Toomey CM, et al. Rest and exercise early after sport-related concussion: a systematic review and meta-analysis. Br J Sports Med. 2023;57(12):762-770. doi: 10.1136/bjsports-2022-106676 [DOI] [PubMed] [Google Scholar]
- 22.Cordingley DM, Cornish SM. Efficacy of aerobic exercise following concussion: a narrative review. Appl Physiol Nutr Metab. 2023;48(1):5-16. doi: 10.1139/apnm-2022-0139 [DOI] [PubMed] [Google Scholar]
- 23.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil. 2016;31(3):215-224. doi: 10.1097/HTR.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 24.Cordingley D, Girardin R, Reimer K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016;25(6):693-702. doi: 10.3171/2016.5.PEDS16139 [DOI] [PubMed] [Google Scholar]
- 25.Bailey C, Meyer J, Briskin S, et al. Multidisciplinary concussion management: a model for outpatient concussion management in the acute and post-acute settings. J Head Trauma Rehabil. 2019;34(6):375-384. doi: 10.1097/HTR.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 26.Kurowski BG, Hugentobler J, Quatman-Yates C, et al. Aerobic exercise for adolescents with prolonged symptoms after mild traumatic brain injury: an exploratory randomized clinical trial. J Head Trauma Rehabil. 2017;32(2):79-89. doi: 10.1097/HTR.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledoux AA, Barrowman NJ, Boutis K, et al. ; Pediatric Emergency Research Canada PedCARE team . Multicentre, randomised clinical trial of paediatric concussion assessment of rest and exertion (PedCARE): a study to determine when to resume physical activities following concussion in children. Br J Sports Med. 2019;53(3):195-195. doi: 10.1136/bjsports-2017-097981 [DOI] [PubMed] [Google Scholar]
- 28.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258. doi: 10.1136/bjsports-2013-092313 [DOI] [PubMed] [Google Scholar]
- 29.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 30.Peterson A, Gabella BA, Johnson J, et al. Multisite medical record review of emergency department visits for unspecified injury of head following the ICD-10-CM coding transition. Inj Prev. 2021;27(S1):i13-i18. doi: 10.1136/injuryprev-2019-043517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guskiewicz KM. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2(1):24-30. doi: 10.1249/00149619-200302000-00006 [DOI] [PubMed] [Google Scholar]
- 32.Ayr LK, Yeates KO, Taylor HG, Browne M. Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J Int Neuropsychol Soc. 2009;15(1):19-30. doi: 10.1017/S1355617708090188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Tang K, Zemek R, et al. ; Pediatric Emergency Research Canada A-CAP Study Team . Factor structure and measurement invariance of post-concussion symptom ratings on the health and behaviour inventory across time, raters, and groups: an A-CAP study. J Int Neuropsychol Soc. 2023;29(4):346-359. doi: 10.1017/S1355617722000340 [DOI] [PubMed] [Google Scholar]
- 34.Davis GA, Purcell L, Schneider KJ, et al. The Child Sport Concussion Assessment Tool 5th Edition (Child SCAT5): background and rationale. Br J Sports Med. 2017;51(11):859-861. doi: 10.1136/bjsports-2017-097492 [DOI] [PubMed] [Google Scholar]
- 35.Broglio SP, Kontos AP, Levin H, et al. National Institute of Neurological Disorders and Stroke and Department of Defense sport-related concussion common data elements version 1.0 recommendations. J Neurotrauma. 2018;35(23):2776-2783. doi: 10.1089/neu.2018.5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien H, Minich NM, Langevin LM, et al. Normative and psychometric characteristics of the health and behavior inventory among children with mild orthopedic injury presenting to the emergency department: Implications for assessing postconcussive symptoms using the Child Sport Concussion Assessment Tool 5th Edition (Child SCAT5). Clin J Sport Med. 2021;31(5):e221-e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed N, Zemek R, Dawson J, et al. Living guideline for pediatric concussion care. 2021. Accessed January 10, 2024. 10.17605/OSF.IO/3VWN9 [DOI]
- 40.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15-26. doi: 10.1136/bjsports-2012-091941 [DOI] [PubMed] [Google Scholar]
- 41.Halstead ME, Walter KD; Council on Sports Medicine and Fitness . Sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597-615. doi: 10.1542/peds.2010-2005 [DOI] [PubMed] [Google Scholar]
- 42.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36(9):1625-1631. [PubMed] [Google Scholar]
- 43.Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for the actical accelerometer. J Sports Sci. 2011;29(8):783-789. doi: 10.1080/02640414.2011.557744 [DOI] [PubMed] [Google Scholar]
- 44.Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. J Phys Act Health. 2011;8(4):587-591. doi: 10.1123/jpah.8.4.587 [DOI] [PubMed] [Google Scholar]
- 45.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681-694. doi: [DOI] [PubMed] [Google Scholar]
- 46.R Core Team . R: A language and environment for statistical computing. Accessed January 16, 2024. http://www.R-project.org/
- 47.van Buuren S, Groothuis-Oudshroon K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 48.Statistics Canada . Average time spent being physically active. Accessed January 10, 2024. 10.25318/1310033901-eng [DOI]
- 49.Ledoux AA, Webster RJ, Clarke AE, et al. Risk of mental health problems in children and youths following concussion. JAMA Netw Open. 2022;5(3):e221235. doi: 10.1001/jamanetworkopen.2022.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krainin BM, Seehusen CN, Smulligan KL, Wingerson MJ, Wilson JC, Howell DR. Symptom and clinical recovery outcomes for pediatric concussion following early physical activity. J Neurosurg Pediatr. 2021;28(6):623-630. doi: 10.3171/2021.6.PEDS21264 [DOI] [PubMed] [Google Scholar]
- 51.Mayer AR, Stephenson DD, Dodd AB, et al. Comparison of methods for classifying persistent post-concussive symptoms in children. J Neurotrauma. 2020;37(13):1504-1511. doi: 10.1089/neu.2019.6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patricios JS, Schneider KJ, Dvorak J, et al. Consensus statement on concussion in sport: the 6th International Conference on Concussion in Sport-Amsterdam, October 2022. Br J Sports Med. 2023;57(11):695-711. doi: 10.1136/bjsports-2023-106898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. LOESS Curve of Unadjusted Observed and Imputed Distribution With 95% CI of Cumulative Moderate to Vigorous Physical Activity (cMVPA) and Health and Behavior Inventory (HBI) at 1, 2, and 4 Weeks
eTable 1. Additional Variables Included in the Imputation Model
eTable 2. HBI Scores Through Time
eTable 3. Contrast for Exploratory Analysis
Data Sharing Statement