Abstract
Isoflavonoids, secondary metabolites derived from the phenylalanine pathway, are predominantly biosynthesized in legumes, especially soybean (Glycine max). They are not only essential for plant responses to biotic and abiotic stresses but also beneficial to human health. In this study, we report that light signaling controls isoflavonoid biosynthesis in soybean. Blue-light photoreceptors (GmCRY1s, GmCRY2s, GmPHOT1s, and GmPHOT2s) and the transcription factors GmSTF1 and GmSTF2 promote isoflavonoid accumulation, whereas the E3 ubiquitin ligase GmCOP1b negatively regulates isoflavonoid biosynthesis. GmPHOT1s and GmPHOT2s stabilize GmSTF1/2, whereas GmCOP1b promotes the degradation of these two proteins in soybean. GmSTF1/2 regulate the expression of approximately 27.9% of the genes involved in soybean isoflavonoid biosynthesis, including GmPAL2.1, GmPAL2.3, and GmUGT2. They also repress the expression of GmBBX4, a negative regulator of isoflavonoid biosynthesis in soybean. In addition, GmBBX4 physically interacts with GmSTF1 and GmSTF2 to inhibit their transcriptional activation activity toward target genes related to isoflavonoid biosynthesis. Thus, GmSTF1/2 and GmBBX4 form a negative feedback loop that acts downstream of photoreceptors in the regulation of isoflavonoid biosynthesis. Our study provides novel insights into the control of isoflavonoid biosynthesis by light signaling in soybean and will contribute to the breeding of soybean cultivars with high isoflavonoid content through genetic and metabolic engineering.
Key words: photoreceptor, light signaling, isoflavonoid, GmSTF, GmBBX4, soybean
GmCRY and GmPHOT photoreceptors promote isoflavonoid accumulation by positively regulating GmSTFs that directly activate a group of genes involved in the isoflavonoid biosynthetic pathway. GmSTF1/2 also form a negative feedback loop with GmBBX4, a negative regulator of isoflavonoid biosynthesis in soybean.
Introduction
Soybean (Glycine max) is a major legume crop grown from temperate to tropical regions worldwide. It provides not only vegetable oil and protein but also a group of bioactive compounds that are beneficial to human health (Graham and Vance, 2003). Isoflavonoids are a class of secondary metabolites that are mainly biosynthesized in legumes (Veitch, 2013). They are essential for responses to biotic and abiotic stress and to the formation of root nodules required for nitrogen fixation (Dixon et al., 2002; Graham et al., 2007; Zanetti et al., 2010; Chu et al., 2017; Kant et al., 2019; Bian et al., 2020; Zhang et al., 2020). In addition, these natural compounds have great nutraceutical and pharmaceutical significance, with anti-osteoporosis, anti-cardiovascular disease, anti-obesity, and anti-cancer effects (Zhou et al., 2019). In current production processes, isoflavonoids are primarily extracted from legumes. Soybean contains about 100 times more isoflavonoids than other legumes (Yu et al., 2003) and is therefore an important source for isoflavonoid extraction and production.
Isoflavonoids are synthesized by a complex biochemical process. Twelve isoflavonoids, namely daidzein, genistein, glycitein, daidzin, genistin, glycitin, 6″-O-acetyldaidzin, 6″-O-acetylgenistin, 6″-O-acetylglycitin, 6″-O-malonyldaidzin, 6″-O-malonylgenistin, and 6″-O-malonylglycitin, have been identified and characterized in soybean (Zeng et al., 2009; Li and Zhang. 2017). Isoflavonoid biosynthesis is regulated by the phenylpropanoid pathway, through which soybean produces naringenin and liquiritigenin. The CYP450 enzyme isoflavone synthase (IFS) catalyzes the hydroxylation of these two flavanones to produce daidzein, genistein, and glycitein. These three substrates are acted upon by uridine diphosphate glycosyltransferase (UGT) and malonyltransferase, leading to production of nine additional isoflavonoid compounds (Zhang et al., 2020). This process is likely controlled by various regulators in response to diverse environmental factors (Li et al., 2021; Yin et al., 2022a, 2022b). According to the SoyBase database (http://www.soybase.org), more than 297 quantitative trait loci (QTLs) related to isoflavonoid content have been identified in soybean. In addition, the transcription factors GmMYB29, GmMYB39, GmMYB100, GmMYB133, GmMYB176, GmbZIP5, and GmZFP7 negatively or positively regulate isoflavonoid accumulation in soybean (Liu et al., 2013; Yan et al., 2015; Chu et al., 2017; Bian et al., 2018; Anguraj Vadivel et al., 2021; Feng et al., 2023).
The biosynthesis and accumulation of secondary metabolites are strongly affected by various environmental factors, including light signals (Fuglevand et al., 1996; Araguirang and Richter, 2022). Plants perceive diverse wavelength-specific light signals using at least five classes of photoreceptors. Phytochromes (phys) sense red and far-red light; cryptochromes (CRYs), phototropins (PHOTs), and ZEITLUPE family members (ZTL, FKF1, and LKP2) absorb ultraviolet (UV)-A and blue light; and UV RESISTANCE LOCUS 8 (UVR8) perceives UV-B light (Galvao and Fankhauser, 2015; Paik and Huq, 2019; Cheng et al., 2021). These photoreceptors are activated by light signals and initiate a set of molecular events that include conformational changes, nucleocytoplasmic partitioning, phosphorylation, ubiquitination, and large-scale transcriptional reprogramming in plant cells (Paik and Huq, 2019; Cheng et al., 2021; Podolec et al., 2021). Light-activated photoreceptors transduce light signals downstream predominantly via PHYTOCHROME INTERACTING FACTORs (PIFs) and CONSTITUTIVELY PHOTOMORPHOGENIC/DE-ETIOLATED/FUSCA (COP/DET/FUS) signaling hubs, both of which are highly active and promote skotomorphogenesis (etiolation) in the dark (Cheng et al., 2021). Upon light irradiation, phys interact with PIFs and trigger phosphorylation, ubiquitination, and subsequent degradation of PIFs within minutes. CRYs inhibit the transcription and accumulation of PIF4 and PIF5 (Ma et al., 2016; Pedmale et al., 2016; Zhai et al., 2020). Concurrently, photoexcited phys and CRYs disrupt the formation of the COP1–SUPPRESSOR OF PHYA-105 (SPA) complex, thereby resulting in suppression of COP1–SPA activity. COP1 is also translocated from the nucleus to the cytoplasm under prolonged light conditions (Hoecker, 2017; Han et al., 2020). Light- and photoreceptor-mediated inactivation of COP1 enables the rapid accumulation of its substrates such as ELONGATED HYPOCOTYL 5 (HY5) and B-BOX PROTEINs (BBXs) that coordinately regulate the expression of one-third of the genes in the plant genome (Song et al., 2020; Xu, 2020). The light signal transduction pathway, which consists of photoreceptors, E3 ligases, and transcription factors, enables appropriate physiological and developmental responses of plants to changing light signals throughout their life cycle (Xu, 2020; Cheng et al., 2021).
Light signaling controls the biosynthesis of numerous secondary metabolites in diverse plant species, such as anthocyanins in Arabidopsis, pear, and apple (Bai et al., 2019a, 2019b; Xing et al., 2023), carotenoids in tomato (Xiong et al., 2019), and artemisinin in Artemisia annua (Fu et al., 2021; Liu et al., 2022). The molecular mechanisms that underlie the control of isoflavonoid biosynthesis by light signals in soybean remain unclear. Here, we report that photoreceptors, E3 ligases, and transcription factors work coordinately to regulate isoflavonoid biosynthesis in soybean. The GmCRY1, GmCRY2, GmPHOT1, and GmPHOT2 photoreceptors positively regulate GmSTFs and isoflavonoid biosynthesis. GmSTF1 and GmSTF2 regulate the transcription of 27.9% of the genes involved in the isoflavonoid biosynthetic pathway to promote isoflavonoid accumulation in soybean. GmSTF1 and GmSTF2 directly associate with DNA cis elements in the promoter regions of GmPAL2.1, GmPAL2.3, and GmUGT2 to activate their transcription. In addition, GmSTF1 and GmSTF2 transcriptionally repress GmBBX4, whose gene product interacts with GmSTF1 and GmSTF2 and inhibits their transcriptional activation activity toward their target genes, thereby negatively regulating isoflavonoid biosynthesis in soybean. Taken together, our results reveal the molecular framework that underlies the regulation of isoflavonoid biosynthesis by the light signal transduction pathway in soybean.
Results
Light signaling is functionally conserved in soybean
Soybean is a paleopolyploid that has undergone two rounds of whole-genome duplication at ∼56.5 million and ∼13 million years ago, resulting in nearly 75% of its genes being present in multiple copies across the genome (Schmutz et al., 2010; Liu et al., 2020). Bioinformatics analysis revealed that the soybean genome contains three copies of GmPHOT1 (GmPHOT1a, GmPHOT1b, and GmPHOT1c) and two copies of GmPHOT2 (GmPHOT2a and GmPHOT2b), GmCOP1 (GmCOP1a and GmCOP1b), and HY5 (GmSTF1 and GmSTF2). To investigate whether light signaling is functionally conserved in soybean, we produced gmphot1s-tm and gmphot2s-dm soybean mutants using CRISPR–Cas9 techniques (Supplemental Figure 1). GmCRY1s and GmCRY2s have been shown to promote light-inhibited soybean seedling development (Lyu et al., 2021). The hypocotyl length and apical hook angles of gmphot1s-tm and gmphot2s-dm were indistinguishable from those of Wm82 (Supplemental Figure 2A–2C), consistent with previous studies showing that PHOT1 and PHOT2 do not control hypocotyl growth in Arabidopsis (Christie, 2007). gmstf1 and gmstf2 single-mutant seedlings displayed hypocotyl phenotypes similar to that of Wm82, whereas gmstfs-dm soybean seedlings exhibited significantly elongated hypocotyls (Supplemental Figure 2D and 2E), suggesting that GmSTF1 and GmSTF2 function redundantly. The apical hook angles of gmstf1, gmstf2, and gmstfs-dm mutant seedlings were comparable to that of Wm82 (Supplemental Figure 2D and 2F). Although the hypocotyl length of gmcop1a and gmcop1b was similar to that of Wm82, their apical hook angles were significantly larger than those of Wm82 grown in darkness for 4 days (Supplemental Figure 2G–2I). These results indicate that GmSTF1/2 promote photomorphogenesis and that GmCOP1a/b act as negative regulators of light signaling. Furthermore, gmpho1s-tm, gmphot2s-dm, gmcry2s-tm, gmcop1a, and gmcop1b accumulated comparable levels of chlorophyll a, chlorophyll b, and total chlorophyll, whereas gmcry1s-qm and gmstfs-dm had significantly lower chlorophyll content than Wm82 when grown under long-day (LD) conditions for 15 days (Supplemental Figure 3). Yeast two-hybrid assays showed that GmCOP1a interacted with GmSTF2 and that GmCOP1b interacted with GmSTF1 (Supplemental Figure 4A). Firefly luciferase complementation imaging (LCI) assays showed that luciferase (LUC) signals were detectable when GmSTF1-LUCN and LUCC-GmCOP1b or GmSTF2-LUCN and LUCC-GmCOP1a were co-expressed in Nicotiana benthamiana leaves incubated in darkness or continuous light for 3 days, suggesting that GmCOP1a/b could interact with GmSTF1 and GmSTF2 in both the dark and light (Supplemental Figure 4B and 4C). Co-immunoprecipitation (co-IP) assays showed that GmCOP1b-FLAG or GmCOP1a-FLAG co-immunoprecipitated with HA-GmSTF1 or HA-GmSTF2, respectively, when transiently co-expressed in N. benthamiana leaves (Supplemental Figure 4D and 4E). Together, these data indicate that light signaling is likely genetically and molecularly conserved in soybean.
Photoreceptors and GmCOP1b regulate isoflavonoid accumulation in soybean
To examine whether photoreceptors affect isoflavonoid biosynthesis, we measured the isoflavonoid content in dry seeds of gmcry1s-qm, gmcry2s-tm, gmphot1s-tm, and gmphot2s-dm. Total isoflavonoid content was significantly lower in gmcry1s-qm, gmcry2s-tm, gmphot1s-tm, and gmphot2s-dm mutant soybean seeds than in those of the corresponding wild type (TL1 or Wm82) (Figure 1A and 1B). The contents of daidzein, genistein, daidzin, and genistin, but not glycitin, were significantly lower in gmcry1s-qm and gmcry2s-tm seeds than in TL1 seeds (Figure 1C and 1D). The contents of daidzin and genistin were significantly lower in gmphot1s-tm and gmphot2s-dm seeds than in Wm82 seeds, whereas the contents of daidzein and glycitin were comparable among gmphot1s-tm, gmphot2s-dm, and Wm82 seeds. The genistein content was higher in gmphot1s-tm but unchanged in gmphot2-dm (Figure 1E and 1F). These data suggest that GmCRY1, GmCRY2, GmPHOT1, and GmPHOT2 photoreceptors promote isoflavonoid accumulation in soybean seeds.
Figure 1.
Isoflavonoid contents of gmcry1s-qm, gmcry2s-tm, gmphot1s-tm, gmphot2s-dm, gmcop1a, and gmcop1b soybean seeds.
(A and B) Total isoflavonoid contents in dry seeds of TL1, gmcry1s-qm, gmcry2s-tm, Wm82, gmphot1s-tm, and gmphot2s-dm soybean.
(C–F) Genistein, daidzein, genistin, daidzin, and glycitin contents in dry seeds of TL1, gmcry1s-qm, and gmcry2s-tm soybean.
(G–I) Total isoflavonoid, glycitin, genistein, daidzein, 6″-O-malonylglycitin, 6″-O-malonyldaidzin, and 6″-O-malonylgenistin contents in dry seeds of Wm82, gmcop1a, and gmcop1b soybean.
(J and K) Transcript levels of GmSTF1 and GmSTF2 in Wm82, gmphot1s-tm, and gmphot2s-dm as determined by qRT–PCR. Wm82, gmphot1s-tm, and gmphot2s-dm were grown under LD conditions (14-h light/10-h dark) at 25°C for 15 days, after which the leaves were collected for RNA extraction and qRT–PCR analysis. Asterisks indicate significant differences (∗P < 0.05) determined by two-tailed Student’s t-test.
(L and M) Immunoblot analysis showing the abundance of GmSTF1/2 in Wm82, gmphot1s-tm, gmphot2s-dm, gmcop1a, and gmcop1b soybean. Wm82 and various soybean mutants were grown under LD conditions (14-h light/10-h dark) at 25°C for 15 days, after which leaves were collected at ZT10 for immunoblot analysis. Arrowheads indicate the specific GmSTF1/2 proteins. gmstfs-dm was used as the negative control. Actin was used as the loading control.
In (A)–(I), data are means ± SE; n ≥ 5. Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test. ns, not significant. The content (μg/g) indicates the amount of specific isoflavones in dry seeds.
The E3 ligase COP1 acts downstream of photoreceptors in the light signal transduction pathway (Hoecker, 2017; Han et al., 2020); we therefore examined whether GmCOP1a and GmCOP1b affected isoflavonoid biosynthesis. Isoflavonoid accumulation was comparable between gmcop1a and Wm82 but was significantly higher in gmcop1b than in Wm82 (Figure 1G). Glycitin, daidzin, genistin, 6″-O-malonylglycitin, 6″-O-malonyldaidzin, and 6″-O-malonylgenistin contents were significantly higher in gmcop1b than in Wm82, but their contents were unaltered in gmcop1a seeds (Figure 1H and 1I), suggesting that GmCOP1b, but not GmCOP1a, inhibits isoflavonoid accumulation in soybean.
Photoreceptors and GmCOP1b control GmSTF accumulation in soybean
Photoreceptors and COP1 precisely control the abundance of HY5 to regulate various light-dependent aspects of plant development (Gangappa and Botto, 2016). UV-A and blue-light photoreceptors GmCRY1s and GmCRY2s have been shown to positively regulate GmSTF1 and GmSTF2, orthologs of Arabidopsis HY5 (Lyu et al., 2021; Ji et al., 2022). We therefore asked whether GmPHOT1s and GmPHOT2s regulated GmSTF1 and GmSTF2 at the transcript and protein levels. Expression of GmSTF1 and GmSTF2 was slightly reduced in gmphot1s-tm but not in gmphot2s-dm (Figure 1J and 1K). GmSTF protein levels were clearly lower in gmphot1s-tm and gmphot2s-dm (Figure 1L), indicating that GmPHOT1s and GmPHOT2s promote GmSTF accumulation in soybean. gmcop1b, but not gmcop1a, showed increased accumulation of GmSTF proteins in the light (Figure 1M). GmSTF protein levels were clearly higher in gmcop1a and gmcop1b than in Wm82 after dark treatment for 2 days (Supplemental Figure 5). These data suggest that GmCOP1b, but not GmCOP1a, negatively regulates GmSTF abundance in the light and that both GmCOP1a and GmCOP1b promote the degradation of GmSTFs in the dark.
GmSTF1 and GmSTF2 promote isoflavonoid biosynthesis in soybean
Orthologs of GmSTF1 and GmSTF2 positively regulate flavonoid biosynthesis in plants such as Arabidopsis, tomato, pear, and apple (Gangappa and Botto, 2016; Liu et al., 2018; Bai et al., 2019a, 2019b; Xing et al., 2023). We therefore measured the isoflavonoid content of gmstfs-dm soybean seeds. The total isoflavonoid content was significantly lower in gmstfs-dm mutant soybean seeds but increased in GmSTF1-YFP and GmSTF2-YFP transgenic soybean seeds (Figure 2A and 2B). The contents of 6″-O-malonylglycitin were comparable in gmstfs-dm and Wm82 seeds, whereas those of glycitin, daidzin, genistin, 6″-O-malonyldaidzin, and 6″-O-malonylgenistin were lower in gmstfs-dm seeds (Figure 2C and 2D). The content of daidzin was comparable in GmSTF1-YFP and TL1 seeds but was significantly higher in GmSTF2-YFP seeds. Both GmSTF1-YFP and GmSTF2-YFP transgenic soybean seeds showed a significant increase in genistin, 6″-O-malonyldaidzin, and 6″-O-malonylgenistin (Figure 2E and 2F). Together, these data suggest that GmSTF1 and GmSTF2 promote isoflavonoid biosynthesis and accumulation in soybean.
Figure 2.
GmSTF1 and GmSTF2 promote isoflavonoid biosynthesis in soybean.
(A and B) Total isoflavonoid contents in dry seeds of Wm82 and gmstfs-dm, TL1, GmSTF1-YFP, and GmSTF2-YFP soybean.
(C and D) Contents of glycitin, daidzin, genistin, 6″-O-malonyldaidzin, 6″-O-malonylgenistin, and 6″-O-malonylglycitin in dry seeds of Wm82 and gmstfs-dm soybean.
(E and F) Contents of glycitin, daidzin, genistin, 6″-O-malonyldaidzin, 6″-O-malonylgenistin, and 6″-O-malonylglycitin in dry seeds of TL1, GmSTF1-YFP, and GmSTF2-YFP soybean. In (A)–(F), error bars represent SE (n ≥ 6). Asterisks indicate significant differences (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) determined by two-tailed Student’s t-test. Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test. The content (μg/g) indicates the amount of specific isoflavones in dry seeds.
(G) Volcano plots showing differentially expressed genes in Wm82 and gmstfs-dm seedlings grown under LD (14-h light/10-h dark) conditions at 25°C for 15 days. Blue dots indicate significantly downregulated genes in gmstfs-dm. Red dots indicate significantly upregulated genes in gmstfs-dm.
(H) Functional category enrichment of the genes regulated in gmstfs-dm vs. Wm82.
(I) Venn diagram showing the overlap between sets of differentially expressed genes and genes related to the isoflavonoid biosynthetic pathway.
(J) Schematic diagram showing the isoflavonoid biosynthetic pathway in soybean. The 29 GmSTF1- and GmSTF2-regulated genes related to isoflavonoid biosynthesis are listed on the left and right sides.
GmSTF1 and GmSTF2 control the expression of genes involved in isoflavonoid biosynthesis
To investigate the molecular mechanism by which GmSTF1 and GmSTF2 regulate isoflavonoid biosynthesis, we performed RNA sequencing (RNA-seq) experiments using Wm82 and the gmstfs-dm mutant grown under LD conditions for 15 days. Ninety-six percent of the sequenced reads from each sample could be uniquely mapped to the Wm82 reference genome (Supplemental Table 1). Pearson correlation analysis showed highly correlated gene expression between biological replicates from each condition (Supplemental Table 2). Our RNA-seq analysis revealed that GmSTF1 and GmSTF2 regulated the expression of approximately 5150 genes in soybean (Figure 2G and Supplemental Table 3), consistent with previous studies showing that HY5 mediates the expression of over 3000 genes in Arabidopsis (Lee et al., 2007; Zhang et al., 2011). We performed qRT–PCR analyses to validate the expression of eight selected differentially expressed genes (Supplemental Figure 6). Gene ontology analysis showed that a group of GmSTF1- and GmSTF2-regulated genes was involved in regulation of flavone metabolic process (Figure 2H). Among these differentially expressed genes, 29 out of 104 genes related to isoflavonoid biosynthesis were regulated by GmSTF1 and GmSTF2 (Kanehisa and Goto, 2000; Dhaubhadel et al., 2008; Wang, 2011; Zhang and Liu, 2015; Yin et al., 2017; Zhang et al., 2020; Figure 2I and 2J; Supplemental Table 4). These genes encode various specific enzymes that catalyze the formation of distinct compounds during the isoflavonoid biosynthetic process (Figure 2J). These data suggest that GmSTF1 and GmSTF2 likely control the transcription of approximately 29 genes involved in isoflavonoid biosynthesis to promote isoflavonoid accumulation in soybean.
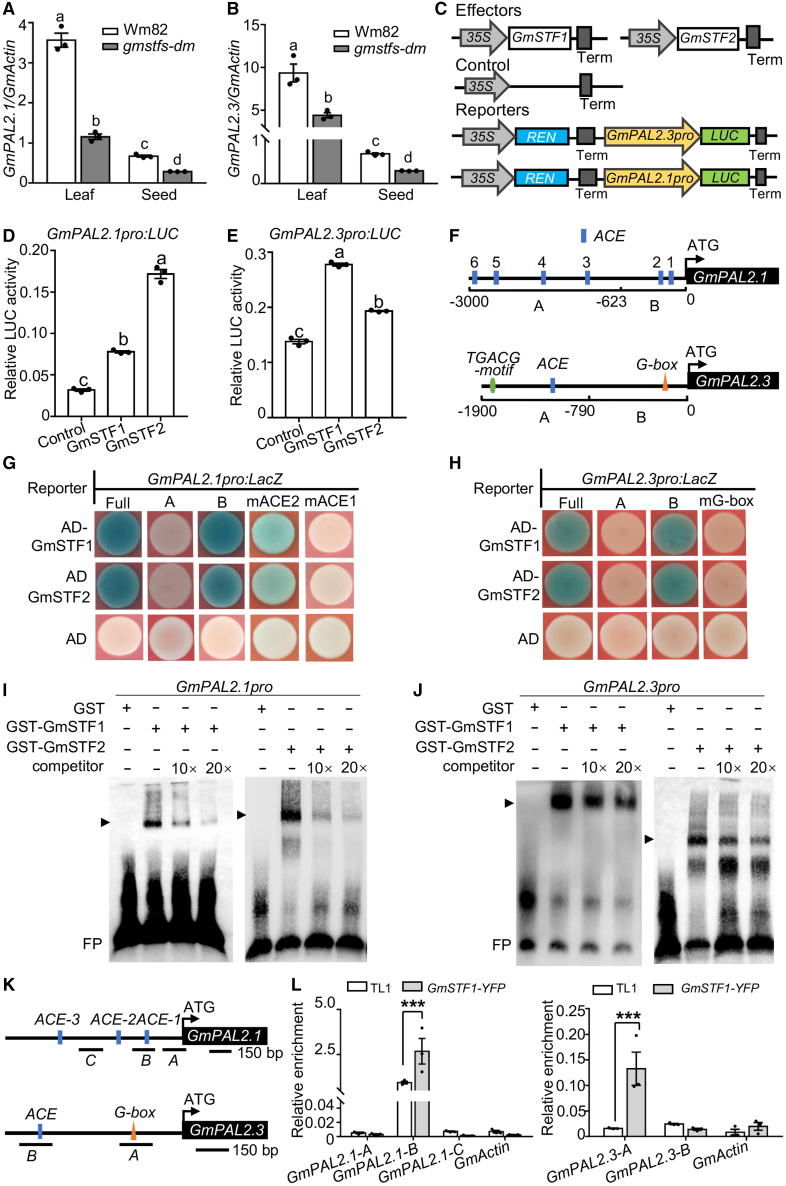
GmSTF1 and GmSTF2 bind to the promoters of GmPAL2.1 and GmPAL2.3 to activate their transcription
Phenylalanine ammonia lyases catalyze the non-oxidative deamination of L-phenylalanine to trans-cinnamate and ammonia (Zhang and Liu, 2015). GmSTF1 and GmSTF2 are bZIP-type transcription factors that directly bind to the promoters of target genes to regulate their transcription. Expression of GmPAL2.1 and GmPAL2.3 was significantly reduced in leaves and seeds of gmstfs-dm soybean (Figure 3A and 3B), indicating that GmSTF1 and GmSTF2 upregulate the transcription of GmPAL2.1 and GmPAL2.3 in soybean. Consistent with this result, GmSTF1 and GmSTF2 activated GmPAL2.1pro:LUC and GmPAL2.3pro:LUC reporters when transiently co-expressed in N. benthamiana leaves (Figure 3C–3E). Yeast one-hybrid assays showed that GmSTF1 and GmSTF2 activated GmPAL2.1pro:LacZ and GmPAL2.3pro:LacZ reporters (Figure 3F–3H). We next divided GmPAL2.1pro and GmPAL2.3pro into two portions (A and B; Figure 3F) and performed additional yeast one-hybrid assays. GmSTF1 and GmSTF2 activated GmPAL2.1pro-B:LacZ and GmPAL2.3pro-B:LacZ, but not GmPAL2.1pro-A:LacZ and GmPAL2.3pro-A:LacZ (Figure 3G and 3H), suggesting that the GmSTF1 and GmSTF2 binding sites reside within the GmPAL2.1pro-B and GmPAL2.3pro-B regions. GmPAL2.1pro-B contains two ACGT-containing cis elements (ACEs), and GmPAL2.3pro-B contains one G-box motif (Figure 3G and 3H). Activation by GmSTF1 and GmSTF2 was completely abolished when the second ACE motif, but not the first one, was mutated in GmPAL2.1pro-B (Figure 3G and Supplemental Figure 7). GmSTF1 and GmSTF2 were unable to activate a GmPAL2.3pro-B-mG-box:LacZ reporter carrying a mutated G-box (Figure 3H and Supplemental Figure 7). These data suggest that the second ACE within GmPAL2.1pro and the G-box within GmPAL2.3pro are required for GmSTF1 and GmSTF2 binding. To verify these results, we performed an in vitro electrophoretic mobility shift assay (EMSA). Recombinant glutathione S-transferase (GST)-GmSTF1 and GST-GmSTF2 were able to bind to GmPAL2.1pro or GmPAL2.3pro DNA subfragments containing an intact ACE or G-box motif, respectively (Figure 3I and 3J). As the amount of non-biotin-labeled DNA probe (competitor) increased, the binding of GmSTF1 and GmSTF2 to the biotin-labeled probe decreased (Figure 3I and 3J). In addition, chromatin immunoprecipitation (ChIP)–qPCR analysis showed that GmSTF1 associated with GmPAL2.1pro and GmPAL2.3pro regions in soybean (Figure 3K–3L). Together, these data indicate that GmSTF1 and GmSTF2 bind directly to the promoters of GmPAL2.1 and GmPAL2.3 to upregulate their expression.
Figure 3.
GmSTF1 and GmSTF2 bind to the promoters of GmPAL2.1 and GmPAL2.3 to activate their transcription.
(A and B) Transcript levels of GmPAL2.1 and GmPAL2.3 in Wm82 and gmstfs-dm leaves and immature seeds grown under LD conditions (14-h light/10-h dark) at 25°C for 80 days. Error bars represent SE (n = 3). Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test.
(C) Schematic representation of various constructs used for the transient transfection assay in Nicotiana benthamiana leaves.
(D and E) Bar graphs showing the activation of GmPAL2.1pro:LUC and GmPAL2.3:pro:LUC reporters by GmSTF1 and GmSTF2. Error bars represent SE (n = 3). Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test.
(F) Schematic representation of the GmPAL2.1 and GmPAL2.3 promoters, showing the locations of the ACE and G-box motifs. The numbers represent the locations of the GmPAL2.1 and GmPAL2.3 promoter regions relative to the translation start site (referred to as position +1).
(G and H) Yeast one-hybrid assays showing that GmSTF1 and GmSTF2 activate GmPAL2.1pro:LacZ and GmPAL2.3pro:LacZ reporters.
(I and J) EMSA showing that GmSTF1 and GmSTF2 bind to GmPAL2.1 and GmPAL2.3 promoter subfragments in vitro. “−” indicates the absence of the corresponding probes or proteins. For GST, “+” indicates that 0.16 pmol is present; for GST-STF1 and GST-STF2, “+” indicates that 0.1 pmol is present; for GmPAL2.1pro and GmPAL2.3pro probes, “+” indicates that 20 fmol is present. Competitor refers to the non-biotin-labeled GmPAL2.1 or GmPAL2.3 probe. FP, free probe. The arrowheads indicate protein–DNA complexes.
(K) Promoter structures of GmPAL2.1 and GmPAL2.3, showing the positions of fragments used for chromatin immunoprecipitation (ChIP)–qPCR.
(L) ChIP–qPCR assays showing that GmSTF1 associates with the GmPAL2.1 and GmPAL2.3 promoters in vivo. Thirty-day-old TL1 and GmSTF1-YFP soybean leaves were used for ChIP assays. Chromatin fragments were immunoprecipitated using GFP-Trap antibodies. The GmActin gene was used as the negative control. Error bars represent SD (n = 3). Asterisks indicate significant differences (∗∗∗P < 0.001) determined by two-tailed Student’s t-test. ns, not significant.
GmSTF1 and GmSTF2 activate GmUGT2
GmUGTs catalyze the conversion of daidzein, genistein, and glycitein to daidzin, glycitin, and genistin in soybean (Dhaubhadel et al., 2008; Yin et al., 2017). Our RNA-seq data revealed that GmSTF1 and GmSTF2 regulate the expression of 18 GmUGT genes (Supplemental Tables 2–4), implying that GmSTF1 and GmSTF2 have a profound impact on the control of GmUGT expression and GmUGT-mediated biological processes in soybean. GmUGT2 expression was significantly reduced in gmstfs-dm leaves and seeds (Figure 4A), indicating that GmSTF1 and GmSTF2 upregulate GmUGT2 transcription. GmSTF1 and GmSTF2 activated the GmUGT2pro:LUC reporter when transiently co-expressed in N. benthamiana leaves (Figure 4B). In addition, GmSTF1 and GmSTF2 activated the GmUGT2pro:LacZ reporter in yeast cells. Yeast one-hybrid assays revealed that GmUGT2pro-A:LacZ, but not GmUGT2pro-B:LacZ, could be activated by GmSTF1 and GmSTF2 (Figure 4C and 4D). Furthermore, recombinant GST-GmSTF1 and GST-GmSTF2, but not GST, could bind to DNA subfragments of the GmUGT2 promoter in vitro. The binding of GST-GmSTF1 and GST-GmSTF2 to the biotin-labeled probe decreased when the amount of non-biotin-labeled probe (competitor) was increased in the reactions (Figure 4E). ChIP–qPCR assays showed that GmSTF1 was significantly enriched at the promoter regions of GmUGT2 (Figure 4F). These data suggest that GmSTF1 and GmSTF2 directly associate with the promoter regions of GmUGT2 to activate its expression at the transcriptional level.
Figure 4.
GmSTF1 and GmSTF2 activate the expression of GmUTG2.
(A) Transcript levels of GmUGT2 in Wm82 and gmstfs-dm leaves and immature seeds grown in LD conditions (14-h light/10-h dark) at 25°C for 80 days. Error bars represent SE (n = 3). Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test.
(B) Bar graphs showing the activation of the GmUGT2pro:LUC reporter by GmSTF1 and GmSTF2. Error bars represent SE (n = 3). Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test.
(C) Schematic representation of the GmUGT2 promoter. The numbers represent the locations of the GmPAL2.1 and GmPAL2.3 promoter regions relative to the translation start site (referred to as position +1).
(D) Yeast one-hybrid assays showing that GmSTF1 and GmSTF2 activate the GmUGT2pro:LacZ reporter.
(E) EMSA showing that GmSTF1 and GmSTF2 bind to GmUGT2 promoter subfragments in vitro. “−” indicates the absence of corresponding probes or proteins. For GST, “+” indicates that 0.16 pmol is present; for GST-STF1 and GST-STF2, “+” indicates that 0.1 pmol is present; for the GmUGT2pro probe, “+” indicates that 20 fmol is present. Competitor indicates the non-biotin-labeled GmUGT2pro probe. FP, free probe. The arrowheads indicate protein–DNA complexes.
(F) ChIP–qPCR assays showing that GmSTF1 associates with the GmUGT2 promoters in vivo. Thirty-day-old TL1 and GmSTF1-YFP soybean leaves were used for the ChIP assays. Chromatin fragments were immunoprecipitated using GFP-Trap antibodies. The GmActin gene was used as the negative control. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗P < 0.05) determined by two-tailed Student’s t-test.
GmSTF1 and GmSTF2 interact with GmBBX4
We next performed yeast two-hybrid screening using BD-STF1 as bait and found that 20 proteins could likely interact with GmSTF1 in yeast cells (Supplemental Table 5). Of these, GmBBX4, which is orthologous to Arabidopsis BBX4, contains two B-box domains at the N terminus and one CCT domain in the C-terminal region. BBX4 is a positive regulator of red-light signaling and anthocyanin biosynthesis in Arabidopsis (Datta et al., 2006; Heng et al., 2019). We performed additional yeast two-hybrid assays and found that both GmSTF1 and GmSTF2 interacted with GmBBX4 in yeast cells (Figure 5A). LCI assays showed that transient co-expression of GmBBX4-LUCN and LUCc-GmSTF1 or GmBBX4-LUCN and LUCc-GmSTF2 produced LUC signals in N. benthamiana leaves. The respective negative control pairs did not produce detectable LUC signals in the same experimental setting (Figure 5B and 5C). We next performed co-IP assays to verify these results. YFP-GmBBX4, but not YFP alone, co-immunoprecipitated with HA-GmSTF1 or HA-GmSTF2 when transiently co-expressed in N. benthamiana leaves (Figure 5D). Together, these data suggest that GmSTF1 and GmSTF2 interact with GmBBX4 in plant cells.
Figure 5.
GmBBX4 interacts with GmSTF1 and GmSTF2 and inhibits their transcriptional activation activity toward their target gene.
(A) Yeast two-hybrid assays showing that GmBBX4 interacts with GmSTF1 and GmSTF2 proteins.
(B and C) LCI assays showing that GmBBX4 interacts with GmSTF1 and GmSTF2 in N. benthamiana leaves. Full-length GmBBX4, GmSTF1, and GmSTF2 were fused to the split N- or C-terminal fragments (LUCN or LUCC) of LUC. LUCN and LUCC were used as negative controls.
(D) Co-IP analysis showing that YFP-GmBBX4 interacts with HA-GmSTF1 or HA-GmSTF2. Total protein was extracted from wild tobacco leaves transiently expressing 35S:YFP-GmBBX4 alone or together with 35S:HA-GmSTF1 or 35S:HA-GmSTF2. The immunoprecipitates were detected using anti-GFP and anti-HA antibodies.
(E and F) Yeast one-hybrid assays showing that GmBBX4 represses transcriptional activation of the GmPAL2.3pro:LacZ reporter by GmSTF1 or GmSTF2. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗∗∗P < 0.001) determined by two-tailed Student’s t-test.
(G and H) Transient transfection assays showing that GmBBX4 represses the transcriptional activation of the GmPAL2.3pro:LUC reporter by GmSTF1 and GmSTF2. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗∗∗P < 0.001) determined by two-tailed Student’s t-test.
(I–K) Transcript levels of GmPAL2.1, GmPAL2.3, and GmUTG2 in Wm82, gmbbx4, and YFP-GmBBX4 #1 and #3 soybean plants. Plants were grown in LD conditions (14-h light/10-h dark) at 25°C for 15 days, after which the leaves were collected for RNA extraction and qRT–PCR analysis. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) determined by two-tailed Student’s t-test.
GmBBX4 inhibits the transcriptional activation activity of GmSTF1 and GmSTF2
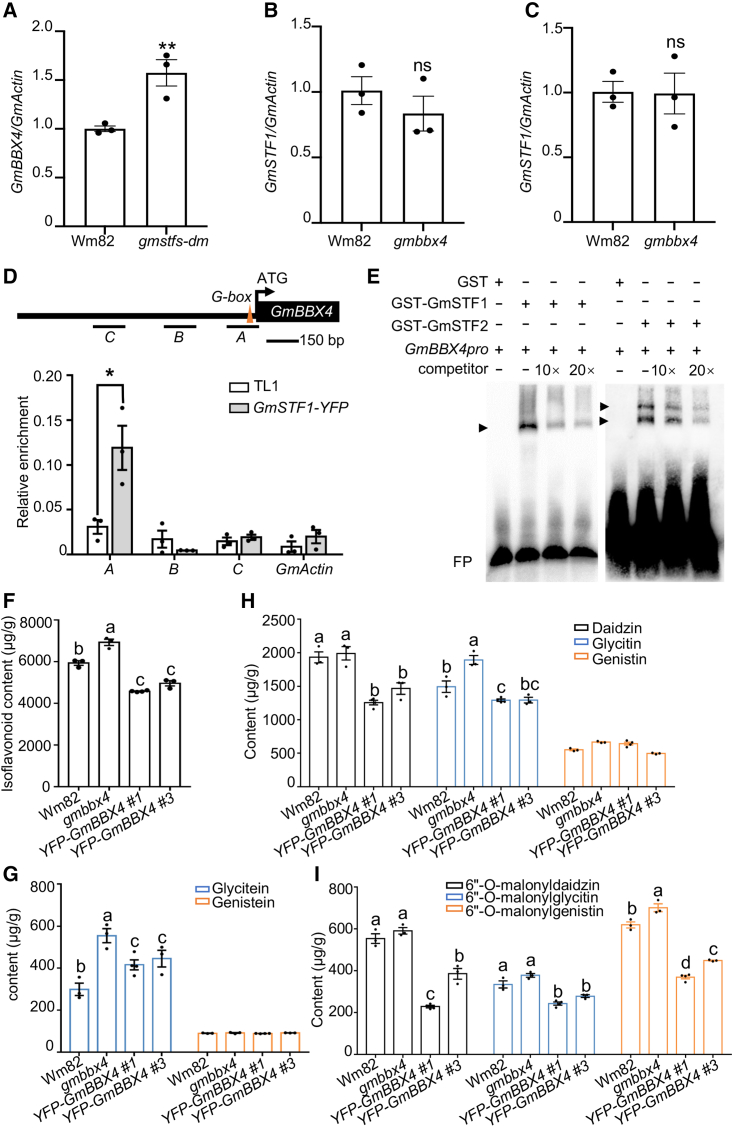
To investigate the biological significance of interaction between GmBBX4 and GmSTF1 or GmSTF2, we performed yeast one-hybrid assays and found that activation of the GmPAL2.3pro:LacZ reporter by GmSTF1 and GmSTF2 was significantly decreased in the presence of GmBBX4 in yeast cells (Figure 5E and 5F). Moreover, activation of the GmPAL2.3pro:LUC reporter by GmSTF1 and GmSTF2 was reduced when they were transiently co-expressed with GmBBX4 in N. benthamiana leaves (Figure 5G and 5H). These data suggest that GmBBX4 inhibits the transcriptional activation activity of GmSTF1 and GmSTF2 toward the target gene. Next, we generated the gmbbx4 mutant using the CRISPR–Cas9 technique and two independent YFP-GmBBX4 (#1 and #3) transgenic soybean lines via Agrobacterium cotyledonary-node transformation. The gmbbx4 mutant carried a 1-bp deletion at position 695 and a 7-bp deletion at positions 709 to 716 from the start codon, which created a premature stop codon (Supplemental Figure 8A and 8B). The transcript levels of GmBBX4 were significantly increased in YFP-GmBBX4 (#1 and #3), and YFP-GmBBX4 protein was detectable by immunoblot analysis (Supplemental Figure 8C and 8D). Hypocotyl length was significantly shorter in gmbbx4 seedlings than in Wm82 seedlings, but YFP-GmBBX4 #1 and YFP-GmBBX4 #3 transgenic lines displayed elongated hypocotyls (Supplemental Figure 8E and 8F). These data suggest that GmBBX4 acts as a negative regulator of light signaling in soybean. Expression of three GmSTF1 and GmSTF2 target genes (GmPAL2.1, GmPAL2.3, and GmUGT2) was significantly increased in gmbbx4 but decreased in the two independent YFP-BBX4 transgenic soybean lines (Figure 6I–6K), indicating that GmBBX4 represses the transcription of these GmSTF target genes, consistent with the finding that GmBBX4 represses the transcriptional activation activity of GmSTFs toward their target genes.
Figure 6.
GmBBX4 negatively regulates isoflavonoid biosynthesis in soybean.
(A) Transcript levels of GmBBX4 in immature seeds of Wm82 and gmstfs-dm soybean grown in LD conditions (14-h light/10-h dark) at 25°C for 80 days. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗∗P < 0.01) determined by two-tailed Student’s t-test.
(B and C) Transcript levels of GmSTF1 and GmSTF2 in immature seeds of Wm82 and gmbbx4 soybean grown in LD conditions (14-h light/10-h dark) at 25°C for 80 days. Error bars represent SE (n = 3). ns, not significant.
(D) ChIP–qPCR assays showing that GmSTF1 associates with the GmBBX4 promoter in vivo. Thirty-day-old TL1 and GmSTF1-YFP soybean leaves were used for the ChIP assays. Chromatin fragments were immunoprecipitated using GFP-Trap antibodies. The GmActin gene was used as the negative control. Error bars represent SE (n = 3). Asterisks indicate significant differences (∗P < 0.05) determined by two-tailed Student’s t-test.
(E) EMSA showing that GmSTF1 and GmSTF2 bind to GmBBX4 promoter subfragments in vitro. “−” indicates absence of the corresponding probes or proteins. For GST, “+” indicates that 0.16 pmol is present; for GST-STF1 and GST-STF2, “+” indicates that 0.1 pmol is present; for the GmBBX4pro probe, “+” indicates that 20 fmol is present. Competitor indicates the non-biotin-labeled GmBBX4pro probe. FP, free probe. Arrowheads indicate protein–DNA complexes.
(F) Total isoflavonoid contents in dry seeds of Wm82, gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3 soybean.
(G) Glycitein and genistein contents in dry seeds of Wm82, gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3 soybean.
(H) Daidzin, glycitin, and genistin contents in dry seeds of Wm82, gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3 soybean.
(I) 6″-O-malonylglycitin, 6″-O-malonyldaidzin, and 6″-O-malonylgenistin contents in dry seeds of Wm82, gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3 soybean.
In (A)–(D), data are means ± SE; n ≥ 3. Letters above the bars indicate significant differences (P < 0.05) determined by one-way ANOVA with Tukey’s post hoc test. The experiments were performed three times with similar results. The content (μg/g) indicates the amount of specific isoflavones in dry seeds.
GmSTF1 and GmSTF2 negatively regulate GmBBX4 transcription in soybean
GmSTF1, GmSTF2, and GmBBX4 are transcription factors that regulate target gene expression. We therefore performed qRT–PCR assays to test whether GmSTF1, GmSTF2, and GmBBX4 affected one another’s transcription in soybean. GmBBX4 transcript levels were significantly increased in the gmstfs-dm mutant (Figure 6A), but GmSTF1 and GmSTF2 expression showed no significant change in gmbbx4 (Figure 6B and 6C), suggesting that GmSTF1 and GmSTF2 inhibit GmBBX4 expression but that GmBBX4 likely does not affect GmSTF1 and GmSTF2 expression in soybean. We next performed ChIP–qPCR experiments and found that GmSTF1 was significantly enriched at promoter region A of GmBBX4 in soybean (Figure 7D). This region contains a G-box cis element that is a potential BBX binding site (Xu et al., 2016, 2018). We therefore performed in vitro EMSA assays using purified recombinant GST-GmSTF1, GST-GmSTF2, and biotin-labeled GmBBX4 promoter DNA subfragments containing an intact G-box. GST-GmSTF1 or GST-GmSTF2 was able to bind to DNA subfragments of the GmBBX4 promoter in vitro. Specifically, two shifted bands were clearly detected in the presence of GST-GmSTF2 but not GST-GmSTF1 (Figure 6E), implying that GST-GmSTF2 may form homodimers that also could bind to the GmBBX4pro probes in vitro. The GST negative control could not bind to the same biotin-labeled DNA subfragments. As the amount of non-biotin-labeled probe increased, binding of GST-GmSTF1 or GST-GmSTF2 to the biotin-labeled probe was reduced (Figure 6E). Thus, these data suggest that GmSTF1 and GmSTF2 directly bind to the promoter of GmBBX4 to inhibit its expression in soybean.
Figure 7.
Photoreceptors control the expression of GmSTF target genes.
(A) Transcript levels of GmPAL2.1, GmPAL2.3, and GmUGT2 in Wm82, gmphot1s-tm, and gmphot2s-dm soybean seeds determined by qRT–PCR.
(B) Transcript levels of GmPAL2.1, GmPAL2.3, and GmUGT2 in TL1, gmcry1s-qm, and gmcry2s-tm soybean seeds determined by qRT–PCR.
(C) A proposed working model for control of isoflavonoid biosynthesis by a photoreceptors–GmSTF–GmBBX4 regulatory module in soybean. Light-activated GmCRY1s, GmCRY2s, GmPHOT1s, and GmPHOT2s stabilize GmSTFs, whereas GmCOP1b negatively controls their abundance in the light. GmSTF1 and GmSTF2 promote isoflavonoid biosynthesis by activating a group of genes involved in the isoflavonoid biosynthetic pathway. GmBBX4 interacts with GmSTFs to repress their transcriptional activation activity. On the other hand, GmSTFs inhibit GmBBX4 at the transcriptional level. Thus, GmBBX4 and GmSTFs form a negative feedback loop that regulates isoflavonoid biosynthesis in soybean.
In (A) and (B), data shown are relative to the control gene GmActin and represent means ± SE of three biological replicates. Asterisks indicate significant differences (∗P < 0.05, ∗∗∗P < 0.001) determined by two-tailed Student’s t-test. The indicated soybean mutants were grown in LD conditions at 25°C for 80 days, after which immature seeds were harvested at the R5 stage for RNA extraction and qRT–PCR analysis.
GmBBX4 negatively regulates isoflavonoid biosynthesis in soybean
To investigate whether GmBBX4 affects isoflavonoid biosynthesis in soybean, we examined the isoflavonoid contents in dry seeds of Wm82, gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3. The total isoflavonoid content was significantly higher in gmbbx4 than in Wm82 but markedly lower in seeds of the two independent YFP-GmBBX4 transgenic lines (Figure 6F). The genistein and genistin contents in gmbbx4, YFP-GmBBX4 #1, and YFP-GmBBX4 #3 were similar to those in Wm82 (Figure 6G and 6H). Compared with those in Wm82, the glycitein, glycitin, and 6″-O-malonylgenistin contents were higher in gmbbx4 but lower in YFP-GmBBX4 #1 and YFP-GmBBX4 #3 (Figure 6G–6I). Although the contents of daidzin, 6″-O-malonyldaidzin, and 6″-O-malonylglycitin were comparable in seeds of gmbbx4 and Wm82, they were significantly lower in seeds of YFP-GmBBX4 #1 and YFP-GmBBX4 #3 (Figure 6H and 6I). These data suggest that GmBBX4 acts as a negative regulator of isoflavonoid biosynthesis in soybean.
Photoreceptors control the expression of GmPAL2.1, GmPAL2.3, and GmUGT2
Because GmCRY and GmPHOT photoreceptors controlled the accumulation of GmSTF and isoflavonoids (Figures 1 and 2; Lyu et al., 2021; Ji et al., 2022), we tested whether these photoreceptors affected the expression of three GmSTF target genes (GmPAL2.1, GmPAL2.3, and GmUGT2) involved in the isoflavonoid biosynthetic pathway. Their transcription was significantly lower in gmphot1s-tm, gmphot2-dm, gmcry1s-qm, and gmcry2s-tm than in Wm82 or TL1 seeds (Figure 7A and 7B). These results suggest that GmCRYs and GmPHOTs positively regulate the transcription of the GmSTF-activated genes GmPAL2.1, GmPAL2.3, and GmUGT2 in soybean.
Discussion
Isoflavonoids, which are primarily synthesized in legumes, have great agricultural, nutraceutical, and pharmaceutical significance (Veitch, 2013). In this study, we showed that light signaling controls isoflavonoid biosynthesis in soybean. UV-A and blue-light photoreceptors (GmCRY1s, GmCRY2s, GmPHOT1s, and GmPHOT2s) positively regulate isoflavonoid biosynthesis by promoting GmSTF accumulation. GmSTF1 and GmSTF2 regulate the expression of approximately 27.9% of the genes involved in isoflavonoid biosynthesis and promote isoflavonoid accumulation in soybean. In addition, GmBBX4, a negative regulator of isoflavonoid biosynthesis, forms a negative feedback loop with GmSTF1/2 to modulate the transcription of GmSTF1/2-controlled isoflavonoid biosynthetic genes and, hence, isoflavonoid accumulation in soybean.
Isoflavonoids not only are essential for plant responses to changing environments but also possess various beneficial properties for human health. Metabolic engineering and synthetic biology strategies have been used to produce specific isoflavonoids such as genistein, daidzein, genistin, and daidzin in Escherichia coli and Saccharomyces cerevisiae (Kim et al., 2009; Trantas et al., 2009; Koirala et al., 2014; Kim, 2019; Liu et al., 2021). However, the incomplete technical system hampers access to industrial-scale production. Isoflavonoid production relies on direct extraction from legumes. Therefore, the isoflavonoid content of soybean seeds needs to be improved to meet the increasing demand for isoflavonoids in the pharmaceutical and nutraceutical industries. Although numerous studies have reported several isoflavonoid-related QTLs, only a few have been cloned and characterized in soybean (Wu et al., 2020; Feng et al., 2023). Distinct environmental factors tightly and significantly regulate these QTLs and genes (Dhaubhadel et al., 2003; Lozovaya et al., 2005; Murphy et al., 2009; Wu et al., 2020).
The light signal transduction pathway has been comprehensively characterized in the model plant Arabidopsis (Cheng et al., 2021; Podolec et al., 2021). Multiple key light-signaling components, such as phys, CRYs, COP1, HY5, and PIFs, are functionally conserved in the regulation of photomorphogenesis in tomato, pea, maize, and rice (Alba et al., 2000; Sullivan and Gray, 2000; Tanaka et al., 2011; Liu et al., 2018; Wu et al., 2019; Yang et al., 2022). Genetic analysis has shown that GmCRY1s, GmCRY2s, and GmSTF1/2 promote photomorphogenic development, whereas GmCOP1a and GmCOP1b repress photomorphogenesis (Lyu et al., 2021; Supplemental Figure 2). Here, GmCRY1s and GmSTF1/2 promoted chlorophyll accumulation in soybean leaves (Supplemental Figure 3). GmCOP1a/b interacted with GmSTF1 or GmSTF2, respectively, at the molecular level (Supplemental Figure 4). These data suggest that light signaling is highly conserved in soybean, supporting the notion that it is evolutionarily conserved in diverse plant species (Han et al., 2020). Both GmCOP1a and GmCOP1b promoted the degradation of GmSTFs in the dark (Supplemental Figure 5), but GmCOP1b, not GmCOP1a, destabilized these proteins in the light (Figure 1M). These results were consistent with observations that both GmCOP1a and GmCOP1b repressed photomorphogenesis in the dark (Supplemental Figure 2G–2I) but that only GmCOP1b inhibited isoflavonoid biosynthesis and accumulation in soybean seeds (Figure 1G–1I). These facts imply that GmCOP1a and COP1b likely have both unique and overlapping functions in diverse physiological and developmental processes of soybean.
Isoflavonoid synthesis is catalyzed by several enzymes in soybean (Zhang et al., 2020). Multiple transcription factors regulate the transcription of specific genes whose encoded enzymes are required for catalyzing the synthesis of different compounds during isoflavonoid production. GmZFP7 upregulates the expression of GmC4H, GmCHS7, GmCHS8, GmCHR1, GmCHR3, and GmIFS2 but downregulates that of GmF3H1 to promote isoflavonoid biosynthesis (Feng et al., 2023). GmMYB29 positively controls the transcription of GmIFS2 and GmCHS8 and increases isoflavonoid accumulation in soybean (Chu et al., 2017). The bZIP-type transcription factors GmSTF1 and GmSTF2, which are close orthologs of HY5, likely controlled 29 of the 104 genes involved in isoflavonoid biosynthesis (Figure 3). Moreover, GmSTF1 and GmSTF2 directly bound to the promoters of two phenylalanine ammonia lyases, GmPAL2.1 and GmPAL2.3, and the uridine diphosphate glycosyltransferase GmUGT2 to activate their transcription in soybean (Figures 4 and 5). Consistent with these results, GmSTF1 and GmSTF2 facilitated isoflavonoid biosynthesis and accumulation in soybean (Figure 2). HY5 accumulation is induced by light irradiation and positively controlled by photoreceptors in Arabidopsis (Osterlund et al., 2000). UV-A and blue-light photoreceptors (GmCRYs and GmPHOTs) promoted GmSTF accumulation and isoflavonoid biosynthesis (Lyu et al., 2021; Ji et al., 2022; Figure 1). In agreement with this result, the transcription of three GmSTF target genes involved in isoflavonoid biosynthesis was positively controlled by GmCRYs and GmPHOTs (Figures 7A and 7B). These findings suggest that GmCRY- and GmPHOT-triggered and GmCOP1b-inhibited isoflavonoid biosynthesis is predominantly dependent on GmSTF abundance and the action of GmSTFs in soybean.
Light-activated GmCRY1s interact with and stabilize GmSTF1 and GmSTF2. GmSTF1 and GmSTF2 directly activate the transcription of GA2 oxidases, leading to the inhibition of the phytohormone GA1 and stem growth (Lyu et al., 2021). In addition, they repress the expression of nodule inception a (GmNINa) and nodulation in soybean (Ji et al., 2022). The present study demonstrated that GmSTF1 and GmSTF2 upregulated the expression of a group of genes involved in isoflavonoid biosynthesis to promote isoflavonoid accumulation (Figures 2, 3, 4, and 5). These facts suggest that GmSTF1 and GmSTF2 likely play pleiotropic roles in diverse physiological and developmental processes of soybean.
GmBBX4 is orthologous to BBX4 in Arabidopsis. BBX4 promotes phyB-mediated signaling and anthocyanin biosynthesis (Datta et al., 2006; Heng et al., 2019), whereas GmBBX4 acted as a negative regulator of light signaling and isoflavonoid biosynthesis in the present work (Figure 6 and Supplemental Figure 8), suggesting that these two close orthologs have functionally diverged. GmBBX4 interacted with GmSTF1 and GmSTF2, inhibiting their transcriptional activation activity toward their target genes (Figure 5). GmSTF1 and GmSTF2 also associated with the GmBBX4 promoter and negatively regulated its transcription (Figure 5). Together, these data suggest that GmBBX4 and GmSTF1/2 form a negative feedback loop that orchestrates isoflavonoid biosynthesis. GmBBX4 acted as a negative regulator of GmSTF1/2 and repressed isoflavonoid biosynthesis (Figures 5 and 6). However, we could not rule out the possibility that GmBBX4 inhibition of isoflavonoid biosynthesis may also occur independently of GmSTF1/2. GmSTF proteins were stabilized upon light irradiation and by light-activated photoreceptors (GmCRY1s, GmCRY2s, GmPHOT1s, and GmPHOT2s) (Lyu et al., 2021; Ji et al., 2022; Figure 1); it is therefore the aforementioned molecular events that most likely occur in the light.
Light signaling, including the activities of photoreceptors, E3 ligases, and transcription factors, modulates isoflavonoid biosynthesis in soybean. GmCRY1, GmCRY2, GmPHOT1, and GmPHOT2 photoreceptors positively regulate isoflavonoid biosynthesis, whereas the E3 ubiquitin ligase GmCOP1b inhibits biosynthesis of these natural compounds. The transcription factors GmSTF1 and GmSTF2, controlled by photoreceptors and GmCOP1b, promote isoflavonoid biosynthesis by activating a group of genes involved in the isoflavonoid biosynthetic pathway. GmBBX4 forms a negative feedback loop with GmSTF1/2 and negatively regulates isoflavonoid biosynthesis in soybean (Figure 7C). Thus, perception of light signals and transduction of light information precisely control isoflavonoid biosynthesis in soybean under natural conditions.
Methods
Plant materials and growth conditions
The gmphot1s-tm, gmphot2s-dm, gmcop1a, gmcop1b, gmstf1, gmstf2, and gmstfs-dm (this study) mutants are in the soybean (Glycine max [L.] Merr.) cultivar Williams 82 (Wm82) background. The gmphot1s-tm, gmphot2s-dm, gmcop1a, and gmcop1b mutants were generated using the CRISPR–Cas9 method. gmstf1 and gmstf2 were segregated from the F2 progeny of a cross between gmstfs-dm and Wm82. gmcry1s-qm, gmcry2s-tm, GmSTF1-YFP, and GmSTF2-YFP transgenic soybean are in the Tianlong 1 (TL1) background (Lyu et al., 2021). Various soybean mutants or transgenic lines were grown under LD conditions (14-h light/10-h dark, light intensity 524 μmol/m2/s) in a greenhouse maintained at 25°C.
Measurement of hypocotyl length and apical angle
To measure the hypocotyl length and apical angle of soybean seedlings, surface-sterilized soybean seeds were kept on sterilized, moist filter paper for 2 days in the dark at 25°C to induce germination. Well-germinated seeds were then planted with the radicle embedded in the soil. The seedlings were grown under continuous white light (524 μmol/m2/s) at 25°C for 3 more days. The hypocotyl length and apical angle of the seedlings were measured using ImageJ software.
Construction of plasmids
To construct plasmids for soybean transformation, the coding DNA sequence (CDS) of GmBBX4 was amplified by PCR using cDNA derived from young seedlings of Wm82 and cloned into the modified JRH0641-FLAG vector, which was driven by the CaMV 35S promoter. To generate CRISPR–Cas9-engineered mutants of the indicated genes, guide RNAs (gRNAs) were designed using the web tool CRISPR-P (http://cbi.hzau.edu.cn/crispr/) (Lei et al., 2014). At least three gRNAs were selected for each target gene and used to construct the CRISPR–Cas9 expression vectors. The efficiency of each candidate gRNA was estimated using the soybean hairy root system (Zhang et al., 2020). To generate pLexA-GmSTF1, pLexA-GmSTF2, pLex-GmCOP1a, and pLexA-GmCOP1b constructs, full-length GmSTF1, GmSTF2, GmCOP1a, and GmCOP1b fragments were amplified by PCR with corresponding primer pairs and then cloned into the EcoRI/XhoI sites of the pLexA vector (BD Clontech). To generate pB42AD-GmSTF1, pB42AD-GmSTF2, and pB42AD-GmBBX4 constructs, full-length GmSTF1, GmSTF2, and GmBBX4 fragments were amplified by PCR with corresponding primer pairs and then cloned into the EcoRI/XhoI sites of the pB42AD vector (BD Clontech). For the yeast one-hybrid experiment, the first class of promoters included the upstream sequences of ATG consisting of 3000 bp of GmPAL2.1pro, 623 bp of GmPAL2.1pro-B, 1900 bp of GmPAL2.3pro, 790 bp of GmPAL2.3pro-B, 1800 bp of GmUGT2pro, and 712 bp of GmUGT2pro-B. The second class of promoters included the upstream sequences of the target gene B region, which consisted of 2376 bp of GmPAL2.1pro-A, 1110 bp of GmPAL2.3pro-A, and 1088 bp of GmUGT2pro-A. These sequences were amplified by PCR using specific primers and cloned into the KpnI/PstI sites of the placZ-2μ vector (Lin et al., 2007). The full-length GmSTF1 and GmSTF2 sequences were amplified by PCR with corresponding primer pairs and cloned into the EcoRI/XhoI sites of the pGEX-4T-1 vector. To produce constructs for the LCI assays, full-length GmSTF1, GmSTF2, GmCOP1a, GmCOP1b, and GmBBX4 sequences were amplified by PCR with corresponding primer pairs and cloned into the KpnI/SalI sites of pCambia1300-nLUC or pCambia1300-cLUC (Chen et al., 2008). For the dual-luciferase reporter assays, the 3000-bp GmPAL2.1, 1900-bp GmPAL2.3, and 1800-bp GmUGT2 promoter sequences upstream of ATG were amplified by PCR with the corresponding primer pairs and cloned into the KpnI/PstI sites of the pGreen0800II-LUC vector. For the co-IP assays, UBQ10:HA-GmSTF1 and UBQ10:HA-GmSTF2 were amplified by PCR and cloned into the EcoRI/KpnI sites of the pCambia1300 vector. The full-length GmCOP1a and GmCOP1b sequences were amplified by PCR with the corresponding primer pairs and cloned into the XbaI/HindIII sites of the pCAMBIA1307-FLAG vector. pCambia1300-35S:P19 was used to suppress post-transcriptional gene silencing (Sparkes et al., 2006; Liu et al., 2010). Specific primers used in these experiments are listed in Supplemental Table 6.
Soybean transformation
The overexpression lines and CRISPR–Cas9-engineered mutants were generated by Agrobacterium cotyledonary-node transformation as described previously (Zhang et al., 1999) with minor modifications. In brief, the seeds were kept in a vacuum desiccator with chlorine gas for 3 h and then sown on germination medium for 8–10 h. The seed coat was gently removed, and two cotyledonary-node explants were prepared by making a vertical slice between the cotyledons. Explants were immersed in Agrobacterium (EHA105) inoculum for 30 min with occasional agitation and then transferred to co-cultivation plates and kept in the dark at 25°C for 5 days. After the co-cultivation period, explants were briefly washed in sterile water four times and in shoot induction liquid medium supplemented with 1.6 mg/l 6-BA, 5 mg/l cefotaxime sodium, and 5 mg/l carbenicillin disodium. Explants were then cultured on shoot initiation medium with the hypocotyl embedded in the medium under a 16-h light/8-h dark photoperiod at 25°C and subcultured biweekly to fresh medium. At the end of the shoot initiation stage, differentiating explants were transferred to shoot elongation medium. The elongated shoots (>3 cm) were transferred to rooting medium without further selection.
Extraction and measurement of isoflavonoids
Isoflavonoid content was determined as described previously (Chu et al., 2017) with minor modifications. Dry seed powder (0.02 g) was weighed into 2.0-ml centrifuge tubes containing 1.0 ml of 80% chromatographic methanol (solid/liquid ratio 1:50). The mixture was vortexed for 1 min, and 50°C ultrasound (frequency 40 kHz, power 300 W) was used to assist extraction for 1 h. After centrifugation at 14 000 g for 10 min at 4°C, the supernatant was passed through a 0.22-μm organic phase needle filter and injected into a water autoinjection vial (2 ml), which was stored at −20°C. Samples were analyzed with an ultra-performance liquid chromatography (UPLC) system (National Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University; DIONEX Ultimate 3000 instrument; ACQUITY UPLC HSS T3 column, 1.8 μm, 2.1 mm × 100 mm, part no. 186003539, serial no. 012631029257). Solvent A was 0.5% aqueous acetic acid, and solvent B was 100% acetonitrile; the solvent system was 0–16 min 15%–26% B (v/v), 16–16.1 min 26%–15% B, and 16.1–18 min 15% B. The solvent flow rate was 4 ml/min, and UV absorption was measured at 260 nm (DAD). The column temperature was set to 40°C, and the injection volume was 2 μl. Nine isoflavonoids were measured: daidzein, genistein, glycitein, daidzin, genistin, glycitin, 6″-O-malonyldaidzin, 6″-O-malonylgenistin, and 6″-O-malonylglycitin.
Firefly luciferase complementation imaging assay
LCI assays were performed as described previously (Chen et al., 2008). The LUCN- and LUCC-fused plasmids were transformed into Agrobacterium strain GV3101, and the indicated transformant pairs were infiltrated into N. benthamiana leaves. A 24-h incubation in the dark at 22°C was followed by an additional 24–36 h incubation under a 16-h light/8-h dark photoperiod unless otherwise indicated. The leaves were then harvested, and 0.33 mM D-luciferin (Yeasen, 40901ES) solution was sprayed onto the leaves. Luciferase activity was measured with the LB 985 NightSHADE Spectrum imaging system (Berthold). The experiments were performed three times, and each data point was obtained from the results of three replicate experiments.
Yeast two-hybrid screening
A cDNA library was constructed using total RNA from a soybean leaf nuclear gene library and screened with the AD Matchmaker Library Construction & Screening Kit (Clontech Laboratories, Mountain View, CA, USA). The full-length coding sequence of GmSTF1 was cloned into the pGBKT7 vector for this assay. Yeast two-hybrid screening was performed using the GAL4-based Matchmaker Gold Yeast Two-Hybrid System (Clontech Laboratories) following the manufacturer’s instructions.
Yeast two-hybrid assay
Yeast two-hybrid assays were performed using the Matchmaker LexA Two-Hybrid System as described in the Yeast Protocols Handbook (BD Clontech). The indicated combinations of pLexA and pB42AD fusion plasmids were co-transformed into yeast strain EGY48 containing the p8op-LacZ plasmid. The empty pLexA and pB42AD vectors were co-transformed in parallel as negative controls. Transformants were selected and grown on SD/−His−Trp−Ura dropout plates at 30°C. The transformants were grown on SD/−His−Trp−Ura dropout plates containing 80 mg/l X-gal for blue color development.
Yeast one-hybrid assay
For the yeast one-hybrid assay, the indicated combinations of AD-fusion effectors and LacZ reporters were co-transformed into yeast strain EGY48, and transformants were selected and grown on SD/−Trp−Ura dropout medium. Yeast transformation and liquid assays were performed as described in the Yeast Protocols Handbook (BD Clontech).
Dual-luciferase reporter system
N. benthamiana plants grown in LD conditions (16-h light/8-h dark) were used for transient transactivation assays. Agrobacterium strain GV3101 cells carrying the 35S:GmSTF1-HA, 35S:GmSTF2-HA, and 35S:YFP-GmBBX4, or GmPAL2.1pro:LUC, GmPAL2.3pro:LUC, GmUGT2pro:LUC, and GmBBX4pro:LUC constructs were transiently infiltrated into N. benthamiana leaves in the indicated combinations. Firefly LUC and Renilla LUC (Ren) were detected using the Dual-LUC Reporter Assay System (Vazyme, DL101) according to the manufacturer’s instructions.
Electrophoretic mobility shift assay
The GmSTF1 or GmSTF2 CDS was cloned into pGEX-4T-1 between the EcoRI and XhoI sites, and the sequenced recombination vectors were transformed and expressed in E. coli BL21 cells. The purified recombinant GST-GmSTF1 and GST-GmSTF2 proteins were used for the EMSA, which was performed using a Light Shift Chemiluminescent EMSA Kit (Thermo Scientific #20148) according to the manufacturer’s protocol. The binding activities of proteins were analyzed using an oligonucleotide labeled with biotin at the 5′ end with the EMSA Probe Biotin Labeling Kit (Beyotime #GS008). For unlabeled probe competition, 10- and 20-fold unlabeled probe was added to the reactions. The specific probes used for in vitro EMSA are listed in Supplemental Table 6.
ChIP–qPCR assay
The ChIP–qPCR assay was performed as described previously (Xu et al., 2016). In brief, seedlings of TL1, GmSTF1-YFP, and GmSTF2-YFP soybean plants were grown under a 14-h light/10-h dark photoperiod (light intensity 524 μmol/m2/s) at 25°C for 30 days. Fresh leaves (1.5 g) were collected and fixed in 30 ml 1% formaldehyde buffer under vacuum conditions for 20 min. Next, 2 ml of 2 M glycine buffer was added to quench crosslinking, followed by an additional 5 min under vacuum. The samples were then ground into a fine powder in liquid nitrogen, and the powder was resuspended in 30 ml of extraction buffer I (0.4 M sucrose, 10 mM Tris–HCl [pH 8.0], 10 mM MgCl2, 5 mM β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], and cOmplete protease inhibitor cocktail [11873580001, Roche, UK]). Nuclei were collected after filtering the solution through two layers of 50-μm Miracloth. After centrifugation for 20 min at 4000 g, pellets were suspended in extraction buffer II (0.25 M sucrose, 10 mM Tris–HCl [pH 8.0], 10 mM MgCl2, 1% [v/v] Triton X-100, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and protease inhibitor cocktail). After centrifugation for 10 min at 14 000 g, pellets were resuspended in extraction buffer III (1.7 M sucrose, 10 mM Tris–HCl [pH 8.0], 2 mM MgCl2, 0.15% [v/v] Triton X-100, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and protease inhibitor cocktail). After centrifugation for 1 h at 14 000 g, the pellets were resuspended in nuclei lysis buffer (50 mM Tris–HCl [pH 8.0], 10 mM EDTA, 1% [w/v] SDS, and protease inhibitor) and sonicated on ice using a sonicator (Bioruptor Pico). The supernatants were diluted 10-fold with ChIP dilution buffer (1.1% [v/v] Triton X-100, 1.2 mM EDTA, 16.7 mM Tris–HCl [pH 8.0], 167 mM NaCl). Immunoprecipitation was performed using anti-GFP antibodies (Abmart) at 4°C overnight. Chromatin that precipitated without the use of antibodies was used as a negative control, and chromatin isolated before precipitation was used as an input control. Immunoprecipitates were eluted twice with 250 ml of fresh elution buffer (1% [w/v] SDS, 0.1 M NaHCO3), and the DNA was purified and resuspended in 50 μl of TE buffer for qPCR analysis. Both immunoprecipitated DNA and input DNA were analyzed by real-time PCR (Roche Light Cycler 480 II). Fold enrichment is presented as a percentage of the input value of the ChIP with specific antibodies in the transgenic gene relative to the ChIP with specific antibodies in the wild type. Specific primers used in this experiment are listed in Supplemental Table 6.
Immunoblot assay
Distinct soybean mutants or transgenic lines were grown for 15 days under LD conditions (14-h light/10-h dark; light intensity 524 μmol/m2/s) in a greenhouse maintained at 25°C. Fresh soybean leaves were ground to a fine powder in liquid nitrogen and homogenized in protein extraction buffer (100 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 0.5% NP-40, 3 mM dithiothreitol, and protease inhibitor cocktail). The supernatants were collected after centrifugation at 14 000 g for 10 min at 4°C. Equal amounts of protein from each sample were boiled in 5× SDS–PAGE loading buffer (250 mM Tris–HCl [pH 6.8], 10% SDS, 50% glycerol, and 0.01% bromophenol blue) for 10 min. The supernatants were separated by 12% SDS–PAGE, followed by semidry transfer to polyvinylidene difluoride membranes (0.45 μm, Immobilon-P). The membranes were incubated with anti-STF1/2 antibody (1:1000) (Lyu et al., 2021), anti-GFP antibody (Abmart, 1:3000), or anti-FLAG antibody (MBL, 1:2000) at 4°C overnight and then incubated with secondary antibody for 1 h at 25°C. The target proteins were detected using the GE Healthcare ECL kit.
Co-immunoprecipitation assay
Agrobacterium strain GV3101 cells carrying the UBQ10:HA-GmSTF1, UBQ10:HA-GmSTF2, 35S:YFP-GmBBX4, 35S:GmCOP1a-FLAG, or 35S:GmCOP1b-FLAG construct were transiently infiltrated into N. benthamiana leaves. After infiltration, the constructs were expressed for 2 days. The leaves were then homogenized in extraction buffer for 15 min on ice. After centrifugation at 16 000 g and 4°C for 10 min, extracts were incubated with 5 μl of anti-HA (MBL), anti-FLAG (MBL), or anti-GFP (Abmart) antibody coupled with 20 μl of Protein-A Sepharose (GE Healthcare) for 3 h at 4°C. The beads were collected by centrifugation at 1000 g and 4°C for 3 min and then washed three times with protein extraction buffer. The precipitates were boiled in 5× SDS protein loading buffer before SDS–PAGE and immunoblot analysis.
RNA extraction and qRT–PCR analysis
Wm82, TL1, gmphot1s-tm, gmphot2-dm, gmcry1s-qm, gmcry2s-tm, gmphybs-dm, and gmstfs-dm were grown under LD conditions (14-h light/10-h dark, light intensity 524 μmol/m2/s) at 25°C for 80 days. The leaves and immature seeds were collected and flash-frozen in liquid nitrogen for subsequent RNA extraction using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, RC112). cDNA was synthesized from 1 μg of total RNA using the HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, R312) according to the manufacturer’s instructions. cDNA was then used for real-time qPCR assays. Quantitative real-time PCR was performed using the LightCycler 480 II detection system (Roche) and ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q511). PCR was performed in triplicate for each sample, and expression levels were normalized to that of a GmActin gene. Specific primers used in this experiment are listed in Supplemental Table 6.
Measurement of chlorophyll content
For analysis of chlorophyll content, 0.1 g of fresh leaves were collected from the indicated 15-day-old soybean plants. Two milliliters of 80% acetone was added, and the mixture was kept in the dark at room temperature overnight. A UV spectrophotometer was used to measure the absorbance of chlorophyll extraction solution at wavelengths of 645, 663, and 470 nm. Chlorophyll contents were calculated according to the following formulas. Chlorophyll a content: Chla = (12.72 × A663) − (2.59 × A645); chlorophyll b content: Chlb = (22.88 × A645) − (4.67 × A663); total chlorophyll content: Chlt = (20.92 × A645) + (8.05 × A663).
Statistical analysis
Statistical analyses were performed in Microsoft Excel, in GraphPad Prism version 7.0, or through an online website (https://astatsa.com/OneWay_Anova_with_TukeyHSD/).
Accession numbers
Gene sequences are available at the Phytozome database (Glycine max Wm82.a2.v1) under the following accession numbers: GmSTF1 (Glyma.18G117100), GmSTF2 (Glyma.08G302500), GmPHOT1a (Glyma.12G074100), GmPHOT1b (Glyma.13G330400), GmPHOT1c (Glyma.15G043600), GmPHOT2a (Glyma.08G264900), GmPHOT2b (Glyma.16G096600), GmBBX4 (Glyma.04G058900), GmCRY1a (Glyma.04G101500), GmCRY1b (Glyma.06G103200), GmCRY1c (Glyma.14G174200), GmCRY1d (Glyma.13G089200), GmCRY2a (Glyma.10G180600), GmCRY2b (Glyma.02G005700), GmCRY2c (Glyma.20G209900), GmCOP1a (Glyma.02G267800), GmCOP1b (Glyma.14G049700), GmPAL2.1 (Glyma.10G058200), GmPAL2.3 (Glyma.13G145000), GmUGT2 (Glyma.16G175300), and GmActin (Glyma.18G290800).
Funding
This work was supported by the Natural Science Foundation of Jiangsu for Distinguished Young Scholars (BK20211525), the National Natural Science Foundation of China (32270256, 31970258), the Core Technology Development for Breeding Program of Jiangsu Province (JBGS-2021-014), the Jiangsu Collaborative Innovation Center for Modern Crop Production (to D.X.), and Nanjing Agricultural University (start-up funding to D.X.).
Author contributions
Z.S., F.Z., H.L., L.C., Y.X., and Z.F. conducted the experiments. X.W. performed bioinformatic analysis. X.L. and B.L. contributed materials. Z.S. and D.X. designed the experiments. D.X., J.G., and J.D. analyzed the data. D.X. wrote the article.
Acknowledgments
We thank Professor Fanjiang Kong for providing gmstfs-dm.
Published: October 10, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Bin Liu, Email: liubin05@caas.cn.
Junyi Gai, Email: sri@njau.edu.cn.
Dongqing Xu, Email: dongqingxu@njau.edu.cn.
Supplemental information
References
- Alba R., Kelmenson P.M., Cordonnier-Pratt M.M., Pratt L.H. The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Mol. Biol. Evol. 2000;17:362–373. doi: 10.1093/oxfordjournals.molbev.a026316. [DOI] [PubMed] [Google Scholar]
- Anguraj Vadivel A.K., McDowell T., Renaud J.B., Dhaubhadel S. A combinatorial action of GmMYB176 and GmbZIP5 controls isoflavonoid biosynthesis in soybean (Glycine max) Commun. Biol. 2021;4:356. doi: 10.1038/s42003-021-01889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araguirang G.E., Richter A.S. Activation of anthocyanin biosynthesis in high light - what is the initial signal? New Phytol. 2022;236:2037–2043. doi: 10.1111/nph.18488. [DOI] [PubMed] [Google Scholar]
- Bai S., Tao R., Tang Y., Yin L., Ma Y., Ni J., Yan X., Yang Q., Wu Z., Zeng Y., et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019;17:1985–1997. doi: 10.1111/pbi.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Tao R., Yin L., Ni J., Yang Q., Yan X., Yang F., Guo X., Li H., Teng Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019;100:1208–1223. doi: 10.1111/tpj.14510. [DOI] [PubMed] [Google Scholar]
- Bian S., Li R., Xia S., Liu Y., Jin D., Xie X., Dhaubhadel S., Zhai L., Wang J., Li X. Soybean CCA1-like MYB transcription factor GmMYB133 modulates isoflavonoid biosynthesis. Biochem. Biophys. Res. Commun. 2018;507:324–329. doi: 10.1016/j.bbrc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- Bian X.H., Li W., Niu C.F., Wei W., Hu Y., Han J.Q., Lu X., Tao J.J., Jin M., Qin H., et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2020;225:268–283. doi: 10.1111/nph.16104. [DOI] [PubMed] [Google Scholar]
- Chen H., Zou Y., Shang Y., Lin H., Wang Y., Cai R., Tang X., Zhou J.M. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.C., Kathare P.K., Paik I., Huq E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021;72:217–244. doi: 10.1146/annurev-arplant-080620-024221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- Chu S., Wang J., Zhu Y., Liu S., Zhou X., Zhang H., Wang C.E., Yang W., Tian Z., Cheng H., et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S., Hettiarachchi G.H.C.M., Deng X.W., Holm M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell. 2006;18:70–84. doi: 10.1105/tpc.105.038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S., Mcgarvey B.D., Williams R., Gijzen M. Isoflavonoid biosynthesis and accumulation in developing soybean seeds. Plant Mol. Biol. 2003;53:733–743. doi: 10.1023/B:PLAN.0000023666.30358.ae. [DOI] [PubMed] [Google Scholar]
- Dhaubhadel S., Farhangkhoee M., Chapman R. Identification and characterization of isoflavonoid specific glycosyltransferase and malonyltransferase from soybean seeds. J. Exp. Bot. 2008;59:981–994. doi: 10.1093/jxb/ern046. [DOI] [PubMed] [Google Scholar]
- Dixon R.A., Achnine L., Kota P., Liu C.-J., Reddy M.S.S., Wang L. The phenylpropanoid pathway and plant defence - A genomics perspective. Mol. Plant Pathol. 2002;3:371–390. doi: 10.1046/j.1364-3703.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhang S., Li J., Pei R., Tian L., Qi J., Azam M., Agyenim-Boateng K.G., Shaibu A.S., Liu Y., et al. Dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. New Phytol. 2023;237:1794–1809. doi: 10.1111/nph.18610. [DOI] [PubMed] [Google Scholar]
- Fu X., Peng B., Hassani D., Xie L., Liu H., Li Y., Chen T., Liu P., Tang Y., Li L., et al. AaWRKY9 contributes to light- and jasmonate-mediated to regulate the biosynthesis of artemisinin in Artemisia annua. New Phytol. 2021;231:1858–1874. doi: 10.1111/nph.17453. [DOI] [PubMed] [Google Scholar]
- Fuglevand G., Jackson J.A., Jenkins G.I. UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell. 1996;8:2347–2357. doi: 10.1105/tpc.8.12.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão V.C., Fankhauser C. Sensing the light environment in plants: photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015;34:46–53. doi: 10.1016/j.conb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Gangappa S.N., Botto J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant. 2016;9:1353–1365. doi: 10.1016/j.molp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Graham P.H., Vance C.P. Legumes: importance and constraints to greater use. Plant Physiol. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T.L., Graham M.Y., Subramanian S., Yu O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonoids suppresses race-specific resistance and hypersensitive cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007;144:728–740. doi: 10.1104/pp.107.097865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Huang X., Deng X.W. The photomorphogenic central repressor COP1: conservation and functional diversification during evolution. Plant Commun. 2020;1 doi: 10.1016/j.xplc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng Y., Jiang Y., Zhao X., Zhou H., Wang X., Deng X.W., Xu D. BBX4, a phyB interacting and -modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis. Proc. Natl. Acad. Sci. USA. 2019;116:26049–26056. doi: 10.1073/pnas.1915149116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017;37:63–69. doi: 10.1016/j.pbi.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Ji H., Xiao R., Lyu X., Chen J., Zhang X., Wang Z., Deng Z., Wang Y., Wang H., Li R., et al. Differential light-dependent regulation of soybean nodulation by papilionoid-specific HY5 homologs. Curr. Biol. 2022;32:783–795.e5. doi: 10.1016/j.cub.2021.12.041. [DOI] [PubMed] [Google Scholar]
- Kant R., Tyagi K., Ghosh S., Jha G. Host alternative NADH:Ubiquinone oxidoreductase serves as a susceptibility factor to promote pathogenesis of Rhizoctonia solani in plants. Phytopathology. 2019;109:1741–1750. doi: 10.1094/PHYTO-02-19-0055-R. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.G. Biological synthesis of genistein in Escherichia coli. J. Microbiol. Biotechnol. 2019;30:770–776. doi: 10.4014/jmb.1911.11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Kim B.G., Jung N.R., Ahn J.H. Production of genistein from naringenin using Escherichia coli containing isoflavone synthase cytochrome P450 reductase fusion protein. J. Microbiol. Biotechnol. 2009;19:1612–1616. doi: 10.4014/jmb.0905.05043. [DOI] [PubMed] [Google Scholar]
- Koirala N., Pandey R.P., Thang D.V., Jung H.J., Sohng J.K. Glycosylation and subsequent malonylation of isoflavonoids in E. coli: strain development, production and insights into future metabolic perspectives. J. Ind. Microbiol. Biotechnol. 2014;41:1647–1658. doi: 10.1007/s10295-014-1504-6. [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Lu L., Liu H.Y., Li S., Xing F., Chen L.L. CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant. 2014;7:1494–1496. doi: 10.1093/mp/ssu044. [DOI] [PubMed] [Google Scholar]
- Li X., Yang C., Chen J., He Y., Deng J., Xie C., Xiao X., Long X., Wu X., Liu W., et al. Changing light promotes isoflavone biosynthesis in soybean pods and enhances their resistance to mildew infection. Plant Cell Environ. 2021;44:2536–2550. doi: 10.1111/pce.14128. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang H. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food Funct. 2017;8:2935–2944. doi: 10.1039/c7fo00342k. [DOI] [PubMed] [Google Scholar]
- Lin R., Ding L., Casola C., Ripoll D.R., Feschotte C., Wang H. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science. 2007;318:1302–1305. doi: 10.1126/science.1146281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Chi C., Jin L.J., Zhu J., Yu J.Q., Zhou Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018;41:1762–1775. doi: 10.1111/pce.13171. [DOI] [PubMed] [Google Scholar]
- Liu H., Li L., Fu X., Li Y., Chen T., Qin W., Yan X., Wu Z., Xie L., Kayani S.L., et al. AaMYB108 is the core factor integrating light and jasmonic acid signaling to regulate artemisinin biosynthesis in Artemisia annua. New Phytol. 2022;237:2224–2237. doi: 10.1111/nph.18702. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhang Y., Tang S., Zhao Q., Zhang Z., Zhang H., Dong L., Guo H., Xie Q. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 2010;61:893–903. doi: 10.1111/j.1365-313X.2009.04109.x. [DOI] [PubMed] [Google Scholar]
- Liu Q., Liu Y., Li G., Savolainen O., Chen Y., Nielsen J. De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat. Commun. 2021;12:6085. doi: 10.1038/s41467-021-26361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yuan L., Xu L., Xu Z., Huang Y., He X., Ma H., Yi J., Zhang D. Over-expression of GmMYB39 leads to an inhibition of the isoflavonoid biosynthesis in soybean (Glycine max. L) Plant Biotechnol. Rep. 2013;7:445–455. [Google Scholar]
- Liu Y., Du H., Li P., Shen Y., Peng H., Liu S., Zhou G.A., Zhang H., Liu Z., Shi M., et al. Pan-genome of wild and cultivated soybeans. Cell. 2020;182:162–176.e13. doi: 10.1016/j.cell.2020.05.023. [DOI] [PubMed] [Google Scholar]
- Lozovaya V.V., Lygin A.V., Ulanov A.V., Nelson R.L., Daydé J., Widholm J.M. Effect of temperature and soil moisture status during seed development on soybean seed isoflavone concentration and composition. Crop Sci. 2005;45:1934–1940. [Google Scholar]
- Lyu X., Cheng Q., Qin C., Li Y., Xu X., Ji R., Mu R., Li H., Zhao T., Liu J., et al. GmCRY1s modulate gibberellin metabolism to regulate soybean shade avoidance in response to reduced blue light. Mol. Plant. 2021;14:298–314. doi: 10.1016/j.molp.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Ma D., Li X., Guo Y., Chu J., Fang S., Yan C., Noel J.P., Liu H. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. USA. 2016;113:224–229. doi: 10.1073/pnas.1511437113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.E., Lee E.A., Woodrow L., Seguin P., Kumar J., Rajcan I., Ablett G.R. Genotype × environment interaction and stability for isoflavone content in soybean. Crop Sci. 2009;49:1313–1321. [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- Paik I., Huq E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019;92:114–121. doi: 10.1016/j.semcdb.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Huang S.S.C., Zander M., Cole B.J., Hetzel J., Ljung K., Reis P.A.B., Sridevi P., Nito K., Nery J.R., et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell. 2016;164:233–245. doi: 10.1016/j.cell.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolec R., Demarsy E., Ulm R. Perception and signaling of ultraviolet-B radiation in plants. Annu. Rev. Plant Biol. 2021;72:793–822. doi: 10.1146/annurev-arplant-050718-095946. [DOI] [PubMed] [Google Scholar]
- Schmutz J., Cannon S.B., Schlueter J., Ma J., Mitros T., Nelson W., Hyten D.L., Song Q., Thelen J.J., Cheng J., et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006;1:2019–2025. doi: 10.1038/nprot.2006.286. [DOI] [PubMed] [Google Scholar]
- Song Z., Bian Y., Liu J., Sun Y., Xu D. B-box proteins: pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020;62:1293–1309. doi: 10.1111/jipb.12935. [DOI] [PubMed] [Google Scholar]
- Sullivan J.A., Gray J.C. The pea light-independent photomorphogenesis1 mutant results from partial duplication of COP1 generating an internal promoter and producing two distinct transcripts. Plant Cell. 2000;12:1927–1938. doi: 10.1105/tpc.12.10.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Itoh H., Sentoku N., Kojima M., Sakakibara H., Izawa T., Itoh J.I., Nagato Y. The COP1 ortholog PPS regulates the juvenile-adult and vegetative-reproductive phase changes in rice. Plant Cell. 2011;23:2143–2154. doi: 10.1105/tpc.111.083436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantas E., Panopoulos N., Ververidis F. Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab. Eng. 2009;11:355–366. doi: 10.1016/j.ymben.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Veitch N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2013;30:988–1027. doi: 10.1039/c3np70024k. [DOI] [PubMed] [Google Scholar]
- Wang X. Structure, function, and engineering of enzymes in isoflavonoid biosynthesis. Funct. Integr. Genom. 2011;11:13–22. doi: 10.1007/s10142-010-0197-9. [DOI] [PubMed] [Google Scholar]
- Wu D., Li D., Zhao X., Zhan Y., Teng W., Qiu L., Zheng H., Li W., Han Y. Identification of a candidate gene associated with isoflavone content in soybean seeds using genome-wide association and linkage mapping. Plant J. 2020;104:950–963. doi: 10.1111/tpj.14972. [DOI] [PubMed] [Google Scholar]
- Wu G., Zhao Y., Shen R., Wang B., Xie Y., Ma X., Zheng Z., Wang H. Characterization of maize phytochrome-interacting factors in light signaling and photomorphogenesis. Plant Physiol. 2019;181:789–803. doi: 10.1104/pp.19.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Sun W., Sun Y., Li J., Zhang J., Wu T., Song T., Yao Y., Tian J. MPK6-mediated HY5 phosphorylation regulates light-induced anthocyanin accumulation in apple fruit. Plant Biotechnol. J. 2023;21:283–301. doi: 10.1111/pbi.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Luo D., Lin A., Zhang C., Shan L., He P., Li B., Zhang Q., Hua B., Yuan Z., et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019;221:279–294. doi: 10.1111/nph.15373. [DOI] [PubMed] [Google Scholar]
- Xu D. COP1- and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020;228:1748–1753. doi: 10.1111/nph.16296. [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang Y., Li J., Lin F., Holm M., Deng X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA. 2016;113:7655–7660. doi: 10.1073/pnas.1607687113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Jiang Y., Li J., Holm M., Deng X.W., Deng X.W. B-box domain protein BBX21 promotes photomorphogenesis. Plant Physiol. 2018;176:2365–2375. doi: 10.1104/pp.17.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Wang B., Zhong Y., Yao L., Cheng L., Wu T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015;89:35–48. doi: 10.1007/s11103-015-0349-3. [DOI] [PubMed] [Google Scholar]
- Yang G., Zhang C., Dong H., Liu X., Guo H., Tong B., Fang F., Zhao Y., Yu Y., Liu Y., et al. Activation and negative feedback regulation of SlHY5 transcription by the SlBBX20/21-SlHY5 transcription factor module in UV-B signaling. Plant Cell. 2022;34:2038–2055. doi: 10.1093/plcell/koac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Hu J., Tian X., Yang Z., Fang W. Nitric oxide mediates melatonin-induced isoflavone accumulation and growth improvement in germinating soybeans under NaCl stress. J. Plant Physiol. 2022;279 doi: 10.1016/j.jplph.2022.153855. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tian X., He X., Yang J., Yang Z., Fang W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.) Plant Physiol. Biochem. 2022;185:123–131. doi: 10.1016/j.plaphy.2022.05.033. [DOI] [PubMed] [Google Scholar]
- Yu O., Shi J., Hession A.O., Maxwell C.A., McGonigle B., Odell J.T. Metabolic engineering to increase isoflavone biosynthesis in soybean seed. Phytochemistry. 2003;63:753–763. doi: 10.1016/s0031-9422(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Yin Q., Shen G., Di S., Fan C., Chang Z., Pang Y. Genome-wide identification and functional characterization of UDP-glucosyltransferase genes involved in flavonoid biosynthesis in Glycine max. Plant Cell Physiol. 2017;58:1558–1572. doi: 10.1093/pcp/pcx081. [DOI] [PubMed] [Google Scholar]
- Zanetti M.E., Blanco F.A., Beker M.P., Battaglia M., Aguilar O.M. A C subunit of the plant nuclear factor NF-Y required for rhizobial infection and nodule development affects partner selection in the common bean-Rhizobium etli symbiosis. Plant Cell. 2010;22:4142–4157. doi: 10.1105/tpc.110.079137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G., Li D., Han Y., Teng W., Wang J., Qiu L., Li W. Identification of QTL underlying isoflavone contents in soybean seeds among multiple environments. Theor. Appl. Genet. 2009;118:1455–1463. doi: 10.1007/s00122-009-0994-5. [DOI] [PubMed] [Google Scholar]
- Zhai H., Xiong L., Li H., Lyu X., Yang G., Zhao T., Liu J., Liu B. Cryptochrome 1 inhibits shoot branching by repressing the self-activated transciption loop of PIF4 in Arabidopsis. Plant Commun. 2020;1 doi: 10.1016/j.xplc.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Xing A., Staswick P., Clemente T.E. The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tissue Organ Cult. 1999;56:37–46. [Google Scholar]
- Zhang H., He H., Wang X., Wang X., Yang X., Li L., Deng X.W. Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 2011;65:346–358. doi: 10.1111/j.1365-313X.2010.04426.x. [DOI] [PubMed] [Google Scholar]
- Zhang P., Du H., Wang J., Pu Y., Yang C., Yan R., Yang H., Cheng H., Yu D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2020;18:1384–1395. doi: 10.1111/pbi.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant. 2015;8:17–27. doi: 10.1016/j.molp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Zhou T., Meng C., He P. Soy isoflavones and their effects on xenobiotic metabolism. Curr. Drug Metabol. 2019;20:46–53. doi: 10.2174/1389200219666180427170213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.