Abstract
We have previously shown that human tear fluid protects corneal epithelial cells against Pseudomonas aeruginosa in vitro and in vivo and that protection does not depend upon tear bacteriostatic activity. We sought to identify the responsible tear component(s). The hypothesis tested was that collectins (collagenous calcium-dependent lectins) were involved. Reflex tear fluid was collected from healthy human subjects and examined for collectin content by enzyme-linked immunosorbent assay (ELISA) and Western blot with antibody against surfactant protein D (SP-D), SP-A, or mannose-binding lectin (MBL). SP-D, but not SP-A or MBL, was detected by ELISA of human reflex tear fluid. Western blot analysis of whole tears and of high-performance liquid chromatography tear fractions confirmed the presence of SP-D, most of which eluted in the same fraction as immunoglobulin A. SP-D tear concentrations were calculated at ∼2 to 5 μg/ml. Depletion of SP-D with mannan-conjugated Sepharose or anti-SP-D antibody reduced the protective effect of tears against P. aeruginosa invasion. Recombinant human or mouse SP-D used alone reduced P. aeruginosa invasion of epithelial cells without detectable bacteriostatic activity or bacterial aggregation. Immunofluorescence microscopy revealed SP-D antibody labeling throughout the corneal epithelium of normal, but not gene-targeted SP-D knockout mice. SP-D was also detected in vitro in cultured human and mouse corneal epithelial cells. In conclusion, SP-D is present in human tear fluid and in human and mouse corneal epithelia. SP-D is involved in human tear fluid protection against P. aeruginosa invasion. Whether SP-D plays other roles in the regulation of other innate or adaptive immune responses at the ocular surface, as it does in the airways, remains to be explored.
The opportunistic gram-negative bacterial pathogen Pseudomonas aeruginosa is a leading cause of human morbidity and mortality from acute pneumonia in patients with immunocompromised states, burn wound infections, chronic respiratory disease with cystic fibrosis, or sight-threatening corneal infections. Corneal infection by P. aeruginosa progresses quickly, can be highly destructive, and is difficult to treat. The most common predisposing factor is contact lens wear, but infection can also follow corneal injury or ocular surface disease. At least two types of P. aeruginosa have been isolated from infections: those that invade epithelial cells (13) and those that cause ExoU-dependent cytotoxicity mediated by the ExsA-regulated type III secretion system (12). Both invasive and cytotoxic strains can target surface cells on intact corneas in vitro (11, 14), but neither can infect healthy corneas in vivo in the absence of surface injury. This suggests that factors found only in vivo are required for protecting the corneal epithelium against these P. aeruginosa virulence mechanisms.
A common assumption is that tear fluid protects the cornea from bacterial infection through the bacteriostatic or bactericidal actions of antimicrobials such as lysozyme, lactoferrin, secretory phospholipase A2, and β-defensins. It was previously reported that whole human tear fluid, which normally bathes the ocular surface, protects corneal epithelial cells in vitro from P. aeruginosa invasion and cytotoxicity (10) and that in vivo it protects the cornea against P. aeruginosa colonization and disease in a murine model of bacterial keratitis (M. Ni, M. Kwong, and S. M. J. Fleiszig, Abstr. Investig. Ophthalmol. Vis. Sci. 44 [Suppl.], 2003, abstr. no. 4757, 2003). However, in vitro protective activity was not dependent upon inhibition of bacterial growth or loss of bacterial motility (10).
Surfactant protein D (SP-D) is part of the collectin (collagenous calcium-dependent lectin) family of innate defense molecules, other family members in humans being SP-A and mannan-binding lectin (MBL) (6). These oligomeric proteins share certain structural and functional traits. For example, they recognize clustered sugar residues common to microbial surfaces including mannose, glucose, l-fucose, and N-acetylglucosamine via acarbohydrate recognition domain and bind those residues in a calcium-dependent fashion (19). Collectins do not readily recognize common mammalian cell carbohydrates, e.g., sialic acid, thereby facilitating self versus non-self discrimination.
Collectin binding to microorganisms (bacteria, viruses, protozoa, etc.) has been associated with microbial aggregation, inhibition of microbial growth, complement activation, stimulation of macrophage cytokine production, and the enhancement of microbial phagocytosis by immune cells (5, 21, 29, 33, 39). However, these antimicrobial effects vary between different microbial genera, species, or strains. For example, SP-D can also reduce microbial phagocytosis by immune cells for Mycobacterium tuberculosis (9) and Pneumocystis carinii (40), and the aggregative and growth inhibitory actions of SP-D are strain dependent (5, 39). However, SP-D-deficient animals are more susceptible to lung infection by Haemophilus influenzae (25) and exhibit delayed clearance of P. carinii (3) and respiratory syncytial virus (24) from the airways. Cystic fibrosis sufferers, who are vulnerable to chronic respiratory infection by P. aeruginosa, exhibit markedly reduced levels of SP-D in their lungs (30).
The effects of SP-D on bacterial invasion of epithelial cells have not been studied. In this study, we hypothesized that SP-D was present in human tear fluid and contributed to tear fluid protection of corneal epithelial cells against P. aeruginosa invasion. This hypothesis was based upon previously published data showing (i) the presence of SP-D in the human lacrimal gland (37) and in the lacrimal gland and tear film of C57BL/6 mice (1) and (ii) that SP-D can interact with gram-negative bacteria via the core region of the bacterial lipopolysaccharide (LPS) (26); the LPS core is involved in P. aeruginosa invasion of and survival within corneal epithelial cells (8, 41).
MATERIALS AND METHODS
Tear collection.
Tear fluid was collected from the lower conjunctival sacs of healthy human volunteers by the capillary tube method previously described (10). This method was approved by the Committee for the Protection of Human Subjects, University of California, Berkeley, Calif. A tear volume of 100 μl was collected over approximately 15 min on each occasion. Collected tears were pooled, frozen, and stored at −20°C for use in experiments.
Cell culture.
Rabbit and human immortalized corneal epithelial cells were cultured on 6-well (for preparation of cell lysates) or 96-well (for gentamicin survival assays) tissue culture plates (Becton Dickinson, Franklin Lake, N.J.), as previously described (12, 28). Cells were fed on alternate days with supplemented hormonal epithelial medium, which has been described previously (20). Cells were used for experiments 3 to 6 days after passage.
Primary cultures of mouse corneal epithelial cells were grown on 6-well tissue culture plates and prepared as previously described (16). Female C57BL/6 mice (8 to 10 weeks old) were obtained from Jackson Laboratories (Bar Harbor, Maine). Gene-targeted C57BL/6 SP-D-deficient mice were generated as described previously (4). All procedures were conducted in accordance with the policies established by Association for the Research in Vision and Ophthalmology and under protocols approved by the institutional animal care and use committee.
Prior to each experiment, wells containing cultured cells were each washed once with phosphate-buffered saline (PBS) to remove residual supplemented hormonal epithelial medium that contains antibiotics.
Bacteria.
P. aeruginosa strain PAK was used. PAK is classified as an invasive clinical isolate, i.e., it invades corneal epithelial cells as demonstrated by gentamicin survival assays (10, 12, 14). Bacterial inocula were prepared from overnight cultures grown on Trypticase soy agar plates at 37°C before suspension in Hank's balanced salt solution (HBSS). Bacteria were initially suspended to a concentration of 108 CFU/ml (determined by a spectrophotometer optical density at 650 nm of 0.1). This suspension was then diluted to a concentration of 106 CFU/ml of HBSS, human tear fluid, or HBSS containing recombinant SP-D for use in experiments. Bacterial numbers were confirmed by viable counts after serial dilution.
Assessment of bacterial invasion.
P. aeruginosa invasion of corneal epithelial cells was quantified by gentamicin exclusion assays as previously described (10, 12). Cultured rabbit corneal epithelial cells were incubated with bacterial suspensions for 3 h at 37°C and then treated with gentamicin solution (200 μg/ml) to kill extracellular bacteria. After being washed to remove the antibiotic, the corneal cells were lysed by exposure to PBS containing Triton X-100 (0.25% [vol/vol]) for 15 min. Viable counts of the cell lysate allowed quantification of previously intracellular bacteria. Trypan blue exclusion assays were used to assess corneal epithelial cell health (15).
Assays of bacterial growth, motility, and aggregation.
The effects of human tear fluid and recombinant SP-D on bacterial growth, mobility, and aggregation were tested as previously described (10). Briefly, bacterial growth and viability were assessed with and without the corneal epithelial cells present. Thus, 40 μl of bacterial suspension, in either HBSS, tear fluid, or HBSS containing recombinant SP-D, was added to the empty wells of tissue culture dishes or to wells containing corneal epithelial cell cultures. Following a 3- or 5-h incubation at 37°C, 5 μl of bacterial suspension was collected for quantification by viable counting after serial dilution. The number of bacteria present in each well at the end of the experiment was compared to the starting inoculum to study bacterial growth and/or killing. Bacterial mobility and aggregation were assessed by phase-contrast light microscopy of the same samples.
Reagents.
Rabbit anti-mouse polyclonal SP-D antibody (4) and recombinant mouse SP-D (31) were used. Rabbit anti-human SP-D antibody and recombinant human SP-D were generously provided by Erika Crouch (Washington University, St. Louis, Mo.). All other reagents were obtained from Sigma (St. Louis, Mo.) unless otherwise stated.
ELISA for SP-D detection.
Wells of a flexible polyvinyl microtiter plate (Dynatech) were coated overnight at room temperature with 50 μl of yeast mannan at 100 μg/ml in TBS (20 mM Tris-HCl, 140 mM NaCl [pH 7.4]) and then blocked for 1 h with 10 mg of bovine serum albumin (BSA) per ml. After this and subsequent steps, plates were washed five times with TBS containing 0.05% (vol/vol) Tween-20 (TBST). For each tear sample, serial twofold dilutions were prepared in TBST containing 5 mg of BSA per ml and either 5 mM CaCl2 (BSA5-TBST-Ca2+) or 5 mM EDTA (BSA5-TBST-EDTA) and applied to coated wells in 50-μl volumes. Dilutions of recombinant human SP-D were prepared similarly to produce a calibration curve, commencing at a concentration of 1 μg/ml in the first well. Plates were incubated overnight and then washed. Binding of SP-D was detected by the addition of rabbit antiserum against human SP-D (dilution, 1:400 in BSA5-TBST-Ca2+) for 3 h followed, after washing, by horseradish peroxidase (HRPO)-conjugated swine anti-rabbit immunoglobulin (Ig) (Dako; 1:400 in the same diluent) for 1 h. After further washing, 100 μl of ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] substrate solution (0.2 mM in 50 mM citrate buffer [pH 4.0] containing 0.004% H2O2) was added to the wells. Color development was stopped after 20 min by the addition of 50 μl of NaF (1.92 mg/ml), and absorbance was read at dual wavelengths of 405 and 450 nm. The concentration of SP-D in samples was determined by reference to the standard curve.
Removal of SP-D from human tear fluid.
Two separate methods were used to deplete tear fluid of SP-D: (i) adsorption with mannan-conjugated Sepharose and (ii) immunoprecipitation with antibody specific for SP-D.
(i) Adsorption with mannan-conjugated Sepharose.
Mannan was coupled to CNBr-activated Sepharose 4B-CL (Pharmacia) according to the manufacturer's instructions to give 10 mg of mannan per ml of packed beads. The gel was stored at 4°C in final wash buffer (0.1 M Tris-HCl, 0.5 M NaCl [pH 8]) with 0.1% NaN3. Since TBS adversely affected corneal cells (data not shown), complement fixation test buffer (veronal-buffered saline [VBS]), which was not toxic, was used as the diluent for adsorption of tears with mannan-Sepharose. VBS (4 mM barbitone, 140 mM NaCl, 1.8 mM MgCl2, 0.25 mM CaCl2) was supplemented with an additional 10 mM CaCl2 for adsorption experiments. An aliquot of mannan-Sepharose beads was washed three times with VBS-10 mM Ca2+, and the pellet of packed beads was gently resuspended in an equal volume of human tear fluid. The mixture was incubated on ice for 2 h with occasional gentle agitation. The mixture was then centrifuged, and the supernatant was taken and further centrifuged in a microfuge (∼10,000 × g) to ensure complete removal of beads from the tear fluid. The procedure resulted in a 1:2 dilution of tears (final concentration of Ca2+, 5 mM); to establish an “unadsorbed” tear control, an aliquot of tears was diluted directly 1:2 in VBS-10 mM Ca2+. The samples were assayed for SP-D by ELISA, as described above, to determine the extent of SP-D removal by the adsorption process.
(ii) Immunoprecipitation with antibody specific for SP-D.
For the removal of SP-D from human tear fluid by immunoprecipitation, volumes of whole tear fluid (100 to 300 μl) were mixed with 5 to 10 μg of rabbit anti-human SP-D antibody and incubated overnight at 4°C with gentle agitation. Complexes of SP-D bound to specific antibody were then removed from the tear fluid with protein A and by standard methods.
High-performance liquid chromatography (HPLC).
Human tear fluid (0.150 to 1 ml) was separated isocratically on an SW 3000 preparative column in 0.5 M NaCl-0.1 M phosphate buffer (pH 5.0) at a flow rate of 2 ml/min with the eluent monitored at 254 nm. Fractions were collected based upon the apparent molecular size in a fashion similar to that detailed previously with an SW 400 analytical column (34). This profile was determined based upon the literature, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and Western blot probing with antibodies specific for various mucin species, secretory IgA, IgG, lactoferrin, free secretory component tear-specific lipochalin, and lysozyme, much of which has been previously detailed. In this study, the high-molecular-mass end of the profile was further subdivided into several fractions. These consisted, in order of decreasing apparent molecular mass, of a sialoglycoprotein-enriched fraction (containing mucins) and front, middle, and tail end of the secretory IgA-enriched fraction. All of the fractions were concentrated on 3-kDa cutoff centrifugal ultrafilters (Filtron, Northborough, Mass.).
SDS-PAGE and Western immunoblotting.
Rabbit and human corneal epithelial cells were grown on six-well tissue culture dishes. Cells were washed once with ice-cold PBS (2 ml) and then lysed with 1 ml of a boiling solution of SDS (1% [wt/vol]), sodium orthovanadate (1 mM), and Tris-HCl (10 mM; pH 7.4). Cells were then scraped off the plastic, and the cell lysate was transferred to a microcentrifuge tube (1.5 ml), and boiled for 5 min. Viscosity was reduced by passaging the lysate five times through a 27-gauge needle. The lysate was centrifuged (14,000 × g; 4°C) for 5 min to remove cell debris, and the supernatant was collected. The protein concentration was measured for each sample with a BCA assay kit (Sigma). Equal amounts of each sample (each equivalent to ∼15 μg of protein) were mixed with double-strength SDS-PAGE sample buffer (under reducing conditions), boiled for 1 min, then subjected to SDS-PAGE (20 mA; 1.5 h) with precast Tris-HCl polyacrylamide gels (10% polyacrylamide; Bio-Rad, Hercules, Calif.). Tear samples collected from human subjects were also subjected to SDS-PAGE but under nonreducing conditions, by mixing an equal volume of whole tear fluid or HPLC-fractionated human tear fluid (see the method described above) with double-strength SDS-PAGE sample buffer without β-mercaptoethanol.
After SDS-PAGE, proteins were transferred to nitrocellulose membranes for detection by Western immunoblotting (30 V overnight at 4°C). Membranes were blocked overnight at 4°C using 5% (wt/vol) skim milk with 0.1% (vol/vol) Tween-20 in Tris-buffered saline. To detect SP-D, membranes were incubated for 1 h at room temperature with primary antibody solution consisting of either rabbit anti-mouse or rabbit anti-human SP-D antibody diluted 1:100 in blocking buffer. After removal of primary antibody solution, membranes were washed six times (5 min per wash) with TBS-Tween 20 (0.1% [vol/vol]) and then exposed for 1 h at room temperature to a secondary antibody (HRPO-conjugated goat anti-rabbit antibody) diluted 1:1,000 in blocking buffer. After removal of the secondary antibody solution, membranes were again washed six times (5 min per wash) with TBS-Tween 20 (0.1% [vol/vol]), and antibody-bound proteins were visualized by enhanced chemiluminescence (NEN, Belmont, Mass.) and with Kodak X-Omat film.
Immunohistochemistry and fluorescence microscopy.
Freshly harvested mouse eyes were immersion fixed in 4% (wt/vol) paraformaldehyde in PBS. After being rinsed several times in 0.1 M PBS, the eyes were cryoprotected by overnight immersion in sucrose solution (30% [wt/vol] in PBS). The mouse eyes were embedded in optimal cutting temperature medium (Ted Pella, Redding, Calif.), frozen at −20°C, and cryosectioned vertically at 10 μm. Cryosections were blocked for 1 h with a solution of PBS containing 1% (wt/vol) BSA, 10% (vol/vol) goat serum, and 0.3% (vol/vol) Triton X-100. After this time, sections were incubated with primary antibody solution (rabbit anti-mouse SP-D antibody diluted 1:100 in blocking buffer) for 1 h at room temperature. After being washed with PBS containing 1% (wt/vol) BSA and 0.2% (vol/vol) Triton X-100, the sections were incubated for 1 h at room temperature with a secondary antibody solution consisting of goat anti-rabbit IgG conjugated to Alexa-Fluor 594 (Molecular Probes, Eugene, Oreg.) diluted 1:5,000 in blocking buffer. Sections were examined with a fluorescence microscope. Images were captured and processed by computer with the Improvision image analysis system.
Statistical analysis.
For gentamicin survival (invasion) assays, at least four wells were used for each group of samples in all experiments, which were repeated at least twice. The Student t test and analysis of variance (ANOVA) were used to analyze the data. P values of <0.05 were considered significant.
RESULTS
SP-D is present within human tear fluid.
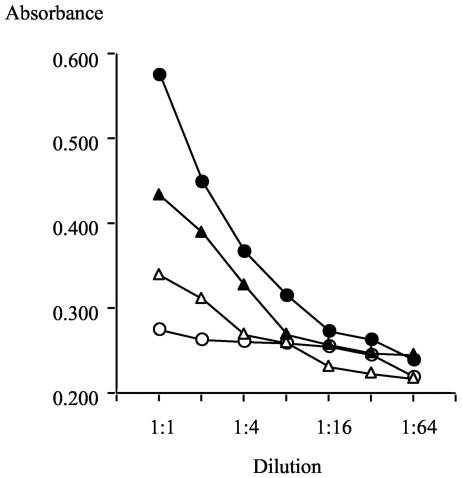
Whole human tear fluid was assayed for the presence of SP-D, SP-A, and MBL by ELISA with mannan as a substrate for collectin binding and in the presence or absence of EDTA to test for calcium dependence of the collectin-mannan interaction. SP-A and MBL were not detected in tear fluid (data not shown), but SP-D was present (Fig. 1), and the reduction of binding in the presence of EDTA confirmed the calcium-dependent binding of tear SP-D to mannan (Fig. 1). There remained, however, a component of tear fluid binding that was not EDTA sensitive. Recombinant human SP-D was included in the same assay (Fig. 1), and the complete calcium dependence of its binding to mannan was verified. By plotting absorbance versus concentration on a linear scale for recombinant human SP-D at concentrations of 1, 0.5, 0.25 and 0.125 μg/ml, a linear calibration curve was obtained (y = 0.292x + 0.290; r = 0.995). From the absorbance values for tears at 1/10, 1/20, and 1/40 dilutions, the concentration of SP-D in tear fluid was calculated to be 4.9, 6.8, and 5.1 μg/ml, respectively. When the absorbance value for each of these tear dilutions was corrected to subtract absorbance contributed by the EDTA-insensitive binding component, the calculated estimates for SP-D in tears became 1.8, 2.5, and 2.1 μg/ml, respectively. Since the nature of the EDTA-insensitive mannan binding is not known but could include a subset of SP-D complexed with other tear components, the concentration of SP-D in this tear fluid sample was concluded to lie within the range of 2 to 5 μg/ml. Similar results were obtained with other human tear fluid samples.
FIG. 1.
Detection of SP-D in human tear fluid by ELISA. Human tear fluid (triangles) and recombinant human SP-D (circles) were titrated on mannan-coated wells in the presence of 5 mM Ca2+ (solid symbols) or 5 mM EDTA (open symbols). The dilution series commenced at 1 μg of recombinant human SP-D/ml and at a 1:10 dilution of tear fluid. The assay was developed with rabbit anti-human SP-D antibody and HRPO-conjugated swine anti-rabbit Ig, as described in Materials and Methods. Human tear fluid was calculated to contain between 2 to 5 μg of SP-D/ml (see text).
Whole human tear fluid and HPLC-fractionated tear fluid were separated by SDS-PAGE under nonreducing conditions to determine if SP-D was present as monomeric, trimeric, or higher-order aggregative forms. Western blot analysis confirmed the presence of SP-D in human tear fluid and showed two tear proteins labeled by the anti-SP-D antibody. The same two SP-D antibody-labeled proteins eluted in fraction 3 of HPLC-separated tears (Fig. 2).
FIG. 2.
Western immunoblot analysis of whole and HPLC-fractionated human tear fluid that was subjected to SDS-PAGE under nonreducing conditions. Rabbit anti-human SP-D antibody detected SP-D as a large aggregate(s) (>200 kDa) in human tear fluid. The same aggregates eluted in HPLC fraction 3.
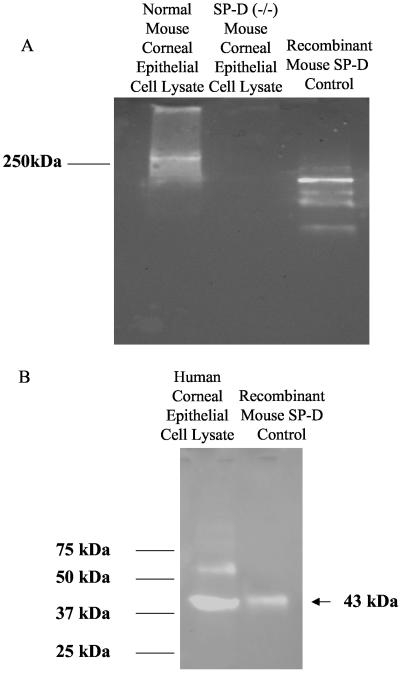
SP-D was recovered from cultured human and mouse corneal epithelial cells.
To determine if SP-D was also present in the cornea, cultured mouse and human corneal epithelial cells were lysed and subjected to SDS-PAGE, and the presence of SP-D was determined by Western immunoblot with recombinant mouse SP-D as a control and rabbit anti-mouse SP-D antibody (cross-reactive with human SP-D). Under nonreducing conditions, SP-D aggregates (molecular mass, >250 kDa) were detected in primary cultured mouse corneal epithelial cells derived from C57BL/6 mice but not in lysates of corneal epithelial cells derived from gene-targeted C57BL/6 SP-D knockout mice (Fig. 3A) (4). SP-D was also detected in human corneal epithelial cell lysates (reducing conditions were used in this instance to demonstrate the SP-D monomer of 43 kDa) (Fig. 3B). The nature of the second protein band (∼62 kDa) detected under reducing conditions in human corneal cell lysates is not known, but it was also present in wild-type, but not knockout, mouse corneal epithelial cell lysates under reducing conditions (data not shown), suggesting that it is SP-D related.
FIG. 3.
Western immunoblots of cell lysates derived from primary cultured mouse corneal epithelial cells (separated by SDS-PAGE under nonreducing conditions) (A) or cultured human corneal epithelial cells (separated by SDS-PAGE under reducing conditions) (B). Purified recombinant mouse SP-D was used as a positive control. Rabbit anti-mouse SP-D antibody detected high-molecular-mass aggregates of SP-D in normal but not SP-D knockout mice (A) and an SP-D monomer (∼43 kDa) and a larger protein (∼62 kDa) in cultured human corneal epithelial cells (B).
SP-D was present within the murine cornea in vivo.
Having demonstrated the presence of SP-D in human tear fluid and in cultured human and mouse corneal epithelial cells, it was then of interest to know whether SP-D could be detected in the cornea in vivo. For this purpose, normal mouse corneas (C57BL/6 mice) were cryosectioned (see Materials and Methods) to 10-μm thickness, stained with rabbit anti-mouse SP-D antiserum, and examined by immunofluorescence microscopy. Control sections were prepared in which the SP-D-specific antibody was not included. Corneas from SP-D-deficient gene-targeted knockout mice were also used as controls. SP-D antiserum was found to label the entire corneal epithelium of normal, but not of SP-D-deficient, mice (Fig. 4).
FIG. 4.
Detection of SP-D in the cornea by immunofluorescence microscopy. (A) Normal mouse cornea viewed by immunofluorescence microscopy (magnification, ×1,200) with (left) and without (right) exposure to rabbit anti-mouse SP-D antiserum (dilution, 1:100). (B) Another experiment in which sections of wild-type (left) and SP-D (−/−) knockout (right) mouse corneas were exposed to the same SP-D-specific antiserum (dilution, 1:100). SP-D was strongly expressed in the corneal epithelium of the wild-type mouse.
Subtraction of SP-D from whole human tear fluid reduced protection against P. aeruginosa invasion of corneal epithelial cells in vitro.
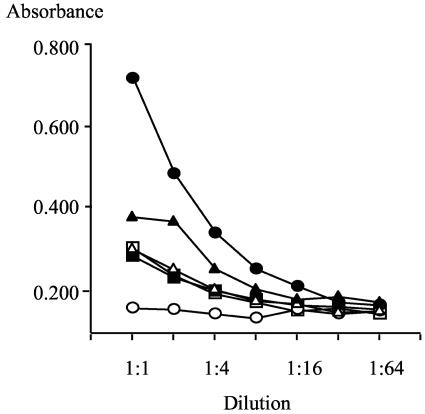
To determine if SP-D contributed to the ability of human tear fluid to protect corneal epithelial cells against P. aeruginosa invasion, SP-D was subtracted from whole human tear fluid, and the effect on tear cytoprotection was examined. Whole human tear fluid was adsorbed with mannan-conjugated Sepharose in the presence of calcium to subtract SP-D. An assay of adsorbed and unadsorbed tear samples by ELISA indicated the presence of ∼2 to 5 μg of SP-D/ml in the unadsorbed tear fluid and showed depletion of SP-D by mannan-Sepharose adsorption (Fig. 5).
FIG. 5.
Subtraction of SP-D from human tear fluid with mannan-conjugated Sepharose. Whole human tear fluid (triangles), mannan-Sepharose adsorbed human tear fluid (squares), and recombinant human SP-D (circles) were titrated on mannan-coated wells in the presence of 5 mM Ca2+ (solid symbols) or 5 mM EDTA (open symbols). The dilution series commenced at 1 μg of recombinant human SP-D/ml and at a 1:10 dilution of the tear fluid samples. The ELISA was developed as described in the legend to Fig. 1. Whole tear fluid contained ∼2 to 5 μg of SP-D/ml, and this was depleted by adsorption with mannan-conjugated Sepharose.
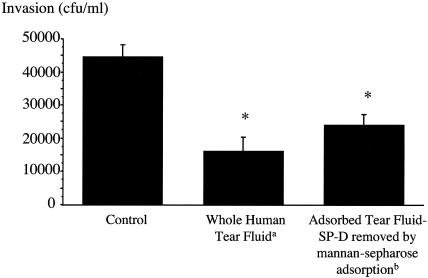
Once subtraction of SP-D was demonstrated, gentamicin survival assays were then used to determine if tear fluid protection of corneal epithelial cells against P. aeruginosa invasion was affected. Unprocessed human tear fluid inhibited invasion of P. aeruginosa strain PAK into corneal epithelial cells, as previously described (10). However, mannan-Sepharose-adsorbed tear fluid (SP-D depleted) showed a reduced ability to protect against P. aeruginosa invasion (P = 0.03; t test) (Fig. 6).
FIG. 6.
Gentamicin survival assay showing that the ability of human tear fluid to protect cultured rabbit corneal epithelial cells against P. aeruginosa invasion (strain PAK) was reduced after SP-D subtraction from tear fluid with mannan-conjugated Sepharose. *, P < 0.05 versus control, by ANOVA; a, tears diluted 1:2 to match the dilution of adsorbed tears due to adsorption procedure (see Materials and Methods); b, 49% increase in bacterial invasion when compared to whole tear fluid (P = 0.03; t test).
This latter finding was consistent with a role for SP-D in tear cytoprotection against P. aeruginosa invasion of epithelial cells, although it does not preclude the involvement of other mannan-binding proteins in tear fluid. To examine further the contribution of SP-D to tear cytoprotection, rabbit anti-human SP-D antibody was used to immunoprecipitate the SP-D from whole human tear fluid. Western immunoblot confirmed subtraction, but not complete removal, of SP-D from tear fluid by this method (data not shown). Gentamicin survival assays showed that SP-D antibody treatment of tear fluid reduced tear protection of corneal epithelial cells against P. aeruginosa invasion (P = 0.04; t test) (Fig. 7).
FIG. 7.
Gentamicin survival assay showing that tear fluid protection against P. aeruginosa strain PAK invasion of cultured rabbit corneal epithelial cells was reduced after SP-D subtraction from tear fluid with rabbit anti-human SP-D antibody.*, P < 0.05 versus control, by ANOVA; a, 39% increase in invasion versus whole tears (P = 0.04; t test).
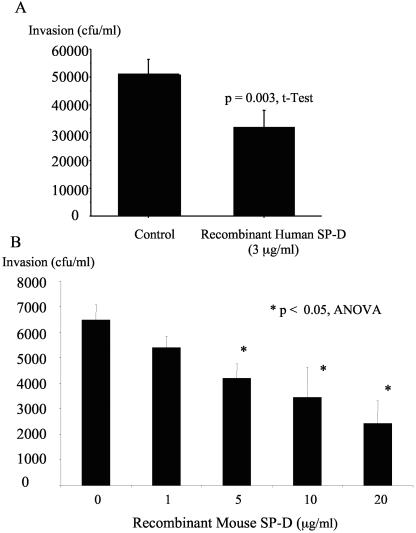
Recombinant SP-D reduced P. aeruginosa invasion of corneal epithelial cells.
Having shown that SP-D depletion from tear fluid reduced its cytoprotective ability against bacterial invasion, the effect of SP-D used alone on P. aeruginosa invasion of corneal epithelial cells was explored. By gentamicin survival assays, recombinant human SP-D (3 μg/ml) reduced corneal epithelial invasion by strain PAK by ∼37% (P = 0.003; t test) (Fig. 8A). Recombinant mouse SP-D also reduced P. aeruginosa invasion of corneal epithelial cells in a dose- dependent manner, with significant inhibition of invasion at 5 μg/ml (35% inhibition), 10 μg/ml (47% inhibition), and 20 μg/ml (62% inhibition) (P < 0.05; ANOVA) (Fig. 8B). SP-D did not significantly inhibit invasion at 1 μg/ml. Trypan blue exclusion assay controls in each experiment confirmed that recombinant SP-D was not toxic to the cultured corneal epithelial cells (data not shown).
FIG. 8.
The effect of recombinant SP-D on P. aeruginosa invasion of cultured rabbit corneal epithelial cells. (A) Human recombinant SP-D inhibited corneal epithelial invasion by P. aeruginosa strain PAK when compared to control tissue culture media (HBSS). (B) Mouse recombinant SP-D significantly inhibited invasion by PAK at 5, 10, and 20 μg/ml (P < 0.05; ANOVA). SP-D at a concentration of 1 μg/ml did not significantly inhibit invasion.
Recombinant mouse SP-D did not affect the growth or swimming motility of P. aeruginosa strain PAK, nor did it aggregate bacteria at concentrations inhibitory to bacterial invasion.
In a previous study with 10 different isolates of P. aeruginosa (including strain PAK used in the present study), we reported that whole human tear fluid could inhibit the growth of some P. aeruginosa strains including PAK but caused the loss of swimming motility and bacterial aggregation-clumping or chain formation in all strains (10). The role of SP-D in these effects of tear fluid was explored. Examination of SP-D-subtracted tears (either by mannan-conjugated Sepharose or immunoprecipitation methods) showed that SP-D depletion did not affect the ability of tear fluid to inhibit the growth of this P. aeruginosa strain. For example, over a 3-h time period, numbers of viable strain PAK growing in control buffer (VBS) increased by 3.925 × 107 CFU/ml, whereas in diluted tear fluid and adsorbed tear fluid (SP-D subtracted), bacterial growth was reduced similarly (bacterial numbers increased by 1.545 × 107 and 1.585 × 107 CFU/ml, respectively). SP-D depletion did not affect tear fluid-mediated inhibition of P. aeruginosa swimming motility or bacterial clumping and aggregation (data not shown).
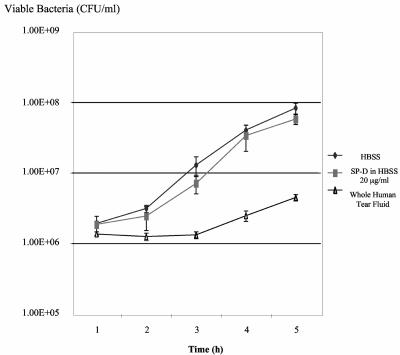
Experiments with recombinant mouse SP-D confirmed those results. The highest concentration of SP-D that was used to inhibit P. aeruginosa strain PAK invasion of corneal epithelial cells (20 μg/ml) (Fig. 8B), did not significantly inhibit the growth of this P. aeruginosa strain over a 5-h time period, compared to HBSS controls (P < 0.05; ANOVA) (Fig. 9). SP-D did not inhibit the swimming motility of P. aeruginosa strain PAK over 5 h (data not shown), nor did it cause observable bacterial chain formation, clumping, or aggregation (Fig. 10).
FIG. 9.
Effect of recombinant mouse SP-D on the growth of P. aeruginosa. Recombinant SP-D (20 μg/ml) in HBSS did not significantly affect the growth of strain PAK over a 5-h time period when compared to a HBSS control. Human tear fluid was bacteriostatic to this strain, as previously reported (10). ⧫, HBSS; ▪, SP-D in HBSS (20 μg/ml); ▴, whole human tear fluid.
FIG. 10.
Light microscopy of P. aeruginosa strain PAK in the presence of control media (HBSS) (A), recombinant mouse SP-D (20 μg/ml) (B), or whole human tear fluid (C). SP-D did not cause significant visible aggregation of this P. aeruginosa strain over a 5-h time period compared to tear fluid (magnification, approximately ×1,500).
DISCUSSION
The data collected in this study demonstrate that SP-D is present within human tear fluid, cultured human and mouse corneal epithelial cells, and the mouse cornea in vivo. They also show that SP-D contributes to tear fluid cytoprotection against corneal epithelial cell invasion by P. aeruginosa by mechanisms not dependent upon inhibition of bacterial growth or swimming motility and without visible bacterial aggregation.
Detection of SP-D, but not SP-A or MBL, in human tear fluid is consistent with the results of previous studies showing the presence of SP-D, but not of SP-A, mRNA and protein in mouse lacrimal gland and tear fluids (1) and in the human lacrimal gland (37). Recent studies have also shown the presence of SP-D in the human female reproductive tract (23). The detection of SP-D in multiple sites outside of the respiratory tract suggests a broader role for collectins in human innate immunity (1, 37). The data presented in this study showing that SP-D contributes to tear protection of epithelial cells against invasion by an opportunistic bacterial pathogen support that hypothesis by suggesting an additional role for this collectin in human innate immunity.
Epithelia are important sites for SP-D synthesis in the lung (6). At least some of the detected tear fluid SP-D is likely to be derived from the lacrimal gland (1, 37). Detection of SP-D in the intact mouse corneal epithelium raises the possibility that some of the tear fluid SP-D could have originated from the ocular surface. The finding that SP-D was also detected in cultured human and mouse corneal epithelial cells that were passaged many times in vitro supports this possibility by showing that corneal epithelial cells are capable of synthesizing SP-D, at least in vitro. Whether SP-D is also synthesized by human corneal epithelial cells in vivo and if it contributes to tear fluid SP-D levels is yet to be determined.
For P. aeruginosa strain PAK, SP-D did not cause obvious aggregation of bacteria, inhibition of bacterial growth, or loss of bacterial motility, thereby excluding these factors as mechanisms for the inhibition of bacterial invasion. Studies of the interaction of P. aeruginosa with monocytes and macrophages have shown that SP-D-enhanced phagocytosis of this bacterium can occur without visible aggregation of the bacteria (5, 32). Interestingly, it has been shown that SP-D can bind avidly to the LPS core and/or O antigen of gram-negative bacteria, e.g., Escherichia coli, Klebsiella pneumoniae, and P. aeruginosa in a calcium-dependent manner (22, 26, 29, 35), and SP-D binding to LPS can inhibit the adhesion of K. pneumoniae to airway epithelial cells in vitro (35). SP-D can also exhibit growth inhibitory effects on certain gram-negative bacteria, especially LPS-rough strains of E. coli and K. pneumoniae (39). SP-D can bind to both LPS rough and smooth strains of P. aeruginosa and enhances their phagocytosis by monocytes (5). Since the LPS core is a ligand for internalization of P. aeruginosa by epithelial cells (41) and is used for survival within those cells after invasion (8), the mechanism by which SP-D inhibits epithelial cell invasion could involve LPS interactions. SP-D has been shown to have opposite effects on P. aeruginosa internalization by macrophages and epithelial cells. Whether those findings reflect different internalization mechanisms between the two cell types is not yet clear. Nevertheless, each would contribute to innate immune defense against this opportunist bacterial pathogen.
Recent studies have shown that SP-D is susceptible to degradation by P. aeruginosa elastase and phagocyte-derived proteases (2, 38). These actions could reduce invasion inhibitory effects of SP-D in vitro or in vivo.
SP-D derived from human tear fluid and primary cultured mouse corneal epithelial cells was found to exist in a high-molecular-mass form(s). In humans, SP-D exists as a trimer (three 43-kDa monomers) but is known to associate to form dodecamers and other high-order forms that could include associations with glycoprotein 340 (GP-340, also known as DMBT1), a putative receptor for SP-D (7, 18, 19), or secretory IgA, which has been shown to associate with MBL (33). Both GP-340 and secretory IgA are present in human tear fluid (17, 27, 36). Thus, the SP-D forms observed in this study could represent aggregates of SP-D or SP-D complexed with GP-340, IgA, or other tear-cellular factors. (Interestingly, tear SP-D eluted in the same HPLC fraction as secretory IgA, an established tear defense against P. aeruginosa [27]).
The detection of SP-D in human tears by mannan-binding ELISA also revealed the presence of an additional component displaying calcium-independent binding to the wells. It is not known if this represents a subset of SP-D complexed with another tear component(s). This tear component was not removed by adsorption with mannan-Sepharose and could contribute to the residual invasion inhibitory activity of the adsorbed tear fluid.
In conclusion, the data collected in this study showed that SP-D was involved in tear fluid protection against P. aeruginosa invasion (10) without contributing to the ability of human tear fluid to inhibit bacterial growth, cause bacterial aggregation, or effect the loss of bacterial motility. Demonstration of SP-D in human tear fluid and corneal epithelium, combined with its ability to inhibit P. aeruginosa invasion of corneal epithelial cells, represents a new development in our understanding of ocular surface innate immunity and in the roles played by SP-D in defending epithelial surfaces against microbial pathogens. Given the multifactorial role(s) of SP-D in the respiratory tract, it will be important to determine the full extent to which SP-D contributes to ocular surface immunity.
Acknowledgments
This work was supported by a research grant from the National Institutes of Health (R01-EY11221) awarded to S.M.J.F.
We gratefully acknowledge Erika Crouch, Washington University, St. Louis, Mo., for providing recombinant human SP-D and rabbit anti-human SP-D antibody.
Editor: V. J. DiRita
REFERENCES
- 1.Akiyama, J., A. Hoffman, C. Brown, L. Allen, J. Edmondson, F. Poulain, and S. Hawgood. 2002. Tissue distribution of surfactant proteins A and D in the mouse. J. Histochem. Cytochem. 50:993-996. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn, J. F., and J. R. Wright. 2004. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J. Biol. Chem. 279:30871-30879. [DOI] [PubMed] [Google Scholar]
- 3.Atochina, E. N., A. J. Gow, J. M. Beck, A. Haczku, A. Inch, H. Kadire, Y. Tomer, C. Davis, A. M. Preston, F. Poulain, S. Hawgood, and M. F. Beers. 2004. Delayed clearance of Pneumocystis carinii infection, increased inflammation, and altered nitric oxide metabolism in lungs of surfactant protein-D knockout mice. J. Infect. Dis. 189:1528-1539. [DOI] [PubMed] [Google Scholar]
- 4.Botas, C., F. Poulain, J. Akiyama, C. Brown, L. Allen, J. Goerke, J. Clements, E. Carlson, A. M. Gillespie, C. Epstein, and S. Hawgood. 1998. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 95:11869-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bufler, P., B. Schmidt, D. Schikor, A. Bauernfeind, E. C. Crouch, and M. Griese. 2003. Surfactant protein A and D differently regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 28:249-256. [DOI] [PubMed] [Google Scholar]
- 6.Crouch, E., and J. R. Wright. 2001. Surfactant proteins a and d and pulmonary host defense. Annu. Rev. Physiol. 63:521-554. [DOI] [PubMed] [Google Scholar]
- 7.Crouch, E. C. 2000. Surfactant protein-D and pulmonary host defense. Respir. Res. 1:93-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, D., T. Kuo, M. Kwong, R. Van, and S. Fleiszig. 2002. Pseudomonas aeruginosa strains with lipopolysaccharide defects exhibit reduced intracellular viability after invasion of corneal epithelial cells. Exp. Eye Res. 75:635-643. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, J. S., D. R. Voelker, J. A. Ufnar, A. J. Dawson, and L. S. Schlesinger. 2002. Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is independent of bacterial agglutination. J. Immunol. 168:1309-1314. [DOI] [PubMed] [Google Scholar]
- 10.Fleiszig, S. M., M. S. Kwong, and D. J. Evans. 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71:3866-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig, S. M., E. J. Lee, C. Wu, R. C. Andika, V. Vallas, M. Portoles, and D. W. Frank. 1998. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 24:41-47. [PubMed] [Google Scholar]
- 12.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig, S. M., T. S. Zaidi, E. L. Fletcher, M. J. Preston, and G. B. Pier. 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62:3485-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig, S. M., T. S. Zaidi, and G. B. Pier. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlett, L., S. Masinick, B. Mezger, R. Barrett, M. Kurpakus, and M. Garrett. 1996. Ultrastructural, immunohistological and biochemical characterization of cultured mouse corneal epithelial cells. Ophthalmic Res. 28:50-56. [DOI] [PubMed] [Google Scholar]
- 17.Hazlett, L. D. 2002. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 21:383-390. [DOI] [PubMed] [Google Scholar]
- 18.Holmskov, U., J. Mollenhauer, J. Madsen, L. Vitved, J. Gronlund, I. Tornoe, A. Kliem, K. B. Reid, A. Poustka, and K. Skjodt. 1999. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc. Natl. Acad. Sci. USA 96:10794-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmskov, U., S. Thiel, and J. C. Jensenius. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547-578. [DOI] [PubMed] [Google Scholar]
- 20.Jumblatt, M. M., and A. H. Neufeld. 1983. Beta-adrenergic and serotonergic responsiveness of rabbit corneal epithelial cells in culture. Investig. Ophthalmol. Vis. Sci. 24:1139-1143. [PubMed] [Google Scholar]
- 21.Keisari, Y., H. Wang, A. Mesika, R. Matatov, L. Nissimov, E. Crouch, and I. Ofek. 2001. Surfactant protein D-coated Klebsiella pneumoniae stimulates cytokine production in mononuclear phagocytes. J. Leukoc. Biol. 70:135-141. [PubMed] [Google Scholar]
- 22.Kuan, S. F., K. Rust, and E. Crouch. 1992. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J. Clin. Investig. 90:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leth-Larsen, R., C. Floridon, O. Nielsen, and U. Holmskov. 2004. Surfactant protein D in the female genital tract. Mol. Hum. Reprod. 10:149-154. [DOI] [PubMed] [Google Scholar]
- 24.LeVine, A. M., J. Elliott, J. A. Whitsett, A. Srikiatkhachorn, E. Crouch, N. DeSilva, and T. Korfhagen. 2004. Surfactant protein-D enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 31:193-199. [DOI] [PubMed] [Google Scholar]
- 25.LeVine, A. M., J. A. Whitsett, J. A. Gwozdz, T. R. Richardson, J. H. Fisher, M. S. Burhans, and T. R. Korfhagen. 2000. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165:3934-3940. [DOI] [PubMed] [Google Scholar]
- 26.Lim, B. L., J. Y. Wang, U. Holmskov, H. J. Hoppe, and K. B. Reid. 1994. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem. Biophys. Res. Commun. 202:1674-1680. [DOI] [PubMed] [Google Scholar]
- 27.Masinick, S. A., C. P. Montgomery, P. C. Montgomery, and L. D. Hazlett. 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Investig. Ophthalmol. Vis. Sci. 38:910-918. [PubMed] [Google Scholar]
- 28.McNamara, N. A., R. Van, O. S. Tuchin, and S. M. Fleiszig. 1999. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 69:483-490. [DOI] [PubMed] [Google Scholar]
- 29.Ofek, I., A. Mesika, M. Kalina, Y. Keisari, R. Podschun, H. Sahly, D. Chang, D. McGregor, and E. Crouch. 2001. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 69:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postle, A. D., A. Mander, K. B. Reid, J. Y. Wang, S. M. Wright, M. Moustaki, and J. O. Warner. 1999. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 20:90-98. [DOI] [PubMed] [Google Scholar]
- 31.Poulain, F. R., J. Akiyama, L. Allen, C. Brown, R. Chang, J. Goerke, L. Dobbs, and S. Hawgood. 1999. Ultrastructure of phospholipid mixtures reconstituted with surfactant proteins B and D. Am. J. Respir. Cell Mol. Biol. 20:1049-1058. [DOI] [PubMed] [Google Scholar]
- 32.Restrepo, C. I., Q. Dong, J. Savov, W. I. Mariencheck, and J. R. Wright. 1999. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 21:576-585. [DOI] [PubMed] [Google Scholar]
- 33.Roos, A., L. H. Bouwman, D. J. van Gijlswijk-Janssen, M. C. Faber-Krol, G. L. Stahl, and M. R. Daha. 2001. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 167:2861-2868. [DOI] [PubMed] [Google Scholar]
- 34.Sack, R. A., B. I. Bogart, A. Beaton, S. Sathe, and G. Lew. 1997. Diurnal variations in tear glycoproteins: evidence for an epithelial origin for the major non-reducible > or = 450 kDa sialoglycoprotein(s). Curr. Eye Res. 16:577-588. [DOI] [PubMed] [Google Scholar]
- 35.Sahly, H., I. Ofek, R. Podschun, H. Brade, Y. He, U. Ullmann, and E. Crouch. 2002. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigens. J. Immunol. 169:3267-3274. [DOI] [PubMed] [Google Scholar]
- 36.Schulz, B. L., D. Oxley, N. H. Packer, and N. G. Karlsson. 2002. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochem. J. 366:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahlman, M. T., M. E. Gray, W. M. Hull, and J. A. Whitsett. 2002. Immunolocalization of surfactant protein-D (SP-D) in human fetal, newborn, and adult tissues. J. Histochem. Cytochem. 50:651-660. [DOI] [PubMed] [Google Scholar]
- 38.von Bredow, C., A. Wiesener, and M. Griese. 2003. Proteolysis of surfactant protein D by cystic fibrosis relevant proteases. Lung 181:79-88. [DOI] [PubMed] [Google Scholar]
- 39.Wu, H., A. Kuzmenko, S. Wan, L. Schaffer, A. Weiss, J. H. Fisher, K. S. Kim, and F. X. McCormack. 2003. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 111:1589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong, S. J., Z. Vuk-Pavlovic, J. E. Standing, E. C. Crouch, and A. H. Limper. 2003. Surfactant protein D-mediated aggregation of Pneumocystis carinii impairs phagocytosis by alveolar macrophages. Infect. Immun. 71:1662-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaidi, T. S., S. M. Fleiszig, M. J. Preston, J. B. Goldberg, and G. B. Pier. 1996. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci. 37:976-986. [PubMed] [Google Scholar]